Abstract
Due to their inexpensive and eco-friendly nature, and existence of manganese in various oxidation states and their natural abundance have attained significant attention for the formation of Mn3O4 nanoparticles (Mn3O4 NPs). Herein, we report the preparation of Mn3O4 nanoparticles using manganese nitrate as a precursor material by utilization of a precipitation technique. The as-prepared Mn3O4 nanoparticles (Mn3O4 NPs) were characterized by using X-ray powder diffraction (XRD), UV–Visible spectroscopy (UV–Vis), High-Resolution Transmission electron microscopy (HRTEM), Field emission scanning electron microscopy (FESEM), Thermal gravimetric analysis (TGA) and Fourier-transform infrared spectroscopy (FT-IR). The antimicrobial properties of the as-synthesized Mn3O4 nanoparticles were investigated against numerous bacterial and fungal strains including S. aureus, E. coli, B. subtilis, P. aeruginosa, A. flavus and C. albicans. The Mn3O4 NPs inhibited the growth of S. aureus with a minimum inhibitory concentration (MIC) of 40 μg/ml and C. albicans with a MIC of 15 μg/ml. Furthermore, the Mn3O4 NPs anti-cancer activity was examined using MTT essay against A549 lung and MCF-7 breast cancer cell lines. The Mn3O4 NPs revealed significant activity against the examined cancer cell lines A549 and MCF-7. The IC50 values of Mn3O4 NPs with A549 cell line was found at concentration of 98 µg/mL and MCF-7 cell line was found at concentration of 25 µg/mL.
Keywords: Mn3O4, Nanoparticles, Antimicrobial, Anticancer activity, A549, MCF-7
1. Introduction
Now a day’s nanotechnology attained a great interest due to the recent developments in the science and engineering fields, nanotechnology can be deliberated as technology of future (Havancsak, 2003, Hulla et al., 2015, Kumar et al., 2020, Poole Jr and Owens, 2003, Weiss, 2010). Nanotechnology developed numerous types of materials at nanoscale level. In current years, quick advancement in the nanotechnology field has facilitated the development of engineered nanoparticles of different kinds, dimensions, and morphologies (Henry, 2005, Kuhlbusch et al., 2011). Nanoparticles are extensive kinds of materials that embrace particulate materials, which have one dimension below 100 nm at least (Cele, 2020). They can maintain physico-chemical properties such as high surface area, conductance, homogeneity, structural stability or distinct optical properties etc., that make them required in materials science and various application fields (Kung and Kung, 2004, Nabi et al., 2020, Nie et al., 2007).
These nanoparticles have made a tremendous impact in the various applications such as catalysis, pharmaceutics, optics, food technology, cosmetics, water treatment, electrochemistry, energy, biomedicine, biosensor cancer treatment, healthcare and drug delivery (Al-Hobaib et al., 2015, Bae et al., 2006, Dreaden et al., 2012, El-Sayed and Kamel, 2020, Kung and Kung, 2003, Mu and Sprando, 2010, Ndlovu et al., 2020). The nanoparticles properties in the medicinal arena were improved greater associated to their bulk counterparts. In this nanomaterial’s metal and metal oxide nanoparticles display distinct physical and chemical properties, particularly metal oxide nanomaterial’s are enormously stimulating in the field of semiconducting constituents and endorse an ideal prospect for the progress of biological activities (Charbgoo et al., 2017, Mirzaei and Darroudi, 2017). In these nanomaterials, trimangnese tetraoxide nanoparticles (Mn3O4) is one of the significant nanoparticles comprise of various crystal structures constructed from octahedral MnO6 were, manganese dioxide (MnO2), dimanganese trioxide (Mn2O3) and trimangnese tetraoxide nanoparticles (Mn3O4) (Deka et al., 2020, Jiang et al., 2020, Roche et al., 2007, Shin et al., 2009, Yang et al., 2010).
There are a diversity of approaches to develop metal/metal oxide NPs, for instance precipitation, hydrothermal, micro-emulsion, sol–gel, and sonochemical technique, which usually provide adequate products for various applications (Ghazal et al., 2020, Medhi et al., 2020, Yadav et al., 2020). Manganese oxides is a mixed oxide material are appropriate for wide range applications such as catalysis, electrochemical, medicinal due to their low cost, eco-friendly nature, existence of manganese in various states and their natural abundance (Hoseinpour and Ghaemi, 2018, Kumar et al., 2017, Wang et al., 2017). The numerous manganese oxidation states consequences in the development of manganese dioxide, dimanganese trioxide, trimanganese tetraoxide. In these, Mn3O4 nanoparticles have encouraged abundant attention owing to their distinctive structural and electronic features with exclusive ion exchange, catalysis, molecular adsorption, magnetic and electrochemical properties. Manganese is deliberated a vital constituent in metabolism has been well controlled by biological systems and Mn3O4 NPs display greater biological properties and low toxicity (Bhattacharya et al., 2019, Khan et al., 2016). Accordingly, Mn3O4 NPs have been competently examined as prospective antibacterial systems because of their synergistic performance. The ions ‘Mn2+’ generates harmless free radicals, which performance a vital part in clinical sicknesses like Alzheimer's, cardiac, diabetes, numerous diseases etc., including cancer, and moreover, nanomedicine is supposed to be a favorable investigation route in the tumors treatment (Khan et al., 2016).
In the current scenario, the major complication in cancer treatment is the partial existence of particular anticancer drugs or nanomedicines along with a lesser amount of competent delivery systems and majority of the currently existing anticancer drugs having poor solubility in aqueous solutions. Consequently, major efforts have been carried out to generate novel anticancer drugs with superior aqueous solubility and effective in cancer treatment. Among them, metal oxide nanoparticles have synergistic and superior physicochemical properties made huge attention in cancer treatment (Behzadi et al., 2019, Khan et al., 2016, Pugazhendhi et al., 2019).
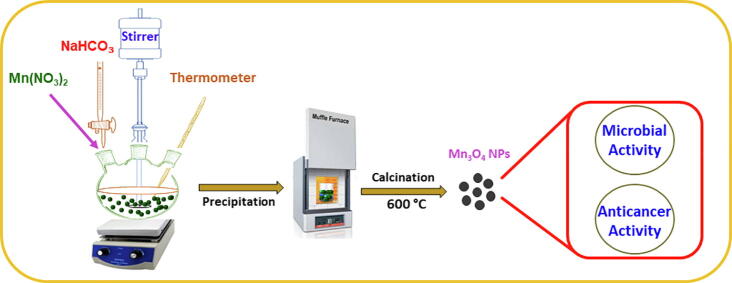
In the current work, we demonstrate a precipitation method for the synthesis Mn3O4 nanoparticles using manganese nitrate as a precursor material by utilization of a precipitation method (Scheme 1). As-synthesized Mn3O4 nanoparticles characterized by several microscopic, spectroscopic and thermal techniques such as HRTEM, FESEM, EDX, UV–Vis, XRD, FT-IR, and TGA. The biological properties of the as-prepared Mn3O4 NPs were investigated against various fungal and bacterial strains comprising S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans and A. flavus. The growth of S. aureus inhibited with a MIC of 40 μg/ml and C. albicans with a MIC of 15 μg/ml using Mn3O4 NPs. Furthermore, the Mn3O4 NPs anti-cancer activity was examined using MTT essay against A549 lung and MCF-7 breast cell lines. The Mn3O4 NPs revealed significant activity against the investigated cancer cell lines A549 and MCF-7.
Scheme 1.
Graphical representation of as-prepared Mn3O4 NPs using sodium bicarbonate and assessment of their antimicrobial and anticancer activity.
2. Materials and methods
2.1. Materials
All chemicals, such as Manganese nitrate, sodium bicarbonate, phosphate buffer saline, dimethyl sulfoxide, and all other materials and organic solvents were procured from Sigma Aldrich (USA).
2.2. Synthesis of Mn3O4 nanoparticles
Mn3O4 was prepared by precipitation method. 0.1 M Manganese nitrate (100 mL) was prepared in distilled water and transferred to a reaction vessel and the heating of the reaction vessel was increased to 90 °C under mechanical stirring. Once the temperature is attained 0.05 M sodium bicarbonate is added dropwise to attain the pH 9. At pH 9 stopped the addition of sodium bicarbonate and heated another 3 h under continuous stirring. After 3 h, the reaction vessel brought down to room temperature and stirred further overnight. The reaction mixture was washed using centrifugation and dried at 70 °C overnight. The attained powder was calcined at 600 °C for12 hours to achieve Mn3O4 nanoparticles.
2.3. Characterization
The as-prepared Mn3O4 NPs are characterized by several approaches. The XRD characterization is carried out using Bruker diffractometer (Cu Kα, (λ = 1.5406 Å)). The spectral characterization was carried out using PerkinElmer UV–Vis spectrometer and Bruker IFS 66 v/S spectrometer are used for UV–Vis and FT-IR spectral analysis, respectively. The microscopic analysis such as FESEM is carried to understand the surface analysis is analysed out by FESEM (JED-2200, JEOL, Tokyo, Japan) and the FESEM sample was fixed onto a stub with carbon‐built dual‐sided adhesive tape and then sputter coated with nearly 10 nm of platinum. For imaging, the sample is focused on perpendicular to the inward electron beam. FESEM pictures were captured using an operational voltage of 5 kV and a working distance of 4.5 mm over several magnifications. HRTEM was performed on a JEOL JEM 2100 PLUS, (USA). The sample for HRTEM analysis was prepared by placing a drop of primary sample on a holy carbon copper grid, which were dried in oven for 6 h at 80C°. HRTEM images are recorded with operating at 200 kV accelerating voltage. Thermo gravimetric analysis was analyzed using Metler Toledo instrument, TGA/DSC1, Im Langacher, Switzerland.
2.4. Antibacterial and antifungal activity of the of the as-synthesized Mn3O4 nanoparticles
The antimicrobial activity of NPs was accomplished using the conventional agar disk diffusion and MIC approaches. Six American Type Culture Collection bacterial and fungal strains, namely E. coli, S. aureus, B. subtilis, P. aeruginosa and two fungal strains C. albicans and A. flavus were acquired from the Microbiology Department, Pharmacy College, King Saud University. All the bacterial and fungal strains were sub-cultured in newly made nutrient broth and post observation of growth was consistent as per 0.5 McFarland turbidity standards. The inoculum was plated on nutrient agar plates by the spread plate procedure and a sterile borer six mm was cast-off to bore four wells in the ready plate. Each wells, 100 µL of 30 µg/mL each of Mn3O4 NPs, gentamycin & fluconazole (50 µg/mL) was added and the fourth well persisted as the ‘-ve’ control. The cultured plates were incubated at 37 ˚C for 24 h to check the inhibition zone of tested bacteria 24 h for bacterial strains and for 48 h for tested fungal strains. All the test were done in triplicates to rule out false positive results
2.5. Epsilometer test for MIC analysis
To determine MIC, Standard McFarland inoculum was prepared using Muller Hilton broth using overnight grown culture of the tested strains. 100 µg/ml Mn3O4 NPs was added to the broth solution containing the standard culture and incubated at optimum temperature and time as for the previous protocol (Hannan et al., 2020). The Muller Hilton agar plates were swabbed with the broth inoculum containing the Mn3O4 NPs. The plates were seeded with E strip containing gradient concentration of standard antibiotic of gentamycin for antibacterial and fluconazole for anti-fungal analysis. After incubation MIC was recorded. All the tests were done in triplicates to rule out false positive results
2.6. Cytotoxic activity Mn3O4 NPs
The as-prepared Mn3O4 NPs was employed to investigate the anticancer activity of human lung and breast A549 and MCF-7 cell lines. Both cell lines cultured in Dulbecco’s Modified Eagle Medium with fetal bovine serum (7%) and each penicillin & streptomycin (1%). The cell culturing was prepared at 37 °C in 5% CO2 supply incubator. The sub culturing was completed at regular intermission for every round of analysis. The exponential growing cells were exploited.
MTT colorimetric assay (3-[4,5-dimethylthiazole-2-y]-2,5-diphenyltetrazolium bromide) method was used to examine the both cancer cell lines. The IC50 values of both A549 and MCF-7 were assessed by the cytotoxicity assessment using Mn3O4 NPs. All cell suspension of about 5 × 104 cells were sowed in a 96-well flat plate comprising Dulbecco’s Modified Eagle Medium with fetal bovine serum (5%) and various concentrations of Mn3O4 NPs: 0, 25, 50, 75 and 100 µg/mL. The mixture was kept in 5% CO2 incubator for a time of 24 h. After incubation period, the subjected cells to a wash serum free medium and a 100 mL of 5 µg/mL of MTT solution was added pursued by incubation period for 4 to 5 h. After post incubation, the both cell lines were again processed and cleaned with buffer phosphate, and dimethyl sulfoxide 100 µL was added to solubilize the unbound formazan. The plates were assessed at an absorbance of 570 nm in a plate reader. The whole tests were performed in triplicates. The IC50 was estimated by the intensity of the color produced formazan dye comparably to the number of viable cells in the well. The IC50 value was defined as the concentration of tested compound needed to inhibit 50% of cells growth.
3. Results
3.1. UV–Visible analysis
Primarily, the Mn3O4 nanoparticle development was examined via UV–Visible analysis, Fig. 1. exhibits the UV–Vis spectra of as-prepared Mn3O4 nanoparticle using manganese nitrate. The UV–Vis spectra of Mn3O4 nanoparticles characteristic peak at ~ 220 nm clearly indicating the Mn3O4 nanoparticle formation.
Fig. 1.
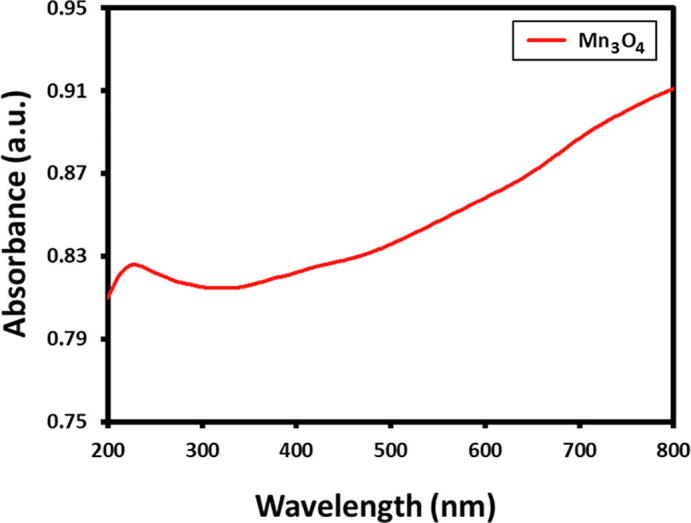
UV–Vis absorption spectra of Mn3O4 nanoparticles.
3.2. XRD analysis
The crystallinity of Mn3O4 NPs attained via precipitation method was identified using XRD analysis as presented in Fig. 2. The as-synthesized Mn3O4 NPs exhibited tetragonal structure (hausmannite, I41/amd) with constant lattice values ‘a’ = 0.5746 nm and ‘c’ = 0.9463 nm. These values are reliable with the previous report attained by other investigator values for Mn3O4 NPs (JCPDS No.24–0734), which is clearly replicated by the existence of fourteen characteristic peaks in the XRD pattern such as, 18.2° (1 0 1), 29.1° (1 1 2), 31.2° (2 0 0), 32.5° (1 0 3), 36.3° (2 1 1), 38.2° (0 0 4), 44.6° (2 2 0), 50.8° (1 0 5), 53.8° (3 1 2), 55.3° (3 0 3), 58.7° (3 2 1), 60.0° (2 2 4), 64.8° (3 1 4), 74.3° (4 1 3). No further additional impurities peaks noticed in XRD analysis i.e. clearly indicating the no other forms of manganese oxides were noticed. Furthermore, the Debye–Scherrer formula was used to estimate average crystallite size (Table 1).
Fig. 2.
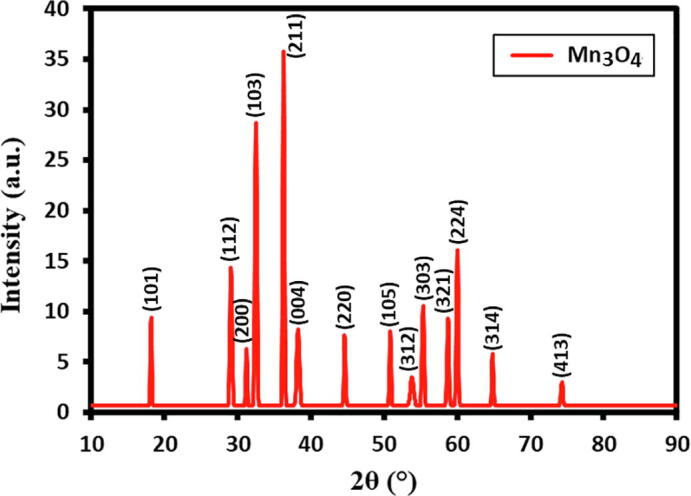
XRD diffractograms of Mn3O4 nanoparticles.
Table 1.
XRD results and crystallographic data of the Mn3O4 nanoparticles.
| 2θ (°) | d space (nm) | h,k,l | a (nm) | c (nm) | Crystal size (D) (nm) |
|---|---|---|---|---|---|
| 18.2 | 0.49 | 101 | 0.5746 | 0.9463 | 45.45258 |
| 29.1 | 0.31 | 112 | 0.5746 | 0.9463 | 31.16673 |
| 31.2 | 0.29 | 200 | 0.5746 | 0.9463 | 47.01039 |
| 32.5 | 0.28 | 103 | 0.5746 | 0.9463 | 31.99853 |
| 36.3 | 0.25 | 211 | 0.5746 | 0.9463 | 40.4086 |
| 38.2 | 0.24 | 004 | 0.5746 | 0.9463 | 23.22652 |
| 44.6 | 0.20 | 220 | 0.5746 | 0.9463 | 38.23237 |
| 50.8 | 0.18 | 105 | 0.5746 | 0.9463 | 42.44687 |
| 53.8 | 0.17 | 312 | 0.5746 | 0.9463 | 20.28826 |
| 55.3 | 0.16 | 303 | 0.5746 | 0.9463 | 33.65284 |
| 58.7 | 0.16 | 321 | 0.5746 | 0.9463 | 35.47193 |
| 60.0 | 0.15 | 224 | 0.5746 | 0.9463 | 37.4525 |
| 64.8 | 0.14 | 314 | 0.5746 | 0.9463 | 42.27171 |
| 74.3 | 0.13 | 413 | 0.5746 | 0.9463 | 37.38172 |
“d” space calculated for tetragonal structure
Where, a, c = Lattice Constants, ‘d’ is Interplanar Spacing, h, k, l = Miller Indices;
Debye–Scherrer formula,
‘D’ is the Crystal size, ‘λ’- is corresponding to wavelength X-ray, and ‘k’dimensionless shape factor, ‘β’ is the line broadening at half the maximum intensity, ‘θ’ is Bragg’s angle.
The average crystallite size is found to be 36 nm, which is little higher when compare to the size of the particles obtained by TEM. This is due the slight deviation from the ideal shape of the particles considered in the Debye–Scherer formula.
3.3. FT-IR analysis
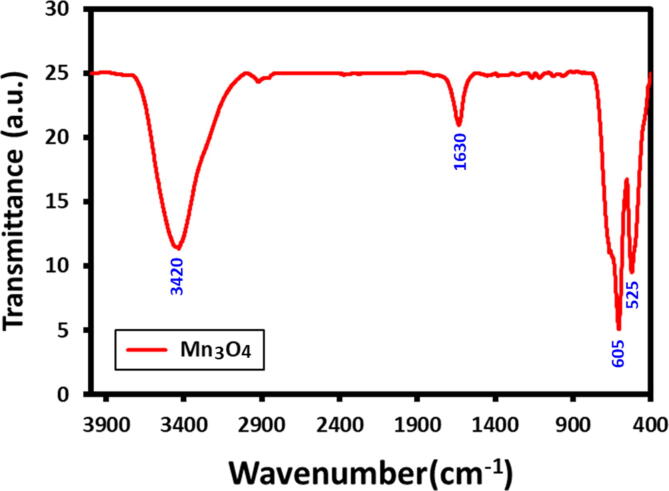
Fig. 3. displays the FTIR spectra of as-synthesized Mn3O4 NPs, the ‘Mn’ and ‘O’ bonding state in the Mn3O4 NPs was investigated with the FTIR analysis. The Mn3O4 FTIR spectra displayed several characteristic peaks at lower region bands of Mn-O comprising the peaks between 400 and 650 cm−1. However, the characteristic peak of Mn-O, stretching modes occurred in the range of 624 cm−1. The significant peak positioned at 624 cm−1 was characteristic of Mn-O stretching modes in tetragonal sites. While the vibrational frequency poisoned at 525 cm−1 associated to the Mn-O distortion vibration. Furthermore, the characteristic narrow band and broad band situated at 3420 and 1600 cm−1 were corresponded to the hydroxy (OH) should be absorbed by the samples or KBr. These FT-IR analysis outcomes are reliable with the evidence achieved from further performed characterization outcomes.
Fig. 3.
FT-IR spectrum of the as-synthesized Mn3O4 nanoparticles.
3.4. TGA analysis
Thermal stability of the Mn3O4 nanoparticles were performed under ‘N2′ atmosphere at a heating rate of 10˚C/min. TGA of the as-synthesized Mn3O4 NPs sample shows thermal stability of the Mn3O4 NPs presented in this Fig. 4. The Mn3O4 NPs shows no significant weight loss in this temperature range to 800 ˚C, as all organic residues have been removed, that discloses the stability of attained Mn3O4 nanoparticles.
Fig. 4.
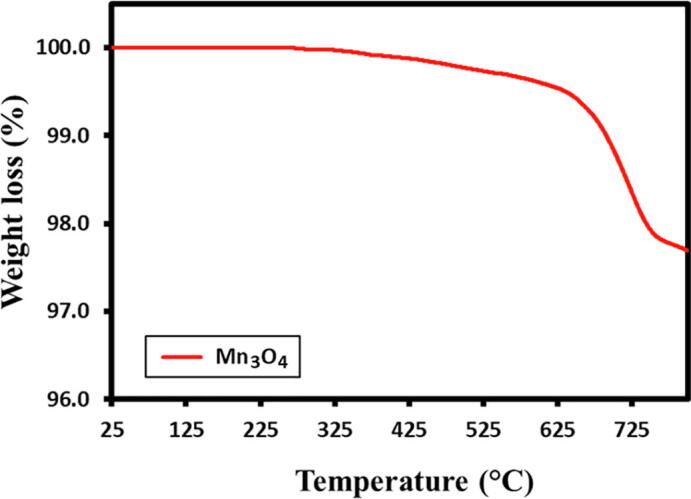
TGA analysis of Mn3O4 nanoparticles.
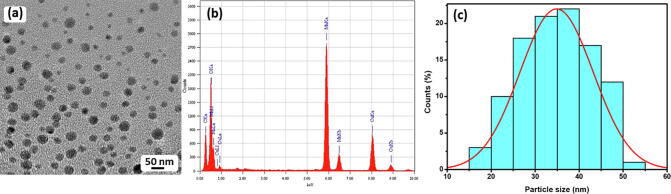
3.5. HRTEM and EDX analysis
The as-synthesized Mn3O4 NPs size and shape was investigated by HRTEM analysis (Fig. 5(a)). The HRTEM results displays the uniformly distributed nanoparticles within a size approximately 35–40 nm. Though, the bulk of the NPs in the HRTEM images are less than 35 nm in size. Moreover, the composition of elements in the as-synthesized Mn3O4 NPs was also determined by energy dispersive X-ray spectroscopy analysis (EDX). The EDX analysis revealed that ‘Mn’ and ‘O’ is the main elements in the attained product, which was determined by the occurrence of strong intense peaks at 0.6, 5.8, and 6.4 keV corresponding to ‘Mn’ and 0.5 keV intense signal belongs to ‘O’, which is due to an optical absorption in this region due to the surface Plasmon resonance (SPR). Consequently, The EDX analysis authorized the development of Mn3O4 NPs (Fig. 5(b)). The particle size distribution graph was attained from TEM image using Image J software. Crystallite size attained from the XRD analysis is approximately similar to the particle size attained from the TEM analysis (Fig. 5(c)).
Fig. 5.
(a) TEM image of the as-synthesized Mn3O4 nanoparticles, (b) energy dispersive X-ray spectroscopy (EDX) of Mn3O4 nanoparticles and (c) particle size distribution graph of Mn3O4 nanoparticles.
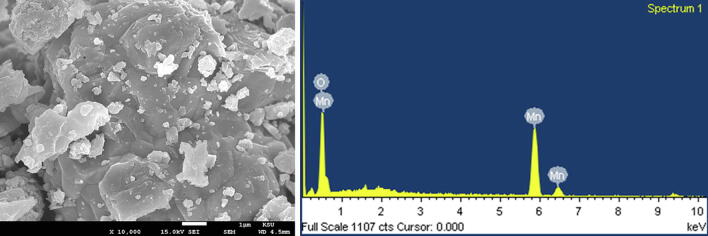
3.6. FESEM and EDX results
The surface structure and size of the as-prepared Mn3O4 NPs were further scrutinized using Field emission Scanning Electron Microscopy (FESEM). Fig. 6, designates that the original surface morphology of the Mn3O4 NPs is flakes with the diameter about below 100 nm. Aggregated Mn3O4 NPs is in the form of flakes. Based on the previously reported literature it can be assumed that the flakes like morphology corresponding to the Mn3O4 NPs are observed, and their diameter varies between 30 and 40 nm. The FESEM results of the Mn3O4 nanoparticles agree with the XRD data. The EDX analysis revealed that ‘Mn’ and ‘O’ is the main elements in the attained product, which was determined by the occurrence of strong intense peaks at 0.6, 5.8, and 6.4 keV corresponding to ‘Mn’ and 0.5 keV intense signal belongs to ‘O’, which is due to an optical absorption in this range due to the SPR. Consequently, The EDX analysis revealed that ‘Mn’ and ‘O’ is the main elements in the attained product, which was determined by the occurrence of strong intense peaks at 0.6, 5.8, and 6.5 keV corresponding to ‘Mn’ and 0.5 keV intense signal belongs to ‘O’. The attained EDX analysis confirmed the formation of Mn3O4 NPs.
Fig. 6.
(a) FESEM image of the as-synthesized Mn3O4 nanoparticles and (b) energy dispersive X-ray spectroscopy of Mn3O4 nanoparticles.
3.7. Antimicrobial activity of the Mn3O4 NPs
The Mn3O4 nanoparticle’s microbial properties of were examined using the disk diffusion method including Gram ‘+ve’ and ‘–ve’ organisms such as E. coli, B. subtilis, S. aureus, and P. aeruginosa. The activity of Mn3O4 was matched with the Gentamycin. For the Gram + ve organisms, Mn3O4 nanoparticles showed a similar zone of inhibition resultant to well-known antibiotic Gentamycin. Mn3O4 NPs shown resistance towards bacterial strains both for E. coli and P. aeruginosa with zone of inhibition 10 mm & 12 mm. Gram + ve bacteria S. aureus & B. subtilis shown susceptibility towards Mn3O4 NPs with zone of inhibition 19 mm & 18 mm respectively. The results from the fungal activity showed mixed results where C. albicans was susceptible with zone of inhibition 18 mm and A. flavus showing intermediate results with zone of inhibition of 13 mm (Table 2).
Table 2.
Zone of Inhibition results of the susceptibility of different microbial strains tested against standard antibiotics.
| Zone of Inhibition (mm) | Mn3O4 NPs | Antibiotic-Gentamycin (30 µg/ml) | Antibiotic-fluconazole (50 µg/ml) |
|---|---|---|---|
| E. coli | 10 mm | 22 mm | – |
| S. aureus | 19 mm | 21 mm | – |
| P. aeruginosa | 12 mm | 22 mm | – |
| B. subtilis | 18 mm | 22 mm | – |
| C. albicans | 18 mm | – | 20 mm |
| A. flavus | 14 mm | – | 20 mm |
Further MIC analysis was determined for the susceptible strains using E-test. This is one the advance and cheapest method to evaluate the inhibitory concentration of the drugs. The MIC of nano with S. aureus was recorded as 40 µg/ml and with C. albicans was recorded to be 15 µg/ml.
3.8. Cytotoxicity results
The Cytotoxicity assessment in terms of IC50 was calculated. As shown in Fig. 7. The IC50 of Mn3O4 NPs with A549 cell lines was found at concentration of 98 µg/mL and IC50 of Mn3O4 NPs with MCF-7 cell lines was found at concentration of 25 µg/mL. More than 80% growth inhibition was observed in concentrations more than 200 µg/mL. Lower concretions of Mn3O4 NPs were working efficient which would be used and compactable in drug delivery systems. Our results clearly indicate that lower concentration of Mn3O4 NPs showing efficient activity towards tested two cell lines.
Fig. 7.

Viability outcome by MTT assay for Mn3O4 NPs.
4. Discussion
Development of antimicrobial agents using metallic nanoparticles been proved to be alternative to conventional antibiotics and the results were promising which is of clinical importance (Singh et al., 2020). The antimicrobial activity of Mn3O4 NPs and the ability of this nano material inhibiting the microbial growth would be considered for drug formulations in treating various infectious diseases. Kim et al., described that nanoparticles display antimicrobial activity in correlation of the size and concentration of NPs (Kim et al., 2007), perhaps lower activity may also due to lower concentration of NPs. The scenario may somehow consider and has to be correlated with other factors like type of microorganism and optimum conditions. The current study on detecting the antimicrobial and anticancer activity of Mn3O4 NPs prepared using precipitation approach. Moreover, the as-prepared Mn3O4 NPs was characterized using various microscopic, spectroscopic and thermal techniques, comprising UV–Vis, FT-IR XRD, HRTEM, FESEM and TGA. The as-synthesized Mn3O4 NPs exhibited tetragonal structure and the average crystallite size is found to be 36 nm, which is little higher when compare to the size of the particles obtained by HRTEM. This is due the slight deviation from the ideal shape of the particles considered in the Debye–Scherer formula. The thermal stability of the Mn3O4 NPs displayed no significant weight loss up to 800 °C that discloses the stability of attained Mn3O4 nanoparticles. The as-prepared Mn3O4 NPs were then scrutinized for antimicrobial activity using disk diffusion process and MIC, however the anticancer activity was examined using human and breast cancer cell lines.
Our antimicrobial results were in agreement with Azhir et al., 2015 where E. coli shown to be sensitive and S. aurues showing susceptibility towards Mn3O4 NPs, whereas reports from (Chowdhury et al., 2009) shown E. coli is susceptible. Packirisamy et al., displayed the same results where B. subtilis is susceptible and E. coli and P. aeruginosa also susceptible as anti-microbial activity increased by increasing the concentration (Packirisamy et al., 2019). The Mn3O4 NPs inhibited the growth of S. aureus with a minimum inhibitory concentration (MIC) of 40 μg/ml and C. albicans with a MIC of 15 μg/ml. The results indicate that the as-prepared Mn3O4 NPs prepared from manganese nitrate using precipitation approach is a favorable contender for usage pharmaceutical industries and food packaging applications.
The application of nanotechnology in modern medicine has extended the permeability in various approaches in better treatment procedures most importantly in cancer treatment (Ivanković et al., 2003). Many of the previous studies has used different methods in producing Mn3O4 NPs to enhance the permeability and also minimize the toxicity as ‘Mn’ is known for the best catalytic activity (Juzenas et al., 2008). Mn3O4 NPs known for its anti-inflammatory (Fu et al., 2020), hepato-protecive (Adhikari et al., 2016) and anti-cancer (Gotić et al., 2009) properties. Our results clearly indicate that lower concentration of Mn3O4 NPs showing efficient activity towards tested two cell lines. In our study Mn3O4 NPs were made and their anticancer activity was evaluated using human lung cell line (A549) and breast cancer cell line (MCF-7). The IC50 investigation accomplished using Mn3O4 NPs. Mn3O4 NPs showed them A549 cell lines were found at concentration of 98 µg/mL and IC50 of Mn3O4 NPs with MCF-7 cell lines was found at concentration of 25 µg/mL. The as-prepared Mn3O4 NPs were found to be effective against the examined cell and demonstrating their potential use against human lung and breast cancer cell lines.
5. Conclusions
Mn3O4 NPs successfully achieved by using precipitation approach. The as-synthesized Mn3O4 nanoparticles are uniformly distributed and well dispersed and tetragonal in shape. The characterization of Mn3O4 nanoparticles was performed by UV–Vis, FT-IR, TGA, XRD, FESEM, HRTEM and EDX analysis. The results have exposed the development of tetragonal shape with an average size of 36 nm. The prepared Mn3O4 nanoparticles were assessed for their biological and anticancer activities. Furthermore, the antibacterial properties, the zone of inhibition, and the minimum inhibitory concentration of Mn3O4 nanoparticles displayed the inhibitory effect against all tested bacteria. Though, the Mn3O4 nanoparticles were found to be selective against S. aureus, & B. subtilis gram positive bacterial strains. Both Gram positive bacteria S. aureus & B. subtilis shown susceptibility towards Mn3O4 nanoparticles with zone of inhibition 19 mm and 18 mm respectively. The results from the antifungal activity exposed mixed results where C. albicans was susceptible with zone of inhibition 18 mm and A. flavus showing intermediate results with zone of inhibition of 13 mm. additionally, Additionally, the anti-cancer activity of Mn3O4 NPs was also studied against A549 lung and MCF-7 breast cancer cell lines by applying MTT assay. The cytotoxicity assessment in terms of IC50 was calculated. As revealed the IC50 of Mn3O4 nanoparticles with A549 was found at concentration of 98 µg/mL and IC50 of Mn3O4 nanoparticles with MCF −7 cell lines was found at concentration of 25 µg/mL.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1441-453.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adhikari A., Polley N., Darbar S., Bagchi D., Pal S.K. Citrate functionalized Mn3O4 in nanotherapy of hepatic fibrosis by oral administration. Future Sci. OA. 2016;2:FSO146. doi: 10.4155/fsoa-2016-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hobaib A.S., Al-sheetan K.M., Shaik M.R., Al-Andis N.M., Al-Suhybani M. Characterization and evaluation of reverse osmosis membranes modified with Ag2O nanoparticles to improve performance. Nanoscale Res. Lett. 2015;10:379. doi: 10.1186/s11671-015-1080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y., Lee D.C., Rhogojina E.V., Jurbergs D.C., Korgel B.A., Bard A.J. Electrochemistry and electrogenerated chemiluminescence of films of silicon nanoparticles in aqueous solution. Nanotechnology. 2006;17:3791. [Google Scholar]
- Behzadi E., Sarsharzadeh R., Nouri M., Attar F., Akhtari K., Shahpasand K., Falahati M. Albumin binding and anticancer effect of magnesium oxide nanoparticles. Int. J. Nanomed. 2019;14:257. doi: 10.2147/IJN.S186428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Swain S., Giri L., Neogi S. Fabrication of magnesium oxide nanoparticles by solvent alteration and their bactericidal applications. J. Mater. Chem.: B. 2019;7:4141–4152. [Google Scholar]
- Cele, T., 2020. Preparation of Nanoparticles, in: Silver Nanoparticles-Health and Safety. IntechOpen.
- Charbgoo F., Ahmad M.B., Darroudi M. Cerium oxide nanoparticles: green synthesis and biological applications. Int. J. Nanomed. 2017;12:1401. doi: 10.2147/IJN.S124855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A.-N., Azam M.S., Aktaruzzaman M., Rahim A. Oxidative and antibacterial activity of Mn3O4. J. Hazard. Mater. 2009;172:1229–1235. doi: 10.1016/j.jhazmat.2009.07.129. [DOI] [PubMed] [Google Scholar]
- Deka K., Guleria A., Kumar D., Biswas J., Lodha S., Kaushik S.D., Dasgupta S., Deb P. Exclusive T2 MRI contrast enhancement by mesoporous carbon framework encapsulated manganese oxide nanoparticles. Curr. Appl. Phys. 2020;20:89–95. [Google Scholar]
- Dreaden E.C., Alkilany A.M., Huang X., Murphy C.J., El-Sayed M.A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed A., Kamel M. Advanced applications of nanotechnology in veterinary medicine. Environ. Sci. Pollut. Res. 2020;27:19073–19086. doi: 10.1007/s11356-018-3913-y. [DOI] [PubMed] [Google Scholar]
- Fu S., He Z., Tang Y., Lan B. Effect of Mn3O4 Nanoparticles on Lipopolysaccharide-Induced Inflammatory Factors in the Human Tendon Cells and Its Mechanism. Int. J. Polym. Sci. 2020 [Google Scholar]
- Ghazal S., Akbari A., Hosseini H.A., Sabouri Z., Forouzanfar F., Khatami M., Darroudi M. Sol-gel biosynthesis of nickel oxide nanoparticles using Cydonia oblonga extract and evaluation of their cytotoxicity and photocatalytic activities. J. Mol. Struct. 2020:128378. [Google Scholar]
- Gotić M., Ivanković S., Musić S., Prebeg T. Synthesis of Mn3O4 nanoparticles and their application to cancer cells. Collect. Czech. Chem. Commun. 2009;74:1351–1360. [Google Scholar]
- Hannan M., Haque M., Mohibbullah M., Dash R., Hong Y.K., Moon I.S. Gelidium amansii Attenuates Hypoxia/Reoxygenation-Induced Oxidative Injury in Primary Hippocampal Neurons through Suppressing GluN2B Expression. Antioxidants. 2020;9 doi: 10.3390/antiox9030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havancsak K. In Nanotechnology at present and its promise for the future. Trans Tech Publ Mater. Sci. Forum. 2003:85–94. [Google Scholar]
- Henry C.R. Morphology of supported nanoparticles. Prog. Surf. Sci. 2005;80:92–116. [Google Scholar]
- Hoseinpour V., Ghaemi N. Green synthesis of manganese nanoparticles: Applications and future perspective–A review. J. Photochem. Photobiol B. 2018;189:234–243. doi: 10.1016/j.jphotobiol.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Hulla J., Sahu S., Hayes A. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015;34:1318–1321. doi: 10.1177/0960327115603588. [DOI] [PubMed] [Google Scholar]
- Ivanković S., Gotić M., Jurin M., Musić S. Photokilling squamous carcinoma cells SCCVII with ultrafine particles of selected metal oxides. J. Sol-Gel Sci. Technol. 2003;27:225–233. [Google Scholar]
- Jiang X., Gray P., Patel M., Zheng J., Yin J.-J. Crossover between anti-and pro-oxidant activities of different manganese oxide nanoparticles and their biological implications. J. Mater. Chem.: B. 2020;8:1191–1201. doi: 10.1039/c9tb02524c. [DOI] [PubMed] [Google Scholar]
- Juzenas P., Chen W., Sun Y.-P., Coelho M.A.N., Generalov R., Generalova N., Christensen I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008;60:1600–1614. doi: 10.1016/j.addr.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Ansari A.A., Khan A.A., Abdulla M., Al-Obeed O., Ahmad R. In vitro evaluation of anticancer and biological activities of synthesized manganese oxide nanoparticles. MedChemComm. 2016;7:1647–1653. [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.-H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kuhlbusch T.A., Asbach C., Fissan H., Göhler D., Stintz M. Nanoparticle exposure at nanotechnology workplaces: a review. Part. Fibre Toxicol. 2011;8:1–18. doi: 10.1186/1743-8977-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Nehra M., Kedia D., Dilbaghi N., Tankeshwar K., Kim K.-H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. 2020;C 106 doi: 10.1016/j.msec.2019.110154. [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh K., Panwar S., Mehta S.K. Green synthesis of manganese oxide nanoparticles for the electrochemical sensing of p-nitrophenol. Int. Nano Lett. 2017;7:123–131. [Google Scholar]
- Kung H.H., Kung M.C. Heterogeneous catalysis: what lies ahead in nanotechnology. Appl. Catal. A. 2003;246:193–196. [Google Scholar]
- Kung H.H., Kung M.C. Nanotechnology: applications and potentials for heterogeneous catalysis. Catal. Today. 2004;97:219–224. [Google Scholar]
- Medhi R., Marquez M.D., Lee T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020;3:6156–6185. [Google Scholar]
- Mirzaei H., Darroudi M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017;43:907–914. [Google Scholar]
- Mu L., Sprando R.L. Application of nanotechnology in cosmetics. Pharm. Res. 2010;27:1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Nabi G., Raza W., Tahir M. Green Synthesis of TiO2 Nanoparticle Using Cinnamon Powder Extract and the Study of Optical Properties. J. Inorg. Organomet. Polym. Mater. 2020;30:1425–1429. [Google Scholar]
- Ndlovu N., Mayaya T., Muitire C., Munyengwa N. Nanotechnology Applications in Crop Production and Food Systems. Int. J. Plant Breed. 2020;7:624–634. [Google Scholar]
- Nie S., Xing Y., Kim G.J., Simons J.W. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- Packirisamy R.G., Govindasamy C., Sanmugam A., Karuppasamy K., Kim H.-S., Vikraman D. Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites. Nanomaterials. 2019;9:1589. doi: 10.3390/nano9111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C.P., Jr, Owens F.J. John Wiley & Sons; 2003. Introduction to nanotechnology. [Google Scholar]
- Pugazhendhi A., Prabhu R., Muruganantham K., Shanmuganathan R., Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B. 2019;190:86–97. doi: 10.1016/j.jphotobiol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Roche I., Chaînet E., Chatenet M., Vondrák J. Carbon-supported manganese oxide nanoparticles as electrocatalysts for the oxygen reduction reaction (ORR) in alkaline medium: physical characterizations and ORR mechanism. J. Phys. Chem. C. 2007;111:1434–1443. [Google Scholar]
- Shin J., Anisur R.M., Ko M.K., Im G.H., Lee J.H., Lee I.S. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew. Chem. Int. Ed. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- Singh A., Gautam P.K., Verma A., Singh V., Shivapriya P.M., Shivalkar S., Sahoo A.K., Samanta S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020;25:e00427. doi: 10.1016/j.btre.2020.e00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yang J., Zhou B., Hu Y., Xing L., Xu F., Shen M., Zhang G., Shi X. Antifouling manganese oxide nanoparticles: synthesis, characterization, and applications for enhanced MR imaging of tumors. ACS Appl. Mater. Interf. 2017;9:47–53. doi: 10.1021/acsami.6b13844. [DOI] [PubMed] [Google Scholar]
- Weiss P.S. ACS Publications; 2010. Nanoscience and nanotechnology: present and future. [DOI] [PubMed] [Google Scholar]
- Yadav V.K., Ali D., Khan S.H., Gnanamoorthy G., Choudhary N., Yadav K.K., Thai V.N., Hussain S.A., Manhrdas S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials. 2020;10:1551. doi: 10.3390/nano10081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zhuang Y., Hu H., Du X., Zhang C., Shi X., Wu H., Yang S. Silica-coated manganese oxide nanoparticles as a platform for targeted magnetic resonance and fluorescence imaging of cancer cells. Adv. Funct. Mater. 2010;20:1733–1741. [Google Scholar]