Abstract
ORCID: 0000–0001-6019–7309. In the treatment of breast cancer, intensity-modulated radiation therapy (IMRT) reportedly reduces the high-dose irradiation of at-risk organs and decreases the frequency of adverse events (AEs). Comparisons with conventional radiotherapy have shown that IMRT is associated with lower frequencies of acute and late-onset AEs. Here, we extended a prospective, observational, single-center study of the safety of IMRT to a second investigating center. Patients scheduled for adjuvant IMRT after partial or total mastectomy were given a dose of 50 Gy (25 fractions of 2 Gy over 5 weeks), with a simultaneous integrated boost in patients having undergone conservative surgery. 300 patients were included in the study, and 288 were analyzed. The median follow-up period was 2.1 years. The 2-year disease-free survival rate [95% CI] was 93.4% [89.2–96.0%]. Most AEs were mild. The most common AEs were skin-related—mainly radiodermatitis [in 266 patients (92.4%)] and hyperpigmentation (in 178 (61.8%)). 35% and 6% of the patients presented with grade 2 acute skin and esophageal toxicity, respectively. Only 4 patients presented with a grade 3 event (radiodermatitis). Smoking (odds ratio) [95% CI] = 2.10 [1.14–3.87]; p = 0.017), no prior chemotherapy (0.52 [0.27–0.98]; p = 0.044), and D98% for subclavicular skin (1.030 [1.001–1.061]; p = 0.045) were associated with grade ≥ 2 acute AEs. In a univariate analysis, the mean dose, (p < 0.0001), D2% (p < 0.0001), D50% (p = 0.037), D95% (p = 0.0005), D98% (p = 0.0007), V30Gy (p < 0.0001), and V45Gy (p = 0.0001) were significantly associated with grade ≥ 1 acute esophageal AEs. In a multivariate analysis, D95% for the skin (p < 0.001), D98% for the subclavicular skin and low D95% for the internal mammary lymph nodes were associated with grade ≥ 1 medium-term AEs. The safety profile of adjuvant IMRT after partial or total mastectomy is influenced by dosimetric parameters.
Trial registration: ClinicalTrials.gov NCT02281149.
Subject terms: Oncology, Cancer
Introduction
Radiotherapy is recommended as an adjuvant treatment for breast cancer after breast-conserving surgery or after mastectomy in patients with node-positive disease; it is associated with significant reductions in the risk of recurrence and long-term cancer mortality1,2. The current standard of care is three-dimensional conformational radiotherapy (3D-CRT). However, dose inhomogeneity may accentuate the likelihood of local recurrence, damage to nearby organs at risk (OAR), and acute and long-term adverse events3,4. The main acute adverse events (defined as those first observed within 90 days of the last radiotherapy session) are erythema, skin desquamation and esophagitis, while late cosmetic and functional adverse events include fibrosis of the skin, lung and deep tissues, breast and chest wall pain, skin hyper/hypopigmentation, telangiectasia, and secondary cancer5–10.
Intensity-modulated radiation therapy (IMRT) has been developed as means of delivering precise doses of radiation to a sometimes complex target volume. A number of clinical trials have shown that when compared with 3D-CRT, IMRT (i) provides good coverage of the target volume, (ii) reduces the delivery of high doses of radiation to OAR, and (iii) is associated with better quality of life (QoL) and lower frequencies of acute and late adverse events after conservative surgery11–15. In Donovan et al.’s randomized study, a change in esthetic breast appearance was less common in the IMRT arm (40%, vs. 58% in the standard arm; p = 0.008). Moreover, there was significantly less fibrosis in patients treated with IMRT13. Mukesh et al. reported a better dose distribution, better overall cosmetic results (OR [95% CI] = 0.68 [0.48–0.96], p = 0.027) and less frequent telangiectasia in the IMRT arm (OR [95% CI] = 0.58 [0.36–0.92], p = 0.038)14. Pignol et al. found an absolute reduction in exudative epithelitis (17 percentage points) in the IMRT group, relative to 2D radiotherapy (31.2% vs. 48%, respectively, p = 0.0002)15. However, these studies did not provide guidance how to evaluate a plan, i.e. they did not describe planning constraints. In view of these shortcomings, we recently initiated a clinical project aimed at (i) evaluating acute and medium-term toxicity in breast cancer patients treated with adjuvant IMRT, and (ii) assessing the association between adverse events and the patients’ clinical, treatment-related and dosimetric characteristics. The project was initially set up as a single-center study at the Centre Oscar Lambret cancer center (Lille, France), and the preliminary results in 114 patients have been reported16; we found that QoL was well maintained, and that acute esophageal toxicity was associated with a number of dosimetric factors.
We report here data collected in 288 patients treated with adjuvant breast radiotherapy in 2 centers. To the best of our knowledge, the correlation between clinical toxicity and dosimetric data has not been previously investigated.
Patients and methods
Study design
This was a two-center, prospective clinical study of the safety of adjuvant IMRT after breast cancer surgery. The primary objective was to describe acute adverse events. The secondary objectives were (i) identify potential prognostic factors for grade ≥ 2 acute adverse events following adjuvant IMRT, (ii) describe long-term adverse events and identify potential prognostic factors for long-term adverse events, (iii) assess QoL and esthetic outcomes, and (iv) evaluate effectiveness (in terms of time to recurrence).
Patients and treatments
Patients were recruited at two cancer centers: the Centre Oscar Lambret (Lille, France) and the Centre Leonard de Vinci (Douai, France). The main inclusion criteria were as follows: age 18 or over, provision of informed consent, histologically proven breast cancer, and adjuvant radiotherapy after partial or total mastectomy, with or without inclusion of the axillary lymph nodes. The main exclusion criteria were metastatic disease, any severe or non-controlled disease that would have compromised participation in the study, and breast-feeding or pregnancy.
The treatment procedure was that used routinely in the investigating centers, and has been described in detail elsewhere17. Briefly, the clinical target volume (CTV) and the OAR were delineated according to the American Society for Radiation Oncology (ASTRO) guidelines until the end of December 201518 and according to the European Society for Radiotherapy and Oncology (ESTRO) guidelines thereafter19–21. A 5 mm margin was added to the CTV to obtain the planning target volume (PTV). The prescribed dose for the breast, chest wall and axillary lymph nodes was 50 Gy (25 fractions of 2 Gy). This dose was delivered over 5 weeks (five irradiations/week). Patients having undergone partial mastectomy received a simultaneous integrated boost (SIB) at the surgical bed of 60 Gy, delivered in 25 fractions (25 fractions × 2.4 Gy). Treatment planning was performed in the helical mode with TomoEdge (Accuray) using a 5 cm field width. The aim was for 95% of the PTV to receive 95% of the prescribed dose. To avoid overdosing during optimization a 3 mm zone is subtracted from the outer contour—resulting in the creation of a "skin volume". The constraints for OAR are specified in Table 1.
Table 1.
Dosimetric constraints for organs at risk.
| Organ at risk | Constraint |
|---|---|
| Spinal cord | D2% < 15 Gy |
| Heart (left breast) | V15 < 20% |
| V20 < 15% | |
| V25 < 10% | |
| Ipsilateral lung | V15 < 50% |
| V20 < 35% | |
| V30 < 20% | |
| V35 < 15% | |
| Contralateral lung | V10 < 50% |
| V12 < 35% | |
| V15 < 20% | |
| Contralateral breast | V5 < 50% |
| V7 < 35% | |
| V10 < 20% | |
| V20 < 15% |
Vx volume receiving × dose (gray); % of the organ at risk.
Outcomes
Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0)22. Acute adverse events were defined as those first observed within 90 days of the last radiotherapy session. We recorded: skin toxicity (radiodermatitis, ulceration, necrosis, telangiectasia, atrophy, hyperpigmentation, and hypopigmentation), esophageal toxicity, and breast, surgical bed or scar induration. Adverse events were recorded weekly during IMRT and then 1 and 6 months and 1, 2, 3, 4 and 5 years thereafter. The purpose of the 1-month consultation was to assess early toxicity more accurately.
Health-related QoL was evaluated using the disease-specific European Organisation for Research and Treatment of Cancer (EORTC) core QoL questionnaire (QLQ-C30) and the additional breast-cancer-specific QLQ-BR-23 module, according to the EORTC manual23,24. The scores were linearly transformed onto a scale ranging from 0 (worst possible QoL) to 100 (best possible QoL). Aesthetic outcomes were rated by the patient and by her physician as poor, moderate, good or excellent.
Statistical analyses
Quantitative variables were expressed as the mean ± standard deviation (SD) or the median (range), and qualitative variables were expressed as the frequency (percentage). Skin toxicity and skin fibrosis were analyzed per treated breast. Esophageal adverse events were analyzed per patient. The clinical and dosimetric variables examined for a putative association with acute or long-term adverse events and the corresponding statistical methods used have been described in detail in our previous report16. The sample size was calculated as follows. Given that the primary endpoint was the occurrence of grade ≥ 2 acute adverse events (considered to be treatment failures), the calculation was performed by organ (skin or esophagus). With regard to prognostic factors of toxicity, at least 10 failures per factor had to be observed. We had planned to study nine prognostic factors for skin adverse events and five for esophageal adverse events. Hence, we expected to observe at least 90 patients with a skin adverse event. According to the literature, around 30% of patients will experience a grade ≥ 2 acute skin adverse event25. Hence, a sample of 300 patients was required to observe 90 skin adverse events with p > 0.05 and a power of 83%.
Ethics
The study was carried out in accordance with the precepts of the Declaration of Helsinki, approved by the local institutional review board (Comité de Protection des Personnes Nord Ouest IV, Lille, France; reference: SC14/03) and registered at ClinicalTrials.gov (NCT02281149). All included patients received information on the study’s objectives and procedures. In line with the French legislation on the analysis of data collected during routine care, patients gave their consent to participation.
Consent to participate
All included individuals gave their informed consent to participation in the study and to analysis of their personal data.
Results
Characteristics of the patients and treatments
A total of 300 patients were included in the study, 12 of whom were subsequently excluded for various reasons: hypofractionated treatment, local progression and metastasis, withdrawal decided by the patient, withdrawal decided by the investigator, a change in treatment center, use of a different radiotherapy machine, and erroneous inclusion. Hence, 288 patients were analyzed (Table 2).
Table 2.
Characteristics of the study population and the tumors on inclusion. Data are quoted as the number (percentage) or the median (range).
| Demographic and health characteristics (n = 288) | |
|---|---|
| Age, years | 55 (32–82) |
| Past or current smokers | 79 (27.4%) |
| Number of packets per year (n = 72) | 17.5 (0.5–51) |
| Duration (years) | 28 (2–47) |
| Current and/or past health conditions | |
| History of heart disease | 94 (23.8%) |
| Current diabetes | 43 (14.9%) |
| Current dyslipidemia | 91 (28.3%) |
| History of respiratory disease | 29 (10.1%) |
| Family history of breast cancer | 122 (42.4%) |
| BMI (kg/m2) | 26.5 (16.5–48.8) |
| Normal weight | 111 (38.8%) |
| Overweight (BMI ≥ 25) | 95 (33.2%) |
| Obesity(BMI ≥ 30) | 80 (28%) |
| WHO score | |
| 0 | 222 (79%) |
| 1 | 58 (20.6%) |
| 2 | 1 (0.4%) |
| Breast size | |
| Small (85A-B, 90A) | 31 (11.6%) |
| Medium (85C, 90B-C, 95A-B) | 76 (28.4%) |
| Large (> 85C, > 90C, > 95B) | 161 (60.1%) |
| Tumor characteristics (n = 288) | |
|---|---|
| Tumor side | |
| Right | 130 (45.1%) |
| Left | 142 (49.3%) |
| Bilateral | 16 (5.6%) |
| Histology | |
| Invasive ductal carcinoma | 217 (75.4%) |
| Invasive lobular carcinoma | 33 (11.4%) |
| Other | 38 (13.2%) |
| In situ component (n = 281) | 116 (41.3%) |
| SBR grade | |
| SBR I | 64 (24.2%) |
| SBR II | 143 (54.2%) |
| SBR III | 57 (21.6%) |
| ER + (n = 286) | 243 (85.0%) |
| PR + (n = 286) | 205 (71.7%) |
| HER2 + (n = 273) | 42 (15.4%) |
| Triple-negative (n = 288) | 28 (9.7%) |
| pT grade (n = 265) | |
| pT1 | 122 (46%) |
| pT2 | 110 (41.5%) |
| pT3 | 30 (11.3%) |
| pT4 | 3 (1.1%) |
| pN grade (n = 275) | |
| pN0 | 60 (21.8%) |
| pN1 | 158 (57.5%) |
| pN2 | 42 (15.3%) |
| pN3 | 15 (5.5%) |
WHO World Health Organization, BMI body mass index, SBR Scarff-Bloom and Richardson.
One hundred and seventy patients (59%) received the SIB. Seven of the 288 patients (2.4%) did not receive the treatment not specified in the study protocol (25 × 2 Gy fractions and in some cases a SIB with 25 × 2.4 Gy fractions). All other patient received between 49.75 and 50.5 Gy in 25 fractions (breast) and (if a SIB was applied) 60 Gy in 25 fractions (surgical bed). The mean ± SD treatment time was 36.8 ± 2.0 days (median 36; range 33–45) (Table 3).
Table 3.
Characteristics of the treatments.
| Treatments | |
|---|---|
| Surgery (n = 288) | |
| Type of surgery | |
| Partial mastectomy | 170 (59.0%) |
| Bilateral partial mastectomy | 7 (2.4%) |
| Lumpectomy | 3 (1.0%) |
| Total mastectomy | 99 (34.4%) |
| Bilateral total mastectomy | 3 (1.0%) |
| Total mastectomy on one side and partial mastectomy on the other | 6 (2.1%) |
| Axillary node dissection | 205 (71.2%) |
| Sentinel lymph node | 176 (61.1%) |
| Chemotherapy (n = 288) | |
| Any type | 209 (72.6%) |
| Adjuvant | 159 (55.2%) |
| Neo-adjuvant | 61 (21.2%) |
| Hormone therapy (n = 283) | |
| Any type | 227 (80.2%) |
| Tamoxifen-based | 90 (31.8%) |
| Radiotherapy | |
| Breast or chest wall PTV (n = 288) | |
| D50% (mean ± SD) (Gy) | 49.8 ± 0.8 |
| D95% (mean ± SD) (Gy) | 47.0 ± 2.9 |
| D2% (mean ± SD) (Gy) | 56.5 ± 4.1 |
| Concomitant boost PTV (n = 167) | |
| D50% (mean ± SD) (Gy) | 59.4 ± 1.0 |
| D95% (mean ± SD) (Gy) | 57.2 ± 1.2 |
| D2% (mean ± SD) (Gy) | 61.2 ± 1.2 |
| Internal mammary chain PTV (n = 258) | |
| D50% (mean ± SD) (Gy) | 49.5 ± 1.8 |
| D95% (mean ± SD) (Gy) | 46.9 ± 3.6 |
| D2% (mean ± SD) (Gy) | 52.1 ± (1.6 |
| Subclavicular* PTV (n = 253) | |
| D50% (mean ± SD) (Gy) | 49.6 ± 1.3 |
| D95% (mean ± SD) (Gy) | 47.1 ± 2.1 |
| D2% (mean ± SD) (Gy) | 51.9 ± 1.1 |
| Supraclavicular** PTV (n = 258) | |
| D50% (mean ± SD) (Gy) | 49.9 ± 1.4 |
| D95% (mean ± SD) (Gy) | 48.2 ± 2.0 |
| D2% (mean ± SD) (Gy) | 52.0 ± 1.1 |
Data are quoted as the n (%) or the mean ± standard deviation (SD).
PTV planning target volume, Dx% dose received by at least x% of the volume.
*2 and 3 areas, according to ESTRO guidelines.
**Area 4, according to ESTRO guidelines.
The median follow-up time (calculated according to the reverse Kaplan–Meier method) was 2.1 years (range 6 months to 4 years). Eleven patients (3.8%) died during the follow-up period. Seven of these deaths were due to disease progression. The 2-year overall survival rate [95% confidence interval (CI)] was 97.8% [94.1–99.2%]. Seventeen cases of disease recurrence were noted (13 metastatic, 3 local + regional + metastatic, and 1 regional + metastatic). In all, 19 patients died or relapsed, giving a 2-year relapse-free survival rate [95% CI] of 93.4% [89.2–96.0%].
Adverse events
The acute and medium-term adverse events observed during the study are summarized in Table 4. Further details of the clinical results will be presented in a subsequent publication. The most common acute adverse events were skin-related; almost all the patients experienced at least one acute skin adverse event (radiodermatitis, ulceration-necrosis, telangiectasia, atrophy, hyperpigmentation, and hypopigmentation)—primarily radiodermatitis [in 266 patients (92.4%)] and hyperpigmentation [in 178 (61.8%)]. Although the majority of these events were non-severe (i.e. no higher than grade 1), 106 patients presented with a grade ≥ 2 event (36.8% [31.2–42.7%]), and 4 presented with a grade 3 event (radiodermatitis in all 4 cases); these were the only grade 3 acute event observed in the study as a whole. The next most frequent types of acute adverse event were (mainly grade 1) esophageal damage and breast edema. Breast fibrosis and chest wall fibrosis were observed in a third of the patients, and almost all events were grade 1. The proportions of patients developing mammary fibrosis and chest wall fibrosis did not differ greatly when comparing the “total mastectomy” and “partial mastectomy” subgroups.
Table 4.
Acute and medium-term adverse events.
| Patients: n = 288 | Acute | Medium-term |
|---|---|---|
| Skin adverse events | 278 (96.5%) | 152 (53.1%) |
| Grade 1 | 172 (59.7%) | 147 (51.0%) |
| Grade 2 | 102 (35.4%) | 2 (0.6%) |
| Grade 3 | 4 (1.4%) | 2 (0.6%) |
| Esophageal adverse events | 138 (47.9%) | 6 (2.1%) |
| Grade 1 | 120 (41.7%) | 6 (2.1%) |
| Grade 2 | 18 (6.3%) | - |
| Edema | 57 (19.8%) | 69 (24.1%) |
| Grade 1 | 55 (19.1%) | 63 (22.0%) |
| Grade 2 | 1 (0.3%) | 5 (1.7%) |
| Grade unknown | 1 (0.3%) | 1 (0.3%) |
| Parietal fibrosis (total mastectomy, n = 120) | 36 (30.0%) | 51 (42.9%) |
| Grade 1 | 34 (28.3%) | 42 (35.3%) |
| Grade 2 | 2 (1.7%) | 9 (7.6%) |
| Breast fibrosis (partial mastectomy, n = 168) | 57 (33.9%) | 68 (40.7%) |
| Grade 1 | 54 (32.1%) | 59 (35.3%) |
| Grade 2 | 2 (1.2%) | 9 (5.4%) |
| Grade unknown | 1 (0.6%) | - |
| Surgical scar fibrosis (total mastectomy, n = 98) | 20 (20.4% %) | 52 (44.1%) |
| Grade 1 | 18 (18.4%) | 45 (38.1%) |
| Grade 2 | 2 (2.0%) | 7 (5.9%) |
| Surgical bed fibrosis (partial mastectomy, n = 165) | 31 (18.8%) | 66 (39.5%) |
| Grade 1 | 29 (17.6%) | 56 (33.5%) |
| Grade 2 | 1 (0.6%) | 10 (6.0%) |
| Grade unknown | 1 (0.6%) | – |
Skin adverse events were defined as radiodermatitis, ulceration-necrosis, telangiectasia, atrophy, hyperpigmentation or hypopigmentation. With regard to surgical bed fibrosis, only fibrosis absent on inclusion or of a higher grade on inclusion was considered.
With regard to the delineation method used (ASTRO: n = 88; ESTRO: n = 200), we observed several statistically significant differences in the incidence of (i) grade ≥ 1 (but not grade ≥ 2) esophageal adverse events (60.2% vs. 42.5%, respectively; p = 0.006), (ii) grade ≥ 1 (but not grade ≥ 2) breast induration in the partial mastectomy group (47.7% vs. 29.0%, respectively; p = 0.024), and (iii) grade ≥ 1 (but not grade ≥ 2) scar induration in the total mastectomy group (34.5% vs. 14.5%, respectively; p = 0.025). The mean total PTV volumes according to the ASTRO or ESTRO guidelines did not differ significantly (1052 cc ± 581 vs. 1085 cc ± 531, respectively, p = 0.65).
The most common medium-term acute adverse events at both the 13-month and 26-month time points affected the skin; at 26 months, the cumulative incidence [95% CI] was 59.2% [52.2–66.3] for grade ≥ 1 events and 1.7% [0.6–4.5] for grade ≥ 2 events. (Table 4). The incidence of fibrosis was higher in the partial mastectomy subgroup (Table 5). No respiratory toxicity was observed.
Table 5.
Cumulative incidence of medium-term adverse events.
| Cumulative incidence [95% CI] of grade ≥ 2 adverse events after IMRT | ||
|---|---|---|
| At 13 months* | At 26 months* | |
| Esophageal adverse events | – | – |
| Skin adverse events | 1.1% [0.4–3.5] | 1.7% [0.6–4.5] |
| Fibrosis, total mastectomy | 4.5% [1.9–10.5] | 7.0% [2.9–16.4] |
| Fibrosis, partial mastectomy | 5.2% [2.6–10.2] | 7.3% [3.5–15.1] |
| Scar fibrosis, total mastectomy | 5.5% [2.5–11.9] | 5.5% [2.5–11.9] |
| Scar fibrosis, partial mastectomy | 5.7% [3.0–10.6] | 7.8% [3.9–15.6] |
*The cumulative incidences are quoted at 13 and 26 months (rather than 12 and 24 months) so as not to underestimate the values, since the annual check-ups often took place slightly later than 12 and 24 months. Skin adverse events were defined as radiodermatitis, ulceration-necrosis, telangiectasia, atrophy, hyperpigmentation or hypopigmentation. With regard to surgical bed fibrosis, only fibrosis absent or of a higher grade on inclusion were considered.
Variables associated with the occurrence of grade ≥ 2 adverse events
In a univariate analysis of the whole population, age, cup size, diabetes, aesthetic score before IMRT, the type of surgery, node irradiation, SIB and the other dosimetric parameters tested were not significantly associated with the occurrence of acute adverse events. Factors positively associated (p < 0.05) with the occurrence of grade ≥ 2 acute adverse events were body mass index (BMI, as a quantitative variable), tobacco smoking, the absence of prior chemotherapy, CTV, PTV, the volume receiving 95% of the dose (V95%), boost CTV, skin volume, and the dose received by 98% of the volume (D98%). After the removal of highly correlated variables, the three variables significantly associated with the occurrence of grade ≥ 2 acute adverse events were (i) tobacco smoking (odds ratio (OR) [95% CI] = 2.10 [1.14–3.87]; p = 0.017), (ii) the absence of prior chemotherapy (0.52 [0.27–0.98]; p = 0.044) and D98% for the subclavicular skin (1.030 [1.001–1.061]; p = 0.045). Concerning breast size, an increment in CTV of 100 cc was associated with an OR [95% CI] = 1.11 (1.04–1.18) for presenting grade ≥ 2 acute skin toxicity (p = 0.003), for patients having undergone by partial mastectomy. In a quartile analysis vs. patients with a breast CTV < 610 cc, a breast CTV volume [610–811] was associated with an OR of 2.75, a CTV volume of [811–1150] was associated with an OR of 1.64, and a CTV volume > = 1150 cc was associated with an OR of 5.96 for presenting grade ≥ 2 acute skin toxicity respectively, p = 0.002. These criteria were not selected for multivariate analysis because they were highly correlated with skin volume.
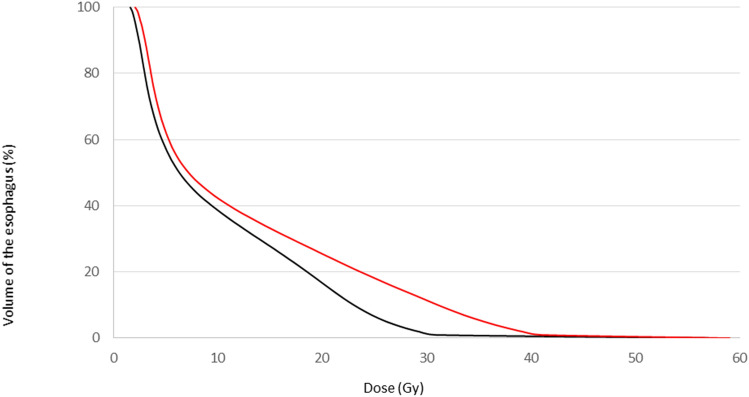
It was not possible to search for prognostic factors for grade ≥ 2 acute esophageal adverse events, given the small number (n = 18); hence, we analyzed grade ≥ 1 events. In a univariate analysis, the following dosimetric parameters were significantly associated with grade ≥ 1 acute esophageal adverse events: the mean dose, (p < 0.0001; Fig. 1), D2% (p < 0.0001), D50% (p = 0.037), D95% (p = 0.0005), D98% (p = 0.0007), V30Gy (p < 0.0001), and V45Gy (p = 0.0001). Lymph node irradiation was the only non-dosimetric parameter significantly associated with grade ≥ 1 acute esophageal adverse events. The significantly prognostic factors identified in the univariate analysis were highly correlated; this prevented us from performing a multivariate analysis of grade ≥ 1 acute esophageal adverse events.
Figure 1.
The mean dose-volume histogram for the esophagus for patients without esophageal toxicity (black line) and patients with grade ≥ 1 acute esophageal adverse events (red line).
Given that only 4 patients experienced grade ≥ 2 medium-term skin adverse events, it was not feasible to look for associated variables; we therefore focused on grade ≥ 1 medium-term adverse events. After univariate and multivariate analyses, the following factors were found to be significant: cup size ≥ C (subhazard ratio [95% CI] 1.51 [1.03–2.22]; p = 0.035), D95% for the skin volume (1.048 [1.021–1.076]; p < 0.001), D98% for the subclavicular skin (0.920 [0.888–0.953]; p < 0.001) and low D95% for the internal mammary lymph nodes (0.978 [0.963–0.992], p = 0.003).
Discussion
In the present prospective, two-center study of 288 patients treated for breast cancer with IMRT (including 225 (78.3%) with axillary node involvement), we found that skin adverse events were very common (observed in 96.5% of the patients) but rarely severe (with only 4 events grade 3 or higher). In a multivariate analysis, smoking and D98% for the subclavicular skin were prognostic factors for grade ≥ 2 skin adverse events, whereas prior chemotherapy was protective. Esophageal adverse events affected 47.9% of the patients but no grade ≥ 3 events were recorded. Dosimetric variables were the only prognostic factors for esophageal adverse events. The esthetic outcome for the breast 12 months post-surgery was generally good or excellent, whether judged the patients or the physicians. The patients’ QoL remained stable or improved over time following IMRT, and the overall and disease-free survival rates at 2 years were over 90%.
Acute adverse events
As noted above, almost all the patients in the present study experienced at least one (mainly mild) acute skin adverse event. In Freedman et al.’s comparative study, the incidence of grade 2 wet desquamation was significantly lower in an IMRT cohort (21%) than in a matched 3D-CRT cohort (38%)26. Pignol et al.’s comparison of 2D-CRT and IMRT produced the same conclusion15.
In fact, most previous studies of IMRT concerned the treatment of a single breast, which limits damage to the esophagus. For bilateral treatment, Ekici et al.’s study of 14 found that 6 had an acute esophageal adverse event (all grade 1)27. Wang et al. studied a larger sample (n = 200) of patients having undergone IMRT after total mastectomy; only 21 patients (10.5%), experienced an acute esophageal adverse event (all but three of which were grade 1 events)28. Aoulad et al.’s study of 292 patients found that 58 (19.9%) experienced a grade 1 or 2 acute esophageal adverse event29. Caudrelier et al. reported that 37% of their patients experienced an acute esophageal adverse event (all grade 1)30. In comparison, our value of 47.9% is high. This might be because a high proportion of our patients displayed axillary node involvement (justifying larger treatment volumes) or because the retrospective design of previous studies possibly led to underestimation of the event frequency. Lastly, our compliance with ASTRO and ESTRO guidelines meant that the esophagus received a higher dose. In our study, we observed more esophageal toxicity using the ASTRO guidelines; this might have been due to the more cranial limit of the ASTRO target volume, relative to the ESTRO guidelines.
In the present study, 59 patients (19.8%) presented with acute breast edema (grade 1 in 57 cases). This incidence is in line with the scarce literature data. Aoulad et al. reported a value of 19.5% for grade ≥ 2 acute breast edema29. Harsolia et al.’s comparison of IMRT and 3D-CRT cohorts treated in the same institution found that the incidence of grade ≥ 2 acute breast edema was significantly lower in the former group (1%, vs. 29% for 3D-CRT, p = 0.02)31.
Medium-term adverse events
The skin was the organ system most frequently concerned by medium-term adverse events (in 53.1% of our patients); this was primarily mild hyperpigmentation. In fact, hyperpigmentation tends to disappear with time, and so our median follow-up period of 2.1 years may have overestimated the long-term frequency of this event. The frequency of medium-term telangiectasia in our study was lower (7.0%) that the value of 31.4% was found in a study of 416 patients treated with 3D-CRT32. However, the results of two randomized studies were contradictory; an advantage of IMRT was reported by Mukesh et al. in the UK14, but not by Pignol et al. in Canada15. Furthermore, our study’s follow-up period was probably too short to assess truly long-term adverse events.
The esthetic outcome in the present study was judged to be “good or excellent” by 86.7% of the physicians and 84.6% of the patients. These results may be compared to the corresponding values of 96% and 88% in the Fox Chase Cancer Center study33. Hence, IMRT appears to be advantageous for the mid-term esthetic outcome, notably relative to 2D-CRT (64)13,14—probably because this measure is correlated with long-term fibrosis, edema and telangiectasia, which are generally less frequent after IMRT 33. However, the Canadian randomized trial failed to evidence a significant difference34.
In the present study, the frequency of medium-term fibrosis was 40.7% for partial mastectomy and 42.9% for total mastectomy. The corresponding frequencies of surgical scar fibrosis were 39.5% and 44.1%, respectively. Even though most of these events were grade 1, these frequencies were higher than in the literature. The Royal Marsden randomized trial of IMRT found 2-year breast fibrosis and surgical bed fibrosis rates of 16% and 37%, respectively13. However, the two other randomized trials did not evidence a long-term difference for IMRT14,15. Our study’s prospective design might have facilitated the detection of grade 1 event with little or no functional or esthetic impact. Secondly, high proportions of our patients had risk factors for the development of fibrosis (overweight, smoking, prior chemotherapy, node involvement, etc.)35,36. In the short term, however, the induration and fibrosis were more related to surgery than to radiotherapy. The assessment of short-term fibrosis probably increased the estimated incidence.
Prognostic factors associated with acute adverse events
The significant variables positively associated with the occurrence of grade ≥ 2 acute adverse events were tobacco use and D98% for the subclavicular skin, whereas the prior chemotherapy was protective. In the literature, high BMI, breast volume, and smoking are confirmed risk factors for acute adverse events29,37–41. Prior chemotherapy is typically found to be a factor associated with toxicity too37,42–44. In our study patients treated intermittently with corticosteroids may have benefitted from the latter’s anti-inflammatory action. Trastuzumab has been found to be protective in some studies39 but not others45. The literature data on dosimetric parameters are far more heterogeneous. Here, the OR [95% CI] for toxicity associated with D98% for the subclavicular skin was 1.030 [1.001–1.061]; p = 0.045). To the best of our knowledge, the present study is the first to have shown a correlation between skin dose and toxicity. Unfortunately, however, the dose delivered to the skin during inverse planning is not an actionable variable.
In the present study, the significant variables positively associated with grade ≥ 1 (rather than grade ≥ 2) medium-term skin adverse events in a multivariate analysis were cup size, D95% for the skin volume, D98% for the subclavicular skin and D95% for the internal mammary lymph nodes. Regardless of the technique (IMRT or 3D-CRT), women with a larger cup size are more exposed to a risk of late skin adverse events46,47.
Study limitations and strengths
The study had a number of strengths. Firstly, this was one of the largest yet studies of IMRT with SIB (n = 170 patients, 59%) as an adjuvant treatment for breast cancer. Secondly, the study’s prospective design produced full, unbiased datasets on adverse events. The fact that the detected events were grade 1 suggests good treatment tolerance in the short and medium terms. Thirdly, the present study is the first to have reported on the correlation between toxicity and dosimetric factors. The study also had some limitations. Firstly, our population was relatively heterogeneous, with total mastectomy vs. breast-conserving surgery, and chemotherapy prior to IMRT, SIB, and axillary lymph node irradiation in some cases but not others. The high proportion of patients with locally advanced disease (with axillary node involvement in 78.3% of cases and prior chemotherapy in 72.6% of cases) and thus greater treatment volumes might explain the incidence of acute and long-term adverse events. We decided not to divide our study population into subgroups because this would have decreased the statistical power. Secondly, our relatively short follow-up period (median: 2.1 years) prevented us from fully assessing the incidence and nature of long-term adverse events in general and the most serious cardiac and respiratory events in particular.
Conclusion
Adjuvant IMRT after partial or total mastectomy is associated with a low incidence of acute and medium-term adverse events. The majority of these events were non-severe, and did not degrade the cosmetic outcome for the breast. Importantly, the safety profile of IMRT is linked to dosimetric parameters (such as D2%, D50%, D95%, D98%, V30Gy and V45Gy) as well as to clinical and disease-related factors.
Acknowledgements
We thank all the patients, physicians, nurses, and data managers who participated in the study. Medical writing support was provided by David Fraser PhD (Biotech Communication SARL) and funded by Centre Oscar Lambret.
Abbreviations
- 2D-CRT
Two-dimensional conformational radiotherapy
- 3D-CRT
Three-dimensional conformational radiotherapy
- AE
Adverse event
- ASTRO
American Society for Radiation Oncology
- BMI
Body mass index
- CI
Confidence interval.
- CTV
Clinical target volume
- D2%
Dose received by 2% of the volume
- D50%
Dose received by 50% of the volume
- D95%
Dose received by 95% of the volume
- D98%
Dose received by 98% of the volume
- EORTC QLQ-BR23
European Organisation for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire
- EORTC QLQ-C30
European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire
- ESTRO
European Society for Radiotherapy and Oncology
- IMRT
Intensity-modulated radiation therapy
- OAR
Organs at risk
- OR
Odds ratio
- PTV
Planning target volume.
- QoL
Quality of life
- SBR
Scarff-Bloom and Richardson
- SD
Standard deviation
- SIB
Simultaneous integrated boost
- V95%
Volume receiving 95% of the dose
- WHO
World Health Organization
Author contributions
D.P. and E.L. contributed to the study conception and design. Data were collected by D.P., B.B., F.L.T., R.B., H.L., A.E., D.C., F.D., and F.C. E.T. performed the statistical analysis. All authors provide critical comments on the versions of the manuscript prior to submission. All authors read and approved the final manuscript.
Funding
The study was funded by Centre Oscar Lambret (Lille, France).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Early Breast Cancer Trialists' Collaborative Group, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 378, 1707–1716 (2011). [DOI] [PMC free article] [PubMed]
- 2.Early Breast Cancer Trialists' Collaborative Group, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet383, 2127–2135 (2014). [DOI] [PMC free article] [PubMed]
- 3.Riou O, Fenoglietto P, Lemanski C, Azria D. Intensity modulated radiotherapy for breast cancer. Cancer Radiother. 2012;16:479–484. doi: 10.1016/j.canrad.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Vicini FA, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:1336–1344. doi: 10.1016/S0360-3016(02)03746-X. [DOI] [PubMed] [Google Scholar]
- 5.Blom Goldman U, Svane G, Anderson M, Wennberg B, Lind P. Long-term functional and radiological pulmonary changes after radiation therapy for breast cancer. Acta Oncol. 2014;53:1373–1379. doi: 10.3109/0284186X.2014.934967. [DOI] [PubMed] [Google Scholar]
- 6.Chandra RA, et al. Radiation therapy risk factors for development of lymphedema in patients treated with regional lymph node irradiation for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015;91:760–764. doi: 10.1016/j.ijrobp.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 8.Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 2016;121:402–413. doi: 10.1016/j.radonc.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. Treatment related impairments in arm and shoulder in patients with breast cancer: A systematic review. PLoS ONE. 2014;9:e96748. doi: 10.1371/journal.pone.0096748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korzets Y, Fyles A, Shepshelovich D, Amir E, Goldvaser H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019;175:531–545. doi: 10.1007/s10549-019-05209-9. [DOI] [PubMed] [Google Scholar]
- 11.Barnett GC, et al. A randomised controlled trial of forward-planned radiotherapy (IMRT) for early breast cancer: Baseline characteristics and dosimetry results. Radiother. Oncol. 2009;92:34–41. doi: 10.1016/j.radonc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Barnett GC, et al. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: Interim results at 2 years. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:715–723. doi: 10.1016/j.ijrobp.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 13.Donovan E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother. Oncol. 2007;82:254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Mukesh MB, et al. Patient reported outcome measures (PROMs) following forward planned field-in field IMRT: Results from the Cambridge Breast IMRT trial. Radiother. Oncol. 2014;111:270–275. doi: 10.1016/j.radonc.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Pignol JP, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 16.Pasquier D, et al. Intensity-modulated radiation therapy with simultaneous integrated boost for locally advanced breast cancer: A prospective study on toxicity and quality of life. Sci. Rep. 2019;9:2759. doi: 10.1038/s41598-019-39469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crop F, et al. Surface imaging, laser positioning or volumetric imaging for breast cancer with nodal involvement treated by helical TomoTherapy. J. Appl. Clin. Med. Phys. 2016;17:200–211. doi: 10.1120/jacmp.v17i5.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartford AC, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for Intensity-modulated Radiation Therapy (IMRT) Am. J. Clin. Oncol. 2012;35:612–617. doi: 10.1097/COC.0b013e31826e0515. [DOI] [PubMed] [Google Scholar]
- 19.Offersen BV, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Offersen BV, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother. Oncol. 2016;118:205–208. doi: 10.1016/j.radonc.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 21.White, J. T. et al. Breast cancer atlas for radiation therapy planning: Consensus definitions. https://www.rtog.org/LinkClick.aspx?fileticket=SQhssxHu7Jg%3d&tabid=227. Accessed 10 February 2020.
- 22.National Cancer Institute Common Terminology Criteria for Adverse Events. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 10 February 2020.
- 23.Fayers, P. M. et al. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Published by: European Organisation for Research and Treatment of Cancer, Brussels 2001. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf.
- 24.Fayers, P., Bottomley, A., Group EQoL., Quality of Life U. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur. J. Cancer. 38(Suppl 4), S125–S133 (2002). [DOI] [PubMed]
- 25.Wojcieszynski AP, Olson AK, Rong Y, Kimple RJ, Yadav P. Acute toxicity from breast cancer radiation using helical tomotherapy with a simultaneous integrated boost. Technol. Cancer Res. Treat. 2016;15:257–265. doi: 10.1177/1533034615574387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman GM, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am. J. Clin. Oncol. 2006;29:66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 27.Ekici K, et al. Is helical tomotherapy-based intensity-modulated radiotherapy feasible and effective in bilateral synchronous breast cancer? A two-center experience. J. BUON. 2016;21:46–52. [PubMed] [Google Scholar]
- 28.Wang Q, Jie W, Liang Z, Wu H, Cheng J. Postmastectomy intensity modulation radiated therapy of chest wall and regional nodes: Retrospective analysis of the performance and complications up for 5 years. Medicine. 2017;96:e7956. doi: 10.1097/MD.0000000000007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoulad N, et al. Acute toxicity of breast cancer irradiation with modulated intensity by tomotherapy((R)) Cancer Radiother. 2017;21:180–189. doi: 10.1016/j.canrad.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Caudrelier J, Meng J, Esche B, et al. IMRT sparing of normal tissues in locoregional treatment of breast cancer. Radiat. Oncol. 2014;9:161. doi: 10.1186/1748-717X-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harsolia A, et al. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1375–1380. doi: 10.1016/j.ijrobp.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Lilla C, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res. Treat. 2007;106:143–150. doi: 10.1007/s10549-006-9480-9. [DOI] [PubMed] [Google Scholar]
- 33.Keller LM, et al. Five-year results of whole breast intensity modulated radiation therapy for the treatment of early stage breast cancer: The Fox Chase Cancer Center experience. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:881–887. doi: 10.1016/j.ijrobp.2012.01.069. [DOI] [PubMed] [Google Scholar]
- 34.Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 35.Bourgier C, et al. Late side-effects after curative intent radiotherapy: Identification of hypersensitive patients for personalized strategy. Crit. Rev. Oncol. Hematol. 2015;93:312–319. doi: 10.1016/j.critrevonc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Back M, Guerrieri M, Wratten C, Steigler A. Impact of radiation therapy on acute toxicity in breast conservation therapy for early breast cancer. Clin. Oncol. 2004;16:12–16. doi: 10.1016/j.clon.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Blanchecotte J, Ruffier-Loubiere A, Reynaud-Bougnoux A, Barillot I. Acute skin toxicity in breast intensity modulated radiotherapy using field in field technique. Cancer Radiother. 2015;19:82–88. doi: 10.1016/j.canrad.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 39.De Langhe S, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711. doi: 10.1186/1471-2407-14-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco P, et al. Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): A prospective case series. J. Cancer Res. Clin. Oncol. 2013;139:1927–1936. doi: 10.1007/s00432-013-1515-0. [DOI] [PubMed] [Google Scholar]
- 41.Shah C, Wobb J, Grills I, Wallace M, Mitchell C, Vicini FA. Use of intensity modulated radiation therapy to reduce acute and chronic toxicities of breast cancer patients treated with traditional and accelerated whole breast irradiation. Pract. Radiat. Oncol. 2012;2:e45–e51. doi: 10.1016/j.prro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Fiorentino A, et al. Intensity modulated radiation therapy with simultaneous integrated boost in early breast cancer irradiation. Report of feasibility and preliminary toxicity. Cancer Radiother. 2015;19:289–294. doi: 10.1016/j.canrad.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Morganti AG, et al. Phase I-II studies on accelerated IMRT in breast carcinoma: Technical comparison and acute toxicity in 332 patients. Radiother. Oncol. 2009;90:86–92. doi: 10.1016/j.radonc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Parekh A, et al. Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: Role of fractionation and treatment volume. Adv. Radiat. Oncol. 2018;3:8–15. doi: 10.1016/j.adro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald MW, Godette KD, Whitaker DJ, Davis LW, Johnstone PA. Three-year outcomes of breast intensity-modulated radiation therapy with simultaneous integrated boost. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:523–530. doi: 10.1016/j.ijrobp.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 46.Moody AM, et al. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother. Oncol. 1994;33:106–112. doi: 10.1016/0167-8140(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 47.Turesson I, Nyman J, Holmberg E, Oden A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 1996;36:1065–1075. doi: 10.1016/S0360-3016(96)00426-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.