Abstract
In an animal models, carbon tetrachloride (CCl4) is a carcinogenic agent that causes liver fibrosis. The current study aims to investigate whether induction in liver-fibrosis by CCl4 in the mouse model could promote the initiation of fibrosis in lymph node and spleen due to sustained increase of inflammatory signals and also aimed to clarify the protective therapeutic effects of propolis. The male mice (BALB/c) were categorized into three experimental sets and each group involved 15 mice. Control group falls into first group; group-II and group-III were injected with CCl4 to induce liver-fibrosis and oral supplementation with propolis was provided in group-III for 4-weeks. A major improvement with hepatic collagen and α-smooth muscle actin (α-SMA) production was aligned with the activation of liver fibrosis from CCl4. Mice treated with CCl4 exhibited collagen deposition towards liver sections, pathological alterations in spleen and lymph node architectures, and a significantly increase the circulation of both T&B cells in secondary lymphoid organs. Mechanically, the secondary lymphoid organs treated with CCl4 in mice exposed a positive growth in α-SMA and collagen expression, increased in proinflammatory cytokine levels and a significant increase in TGF-β, NO and ROS levels. A manifest intensification in the expression of Nrf2, COX-2, and eNOS and upregulation of ASK1 and P38 phosphorylation. Interestingly, addition of propolis-treated CCl4 mice, substantially suppressed deposition of liver collagen, repealed inflammatory signals and resorted CCl4-mediated alterations in signaling cascades, thereby repairing the architectures of the secondary lymphoid organs. Our findings revealed benefits of propolis against fibrotic complications and enhancing secondary lymphoid organ architecture.
Keywords: CCl4, Inflammation, Liver fibrosis, Oxidative stress, Propolis, Secondary lymphoid organs
1. Introduction
The immune system defines the molecules, cells, and tissues involved in host defenses that are crucial in defending animals from external non-self-invaders. This vital system comprises lymphoid organs, barriers, leukocytes, and proteins, such as antibodies and complement components (Blach-Olszewska and Leszek, 2007). The liver controls immune homeostasis by couple of mechanisms. Initially, it plays a key role in immune response, protecting by its dual blood supply against blood-borne pathogens, ultimately eliminating the pervasive transmission of nutritional antigens and microbes from the gut (Albillos et al., 2014). Secondly, a proliferation of simple compounds essential for an adequate immune response leads to homeostasis of the immune response (Racanelli and Rehermann, 2006). The liver executes its antimicrobial-monitoring role across various coordinated inhabitants by antigen-presenting cells (APCs) with lymphocytes that specifically screen for general and gut-derived pathogen. The APCs involves both Kupffer and sinusoidal endothelial cells from liver and dendritic cells (Gregory et al., 2002). Furthermore, liver encompasses T and B-cells of lymphocytes via the inhabitant and transiting population by the adaptive immune response of parenchyma and portal tracts. However, liver is abundant in NK-cells and unusual lymphocytes that are involved in in the liver’s innate immune responses (Schildberg et al., 2008). Bone-marrow, lymph-nodes, appendix, spleen and thymus are some of the immune system organs (Blach-Olszewska and Leszek, 2007). The spleen is the major organs of secondary-lymphoid gland which has a wide range of immunological activities, including hematopoiesis roles and consent of RBCs. Spleens physical structure enables the filtration of pathogens and abnormal cells from the blood and promotes low-probability interactions between APCs and lymphocytes. Spleen-specific APCs control the response of T and B cells to these blood antigen targets (Lewis et al., 2019). Lymph nodes are controlled by lymphoid organs that contain lymphocytes within a fine reticular stroma. Lymph nodes act as tissue filters and are sites of lymphocyte origin and development for normal physiological functions (Elmore and Bouknight, 2017).
Carbon tetrachloride (CCl4) is a poly-chlorinated hydro-carbon and an ozone-depleting substance which is used as a liquid-solvent for decades as an intermediate material. CCl4 exposure damages many organs. While in most animal species the primary-organ for CCl4-induced liver toxicity, evidence indicates the affect of immune system (Guo et al., 2000). Propolis is a natural adhesive agent produced from plants by honeybees. Closing splits in bees is used and preventing diseases for the bee population. Propolis has a strong tradition in natural medicine and possesses antimicrobial, antioxidant, cellular-strengthening, immunomodulatory, and antitumor activity (Badr et al., 2019). The current study aims to explore the defensive effect of propolis against CCl4-mediated immunomodulation in a mouse model.
2. Materials and methodologies
2.1. Propolis preparation
Propolis honey was purchased since Etman Hives, Shabshir Al Hissah, Al Gharbiyah, Egypt and prepared in our laboratory (Badr et al., 2019). Abundant evidence in our lab was obtained through various animal-studies, showed the regular dosage of 50–250 mg/kg of ethanol-soluble propolis derivatives did not cause noxious-effects and appropriate conc. of 100 mg/g of body-weight in the ethanol-soluble bee propolis derivatives to the fibrotic mice in our experiment.
2.2. CCl4
Sigma chemicals has provided the CCl4 to induce chronic liver-fibrosis in the mice. Using the olive oil, CCl4 was liquified in 1:9 ratio to obtain a 10% CCl4 solution that was injected intraperitoneally into mice twice a week for 6 weeks.
2.3. Experimental design and dosages
We bought mostly from the Theodor Bilharz research institute in Egypt, a group of 45 male adult BALB/C mice weighing between 25 and 30 g. The mice were caged around 25 °C under 12 h of each in day and night (Badr et al., 2011). Mice were acclimatized for one week, with access to a pelleted towards diet cum water before being bifurcated in three-categories. Fifteen mice were categorized into each group. Group-I is a control-group of mice; Group-II is treated with CCl4 and Group-III is treated with both CCl4 and orally supplemented with propolis (group III; CCl4 + propolis-treated group). The CCl4 (1µ by 10% of CCl4/g body weight, injected twice a week for six weeks) cause intraperitoneal liver fibrosis in Groups II &III.
The control group mice were vaccinated intraperitoneally with the vehicle only (1 ml of olive oil/kg body-weight, twice a week for about six-weeks). After the induction of liver-fibrosis (using CCl4 for six weeks), mice in Group-III received oral supplementation with 100 μl of 50% ethanol-soluble propolis derivative (100 mg/kg body weight/day) for additional for four weeks, and the mice in groups I and II received 100 µl of 50% ethanol per day (as a vehicle) through oral gavage for additional four weeks.
All research was completed in agreement through internationally accepted principles care of lab-animals (based on US and Europe guidelines). Ethical approval and protocol procedure were obtained from Assiut$ university.
2.4. Histological evaluation
Mice tissues of the liver, spleen, and lymph nodes were fixed with formal alcohol, embedded and desiccated, into five-µm of paraffin-blocks, blemished with hematoxylin and eosin of Sirius-red according to standard protocols.
2.5. Immunohistochemistry
The paraffin units of both spleen and lymph nodes were immobile complete-night in a freshly prepared alcohol solution (Mohany et al., 2012). The dehydrated samples were arranged as paraffin-blocks and sections were blemished for IHC using main antibodies anti-CD3 and anti-CD20 to identify T&B-cells. Tissue samples were washed in xylene, rehydrated in 70% ethanol, submerged in distilled water for 5 mins. Then, they were boiled in citrate-buffer for 2/3rd hour to unmask epitopes. Further stage, the slides were cleaned in water, tracked by phosphate-buffered saline (PBS). The IHC technique were performed as per the Badr et al. (2019) studies.
2.6. Measuring the reactive oxygen species (ROS) levels
The ROS levels in spleen and lymph node tissue-lysates were determined using H2DCFDA, where the oxidation of H2DCFDA to 2ʹ-7ʹdichlorofluorescein (DCF) was used to quantify the levels of H2O2. Briefly, following the cellular absorption of H2DCFDA and its acetomethyl ester, non-specific cellular-esterase’s eliminate the lipophilic groups and produce an electric composite that is trapped within cell. ROS-oxidation of H2DCFDA transforms the molecule into extremely fluorescent DCF. The DCF fluorescence is determined at 498 nm and 522 nm for excitation and emission, respectively (Badr et al., 2013).
2.7. Measuring nitric oxide (NO) levels
The concentration of NO2− in peritoneal exudate was calculated by the Griess reaction as an indication of NO development. Briefly, 100 μl of spleen and lymph node tissue lysates and 100 μl of Griess substance (2%mixture of sulfanilamide in 5% phosphoric acid and 0.2% N-(1-naphthyl) ethylenediamine hydrochloride) were assorted into the 96-well plate of enzyme-linked immunosorbent assay (ELISA).
2.8. ELISA
Proinflammatory cytokines levels such as IL-1β, TGF-β and IL-6 were measured by ELISA in spleen and lymph nodes tissue lysates (Sayed et al., 2010).
2.9. Western blot analysis
The complete tissue-lysates were organized via radioimmunoprecipitation assay-buffered liver, spleen, and lymph node tissues isolated after control mice. The lysates were centrifuged around 15mins at 4 °C for16 kg. Protein assay kit was used in this study and lysates was stored for further western blot analysis. The SDS-PAGE analysis was done based on Khan et al. (2016) studies. The western blot analysis was performed as described previously (Badr et al., 2013, Badr et al., 2012, Badr et al., 2010).
2.10. Statistical analysis
Normality using the Anderson-Darling test and homogenous variances were tested for results. The statistical data were expressed as mean and standard deviation and Anova analysis was performed (Khan et al., 2019). P value is confirmed as < 0.05. Statistical analysis was carried out using GraphPad Prism v5 statistical software (Graph Pad Software, CA, USA).
3. Results
3.1. Treatment of fibrotic mice with propolis repaired the pathological alterations of liver and collagen deposition
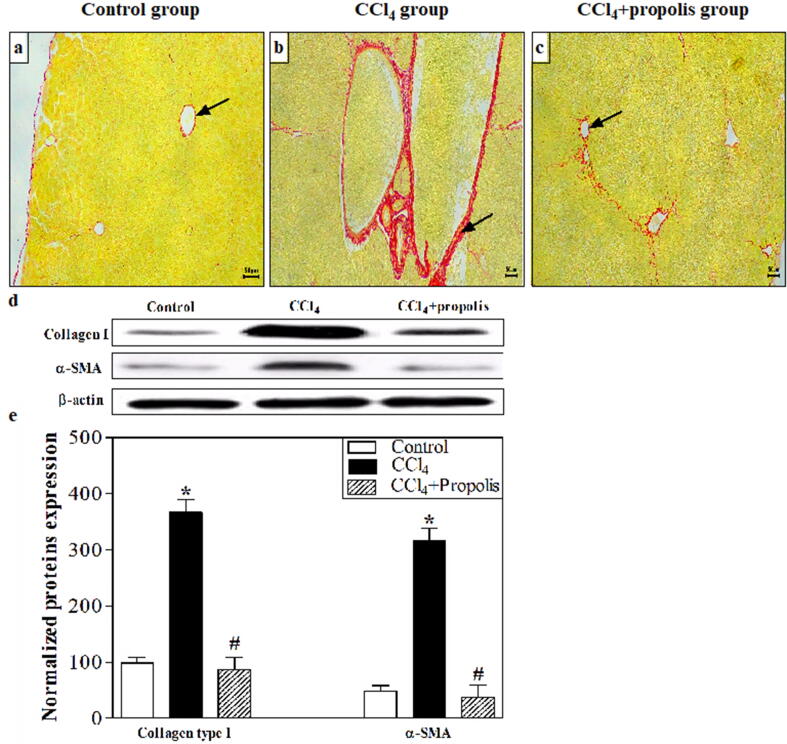
The key cause of collagen deposition in the diseases liver is activated hepatic stellate cells (HSCs). Following CCl4-induced liver fibrosis, collagen deposition in the three groups was monitored by staining liver sections with the collagen stain Sirius red. Only a small amount of collagen was present in the control mice in the portal area and central veins (Fig. 1A). Compared with the control group, abundant collagen fibers surrounded the central vein in the CCl4-preserved assembly (Fig. 1B). Interestingly, a great change in histoarchitecture was detected in CCl4 + propolis-treated groups (Fig. 1C) linked with the CCl4-treated group$. We also used western blotting to assess the expression of α-SMA (an HSC activation predictor), collagen type I, and β-actin (loading control) in liver samples from the three groups (Fig. 1D). Mice treated with CCl4 has demonstrated a substantial growth in expressing type-I collagen and α-SMA in comparison. When CCl4-treated mice were orally supplemented with propolis, there was a significant reduction in the appearance of collagen type-I and α-SMA to almost the same closes as that of the control mice. Fig. 1E shows the accumulated data from five mice in each group. The treated mice thru CCl4 disclosed a substantial growth in collagen type-I and α-SMA expression relative to the control mice (P < 0.05). Fascinatingly, when CCl4-treated via the mice were orally supplemented with propolis, they displayed an important downregulation of collagen type-I and α-SMA expression associated with the CCl4-treated mice (P < 0.05).
Fig. 1.
Propolis treatment mitigated hepatic fibrosis induced by CCl4 in our mouse model. Histological changes in collagen distribution were assessed by staining liver sections with Sirius red according to a standard protocol. (A–C). All images were taken at 100× magnification and are representative of samples from mice in each group. The liver sections of the control group showed normal collagen fibers distribution (A). The CCl4-treated group showed extensive collagen deposition around the central vein (arrow) (B). The collagen fibers in the CCl4 + propolis-treated group were small and delicate, suggesting that the liver had repaired itself. Western blotting analysis of the liver tissue lysates was performed using antibodies against collagen type I, α-SMA, and β-actin (loading control). The protein bands from one representative experiment are shown (D). The expression of collagen type I and α-SMA was normalized to total protein levels of β-actin and expressed as the means ± SEM of the collagen type I- and α-SMA-normalized values. The accumulated data of five mice from each group are shown for the expression of normalized collagen type I and α-SMA from control mice (open bar), CCl4-treated mice (closed black bar), and CCl4 + propolis-treated mice (hatched bar) (E). *P < 0.05 for CCl4-treated mice vs. control mice. #P < 0.05 for CCl4 + propolis-treated mice vs. CCl4-treated mice. +P < 0.05 for CCl4 + propolis-treated mice vs. control mice (ANOVA with Tukey’s post-hoc test).
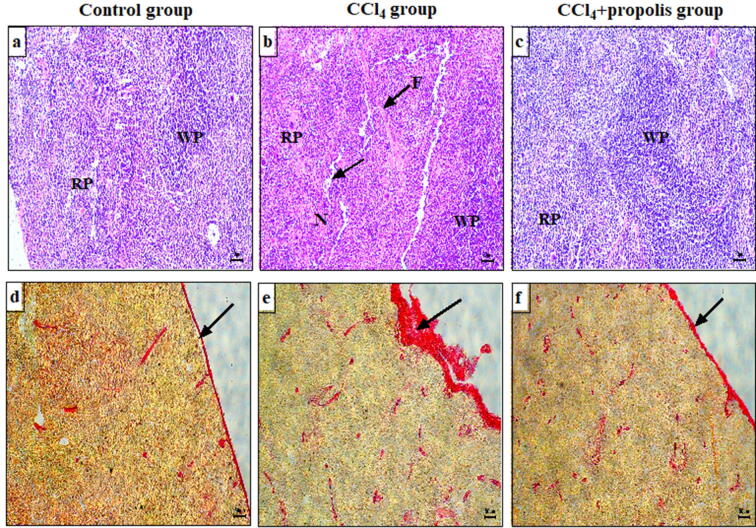
3.2. Treatment of CCl4-induced fibrosis in mice with oral propolis supplementation decreased pathogenic changes in spleen tissue
We used H&E and Sirius red staining to evaluate the histopathological alterations in spleen sections (as a secondary immune organ) from the three mouse groups after inducing fibrosis by CCl4. The spleen sections of the control group showed a normal histological appearance. The white pulp was made up of concentrated lymphocytes and central arteries, and the red pulp contained splenic cords and sinusoids (Fig. 2A). The spleen sections in the CCl4-treated group showed degenerative changes characterized by fibrosis, lymphocytic necrosis, and lymphocyte depletion and marked fibrosis in the red and white pulp (Fig. 2B). The CCl4 + propolis-treated group showed partial restoration of the white and red pulp to typical splenic histoarchitecture similar to that of the control group (Fig. 2C). We used a Sirius red staining method to identify collagen fiber deposition in the spleen sections of the CCl4-treated groups. The collagen fibers in the spleen capsule sections in the control group were normally distributed (Fig. 2D). Collagen deposition and the establishment of fibrosis were observed in the spleen capsule in the CCl4-treated group (Fig. 2E). Interestingly, the CCl4 + propolis-treated group showed less collagen deposition in the spleen capsule, and the structure was preserved, similar to the control group (Fig. 2F).
Fig. 2.
Oral propolis supplementation improved the spleen histoarchitecture in fibrotic mice. Histopathological changes in spleen tissue sections were assessed following staining with H&E and Sirius red using standard protocols. All images were taken at 100× magnification and are representative samples from mice in each group. Spleen sections from the control group, CCl4-treated group, and CCl4 + propolis group stained with H&E (A–C, respectively) and Sirius red (D–F, respectively) for collagen deposition. RP: red pulp; WP: white pulp; F: fibrosis; N: necrosis.
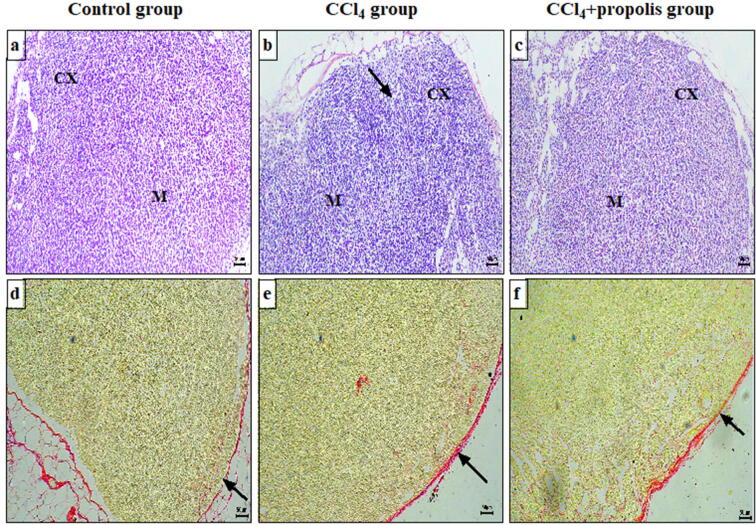
3.3. Propolis supplementation enhanced the toxic effects of CCl4 on lymph node histology
We investigated the effect of propolis on lymph nodes (secondary immune organs). Microscopic examination of lymph node sections stained with H&E from the control group revealed a normal histological appearance of the cortex, divided into the outer cortex and inner cortex (paracortex), and medulla and normal lymphocyte populations in the outer cortex, paracortex, and medulla (Fig. 3A). The lymph node sections from the CCl4-treated group displayed degenerative changes, reflected by necrosis and a decreased lymphocyte population in the cortex and medulla compared with the control group (Fig. 3B). The CCl4 + propolis group displayed a relatively regular outer cortex, paracortex, and medulla, with a decreased lymphocyte population in the cortex and medulla compared with the CCl4-treated group (Fig. 3C). The lymph node sections were also stained with Sirius red for the selective staining of collagen fibers. The lymph node sections of the control group showed marginal collagen fiber content in the capsule (Fig. 3D). Those of the CCl4-treated group showed a relative increase in capsule collagen fibers (Fig. 3E). Interestingly, the CCl4 + propolis group showed less collagen deposition in the lymph node capsules compared with the CCl4-treated group (Fig. 3F).
Fig. 3.
The effect of CCl4 and propolis on lymph node architecture in our fibrotic mouse model. Lymph node sections from the three mouse groups were stained with H&E and Sirius red, which selectively stains collagen fibers red. All images were taken at 100× magnification and are representative of samples from mice in each group. Lymph node sections from the control, CCl4-treated, and CCl4 + propolis-treated groups stained with H&E for the investigation of histopathological alterations (A–C, respectively) and Sirius red for collagen deposition (D–F, respectively). CX: cortex; M: medulla.
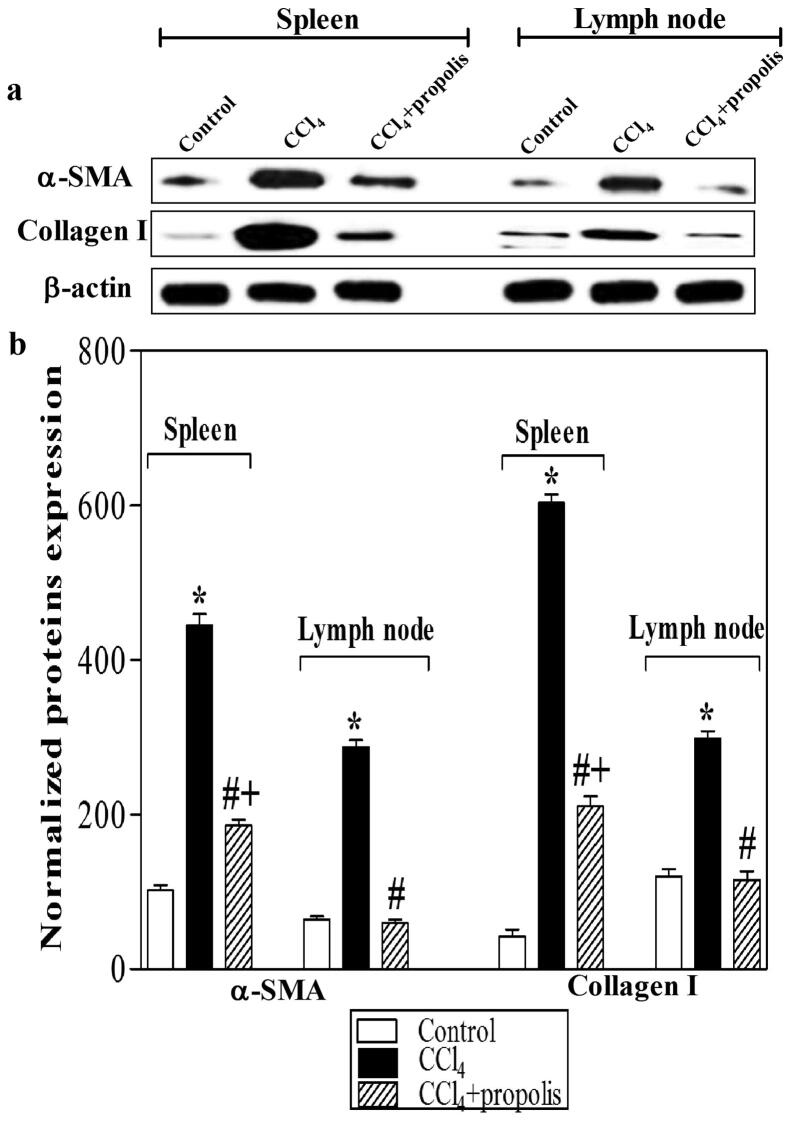
3.4. Propolis reduced myofibroblastic marker expression in secondary lymphoid organs of fibrotic mice
Most antifibrotic therapies prevent the activation, proliferation, or synthetic products of HSCs. Activated HSCs are the main source of collagen deposition during fibrogenesis. We used western blot analysis of tissue lysates of the secondary lymphoid organs (spleen and lymph nodes) to monitor the influence of propolis on the expression of α-SMA and collagen type I (Fig. 4A). The results indicated that CCl4 mediated the overexpression of α-SMA and collagen type I in the spleen and lymph nodes. The CCl4 + propolis-treated group showed a significant reduction in α-SMA and Type I collagen expression. The CCl4-treated group displayed significant overexpression of α-SMA and collagen type I compared with the control group. Interestingly, the CCl4 + propolis-treated group demonstrated a significant decrease in the expression of collagen type I and α-SMA relative to the CCl4- treated group (Fig. 4B).
Fig. 4.
Propolis reduced myofibroblastic marker expression in secondary immune organs. Lysates of spleen and lymph node tissues were subjected to western blotting analysis using antibodies against α-SMA, collagen type I, and β-actin. The protein bands from one representative experiment are shown for the expression of α-SMA, collagen type I, and β-actin (loading control) (A). α-SMA and collagen type I expression was normalized to the total protein levels of β-actin. The results are expressed as the means ± SEM of the normalized values of α-SMA and collagen type I. The accumulated data of five mice from each group are shown in (B) for the expression of normalized α-SMA and collagen type I from the control mice (open bar), CCl4-treated mice (closed black bar), and CCl4 + propolis-treated mice (hatched bar). *P < 0.05 for CCl4-treated mice vs. control mice. #P < 0.05 for CCl4 + propolis-treated mice vs. CCl4-treated mice (ANOVA with Tukey’s post-hoc test).
3.5. Propolis supplementation enhanced the altered distribution of CCl4-mediated T and B cells in spleen and lymph node tissues
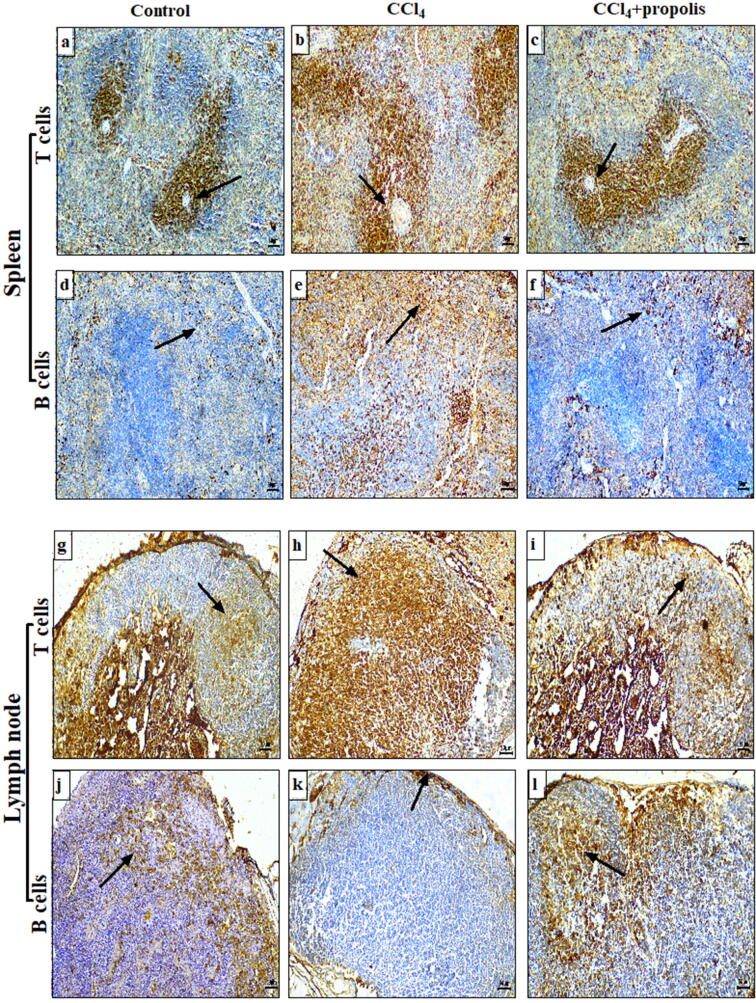
We examined the effect of CCl4 on the distribution of T and B cells in the spleen and lymph nodes (secondary lymphoid organs) of the three mouse groups using anti-CD3 and anti-CD20 antibodies to monitor T and B cells, respectively. The spleen sections from the control mice showed the moderate distribution of T cells between the red pulp and the white pulp periarterial lymphatic sheaths (Fig. 5A), while the CCl4-treated mice showed a marked increase in the number of T cells in both regions (Fig. 5B). The T cell distribution in the red pulp and the periarterial lymph sheaths of the white pulp in the CCl4 + propolis-treated mice was significantly restored to close to that observed in the control mice (Fig. 5C). The B cells tended to be clustered in the red pulp and marginally scattered throughout the white pulp of the control mice spleen sections (Fig. 5D), whereas there was increased clustering of B cells in the white pulp and widespread dispersion of these cells throughout the red pulp in the CCl4-treated group (Fig. 5E). The CCl4 + propolis-treated mice showed the restored distribution of B cells in the white and red pulp that was close to that observed in the control mice (Fig. 5F). Moderate amounts of T cells were observed in the lymph node tissue from the control group, with the T cells dispersed in the outer cortex lymphoid follicles with normal distribution in the inner cortex (paracortical zone) (Fig. 5G). There was a significant increase in the distribution of T cells in the outer cortex lymphoid follicles and in paracortical lymph node region in the CCl4-treated group (Fig. 5H) compared with the control mice. The lymph node sections of the CCl4 + propolis-treated group showed an apparent restoration of T cell distribution in the outer cortex lymphoid follicles and the paracortical zone, with patterns identical to those observed in the control group (Fig. 5I). Furthermore, the B cells in the control lymph node sections were normally distributed in the outer cortex lymphoid follicles (Fig. 5J). The lymph node sections prepared from the CCl4-treated group showed a marked reduction in the B cell distribution in the outer cortex lymphoid follicles (Fig. 5K). Interestingly, the B cell distribution in the lymph nodes of the CCl4 + propolis-treated group was relatively normal in the outer cortex lymphoid follicles (Fig. 5L).
Fig. 5.
Effects of CCl4 and propolis on T and B cell distribution in the spleen and lymph nodes of mice with CCl4-induced hepatic fibrosis. Spleen sections from the control, CCl4-treated, and CCl4 + propolis-treated groups were immunohistochemically stained with anti-CD3 (A–C, respectively) and anti-CD20 (D–F, respectively) and then detected by immunohistochemical analysis (Immunoperoxidase × 100). Lymph node sections from the control, CCl4-treated, and CCl4 + propolis-treated groups were immunohistochemically stained with anti-CD3 (G–I, respectively) and anti-CD20 (J–L, respectively) and then detected by immunohistochemical analysis (Immunoperoxidase × 100).
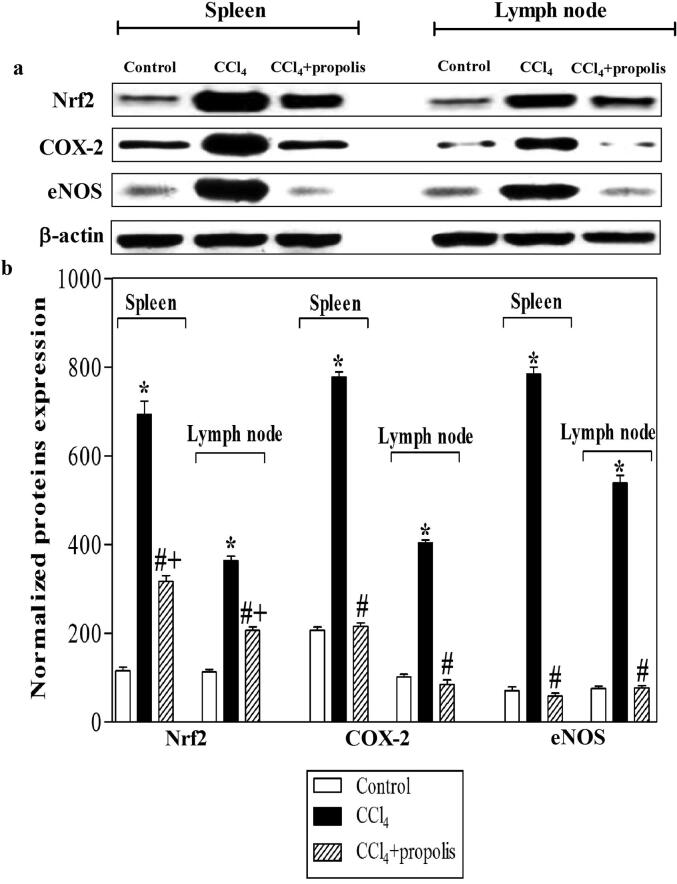
3.6. Oral propolis supplementation recovered Nrf2, COX-2, and eNOS expression in fibrotic mice
Nrf2, COX-2, and eNOS are the major regulators of the cellular protection mechanism against oxidative stress. Thus, we used western blot analysis of spleen and lymph node lysates to investigate the impact of propolis supplementation on the expression of Nrf2, COX-2, and eNOS in mice with CCl4-induced liver fibrosis. (Fig. 6A). The CCl4-treated group demonstrated increased expression of Nrf2, COX-2, and eNOS relative to that of the control group. Interestingly, the CCl4-treated mice that received propolis supplementation showed decreased levels of Nrf2, COX-2, and eNOS expression (Fig. 6B). The CCl4-treated group showed a marked increase in Nrf2, COX-2, and eNOS expression compared with the control group. The CCl4 + propolis-treated group showed significantly decreased expression levels of Nrf2, COX-2, and eNOS compared with the CCl4-treated group.
Fig. 6.
Impact of propolis treatment on the expression of Nrf2, COX-2, and eNOS in secondary immune organs of mice with CCl4-induced hepatic fibrosis. The spleen and lymph node lysates of the three mouse groups were prepared for western blotting using antibodies against Nrf2, COX-2, eNOS, and β-actin. The protein bands from one representative experiment are shown for the expression of Nrf2, COX-2, eNOS, and β-actin (A). The expression of Nrf2, COX-2, and eNOS was normalized to the total protein levels of β-actin. The accumulated data of five mice from the control group (open bars), CCl4-treated (closed black bars), and CCl4 + propolis-treated (hatched bars) groups, respectively, are expressed as the means ± SEM of the normalized value of each parameter (B). *P < 0.05 for CCl4-treated mice vs. control mice. #P < 0.05 for CCl4 + propolis-treated mice vs. CCl4-treated mice. +P < 0.05 for CCl4 + propolis-treated mice vs. control mice (ANOVA with Tukey’s post-hoc test).
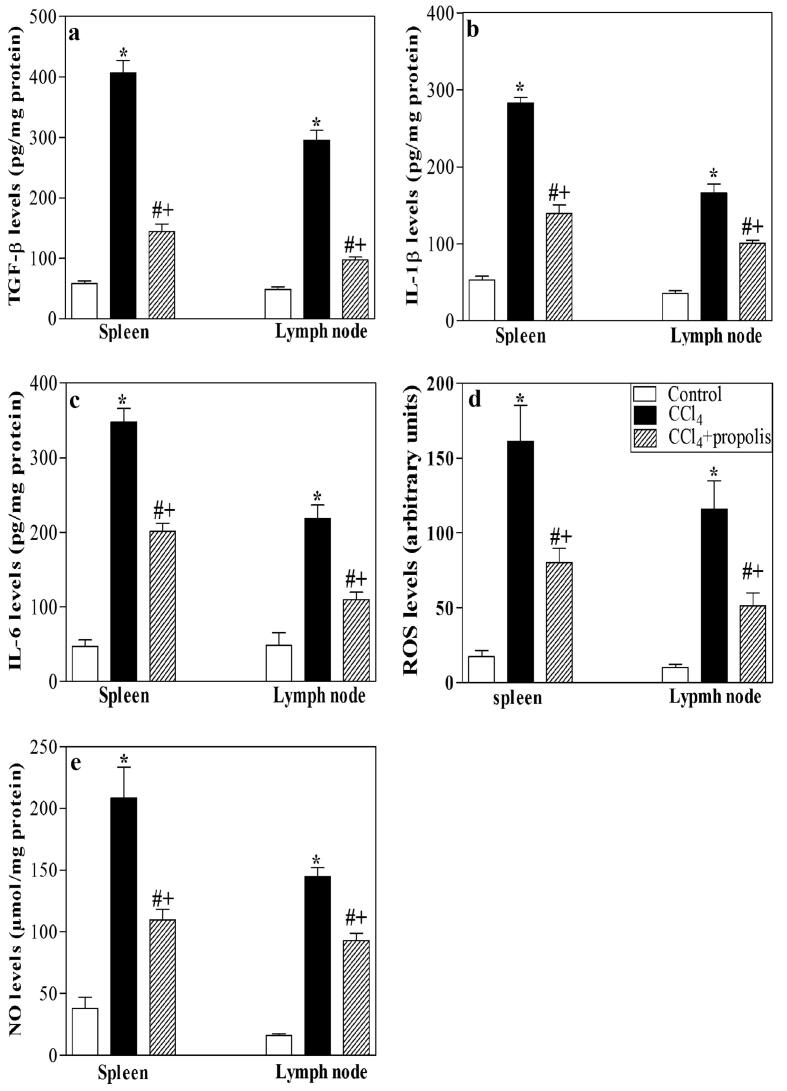
3.7. Treatment of fibrotic mice with propolis restored the levels of TGF-β, proinflammatory cytokines, ROS, and NO in lymphoid tissues
Induction of liver fibrosis by CCl4 resulted in the inflammation of secondary lymphoid organs and a chronic oxidative stress condition. CCl4 is a commonly used hepatotoxin in animal models of liver fibrosis. It is activated by CYP450 in the liver to form trichloromethyl and trichloromethyl peroxy radicals. CCl4 may also induce Kupffer cells to generate ROS and then destroy liver cells, resulting in an aberrant elevation of TGF-β, proinflammatory cytokines (IL-1β and IL-6), ROS, and NO levels. Liver macrophages, including Kupffer cells, generate TGF-β to activate HSCs, which are important regulators of liver fibrosis (Fararh et al., 2016). Therefore, we used ELISA to examine the effect of propolis on TGF-β levels after CCl4-induced liver fibrogenesis in lysates of the spleen and lymph nodes. The TGF-β level in the CCl4-treated group was significantly increased relative to the control group (Fig. 7A). Supplementing the CCl4-treated group with propolis significantly reduced the TGF-β levels. Additionally, ELISA was implemented in spleen and lymph node lysates from the three animal groups (Fig. 7B, C). Amassed data from five mice in each group showed that CCl4 induced a substantial growth in the levels of these proinflammatory cytokines relative to the control group. The supplementation of CCl4-treated mice with propolis considerably decreased the levels of proinflammatory cytokines. We also used ELISA to determine the ROS levels in spleen and lymph node lysates from the three mouse groups (Fig. 7D). The ROS levels in the liver tissue lysates from CCl4-treated mice were significantly increased compared with the control mice. Most interestingly, the liver tissue lysates from the CCl4-treated mice that received propolis supplementation showed significant restoration of the chronic oxidative stress condition alterations induced by CCl4 by decreasing the ROS levels (Fig. 7E) found in the control group.
Fig. 7.
Influence of propolis on the levels of TGF-β, IL-1β, IL-6, ROS, and NO in secondary immune organs of fibrotic mice. The levels of TGF-β (A), IL-1β (B), IL-6 (C), ROS (D), and NO (E) were measured in the spleen and lymph node lysates of the control (open bars), CCl4-treated (closed black bars), and CCl4 + propolis-treated (hatched bars) groups. The accumulated data from five mice of each group are expressed as the means ± SEM and were analyzed by ANOVA, followed by Tukey’s post-hoc test. Differences were considered statistically significant at *P < 0.05 for CCl4-treated mice vs. control mice, #P < 0.05 for CCl4 + propolis-treated mice vs. CCl4-treated mice, and +P < 0.05 for CCl4 + propolis-treated mice vs. control mice.
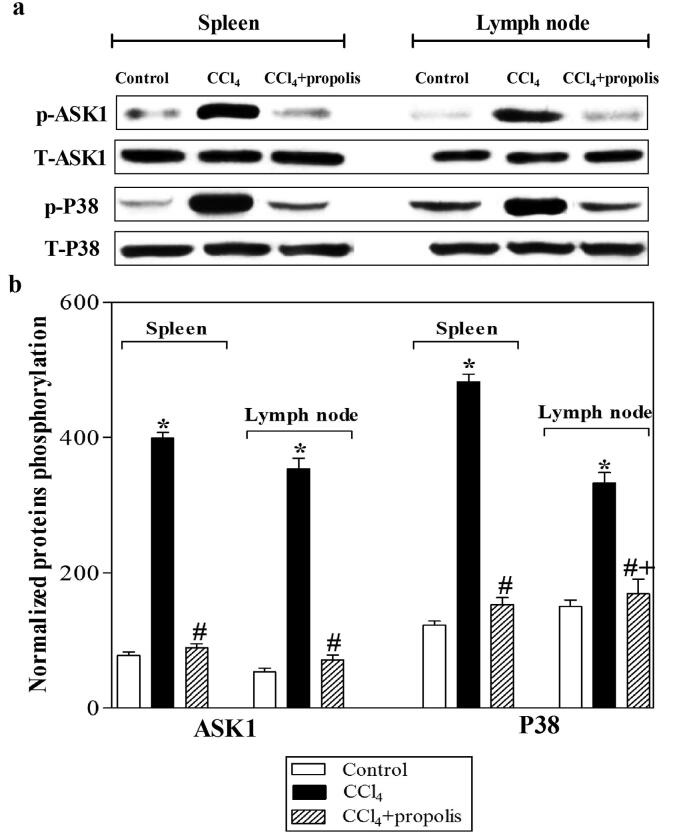
3.8. Propolis inhibits ASK1 and P38 phosphorylation induced by CCl4 treatment
Liver fibrosis is a specific disorder resulting from inflammation. HSCs are the focal point of the fibrogenic response, which results in the expression of different inflammatory factors, including COX-2, IL-1, IL-6, tumor necrosis factor-α (TNF-α), and TGF-β. In turn, these factors activate HSCs in a process based on p38 mitogen-activated protein kinases (MAPKs). ASK1 is one of several mitogen-activated protein kinase kinase kinases (MAP3Ks) that are activated in response to proinflammatory stimuli, ROS, and other cellular stresses. ASK1 then activates the c-Jun N-terminal kinase (JNK) and p38 MAPK pathway. Activation of these pathways induces cellular responses such as apoptosis, differentiation, cell survival, and production of inflammatory cytokines. ASK1-deficient mice have reduced CCl4-induced hepatic fibrosis, and TGF-β expression, which is responsible for hepatic fibrosis. The analysis of ASK1-deficient mice has also demonstrated the requirement of the ASK1-p38 pathway for immune responses. Liver fibrosis may be caused by inflammatory and stress factors. Thus, we used western blot analysis to investigate the impact of propolis supplementation on ASK1 and P38 phosphorylation following CCl4 induction of liver fibrosis. We used antibodies that recognized phosphorylated ASK1 (p-ASK1), total ASK1 (T-ASK1), phosphorylated P38 (p-P38), and total P38 (T-P38) (Fig. 8A). The CCl4-treated group showed a significant increase in p-ASK1 and p-P38 compared with the control group. Interestingly, the supplementation of propolis to CCl4-treated mice decreased p-ASK1 and p-P38. The levels of p-ASK1 and p-P38 were normalized to the levels of T-ASK1 and T-P38, respectively. (Fig. 8B). The CCl4-treated group exhibited a significant increase in p-ASK1 and p-P38 relative to the control group. When propolis was administered to the CCl4-treated group, the levels of p-ASK1 and p-P38 in the CCl4 + propolis group were significantly reduced compared with the CCl4-treated group.
Fig. 8.
Propolis enhances the phosphorylation of ASK1 and P38 in spleen and lymph node tissues of mice with CCl4-induced hepatic fibrosis. Lysates of spleen and lymph node tissue samples collected from mice in each group were subjected to western blotting using antibodies recognizing p-ASK1 and p-P38 and the total relevant proteins T-ASK1 and T-P38. The protein bands from one representative experiment are shown (A). The phosphorylation of ASK1 and P38 was normalized to the total relevant protein levels, and accumulated data of five mice from the control (open bars), CCl4-treated (closed black bars), and CCl4 + propolis-treated (hatched bars) groups are expressed as the means ± SEM of the normalized value of each parameter (B). *P < 0.05 for CCl4-treated mice vs. control mice. #P < 0.05 for CCl4 + propolis-treated mice vs. CCl4-treated mice. +P < 0.05 for CCl4 + propolis-treated mice vs. control mice (ANOVA with Tukey’s post-hoc test).
4. Discussion
Liver is one of the largest organs in the human body which performs many vital functions, such as eliminating damaged red blood cells from the blood in conjunction with the spleen, processing bile, synthesizing coagulation factors, and storing vitamins, minerals, proteins, fats, and dietary glucose (Fararh et al., 2016). Liver fibrosis is a natural process which ensues in response to hepatic injuries such as toxicity or drug exposure. Studies of liver fibrosis over the past 2 decades have improved knowledge of the condition’s molecular pathogenesis, which enabled the identification of potentially effective drugs to treat liver fibrosis. CCl4 is widely used as hepatotoxin in hepatopathy studies (Geetha et al., 2008) and causes liver cirrhosis in animals that is similar to human liver cirrhosis (Lee et al., 2007). This study investigated the immunotherapy properties of propolis against CCl4-mediated immune toxicity. The current study results confirmed CCl4 as persuaded histological alterations in liver architecture, characterized by the abundant deposition of collagen fibers surrounding the central vein, which was in agreement with Bataller and Brenner (Bataller and Brenner, 2005), who reported that the production of extracellular matrix by the liver increased approximately 6-fold in the advanced stages of fibrosis. Hepatic injury results in HSC propagation and revolution into a myofibroblast-like phenotype in a process called activation. The activated HSCs express α-SMA and procollagen-I and are the main source of collagen I, III, and IV and other fibrosis-deposited matrix proteins (Friedman, 1993). Accordingly, most antifibrotic therapies are designed to prevent HSC activation, proliferation, or synthesis of products (Nguyen-Lefebvre et al., 2018). Our results disclosed lowered chronic inflammation, with a decrease in type I & III-collagen deposition (Montes and Junqueira, 1991), and decreased α-SMA expression in hepatic tissue in the CCl4-treated group supplemented with propolis. Similarly, CCl4 mediated the overexpression of α-SMA & type-I collagen in spleen and lymph nodes, while supplementing the CCl4-treated groups with propolis markedly downregulated α-SMA and collagen type-I appearance. Likewise, CCl4 treatment encouraged collagen deposition and spleen fibrosis, while propolis supplementation restored normal splenic histoarchitecture (Abraham and Wilfred, 1999). Lipid peroxidation can arise in tissues apart from liver, especially in kidney, testicles, spleen, and also in lungs (Casini and Benedetti, 1978). It was documented that CCl4 radicals bound to cellular lipids in extrahepatic tissues of rats, albeit to a lesser extent than in the liver. Additionally, CCl4 toxicity had been shown to alter the morphology of splenic macrophages by modulating macrophage differentiation (Chakraborty and Sengupta, 2012). Moreover, it has been shown that apitherapy had a beneficial effect on the histoarchitecture of the liver and spleen, in line with our findings (Andritoiu et al., 2014). In this context, it has been observed that the appearance of the spleen mostly returns to normal after propolis treatment (AlGabbani et al., 2017). The lymph node sections of the CCl4-treated group in our study showed degenerative changes in the cortex and medulla characterized by necrosis and depletion of the lymphocyte population. Our results correlated with Doi et al. (1991) that might have been a secondary effect of induced hepatic injury (Faroon, 2005). Our study results showed that propolis supplementation restored the histoarchitecture and induced an increase in in the lymphocyte populations in the splenic cortex and medulla. These results were arrangement with Sforcin et al. (2005), who conveyed flavonoids possessed anti-inflammatory activity and confirmed that the tissue and regenerative effect improved damage to immune system organs (Elshama et al., 2016). Our study results exhibited a growth clustering of B cells in white pulp and a large dispersion of B-cells in red pulp of spleen of mice treated with CCl4. Similarly, there was a marked increase in the number of T and B cells in the outer cortex lymphoid follicles and the paracortical region. Such results were in line with Jirova et al. (1996), who found that T-cell based areas showed significant activation of lymphoid tissue. The morphological examination of spleen and lymph nodes after CCl4 treatment showed stimulated B cell areas. Interestingly, propolis therapy recovered the circulation of T and B cells in the lymphoid follicles and white and red splenic pulps. Hayriye, 2018 reported that caffeic acid phenethyl ester, a propolis component, possessed immune-modulatory properties.
Though the accurate role of propolis action in immune-cells remain simple, flavonoids are capable of stimulating B and T-cells (Wolska et al., 2019).
Nrf2 mediates the activity of cleansing and antioxidant enzymes in CCl4-induced liver fibrosis by regulating stage II. Thus, Nrf2 activation is crucial in treating hepatic fibrosis (Zou et al., 2017). COX-2 was recently identified as an immediate early gene linked to inflammation, cell formation, differentiation, apoptosis prevention, and tumorigenesis. Evidence suggests that NO produced by eNOS improves COX-2 activity (Rahman et al., 2001). Our study results showed a significant increase in the expression of Nrf2, COX-2, and eNOS in the CCl4-treated group, which was in accordance with Domitrović et al. (2012), who claimed that the presence of COX-2 and eNOS in the nucleus indicated their involvement in the control of certain nuclear functions. Our analysis was also in contract with Yang et al. (2014), who showed the upregulation of Keap1 and Nrf2 mRNA and CCl4-induced liver fibrosis in rats. However, our results were against the results by Said et al. (2018), who reported that CCl4 significantly downregulated Nrf2 expression. Our study results showed that the supplementation of CCl4-treated animals with propolis decreased COX-2 and eNOS expression. Furthermore, propolis administration also contributes to decreasing the amount of nitric oxide synthetase. The CCl4-treated animals who received propolis supplementation in our study showed decreased Nrf2 expression, which was in disagreement with Mujica et al. (2017), who reported that propolis could activate Nrf2 and improve the antioxidant ability of the cells.
TGF-β signaling plays a major role in maintaining liver homeostasis, terminal differentiation, and hepatocyte apoptosis. TGF-β is regulated under conditions of liver damage and controls parenchymal cells, inflammatory cells, and HSCs (Karkampouna et al., 2016). TGF-β1 plays a crucial role in regulating immune homeostasis and inflammation within the immune system (Tamayo Revuelta et al., 2018). Our study results showed a significant increase in the level of TGF-β in the CCl4-treated group. Supplementing the CCl4-treated group with propolis significantly reduced the level of TGF-β in spleen and lymph node tissue lysates. These results were in accordance with Said et al. (2018), who reported that treatment with pinocembrin (a flavanone abundant in honey and propolis) mitigated the increased levels of the prominent CCl4-induced pro-fibrogenic cytokine TGF-β.
Cytokines are polypeptides with a broad range of regulatory, inflammatory, metabolic, hematopoietic, and immunological properties. The liver represents a major site for the synthesis and clearance of several cytokines (Rey et al., 2018). IL-1 is a proinflammatory cytokine that plays a crucial role in chronic and acute inflammation. IL-1 refers to two different cytokines, of which one is mainly secreted (IL-1β) and the other intracellular (IL-1α), that bind to the same receptor (Meier et al., 2019). Improving the clearance and signaling of hepatic IL-6 partially improves the liver function of patients with liver cirrhosis (Prystupa et al., 2015). The impaired hepatic elimination of IL-6 may explain the elevated levels of systemic IL-6 in patients with liver cirrhosis. The toxicity of CCl4 is mediated by many signaling pathways, such as TGF-α and NO, leading to inflammation and apoptosis, while TGF-α and -β induce fibrosis (Weber et al., 2003). Our results showed that the NO level in the CCl4-treated group was significantly increased. Oral supplementation of the CCl4-treated mice with propolis improved the NO level to a normal value. The antioxidant and anti-inflammatory effects of propolis are due to its high content of polyphenols, flavonoids, and phenolic acids, which serve as reducing agents, inhibitors of lipid peroxidation, and free radical scavengers (Nna et al., 2018). Our results revealed that the CCl4-treated group exhibited a growth in phosphorylation of ASK1 and P38, while supplementation of CCl4-treated animals with propolis decreased.
5. Conclusion
Distinctive immune-cells are plentiful in the liver which is an important lymphoid organ. Liver-fibrosis is a chief challenge of global health and the level of fibrosis characterizes the foremost risk-factors for the progression of other diseases such as hepatocellular carcinoma (HCC). Anti-fibrotic therapies to inhibit deterioration of liver failure and HCC progression have also been tremendously unmet. Propolis exhibited anti-fibrotic therapeutic effects against liver fibrosis. Our results conclude that induction of liver fibrosis by CCl4 mediated inflammatory and fibrotic signals which provoke disruption of physiological architectures of the spleen and lymph node. Supplementation of CCl4-treated mice with-propolis significantly lowered the free-radical-levels and proinflammatory cytokines; the expression of α-SMA, collagen, Nrf2, COX-2, and eNOS; and the phosphorylation of ASK1 and P38. Hence, propolis abolished the pro-fibrogenic signals mediated by CCl4 and inhibited the life-threatening complications of liver fibrosis.
CRediT authorship contribution statement
Eman Abdo Sayed: Methodology, Formal analysis, Investigation. Gamal Badr: Supervision, Validation, Visualization, writing original draft. Khadiga Abdel-Hameed Hassan: Validation, Supervision, Investigation. Hanan Waly: Methodology, Formal analysis, writing. Betul Ozdemir: Formal analysis, Data curation. Mohamed Mahmoud: Data curation, Writing-reviewing and editing, Funding. Salman Alamery: Project administration, Writing-reviewing and editing, Funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Laboratory of Immunology, Faculty of Science, Assiut University, for supporting this research. Additionally, the authors would also like to extend their gratitude to the King Saud University (Riyadh, Saudi Arabia) for the funding of this research through Researchers Supporting Project number RSP-2020/241.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abraham P., Wilfred G. Oxidative damage to the lipids and proteins pf the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin. Chim. Acta. 1999;289:177–179. doi: 10.1016/s0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- AlGabbani Q., Mansour L., Elnakady Y.A., Al-Quraishy S., Alomar S., Al-Shaebi E.M., Abdel-Baki A.A.S. In vivo assessment of the antimalarial and spleen-protective activities of the Saudi propolis methanolic extract. Parasitol. Res. 2017;116:539–547. doi: 10.1007/s00436-016-5318-5. [DOI] [PubMed] [Google Scholar]
- Andritoiu C.V., Andritoiu V., Cuciureanu M., Nica-Badea D., Bibire N., Popa M. Effect of apitherapy products against carbon tetrachloride-induced toxicity in Wistar rats. Rom. J. Morphol. Embryol. 2014:835–847. [PubMed] [Google Scholar]
- Badr G., Al-Sadoon M.K., El-Toni A.M., Daghestani M. Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFκB and ERK signaling. Lipids Health Dis. 2012;11:1–10. doi: 10.1186/1476-511X-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G., Mahmoud M.H., Farhat K., Waly H., Al-Abdin O.Z., Rabah D.M. Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids Health Dis. 2013;12:37. doi: 10.1186/1476-511X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G., Mohany M., Metwalli A. Effects of undenatured whey protein supplementation on CXCL12-and CCL21-mediated B and T cell chemotaxis in diabetic mice. Lipids Health Dis. 2011;10:203. doi: 10.1186/1476-511X-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G., Saad H., Waly H., Hassan K., Abdel-Tawab H., Alhazza I.M., Ahmed E.A. Type I interferon (IFN-α/β) rescues B-lymphocytes from apoptosis via PI3Kδ/Akt, Rho-A, NFκB and Bcl-2/BclXL. Cell Immunol. 2010;263:31–40. doi: 10.1016/j.cellimm.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Badr G., Sayed E.A., Waly H., Hassan K.A., Mahmoud M.H., Selamoglu Z. The therapeutic mechanisms of propolis against CCl4-mediated liver injury by mediating apoptosis of activated hepatic stellate cells and improving the hepatic architecture through PI3K/AKT/mTOR, TGF-β/Smad2, Bcl2/BAX/P53 and iNOS signaling pathways. Cell Physiol. Biochem. 2019;53:301. doi: 10.33594/000000140. [DOI] [PubMed] [Google Scholar]
- Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blach-Olszewska Z., Leszek J. Mechanisms of over-activated innate immune system regulation in autoimmune and neurodegenerative disorders. Neuropsychiatr. Dis. Treat. 2007;3:365. [PMC free article] [PubMed] [Google Scholar]
- Casini A.F., Benedetti A. Free radical damage produced by carbon tetrachloride in the lipids of various rat tissues. Agents Actions. 1978;8:303–310. doi: 10.1007/BF01966620. [DOI] [PubMed] [Google Scholar]
- Chakraborty B., Sengupta M. Supporting the immune system through functional modulation of carbon tetrachloride intoxicated splenic macrophages by administering Tinospora cordifolia. J. Appl. Pharm. 2012;2:117. [Google Scholar]
- Doi K., Kurabe S., Shimazu N., Inagaki M. Systemic histopathology of rats with CCl4-induced hepatic cirrhosis. Lab. Anim. 1991;25:21–25. doi: 10.1258/002367791780808121. [DOI] [PubMed] [Google Scholar]
- Domitrović R., Jakovac H., Marchesi V.V., Vladimir-Knežević S., Cvijanović O., Tadić Ž., Romić Ž., Rahelić D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012;33:1260–1270. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S.A., Bouknight S.A. Immunopathology in Toxicology and Drug Development. Humana Press; Cham: 2017. Lymph node; pp. 59–79. [Google Scholar]
- Elshama S.S., Hussein Osman H.E., El-Kenawy A. Effect of propolis on immunotoxicity induced by phenol subchronic use in adult Albino rats. Iran. J. Pharmacol. 2016;14:41–50. [Google Scholar]
- Fararh K.M., Farid A.S., Azzam I.M., Sultan A.A. The hepato-protective Effect of Stem Cells and Levamisole against Carbontetrachloride induced Liver Fibrosis. BVMJ. 2016;31:149–157. [Google Scholar]
- Faroon, O., 2005. Toxicological profile for carbon tetrachloride. ATSDR. [PubMed]
- Friedman S.L. The cellular basis of hepatic fibrosis–mechanisms and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Geetha S., Jayamurthy P., Pal K., Pandey S., Kumar R., Sawhney R.C. Hepatoprotective effects of sea buckthorn (Hippophae rhamnoides L.) against carbon tetrachloride induced liver injury in rats. J. Sci. Food Agric. 2008;88:1592–1597. [Google Scholar]
- Gregory S.H., Cousens L.P., van Rooijen N., Döpp E.A., Carlos T.M., Wing E.J. Complementary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J. Immunol. 2002;168:308–315. doi: 10.4049/jimmunol.168.1.308. [DOI] [PubMed] [Google Scholar]
- Guo T.L., McCay J.A., Brown R.D., Musgrove D.L., Germolec D.R., Butterworth L., Munson A.E., White K.L., Jr Carbon tetrachloride is immunosuppressive and decreases host resistance to Listeria monocytogenes and Streptococcus pneumoniae in female B6C3F1 mice. Toxicol. 2000;154:85–101. doi: 10.1016/s0300-483x(00)00327-9. [DOI] [PubMed] [Google Scholar]
- Hayriye A.L.P. Effects of propolis on immune system. Anadolu Ege Tarımsal Araştırma Enstitüsü Dergisi. 2018;28:99–104. [Google Scholar]
- Jirova D., Sperlingova I., Halaskova M., Bendova H., Dabrowska L. Immunotoxic effects of carbon tetrachloride–the effect on morphology and function of the immune system in mice. Cent. Eur. J. Publ. Heal. 1996;4:16–20. [PubMed] [Google Scholar]
- Karkampouna S., Goumans M.J., Ten Dijke P., Dooley S., Kruithof-de Julio M. Inhibition of TGFβ type I receptor activity facilitates liver regeneration upon acute CCl4 intoxication in mice. Arch. Toxicol. 2016;90:347–357. doi: 10.1007/s00204-014-1436-y. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Jahan P., Hasan Q., Rao P. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab. Syndr. 2019;13:688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Vattam K.K., Jahan P., Hasan Q., Rao P. Importance of glucokinase -258G/A polymorphism in Asian Indians with post-transplant and type 2 diabetes mellitus. Intractable Rare Dis. Res. 2016;5:25–30. doi: 10.5582/irdr.2015.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.Y., Chang H.H., Chen J.H., Hsueh M.L., Kuo J.J. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J. Ethnopharmacol. 2007;109:318–324. doi: 10.1016/j.jep.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Lewis S.M., Williams A., Eisenbarth S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier R.P., Meyer J., Montanari E., Lacotte S., Balaphas A., Muller Y.D., Clément S., Negro F., Toso C., Morel P., Buhler L.H. Interleukin-1 receptor antagonist modulates liver inflammation and fibrosis in mice in a model-dependent manner. Int. J. Mol. Sci. 2019;20:1295. doi: 10.3390/ijms20061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohany M., El-Feki M., Refaat I., Garraud O., Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J. Toxicol. Sci. 2012;37:1–11. doi: 10.2131/jts.37.1. [DOI] [PubMed] [Google Scholar]
- Montes G.S., Junqueira L.C.U. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem. Inst. 1991;86:1–11. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- Mujica V., Orrego R., Pérez J., Romero P., Ovalle P., Zúñiga-Hernández J., Arredondo M., Leiva E. The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evid. Based Compl. Alternat. Med. 2017 doi: 10.1155/2017/4272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Lefebvre A.T., Ajith A., Portik-Dobos V., Horuzsko D.D., Arbab A.S., Dzutsev A., Sadek R., Trinchieri G., Horuzsko A. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J. Clin. Investig. 2018;128:4870–4883. doi: 10.1172/JCI98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nna V.U., Bakar A.B.A., Lazin M.R.M.L.M., Mohamed M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin–induced diabetic rats. Food Chem. Toxicol. 2018;120:305–320. doi: 10.1016/j.fct.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Prystupa A., Kiciński P., Sak J., Boguszewska-Czubara A., Toruń-Jurkowska A., Załuska W. Proinflammatory cytokines (IL-1α, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol. Res. Pract. 2015 doi: 10.1155/2015/532615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racanelli V., Rehermann B. The liver as an immunological organ. Hepatol. 2006;43:54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Dhar D.K., Yamaguchi E., Maruyama S., Sato T., Hayashi H., Ono T., Yamanoi A., Kohno H., Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin. Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- Rey I., Dairi L.B., Siregar G.A., Zain L.H. Serum level of IL-6 in liver cirrhosis patients. IOP Conf. Series: Environ. Earth Sci. 2018;125 [Google Scholar]
- Said M.M., Azab S.S., Saeed N.M., El-Demerdash E. Antifibrotic mechanism of pinocembrin: impact on oxidative stress, inflammation and TGF-β/Smad inhibition in rats. Ann. Hepatol. 2018;17:307–317. doi: 10.5604/01.3001.0010.8661. [DOI] [PubMed] [Google Scholar]
- Sayed D., Badr G., Maximous D., Mikhail N.N.H., Abu-Tarboush F., Alhazza I.M. HLA-G and its relation to proliferation index in detection and monitoring breast cancer patients. Tissue Antigens. 2010;75:40–47. doi: 10.1111/j.1399-0039.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- Schildberg F.A., Hegenbarth S.I., Schumak B., Limmer A., Knolle P.A. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur. J. Immunol. 2008;38:957–967. doi: 10.1002/eji.200738060. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M., Orsi R.O., Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J. Ethnopharmacol. 2005;98:301–305. doi: 10.1016/j.jep.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Tamayo Revuelta, E., Álvarez Sainz de la Maza, P., Merino Pérez, R., 2018. TGFB superfamily members as regulators of B cell development and function-implications for autoimmunity. [DOI] [PMC free article] [PubMed]
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Wolska K., Gorska A., Antosik K., Lugowska K. Immunomodulatory effects of Propolis and its components on basic immune cell functions. Indian J. Pharm. Sci. 2019;81:575–588. [Google Scholar]
- Yang J.J., Tao H., Hu W., Liu L.P., Shi K.H., Deng Z.Y., Li J. MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis. Cell. Signal. 2014;26:2381–2389. doi: 10.1016/j.cellsig.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Zou L., Chen S., Li L., Wu T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp. Toxicol. Pathol. 2017;69:451–460. doi: 10.1016/j.etp.2017.04.001. [DOI] [PubMed] [Google Scholar]