Abstract
Some nanoscale morphologies of titanium oxide nanostructures blend with gold nanoparticles and act as satellites and targeted weapon methodologies in biomedical applications. Simultaneously, titanium oxide can play an important role when combined with gold after blending with polyethylene glycol (PEG). Our experimental approach is novel with respect to the plasmonic role of metal nanoparticles as an efficient PDT drug. The current experimental strategy floats the comprehensive and facile way of experimental strategy on the critical influence that titanium with gold nanoparticles used as novel photosensitizing agents after significant biodistribution of proposed nanostructures toward targeted site. In addition, different morphologies of PEG-coated Au-doped titanium nanostructures were shown to provide various therapeutic effects due to a wide range of electromagnetic field development. This confirms a significantly amplified population of hot electron generation adjacent to the interface between Au and TiO2 nanostructures, leading to maximum cancerous cell injury in the MCF-7 cell line. The experimental results were confirmed by applying a least squares fit math model which verified our results with 99% goodness of fit. These results can pave the way for comprehensive rational designs for satisfactory response of performance phototherapeutic model mechanisms along with new horizons of photothermal therapy (HET) and photodynamic therapy (HET) operating under visible and near-infrared (NIR) light.
Keywords: PEG-coated Au-doped Titanium oxide (TiO2) nanostructures, Photodynamic therapy (PDT), Photothermal therapy/(HET), MCF-7 cancerous cells
Abbreviations: NRA, Neutral red assay; PEG, Polyethylene glycol; RMSE, Root mean square error; SPR, Surface plasmon resonance; TEM, Transmission electron microscope
1. Introduction
Since the discovery of its tremendous photocatalytic performance in water splitting under ultraviolet (UV) light, titanium dioxide (TiO2) has garnered significant attention (Fujishima et al., 2008, Chen and Selloni, 2014, Zhang et al., 2014). Comprehensive research on the fabrication, structure, and applications of TiO2 nanostructures (Devi and Kavitha, 2013, Dozzi and Selli, 2013, Xu et al., 2014) have been carried out. The functionalization of TiO2 nanostructured materials has been applied to nanobiotechnology in drug delivery systems, photo thermal therapy, bone scaffolds, vascular stents, and biosensors. Recently, nano-TiO2 scaffolds have been shown to increase the speed of apatite formation and enhance cell adhesion, proliferation, and differentiation. (Primo et al., 2011, Cushing et al., 2012, Cushing et al., 2015, Li et al., 2015, Mubeen et al., 2013).
Similarly, gold nanomaterials (Au NMTs) have proven to be useful in the field of medical nanotechnology by providing light at the nanoscale and being incorporated into biological systems (Govorov et al., 2013, Furube et al., 2007). Most of these applications are based on an optical phenomenon such as localized surface plasmon resonance, which is highly tunable by the size and morphology of the nanoparticle (Daniel and Astruc, 2004). It can be regulated to specifically absorb or scatter light at specific wavelengths between the visible and near-infrared (NIR) range of the light spectrum. For maximum penetration in the visible-NIR range for therapeutic tissue, NIR is the most appropriate region. Compared with conventional therapeutic materials, gold nanostructures provide several advantages for biomedical applications, including high biocompatibility and non-cytotoxicity toward healthy tissues, passive accumulation at tumor sites due to enhanced permeability and retention effects (Liz-Marzán, 2006, Kochuveedu et al., 2012), and ease of bioconjugation via Au-TiO2 bonding to provide increased stability and active tumor targeting at the nanoscale (Oros-Ruiz et al., 2011, Murdoch et al., 2011). Metal nanoparticles and hybrid metal/TiO2 have been utilized in the development of new band gaps suitable for cancer therapy and other relevant applications. In addition, due to the significant research contributions of gold embedded with TiO2, the hybrid form has become renowned in biosensors, optical sensors (Li et al., 2015, Hu et al., 2014, Kodiyath et al., 2014, Alvarez-Puebla et al., 2010), and absorption components in solar cells and gas sensing systems (Stöber et al., 1968).
Most applications utilizing optical resonances in the visible region of the electromagnetic spectrum are due to surface plasmon resonance (SPR) absorption. NP dispersion depends on plasmon resonance frequency, while this frequency depends on size, morphology, shape, and distribution, as well as the particular dielectric characteristics of the surrounding dispersion medium (Sclafani and Herrmann, 1996, Xu et al., 2011). Consequently, oscillations induced by electromagnetic radiation on the conductive surface electrons of the nanoparticles can alter the position and intensity of the SPR with changes in volume fraction, size and morphology of the grown novel nanoparticles. Furthermore, particle interaction dependency may occur for high densities of nanostructure volume fraction (≥17%), and red shifting of SPR was detected with increasing particle size (Zuber et al., 2016). For dielectric media doping, milieu and ion implantation via manifold process are essential. Also, doping with magnetic and other transition metals to extend the technology has been the subject of research over the past few years (Kaur et al., 2018, Tedstone et al., 2016, He et al., 2018).
Simple and common superficial synthetic methods can be adopted due to the outstanding colloidal stability of these nanostructures. For the purpose of electron plasmonic response and electrochemical response of Au interfaces with TiO2 hybrid hierarchical architectures consisting of various morphologies, the process must be controlled via synthesis (concentration, temperature, irradiation time etc.), which allows for better understanding of this innovative strategy. Moreover, the numerical simulation response of the electron injection mechanism from gold to TiO2 has been uncovered. In this work, the mechanism of energy band gap electron transfer has been explored.
2. Materials and methods
2.1. 1. Synthesis procedure of titanium oxide nanoparticles
Titanium butoxide, oleic acid, oleyl amine, and absolute ethanol were purchased from Sigma Aldrich chemicals. TiO2 nanospheres were synthesized by a sol gel method. In this composition, titanium butoxide, (4.5 g), oleic acid (90%) (4.5 g), oleyl amine (80–90%) (4.3 g), and absolute ethanol (3.3 g) were added into a beaker and sonicated for few minutes. Then, the mixture was stirred for 20 min. The aqueous solution was poured into a hydrothermal Teflon reactor and 4 mL of DI water and 10 mL of ethanol were added into the empty space of the autoclave lining. The vessel was placed into a furnace at 200 ⁰C for 24 h until a white precipitate of titanium nanoparticles formed. The nanoparticles were then washed twice with ethanol in a centrifuge at 11000 rpm for 10 min at room temperature. Particle dispersions were achieved by adding 95% n-hexane C6H14, and a non-polar chain was attained by mixing in cyclohexane C6H12. Each mixture was stored at room temperature until the white precipitate settled.
2.1.1. Deposition of gold nanostructures on TiO2 nanoparticles
By adopting the seed growth mechanism, various morphologies of gold nanostructures were preferred using various ratios of benzyl-hexadecyl trimethyl ammonium chloride and CTAB. Using this simple technique, the gold content of the growth solution was used to grow NRs of ultrafine structures by adopting the same seed growth procedure as attempted by Babak Nikoobakht (Lkhagvadulam et al., 2013).
2.1.1.1. Capping polyethylene glycol (PEG) –Co- Au-doped TiO2 nanostructures
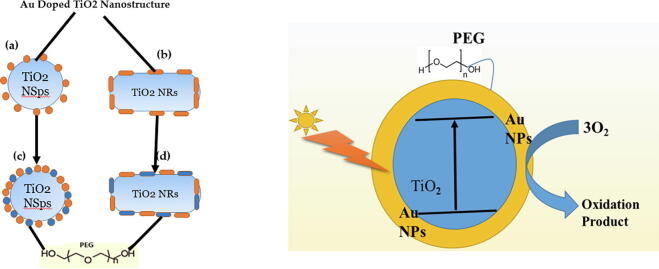
350 mg of PEG (200 µmol of PEG-2000) was added into 85 mL of Au-doped TiO2 nanostructures for 4 to 5 h of vigorous stirring at room temperature. After the reaction was completed, the final product was frozen and dried. This form could be analyzed with various techniques. The fabrication of TiO2 nanostructures with Au hierarchy is presented in Fig. 1. Fig. 1 (a) describes the schematic of Au nanospheres capped on the TiO2 nanostructure. Fig. 1(b) illustrates the schematic of Au nanorods capped on the TiO2 nanostructure, and Fig. 1(c) describes the PEG-coated Au nanospheres capped on the TiO2 nanostructure. Fig. 1(d) describes the schematic of PEG-coated Au nanorods capped on TiO2 nanostructure Fig. 1(e) depicts the newly developed band gap of PEG-capped Au-doped TiO2, and the corresponding hot electron generation enhancement in the Fermi sea of Au-doped TiO2 nanostructures. The concept of the mutual effect of hot electrons/PTT and PDT is novel. As long as the concentration of PEG-coated Au-doped titania increases, the loss in cell viability significantly decreases which is an indication of remarkable drug delivery toward a targeted cancerous site.
Fig. 1.
Illustration of the deposition of (a) Au nanospheres doped TiO2 nanostructures; (b) Au nanorods doped TiO2 nanostructure; (c) PEG-coated Au nanospheres doped TiO2 nanostructure; (d) PEG-coated Au nanorods doped TiO2 nanostructure; (e) Photoexcitation mechanism of PEG coated Au doped TiO2 nanostructure after laser exposure.
2.2. Cell culturing of MCF-7 cell line and labeling for PEG-coated Au-doped TiO2 nanostructures
The MCF-7 cell line (breast carcinoma) was individually cultured in 25 cm2 plastic tissue flasks. The flasks contained a minimum essential medium with bicarbonate ions (Hanks salts), 10% FBS, and 2 mM glutamine along with nonessential amino acids. The cells were incubated for 48 h to allow the cells to stick to the flask and controlled parameters were maintained at 37 °C in moist air. The 70–80% seeded cells were harvested by 0.25% trypsin. The breast carcinoma cells culture and 96-well plates containing 2% fetal bovine serum were used in the presence of 5% CO2 0–400 μg/mL of a dispersed solution of PEG-coated Au-doped TiO2 nanostructures added to each row of the 96-well plates for hot electron therapy and PDT analysis (Atif et al., 2019).
2.3. PDT of PEG-coated Au-doped TiO2 nanostructures
Photodynamic efficacy of the nanoparticles was conducted after suitable irradiation at the optimal wavelength of light and exposure of PEG-coated AuNP-coated TiO2 nanoparticles into MCF-7 cells by optimizing incubation time (0–200 min). In these experiments, UV contributions were screened out, and the degree of TiO2 photosensitization was confirmed. The 96-well plates were arranged in eight columns, and each column contained 4 wells. The final two columns were used as standards. Moreover, 100–400 μg/mL of a dispersed solution of PEG-coated Au-doped TiO2 nanoparticles were added to each row of the 96-well plates for hot electron therapy and PDT analysis. The incubation time was fixed for 0–200 min. The PEG-coated Au-doped TiO2 nanoparticles uptake was recorded. The neutral red assay (NRA) is a conventional procedure that precisely determines cell viability by using a microplate reader. Various PDTs were performed after preparing and labeling of solutions of different concentrations of PEG-coated Au-doped TiO2 into the MCF-7 cellular model (Iqbal et al., 2019aa, Iqbal et al., 2019bb).
2.4. NRA assessment of peg-coated-au-doped TiO2 nanostructures
The NRA is a conventional procedure that precisely determines cell viability by using a microplate reader. Various PDT optimized parameters were performed after preparing and labeling solutions of different concentrations of PEG-co-Au-doped TiO2 nanostructures into the MCF-7 cellular model. In the first step, the cells were seeded in 96-well plates and exposed to different concentrations of dispersed PEG-co-Au-doped TiO2 nanostructures in the absence and presence of laser light exposure (UV light (200 nm to 240 nm of light wavelength) and visible region of light (630 nm of wavelength)). After 24 h of cell incubation with PEG-co-Au-doped TiO2 nanostructures at an optimal concentration, 50 μL of NRA (50 mg/mL) was incorporated in the treated cultured plate and incubated for 3 h (Fakhar-e-Alam et al., 2020). The medium was removed and the cells were washed with 40% formaldehyde and 10% CaCl2 (v/v, 4:1). In the next step, a complex of 45% ethanol and 15% acetic acid (1:1) was assimilated to extract NR. In a further step, the NR-mixed plate was shaken and kept free for 15 min. The absorbance was then examined at 510 nm. The quantification of solubilized dye was statistically analyzed with the living cell numbers according to the % cell viability formula (Iqbal et al., 2019). Solution uptake/absorbance was assessed by selecting a filter compatible with the microplate reader.
3. Results
3.1. Material characterization of PEG-coated Au-doped TiO2 nanostructures
We have carried out the fundamental concept of PEG-coated gold-titanium nanostructures for treatment via hot electron therapy/photo thermal therapy. Optimizing the Au-doped TiO2 nanostructures with respect to morphology, size, optical absorption band, and purity is a major challenge. The surface morphology of the prepared composites was analyzed by a JEM 1230 transmission electron microscope (TEM).
3.2. Morphology analysis
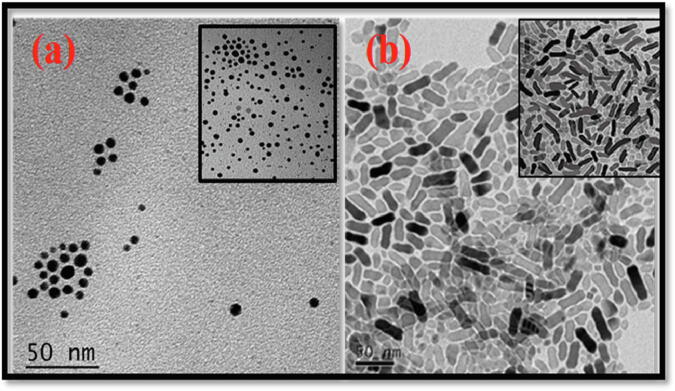
The morphology of TiO2 nanospheres and Au-doped TiO2 nanospheres were confirmed using TEM, as depicted in Fig. 2. TEM images confirmed immobilization and homogeneous distribution of the nanospheres (5–10 nm) and nanorods (15–20 nm) respectively.
Fig. 2.
TΕM images of differently shaped PEG-coated Au-doped TiO2 nanostructures (a) nanospheres (b) nanorods.
3.3. PDT combined with HET of MCF-7 cells exposed with nanostructures
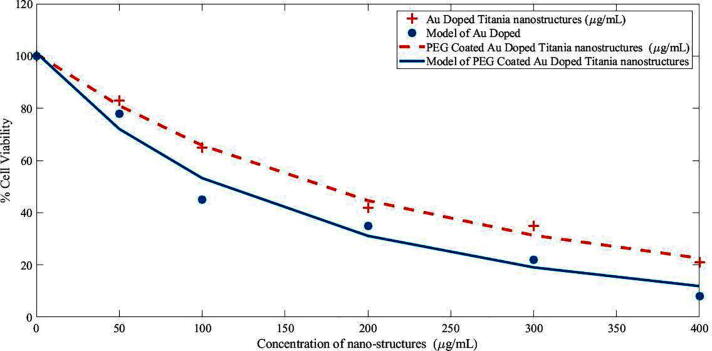
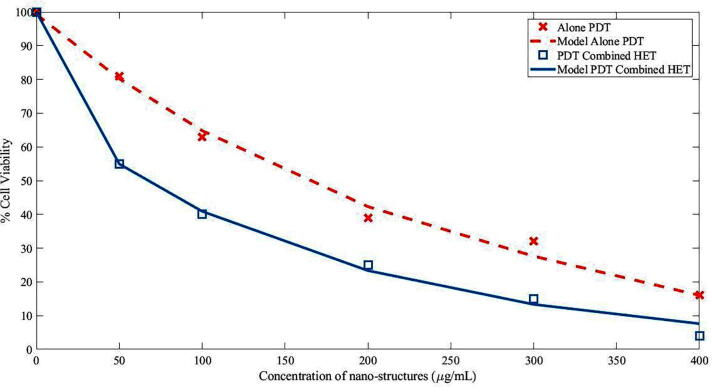
A boosted form of the effective injection of generated hot electrons via photothermal therapy across the Schottky barrier to the conduction band of TiO2 triggers the formation of excitons and the successive liberation of essential radical species which is beneficial toward reactive oxygen species liberation which leads to cell death (Fakhar-e-Alam et al., 2020). Even though the peak intensity of the indigenous fields created by the surface plasmon exponentially decay with distance, the contact between the Au and TiO2 allows for an efficient interaction between both components. In addition, the robust scattering of Au NSTs may lead to enhanced optical concentration and thus, an enhanced light absorption of TiO2 (Kovacs et al., 2015). Fig. 3 shows cell viability graph of MCF-7 vells exposed with Gold doped Titania and PEG coated Gold doped Titania. Fig. 4 shows percentage cell viability graph of MCF-7 Cells after treatment with PDT and PDT along HET.
Fig. 3.
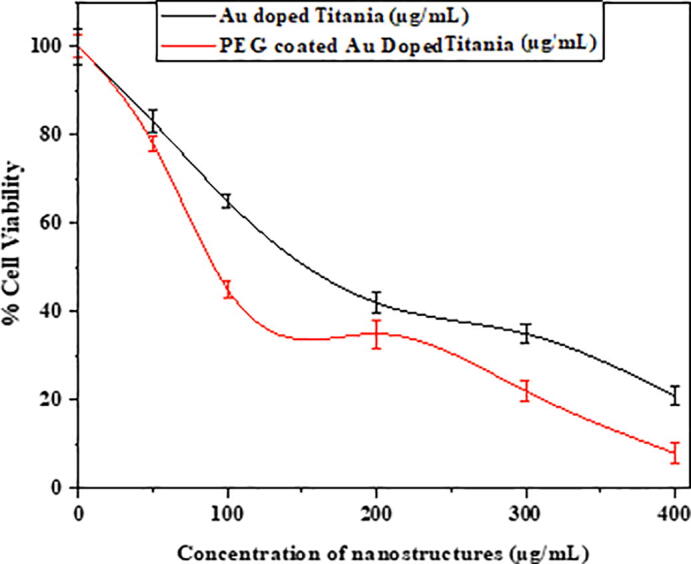
Cell viability graph of MCF-7 cells exposed with gold-doped Titania and PEG-coated gold-doped Titania.
Fig. 4.
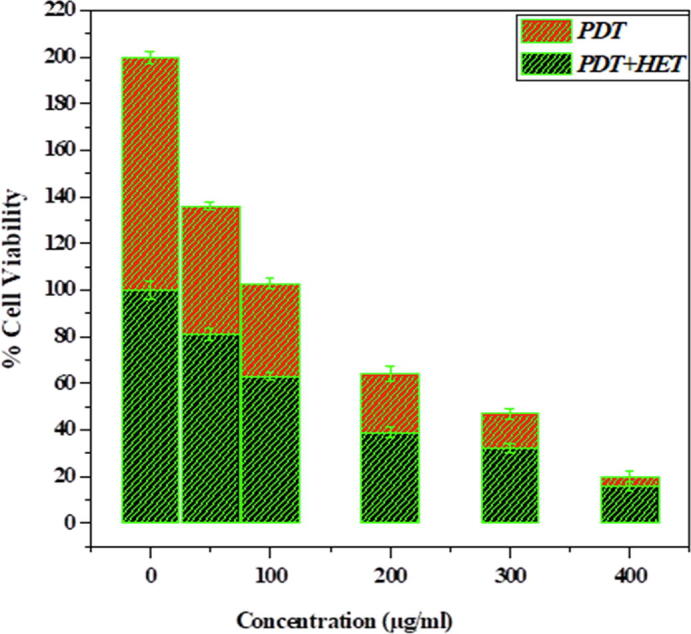
%Cell Viability graph of MCF-7 Cells after treatment with PDT and PDT along HET.
3.4. Mathematical modeling
Data was obtained from experimental studies by varying the concentration (µg/mL) of nanostructures and measuring the % cell viability. The experiment was repeated for Au-doped titania µg/mL, PEG-coated Au-doped titania µg/mL, PDT only, and PDT + HET (combined therapy effect) and the data is shown in Tables 1. Then, a mathematical model was used to fit the data (Iqbal et al., 2019aa, Iqbal et al., 2019bb). It was proposed to have a bi-exponential model as presented in equation 1 by observing the nature of the data with respect to the increasing concentration (Fakhar-e-Alam et al., 2020, Iqbal et al., 2019).
Table 1.
Estimation of constants used in equation 1 based on the least square error method, along with goodness of fit parameters.
| a | b | c | d | SSE | R-square | Adjusted R-square | RMSE | |
|---|---|---|---|---|---|---|---|---|
| Au | 47 | −0.00665 | 53.75 | −0.00256 | 28.17 | 0.9939 | 0.9847 | 3.753 |
| PEG | 26.42 | −0.01464 | 74.76 | −0.004614 | 143.3 | 0.9763 | 0.9409 | 8.464 |
| PDT | 99.28 | −0.004266 | −1.8e-15 | 0.08659 | 34.55 | 0.9932 | 0.983 | 4.157 |
| PDT + HET | 28.41 | −0.0701 | 71.59 | −0.005605 | 19.4 | 0.9968 | 0.9919 | 3.114 |
(eq 1)
The careful selection of a mathematical function able to accurately represent the data was based on various parameters of goodness of fit like SSE, R-squared values, adjusted R-squared values, and root mean square error (RMSE). Other functions are likely to present poor values for goodness of fit. Moreover, having a bi-exponential model for representation of all the experiments had provided ease of finding out the best therapy and a quantitative benchmark in general. Therefore, using equation 1 shows a comparison for which therapy is better for the patient (Fig. 5) (Xu et al., 2007).
Fig. 5.
Modeling of %cell viability graph of MCF-7 cells exposed with gold-doped titania and PEG-coated gold-doped titania nanostructures.
It can be observed that “b” and “d” are coefficients representing the decay constant in the bi-exponential model. The values of “b” are more important and play a vital role in deciding the decay factor and, hence, the shape of the tapering at lower values of concentration, while the negative sign shows that % cell viability decreases with increasing concentration (µg/mL) (Fig. 6).
Fig. 6.
Modeling of %cell viability graph of MCF-7 cells after treatment with PDT and PDT along HET.
4. Discussion
Experimentally, titania nanospheres (~200 nm) were successfully synthesized and by applying a controlled chemical reaction, various nanomorphologies of Au nanostructures were generated on the surface of the 200 nm-nanosphere of TiO2. PEG was encapsulated on the surface of the Au composite TiO2 nanospheres after the final layer. In this experiment, the role of the outer layer of organic material (PEG) was significant relative to stability and valuable nanomaterial delivery through delicate blood vessels. From the TEM image, it is obvious that Au nanostructures retain their structural integrity throughout the assembly process, and the crystalline nature of the TiO2 inner layer was confirmed by HRTEM analysis. HRTEM images (lower panel of Fig. 2) clearly showed that Au dopant was well distributed and decorated on the surface of host TiO2 NPs. High resolution TEM images also demonstrated that TiO2 NPs are highly crystalline, which matches well with the (1 0 1) plane of powdered TiO2 (Moosavi et al., 2016, Tisdale et al., 2010).
The random morphology of the Au nanostructures was oriented into solution and photo energy production was shown to be dependent on size, shape, and anisotropy of the nanomaterial.
Cell viability loss in MCF-7 cells were employed by exposing with individual Au-doped titanium nanomorphology and PEG-capsulated gold-doped titania in Fig. 3. The concept of mutual effect of hot electrons and PDT is novel (Clavero, 2014, Qiu et al., 2018). As long as the concentration of PEG-coated Au-doped titania increases, the loss in cell viability will significantly decrease which is indicative of remarkable drug delivery toward targeted/cancerous site. This has been observed after Au-doped titania NSTs formation with and without PEG, and the biodistribution results were compared. Beyond 100 µg/mL NPs concentration, significant loss in cell viability was assessed, which is about 79% in the cases of gold and TiO2 nanostructures at 400 µg/mL, and this loss reaches 92% at PEG-coated gold nanostructures with TiO2 at 400 µg/mL. Similar therapeutic studies were conducted toward a malignant cell model (Atif et al., 2020, Atif et al., 2016).
The aim of our experimental approach was to discriminate the possibility of individual and mutual effect of photothermal therapy and photodynamic therapy. It had been evaluated that the absorption spectra of gold lied in the region of 525 nm 560 nm, as long as the size of Au NSTs increases the absorption peak shifted to longer wavelengths. However, for Au-doped titania, two types of therapies were noted in the case of PEG-coated Au-doped titania under 525 nm and (525 and 560 nm) of light wavelength. It was determined that terms of only 525 nm of red-light exposure toward PEG-coated Au-doped TiO2 exposed MCF-7 breast cancer cells, only photodynamic therapy effects were recorded as depicted in Fig. 3 (Atif et al., 2016). Because of this, the cell viability loss went from 50% to 90% when NPs were exposed to 100 µg/mL to 400 µg/mL of PEG-coated Au-doped TiO2, respectively. Although, in the case of two wavelength laser additions in a single shot (525 and 560 nm), PDT and photothermal therapy were confirmed. In the case of the combined effects of PDT with HET, ~98% loss in cell viability were measured, which is quite significant and was confirmed by applying multiple reliable tests, as shown in Fig. 4, Fig. 5.
The combination of photodynamic therapy and photothermal therapy (PDT and HET) plays a vital role in the successful treatment of cancer patients, and these experimental results have validated this novel achievement. It was further observed that the value of “b” is highest for PDT + HET (Fig. 6), which clearly indicated this as the optimal therapy compared to others listed in the table. The model also showed excellent goodness of fit values of R-squared, Adjusted R-squared, RMSE, and SSE (Iqbal et al., 2020). In summary, this novel form has 98.5% malignant cell killing potential as confirmed by experimental data.
5. Conclusions
We have demonstrated the role of gold-doped titania (Au-doped TiO2) toward biomedical applications. It was shown that the morphology of Au on the titania surface played a significant role in the efficacy of the combined photothermal and photodynamic therapies. The morphology and crystallite of different hybrid forms were confirmed by applying TEM. In addition, problems of drug uptake resistance and drug release were advocated by incorporating the PEGylated form of gold-doped TiO2 which showed enhanced quantities of ligands toward the targeted site.
In this article, a new strategy of hot electron generation synergistic coupling with powerful PDT treatment modality was introduced toward the vitro/MCF-7 model. It was concluded that this synergistic approach toward malignant cell killing potential up to 98.5% was confirmed by experimental data analysis. In the light of current experimental findings, it is expected that in the near future, this combined therapeutic effect will be preferable over all traditional/conventional techniques. Additionally, the experimental results agreed well with a least squares fit math model.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group number RGP-293. The author would like to specially thank the Higher Education Commission (HEC) Pakistan for the financial support under NRPU Grant No. 8056/Punjab/ NRPU/R&D/HEC/ 2017. The research was supported by the National Natural Science Foundation of China (Grant Nos. 11971142, 11871202, 61673169, 11701176, 11626101, 11601485).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
M. Fakhar-e-Alam, Email: fakharphy@gmail.com.
Yu-Ming Chu, Email: chuyuming2005@126.com.
Hijaz Ahmad, Email: hijaz555@gmail.com.
References
- Fujishima A., Zhang X., Tryk D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008;63:515–582. doi: 10.1016/j.surfrep.2008.10.001. [DOI] [Google Scholar]
- Chen X., Selloni A. Introduction: titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014;114:9281–9282. doi: 10.1021/cr500422r. [DOI] [PubMed] [Google Scholar]
- Zhang G., Kim G., Choi W. Visible light driven photocatalysis mediated via ligand-to-metal charge transfer (LMCT): an alternative approach to solar activation of Titania. Energy Environ. Sci. 2014;7:954–966. doi: 10.1039/c3ee43147a. [DOI] [Google Scholar]
- Devi L.G., Kavitha R. A review on Nonmetal ion doped Titania for the photocatalytic degradation of organic pollutants under UV/solar Light: role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B. 2013;140–141(559–587):559–587. doi: 10.1016/j.apcatb.2013.04.035. [DOI] [Google Scholar]
- Dozzi M.V., Selli E. Doping TiO2 with P-block elements: effects on photocatalytic activity. J. Photochem. Photobiol. C. 2013;14:13–28. doi: 10.1016/j.jphotochemrev.2012.09.002. [DOI] [Google Scholar]
- Xu H., Ouyang S., Liu L., Reunchan P., Umezawa N., Ye J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A. 2014;2:12642–12661. doi: 10.1039/C4TA00941J. [DOI] [Google Scholar]
- Primo A., Corma A., García H.T. Titania Supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011;13:886–910. doi: 10.1039/C0CP00917B. [DOI] [PubMed] [Google Scholar]
- Cushing S.K., Li J., Meng F., Senty T.R., Suri S., Zhi M., Li M., Bristow A.D., Wu N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012;134:15033–15041. doi: 10.1021/ja305603t. [DOI] [PubMed] [Google Scholar]
- Cushing S.K., Li J., Bright J., Yost B.T., Zheng P., Bristow A.D., Wu N. Controlling plasmon-induced resonance energy transfer and hot electron injection processes in Metal@TiO2 core–shell nanoparticles. J. Phys. Chem. C. 2015;119:16239–16244. doi: 10.1021/acs.jpcc.5b03955. [DOI] [Google Scholar]
- Li J., Cushing S.K., Meng F., Senty T.R., Bristow A.D., Wu N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Phot. 2015;9:601–607. doi: 10.1038/nphoton.2015.142. [DOI] [Google Scholar]
- Mubeen S., Lee J., Singh N., Krämer S., Stucky G.D., Moskovits M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nano. 2013;8:247–251. doi: 10.1038/nnano.2013.18. [DOI] [PubMed] [Google Scholar]
- Govorov, A.O., Zhang, H., Gun’ko, Y.K., 2013. Y. K Theory of photoinjection of hot plasmonic carriers from metal nanostructures into semiconductors and surface molecules. J. Phys. Chem. C. 117, 16616–16631. DOI: 10.1021/jp405430m.
- Furube A., Du L., Hara K., Katoh R., Tachiya M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007;129:14852–14853. doi: 10.1021/ja076134v. [DOI] [PubMed] [Google Scholar]
- Daniel M.C., Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Liz-Marzán L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir. 2006;22:32–41. doi: 10.1021/la0513353. [DOI] [PubMed] [Google Scholar]
- Kochuveedu S.T., Kim D.-P., Kim D.H. Surface-plasmon-induced visible light photocatalytic activity of TiO2 nanospheres decorated by Au nanoparticles with controlled configuration. J. Phys. Chem. C. 2012;116:2500–2506. doi: 10.1021/jp209520m. [DOI] [Google Scholar]
- Oros-Ruiz S., Pedraza-Avella J.A., Guzmán C., Quintana M., Moctezuma E., del Angel G., Gómez R., Pérez E. Effect of gold particle size and deposition method on the photodegradation of 4-chlorophenol by Au/TiO2. Top. Catal. 2011;54:519–526. doi: 10.1007/s11244-011-9616-y. [DOI] [Google Scholar]
- Murdoch M., Waterhouse G.I., Nadeem M.A., Metson J.B., Keane M.A., Howe R.F., Llorca J., Idriss H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011;3:489–492. doi: 10.1038/nchem.1048. [DOI] [PubMed] [Google Scholar]
- Li A., Zhang P., Chang X., Cai W., Wang T., Gong J. Gold Nanorod@TiO2 yolk-shell nanostructures for visible-light-driven photocatalytic oxidation of benzyl alcohol. Small. 2015;11:1892–1899. doi: 10.1002/smll.201403058. [DOI] [PubMed] [Google Scholar]
- Hu C., Zhang X., Li X., Yan Y., Xi G., Yang H., Bai H. Au photosensitized TiO2 ultrathin nanosheets with 001 exposed facets. Chemistry. 2014;20:13557–13560. doi: 10.1002/chem.201403428. [DOI] [PubMed] [Google Scholar]
- Kodiyath R., Manikandan M., Liu L., Ramesh G.V., Koyasu S., Miyauchi M., Sakuma Y., Tanabe T., Gunji T., Duy Dao T., Ueda S., Nagao T., Ye J., Abe H. Visible-light photodecomposition of acetaldehyde by TiO2-coated gold nanocages: plasmon-mediated hot electron transport via defect states. Chem. Commun. 2014;50:15553–15556. doi: 10.1039/c4cc06229a. [DOI] [PubMed] [Google Scholar]
- Alvarez-Puebla R., Liz-Marzán L.M., García de Abajo F.J., Light F.J. Light Concentration at the nanometer scale. J. Phys. Chem. Lett. 2010;1:2428–2434. doi: 10.1021/jz100820m. [DOI] [Google Scholar]
- Stöber W., Fink A., Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968;26:62–69. doi: 10.1016/0021-9797(68)90272-5. [DOI] [Google Scholar]
- Sclafani A., Herrmann J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of Titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996;100:13655–13661. doi: 10.1021/jp9533584. [DOI] [Google Scholar]
- Xu M., Gao Y., Moreno E.M., Kunst M., Muhler M., Wang Y., Idriss H., Wöll C. Photocatalytic activity of bulk TiO2 anatase and rutile single crystals using infrared absorption spectroscopy. Phys. Rev. Lett. 2011;106 doi: 10.1103/PhysRevLett.106.138302. [DOI] [PubMed] [Google Scholar]
- Zuber A., Purdey M., Schartner E., Forbes C., van der Hoek B., Giles D., Abell A., Monro T., Ebendorff-Heidepriem H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuators B. 2016;227:117–127. doi: 10.1016/j.snb.2015.12.044. [DOI] [Google Scholar]
- Kaur H., Kumar S., Verma N.K., Singh P. Role of pH on the photocatalytic activity of TiO2 tailored by W/T mole ratio. J. Mater. Sci. Mater. Electron. 2018;29:16120–16135. doi: 10.1007/s10854-018-9701-0. [DOI] [Google Scholar]
- Tedstone A.A., Lewis D.J., O’Brien P.O. Synthesis, properties and applications of transition metal-doped layered transition metal dichalcogenides. Chem. Mater. 2016;28:1965–1974. doi: 10.1021/acs.chemmater.6b00430. [DOI] [Google Scholar]
- He C., Cheng M., Zhang W.X. Tunable electronic and magnetic properties of transition metals doped antimonene: a first-principles study. Mater. Res. Exp. 2018;5 doi: 10.1088/2053-1591/aacdd7. [DOI] [Google Scholar]
- Lkhagvadulam B., Kim J.H., Yoon I., Shim Y.K. Photodynamic activity of gold nanoparticles conjugate of water soluble purpurin-18-N-methyl-D-glucamine. BioMed Res. Int. 2013:720759. doi: 10.1155/2013/720579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif M., Iqbal S., Fakhar-E-Alam M., Ismail M., Mansoor Q., Mughal L., Aziz M.H., Hanif A., Farooq W.A. Manganese-doped cerium oxide nanocomposite induced photodynamic therapy in MCF-7 cancer cells and antibacterial activity. BioMed Res. Int. 2019;2019:1–13. doi: 10.1155/2019/7156828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, S., Fakhar-e-Alam, M., Atif, M., Amin, N., Alimgeer, K.S., Ali, A., Aqrab-ul-Ahmad, Hanif, A., Aslam Farooq, W., 2019. Structural, morphological, antimicrobial, and in vitro photodynamic therapeutic assessments of novel Zn+ 2-substituted cobalt ferrite nanoparticles. Results Phys. 15. DOI: 10.1016/j.rinp.2019.102529, http://www.ncbi.nlm.nih.gov/pubmed/102529.
- Iqbal S., Fakhar-e-Alam M., Akbar F., Shafiq M., Atif M., Amin N., Ismail M., Hanif A., Farooq W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019;1189:203–209. doi: 10.1016/j.molstruc.2019.04.041. [DOI] [Google Scholar]
- Fakhar-e-Alam, M., Aqrab-ul-Ahmad, Atif, M., Alimgeer, K.S., Suleman Rana, M., Yaqub, N., Aslam Farooq, W., Ahmad, H., 2020. Synergistic effect of TEMPO-coated TiO2 nanorods for PDT applications in MCF-7 cell line model. Saudi J. Biol. Sci.. DOI: 10.1016/j.sjbs.2020.09.027. [DOI] [PMC free article] [PubMed]
- S. Iqbal, S., Fakhar-e-Alam, M., Atif, M., Ahmed, N., -ul-Ahmad, A., Amin, N., Alghamdi, R., Hanif, A., Farooq, W., 2019. Empirical modeling of Zn/ZnO nanoparticles decorated/conjugated with fotolon (chlorine e6) based photodynamic therapy towards liver cancer treatment. Micromachines. 10, 60. DOI: 10.3390/mi10010060. [DOI] [PMC free article] [PubMed]
- Kovacs G., ZsoltPap C. Cotet, Veronica C. Lucian Baia and V. Danciu, photocatalytic, morphological and structural properties of the TiO2-SiO2-Ag porous structures based system. Materials. 2015;8:1059–1073. doi: 10.3390/ma8031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Sun, Y., Zhao, Y., Huang, J., Chen, C., Jiang, Z. Photocatalytic inactivation effect of gold-doped TiO2 (Au/TiO2) nanocomposites on human colon carcinoma LoVo cells; Hindawi publishing corporation. Int. J. Photoenergy Volume (2007), 1-7, Article ID 97308. DOI: 10.1155/2007/97308.
- Moosavi, M.A., Sharifi, M., Ghafary, S.M. Zahra M. A. p., Alireza K., Marvesh R., Sadaf H., Marek J. L., Thomas K., Saeid G., 2016. Photodynamic N-TiO2 nanoparticle treatment induces controlled ros-mediated autophagy and terminal differentiation of leukemia cells, Sci. Rep. [DOI] [PMC free article] [PubMed]
- Tisdale W.A., Williams K.J., Timp B.A., Norris D.J., Aydil E.S., Zhu X.-Y. Hot-electron transfer from semiconductor nanocrystals. Science. 2010;328:1543–1547. doi: 10.1126/science.1185509. [DOI] [PubMed] [Google Scholar]
- Clavero C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics. 2014;8:95–103. doi: 10.1038/nphoton.2013.238. [DOI] [Google Scholar]
- Qiu H., Tan M., Ohulchanskyy T.Y., Lovell J.F., Chen Guanying. Recent progress in Upconverstion photodynamic therapy. Nanomaterials. 2018;8:344. doi: 10.3390/nano8050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif M., Devanesan S., AlSalhi M.S., Masilamani V., Saleem M.N.A., AlShebly M., Farhat K., Hussain I., Alimgeer K.S. An experimental and algorithm-based study of the spectral features of breast cancer patients by a photodiagnosis approach. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101851. [DOI] [PubMed] [Google Scholar]
- Atif, M., Fakhar-e-Alam, M., Abbas, N., Siddiqui, M. A., Ansari, A. A., Al-Khedhairy, A. A., Wang, Z. M., 2016. In vitro cytotoxicity of mesoporous SiO2@Eu(OH)3 core-shell nanospheres in MCF-7, Hindawi. J. Nanomater. 6, Article ID 7691861.
- Iqbal S., Fakhar-e-Alam M., Atif M., Amin N., Ali A., Shafiq M., Ismail M., Hanif A., Farooq W.A. Photodynamic therapy, facile synthesis, and effect of sintering temperature on the structure, morphology, optical properties, and anticancer activity of Co3O4 nanocrystalline materials in the HepG2 cell line. J. Photochem. Photobiol. A. 2020;386 doi: 10.1016/j.jphotochem.2019.112130. [DOI] [Google Scholar]