Abstract
Physiological systems vary in a day-night manner anticipating increased demand at a particular time. Heart is no exception. Cardiac output is primarily determined by heart rate and unsurprisingly this varies in a day-night manner and is higher during the day in the human (anticipating increased day-time demand). Although this is attributed to a day-night rhythm in post-translational ion channel regulation in the heart’s pacemaker, the sinus node, by the autonomic nervous system, we investigated whether there is a day-night rhythm in transcription. RNAseq revealed that ~ 44% of the sinus node transcriptome (7134 of 16,387 transcripts) has a significant day-night rhythm. The data revealed the oscillating components of an intrinsic circadian clock. Presumably this clock (or perhaps the master circadian clock in the suprachiasmatic nucleus) is responsible for the rhythm observed in the transcriptional machinery, which in turn is responsible for the rhythm observed in the transcriptome. For example, there is a rhythm in transcripts responsible for the two principal pacemaker mechanisms (membrane and Ca2+ clocks), transcripts responsible for receptors and signalling pathways known to control pacemaking, transcripts from genes identified by GWAS as determinants of resting heart rate, and transcripts from genes responsible for familial and acquired sick sinus syndrome.
Subject terms: Physiology, Cardiology
Introduction
Resting heart rate is associated with cardiovascular health: an elevated resting heart rate is an independent risk factor for cardiovascular mortality and morbidity even in healthy individuals1–3, whereas a slow heart rate can compromise cardiac output and even lead to heart failure4–7. Heart rate is affected by many factors such as pregnancy8, development9 and ageing10, physical activity, long-term physical training11 and disease12. Another factor is the time of day and night—the resting heart rate oscillates from day to night and is lower at night in the case of diurnal species such as the human13. Clinically, this is important because bradyarrhythmias (slow heart rhythms because of sinus bradycardia or atrioventricular block) occur at night in humans (diurnal) and during the day in rats (nocturnal)13–15. This is particularly evident in veteran athletes, who have nocturnal pauses between heart beats; the longest documented nocturnal pause is 15 s14. Based principally on heart rate variability e.g.16, but also in part on autonomic blockade17, the day-night rhythm in heart rate is attributed to high vagal tone during sleep and changes in ionic conductances (as a result of a post-translational regulation of the corresponding ion channels) in the pacemaker of the heart, the sinus node. However, it is possible that the day-night rhythm in heart rate is the result of transcriptional changes in the sinus node. Most day-night rhythms are the result of a circadian clock and, whereas there is a master circadian clock in the suprachiasmatic nucleus, there are peripheral clocks in peripheral tissues. The heart is known to have its own circadian clock, and 6–13% of the transcriptome of the mouse heart (likely to be exclusively or mainly the ventricles) has been reported to vary in a day-night manner, presumably under the control of the local clock in the heart18–21 or the master circadian clock in the suprachiasmatic nucleus. In ventricular muscle, a day-night rhythm in 10 ion channel transcripts has been reported13,22. As well as ion channel expression, a day-night rhythm has been reported in metabolism in the heart e.g.23.
The aim of the present study was to measure the transcriptome of the sinus node using RNAseq and determine whether there is a functional circadian clock and a day-night rhythm in pacemaker genes in the sinus node. Because RNAseq yields the whole transcriptome of a tissue, a secondary aim was to determine if other systems in the sinus node cell, especially those which may impact pacemaking, show a day-night rhythm. The data show an all-pervasive day-night rhythm in the transcriptome of the pacemaker of the heart, the sinus node—there is a day-night rhythm in pacemaker genes and in many other systems as well.
Results
In total, 55,450 transcripts were identified in the mouse sinus node. Expression of the transcripts was measured at six time points (at 4 h intervals) over 24 h. At each time point, expression was measured in three mice. The average expression of each transcript over 24 h was calculated and it varied from 910,910 to 0 normalised reads. The average expression of 16,387 transcripts with an expression greater than 10 normalised reads is plotted in Fig. S1A in the Data Supplement. It was assumed that transcripts poorly expressed do not play a functional role in the sinus node cell, and transcripts with an average expression of less than 10 normalised reads were arbitrarily excluded from further analysis. JTK Cycle software was developed by Hughes et al.24 to test whether a variable shows a statistically significant day-night rhythm. The 16,387 transcripts plotted in Fig. S1A were analysed using JTK Cycle and Fig. S1B shows that of these 7134 transcripts (~ 44%) showed a significant day-night rhythm (permutation-based P value < 0.05). Figure S1C shows the amplitude of the day-night variation, i.e. deviation from the mean; the day-night variation from day to night (2 × amplitude) could be substantial. Figure S1 demonstrates that the sinus node transcriptome has a day-night rhythm.
Functioning circadian clock in the sinus node
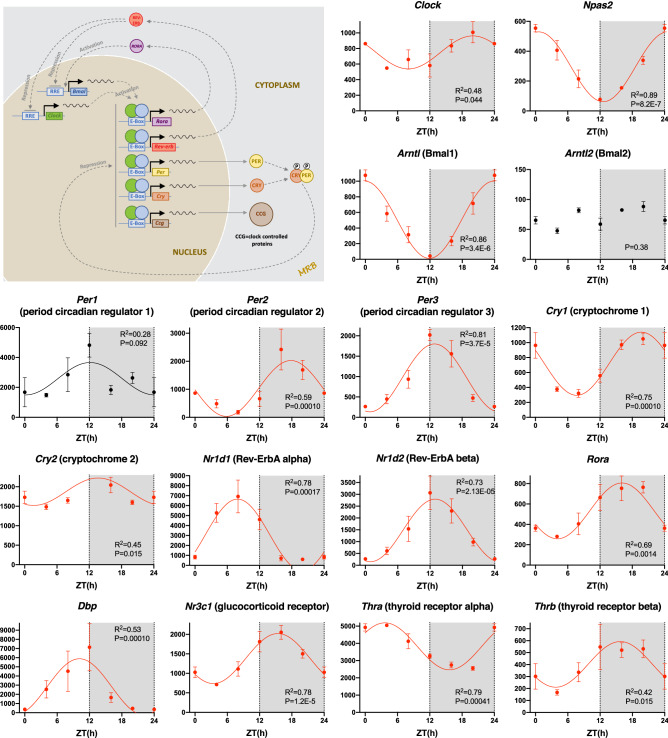
Figure 1 shows a schematic diagram of the circadian clock. There are two feedback loops. First, CLOCK and BMAL1 proteins dimerise and activate transcription of period (Per1, Per2, and Per3) and cryptochrome (Cry1 and Cry2) genes. PER and CRY proteins then dimerise and repress their own transcription by inhibiting the activity of the CLOCK:BMAL1 dimer. Secondly, CLOCK:BMAL1 dimer activates the transcription of Nr1d1 (and Rora). NR1D1 then represses transcription of Bmal1 (whilst RORA activates it). All components of the circadian clock are present in the sinus node, including paralogs of Clock and Bmal1 (Npas2 and Bmal2), and all show significant day-night rhythms except Bmal2 (poorly expressed) and Per1 (Fig. 1). Table 1 shows that of 19 circadian clock transcripts present, ~ 79% showed a significant day-night rhythm. It is concluded that there is a functioning circadian clock in the sinus node and this could be responsible for the day-night rhythm in other transcripts, selected examples of which are shown below.
Figure 1.
Circadian clock. Abundance (normalised counts) of circadian clock transcripts and some potentially related transcripts is shown over 24 h. The inset shows a schematic diagram of the circadian clock. In this and similar figures: gene name and common name (in parenthesis) given; mean ± SEM transcript abundance shown (n = 3) at six ZT time points over 24 h (24 h data repeat of 0 h data); the permutation-based P value (corrected for multiple testing) from JTK Cycle for significance of a day-night rhythm is given; significant transcripts (permutation-based P value < 0.05) are shown in red and have been fitted with a sine wave by a least squares fitting method and the R2 value given; transcripts showing a trend towards significance (permutation-based P value > 0.05 and < 0.1) are shown in black and have again been fitted with a sine wave by a least squares fitting method and the R2 value given; non-significant transcripts (permutation-based P value > 0.1) are shown in black and have not been fitted with a sine wave.
Table 1.
Summary of the percentage of transcripts showing a significant day-night rhythm in different groups and pathways. HGNC, HUGO Gene Nomenclature Committee; MGI, MGI Gene Ontology Browser; RGD, Rat Genome Database.
| Group of transcripts | Source of transcripts | Total number of transcripts with > 10 normalised reads | Number showing significant day-night rhythm | Percentage showing significant day-night rhythm |
|---|---|---|---|---|
| Histone acetyltransferases | HGNC | 15 | 12 | 80.0% |
| Circadian clock | Custom selection | 19 | 15 | 78.9% |
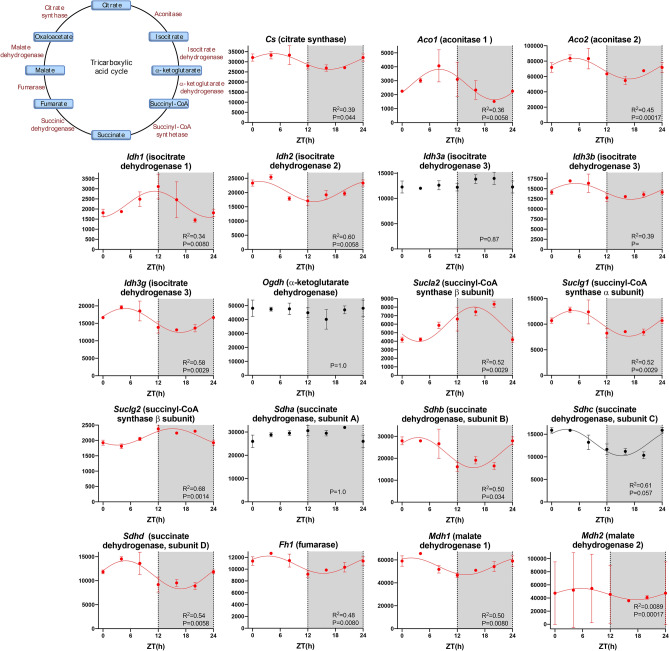
| Citric acid cycle | RGD | 30 | 20 | 66.7% |
| Eukaryotic initiation factors | Custom selection | 55 | 35 | 63.6% |
| CaMKII pathway | Kreusser and Backs (2014) | 19 | 12 | 63.2% |
| Ca2+clock pacemaker mechanism | Custom selection | 21 | 13 | 61.9% |
| Glycolysis pathway | RGD | 54 | 32 | 59.3% |
| Autonomic receptors and their pathways | Custom selection | 31 | 18 | 58.1% |
| Mitochondrial transporters (Slc25 transcripts) | – | 40 | 23 | 57.5% |
| Histone deacetylases | Wikipedia | 17 | 9 | 52.9% |
| RNA degradation pathway | Houseley and Tollervey79 | 38 | 20 | 52.6 |
| Transcription factors | Custom selection | 703 | 347 | 49.4% |
| Fatty acid β-oxidation | MGI | 61 | 30 | 49.2% |
| Ubiquitin and proteasome | Custom selection | 46 | 22 | 47.8% |
| G-protein α β and γ subunits | HGNC | 25 | 12 | 48.0% |
| All transcripts | – | 16,387 | 7134 | 43.5% |
| Electron transport chain | MGI | 74 | 32 | 43.2% |
| RNA polymerases | Custom selection | 26 | 11 | 42.3% |
| Solute carriers (Slc transcripts) | – | 278 | 114 | 41.0% |
| Ca2+ ion transport | MGI | 348 | 128 | 36.8% |
| G-protein coupled receptors (Gpr genes) | – | 52 | 17 | 32.7% |
| Extracellular matrix | MGI | 378 | 122 | 32.3% |
| Ion channel subunits | HGNC | 199 | 64 | 32.2% |
| Gap junction subunits | Custom selection | 13 | 2 | 15.4% |
| microRNAs | – | – | 67 | – |
Circadian clocks in peripheral tissues are known to be entrained to the master circadian clock in the suprachiasmatic nucleus via neurohumoral regulation. Potential entrainment signals have been suggested to be plasma glucocorticoids25 and thyroid hormone26 as well as the autonomic nervous system25. It is therefore interesting that transcripts for glucocorticoid and thyroid hormone receptors (Fig. 1) as well as receptors for autonomic transmitters (see below) all showed significant day-night rhythms.
Day-night rhythm in pacemaker genes
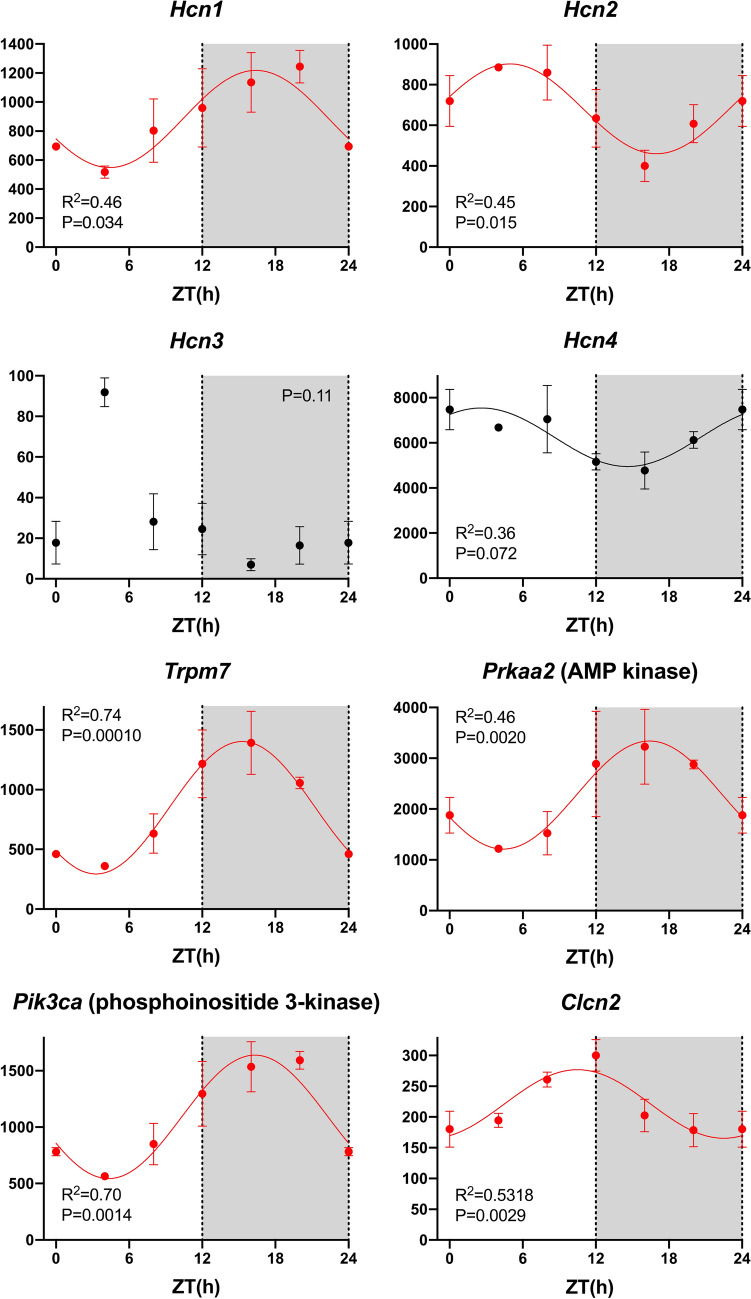
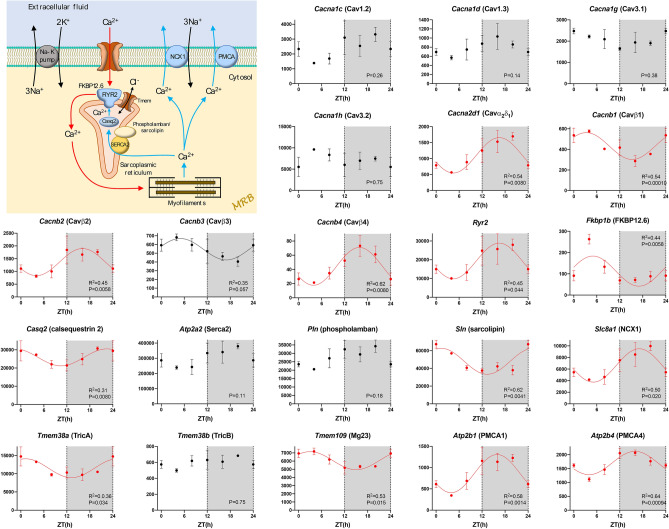
Pacemaking in the sinus node is widely regarded as the result of two mechanisms, the membrane and Ca2+ clocks27,28. The main contributor to the membrane clock are the HCN (funny) channels. All four Hcn isoform transcripts were present and Hcn4 > > Hcn1≈Hcn2 > > Hcn3 as expected (Fig. 2). Hcn1 and Hcn2 showed significant day-night rhythms, whereas Hcn3 did not and Hcn4 showed a trend towards a rhythm (P = 0.072) (Fig. 2). Using quantitative PCR, we have previously shown a qualitatively similar, but significant, day-night rhythm in Hcn4 in the mouse sinus node13. TRPM7 (a divalent-permeant channel-kinase)29, AMP kinase30 and phosphoinositide 3-kinase31 have been shown to regulate funny channels and/or current, and their transcripts all showed significant day-night rhythms (Fig. 2). CLCN2 is an ion channel responsible for a Cl- current that functions in a similar way to the funny current and has been shown to contribute to pacemaking32, and Clcn2 too showed a significant rhythm (Fig. 2). Figure 3 shows a schematic diagram of intracellular Ca2+-handling and, therefore, the main components of the Ca2+ clock: surface membrane Ca2+ channels and their accessory subunits, the sarcoplasmic reticulum (SR) Ca2+ release channel (RYR2), FKBP12.6, the SR Ca2+ pump (SERCA2), phospholamban/sarcolipin, calsequestrin 2, and the Na+-Ca2+ exchanger (NCX1). For completeness, the diagram also shows SR Cl- channels (which balance charge associated with Ca2+ movements in and out of the SR)33 and surface membrane Ca2+ pumps (PMCAs) (Fig. 3). Not all, but many, of the corresponding transcripts showed a significant day-night rhythm (Fig. 3). Two of the most important players in the Ca2+ clock are RYR2 and NCX1 and transcripts for both showed prominent and significant day-night rhythms (Fig. 3). Table 1 shows that of 21 transcripts involved in the Ca2+ clock, ~ 62% showed a significant day-night rhythm. It is concluded that there is a day-night rhythm in transcript expression for many of the key players involved in pacemaker activity in the sinus node.
Figure 2.
Membrane clock pacemaker mechanism. Abundance (normalised counts) of pacemaker ion channel transcripts and some potentially related transcripts is shown over 24 h. See Fig. 1 legend for further details.
Figure 3.
Ca2+ clock pacemaker mechanism. Abundance (normalised counts) of Ca2+ clock transcripts is shown over 24 h. The inset shows a schematic diagram of the Ca2+ clock. See Fig. 1 legend for further details.
Other ion channels, gap junction channels and the Na+-K+ pump
The HGNC website provides a list of 328 ion channel subunits, of which 199 were present in the RNAseq dataset. Of these ~ 32% showed a significant day-night rhythm—listed in Table S1. Examples are shown in Fig. S2. One example, the TASK-1 channel, is highly abundant and its transcript showed a significant day-night rhythm and yet the role of this channel in the sinus node is not known. In the case of surface membrane ion channels, the day-night rhythm could potentially impact pacemaking. Transcripts for twelve gap junction subunits were identified; Gja5 (Cx40) showed a significant day-night rhythm and Gja1 (Cx43; P = 0.092) and Cx45 (Gjc1; P = 0.072) showed a trend towards one (Fig. S3). In addition, TMEM65, which interacts with and functionally regulates Cx4334, showed a significant day-night rhythm (Fig. S3). Gap junctions, by controlling the interaction of the pacemaking sinus node with the hyperpolarized non-pacemaking neighbouring atrial muscle, affect pacemaking35 and, therefore, a day-night rhythm in connexin protein could potentially impact pacemaking. Ionic currents are dependent on ionic gradients across the cell membrane set up by the Na+-K+ pump. There are three Na+-K+ pump α-subunits and transcripts for all three tended to or showed a significant day-night rhythm (Fig. S4). The Na+-K+ pump is regulated by phospholemman36 and its transcript too showed a significant day-night rhythm (Fig. S4). Is Na+-K+ pump expression (and therefore activity) greater during the awake period when the heart rate is higher in order to counter the greater movement of ions through ion channels across the cell membrane?
Autonomic receptors and downstream pathways
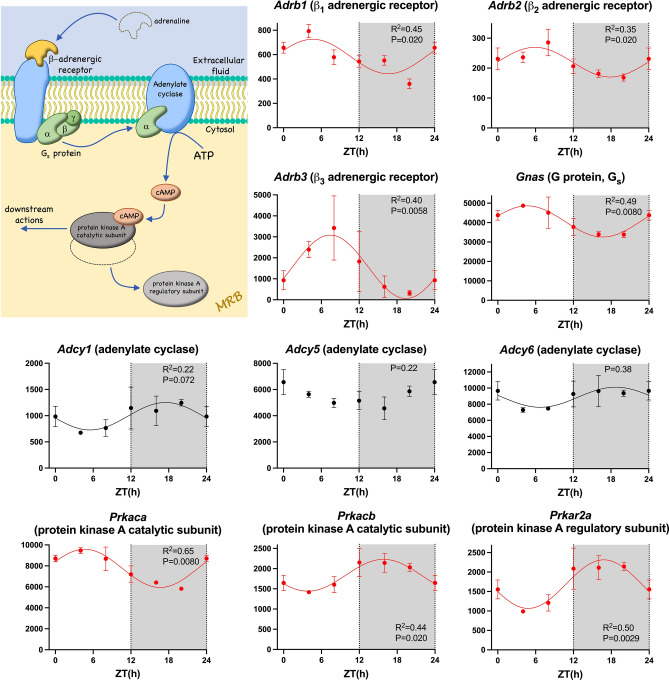
Heart rate is regulated by the autonomic nervous system. The day-night rhythm in heart rate is widely attributed to a day-night rhythm in the autonomic innervation of the heart: high vagal tone during sleep resulting in rapid changes in ionic conductances in the sinus node and thus heart rate. However, curiously, transcripts for some of the autonomic receptors and their immediate downstream mediators showed a day-night rhythm. Binding of catecholamine to the α1 adrenergic receptor activates phospholipase C via a G protein (Gq) and this ultimately results in the activation of the IP3 receptor and protein kinase C (Fig. S5). Of the three α1 adrenergic receptor transcripts expressed, the α1B adrenergic receptor transcript was the most abundant and this showed a significant day-night rhythm (Fig. S5). Many of the downstream transcripts including transcripts for the α subunit of Gq, phospholipase C, two IP3 receptor isoforms and protein kinase C, also showed a significant day-night rhythm (Fig. S5). Binding of catecholamine to the β adrenergic receptor activates protein kinase A via a G protein (Gs) and adenylate cyclase. β1, β2 and β3 adrenergic receptor transcripts were present; surprisingly, whereas the β1 adrenergic receptor is considered the most important in the heart, the β3 adrenergic receptor transcript was the most abundant. Transcripts for β1, β2 and β3 adrenergic receptors, the α subunit of Gs, and the catalytic subunit of protein kinase A showed a significant day-night rhythm (Fig. 4). Binding of ACh to the M2 receptor activates the ACh-activated K+ channel via a G protein (Gi), and transcripts for the receptor, the α subunit of Gi, and one of the two subunits making up the channel (Kir3.1) showed a significant day-night rhythm (Fig. S6). The same pathway is activated by the adenosine A1 receptor37 and this too showed a significant day-night rhythm (Fig. S6).
Figure 4.
β-adrenergic receptor pathway. Abundance (normalised counts) of β-adrenergic receptor pathway transcripts is shown over 24 h. The inset shows a schematic diagram of the β-adrenergic receptor pathway. Transcripts for two other protein kinase A subunits, Prkar1b and Prkar2b, also showed a significant circadian rhythm (data not shown). See Fig. 1 legend for further details.
Signalling pathways
There are many signalling pathways in the heart and just a few examples were investigated. Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays an important role in the heart and the sinus node. Whereas acute activation of CaMKII results in phosphorylation of downstream targets by the kinase38, chronic activation results in transcriptional remodelling39. For example, constitutive activation of CaMKII (by oxidation) in heart failure is reported to be responsible for sinus node disease common in heart failure40. Figure S7 shows a schematic diagram of the pathway (based on Kreusser and Backs39) by which CaMKII regulates gene transcription. Transcripts for CaMKII (Camk2d) and many of its downstream mediators showed a significant rhythm (Fig. S7). Mitogen-activated protein kinases (MAP kinases) are involved in cardiac development, physiological adaptation and pathological manifestation41. They act on ion channels42. They form a three-tiered kinase cascade in which a MAP kinase kinase kinase activates a MAP kinase kinase, which in turn activates a MAP kinase41. Transcripts for some kinases in all three tiers of the cascade showed significant rhythms (Figs. S8-S10). Nitric oxide (NO) is an important regulator of the cardiovascular system and in part NO is derived from NO synthases (NOSs)43. Of the three NOS transcripts present, Nos3 (inducible NOS, iNOS) showed a significant day-night rhythm (data not shown). Any signalling pathway impacting surface membrane ion channels or intracellular Ca2+ handling has the potential to affect pacemaking.
Myofilaments
Some of the transcripts for the contractile apparatus of the sinus node myocyte showed a significant day-night rhythm, including transcripts for myosin light chain 4 and titin, which are linked to sick sinus syndrome44,45. In addition, transcripts for actin, tropomyosin 1 and troponin I showed significant day-night rhythms (Fig. S11).
Metabolism
It is well known that metabolism shows a day-night rhythm, including in the heart e.g.23. The RNAseq data suggests that this is also true of the sinus node. ~ 70–90% of cardiac ATP is produced by the oxidation of fatty acids, which are transported into the mitochondria as acyl-CoA46. Carnitine palmitoyltransferases I and II and translocase are involved in the transport and transcripts for carnitine palmitoyltransferase II and translocase showed a significant day-night rhythm (Fig. S12). The long-chain acyl-CoA enters the fatty acid β-oxidation pathway. ~ 49% of the transcripts for the fatty acid β-oxidation pathway showed a significant day-night rhythm (Table 1)—examples are shown in Fig. S13. The acetyl-CoA then enters the citric acid cycle to generate ATP. Glycolysis is the metabolic pathway that converts glucose into pyruvate; the pyruvate is then converted to acetyl-CoA, which again then enters the citric acid cycle to generate ATP. ~ 59% of the transcripts for the glycolysis pathway showed a significant day-night rhythm (Table 1)—examples are shown in Fig. S14. ~ 67% of the transcripts for the citric acid cycle itself showed a significant day-night rhythm (Table 1)—examples are shown in Fig. 5. The NADH generated by the citric acid cycle is fed into the oxidative phosphorylation pathway—involving the electron transport chain—to form ATP. ~ 43% of the electron transport chain transcripts showed a significant day-night rhythm (Table 1)—examples are shown in Fig. S15. The day-night rhythm in metabolism has the potential to impact pacemaking, because AMP kinase (Fig. 2) both conserves cellular energy homeostasis and also affects the heart rate via the pacemaker funny current carried by HCN channels30. For example, ATPIF1 is an inhibitor of mitochondrial ATPase, the engine of oxidative phosphorylation, and consequently a regulator of energy metabolism47. ATPIF1 has a link to AMP kinase48 and Atpif1 showed a trend of a day-night rhythm (P = 0.072; Fig. S15).
Figure 5.
Citric acid cycle. Abundance (normalised counts) of citric acid cycle transcripts is shown over 24 h. The inset shows a schematic diagram of the citric acid cycle. See Fig. 1 legend for further details.
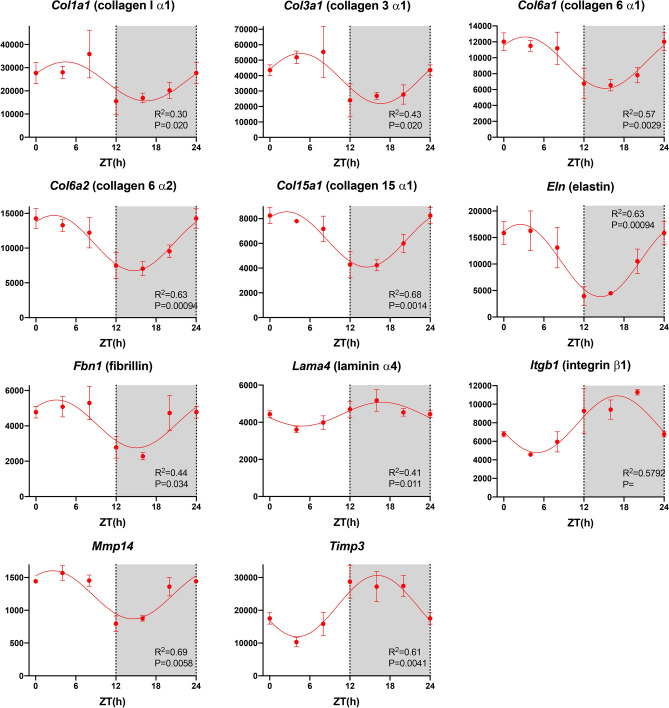
Extracellular matrix
Of 378 transcripts involved in the extracellular matrix, ~ 32% showed a significant day-night rhythm (Table 1)—examples are shown in Fig. 6. The extracellular matrix, as well as being essential for the structural integrity of the heart, is a load that the contractile apparatus must deform in the cardiac cycle—it is therefore an energetic cost. Could the extracellular matrix change from day to night as the heart rate and cardiac output change to obtain the necessary structural integrity at the least energetic cost? Proliferation of the extracellular matrix has long been linked to sinus node dysfunction.
Figure 6.
Extracellular matrix. Abundance (normalised counts) of example extracellular matrix transcripts is shown over 24 h. See Fig. 1 legend for further details.
Immune system
The immune system is known to show a circadian rhythm49. The class I major histocompatibility complex (HMC) of a tissue presents self-antigens to cytotoxic T-cells of the immune system and ultimately prevents the animal’s immune system targeting its own cells, whereas the class II MHC presents pathogen-derived proteins ultimately resulting in the elimination of infected cells by the immune system. The class II MHC is linked to heart failure50,51. In the sinus node, two transcripts involved with the class I MHC (H2-k1 and H2-q1052), a transcript for a class I MHC-like molecule (Cd1d153), two transcripts potentially involved with the class I MHC (Rpp21 and Trim39) and one transcript (H2-dmb252) involved with the class II MHC showed a significant day-night rhythm (Fig. S16). Respiratory (or oxidative) burst is the rapid release of reactive oxygen species (ROS), superoxide anion, and hydrogen peroxide. Macrophages and neutrophils are especially implicated in the respiratory burst. They are phagocytic, and the respiratory burst is vital for the subsequent degradation of internalised bacteria or other pathogens. This is an important aspect of immunological defence. There was a significant day-night rhythm in Cybb (NOX2) encoding the major component of NADPH oxidase, which plays a key role in the respiratory burst (Fig. S16). Interleukin 33 is an alarmin (type of cytokine) and is released under conditions of stress to induce protective measures54. For example, it has been shown to antagonise cardiac hypertrophy and remodelling in mice subject to transverse aortic constriction54. The transcript for interleukin 33 (Il33) showed a significant day-night rhythm (Fig. S16) raising the possibility that the heart’s resistance to stress may also show a day-night rhythm. Heart disease is frequently associated with sinus node dysfunction55 and the immune system has been linked to the adverse remodelling of the diseased heart50. Other transcripts linked to the immune system also showed a significant day-night rhythm and are illustrated in Fig. S17 or listed in Table S2.
GWAS-identified genes associated with resting heart rate
Various genome-wide association studies (GWAS) have been carried out to identify genetic variants (and therefore genes) affecting the resting heart rate. The majority of these genes have not previously been identified as involved in pacemaking. Of the genes identified by these GWAS studies, transcripts for 46 showed a significant day-night rhythm (P < 0.05) and, therefore, could potentially contribute to a day-night rhythm in pacemaking—the transcripts are listed in Table S2 together with transcripts which showed a trend of a day-night rhythm (0.1 > P > 0.05). In most cases, the nature of the relationship between the gene and pacemaking is unknown. In a few cases, there is a plausible link to pacemaking (cAMP-dependent protein kinase type II-alpha regulatory subunit, Prkar2a—Fig. 4; muscarinic M2 receptor, Chrm2—Fig. S6; acetylcholine esterase, Ache—Fig. S6; titin, Ttn—Fig. S1144; Hcn4—Fig. 2; Cx43, Gja1—Fig. S3; desmoplakin, Dsp—Fig. S356). Some genes are related to ion channels, ion transport, receptors or a signalling pathway (Alg10, Slc12a9, Calcrl, Gng11, Map3k10) and, therefore, a relationship with pacemaking is not implausible. Some genes are involved in transcription, translation or degradation of mRNA/protein and could potentially be involved with pacemaker genes (Mkln1, Klhl42, Canx, Ppargc1a, Tbx20, Rnf220, Ppil1, Ddx17, Srrt, Srebf1, Ufsp1, Cby1, Cdc23). Intriguingly, one of the genes, Gtpbp1, promotes degradation of target mRNA species and plays a role in the regulation of circadian mRNA stability57. In some cases, a link with pacemaking can be speculated on: Met is a transcript for a receptor tyrosine kinase, which is known to target phosphoinositide 3-kinase58, which in turn is known to target HCN431. Ephb4 is a transcript for another receptor tyrosine kinase, and could it act in a similar way? Inositol hexakisphosphate kinase 1 (Ip6k1) has a link to AMP kinase59, which is known to regulate HCN430.
Transcription, translation, and mRNA and protein degradation
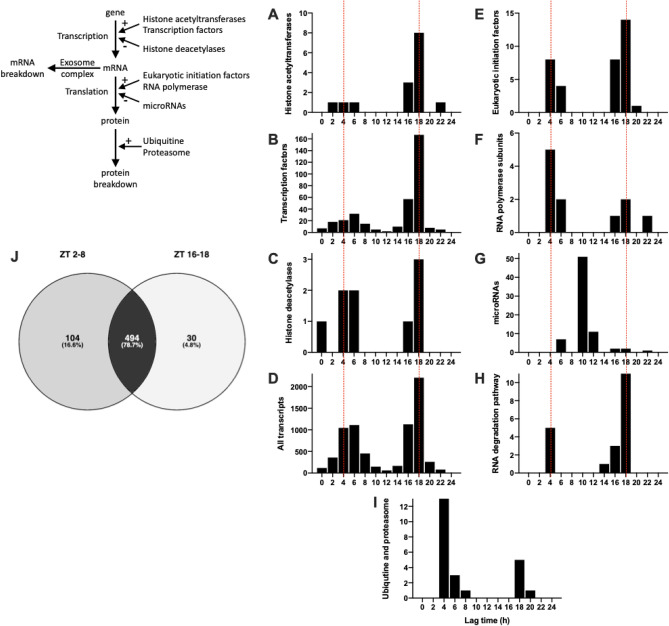
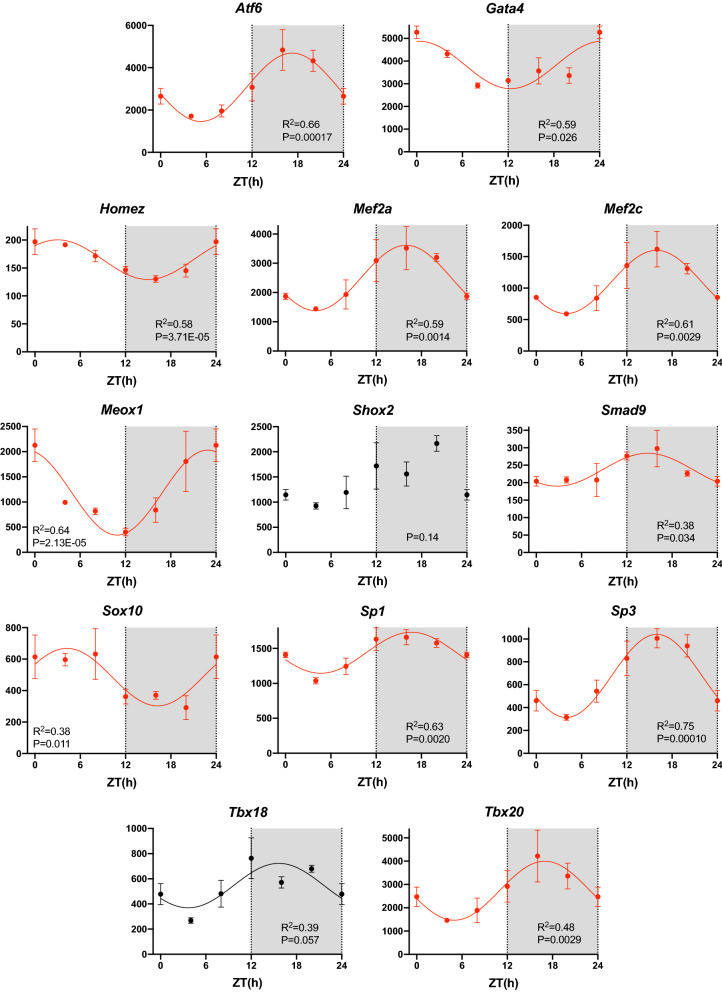
Figure 7D shows the lag time of the 7134 transcripts showing a significant day-night rhythm. The lag time corresponds to the maximum expression during the 24 h period. There are two lag time peaks, one in the day (~ ZT 4–6) and one at night (~ ZT 18), the most prominent being the one at night (Fig. 7D). This suggests that there are two peaks in the process of transcription. Transcription is controlled by histone acetyltransferases (HATs), which make chromatin accessible for transcription, and transcription factors, which drive transcription. Fifteen HAT transcripts were identified and 80% showed a significant day-night rhythm (Table 1). Recently, Zhou et al.60 have published an atlas of 941 mouse transcription factors. Of these, transcripts for 703 were identified in the sinus node and ~ 49% showed a significant day-night rhythm (Table 1)—examples are shown in Fig. 8. The chosen examples include transcripts for transcription factors known to be involved in determining the sinus node pacemaking phenotype (Tbx1861), sinus node development (Mef2c62), cardiac development (Homez, Sp3, Gata163–65), cardiac development and adult function (Tbx2066), cardiac development and possibly the circadian clock (Nrd1d267,68) and cardiac disease (Atf6, Meox169,70). Sp1 is a regulator of many cardiac genes including Serca271 and genes involved in metabolism72. Smad signalling has been linked to cardiac remodelling following myocardial infarction73. Sox10-positive cardiomyocytes of neural crest origin contribute to myocardial regeneration in the Zebrafish74. Mef2 directly targets HCN475. Shox2 is essential for the differentiation of cardiac pacemaker cells76. The oscillating HAT and transcription factor transcripts peaked at approximately the same time during the day and night (~ ZT 4 and 18; Fig. 7A,B) as all transcripts (Fig. 7D). Could these be responsible for the two peaks in transcription (Fig. 7D)? Histone deacetylases (HDACs) close chromatin to transcription—of 17 HDAC transcripts identified, ~ 53% showed a significant day-night rhythm (Table 1). Again HDAC transcripts showed the same two peaks (Fig. 7C). This is not unexpected—if transcription occurs primarily in two relatively brief spurts, the HATs and HDACs are expected to be present at roughly the same times. Intuitively, HDACs are expected to peak later than HATS to provide a time window when chromatin is accessible for transcription, but perhaps such a time window will only be evident with more frequent sampling than once every 4 h. Translation is governed by eukaryotic initiation factors (which guide transcripts to the ribosomes) and RNA polymerases. Of 55 transcripts for eukaryotic initiation factors identified, ~ 66% showed a significant day-night rhythm (Table 1). Of 26 RNA polymerase transcripts identified, ~ 42% showed a significant day-night rhythm (Table 1). It was only transcripts for RNA polymerase 2 (responsible for transcribing precursors of mRNA and most snRNA and microRNA) and 3 (responsible for transcribing housekeeping genes: 5S ribosomal RNA, tRNA and other small RNAs) which showed day-night rhythms—RNA polymerase 1 (responsible for transcribing ribosomal RNA) did not. Once again, transcripts for eukaryotic initiation factors and RNA polymerases showed the same two peaks (Fig. 7E,F). microRNAs are short non-coding RNAs—they downregulate the expression level of transcripts (and thereby downregulate translation) or they directly downregulate translation of transcripts. In a separate study, we measured the expression of microRNAs at four time points at 6 h intervals over 24 h using qPCR77. 74 microRNAs showed a significant day-night rhythm and unlike transcription factors and all transcripts showed a single peak at ~ ZT 10 (Fig. 7G). microRNAs are, therefore, in antiphase to transcription factors etc. This is not unexpected, because whereas transcription factors upregulate transcription/translation, microRNAs downregulate them.
Figure 7.
Transcription, translation, and mRNA transcript and protein breakdown. (A–I), histogram of lag times (times of peak transcript abundance) of different groups of transcripts involved in transcription, translation, and mRNA transcript and protein breakdown. (J), Venn diagram of pathways involving the transcripts peaking during the day and night; data were analysed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)103. Inset, schematic diagram of the cycle of transcription, translation, and RNA and protein degradation.
Figure 8.
Transcription Factors. Abundance (normalised counts) of example transcription factor transcripts is shown over 24 h. See Fig. 1 legend for further details.
Two peaks in transcripts during the day and at night have been seen before in mouse heart (presumably dominated by ventricular muscle) by Zhang et al.20 However, there are important differences between the two studies: in ventricle, 1335 oscillating transcripts were identified, which Zhang et al.20 reports is consistent with the 3–10% of the transcriptome oscillating in different tissues. In contrast, in the present study of the sinus node 7134 oscillating transcripts (~ 44% of the transcriptome) were identified. This is a much higher percentage than in ventricle and other tissues and reasons for this are speculated on in the Discussion. In addition, the segregation into two peaks is much more marked in sinus node (Fig. 7D) than in ventricle20. In ventricle, Zhang et al.20 argued that the transcripts in the two peaks were different in nature and the biphasic distribution of transcripts was under the control of the transcription factor, Klf15. However, no evidence of this was found in the sinus node: the types of transcripts said to be restricted to one peak in ventricle were not restricted to the same peak in the sinus node, and analysis of all significantly rhythmic transcripts in the two peaks in the sinus node by Ingenuity Pathway Analysis (IPA) software revealed no pattern and the enriched pathways in the two peaks were mostly similar (494 of 628 pathways enriched in the peaks were common to both; Fig. 7J). Furthermore, Klf15, did not show a significant day-night rhythm in the sinus node (P = 0.22; data not shown). There is further discussion about the phasing of transcripts in the Supplementary Information.
The potential targets of the 74 oscillating microRNAs were investigated using IPA, which utilises TargetScan predictions, experimentally validated TarBase and miRecords targets and manual curations from the literature. Experimentally observed targets as well as high and moderate confidence predictions were considered, although it is acknowledged that there is some likelihood of false positive predictions resulting from these analyses. Oscillating microRNAs were screened against the rhythmic transcripts using the microRNA target filter function in IPA, with a mouse species filter applied. Of the 7134 oscillating transcripts, 4982 (70%) were predicted to be targeted by 57 of the oscillating microRNAs.
It is assumed that the day-night rhythms in transcripts are the result of the oscillating transcription factors and microRNAs. In total, 16,387 transcripts were detected and 703 transcription factors were detected. In a previous study we have detected 715 microRNAs in the mouse sinus node78. Therefore, the ratio of transcription factors:transcripts is ~ 1:23 and the ratio of microRNAs:transcripts is 1:23. There are 7134 oscillating transcripts, 347 oscillating transcription factors and a minimum of 74 oscillating microRNAs—therefore the ratio of oscillating transcription factors:oscillating transcripts is ~ 1:21 and the ratio of oscillating microRNAs:oscillating transcripts is ~ 1:96.
At steady-state, RNA and protein degradation has to match transcription and translation. The many pathways of RNA degradation were reviewed by Houseley and Tollervey79; 38 components of these pathways were identified and ~ 53% showed a significant day-night rhythm (Table 1). Proteins are tagged for degradation by ubiquitination catalysed by ubiquitin ligases. Once ubiquinated, the protein is degraded by the proteasome. 47 transcripts for this pathway were identified and ~ 49% showed a significant day-night rhythm (Table 1). Once again these showed the same two peaks (Fig. 7H,I).
Discussion
The sinus node has two clocks, the membrane and Ca2+ clocks, operating on a time scale of seconds, and which are responsible for the rhythmic beating of the heart. For the first time, this study has shown that the sinus node has another clock, the circadian clock, operating on a time scale of days and which could be responsible for, or at least involved in, a rhythmic change in the transcriptome of the sinus node. ~ 44% of the transcriptome of the sinus node is changing in a day-night manner and the day-night rhythm is all-pervasive affecting all systems looked at including the membrane and Ca2+ clocks, neurohumoral receptors, important signalling pathways, metabolism and extracellular matrix. The interested reader is likely to find other systems affected—a list of all transcripts together with the permutation-based P value from JTK Cycle for a day-night rhythm is available as part of the Supplementary Data (AllTranscripts.xlsx).
Day-night rhythm in heart rate
Based on heart rate variability and autonomic blockade, the day-night rhythm in heart rate is currently attributed to changes in the autonomic innervation of the heart and in particular to high vagal tone at night in the case of the human13. According to this hypothesis, ACh released from vagal nerve endings binds to muscarinic M2 receptors and activates the ACh-activated K+ channel, and this causes the slowing of heart rate at night. However, we have argued that heart rate variability cannot be used to measure autonomic innervation of the heart80, and data from autonomic blockade has been reported to both block and have no discernible effect on the day-night rhythm in heart rate13. However, although there is doubt concerning the evidence for a day-night rhythm in autonomic innervation of the heart, it is clear that there is a day-night rhythm in the plasma level of catecholamine (presumably coming from the adrenal medulla under the action of the sympathetic nervous system), which is higher during the day in the human81. Therefore, it is possible that a day-night rhythm in the autonomic nervous system is responsible for the day-night rhythm in heart rate via rapid regulation of ionic conductances. This study does not resolve this controversy, but it does show that at the transcript level the two major pacemaking mechanisms of the sinus node, the membrane and Ca2+ clocks, show a profound day-night rhythm (Figs. 2,3). Therefore, it is possible that there is a day-night rhythm in pacemaking as a result of changes in gene transcription in the sinus node. In another study, we have shown that there is indeed an intrinsic day-night rhythm in both funny current density and pacemaking82. However, although an intrinsic day-night rhythm in pacemaking could be responsible for the day-night rhythm in heart rate, it may only be responsible for a day-night rhythm in ‘pacemaker reserve’ so that the sinus node is prepared to deliver higher heart rates during the awake period when called upon to do so by the autonomic nervous system. The final answer to the controversy of whether the day-night rhythm in heart rate is the result of the post-translational regulation of ion channels by the autonomic nervous system or transcriptional changes is unlikely to be simple. This study has shown that there is a day-night rhythm in expression of autonomic receptors (adrenergic and muscarinic receptors; Figs. 4, S5 and S6) and, therefore, there may be a day-night rhythm in the responsiveness to the autonomic receptor stimulation. Another possibility is that the autonomic nervous system is involved, but in a different way to that originally conceived: Tong et al.83,84 have shown that autonomic blockade abolishes the day-night rhythm in the expression of various K+ channels and connexin subunits in the ventricles (see below for further comment).
Day-night rhythm in the transcriptome
Based on the use of Affymetrix GeneChip oligonucleotide arrays, Storch et al.21 estimated that about 10% of the mouse liver transcriptome shows a significant day-night rhythm; they detected 4,805 transcripts in the liver of which 575 transcripts oscillated with a day-night rhythm, but they attributed 16% of these to noise rather than a genuine day-night rhythm. Using Affymetrix GeneChip oligonucleotide arrays, Martino et al.18 detected 12,488 transcripts in mouse heart (likely to be exclusively or mainly the ventricles based on tissue mass), of which 1,634 (~ 13%) showed a significant day-night rhythm (based on use of COSOPT) during a normal 12 h light:12 h dark lighting regime. In a more recent study, using RNAseq, Zhang et al.20 identified 1,335 transcripts (based on use of JTK Cycle) showing a significant day-night rhythm in mouse heart (presumably ventricle) during a normal 12 h light:12 h dark lighting regime. In a variety of studies on mouse heart (presumably ventricle), 6–13% of the transcriptome has been reported to vary in a day-night manner18–21. Using both RNAseq and Affymetric MoGene oligonucleotide arrays, Zhang et al.19 looked at the day-night rhythm in the transcriptome in 12 mouse organs; they reported that the transcripts oscillating varied from 3% in the hypothalamus, 6% in the heart and 16% in the liver. In the present study, 16,387 transcripts were selected, of which 7134 (~ 44%) showed a significant day-night rhythm and the fraction of oscillating genes is clearly much higher than in other studies. The data from the present study are robust and it is concluded that the difference is a tissue difference. The reason why the fraction of transcripts under day-night control is large in the sinus node can only be speculated on. One possibility is the importance of heart rate for an organ that is continuously beating every ~ 1 s throughout life; cardiac output is primarily determined by variation in heart rate rather than by variation in stroke volume. The pacemaker activity of the sinus node has to be tuned for higher heart rates during the day in the human and this perhaps not only involves a day-night rhythm in the membrane and Ca2+ clock pacemaker mechanisms, but also in closely associated systems involving receptors and signalling for example. Presumably because the heart is continuously active, O2 utilisation per 100 g of tissue is highest for the heart. Because work carried out by the heart is primarily determined by the heart rate, O2 utilisation will be primarily determined by the heart rate. Perhaps for this reason, pacemaking and metabolism have to be controlled together including in a day-night manner and there have to be links between the two; AMP kinase could be one of these links30,85. For a similar reason, perhaps pacemaking and the extracellular matrix have to be controlled together to tune the extracellular matrix for the higher heart rate and pressures during the day in the human.
Day-night rhythm in transcription, translation, and mRNA and protein degradation
Transcripts changing in a day-night manner peaked either during the day (~ ZT 4–6) or during the night (ZT 18) (Fig. 7D). This pattern has been seen before: in mouse liver and heart (presumably ventricle) there were peaks at ZT 6–14 and ZT 2021, and in other studies of mouse heart (presumably ventricle) there were peaks at ZT 1 and ZT 1918 or ~ ZT 1 and ZT 1720. However, in all of these cases the day-time peak was larger than the night-time peak, whereas the opposite was true in the case of the sinus node (Fig. 7D). The present study has shown the likely immediate cause of this pattern. Transcripts for the transcription apparatus (HATs, transcription factors, HDACs) peaked at ~ ZT 4–6 and then at ZT 18 and this ultimately was likely to be responsible for the peak in transcripts at these two time points (Fig. 7). Transcripts for the translation apparatus (eukaryotic initiation factors, RNA polymerases) also peaked at the same time points and therefore generation of protein is expected to peak at the same time points (Fig. 7). Finally, transcripts for the apparatus for the breakdown of both transcripts and proteins (RNA degradation pathway, ubiquitine and proteasome) also peaked at the same two times (Fig. 7). However, what this study does not address is how these day-night rhythms impact on the level of proteins. This will depend on the life time of the protein, which can vary from minutes to years86. If the lifetime of the protein is short, there will be a day-night rhythm in the protein, but if it is longer than 24 h this will not be the case.
Potential systemic regulators
The master circadian clock in the suprachiasmatic nucleus is entrained to light via the eyes and neuronal circuitry, and peripheral circadian clocks like that in the sinus node are entrained by the master clock and other physiological stimuli. The peripheral clocks are entrained by neurohumoral factors and systemic regulators, including the autonomic nervous system87, corticosteroids87,88 and possibly thyroid hormone89. Presumably the same is true of the sinus node. It is also possible that these same neurohumoral factors and systemic regulators may directly affect pacemaking. It is interesting that transcripts for receptors for catecholamines, ACh, corticosteroids and thyroid hormone all showed day-night rhythms (Figs. 1, 4, S5 and S6). Spoor and Jackson90 reported that the heart rate response of isolated atria of the rat (nocturnal like the mouse) to ACh is greater during the day than at night, suggesting that the day-night rhythm in the muscarinic pathway (Fig. S6) has a functional corollary, but perhaps lagging behind mRNA by ~ 12 h. Also Peliciari-Garcia et al.89 reported greater triiodothyronine sensitivity (induction of transcript levels) at the end of the night. Therefore, it is possible that the effects of the autonomic nervous system, corticosteroids and thyroid hormone on the sinus node will not just depend on the known day-night rhythms of the autonomic nervous system, corticosteroids and thyroid hormone—they may also depend on the responsiveness of the sinus node to the factors.
Genes underlying familial and acquired sick sinus syndrome
Familial sick sinus syndrome has been linked to mutations in Hcn491, and acquired sick sinus syndrome in ageing92, heart failure93, atrial fibrillation94, diabetes95, pulmonary hypertension96 and even athletes11 has been linked to a downregulation of Hcn4. If there is a day-night rhythm in Hcn4 (P = 0.072), this may impact sick sinus syndrome. For example, athletes have a sinus bradycardia as a result of a downregulation of Hcn411,78 and the bradycardia is most marked at night and athletes can have long nocturnal pauses between heart beats at night14. Familial sick sinus syndrome has also been linked to mutations in KvLQT1 (Kcnq1), Kir2.1 (Kcnj2), and calsequestrin 2 (Casq2)27, all of which show a significant day-night rhythm (Kcnq1, P = 0.015; Kcnj2, P = 0.00017; Ryr2, P = 0.044; Casq2, P = 0.0080). Once again, the day-night rhythm in the ion channels may impact the phenotype caused by the mutation. Recently, Mesirca et al.97 have suggested block of the ACh-activated K+ channel to treat sick sinus syndrome. Figure S6 shows that the Kir3.1 subunit of the channel shows a significant day-night rhythm, and this may impact the effect of channel block.
Conclusion
In conclusion, there is an all-pervasive day-night rhythm in the transcriptome of the pacemaker of the heart, the sinus node. Whether this is responsible for the day-night rhythm in the heart rate or whether it prepares the pacemaker for the demands placed on it during the awake period remains to be determined.
Methods
Care and use of laboratory animals conformed to the UK Animals (Scientific Procedures) Act 1986 and Directive 2010/63/EU of the European Parliament. Ethical approval for all experimental procedures was granted by the University of Manchester Animal Welfare and Ethical Review body. 12–14 week old adult male C57bl/6j mice were maintained in a 12 h light:12 h dark cycle. The work flow is shown below:
Biopsies were collected from the sinus node as we have done previously in many other studies (e.g. Linscheid et al.98). We have previously reported the anatomy of the mouse sinus node99 and this dictated the position of the biospies. We collected biopsies from the smooth intercaval region between the superior and inferior vena cavae centred on the bifurcation of the sinus node artery. The high expression of sinus node markers (e.g. Hcn4) and absence of atrial markers (e.g. atrial natriuretic peptide) have confirmed the nature of the biopsies. Biopsies were collected at six time points at four hourly intervals over the 24 h period: at zeitgeber time (ZT) 0, 4, 8, 12, 16 and 20 h. At each time point, biopsies were collected from three mice. RNA was isolated from the sinus node as described previously78. Quantity and integrity of the RNA samples were measured using a 2200 TapeStation (Agilent Technologies) to ensure their suitability. Subsequently, TruSeq Stranded mRNA assays (Illumina) were used in order to produce libraries of more stable, single-stranded cDNA as follows. Total RNA was purified to polyadenylated mRNA via magnetic separation technology, which works through hybridisation of covalent interactions of oligo d(T)25 to poly (A) regions present in most eukaryotic mRNA. The mRNA sequences were fragmented into parts via divalent cations at higher temperature, and random primers were used to reverse transcribe the mRNA fragments into single-stranded cDNA. DNA polymerase and RNase H mediated the synthesis of the second cDNA strand produced from RNA oligonucleotides, originating from the 5´ end of the mRNA. The final cDNA library was generated by an addition of a single ‘A’ base, binding of adapters to the fragments and purification and enrichment via a PCR reaction. The cDNA libraries were incorporated into a multiplex system using the adapters; they were then pooled and clustered using a cBlot instrument (Illumina). Optical flow-cells containing the mRNA samples were then paired-end sequenced and mRNA was quantified through repeating 76 cycles twice, using a HiSeq4000 instrument (Illumina). Unmapped paired-end sequences from an Illumina HiSeq4000 sequencer were tested by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Sequence adapters were removed, and reads were quality trimmed using Trimmomatic_0.36100. The reads were mapped against the reference mouse genome (mm10/GRCm38) and counts per gene were calculated using annotation from GENCODE M21 (http://www.gencodegenes.org/) using STAR_2.5.3101. Normalisation was carried out using DESeq2_1.18.1102. DESeq2 performs an internal normalisation in which a geometric mean is calculated for each gene across all samples. The counts for a gene in each sample is then divided by this mean. The median of these ratios in a sample is the size factor for that sample. This procedure corrects for library size and RNA composition bias, which can arise for example when only a small number of genes are very highly expressed in one experimental condition but not in the other. In figures, the mean ± SEM transcript expression (normalised counts) from the three mice at each time point is plotted. To guide the eye, the data for ZT 0 are also shown for ZT 24 and the data may have been fitted with a sine wave (based on all time points including ZT 24); if fitted, the R2 value for the fitted curve is shown in figures. JTK Cycle24 was used to test whether a transcript showed a significant day-night rhythm (based on the data at ZT 0, 4, 8, 12, 16 and 20 (but not ZT 24); permutation-based P values are shown in figures. In figures, transcripts showing a significant day-night rhythm (permutation-based P value < 0.05) are shown in red and are fitted with a sine wave, transcripts showing a trend of a day-night rhythm (permutation-based P value > 0.05 but < 0.1) are shown in black and are fitted with a sine wave, and transcripts not showing a significant day-night rhythm (permutation-based P value > 0.1) are shown in black and are not fitted with a sine wave. IPA was used to identify potential targets of microRNAs.
Supplementary Information
Acknowledgements
This work was supported by a British Heart Foundation programme grant to MRB (PG/15/16/31330), a Fondation Leducq grant to MRB, HD and others (TNE FANTASY 19CV03), a British Heart Foundation Intermediate Fellowship to AD (FS/19/1/34035) and a British Heart Foundation Ph.D. Studentship to CA. We thank Andy Hayes and Leo Zeef of the Genomic Technologies and Bioinformatics Facilities at the University of Manchester for providing support with regard to RNAseq.
Author contributions
Y.W. isolated and prepared tissue samples for RNAseq. C.A. carried out the microRNA study (isolated and prepared tissues samples, conducted qPCR measurements and analysed the data). G.H. initiated and oversaw the project. H.D. raised funding and pioneered techniques. A.D. raised funding and directed and supervised work. M.R.B. initiated and designed the project, raised funding, directed and supervised work, analysed the RNAseq data, generated all graphics, prepared figures and wrote and revised the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/6/2021
The version of this Article previously published contained a lower resolution graphic within the image of Figure 4. This has now been corrected in the PDF and HTML versions of the Article.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82202-7.
References
- 1.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M, Heart Rate Working, G. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 2.Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2010;159:612–619.e613. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Shen X, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. Can. Med. Assoc. J. 2016;188:E53–E63. doi: 10.1503/cmaj.150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabritz L, Kirchhof P, Fortmüller L, Auchampach JA, Baba HA, Breithardt G, Neumann J, Boknik P, Schmitz W. Gene dose-dependent atrial arrhythmias, heart block, and brady-cardiomyopathy in mice overexpressing A3 adenosine receptors. Cardiovasc. Res. 2004;62:500–508. doi: 10.1016/j.cardiores.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caliskan K, Balk AH, Jordaens L, Szili-Torok T. Bradycardiomyopathy: The case for a causative relationship between severe sinus bradycardia and heart failure. J. Cardiovasc. Electrophysiol. 2010;21:822–824. doi: 10.1111/j.1540-8167.2009.01704.x. [DOI] [PubMed] [Google Scholar]
- 6.Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, Postma AV, van der Bilt IA, Baars MJ, van Haelst PL, Caliskan K, Hoedemaekers YM, Le Scouarnec S, Redon R, Pinto YM, Christiaans I, Wilde AA, Bezzina CR. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J. Am. Coll. Cardiol. 2014;64:745–756. doi: 10.1016/j.jacc.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji Y, Opthof T, Yasui K, Inden Y, Takemura H, Niwa N, Lu Z, Lee JK, Honjo H, Kamiya K, Kodama I. Ionic mechanisms of acquired QT prolongation and torsades de pointes in rabbits with chronic complete atrioventricular block. Circulation. 2002;106:2012–2018. doi: 10.1161/01.CIR.0000031160.86313.24. [DOI] [PubMed] [Google Scholar]
- 8.El Khoury N, Mathieu S, Marger L, Ross J, El Gebeily G, Ethier N, Fiset C. Upregulation of the hyperpolarization-activated current increases pacemaker activity of the sinoatrial node and heart rate during pregnancy in mice. Circulation. 2013;127:2009–2020. doi: 10.1161/CIRCULATIONAHA.113.001689. [DOI] [PubMed] [Google Scholar]
- 9.Abd Allah ESH, Tellez JO, Nelson T, Monfredi O, Boyett MR, Dobrzynski H. Changes in the expression of ion channels, connexins and Ca2+-handling proteins in the sinoatrial node during postnatal development. Exp. Physiol. 2011;96:426–438. doi: 10.1113/expphysiol.2010.055780. [DOI] [PubMed] [Google Scholar]
- 10.Yanni J, Tellez JO, Maczewski M, Sutyagin PV, Mackiewicz U, Atkinson A, Inada S, Beresewicz A, Billeter R, Dobrzynski H, Boyett MR. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sinoatrial node pacemaking. Exp. Physiol. 2011;96:1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza A, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM, Boyett MR. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Commun. 2014;5:3775. doi: 10.1038/ncomms4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanni J, D’Souza A, Wang Y, Li N, Hansen BJ, Zakharkin SO, Smith M, Hayward C, Whitson BA, Mohler PJ, Janssen PML, Zeef L, Choudhury M, Zi M, Cai X, Logantha SJRJ, Nakao S, Atkinson A, Petkova M, Doris U, Ariyaratnam J, Cartwright EJ, Griffiths-Jones S, Hart G, Fedorov VV, Oceandy D, Dobrzynski H, Boyett MR. Silencing miR-370-3p rescues funny current and sinus node function in heart failure. Sci. Rep. 2020;10:11279. doi: 10.1038/s41598-020-67790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black N, D'Souza A, Wang Y, Piggins H, Dobrzynski H, Morris G, Boyett MR. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 2019;16:298–307. doi: 10.1016/j.hrthm.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northcote RJ, Canning GP, Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br. Heart J. 1989;61:155–160. doi: 10.1136/hrt.61.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otsuka K, Ikari M, Ichimaru Y, Saito H, Kawakami T, Otsuka K, Kaba H, Seto K. Experimental study on the relationship between cardiac arrhythmias and sleep states by ambulatory ECG-EEC monitoring. Clin. Cardiol. 1986;9:305–313. doi: 10.1002/clc.4960090702. [DOI] [PubMed] [Google Scholar]
- 16.Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ. Robust circadian rhythm in heart rate and its variability: Influence of exogenous melatonin and photoperiod. J. Sleep Res. 2007;16:148–155. doi: 10.1111/j.1365-2869.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 17.West AC, Smith L, Ray DW, Loudon ASI, Brown TM, Bechtold DA. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 2017;8:417. doi: 10.1038/s41467-017-00462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J. Mol. Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. U.S.A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, Liao X, Coller J, Jain Mukesh K. KLF15 establishes the landscape of diurnal expression in the heart. Cell Reports. 2015;13:2368–2375. doi: 10.1016/j.celrep.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 21.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 22.Durgan DJ, Young ME. The cardiomyocyte circadian clock: Emerging roles in health and disease. Circ. Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young ME. Circadian control of cardiac metabolism: Physiologic roles and pathologic implications. Methodist DeBakey Cardiovasc. J. 2017;13:15–19. doi: 10.14797/mdcj-13-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: An efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms. 2010;25:372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardman JA, Haslam IS, Farjo N, Farjo B, Paus R. Thyroxine differentially modulates the peripheral clock: Lessons from the human hair follicle. PLoS ONE. 2015;10:e0121878. doi: 10.1371/journal.pone.0121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrzynski H, Anderson RH, Atkinson A, Borbas Z, D'Souza A, Fraser JF, Inada S, Logantha SJRJ, Monfredi O, Morris GM, Moorman AFM, Nikolaidou T, Schneider H, Szuts V, Temple IP, Yanni J, Boyett MR. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 2013;139:260–288. doi: 10.1016/j.pharmthera.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Lakatta EG, DiFrancesco D. What keeps us ticking: A funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, Mangoni ME, Clapham DE. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E3037–3046. doi: 10.1073/pnas.1311865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yavari A, Bellahcene M, Bucchi A, Sirenko S, Pinter K, Herring N, Jung JJ, Tarasov KV, Sharpe EJ, Wolfien M, Czibik G, Steeples V, Ghaffari S, Nguyen C, Stockenhuber A, Clair JRS, Rimmbach C, Okamoto Y, Yang D, Wang M, Ziman BD, Moen JM, Riordon DR, Ramirez C, Paina M, Lee J, Zhang J, Ahmet I, Matt MG, Tarasova YS, Baban D, Sahgal N, Lockstone H, Puliyadi R, de Bono J, Siggs OM, Gomes J, Muskett H, Maguire ML, Beglov Y, Kelly M, Dos Santos PPN, Bright NJ, Woods A, Gehmlich K, Isackson H, Douglas G, Ferguson DJP, Schneider JE, Tinker A, Wolkenhauer O, Channon KM, Cornall RJ, Sternick EB, Paterson DJ, Redwood CS, Carling D, Proenza C, David R, Baruscotti M, DiFrancesco D, Lakatta EG, Watkins H, Ashrafian H. Mammalian gamma2 AMPK regulates intrinsic heart rate. Nat. Commun. 2017;8:1258. doi: 10.1038/s41467-017-01342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin RZ, Lu Z, Anyukhovsky EP, Jiang YP, Wang HZ, Gao J, Rosen MR, Ballou LM, Cohen IS. Regulation of heart rate and the pacemaker current by phosphoinositide 3-kinase signaling. J. Gen. Physiol. 2019;151:1051–1058. doi: 10.1085/jgp.201812293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZM, Prasad C, Britton FC, Ye LL, Hatton WJ, Duan D. Functional role of CLC-2 chloride inward rectifier channels in cardiac sinoatrial nodal pacemaker cells. J. Mol. Cell. Cardiol. 2009;47:121–132. doi: 10.1016/j.yjmcc.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeshima H, Venturi E, Sitsapesan R. New and notable ion-channels in the sarcoplasmic/endoplasmic reticulum: Do they support the process of intracellular Ca2+ release? J. Physiol. 2015;593:3241–3251. doi: 10.1113/jphysiol.2014.281881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, Abbasi C, Lazic S, Teng ACT, Wang D, Dubois N, Ignatchenko V, Wong V, Liu J, Araki T, Tiburcy M, Ackerley C, Zimmermann WH, Hamilton R, Sun Y, Liu PP, Keller G, Stagljar I, Scott IC, Kislinger T, Gramolini AO. Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function. Nat. Commun. 2015;6:8391. doi: 10.1038/ncomms9391. [DOI] [PubMed] [Google Scholar]
- 35.Inada S, Zhang H, Tellez JO, Shibata N, Nakazawa K, Kamiya K, Kodama I, Mitsui K, Dobrzynski H, Boyett MR, Honjo H. Importance of gradients in membrane properties and electrical coupling in sinoatrial node pacing. PLoS ONE. 2014;9:e94565. doi: 10.1371/journal.pone.0094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavlovic D, Fuller W, Shattock MJ. Novel regulation of cardiac Na pump via phospholemman. J. Mol. Cell. Cardiol. 2013;61:83–93. doi: 10.1016/j.yjmcc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Mustafa, S. J., Morrison, R. R., Teng, B. & Pelleg, A. Adenosine receptors and the heart: Role in regulation of coronary blood flow and cardiac electrophysiology. Handb. Exp. Pharmacol. 193, 161–188 (2009). [DOI] [PMC free article] [PubMed]
- 38.Wu, Y. & Anderson, M. CaMKII in sinoatrial node physiology and dysfunction. Front. Pharmacol.5, 48 (2014). [DOI] [PMC free article] [PubMed]
- 39.Kreusser MM, Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J. Clin. Investig. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol. Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury SK, Liu W, Zi M, Li Y, Wang S, Tsui H, Prehar S, Castro S, Zhang H, Ji Y, Zhang X, Xiao R, Zhang R, Lei M, Cyganek L, Guan K, Millar CB, Liao X, Jain MK, Boyett MR, Cartwright EJ, Shiels HA, Wang X. Stress-activated kinase mitogen-activated kinase kinase-7 governs epigenetics of cardiac repolarization for arrhythmia prevention. Circulation. 2017;135:683–699. doi: 10.1161/CIRCULATIONAHA.116.022941. [DOI] [PubMed] [Google Scholar]
- 43.Liu VWT, Huang PL. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y-B, Luo J-W, Jiang F, Liu G. Genetic analysis of sick sinus syndrome in a family harboring compound CACNA1C and TTN mutations. Mol. Med. Rep. 2018;17:7073–7080. doi: 10.3892/mmr.2018.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng, W. H., Li, M. X., Li, H. L., Tang, K., Zhuang, J. H., Zhang, J. G., Xiao, J. J., Jiang, H., Li, D. L., Yu, Y. C., Sham, P. C., Nattel, S. & Xu, Y. W. Dysfunction of myosin light-chain 4 (MYL4) leads to heritable atrial cardiomyopathy with electrical, contractile, and structural components: Evidence from genetically-engineered rats. J. Am. Heart Assoc.6, 11 (2017). [DOI] [PMC free article] [PubMed]
- 46.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Bermúdez J, Cuezva JM. The ATPase inhibitory factor 1 (IF1): A master regulator of energy metabolism and of cell survival. Biochim. Biophys. Acta Bioenerg. 2016;1857:1167–1182. doi: 10.1016/j.bbabio.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Yang K, Long Q, Saja K, Huang F, Pogwizd SM, Zhou L, Yoshida M, Yang Q. Knockout of the ATPase inhibitory factor 1 protects the heart from pressure overload-induced cardiac hypertrophy. Sci. Rep. 2017;7:10501–10501. doi: 10.1038/s41598-017-11251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labrecque N, Cermakian N. Circadian clocks in the immune system. J. Biol. Rhythms. 2015;30:277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- 50.Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: Friendly guests or unwelcome visitors? Am. J. Physiol. Heart Circulatory Physiol. 2019;317:H124–H140. doi: 10.1152/ajpheart.00028.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K, Karoor V. Role of inflammatory cell subtypes in heart failure. J. Immunol. Res. 2019;2019:2164017. doi: 10.1155/2019/2164017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiina T, Blancher A, Inoko H, Kulski JK. Comparative genomics of the human, macaque and mouse major histocompatibility complex. Immunology. 2017;150:127–138. doi: 10.1111/imm.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundararaj S, Zhang J, Krovi SH, Bedel R, Tuttle KD, Veerapen N, Besra GS, Khandokar Y, Praveena T, Le Nours J, Matsuda JL, Rossjohn J, Gapin L. Differing roles of CD1d2 and CD1d1 proteins in type I natural killer T cell development and function. Proc. Natl. Acad. Sci. 2018;115:E1204. doi: 10.1073/pnas.1716669115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghali R, Altara R, Louch WE, Cataliotti A, Mallat Z, Kaplan A, Zouein FA, Booz GW. IL-33 (Interleukin 33)/sST2 axis in hypertension and heart failure. Hypertension. 2018;72:818–828. doi: 10.1161/HYPERTENSIONAHA.118.11157. [DOI] [PubMed] [Google Scholar]
- 55.Bloch Thomsen PE, Jons C, Raatikainen MJ, Moerch Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Gang UJ, Hoest N, Boersma LV, Platou ES, Becker D, Messier MD, Huikuri HV. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: The Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010;122:1258–1264. doi: 10.1161/CIRCULATIONAHA.109.902148. [DOI] [PubMed] [Google Scholar]
- 56.Mezzano V, Liang Y, Wright AT, Lyon RC, Pfeiffer E, Song MY, Gu Y, Dalton ND, Scheinman M, Peterson KL, Evans SM, Fowler S, Cerrone M, McCulloch AD, Sheikh F. Desmosomal junctions are necessary for adult sinus node function. Cardiovasc. Res. 2016;111:274–286. doi: 10.1093/cvr/cvw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SH, Lee KH, Kim DY, Kwak E, Kim S, Kim KT. Rhythmic control of mRNA stability modulates circadian amplitude of mouse Period3 mRNA. J. Neurochem. 2015;132:642–656. doi: 10.1111/jnc.13027. [DOI] [PubMed] [Google Scholar]
- 58.Hervieu A, Kermorgant S. The role of PI3K in met driven cancer: A recap. Front. Mol. Biosci. 2018;5:86–86. doi: 10.3389/fmolb.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Q, Ghoshal S, Rodrigues A, Gao S, Asterian A, Kamenecka TM, Barrow JC, Chakraborty A. Adipocyte-specific deletion of Ip6k1 reduces diet-induced obesity by enhancing AMPK-mediated thermogenesis. J. Clin. Investig. 2016;126:4273–4288. doi: 10.1172/JCI85510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Liu M, Xia X, Gong T, Feng J, Liu W, Liu Y, Zhen B, Wang Y, Ding C, Qin J. A mouse tissue transcription factor atlas. Nat. Commun. 2017;8:15089. doi: 10.1038/ncomms15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vong L, Bi W, O'Connor-Halligan KE, Li C, Cserjesi P, Schwarz JJ. MEF2C is required for the normal allocation of cells between the ventricular and sinoatrial precursors of the primary heart field. Dev. Dyn. 2006;235:1809–1821. doi: 10.1002/dvdy.20828. [DOI] [PubMed] [Google Scholar]
- 63.Xuan C, Jia KG, Wang BB, Bai XY, Gao G, Yang Q, Wang XL, Liu XC, Ma X, He GW. Identification of two novel mutations of the HOMEZ gene in Chinese patients with isolated ventricular septal defect. Genet. Test. Mol. Biomark. 2013;17:390–394. doi: 10.1089/gtmb.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Loo PF, Mahtab EAF, Wisse LJ, Hou J, Grosveld F, Suske G, Philipsen S, Gittenberger-de Groot AC. Transcription factor Sp3 knockout mice display serious cardiac malformations. Mol. Cell. Biol. 2007;27:8571. doi: 10.1128/MCB.01350-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caprioli A, Koyano-Nakagawa N, Iacovino M, Shi X, Ferdous A, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 represses Gata1 gene expression and modulates the cellular fate of cardiac progenitors during embryogenesis. Circulation. 2011;123:1633–1641. doi: 10.1161/CIRCULATIONAHA.110.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OWJ, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- 67.Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput. Biol. 2008;4:e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Priest, J. R., Vogler, G., Nurnberg, S., Portman, M., Gelb, B. D., Ashley, E. A., Bodmer, R. & Quertermous, T. The transcriptional repressor NR1D2 is associated with congenital heart disease and plays an evolutionarily conserved role in cardiac development. Circulation132, Abstract 16484 (2015).
- 69.Blackwood EA, Hofmann C, Santo Domingo M, Bilal AS, Sarakki A, Stauffer W, Arrieta A, Thuerauf DJ, Kolkhorst FW, Muller OJ, Jakobi T, Dieterich C, Katus HA, Doroudgar S, Glembotski CC. ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, Rheb. Circ. Res. 2019;124:79–93. doi: 10.1161/CIRCRESAHA.118.313854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu D, Wang J, Li J, Guan F, Zhang X, Dong W, Liu N, Gao S, Zhang L. Meox1 accelerates myocardial hypertrophic decompensation through Gata4. Cardiovasc. Res. 2018;114:300–311. doi: 10.1093/cvr/cvx222. [DOI] [PubMed] [Google Scholar]
- 71.Takizawa T, Arai M, Tomaru K, Koitabashi N, Baker D, Periasamy M, Kurabayashi M. Transcription factor Sp1 regulates SERCA2 gene expression in pressure-overloaded hearts: A study using in vivo direct gene transfer into living myocardium. J. Mol. Cell. Cardiol. 2003;35:777–783. doi: 10.1016/S0022-2828(03)00122-6. [DOI] [PubMed] [Google Scholar]
- 72.Flesch M. On the trail of cardiac specific transcription factors. Cardiovasc. Res. 2001;50:3–6. doi: 10.1016/S0008-6363(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 73.Duan Y, Zhu W, Liu M, Ashraf M, Xu M. The expression of Smad signaling pathway in myocardium and potential therapeutic effects. Histol. Histopathol. 2017;32:651–659. doi: 10.14670/HH-11-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sande-Melón M, Marques IJ, Galardi-Castilla M, Langa X, Pérez-López M, Botos M-A, Sánchez-Iranzo H, Guzmán-Martínez G, Ferreira Francisco DM, Pavlinic D, Benes V, Bruggmann R, Mercader N. Adult sox10+ cardiomyocytes contribute to myocardial regeneration in the zebrafish. Cell Rep. 2019;29:1041–1054. doi: 10.1016/j.celrep.2019.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuratomi S, Ohmori Y, Ito M, Shimazaki K, Muramatsu S, Mizukami H, Uosaki H, Yamashita JK, Arai Y, Kuwahara K, Takano M. The cardiac pacemaker-specific channel Hcn4 is a direct transcriptional target of MEF2. Cardiovasc. Res. 2009;83:682–687. doi: 10.1093/cvr/cvp171. [DOI] [PubMed] [Google Scholar]
- 76.Espinoza-Lewis RA, Yu L, He F, Liu H, Tang R, Shi J, Sun X, Martin JF, Wang D, Yang J, Chen Y. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009;327:376–385. doi: 10.1016/j.ydbio.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson C, Forte G, Wilson C, Oceandy D, Boyett MR, D’Souza A. MicroRNA control of the circadian rhythm in heart rate. Proc. Physiol. Soc. 2019;45:C06. [Google Scholar]
- 78.D'Souza A, Pearman CM, Wang Y, Nakao S, Logantha S, Cox C, Bennett H, Zhang Y, Johnsen AB, Linscheid N, Poulsen PC, Elliott J, Coulson J, McPhee J, Robertson A, da Costa Martins PA, Kitmitto A, Wisloff U, Cartwright EJ, Monfredi O, Lundby A, Dobrzynski H, Oceandy D, Morris GM, Boyett MR. Targeting miR-423-5p reverses exercise training-induced HCN4 channel remodeling and sinus bradycardia. Circ. Res. 2017;121:1058–1068. doi: 10.1161/CIRCRESAHA.117.311607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 80.Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR. Biophysical characterisation of the under-appreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D'Souza, A., Wang, Y., Anderson, C., Bucchi, A., Baruscotti, M., Olieslagers, S., Mesirca, P., Johnsen, A. B., Mastitskaya, S., Ni, H., Zhang, Y., Black, N., Cox, C., Wegner, S., Bano-Otalora, B., Petit, C., Gill, E., Logantha, S. J., Dobrzynski, H., Ashton, N., Hart, G., Zhang, R., Zhang, H., Cartwright, E. J., Wisloff, U., Mangoni, M. E., Da Costa Martins, P., Piggins, H. D., DiFrancesco, D. & Boyett, M. R. A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate. Heart Rhythm(in press) (2020). [DOI] [PMC free article] [PubMed]
- 83.Tong M, Watanabe E, Yamamoto N, Nagahata-Ishiguro M, Maemura K, Takeda N, Nagai R, Ozaki Y. Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart. Biol. Rhythm Res. 2013;44:519–530. doi: 10.1080/09291016.2012.704801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tong MQ, Wang SS, Pang YY, Zhou Y, Cui HB, Ruan LM, Su J, Chen XM. Circadian expression of connexins in the mouse heart. Biol. Rhythm Res. 2016;47:631–639. doi: 10.1080/09291016.2016.1174404. [DOI] [Google Scholar]
- 85.Salt IP, Hardie DG. AMP-activated protein kinase: An ubiquitous signaling pathway with key roles in the cardiovascular system. Circ. Res. 2017;120:1825–1841. doi: 10.1161/CIRCRESAHA.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toyama BH, Hetzer MW. Protein homeostasis: Live long, won't prosper. Nat. Rev. Mol. Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Astiz M, Heyde I, Oster H. Mechanisms of communication in the mammalian circadian timing system. Int. J. Mol. Sci. 2019;20:343. doi: 10.3390/ijms20020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pezük P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153:4775–4783. doi: 10.1210/en.2012-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peliciari-Garcia RA, Prévide RM, Nunes MT, Young ME. Interrelationship between 3,5,3′-triiodothyronine and the circadian clock in the rodent heart. Chronobiol. Int. 2016;33:1444–1454. doi: 10.1080/07420528.2016.1229673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spoor RP, Jackson DB. Circadian rhythms: Variation in sensitivity of isolated rat artria to acetylcholine. Science. 1966;154:782. doi: 10.1126/science.154.3750.782. [DOI] [PubMed] [Google Scholar]
- 91.Baruscotti M, Bucchi A, Milanesi R, Paina M, Barbuti A, Gnecchi-Ruscone T, Bianco E, Vitali-Serdoz L, Cappato R, DiFrancesco D. A gain-of-function mutation in the cardiac pacemaker HCN4 channel increasing cAMP sensitivity is associated with familial inappropriate sinus tachycardia. Eur. Heart J. 2015;38:280–288. doi: 10.1093/eurheartj/ehv582. [DOI] [PubMed] [Google Scholar]
- 92.Du J, Deng S, Pu D, Liu Y, Xiao J, She Q. Age-dependent down-regulation of hyperpolarization-activated cyclic nucleotide-gated channel 4 causes deterioration of canine sinoatrial node function. Acta Biochim. Biophys. Sin. 2017;49:400–408. doi: 10.1093/abbs/gmx026. [DOI] [PubMed] [Google Scholar]
- 93.Zicha S, Fernandez-Velasco M, Lonardo G, L'Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc. Res. 2005;66:472–481. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 94.Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P, Wang Z, Kuo CT, Nattel S. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: A molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Wang Y, Yanni J, Qureshi MA, Logantha S, Kassab S, Boyett MR, Gardiner NJ, Sun H, Howarth FC, Dobrzynski H. Electrical conduction system remodeling in streptozotocin-induced diabetes mellitus rat heart. Front. Physiol. 2019;10:826. doi: 10.3389/fphys.2019.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamanushi, T. T., Yanni, J., Dobrzynski, H., Kabuto, H. & Boyett, M. R. Changes in ion channel expression in right-sided congestive heart failure. J. Mol. Cell. Cardiol.48 Supplement (S73) (2010).
- 97.Mesirca P, Bidaud I, Briec F, Evain S, Torrente AG, Le Quang K, Leoni AL, Baudot M, Marger L, Chong Chung You, A., Nargeot, J., Striessnig, J., Wickman, K., Charpentier, F. & Mangoni, M. E. G, protein-gated IKACh channels as therapeutic targets for treatment of sick sinus syndrome and heart block. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E932–E941. doi: 10.1073/pnas.1517181113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Linscheid N, Logantha S, Poulsen PC, Zhang S, Schrolkamp M, Egerod KL, Thompson JJ, Kitmitto A, Galli G, Humphries MJ, Zhang H, Pers TH, Olsen JV, Boyett M, Lundby A. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat. Commun. 2019;10:2889. doi: 10.1038/s41467-019-10709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: Structure and expression of HCN channels. Cardiovasc. Res. 2007;73:729–738. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 100.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krämer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.