Summary
Hemorrhagic shock induces an aberrant immune response characterized by simultaneous induction of a proinflammatory state and impaired host defenses. The objective of this study was to evaluate the impact of conditionally immortalized neutrophil progenitors (NPs) on this aberrant immune response. We employed a mouse model of hemorrhagic shock, followed by the adoptive transfer of NPs and subsequent inoculation of Staphylococcus aureus to induce pneumonia. We observed that transplant of NPs decreases the proportion of host neutrophils that express programmed death ligand 1 and intercellular adhesion molecule 1 in the context of prior hemorrhage. Following hemorrhage, NP transplant decreased proinflammatory cytokines in the lungs, increased neutrophil migration into the airspaces, and enhanced bacterial clearance. Further, hemorrhagic shock improved NP engraftment in the bone marrow. These results suggest that NPs hold the potential for use as a cellular therapy in the treatment and prevention of secondary infection following hemorrhagic shock.
Keywords: hemorrhagic shock, pneumonia, myeloid progenitors, neutrophil priming, Staphylococcus aureus, PD-L1, ICAM-1, bone marrow engraftment, cell therapy
Highlights
-
•
Myeloid progenitors restore a competent inflammatory response to pneumonia
-
•
Progenitor transplantation promotes clearance of secondary S. aureus pneumonia
-
•
Hemorrhagic shock enhances engraftment of transplanted myeloid progenitors
In this article, Cohen and colleagues evaluate the impact of myeloid progenitor transplantation in a “two-hit” mouse model of critical illness. The authors show that progenitor transplantation reverses a dysfunctional host response to secondary Staphylococcus aureus pneumonia induced by prior hemorrhagic shock.
Introduction
Trauma and hemorrhage result in immune dysregulation caused by simultaneous aberrant inflammation and immunosuppression, leading to ineffective bacterial clearance and acute respiratory distress syndrome (ARDS) (Dewar et al., 2009; Gannon et al., 2004; Leliefeld et al., 2016; Vincent et al., 2009; Ware and Matthay, 2000). Trauma is independently associated with development of an infectious complication, most commonly pneumonia (Vincent et al., 2009). Staphylococcus aureus is the most common bacterial pathogen in intensive care unit patients, with methicillin-resistant S. aureus (MRSA) accounting for 20%–40% of all nosocomial pneumonias (Rubinstein et al., 2008; Vincent et al., 2009).
Alterations in neutrophil function have been purported to contribute to immune dysfunction following trauma by a multitude of mechanisms. These include the release of suppressive neutrophils from the bone marrow into circulation, resulting in decreased T cell proliferation (Darcy et al., 2014) and the induction of T cell apoptosis in a programmed death ligand 1 (PD-L1)-dependent fashion, resulting in immunosuppression and increasingly severe sepsis (Patera et al., 2016; Patil et al., 2018; Wang et al., 2015). Coinciding with the induction of immunosuppression in response to hemorrhage is an overly exuberant inflammatory response due in part to the contributions of neutrophil priming, a condition in which previous insults result in a heightened response to subsequent stimuli (Ayala et al., 2002; Yao et al., 2015; Zhao et al., 2014). Characteristic of neutrophil priming is an upregulation of intercellular adhesion molecule 1 (ICAM-1) (Yao et al., 2015).
In rodent models of hemorrhagic shock, it has been demonstrated that, in addition to suppressive neutrophil mobilization, there is an increase in hematopoietic progenitor cell (HPC) mobilization from the bone marrow, which may serve to modulate the inflammatory response to hemorrhage (Francis et al., 2019; Xiang et al., 2012). In fact, injection of bone marrow-derived hematopoietic stem cells (HSCs) has been shown to decrease mortality and organ dysfunction in response to cecal ligation and puncture (Mei et al., 2010; Nemeth et al., 2009).
When HPCs are mobilized from the bone marrow, there may exist an opportunity for engraftment of transplanted myeloid progenitor cells into the newly available niche space to restore a pool of normally functioning neutrophils after hemorrhage. Indeed, it has been demonstrated in human trauma patients that, following transfusion, up to 70% of patients will develop microchimerism from donor HPCs contaminating the transfused blood (Lee et al., 1999; Utter et al., 2004).
HOXB8, a transcription factor that is upregulated in myeloid leukemias, promotes self-renewal of HPCs and blocks their differentiation to terminal myeloid leukocytes (Wang et al., 2006). Myeloid progenitor cell lines can be derived from murine bone marrow HPCs through conditional expression of HOXB8 (Wang et al., 2006). Once HOXB8 expression is relieved in these progenitor cell lines, cells differentiate into terminal granulocytic or monocytic leukocytes (Wang et al., 2006). Neutrophil progenitors (NPs) that are conditionally immortalized through enforced expression of HOXB8 hold the potential to act as a therapy in which neutrophils derived in vivo from transplanted donor NPs mediate host defense function. However, prior to employing NPs in this capacity, it is essential to elucidate the acute host response to NP transplant. In the present study, we aim to evaluate the host response to the transplant of murine NPs using a mouse model of hemorrhagic shock and secondary respiratory infection with S. aureus.
Results
Characterization of Conditionally Immortalized NPs
HOXB8-conditional myeloid progenitor cell lines and neutrophils differentiated from them have previously been characterized by microarray and functional analyses (Salmanidis et al., 2013; Saul et al., 2019; Wang et al., 2006). We independently established a GFP-expressing HOXB8-conditional progenitor cell line that is committed to the neutrophil lineage in vitro (Wilson et al., 2017). We adoptively transferred these progenitors into mice, analyzed peripheral blood 7 days after adoptive transfer, and found that the donor-derived cells were committed to the neutrophil lineage in vivo (Figure S1A); we therefore call these cells NPs. NPs were analyzed by flow cytometry to determine their maturation state prior to differentiation based on the classification system proposed by Evrard et al. (2018). We observed that our NP cell line expressed cKit, cluster of differentiation (CD) 34, and CXCR4. There were two distinct populations with expression of CD11b, CD16/32, and Ly6G. NPs had variable expression levels of CD101, and there was a subpopulation of NPs that had low levels of CXCR2 expression (Figure S1B). These findings are consistent with our generated line of NPs being most similar to PreNeus. During in vitro differentiation induced by granulocyte colony-stimulating factor (G-CSF), we observed loss of cKit and CXCR4 expression, acquisition of high Ly6G expression, and stable cell surface levels of CD11b (Figure S1C).
Effects of NP Transplant on the Host Response to Hemorrhagic Shock and Infection
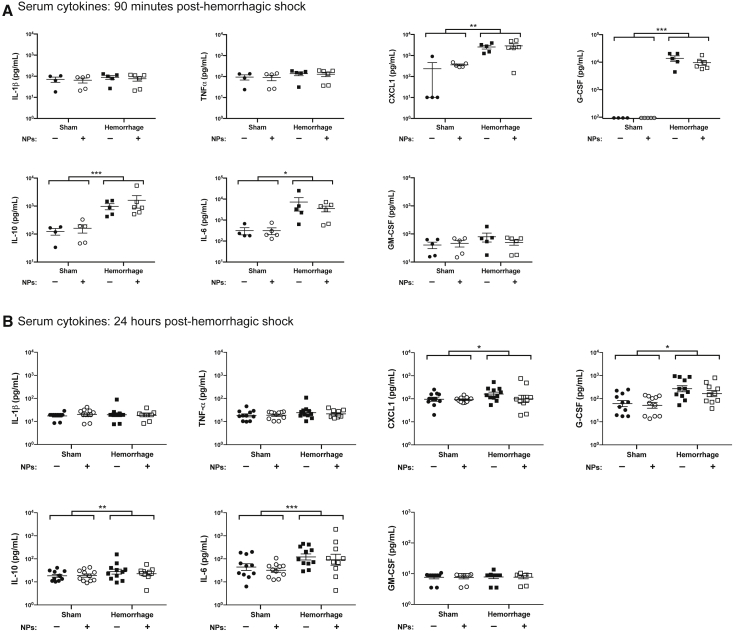
Hemorrhagic shock induces immune dysfunction that results in enhanced susceptibility to secondary infection (Ayala et al., 2002; Lee et al., 2019). To elucidate the impact of NPs on this immune dysfunction, we analyzed circulating cytokines 90 min and 24 h after hemorrhage. At both time points, hemorrhaged mice had elevated levels of CXCL1, G-CSF, interleukin (IL)-6, and IL-10 compared with sham mice (Figure 1). The hemorrhage-induced increase in these cytokines was not influenced by NP transplant (Figure 1).
Figure 1.
NP Transplant Does Not Affect Systemic Cytokines Induced by Hemorrhagic Shock
Mice were subjected to either hemorrhagic shock or sham procedure, and then received transplant with or without NPs. At (A) 90 min or (B) 24 h after resuscitation, serum was collected and analyzed for the concentration of IL-6, CXCL1, TNFα, IL-1β, IL-10, G-CSF, and GM-CSF. Data were acquired across at least three independent experiments, with duplicate measures of each sample/cytokine. Data are expressed as means ± SEM. Data were fit to a lognormal distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
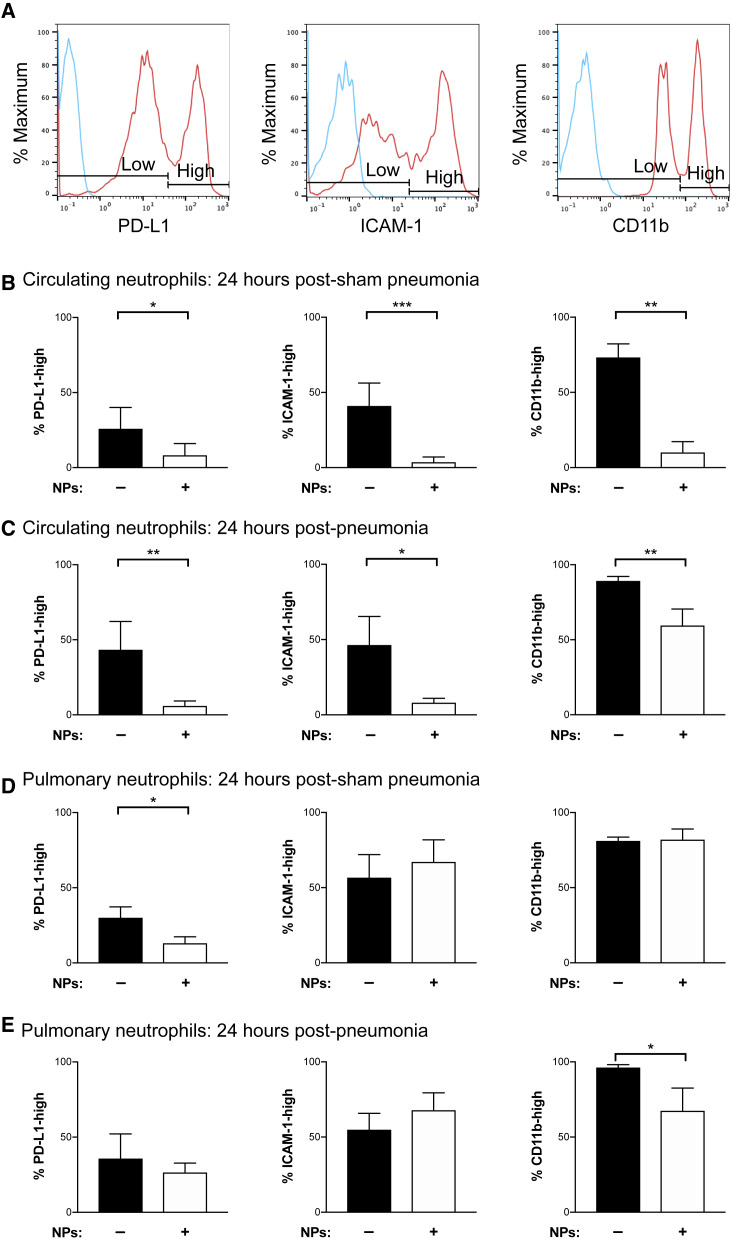
Forty-eight hours following hemorrhagic shock, S. aureus or sham pneumonia was induced. At 24 h post infection, we analyzed circulating neutrophils and the neutrophils in the lung homogenate by flow cytometry. At this early time point, there was only a small degree of donor NP-derived neutrophil chimerism in the peripheral blood (Figure S2A) and lung tissue (Figure S2B), suggesting that the effects noted in surface protein expression were driven by alterations in host neutrophils. To characterize the impact of hemorrhage and infection on neutrophils, we analyzed PD-L1, ICAM-1, and CD11b surface expression to delineate high and low expression subsets (Figure 2A). Adoptive transfer of NPs reduced the proportion of highly expressing PD-L1 neutrophils in circulation and in the lung homogenate in the absence of pneumonia (Figures 2B and 2D). However, NPs did not attenuate pulmonary neutrophil PD-L1 expression in the presence of hemorrhage and pneumonia (Figure 2E). These data indicate that hemorrhagic shock induces an immunosuppressive neutrophil phenotype that is reversed by the transplant of NPs. Interestingly, in mice not subjected to hemorrhagic shock, NPs increased the fraction of circulating host neutrophils with high expression of PD-L1 (Figure S3A).
Figure 2.
Transplant of NPs Decreases the Proportion of Suppressive, Primed, and Activated Neutrophils
Flow cytometry analysis of PD-L1, ICAM-1, and CD11b expression by peripheral blood and pulmonary neutrophils. Mice were subjected to hemorrhagic shock with or without adoptive transfer of progenitor cells followed by induction of S. aureus pneumonia 48 h after hemorrhage.
(A) Representative plots of neutrophil expression levels of PD-L1, ICAM-1, and CD11b by mean fluorescence intensity. Gating is shown for delineating high and low expression profiles. Neutrophils were identified by Ly6G expression.
(B–E) The mean proportion of (B, C) peripheral blood and (D, E) lung neutrophils expressing PD-L1, ICAM-1, and CD11b in mice subjected to hemorrhage and either (B, D) sham pneumonia or (C, E) S. aureus pneumonia. All measurements were made at 24 h post infection (or sham pneumonia). Data were acquired across at least three independent experiments, with duplicate measures of each sample (n = 5–10 mice per group; see Figure S3). Data are presented as the percentage of host neutrophils expressing a given surface protein. Data were fit to a binomial distribution (binomial mean ± SEM is shown), analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Figure S3 for the complete dataset that includes mice subjected to sham hemorrhage procedure.
In addition to immunosuppression, there is simultaneous induction of a hyperinflammatory state due in part to neutrophil priming, marked by ICAM-1 expression, which can progress to ARDS (Ayala et al., 2002; Fan et al., 1998; Lomas-Neira et al., 2012; Yao et al., 2015). To further investigate whether NPs affect the aberrant immune response to hemorrhage, we analyzed the proportion of primed neutrophils by probing changes in ICAM-1 expression. In mice receiving prior hemorrhage, the transplant of NPs decreased the proportion of ICAM-1-high-expressing neutrophils in circulation in the presence or absence of pneumonia (Figures 2B and 2C). In the lung homogenate, we observed a wide variability in primed neutrophils and did not resolve an effect of NP transplant in either the absence or presence of secondary pneumonia (Figures 2D and 2E).
As a marker of neutrophil activation, we analyzed the expression level of CD11b, whose surface expression increases with release of intracellular granules. Consistent with observed changes in ICAM-1 expression, NP transplant resulted in a reduction in the proportion of circulating, degranulated neutrophils in hemorrhaged mice in the absence or presence of pneumonia (Figures 2B and 2C). NPs only had an effect on the proportion of neutrophils highly expressing CD11b in the lung homogenate in the presence of pneumonia (Figure 2E). There was no independent effect of NPs on PD-L1, ICAM-1, and CD11b expression in mice that underwent sham hemorrhage, with the exception of inducing PD-L1-expressing neutrophils in the blood in the absence of pneumonia (Figures S3A and S3B). These data suggest that, following hemorrhagic shock and adoptive transfer of NPs, host neutrophils maintain the ability to activate at the site of infection while having an attenuated premature activation in circulation. Taken together, these data suggest that NP transplant may diminish neutrophil priming and activation in response to hemorrhage, with or without infection.
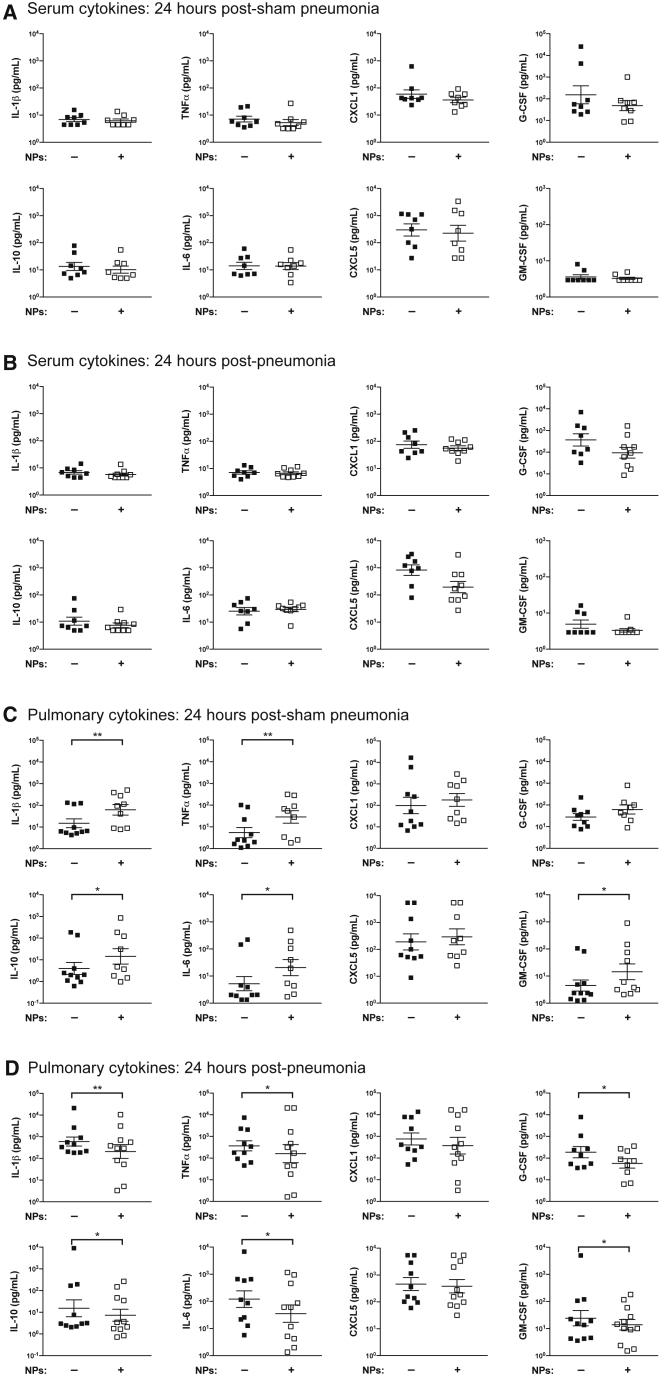
NP Transplant Modulation of the Cytokine Milieu
In order to characterize the effect of NP transplant on the inflammatory response to hemorrhagic shock and secondary infection, we analyzed a series of cytokines in the serum and lungs of mice 24 h after induction of S. aureus pneumonia. Transplant of NPs had no effect on the serum cytokine profile across all conditions (Figures 3A and 3B). In the lungs of mice receiving prior hemorrhagic shock, NP transplant increased the concentrations of IL-1β, tumor necrosis factor alpha (TNFα), IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the absence of secondary infection, suggesting the induction of a proinflammatory environment (Figure 3C). However, IL-10, an anti-inflammatory cytokine, is also elevated in hemorrhaged mice receiving NPs (Figure 3C). There is a paradoxical effect of NP transplant in mice subjected to both hemorrhage and pneumonia on IL-1β, TNFα, IL-6, GM-CSF, G-CSF, and IL-10, in which lower concentrations of these lung cytokines are observed, indicating a less exuberant response to secondary infection (Figure 3D). There were no alterations induced by NP transplant observed in the cytokine profile of the mice subjected to sham hemorrhage (Figures S4A and S4B). Taken together, these data indicate that the effects of NP transplant on the lung microenvironment depend on the presence (NPs are anti-inflammatory) or absence (NPs are proinflammatory) of S. aureus pneumonia.
Figure 3.
NP Transplant Modulates the Pulmonary Cytokine Microenvironment without Altering the Serum Cytokine Profile
S. aureus pneumonia was induced 48 h after hemorrhagic shock, with or without adoptive transfer of NPs. Concentrations of IL-6, CXCL1, TNFα, IL-1β, IL-10, G-CSF, GM-CSF, and CXCL5 in (A) the serum 24 h after induction of sham pneumonia, (B) the serum 24 h after induction of pneumonia, (C) the lung homogenate 24 h post sham pneumonia, and (D) the lung homogenate 24 h post pneumonia, were measured by cytometric bead array. Data were acquired across at least three independent experiments, with duplicate measures of each sample/cytokine. Data are expressed as means ± SEM. Data were fit to a lognormal distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗p < 0.01.
See also Figure S4 for the complete dataset that includes mice subjected to sham hemorrhage procedure.
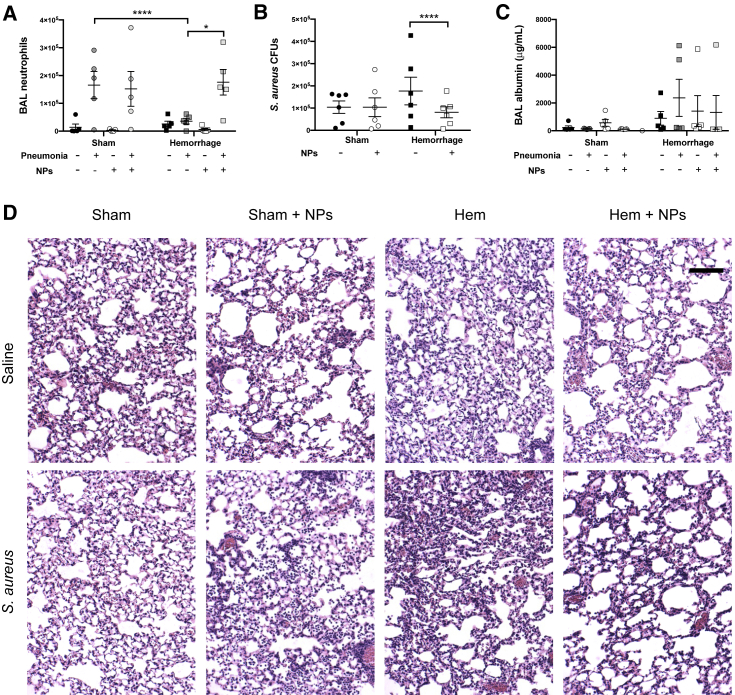
Neutrophil Recruitment and S. aureus Clearance are Modulated by NP Transplant
Twenty-four hours post infection, we observed that, in response to S. aureus pneumonia, prior hemorrhage attenuates neutrophil migration, which is rescued by the adoptive transfer of NPs (Figure 4A). Since neutrophil entry into the airspaces is necessary for clearance of respiratory infection, we assessed the impact of NP transplant on S. aureus colony forming units (CFUs) in the lungs. In mice subjected to sham hemorrhage prior to infection, there was no effect of NP transplant on bacterial burden (Figure 4B). In contrast, we observed a significant reduction in S. aureus CFUs in the lungs of hemorrhaged mice that received NPs (Figure 4B). These data suggest that the rescue of hemorrhage-suppressed host neutrophil recruitment by transplant of NPs promotes clearance of S. aureus from the lungs.
Figure 4.
NP Transplant Improves Neutrophil Recruitment and Bacterial Clearance without Worsening Acute Lung Injury
S. aureus pneumonia was induced 48 h after hemorrhagic shock or sham hemorrhage, with or without adoptive transfer of NPs.
(A) The total number of neutrophils recruited to the alveolae were quantified by flow cytometry analysis of BAL fluid (n = 5 mice per condition) 24 h after infection. Neutrophils were identified by Ly6G expression.
(B) Twenty-four hours post infection with S. aureus, the degree of bacterial burden in the lung homogenate was measured by plating serial dilutions of digested lung in triplicate. Data are expressed as mean CFUs ± SEM (n = 6 mice per condition). Mice that did not receive S. aureus did not have detectable CFUs and were used to confirm specificity of the assay. CFU data were taken from distinct samples.
(C) The albumin content of the BAL fluid was measured by ELISA 24 h post infection (n = 5 mice per condition). BAL neutrophils and albumin represent a repeated measure of the same sample. Data are expressed as mean albumin content ±SEM.
(D) Representative images of lung sections stained with hematoxylin and eosin. Images were captured and selected by a blinded observer. Scale bar is equal to 100 μm. Data were acquired across three independent experiments (n = 3 mice per condition). Histologic data were acquired from distinct samples. Data were fit to a lognormal distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Given the shift toward a more proinflammatory environment in response to hemorrhage and NP transplant, we sought to investigate the effect of NPs on lung injury (Fan et al., 1998; Grommes and Soehnlein, 2011). At 24 h post infection, we found no differences in the albumin content of the bronchoalveolar lavage (BAL) when comparing between the experimental variables (Figure 4C). Next, we performed hematoxylin and eosin staining on fixed lung sections to assess lung injury. Mice that received NPs had decreased lung cellularity and alveolar congestions in the context of hemorrhage (Figure 4D).
There were no differences in the degree of weight change in mice that did or did not receive NPs at 24 h post hemorrhage (Figure S2C), suggesting a similar fluid status following their resuscitation. Similarly, at 24 h post infection, the degree of weight change was not affected by the transplant of NPs when comparing with matched controls, suggesting NPs are insufficient to rescue acute weight loss in mice with S. aureus pneumonia (Figure S2D). Taken together, these data indicate that, in hemorrhaged mice with secondary S. aureus pneumonia, transplant of NPs promotes neutrophil recruitment and clearance of a secondary infection without developing an acute lung injury.
In Vivo Proliferation of NPs
To better understand the fate and behavior of the adoptively transferred NPs, we performed staining with the cell proliferation dye carboxyfluorescein succinimidyl ester (CFSE) prior to transplant of the NPs into naive mice and then performed flow cytometry on days 1, 3, and 5 post transplant (Figure S5A). On a per-cell basis, decreasing CFSE intensity indicates cytoplasmic splitting due to mitosis and is therefore an indicator of proliferation in vivo. Analyzing only the donor NP-derived cells, we observed decreasing CFSE intensity on a per-cell basis in the blood, bone marrow, lungs, and spleen (Figures S5B–S5E), indicating that transplanted NPs have a high proliferative potential in vivo.
Hemorrhagic Shock Promotes NP Engraftment
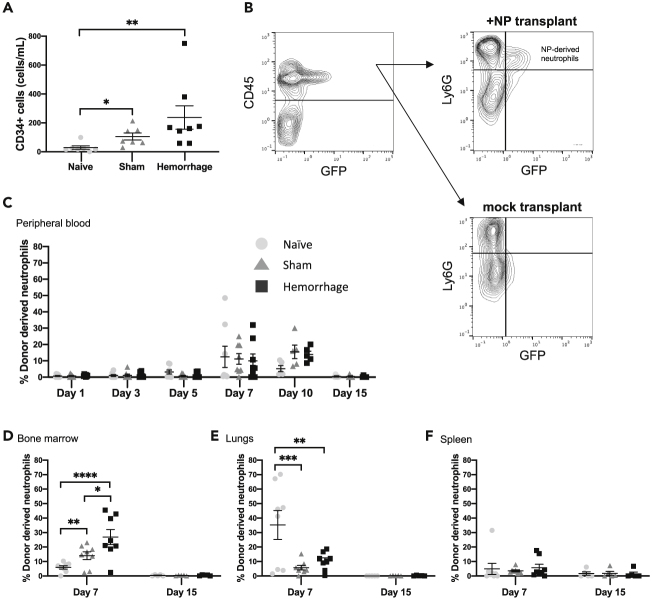
Previous work has indicated that hemorrhagic shock mobilizes HPCs from the bone marrow (Lee et al., 1999; Utter et al., 2004; Xiang et al., 2012). We postulated that HPC mobilization may be able to serve as myeloconditioning for NP engraftment. To determine whether hemorrhagic shock induces the mobilization of HPCs into the circulation, we quantified the concentration of CD34+ cells in peripheral blood of naive mice, or those subjected to hemorrhage or sham procedure (in the absence of NP transplant). Twenty-four hours after hemorrhagic shock or sham procedure, we observed an increase in host progenitors in the circulation when compared with naive mice (Figure 5A).
Figure 5.
Hemorrhage Enhances Transient Bone Marrow Engraftment of NPs
Mice were subjected to either hemorrhagic shock, sham hemorrhage, or no procedure (naive). In (B)–(F), mice then received transplant with or without NPs.
(A) Twenty-four hours post hemorrhage, the degree of CD34+ cells mobilized into the circulation was analyzed by flow cytometry. Data are expressed as the mean number of CD34+ cells per milliliter ± SEM. Data were fit to a lognormal distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons.
(B and C) (B) Representative gating strategy for determining the degree of donor-derived neutrophil chimerism. Leukocytes were identified by CD45 expression, then neutrophils were identified by Ly6G expression. Donor-derived neutrophils were then distinguished from host by GFP expression, with gating performed according to mice receiving mock transplant. The degree of neutrophil chimerism in the (C) peripheral blood (n = 6 for hemorrhage day 5, n = 7 for sham day 5, n = 5 mice per condition for days 10 and 15, n = 8 for the remaining conditions). All flow cytometry data taken from blood represent a repeated measure of the same mice over time from day 1–7 and then a second cohort of mice from day 10–15.
(D–F) (D) Bone marrow (n = 8 mice per condition), (E) lungs (n = 8 mice per condition), and (F) spleen (n = 8 mice per condition) was determined over the course of 15 days. Each replicate of bone marrow, lungs, and spleen flow cytometry data represents repeated measures of the same mouse in different tissues. Distinct mice were used for the 7- and 15-day timepoints. Data are expressed as mean percentage of donor-derived neutrophils ±SEM. Data were fit to a binomial distribution, analyzed by GLMs, and the Holms test was used to adjust for multiple comparisons. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
We confirmed that NPs differentiated in vivo into mature neutrophils (Ly6Ghigh) by probing GFP-positive donor-derived cells in the circulation for expression of Ly6G (Figure S1A). To quantify the fraction of all neutrophils that were derived from donor NPs, we analyzed peripheral blood and tissues for the presence of GFP-expressing neutrophils according to the schematic in Figure 5B. In the peripheral blood, we observed an increasing degree of donor NP-derived neutrophil chimerism over the course of the first 7–10 days across all groups, which returned to baseline by day 15 (Figure 5C). Correlating with CD34+ cell mobilization, there were more donor-derived neutrophils in the bone marrow at day 7 in mice subjected to hemorrhagic shock compared with both sham and naive mice (Figure 5D). There were also more donor-derived bone marrow neutrophils in sham hemorrhaged mice compared with naive mice (Figure 5D). The reverse was true in the lungs, with a higher degree of donor-derived neutrophil chimerism in naive mice compared with their sham or hemorrhaged counterparts (Figure 5E). There were no differences across groups in the spleen (Figure 5F). Donor NP-derived neutrophils were absent in these tissues by day 15 (Figures 5D–5F). Finally, hemorrhage and adoptive transfer of NPs had no effect on the absolute neutrophil count (ANC) in peripheral blood between day 1 and day 15 (Figure S5F). These data indicate that hemorrhagic shock and the subsequent mobilization of host progenitor cells improves bone marrow engraftment of transplanted NPs and production of mature donor-derived neutrophils.
Discussion
Despite advances in critical care, secondary infections continue to be a large source of morbidity and mortality (Lord et al., 2014; Manderscheid et al., 2004; Vincent et al., 2009). In this current study, we employed a mouse model of hemorrhagic shock followed by the adoptive transfer of HOXB8-dependent conditionally immortalized NPs and subsequent respiratory inoculation with S. aureus. We demonstrate that transplant of NPs following hemorrhagic shock decreases the proportion of host neutrophils with a suppressive phenotype, attenuates neutrophil priming, enhances neutrophil migration into the alveolae, and improves bacterial clearance in response to S. aureus pneumonia.
We posit that suppression of neutrophil priming may be central to the mechanism by which NPs attenuate the aberrant host response following hemorrhagic shock. Previous data have demonstrated that, in response to hemorrhage and polymicrobial sepsis, there is an increase in pulmonary IL-6, mediated by primed neutrophils (Ayala et al., 2002). High serum IL-6 concentration correlates with worsening morbidity and mortality in both mouse models and human trauma patients (Cuschieri et al., 2010; Remick et al., 2002). Here, we observed a decrease in neutrophil priming, as indicated by ICAM-1 expression, in response to hemorrhage when NPs were administered, and a simultaneous decrease in IL-6 concentration in the pulmonary microenvironment in response to hemorrhage and pneumonia.
Consistent with data demonstrating ICAM-1 regulating PD-L1 expression (Zhao et al., 2014), we observed a decrease in the proportion of circulating neutrophils expressing high levels of PD-L1 in response to NPs in hemorrhaged mice coinciding with decreased ICAM-1 on neutrophils. The exception to this relationship may be in the lungs, where we observed divergence in the fraction of neutrophils expressing high levels of ICAM-1 and PD-L1, specifically in response to NPs given to mice subjected to hemorrhage and infection. A possible interpretation of these data is that suppression of IL-6 in the lung microenvironment may be sufficient to attenuate pulmonary immunosuppressive neutrophils, independent of the factors driving pulmonary neutrophil priming. This would be consistent with a recent study suggesting a mechanistic link between IL-6 and PD-L1 expression in tissues (Chan et al., 2019). High levels of PD-L1 expression are known to portend a poor prognosis in sepsis, likely due to its induction of T cell apoptosis (Patera et al., 2016; Wang et al., 2015). The lower proportion of PD-L1-expressing neutrophils and, by extension, decreased immunosuppression in combination with improved neutrophil migration is likely to be at least partially responsible for the improvement in bacterial clearance noted here.
The attenuated neutrophil priming in mice receiving NPs may also be responsible for improved host neutrophil migration into infected airspaces. Neutrophil sequestration in the pulmonary capillaries is facilitated by neutrophil stiffening, which is potentiated by priming (Ekpenyong et al., 2017; Wilson et al., 2017; Yoshida et al., 2006). However, sequestration may not necessarily equate to transmigration out of the pulmonary capillaries and into the alveolae. In fact, we recently demonstrated that β2 integrin activation, which is potentiated by priming, restricts neutrophil migration into the alveolae and that there is attenuated neutrophil migration in response to hemorrhagic shock and Pseudomonas aeruginosa pneumonia (Lee et al., 2019; Wilson et al., 2017). In addition, our data indicate that NP transplant reduces neutrophil surface levels of CD11b, a subunit of the β2 integrin macrophage antigen 1, which may serve to attenuate neutrophil sequestration in the pulmonary capillaries. Taken together, these data offer a potential mechanism by which priming may decrease neutrophil migration into the airspaces and therefore may explain how NP transplant restores neutrophil entry into the alveolae.
Finally, decreased neutrophil priming is most likely responsible for the improvement in the histological appearance of the lungs from mice that received NPs. However, the protective nature of NPs on lung injury and systemic inflammation is incomplete as there was a similar increase in BAL albumin concentration and weight loss in the context of hemorrhage and pneumonia with or without the addition of NPs.
Interestingly, in hemorrhaged mice, we observed a paradoxical effect of NP transplant on the cytokine profile in the pulmonary microenvironment with and without S. aureus pneumonia. In the context of hemorrhage without pneumonia, proinflammatory cytokine concentrations were increased in the lung microenvironment of mice receiving NPs. IL-10 was similarly increased in sham mice receiving NPs, suggesting at least some degree of regulation to the proinflammatory milieu. This regulated proinflammatory environment may improve trafficking of immune cells to the lung following hemorrhage, resulting in a harsher environment for S. aureus and contributing to the noted improvement in bacterial clearance.
When S. aureus pneumonia is added as a second hit, we observed a decrease in IL-1β, TNFα, IL-6, G-CSF, GM-CSF, and IL-10 in response to NP transplant, suggesting a more muted proinflammatory response to infection, which is expected given the decrease in neutrophil priming. IL-6, G-CSF, and GM-CSF are potent regulators of emergency granulopoiesis (Manz and Boettcher, 2014). Reduced concentrations of these cytokines produced by NP transplant suggests a less robust granulopoietic response to infection. In this case, NPs may essentially be functioning as a replacement for the normal increase in granulopoiesis and mobilization of progenitors from the bone marrow (Badami et al., 2007; Francis et al., 2012; Manz and Boettcher, 2014).
Our data are consistent with a previous report utilizing bone marrow-derived mesenchymal stem cells (BMSCs) in the prevention of bleomycin-induced lung injury (Rojas et al., 2005). Similar to what we demonstrate here, the intravenous injection of BMSCs results in a significant reduction in lung IL-1β production (Rojas et al., 2005). Further corollaries are seen in a mouse model of polymicrobial sepsis and peritonitis by cecal ligation and puncture where BMSCs have been demonstrated to reduce IL-1β, TNFα, IL-6, and IL-10 in the circulation 1 day following adoptive transfer, resulting in improved bacterial clearance and survival from sepsis (Mei et al., 2010; Nemeth et al., 2009). In the current study, the reduction of IL-1β, TNFα, IL-6, and IL-10 in response to NPs was limited to the lung microenvironment 24 h post infection, which may reflect the differences in the cell type or infectious model. Taken together, NPs offer a readily available, culture-derived, off-the-shelf alternative to HSCs with the ability to recapitulate the immunomodulatory benefits of a stem cell transplant in sepsis. In theory, NPs could be banked, either as a frozen stock or in culture, and incorporated into the blood product resuscitation of a trauma patient to prevent the sequelae of hemorrhagic shock.
Following the acute phase of hemorrhage and pneumonia, transplanted NPs engraft in the bone marrow and produce mature neutrophils over the course of 7–10 days, which are then cleared by day 15. Consistent with previous reports, hemorrhagic shock serves to mobilize CD34+ progenitors into circulation (Badami et al., 2007; Francis et al., 2012; Xiang et al., 2012). In the context of AMD3100 administration, mobilization of progenitors from the bone marrow into circulation has been shown to be sufficient myeloconditioning for HSC transplant (Chen et al., 2006). These data may offer an explanation for the leukocyte microchimerism that has been described in trauma patients who received non-leukocyte-reduced blood (Lee et al., 1999; Utter et al., 2004). Even in the absence of progenitor mobilization, we observed donor NP-derived neutrophils in the bone marrow that are likely the result of NPs taking up residence in the excess niche space present in bone marrow at steady state (Shimoto et al., 2017; Stewart et al., 1993). With genetic modifications, NPs may offer the potential for use in the treatment or prevention of acute bacterial pneumonia in patients with or without prior hemorrhagic shock. Future studies are warranted to probe the direct involvement of neutrophils derived from transplanted NPs in host defense.
In conclusion, NPs are a culture-derived, off-the-shelf cellular product that attenuates the aberrant immune response following hemorrhagic shock and improves host neutrophil recruitment and bacterial clearance. Transplanted NPs go on to produce a pool of mature neutrophils in vivo, holding the potential to act as a cellular therapy for acute bacterial infections.
Experimental procedures
Animals
C57BL/6 mice 8–10 weeks old were used for all experiments (The Jackson Laboratory, Bar Harbor, ME). Experiments were conducted in accordance with Public Health Service guidelines for animal care and use. Water and standard chow were available ad libitum.
Creation and Culture of Conditionally Immortalized NPs
A tamoxifen-inducible HOXB8 oncogene (a gift from Paul Ekert) with hygromycin resistance cassette was transduced into bone marrow hematopoietic stem and progenitor cells (HSPCs) isolated from C57BL/6 mice as described previously to produce conditionally immortalized NPs (Salmanidis et al., 2013; Wang et al., 2006). The resulting cells are stably immortalized as granulocyte progenitor cells in the presence of tamoxifen-induced HOXB8 expression. Expression of a fluorescent tag was induced by transduction of either pMIG (gifted from William Hahn, Addgene plasmid # 9044; http://n2t.net/addgene:9044; RRID:Addgene_9044) lentiviral vector with pLSSmKate2-N1 (gifted from Vladislav Verkhusha, Addgene plasmid # 31867; http://n2t.net/addgene:31867; RRID:Addgene_31867) insert on an SV40 promotor or pLJM1-EGFP (gifted from David Sabatini, Addgene plasmid # 19319; http://n2t.net/addgene:19319; RRID:Addgene_19319) lentiviral plasmids on a CMV promoter. Cells were cultured in Opti-MEM containing GlutaMax (Thermo Fisher, Waltham, MA), 30 μM beta-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), 10% fetal bovine serum (Gemini Bio Products, West Sacramento, CA), penicillin/streptomycin (Gibco, Dun Laoghair, Co Dublin, Ireland), and non-essential amino acids (Gibco) at 37°C with 5% CO2 in a humidified incubator. Media were supplemented with 50 ng/mL recombinant murine stem cell factor (BioLegend, San Diego, CA) and 100 nM Z-4-hydoxytamoxifen (Tocris Bioscience, Bristol, UK) to maintain cells in a progenitor state.
Hemorrhagic Shock and Resuscitation
A fixed-pressure, non-lethal murine model of hemorrhagic shock was employed for this study as previously described (Lomas-Neira et al., 2012). Briefly, mice were anesthetized with isoflurane and bilateral femoral arteries were cannulated. One catheter was connected to a blood pressure monitor (Blood Pressure Analyzer 400, Digi-Med, Louisville, KY) and the other was used to withdraw blood until the mean arterial pressure was 35 ± 5 mm Hg. Hypotension was maintained for 90 min with the mouse secured on a 37°C water-circulating heating pad (T/Pump, Stryker, Kalamazoo, MI). Subsequently, mice were resuscitated with four times the withdrawn blood volume of lactated Ringer, the catheters were removed, and the incisions were sutured. Sham mice had their femoral artery ligated and incisions sutured without cannulation or blood withdrawal. A single 0.1-μg/g dose of subcutaneous buprenorphine was administered for pain control for all mice in the study.
Treatment of Hemorrhagic Shock with NP Transplant
One-hundred million NPs expressing either pLSS-mKate2-N1 or EGFP fluorescent marker were washed extensively in PBS and then resuspended in 350 μL of 0.9% normal saline. Immediately following resuscitation from hemorrhagic shock or completion of sham hemorrhage, cells were transferred by retro-orbital injection. Sham adoptive transfer was accomplished by the retro-orbital injection of 350 μL of 0.9% normal saline alone.
Acute Respiratory infection
Methicillin-resistant S. aureus strain USA300 was cultured to log phase in tryptic soy broth. Bacteria were then washed and resuspended in 0.9% normal saline. The number of CFUs was determined by a standard curve of optical densities at 600 nm. Mice were anesthetized with vaporized isoflurane and placed on an intubation stand (Braintree Scientific, Braintree, MA). Oropharyngeal inhalation was performed by pipetting either 50 μL of 1 × 108 CFU S. aureus or saline alone into the oropharynx and then allowing inspiration of the bolus. Infections were performed 48 h following hemorrhage and NP transplant. Mice were weighed prior to infection and then every 24 h thereafter until they were euthanized.
Quantification of Cytokines, Chemokines, and Bacterial Burden
Twenty-four hours after oropharyngeal S. aureus or saline inhalation, mice were euthanized by intraperitoneal injection of Fatal Plus euthanasia solution. Immediately following euthanasia, mouse whole blood was collected by cardiac puncture and serum was separated from the cellular components of blood by centrifugation. All lobes of the lungs were dissected free and dissociated in gentleMACS C tubes on a gentleMACS dissociator (Miltenyi, Auburn, CA) in a solution of in 2 mg/mL collagenase V (Sigma-Aldrich, St. Louis, MO) in Hanks Buffered Saline Solution and digested at 37°C for 40 min. The S. aureus burden was calculated by plating 10 μL of unfiltered serial dilutions of digested lungs in triplicate on tryptic soy agar plates. The digested lung was then filtered through a 70-μm cell strainer (Falcon, Corning, NY) and centrifuged to remove the cellular components. For determining the cytokine profile of serum 24 h after hemorrhage, blood was collected by saphenous vein puncture and separated by centrifugation. The lung and serum cytokine and chemokine profile were determined by cytometric bead array assay for multiplexed assessment of murine IL-6, CXCL1, TNFα, IL-1β, IL-10, G-CSF, GM-CSF, and CXCL5 per the manufacturer's protocol (BioLegend).
Flow Cytometry
All flow cytometry samples were analyzed on a MACSQuant Analyzer 10 flow cytometer (Miltenyi). Post-acquisition analysis was performed with FlowJo software (Becton Dickinson, Franklin Lakes, NJ). All antibodies were purchased from BioLegend unless otherwise noted. Antibodies used in these experiments were as follows: fluorescein isothiocyanate (FITC) anti-cKIT (clone 2B8), PE anti-CXCR4 (clone FAB2/651P, R&D Systems, Minneapolis, MN), PerCP anti-CD44 (clone IM7), PE/Cy7 anti-VLA4 (clone RL-2), AF647 anti-CXCR2 (clone SA045E1), PE anti-CD11b (clone M1/70), PE/Cy7 anti-CD34 (clone HM34), antigen-presenting cell (APC) anti-CD16/32 (clone 93), BV421 anti-Ly6G (clone 1A8), PE/Cy7 anti-CD101 (clone Moushi101, Invitrogen, Carlsbad, CA), APC anti-CD45 (clone 30-F11), APC/fire anti-ICAM-1 (clone YN1/1.7.4), and APC anti-PD-L1 (clone 10F.9G2). For blood samples, red blood cells were lysed in 1× red blood cell lysis buffer (BioLegend) for 10 min at room temperature prior to staining. Cells were stained with a 1:200 dilution of specific antibodies in PBS with 5 mM EDTA and 1% FBS for 30 min on ice and washed once and analyzed immediately. ANC was determined by density of Ly6G-expressing cells.
Characterization of BAL
BAL was performed 24 h following S. aureus or saline inoculation by isolation of the right lung and installation and withdrawal of 1-mL aliquots of ice-cold PBS with 5 mM EDTA and 0.5% BSA three times. Cells were stained with anti-CD45 and anti-Ly6G and analyzed as described above. BAL albumin content was determined by a mouse albumin ELISA kit (abcam, Cambridge, UK) per manufacturer specifications.
Histologic Analysis
Twenty-four hours after saline or S. aureus inoculation, mice were euthanized as described above. The left lung was isolated and fixed by gravity infusion of 10% phosphate buffered formalin. Tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin. Histologic examination was performed on an Olympus BX60 microscope with a Chameleon3 color camera (FLIR Integrated Imaging Solutions, Richmond, BC, Canada). Representative images of slides were acquired and selected by a blinded observer (n = 3 per condition).
Determination of the Degree of Chimerism
Following hemorrhage and adoptive transfer of NPs, serial blood draws were performed on days 1, 3, 5, 7, 10, and 15 post transplant by saphenous vein puncture. Blood was prepared and stained with anti-CD45 and anti-Ly6G as described above to identify neutrophils. Mouse spleen, bone marrow, and lungs were examined at days 7 and 15 post transplant. Lungs were collected and digested in collagenase as described above. Bone marrow was collected by isolating the right tibia and femur, resecting the ends of the bone, and flushing with 5 mL of cold PBS with 1% FBS and 5 mM EDTA through the medullary cavity and a 700μm cell strainer (Falcon). The spleen was collected and pressed through a 70-μm cell strainer (Falcon) and rinsed in 5 mL of cold PBS with 1% FBS and 5 mM EDTA to form a single-cell suspension. The degree of chimerism was then determined by flow cytometry of tissue samples, expressed as the percentage of mature neutrophils that were expressing fluorescent marker.
Quantification of Proliferation
One-hundred million NPs were stained with CFSE cell division tracker dye (BioLegend) per the manufacturer's recommended procedure and were adoptively transferred into naive mice by retro-orbital injection. Blood, bone marrow, lung, and spleen were examined on days 1, 3, and 5 by flow cytometry. Analyzing only CFSE+ cells, the mean fluorescence intensity was determined in each tissue to determine the degree of cell division over time.
Statistical Analysis
All statistical analysis was conducted using SAS 9.4 (The SAS Institute, Cary, NC). Generalized linear models (GLMs) were used (GLIMMIX procedure) throughout. GLMs are the broader class of models of which ANOVA, regression, and t test are special cases, but that permit modeling based on distributions other than the Gaussian (normal) distribution. The distribution chosen was tailored to the nature of the data being analyzed, with the binomial distribution used to model fractions of cells and the lognormal distribution used for cytokine concentrations. The means and 95% confidence intervals of groups and comparisons were expressed after inverting the link function of the model (binomial) or exponentiating (lognormal) to return then to the expected scales for graphing and expressing effects. Factorial model structures were used to mirror experiments crossing two or more factors and included the appropriate higher-order hypothesis tests (four or eight group comparisons) in addition to select pairwise comparisons. An alpha of 0.05 was maintained across all hypothesis tests within each analysis using the Holm test to adjust each p value.
Study Approval
Experiments in this study were approved by the Lifespan Animal Welfare Committee (approval # 5017-19, Office of Laboratory Animal Welfare Assurance #A3922-01).
Authors contribution
J.T.C. designed the research studies, conducted experiments, acquired and analyzed data, and wrote the manuscript. M.D. conducted experiments, and acquired and analyzed data. J.T.M. designed the research studies, analyzed data, and wrote the manuscript. R.Z. conducted experiments. C.T.L. designed the research studies, analyzed data, and wrote the manuscript.
Conflicts of interests
The authors have declared that no competing interests exist.
Acknowledgments
This research was supported by NIH (GM124911 to C.T.L. and GM065085 to J.T.C.).
Published: January 21, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.12.014.
Supplemental information
References
- Ayala A., Chung C.S., Lomas J.L., Song G.Y., Doughty L.A., Gregory S.H., Cioffi W.G., LeBlanc B.W., Reichner J., Simms H.H. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am. J. Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badami C.D., Livingston D.H., Sifri Z.C., Caputo F.J., Bonilla L., Mohr A.M., Deitch E.A. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J. Trauma. 2007;63:596–600. doi: 10.1097/TA.0b013e318142d231. discussion 600-592. [DOI] [PubMed] [Google Scholar]
- Chan L.C., Li C.W., Xia W., Hsu J.M., Lee H.H., Cha J.H., Wang H.L., Yang W.H., Yen E.Y., Chang W.C. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Invest. 2019;129:3324–3338. doi: 10.1172/JCI126022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Larochelle A., Fricker S., Bridger G., Dunbar C.E., Abkowitz J.L. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107:3764–3771. doi: 10.1182/blood-2005-09-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri J., Bulger E., Schaeffer V., Sakr S., Nathens A.B., Hennessy L., Minei J., Moore E.E., O'Keefe G., Sperry J. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34:346–351. doi: 10.1097/SHK.0b013e3181d8e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy C.J., Minigo G., Piera K.A., Davis J.S., McNeil Y.R., Chen Y., Volkheimer A.D., Weinberg J.B., Anstey N.M., Woodberry T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit. Care. 2014;18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D., Moore F.A., Moore E.E., Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912–918. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Ekpenyong A.E., Toepfner N., Fiddler C., Herbig M., Li W., Cojoc G., Summers C., Guck J., Chilvers E.R. Mechanical deformation induces depolarization of neutrophils. Sci. Adv. 2017;3:e1602536. doi: 10.1126/sciadv.1602536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard M., Kwok I.W.H., Chong S.Z., Teng K.W.W., Becht E., Chen J., Sieow J.L., Penny H.L., Ching G.C., Devi S. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48:364–379 e368. doi: 10.1016/j.immuni.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Fan J., Marshall J.C., Jimenez M., Shek P.N., Zagorski J., Rotstein O.D. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J. Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- Francis W.R., Bodger O.G., Pallister I. Altered leucocyte progenitor profile in human bone marrow from patients with major trauma during the recovery phase. Br. J. Surg. 2012;99:1591–1599. doi: 10.1002/bjs.8919. [DOI] [PubMed] [Google Scholar]
- Francis W.R., Ireland R.E., Spear A.M., Jenner D., Watts S.A., Kirkman E., Pallister I. Flow cytometric analysis of hematopoietic populations in rat bone marrow. Impact of trauma and hemorrhagic shock. Cytometry A. 2019;95:1167–1177. doi: 10.1002/cyto.a.23903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon C.J., Pasquale M., Tracy J.K., McCarter R.J., Napolitano L.M. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Cohen J.T., Wilson Z.S., Zhao R., Lomas-Neira J., Chung C.S., Chen Y., Jamieson A.M., Ayala A., Lefort C.T. Hemorrhage attenuates neutrophil recruitment in response to secondary respiratory infection by Pseudomonas aeruginosa. Shock. 2019;52:506–512. doi: 10.1097/SHK.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.H., Paglieroni T., Ohto H., Holland P.V., Busch M.P. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–3139. [PubMed] [Google Scholar]
- Leliefeld P.H., Wessels C.M., Leenen L.P., Koenderman L., Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care. 2016;20:73. doi: 10.1186/s13054-016-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas-Neira J., Perl M., Venet F., Chung C.S., Ayala A. The role and source of tumor necrosis factor-alpha in hemorrhage-induced priming for septic lung injury. Shock. 2012;37:611–620. doi: 10.1097/SHK.0b013e318254fa6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J.M., Midwinter M.J., Chen Y.F., Belli A., Brohi K., Kovacs E.J., Koenderman L., Kubes P., Lilford R.J. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderscheid P.A., Bodkin R.P., Davidson B.A., Jensen E., Russo T.A., Knight P.R. Bacterial clearance and cytokine profiles in a murine model of postsurgical nosocomial pneumonia. Clin. Diagn. Lab Immunol. 2004;11:742–751. doi: 10.1128/CDLI.11.4.742-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz M.G., Boettcher S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- Mei S.H., Haitsma J.J., Dos Santos C.C., Deng Y., Lai P.F., Slutsky A.S., Liles W.C., Stewart D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- Nemeth K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patera A.C., Drewry A.M., Chang K., Beiter E.R., Osborne D., Hotchkiss R.S. Frontline Science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J. Leukoc. Biol. 2016;100:1239–1254. doi: 10.1189/jlb.4HI0616-255R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil N.K., Luan L., Bohannon J.K., Hernandez A., Guo Y., Sherwood E.R. Frontline Science: anti-PD-L1 protects against infection with common bacterial pathogens after burn injury. J. Leukoc. Biol. 2018;103:23–33. doi: 10.1002/JLB.5HI0917-360R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick D.G., Bolgos G.R., Siddiqui J., Shin J., Nemzek J.A. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Rojas M., Xu J., Woods C.R., Mora A.L., Spears W., Roman J., Brigham K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein E., Kollef M.H., Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008;46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- Salmanidis M., Brumatti G., Narayan N., Green B.D., van den Bergen J.A., Sandow J.J., Bert A.G., Silke N., Sladic R., Puthalakath H. Hoxb8 regulates expression of microRNAs to control cell death and differentiation. Cell Death Differ. 2013;20:1370–1380. doi: 10.1038/cdd.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul S., Castelbou C., Fickentscher C., Demaurex N. Signaling and functional competency of neutrophils derived from bone-marrow cells expressing the ER-HOXB8 oncoprotein. J. Leukoc. Biol. 2019;106:1101–1115. doi: 10.1002/JLB.2A0818-314R. [DOI] [PubMed] [Google Scholar]
- Shimoto M., Sugiyama T., Nagasawa T. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood. 2017;129:2124–2131. doi: 10.1182/blood-2016-09-740563. [DOI] [PubMed] [Google Scholar]
- Stewart F.M., Crittenden R.B., Lowry P.A., Pearson-White S., Quesenberry P.J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566–2571. [PubMed] [Google Scholar]
- Utter G.H., Owings J.T., Lee T.H., Paglieroni T.G., Reed W.F., Gosselin R.C., Holland P.V., Busch M.P. Blood transfusion is associated with donor leukocyte microchimerism in trauma patients. J. Trauma. 2004;57:702–707. doi: 10.1097/01.ta.0000140666.15972.37. discussion 707-708. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- Wang G.G., Calvo K.R., Pasillas M.P., Sykes D.B., Hacker H., Kamps M.P. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods. 2006;3:287–293. doi: 10.1038/nmeth865. [DOI] [PubMed] [Google Scholar]
- Wang J.F., Li J.B., Zhao Y.J., Yi W.J., Bian J.J., Wan X.J., Zhu K.M., Deng X.M. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: an animal study and a prospective case-control study. Anesthesiology. 2015;122:852–863. doi: 10.1097/ALN.0000000000000525. [DOI] [PubMed] [Google Scholar]
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Wilson Z.S., Ahn L.B., Serratelli W.S., Belley M.D., Lomas-Neira J., Sen M., Lefort C.T. Activated beta2 integrins restrict neutrophil recruitment during murine acute pseudomonal pneumonia. Am. J. Respir. Cell Mol. Biol. 2017;56:620–627. doi: 10.1165/rcmb.2016-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M., Yuan Y., Fan L., Li Y., Li A., Yin L., Scott M.J., Xiao G., Billiar T.R., Wilson M.A. Role of macrophages in mobilization of hematopoietic progenitor cells from bone marrow after hemorrhagic shock. Shock. 2012;37:518–523. doi: 10.1097/SHK.0b013e318249b81d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Matsushima H., Ohtola J.A., Geng S., Lu R., Takashima A. Neutrophil priming occurs in a sequential manner and can be visualized in living animals by monitoring IL-1beta promoter activation. J. Immunol. 2015;194:1211–1224. doi: 10.4049/jimmunol.1402018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Kondo R., Wang Q., Doerschuk C.M. Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats. Am. J. Respir. Crit. Care Med. 2006;174:689–698. doi: 10.1164/rccm.200502-276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.J., Yi W.J., Wan X.J., Wang J., Tao T.Z., Li J.B., Wang J.F., Deng X.M. Blockade of ICAM-1 improves the outcome of polymicrobial sepsis via modulating neutrophil migration and reversing immunosuppression. Mediators Inflamm. 2014;2014:195290. doi: 10.1155/2014/195290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.