Abstract
Bmp plays an important role in cardiomyocyte differentiation, but the function of Smad4 in Bmp signaling remains elusive. Here, we show that disruption of the Smad4 gene in cardiac progenitors expressing Sfrp5 led to embryonic lethality with hypoplastic heart formation. Although the expression of Nkx2-5 is regulated by Bmp signaling, expression of Nkx2-5 was weakly detected in the mutant heart. However, the nuclear translocation of Nkx2-5 was impaired. Expression of CK2 or PP1, which could alter the phosphorylation status of the NLS of Nkx2-5, was not affected, but Nkx2-5 was found to bind to Smad4 by co-immunoprecipitation experiments. Introduction of Smad4 into cells derived from Smad4 conditional knockout embryonic hearts restored the nuclear localization of Nkx2-5, and exogenous Nkx2-5 failed to translocate into the nucleus of Smad4-depleted fibroblasts. These results suggest that Smad4 plays an essential role in cardiomyocyte differentiation by controlling not only transcription but also the nuclear localization of Nkx2-5.
Subject terms: Differentiation, Heart development
Introduction
The heart is the first organ to establish a blood circulatory system required for the subsequent development of other organs. Decades of research have revealed that intricate signaling networks, including those involving members of the Wnt, Bmp, and Fgf pathways, play key roles in heart development because perturbation of these secreted factors has been shown to result in a wide range of defects in embryonic and adult hearts1–5. Understanding how signaling networks regulate heart development is critical for elucidating mechanisms underlying congenital heart diseases and for developing novel approaches in regenerative cardiovascular medicine.
Bmp signaling is involved in fate decisions of multiple cell lineages of cardiomyocytes through the transcriptional regulation of cardiac-specific transcription factors, such as GATA4, Mef2c, and Nkx2-56–13. Bmp signaling has been reported to directly regulate transcription of the Nkx2-5 gene, which encodes one of the earliest and most important cardiac transcription factors14,15, given the presence of Smad binding sites in the enhancer region of the Nkx2-5 gene13. Smad4 is a common-mediator Smad (Co-Smad). Once the Bmp receptor is activated by ligand binding, Smad1/5/8 is phosphorylated and forms a complex with Smad4. This complex then translocates into the nucleus and regulates the transcription of target genes, suggesting that Smad4 plays a central role in Bmp signaling.
Smad4-deficient mouse embryos die by E7.5 due to impaired gastrulation, suggesting that Smad4 is required for mesodermal formation16. An analysis of cardiomyocyte-specific Smad4-KO mice revealed that Smad4 functions in the proliferation and differentiation of cardiomyocytes, as reflected in the thinner ventricles in both trabecular and compact hearts17. However, the function of Smad4 during the process of cardiomyocyte differentiation remains unknown.
Wnt proteins are secreted proteins that play a key role in differentiation during the development of many organs, including the heart18–20. In a previous study, we reported that Sfrp5 (a decoy receptor of the Wnt ligand) is a marker of cardiac progenitor cells, which form the first heart field (FHF) and later contribute to the left ventricle (LV), both atria, sinus venous (SV), and a part of the outflow tract (OFT), but not to the right ventricle (RV)21. Thus, mice that express Cre in Sfrp5-expressing cells could serve as a tool to generate mice that specifically lack the Smad4 gene in cardiac progenitor cells.
In the present study, we provide evidence that Smad4 forms a complex with Nkx2-5 to control the nuclear localization of Nkx2-5, and that this in turn promotes the differentiation of cardiac progenitors and heart morphogenesis. To examine specific functions of Smad4 in cardiogenesis, we generated a Smad4-conditional knock-out (Smad4-cKO) model by crossing Smad4floxed/floxed mice with Sfrp5-Cre mice. Smad4-cKO mice exhibited severe cardiac hypoplasia with inhibited myocardial cell differentiation due to the down-regulation of Nkx2-5 expression and loss of nuclear localization of Nkx2-5. These findings suggest that, in addition to a mediator of Bmp signaling, Smad4 could be responsible for cardiac differentiation by binding to Nkx2-5 and regulating its nuclear localization.
Results
Smad4 is required for proper cardiogenesis
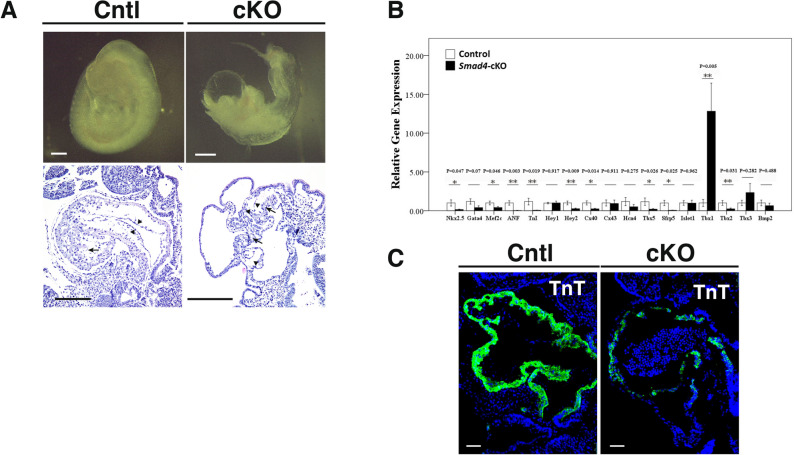
In order to explore the function of Smad4 in cardiomyocyte differentiation, we disrupted the Smad4 gene in Sfrp5-expressing cardiac progenitor cells. Specifically, Sfrp5 Cre/Cre; Smad4 del/+ mice were crossed with Smad4f./f mice21. Compared to the littermate controls (Sfrp5 Cre/+; Smad4del/+), Smad4-cKO mice (Sfrp5 Cre/+; Smad4 del/del) were embryonic lethal at around E8.5, with a hypoplastic heart tube and edema of the pericardium (Fig. 1A). The looping process has already begun but stopped in the middle of the process in the mutant hearts (Supplementary Fig. S1). Histological analysis showed that both trabecular and compact myocardial layers in the mutant heart were much thinner than those in the control. This suggests that Smad4 is required for heart development.
Figure 1.
Disruption of the Smad4 gene in Sfrp5-expressing cells leads to hypoplastic heart formation and impaired cardiac differentiation. (A,B) Gross appearance (A) and Hematoxylin and Eosin staining (B) of sagittal sections of Smad4 mutant (Sfrp5 Cre/+; Smad4 del/del) and littermate control (Sfrp5 Cre/+; Smad4del/+) embryos dissected at E9.5. Scale bar = 100 μm. (C) Relative gene expression levels of key factors involved in cardiogenesis. n = 9 and 7 for control and Smad4-cKO, respectively. Data are presented as mean ± SEM. (D) Immunofluorescent staining for cTnT (green) in control and Smad4 mutant embryos at E9.5. Samples were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) to visualize nuclei (blue). Scale bar = 50 μm.
Since Bmp signaling regulates many genes involved in myocardial differentiation, we assessed the expression of downstream target genes by RT-PCR to gain insight into the potential cause of the hypoplastic heart in Smad4-cKO embryos. Transcription factors required in the myocardial differentiation process, including Nkx2-5, Gata4, and Mef2c, as well as myocardial components, such as Troponin-I and Anf, were down-regulated in Smad4-cKO hearts relative to control hearts. The expression of FHF markers, including Hcn4, Tbx5, and Sfrp5, was decreased, and the expression of SHF markers, such as Tbx1 and Islet1, were unaffected or increased, in Smad4-cKO hearts relative to control hearts (Fig. 1B). Immunofluorescent staining of cTnT also revealed impaired cardiac differentiation in Smad4-cKO hearts (Fig. 1C). These observations suggested that Bmp signaling through Smad4 is required for proper cardiogenesis by regulating the expression of cardiac-specific transcription factors and/or cardiomyocyte-specific components.
Smad4 regulates the expression and nuclear localization of Nkx2-5
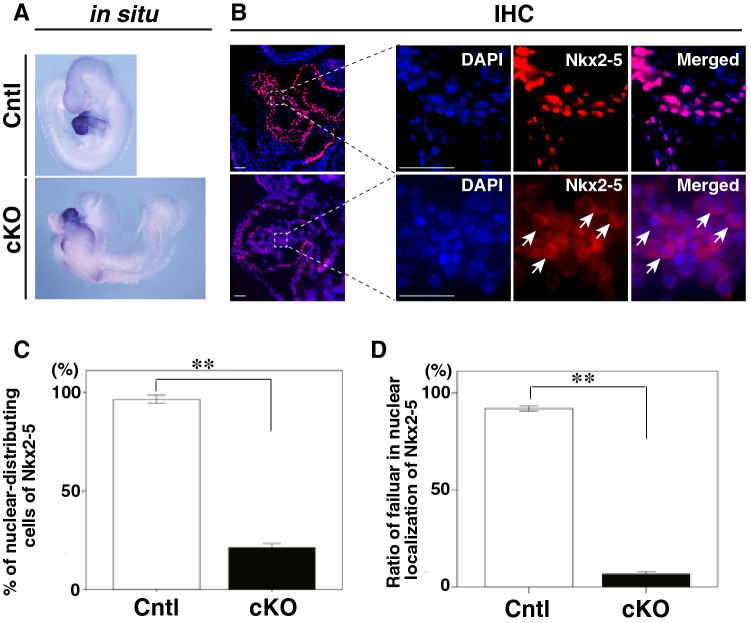
Since many genes encoding cardiac-specific transcription factors, including Nkx2-5, are down-regulated in Smad4-cKO hearts, and Nkx2-5 has been suggested to be regulated by Bmp signaling through Smad-binding regions22, we hypothesized that lack of Nkx2-5 function might explain the impaired cardiac differentiation in Smad4-cKO embryos. In a previous study, Nkx2-5, a homeobox transcription factor, was reported to be a key factor in heart progenitor specification and differentiation by regulating the transcription of multiple cardiac-specific genes, including Gja5 (Cx40), Mef2c, Hey2, Nppa (ANF), and Isl115. Since Smad4 is required for Nkx2-5 gene expression as it binds its upstream enhancer region in vivo13, we expected that Nkx2-5 expression would be low or absent in Smad4-cKO hearts. However, Nkx2-5 was expressed, albeit weakly (Fig. 1B), but detectable in Smad4-cKO hearts by in situ hybridization (ISH) (Fig. 2A). To confirm whether the Nkx2-5 protein was translated, immunofluorescent staining was performed and revealed that Nkx2-5 protein was weakly expressed (Fig. 2B, C). Interestingly, Nkx2-5 was present mainly in the nuclei of control heart cells, while they were found outside the nuclei of Smad4-cKO heart cells (Fig. 2B, D), suggesting that the loss of Smad4 prevented the nuclear localization of Nkx2-5.
Figure 2.
Lack of nuclear localization of Nkx2-5 in Smad4-cKO embryonic hearts. (A) Whole-mount ISH of control and Smad4-cKO embryos with a probe for Nkx2-5 at E9.5. (B) Immunofluorescent staining of sagittal sections of control and Smad4-cKO embryos at E9.5 using anti-Nkx2-5 antibodies (red) and DAPI for detection of nuclei (blue). Arrows indicate the lack of nuclear localization of Nkx2-5. H, heart. Scale bar = 100 μm. (C and D) Percentage of Nkx2-5 protein distributing cell in control and Smad4-cKO embryonic hearts (C) and the percentage of cells showing nuclear localization of Nkx2-5 in control and Smad4-cKO embryonic hearts (D). Data were collected from 10 sections for each embryo (n = 3 and 3 for control and Smad4-cKO, respectively). Data are presented as mean ± SEM, **P < 0.01 (Mann–Whitney U test).
Impaired nuclear localization of Nkx2-5 in Smad4-cKO hearts is not a result of alterations in the phosphorylation status of Nkx2-5
The nuclear translocation of a transcription factor is often determined by the phosphorylation state of its NLS domain. Nkx2-5 contains a highly conserved NLS domain, and phosphorylation of serine 163 (Ser163) by Casein kinase 2 (CK2), a ubiquitously expressed and constitutively active Ser/Thr protein kinase23, is required for nuclear translocation. On this basis, we hypothesized that CK2 expression could be induced by Bmp signaling through Smad4 and examined this by qPCR and in situ hybridization. The expression level of CK2α was decreased, but clearly present, in Smad4-cKO hearts relative to control hearts (Supplementary Fig. S2A, S2B). We used Csnk2a1 as a probe for the CK2 gene because the CK2 catalytic subunit α, rather than its other regulatory subunits, is expressed dominantly in the mouse embryonic heart24. Moreover, luciferase assays showed no activation of the upstream region of the CK2α gene upon stimulation of Bmp signaling (Supplementary Fig. S2C, S2D), suggesting that CK2 expression was not mainly regulated by Smad4.
We next determined whether excessive dephosphorylation of Nkx2-5 by the β-isoform of protein phosphatase 1 (PP1β) might explain the impaired nuclear localization of Nkx2-5. Since the regulatory subunit of PP1β, myosin phosphatase targeting subunit 1 (Mypt1), has been reported to be responsible for the nuclear exclusion of Nkx2-5 through direct interaction25, we expected Mypt1 to be dominantly localized in the nuclei and thereby prevent Nkx2-5 nuclear localization. However, in both control and Smad4-cKO hearts, Mypt1 was robustly expressed outside the nuclei (Supplementary Fig. S3). We also tested the possibility that the expression of protein phosphatase 1 regulatory subunit 3c (Ppp1r3c), a regulatory subunit of PP1 specifically expressed in the heart, might be increased in Smad4-cKO hearts. Here too, no difference was noted in the level of expression of Ppp1r3c by RT-PCR and ISH in Smad4-cKO and control hearts (Supplementary Fig. S4A, S4B), suggesting that Smad4 is not essential for Ppp1r3c expression. Taken together, these findings suggest that the phosphorylation state of the Nkx2-5 NLS might not be regulated by Bmp/Smad4 signaling.
Smad4 regulates Nkx2-5 nuclear localization via protein interaction
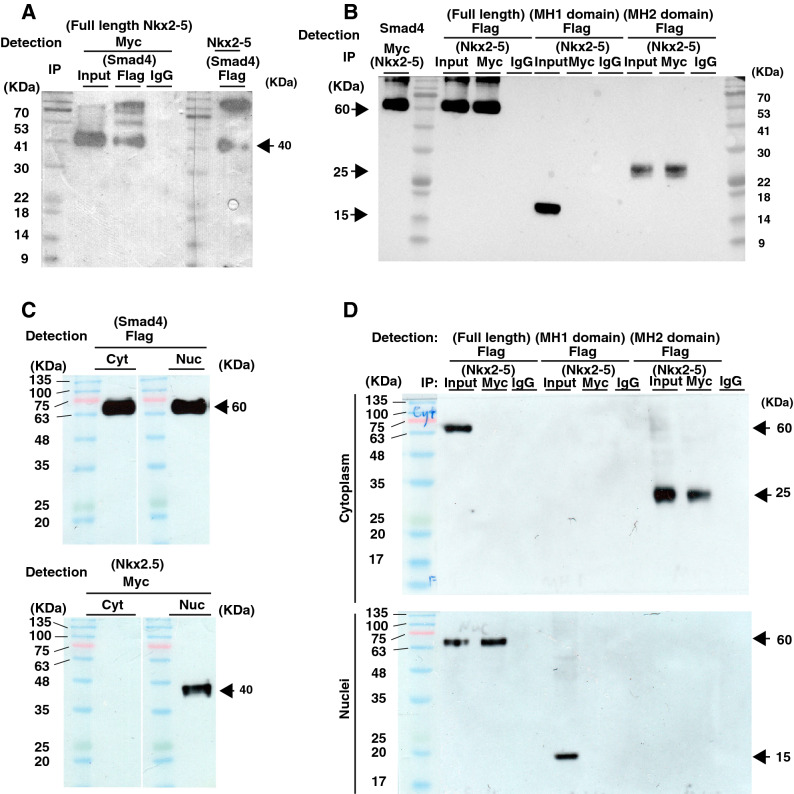
Since phosphorylated Smad1/5/8 forms a complex with Smad4 to translocate into the nucleus and regulate the transcription of target genes, we hypothesize that a protein complex containing both Smad4 and Nkx2-5 might similarly be involved in the proper nuclear translocation of Nkx2-5. Immunoprecipitation (IP) experiments using COS-7 cells demonstrated an interaction between Smad4 and Nkx2-5 (Fig. 3A, B).
Figure 3.
Interaction between Nkx2-5 and Smad4 via the MH2 domain. (A) Myc-tagged Nkx2-5 was co-immunoprecipitated with Flag-tagged Smad4 and detected using anti-Nkx2-5- and anti-Myc-antibodies. (B) Flag-tagged Smad4, and MH1- and MH2- deleted versions, were co-immunoprecipitated with Myc-tagged Nkx2-5 and detected using anti-Smad4 and Flag-antibodies. Nkx2-5 interacts with the Flag-tagged MH2 domain but not the MH1 domain. (C) Subcellular distribution of Flag-tagged Smad4 (upper) and Myc-tagged Nkx2-5 (lower) was determined by western blotting for extracts subcellular fractionated from cytoplasm or nuclei. (D) Interaction of Myc-Nkx2-5 to Flag-Smad4-full length, -MH1, or -MH2 is tested by Co-immunoprecipitation of extracts subcellular fractionated from cytoplasm or nuclei.
Smad4 has two functional domains, MH1 and MH2, both of which bind to distinct proteins, such as importins and exportins. To gain insight into whether Nkx2-5 binds to either of these domains, we performed additional IP experiments with MH1- or MH2-deleted Smad4. As shown in Fig. 3B, the MH2 domain of Smad4 is responsible for binding to Nkx2-5 (Fig. 3B). These results indicate that Smad4 interacts with the MH-2 domain of Nkx2-5 to regulate Nkx2-5 nuclear translocation.
We then tested subcellular localization of Smad4 and Nkx2-5 by western blot analysis of subfractionated cellular compartments of COS-7 cells, which were transfected with their expression vectors (Fig. 3C). Smad4 was found in both cytoplasm and nucleus, while Nkx2-5 was detected only in the nucleus, suggesting that nuclear distribution of Nkx2-5 is occurred not only in cardiomyocytes but also in the fibroblasts. This observation gave rise to a question if the distribution of Nkx2-5 is controlled by Smad4. We performed IP experiments with subcellular compartments (Fig. 3D). We found that Nkx2-5 forms a complex with full length of Smad4 in nuclei or MH2 domain in cytoplasm, suggesting that interaction of full length of Smad4, including MH1 domain for translocation and MH2 domain for binding to Nkx2-5, is required for nuclear localization.
Smad4 is essential for the nuclear localization of Nkx2-5
We further examined whether the introduction of Smad4 would be sufficient to restore the nuclear localization of Nkx2-5 by immunohistochemical analysis. Smad4-cKO heart cells, which express exogenous Flag-tagged Smad4 showed a significant increase in the ratio of cells with Nkx2-5 localized in the nucleus (Fig. 4A, B), suggesting that Smad4 is required for the nuclear localization of Nkx2-5.
Figure 4.
Introduction of Smad4 rescues the nuclear localization of Nkx2-5 in mutant cells and depletion of the Smad4 gene blocks the nuclear translocation of Nkx2-5 in fibroblast cells. (A) Double immunohistochemistry with anti-Nkx2-5 (red) and anti-Flag (green) antibodies. Cells from Smad4-cKO embryonic hearts were transfected with an empty vector (pDEF) as a control (upper panels) or Flag-tagged Smad4 expression vector (lower panels). Arrows show the failure of Nkx2-5 to localize to the nucleus. DAPI was used to stain nuclei. (B) Percentage of cells showing nuclear localization of Nkx2-5. Nuclear localization of Nkx2-5 was significantly increased in the presence of Smad4, compared to cells transfected with empty vector. Data were collected from 20 slides for each experiment (n = 4 and 4 for control and Smad4-cKO, respectively). Data are presented as mean ± SEM. *P < 0.01 (Mann–Whitney U test). (C) Double immunohistochemistry with anti-Myc (red) and anti-Smad4 (green) antibodies. Fibroblast cells from Smad4f./f embryos were transfected with the Myc-Nkx2-5 expression vector and showed nuclear localization of Nkx2-5. After depleting Smad4 by transfecting fibroblasts with Cre expression vector twice, Nkx2-5 was no longer localized to the nucleus. DAPI was used to stain nuclei. (D) The proportion of non-nuclear localization of Nkx2-5 in control (transfected with Myc-Nkx2-5) and Smad4-cKO (transfected with Myc-Nkx2-5 after two rounds of Cre expression vector transfection) cells. Data were collected from 20 slides for each experiment (n = 3 and 3 for control and Smad4-cKO, respectively). Data are presented as mean ± SEM. **P < 0.01 (Mann–Whitney U test).
Since our results indicate that Smad4 seems required for the nuclear translocation of Nkx2-5, we next tested whether exogenous Nkx2-5 would localize to the nucleus of not only cardiomyocytes but also in other types of cells that express CK2 and Smad4, such as fibroblasts. In primary cultures of fibroblasts derived from Smad4f./f embryos transfected with an expression vector for Nkx2-5, Nkx2-5 was able to enter the nucleus. This suggests that the nuclear translocation of Nkx2-5 is not limited to a specific cell type. We also tested whether Smad4 is required for the nuclear translocation of Nkx2-5. After transfecting fibroblasts derived from Smad4f./f embryos with a Cre expression vector to abolish Smad4 expression, the cells were further transfected with an expression vector for Nkx2-5. As shown in Fig. 4C, D, Nkx2-5 failed to enter the nucleus in the absence of Smad4 expression, suggesting that Smad4 is required for the nuclear localization of the Nkx2-5.
Discussion
In this study, we successfully generated mice that have a deletion of the Smad4 gene in cardiac progenitor cells by crossing Sfrp5-Cre mice with Smad4f./f mice. These mice showed hypoplastic heart tubes with disruption of cardiomyocyte differentiation, suggesting that Smad4 is involved in cardiac development. Bmp/Smad4 signaling has been suggested to regulate the transcription of cardiac-specific transcription factors8. We found that expression of such transcription factors, especially those related to the FHF but not SHF, were down-regulated in Smad4-cKO hearts (Fig. 1B). This suggests that disruption of the Smad4 gene in Sfrp5-expressing cells, which are predominantly cardiac progenitor cells for the FHF, resulted in the downregulation of cardiac-specific genes and may explain the hypoplastic heart tubes observed in Smad4-cKO embryos.
Nkx2-5 transcription was not completely eliminated in Smad4-cKO hearts, suggesting that Bmp signaling through Smad4 is likely not required for the expression of Nkx2-5. Since the heart tube itself was formed in Smad4-cKO embryos, Bmp/Smad4 does not appear to be required for cardiac cell fate decisions. One possible explanation is that the disruption of Smad4 was incomplete and residual Smad4 could have activated the transcription of Nkx2-5. However, Sfrp5 is expressed in cardiac development before the Nkx2-5 gene, and Smad4 disruption in the early epiblast using Sox2-Cre results in rudimentary heart formation and the expression of cardiac-specific markers, including Nkx2-526. Moreover, the loss of type1-Bmp receptor in early mesoderm progenitors using Mesp1-Cre also results in the formation of a hypoplastic heart7. Thus, Bmp/Smad4 signaling might not be a trigger for initial cardiac cell specification.
Disruption of Smad4 resulted in the failure of Nkx2-5 to localize to the nucleus (Fig. 5) in both cardiomyocytes and fibroblasts. The NLS sequence in the homeobox of Nkx2-5 is required for nuclear translocation, which is controlled by regulating the phosphorylation state of a serine residue near the NLS by CK2 and PP1β23,25. In our experiments, however, the expression of CK2 and PP1β were not transcriptionally controlled by Bmp/Smad4. Since CK2 and PP1β are extensively involved in fundamental metabolic processes, they may be constitutively expressed ubiquitously to serve as housekeeping genes. This suggests that CK2 is likely always in a position to phosphorylate Nkx2-5, implying that phosphorylation of Nkx2-5 is unlikely to be involved in cardiomyocyte fate decisions. Moreover, Smad4 is ubiquitously expressed after the early definitive endoderm and mesoderm are formed27, suggesting that the nuclear localization of Nkx2-5 should be inevitable once Smad4 is synthesized. Consequently, nuclear localization might be a prerequisite for transcription factors, such as Nkx2-5, to play their respective roles in cardiac differentiation.
Figure 5.
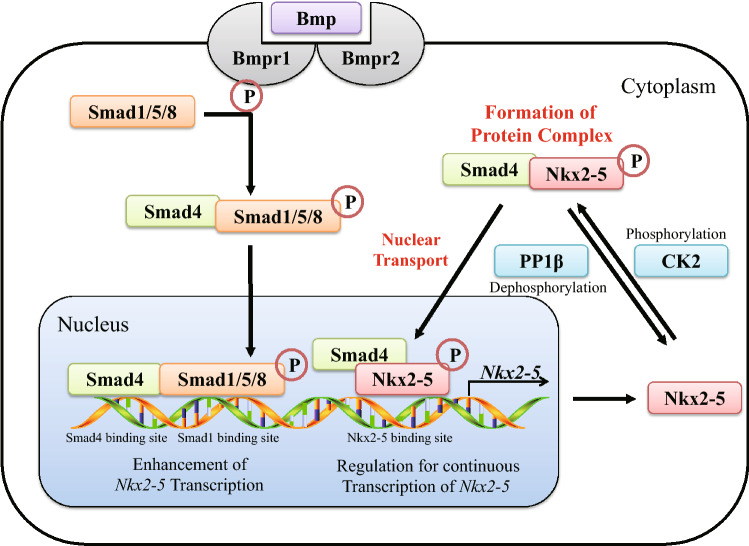
Schematic of Smad4 regulation of cardiac-specific gene expression and nuclear localization of Nkx2-5. Bmp activates its receptor, which then phosphorylates the Smad1/5/8 complex. In turn, the Smad1/5/8 complex binds to Smad4, which subsequently translocates into the nucleus to enhance Nkx2-5 transcription. Nkx2-5 protein is phosphorylated by CK2 and forms a protein complex with Smad4. This complex translocates into the nucleus and regulates Nkx2-5 transcription, thereby maintaining Nkx2-5 expression. Through this mechanism, Smad4 plays a decisive role in cardiac development and myocardial differentiation.
Nkx2-5 interacted with the MH2 domain of Smad4. Smad4 is known to bind to nuclear transporter proteins, such as Importin, via the MH1 domain, and bind to Exportin (Chromosome Region Maintenance 1; CRM1) via its linker region28–33. The MH2 domain, however, is implicated in transcriptional activation and interactions with other Smad proteins to form homo- and heteromeric complexes. This suggests that Nkx2-5 could behave similarly to other Smad proteins with regard to its nuclear localization (Fig. 5). In the nucleus, we speculate that the interaction between Smad4 and Nkx2-5 persists and allows for the transcriptional regulation of genes involved in heart development. For instance, Nkx2-5 regulates its own continuous expression13,34. Moreover, GATA4 reportedly binds to an upstream region of the Nkx2-5 transcriptional start site and controls Nkx2-5 expression in cooperation with Smad1/422. Some studies have also reported an interaction between GATA4 and Nkx2-535,36. Thus, after the Smad4-Nkx2-5 complex translocates into the nucleus, a complex containing Smad4, Nkx2-5, and GATA4 may allow for the sustained expression of Nkx2-5, thereby acting as a maintenance mechanism for mature cardiomyocytes.
Our results provide evidence that Smad4 is required for the transcription of several cardiac-specific genes, despite the fact that initial tube formation occurs and residual Nkx2-5 expression remains in Smad4-cKO embryonic hearts. This suggests that while Bmp/Smad signaling does not serve as a trigger for myocardial differentiation, it can promote cardiac differentiation by controlling the nuclear localization of Nkx2-5 via Smad4. Hence, Smad4 could function not only as a mediator of Bmp signaling for the transcriptional regulation of cardiac-specific genes but also as a regulator of the nuclear localization of Nkx2-5 to ensure proper cardiac differentiation.
Methods
Mice
The Sfrp5 Cre/+; Smad4 del/del mice with a C57Bl6 background were obtained by crossing Sfrp5 Cre/Cre; Smad4 del/+ mice with Smad4 floxed/floxed mice, which carry the Smad4 gene flanked by loxP recognition sites. Generation of the Sfrp5 Cre/+ line was described previously21. The Smad4 floxed/floxed line was a gift from Dr. CX Deng37. All procedures had local approval by the research ethics committee of Hiroshima University (A18-109–2) and conformed to the NIH Guide for the Care and Use of Laboratory Animals and all study carried out in compliance with the ARRIVE guidelines. Mouse tissues for all experiments were isolated after euthanizing mice by CO2 asphyxiation.
Ex vivo rescue experiment
Hearts were dissected from Smad4-cKO embryos at E9.5. Cardiomyocytes were harvested by trypsinization with 0.1% (w/v) Trypsin (Nacalai Tesque, Kyoto, Japan, 32778-05) for 2 min, seeded onto 24 mm × 24 mm glass plates, and cultured in 6-well tissue culture plates. A plasmid containing Smad4 cDNA (Flag-tagged) or an empty vector (pDEF) was transfected using 3.23% (w/v) Polyethylenimine (PEI Max, Cosmobio, Tokyo, Japan, 24765-2) on the following day. The immunofluorescent analysis was performed 24 h after transfection to detect the cellular distribution of Nkx2-5.
Fibroblast cells were obtained from Smad4f./f embryos using the same method described above and cultured in 6-well tissue culture plates. To delete Smad4, plasmids containing Cre cDNA were transfected into the fibroblasts twice using 3.23% (w/v) PEI.
Cell culture
COS-7 cells were maintained in DMEM (Life Technologies, Carlsbad, CA, 11995-065) containing 4.5 g/L D-glucose supplemented with 1 mM sodium pyruvate, 4 mM L-Glutamine, and 10% (v/v) fetal bovine serum (CELLect, Kefar Sava, Israel, 2916754) at 37 °C and 5% CO2.
Transfection
COS-7 and fibroblast cells were transfected with expression plasmids, including Flag-tagged Smad4, MH1, or MH2 (a gift from Dr. T. Imamura, Ehime University) and Myc-tagged Nkx2-5 (a gift from Dr. Koshiba, Toyo University) using 3.23% (w/v) polyethyleneimine (PEI Max, Cosmobio, Tokyo, Japan, 24765-2) following the same steps described above for the rescue experiment.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described previously21. Embryos were dissected at E9.5 and fixed with 4% paraformaldehyde in PBS for durations of 1 h to overnight. After dehydration using a methanol series, embryos were rehydrated with 0.1% Tween 20 in PBS (PBST), bleached with 6% H2O2 in PBS, treated with Protease K, and then hybridized with digoxygenin (DIG)-labeled probes at 68ºC overnight. Embryos were then washed with 5 × and 2 × SSC/50% formamide and PBST. To detect DIG-labelled probes, embryos were incubated with anti-DIG antibodies after treatment with blocking reagent for 1 h, and the signal was developed with BM purple AP substrate (Roche, Basel, Switzerland).
Real-time PCR
Real-time PCR was performed as previously described21. Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA). Reverse transcription was performed with the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). Real-time PCR was conducted using SYBR Premix Ex Taq II (Kapa Biosystems Inc., Wilmington, MA). Intensities of PCR products were measured and analyzed using Mini-Opticon (Bio-Rad, Hercules, CA). Amplification conditions were: 5 s at 95 °C, 20 s at 60 °C, and 15 s at 72 °C for 49 cycles. G3pdh was amplified as an internal control. Primers used in the present study are shown in Supplementary Table S1.
Immunohistochemistry
Embryos were embedded in optimum cutting temperature (OCT) compound (Sakura Tissue-Tek, Tokyo, Japan, 4583) after fixation and cut into 10–14 μm sections. After washing with PBS and blocking with 1% BSA in PBS for 1 h, sections were incubated with 500 × diluted primary antibodies, including anti-Smad4 (Santa Cruz, Dallas, TX, sc-7966), anti-Mypt1 (Proteintech, Rosemont, IL, 22117-1-AP), anti-Flag (DYKDDDDK)-Tag (Sigma Aldrich, St. Louis, MO, F3165 or Cell Signaling Technology, Danvers, MA, 8146S), anti-Myc Tag (Cell Signaling Technology 2278S), anti-Nkx2-5 (Abcam, Cambridge, UK, ab35842 or ab97355), and anti-TnT (Thermo, Waltham, MA, MS-295-P0) at 4 °C overnight. Sections were then incubated with 500 × diluted secondary antibodies conjugated with Alexa Fluor 488 or 555 (Molecular Probes, Eugene, OR). Nuclei were stained with 4, 6-diamidino-2-phenylindole (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
Nuclear and cytoplasmic protein extraction
COS-7 cells, transfected expression vectors, were collected after 24 h, and then their nuclei and cytoplasm fractions were separated. Their containing proteins were harvested by using a NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA, 78833) following the instruction protocols and stored at – 80 °C for further use.
Western blotting
Cultured cells were washed with cold PBS and lysed on ice with Sample buffer (100 mM DTT, 50 mM Tris/HCl pH 8.0, and 0.1% (w/v) SDS). After centrifugation at 15,500 rpm for 10 min at 4 °C, supernatant fractions were collected and analyzed by SDS-PAGE and immunoblotting.
Immunoprecipitation
Cultured cells were washed with cold PBS and lysed on ice with RIPA buffer (150 mM NaCl, 5 mM EDTA pH 8.0, 50 mM Tris/HCl pH 8.0, 1% (v/v) IGEPAL CA-630, 0.1% (w/v) SDS, and 0.5% (w/v) sodium deoxycholate) containing phenylmethylsulfonyl fluoride (PMSF, Tocris Bioscience, Bristol, UK) for 30 min. After centrifugation at 15,000 rpm for 10 min at 4 °C, supernatants were collected and used for immunoprecipitation (IP). IP was performed with the Immunoprecipitation Starter Pack (GE Healthcare, Chicago, IL) according to the manufacturer’s instructions, using 100 μl column resin conjugated with 5 μg anti-Flag antibody, 5 μg anti-Myc antibody, 5 μg anti-Smad4 antibody, 5 μg anti-Nkx2-5 antibody, or 100 μg IgG, and then analyzed by immunoblotting after SDS-PAGE. Signal detection was performed with the LuminoGraphI (ATTO, Amherst, NY, WSE-6100H-CSP) system.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed using one-way analysis of variance (ANOVA) with the Tukey–Kramer post hoc test for multiple comparisons or the nonparametric Mann–Whitney U test to compare two independent populations. Statistical analyses were performed using SPSS 20.0 software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. https://www.ibm.com/analytics/spss-statistics-software). *P < 0.05 was considered statistically significant.
Supplementary Information
Acknowledgments
We thank Dr. Fujii for his technical assistance and members of the Saga laboratory at the National Institute of Genetics for their support. We also appreciate members of the Yoshizumi laboratory for their help and the Natural Science Centre for Basic Research and Development (N-BARD) at Hiroshima University for allowing the use of their facility. We also thank Dr. T. Imamura from Ehime University for sharing Flag-tagged Smad4, MH1, and MH2, Dr. Koshiba from Toyo University for the Myc-tagged Nkx2-5 plasmid, and Dr. CX Deng from the National Institutes of Health for the Smad4f./f mice.
Author contributions
W.H., M.Y., and H.K. designed experiments, W.H., A.D., K.K., S.S., D.O., and M.S, performed experiments and collected Data, W.H., M.Y., M.I., and H.K. analyzed and interpreted Data, W.H., and H.K wrote the manuscript, All authors reviewed the manuscript.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (16K08443). W. H. is financially supported by the Natural Science Foundation of Liaoning Province (2019-MS-386) and the Scientific Research Project of Liaoning Provincial Department of Education (JC2019007).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masao Yoshizumi, Email: yoshizum-tky@umin.ac.jp.
Hiroki Kokubo, Email: hkokubo@hiroshima-u.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82954-2.
References
- 1.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circ. Res. 2009;104:933–942. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- 3.Spater D, Hansson EM, Zangi L, Chien KR. How to make a cardiomyocyte. Development. 2014;141:4418–4431. doi: 10.1242/dev.091538. [DOI] [PubMed] [Google Scholar]
- 4.Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JA, Franklin H. Epstein lecture. Cardiac development and implications for heart disease. N. Engl. J. Med. 2010;363:1638–1647. doi: 10.1056/NEJMra1003941. [DOI] [PubMed] [Google Scholar]
- 6.Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc. Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Andree B, Duprez D, Vorbusch B, Arnold HH, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 1998;70:119–131. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 10.Schlange T, Andree B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech. Dev. 2000;91:259–270. doi: 10.1016/s0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 11.Zheng M, et al. Bone morphogenetic protein2 enhances the expression of cardiac transcription factors by increasing histone H3 acetylation in H9c2 cells. Mol. Med. Rep. 2013;7:953–958. doi: 10.3892/mmr.2013.1266. [DOI] [PubMed] [Google Scholar]
- 12.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 13.Lien CL, McAnally J, Richardson JA, Olson EN. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev. Biol. 2002;244:257–266. doi: 10.1006/dbio.2002.0603. [DOI] [PubMed] [Google Scholar]
- 14.Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prall OW, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirard C, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, et al. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ. Res. 2007;101:277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 19.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ. Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 20.Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol. Metab. 2012;23:628–636. doi: 10.1016/j.tem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Fujii M, et al. Sfrp5 identifies murine cardiac progenitors for all myocardial structures except for the right ventricle. Nat. Commun. 2017;8:14664. doi: 10.1038/ncomms14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CO, 3rd, et al. The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J. Biol. Chem. 2004;279:10659–10669. doi: 10.1074/jbc.M301648200. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara H, Izumo S. Identification of the in vivo casein kinase II phosphorylation site within the homeodomain of the cardiac tisue-specifying homeobox gene product Csx/Nkx2.5. Mol. Cell. Biol. 1999;19:526–536. doi: 10.1128/mcb.19.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou DY, et al. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan T, et al. Myosin phosphatase modulates the cardiac cell fate by regulating the subcellular localization of Nkx2.5 in a Wnt/Rho-associated protein kinase-dependent pathway. Circ. Res. 2013;112:257–266. doi: 10.1161/CIRCRESAHA.112.275818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- 27.Luukko K, Ylikorkala A, Makela TP. Developmentally regulated expression of Smad3, Smad4, Smad6, and Smad7 involved in TGF-beta signaling. Mech. Dev. 2001;101:209–212. doi: 10.1016/s0925-4773(00)00556-6. [DOI] [PubMed] [Google Scholar]
- 28.Pierreux CE, Nicolas FJ, Hill CS. Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol. Cell. Biol. 2000;20:9041–9054. doi: 10.1128/mcb.20.23.9041-9054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baas R, et al. Quantitative proteomics of the SMAD (suppressor of mothers against decapentaplegic) transcription factor family identifies importin 5 as a bone morphogenic protein receptor SMAD-specific importin. J. Biol. Chem. 2016;291:24121–24132. doi: 10.1074/jbc.M116.748582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun DA, et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat. Genet. 2016;48:457–465. doi: 10.1038/ng.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Z, Latek R, Lodish HF. An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity. Oncogene. 2003;22:1057–1069. doi: 10.1038/sj.onc.1206212. [DOI] [PubMed] [Google Scholar]
- 32.Baburajendran N, Jauch R, Tan CY, Narasimhan K, Kolatkar PR. Structural basis for the cooperative DNA recognition by Smad4 MH1 dimers. Nucleic Acids Res. 2011;39:8213–8222. doi: 10.1093/nar/gkr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makkar P, Metpally RP, Sangadala S, Reddy BV. Modeling and analysis of MH1 domain of Smads and their interaction with promoter DNA sequence motif. J. Mol. Gr. Model. 2009;27:803–812. doi: 10.1016/j.jmgm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 34.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5632–5637. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jumppanen M, et al. Synthesis, identification, and structure-activity relationship analysis of GATA4 and NKX2-5 protein–protein interaction modulators. J. Med. Chem. 2019;62:8284–8310. doi: 10.1021/acs.jmedchem.9b01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinnunen SM, et al. Cardiac actions of a small molecule inhibitor targeting GATA4-NKX2-5 interaction. Sci. Rep. 2018;8:4611. doi: 10.1038/s41598-018-22830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.