Abstract
Camellia oleifera Abel. (C. oleifera) is a cultivable plant with important economic value. It is very helpful for the scientific utilization, cultivation and preservation of germplasm resources through evaluating the genetic diversity. In this study, we estimated the genetic relationship of 150 accessions of C. oleifera using morphological and economic traits, as well as SSR molecular marker. Through the variation and cluster analysis of 17 morphological and economic traits, the germplasm was divided into a candidate core breeding group with higher economic traits and a core breeding group with higher morphological traits. The genetic similarity coefficients of SSR markers ranged from 0.05 to 0.91, and the germplasm materials were divided into five groups. The results demonstrated that C. oleifera germplasms perform a rich genetic variation. This is the first report to evaluate the genetic diversity of different C. oleifera germplasms using the morphological and economic traits, together with SSR molecular marker, and the results allow us to find evidence for the origin of varieties, establish core breeding populations and its fingerprint.
Keywords: Camellia oleifera, genetic diversity, phenotypic traits, SSR
Introduction
Camellia oleifera Abel. (C. oleifera), belonging to genus Camellia in the family Theaceae, originates from China and is one of the world’s four major oil tree species (together with oil palm, olive and coconut) (Chen et al. 2015). It has been cultivated in south-central and southern China for more than 2,000 years (Zhu et al. 2020), due to its major product (nearly 50% of dry kernel weight), tea oil, is an edible oil known as “eastern olive oil” with high nutritional value and health benefits. The crop covers a huge area of approximately 4.4 million hectares with abundant resources and an annual output of over 2.4 million tons of seeds, and its oil yield is more than 0.6 million tons, and is distributed in the Hunan, Jiangxi, Guangxi, Fujian, Hainan and provinces (Liu et al. 2018). Recently, C. oleifera has drawn growing attention because of its highly commercial, medic, cosmetic and ornamental value. In addition, C. oleifera species are viewed as important germplasm resources with valuable gene pool for the development of new varieties of oil tree species.
Due to the diversity of C. oleifera germplasm resources, a variety of excellent individual plants, clones and families have been bred and cultivated in China. Phenotypic diversity is a comprehensive manifestation of the interaction between biological genetics and environmental factors, which is one of the major topics of genetic diversity research (Costa et al. 2017). Detecting genetic variation from phenotypic traits is a simple and easy method, which can reveal the magnitude of genetic variation to a certain extent, and the assessment of phenotypic variation of different individuals also will be beneficial to the management and utilization of germplasm (Zhou et al. 2015).
Generally, there is a lot of redundancy of C. oleifera germplasms in the circumstance that the morphology of C. oleifera varies under the cooperation of genetic background and environment, which due partly to same varieties with different names. Moreover, the absence of flowers and fruits for the majority of the year in C. oleifera, especially slight changes of leaf shape exhibits. makes it difficult to distinguish the varieties based on the morphology of leaves, flowers and fruits.
Commonly, the genetic diversity studies of plant germplasm resources are capable of reducing the redundancy of germplasm conservation and promoting the construction of core germplasm resources, which is important for the effective utilization of genetic resources in plant breeding (Verma et al. 2019). Nowadays, the techniques in genetic diversity research at the DNA level is contributed to understand the genetic relationship and distribution. Although the studies on molecular biology of C. oleifera has been brought for nearly twenty years, there is still few reports about it. The random amplified polymorphic DNA (RAPD) markers were firstly applied to analysis of genetic diversity of 32 superior hybrid varieties and wild type varieties of C. oleifera in 2003 (Zhang 2003). In 2007, sixteen the inter-simple sequence repeat (ISSR) primers were developed and used for analysis of genetic diversity of 10 superior clone of C. oleifera (Zhang et al. 2007). The earliest report about the application of Sequence-related amplified polymorphism (SRAP) markers in C. oleifera identified the genetic difference and evaluated the relationship of 48 individuals from 5 different regions (Zhang et al. 2011). Among these genetic techniques, the simple sequence repeat (SSR) is one of the powerful molecular marker technologies established in 1985 (Jeffreys et al. 1985). Over the past decade, the researches that SSR molecular marker used in Camellia genus have been reported. Liu et al. (Liu et al. 2011) optimized the SSR-PCR reaction system and proved the inter-species versatility of microsatellite loci in Camellia genus. Huang first applied SSR markers to study the genetic structure and inter-species hybrid introgression between C. oleifera and C. meiocarpa (Huang 2013). However, the limited genetic studies have been performed on the genetic evaluation of C. oleifera species over 100 accessions from various series using SSR markers.
At present, to the best of our knowledge, the combined phenotypic characteristics and SSR markers has not been applied in C. oleifera species for its genetic diversity assessment. Therefore, in this study, the genetic diversity analysis of C. oleifera germplasm materials was performed using phenotypic characteristics and SSR markers. The results indicate that comprehensive utilization of phenotypic characteristics and SSR markers can be efficient in the analysis of genetic diversity in C. oleifera for plant breeding.
Materials and Methods
Plant materials
A collection of 150 accessions of C. oleifera was used in this study (Table 1). All these accessions were planted in the experimental field at the National Camellia Oleifera Germplasm Resource Preservation Center, Hunan, China. The germplasm materials from H001 to H029 were ancient C. oleifera trees that living for over three hundred years. The rest germplasm materials were either collected or bred by Hunan Academy of Forestry then were grafted to scions and planted in field for about fifteen years.
Table 1.
Information of C. oleifera germplasm materials in this study
| No. | Name | Origin | Location | No. | Name | Origin | Location |
|---|---|---|---|---|---|---|---|
| H001 | YC | Hengnan, Hunan | E 112°68′, N 26°74ʹ | H076 | XL197 | Yongzhou, Hunan | E 113°35ʹ, N 27°00ʹ |
| H002 | YC | Hengnan, Hunan | E 112°68ʹ, N 26°74ʹ | H077 | XL210 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ |
| H003 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H078 | XL218 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H004 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H079 | XL228 | Yongzhou, Hunan | E 113°35ʹ, N 27°00ʹ |
| H005 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H080 | XL258 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H006 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H081 | XL349 | Youxian, Hunan | E 113°35ʹ, N 27°00ʹ |
| H007 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H082 | XL352 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H008 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H083 | XL371 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H009 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H084 | XL374 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H010 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H085 | XL376 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H011 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H086 | XL380 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H012 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H087 | GY11 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ |
| H013 | YC | Shimen, Hunan | E 111°38ʹ, N 29°58ʹ | H088 | GY12 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ |
| H014 | YC | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H089 | GY13 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ |
| H015 | YC | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H090 | GY14 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ |
| H016 | YC | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H091 | DZ1 | Pingjiang, Hunan | E 113°58ʹ, N 28°70ʹ |
| H017 | YC | Zhangjiajie, Hunan | E 110°55ʹ, N 29°35ʹ | H092 | ChaL466 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ |
| H018 | YC | Zhangjiajie, Hunan | E 110°55ʹ, N 29°35ʹ | H093 | ChaL74-14 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ |
| H019 | YC | Zhangjiajie, Hunan | E 110°55ʹ, N 29°35ʹ | H094 | ChaL75-1 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ |
| H020 | YC | Zhangjiajie, Hunan | E 110°55ʹ, N 29°35ʹ | H095 | ChaL75-3 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ |
| H021 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H096 | ChaL77-6 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ |
| H022 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H097 | DP7101 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ |
| H023 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H098 | GuiY7810 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ |
| H024 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H099 | JT22 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ |
| H025 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H100 | XT11 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ |
| H026 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H101 | YJT1 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ |
| H027 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H102 | Tian117 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H028 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H103 | TG151 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ |
| H029 | YC | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H104 | HH1 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ |
| H030 | XL1 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H105 | HH2 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ |
| H031 | XL3 | Hengyang, Hunan | E 112°74ʹ, N 27°23ʹ | H106 | HH3 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ |
| H032 | XL4 | Youxian, Hunan | E 113°35ʹ, N 27°00ʹ | H107 | HH4 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ |
| H033 | XL5 | Youxian, Hunan | E 113°35ʹ, N 27°00ʹ | H108 | HH6 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ |
| H034 | XL9 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H109 | JY7805 | Jiangyong, Hunan | E 111°34ʹ, N 25°27ʹ |
| H035 | XL11 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H110 | Zao7722 | Chenzhou, Hunan | E 113°01ʹ, N 25°78ʹ |
| H036 | XL12 | Huaihua, Hunan | E 110°04ʹ, N 27°58ʹ | H111 | Zao7730 | Chenzhou, Hunan | E 113°01ʹ, N 25°78ʹ |
| H037 | XL13 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ | H112 | Zao7801 | Chenzhou, Hunan | E 113°01ʹ, N 25°78ʹ |
| H038 | XL14 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H113 | G6 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H039 | XL15 | Ningyuan, Hunan | E 111°94ʹ, N 25°57ʹ | H114 | G68 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H040 | XL19 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ | H115 | G70 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H041 | XL26 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H116 | GS84-8 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H042 | XL27 | Yongzhou, Hunan | E 111°59ʹ, N 26°46ʹ | H117 | GW1 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H043 | XL32 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H118 | GW2 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H044 | XL67 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H119 | GW5 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H045 | XL69 | Youxian, Hunan | E 113°35ʹ, N 27°00ʹ | H120 | GW11 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H046 | XL75 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H121 | GW16 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H047 | XL78 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H122 | GW83-4 | Nanchang, Jiangxi | E 115°94422, N 28°54538 |
| H048 | XL81 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H123 | GX46 | Xingguo, Jiangxi | E 115°36314, N 26°33779 |
| H049 | XL82 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H124 | GX48 | Xingguo, Jiangxi | E 115°36314, N 26°33779 |
| H050 | XL97 | Youxian, Hunan | E 113°35ʹ, N 27°00ʹ | H125 | GY6 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H051 | XL102 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H126 | CL3 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H052 | XL103 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H127 | CL4 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H053 | XL104 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H128 | CL21 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H054 | XL105 | Miluo, Hunan | E 112°37ʹ, N 28°58ʹ | H129 | CL23 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H055 | XL106 | Yiyang, Hunan | E 110°04ʹ, N 27°58ʹ | H130 | CL27 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H056 | XL107 | Huaihua, Hunan | E 113°07ʹ, N 28°81ʹ | H131 | CL40 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H057 | XL108 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H132 | CL53 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H058 | XL109 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H133 | CL55 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H059 | XL110 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H134 | CL156 | Jiangxi | E 113°34ʹ–118°28ʹ, N 24°29ʹ–30°04ʹ |
| H060 | XL115 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H135 | HB39 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H061 | XL117 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H136 | HB361 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H062 | XL123 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H137 | HB424 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H063 | XL131 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H138 | EY1 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H064 | XL133 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H139 | EY2 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H065 | XL134 | Yiyang, Hunan | E 112°37ʹ, N 28°58ʹ | H140 | EY5 | Hubei | E 108°47ʹ–114°15ʹ, N 24°38–30°08ʹ |
| H066 | XL141 | Hengyang, Hunan | E 112°74ʹ, N 27°23ʹ | H141 | CR2 | Cenxi, Guangxi | E 110°9949, N 22°91828 |
| H067 | XL152 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H142 | CR3 | Cenxi, Guangxi | E 110°9949, N 22°91828 |
| H068 | XL156 | Hengyang, Hunan | E 112°74ʹ, N 27°23ʹ | H143 | CR11 | Cenxi, Guangxi | E 110°9949, N 22°91828 |
| H069 | XL163 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H144 | DBS1 | Anhui | E 114°54ʹ–119°37ʹ, N 29°41ʹ–34°38ʹ |
| H070 | XL169 | Hengyang, Hunan | E 112°74ʹ, N 27°23ʹ | H145 | DBS2 | Anhui | E 114°54ʹ–119°37ʹ, N 29°41ʹ–34°38ʹ |
| H071 | XL171 | Miluo, Hunan | E 113°07ʹ, N 28°81ʹ | H146 | DBS3 | Anhui | E 114°54ʹ–119°37ʹ, N 29°41ʹ–34°38ʹ |
| H072 | XL183 | Changsha, Hunan | E 112°99ʹ, N 28°26ʹ | H147 | DBS4 | Anhui | E 114°54ʹ–119°37ʹ, N 29°41ʹ–34°38ʹ |
| H073 | XL185 | Chaling, Hunan | E 113°54ʹ, N 26°78ʹ | H148 | Min43 | Fujian | E 115°40–120°30, N23°30–28°20 |
| H074 | XL190 | Hengdong, Hunan | E 112°95ʹ, N 27°08ʹ | H149 | Min48 | Fujian | E 115°40–120°30, N23°30–28°20 |
| H075 | XL192 | Zhuzhou, Hunan | E 113°17ʹ, N 27°86ʹ | H150 | Min60 | Fujian | E 115°40–120°30, N23°30–28°20 |
Measurement of quantitative traits
Nine branches and leaves of each C. oleifera germplasm materials were applied to the measurement of morphological traits. Fifteen fruits together with their seeds of each C. oleifera germplasm materials were applied to the measurement of morphological and economic traits.
DNA extraction and PCR analysis
Genomic DNA was extracted from the young leaves of C. oleifera by a modified cetyltrimethyl ammonium bromide (CTAB) method (Huang et al. 2018). A set of 50 pairs of SSR primers (Chen et al. 2016, Zhou et al. 2017) were initially screened, and 9 pairs of primers with good, clear banding patterns were selected for analysis of genetic diversity (Table 4). SSR amplification was performed as follows: the reaction conditions were 50 ng of template DNA, 0.8 μL of each primer at 10 μM and 10 μL 2 × Taq PCR Master Mix in a total reaction volume of 20 μL. The reaction mixture was initially denatured at 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, annealing temperature according to Table 2 for 45 s, and 72°C for 2 min; and a final extension at 72°C for 10 min. Fluorescently labeled PCR products were analyzed concurrently with the GeneScanTM-500LIZTM Size Standard on an ABI 3730XL sequencer, and sizes were determined with GENEMAPPER version 4.0. Due to the putatively polyploid character of C. oleifera, allele frequencies, polymorphism information content (PIC) were calculated using the methods described by Huang (2013).
Table 4.
SSR primers used in this study
| Primer | Forward sequence | Reverse sequence | Repeat unit | Product size (bp) | Number of alleles/polymorphic alleles | Number of genotypes | Polymorphism information content |
|---|---|---|---|---|---|---|---|
| NJFUC53 | TGCCCTAAGTGTCATTC | CAGGGATGATATTGTTTCT | (AAAAT)5 | 157–217 | 10/10 | 41 | 0.782 |
| NJFUC57 | ATAGGTCTTTGTCTGGTT | ATGTAGAGGAAGACTGGA | (TC)10 | 223–275 | 25/25 | 55 | 0.623 |
| NJFUC243 | TGTATGGTTTGGCTCG | GGTTGGCAAGATGAGA | (AGA)10 | 161–251 | 28/28 | 138 | 0.937 |
| NJFUC251 | AATCAACCAAGCGTAC | AGATCCTCCAAACTCC | (TAT)7 | 93–174 | 28/28 | 112 | 0.913 |
| NJFUC273 | ATCTGTAGCTTAATTCTAG | ATTTTCTGGAGCATCT | (AAAAC)7 | 290–350 | 12/12 | 78 | 0.588 |
| NJFUC600 | GTCTTGGCTATCATTTT | TTTCCTATTGACCTCC | (AT)7 | 222–250 | 11/11 | 23 | 0.588 |
| NJFUC653 | ATCGACCTTTGTTGGG | ATTGAAGCTGGCATTT | (TCGGC)3 | 180–210 | 5/5 | 15 | 0.677 |
| NJFUC787 | GTGGCTCAATAAGGAT | CATTACACCGTCTTCAT | (AT)7 | 140–232 | 15/15 | 77 | 0.887 |
| NJFUC833 | GTGGGTTACGGGTTTA | CGGGACAAGTTCAGTT | (AAT)5 | 213–252 | 11/11 | 32 | 0.746 |
Table 2.
Quantitative traits of C. oleifera
| Quantitative traits | Mean ± sd | Range | CV |
|---|---|---|---|
| Shoot length/cm | 15.01 ± 3.59 | 6.22–26.94 | 23.90% |
| Shoot diameter/mm | 2.85 ± 0.42 | 1.45–4.07 | 14.65% |
| Leaf area/cm2 | 12.87 ± 3.01 | 6.77–24.23 | 23.40% |
| Leaf length/cm | 5.39 ± 0.83 | 3.64–7.64 | 15.45% |
| Leaf average width/cm | 2.32 ± 0.29 | 1.46–3.18 | 12.44% |
| Leaf maximum width/cm | 3.14 ± 0.41 | 2.00–4.32 | 12.93% |
| Fruit height/cm | 37.17 ± 4.92 | 25.63–50.9 | 13.24% |
| Fruit diameter/cm | 36.53 ± 5.44 | 23.65–50.56 | 14.89% |
| Fruit weight/g | 26.83 ± 10.63 | 7.52–62.10 | 39.60% |
| Peel thickness/mm | 5.07 ± 1.06 | 2.72–8.21 | 20.97% |
| Seed height/cm | 23.84 ± 2.91 | 17.69–34.41 | 12.20% |
| Seed diameter/cm | 19.87 ± 2.71 | 12.26–27.38 | 13.66% |
| Fresh seed rate/% | 42.31 ± 6.85 | 24.74–56.56 | 16.18% |
| Dry seed rate/% | 21.62 ± 4.68 | 12.08–34.11 | 21.66% |
| Dry kernel rate/% | 13.06 ± 3.89 | 5.06–23.89 | 29.80% |
| Dry kernel oil content/% | 44.54 ± 6.52 | 23.10–57.68 | 14.63% |
| Fresh fruit oil producrion rate/% | 5.98 ± 2.40 | 1.49–12.91 | 40.11% |
Data analysis
SSR amplified bands in the gel were manually scored as present (1) or absent (0). Only the consistently reproducible bands were scored, and the smeared and weak bands were excluded. The resulting 1/0 data matrix was used to assess the level of genetic diversity of C. oleifera. The obtained genetic distance matrix was then used to perform the cluster analysis and construct the un-weighted pair-group method with arithmetic average (UPGMA) dendrogram using NTsys-pc 2.1 software (Rohlf 2000).
Results
Variation of quantitative traits
Quantitative traits of morphological and economic characteristics such as size and weight of fruits and seeds, as well as productions of fruits, seeds and oil were measured and analyzed in this study (Fig. 1). As shown in Table 2, the seventeen quantitative traits of 150 C. oleifera germplasm materials showed relatively high variation levels, due to the change fold of maximum compare to minimum in each quantitative traits ranged from 1.95 to 8.72, while their coefficient of variation (CV) ranged from 12.20% to 40.11%.
Fig. 1.

Pictures of the shape variation of leaves, fruits and seeds among accessions.
The heatmap was established with normalized quantitative traits (Fig. 2). The clustering results showed that 150 germplasm materials could be divided into two groups. The first group consists of 55 germplasms with relatively high levels of economic traits than the other 95 germplasms from the second group, as well as relatively small sizes of leave, fruits and seeds.
Fig. 2.
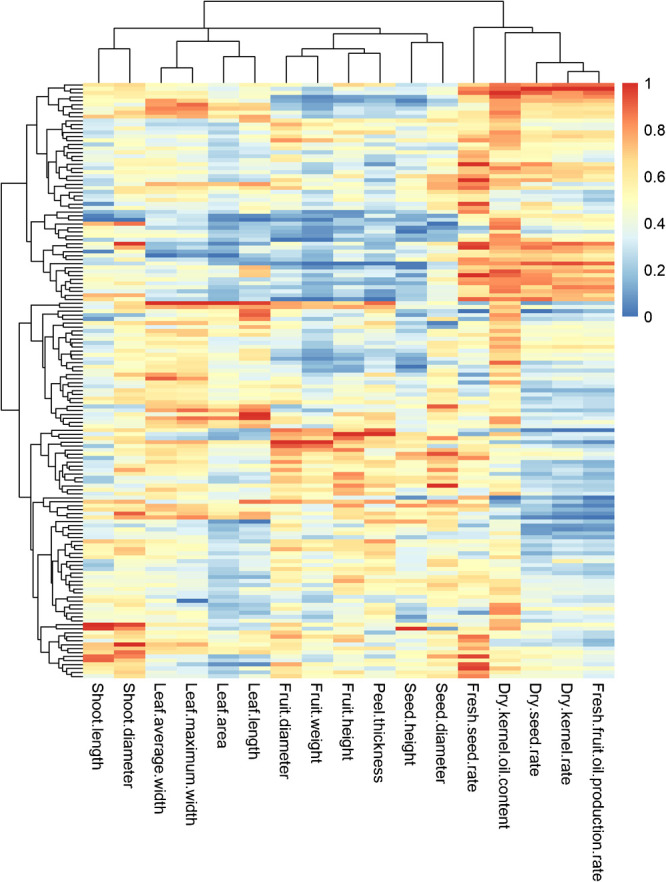
The heatmap of normalized quantitative traits of 150 C. oleifera germplasms.
We conducted a correlation analysis with 17 quantitative traits among 150 C. oleifera germplasms, the results showed that the correlation of these quantitative traits divided into three clusters (Fig. 3). Cluster I consisted with 5 economic characteristics that refer to the fruits, seeds and oil yield. Cluster II included 6 morphological characteristics described the sizes of fruits and seeds. The rest 6 morphological characteristics that involved in the size of leaves and growth of branches constituted Cluster III.
Fig. 3.
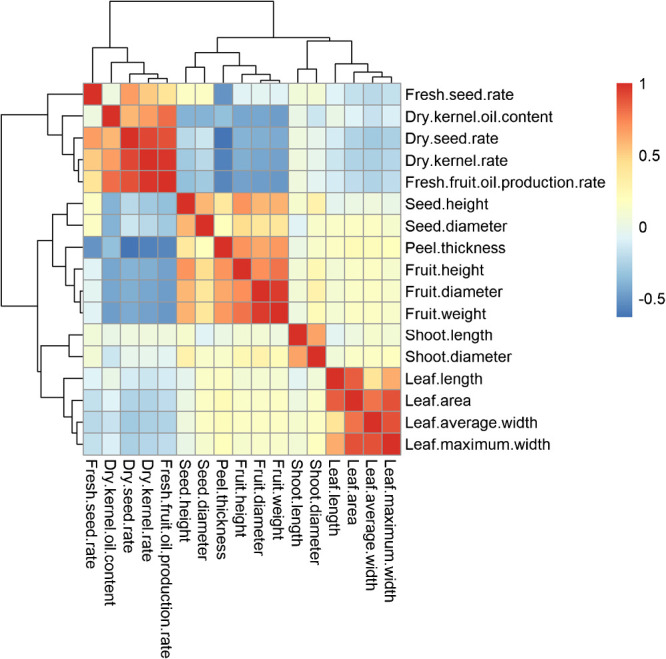
The correlation analysis of quantitative traits of 150 C. oleifera germplasms.
The principal component analysis (PCA) was carried out with seventeen quantitative traits among 150 C. oleifera germplasms. The results showed that the first three principal component (PCs) explained 68.33% of the variations in total (Table 3).
Table 3.
The principal component analysis in the C. oleifera germplasm collection
| Principal components | PC1 | PC2 | PC3 |
|---|---|---|---|
| Standard deviation | 6.25 | 3.05 | 2.31 |
| Proportion of variance | 36.77% | 17.96% | 13.60% |
| Cumulative proportion | 36.77% | 54.73% | 68.33% |
| Traits | Eigen vectors | ||
| Shoot length | 0.05 | 0.01 | 0.44 |
| Shoot diameter | 0.25 | –0.01 | 0.57 |
| Leaf area | 0.44 | 0.84 | 0.23 |
| Leaf length | 0.30 | 0.70 | 0.19 |
| Leaf average width | 0.45 | 0.72 | 0.18 |
| Leaf maximum width | 0.42 | 0.83 | 0.20 |
| Fruit height | 0.76 | –0.32 | 0.29 |
| Fruit diameter | 0.76 | –0.31 | 0.29 |
| Fruit weight | 0.79 | –0.32 | 0.26 |
| Peel thickness | 0.77 | –0.09 | –0.13 |
| Seed height | 0.57 | –0.43 | 0.43 |
| Seed diameter | 0.47 | –0.18 | 0.37 |
| Fresh seed rate | –0.37 | –0.25 | 0.64 |
| Dry seed rate | –0.80 | –0.04 | 0.52 |
| Dry kernel rate | –0.82 | 0.01 | 0.46 |
| Dry kernel oil content | –0.69 | 0.23 | 0.12 |
| Fresh fruit oil production rate | –0.84 | 0.08 | 0.40 |
PCA also showed that the size of leaves and fruits, together with economic traits had a significant contribution of the total variation. However, the germplasm materials could not be grouped clearly based on principal component in three-dimensional space (Fig. 4).
Fig. 4.
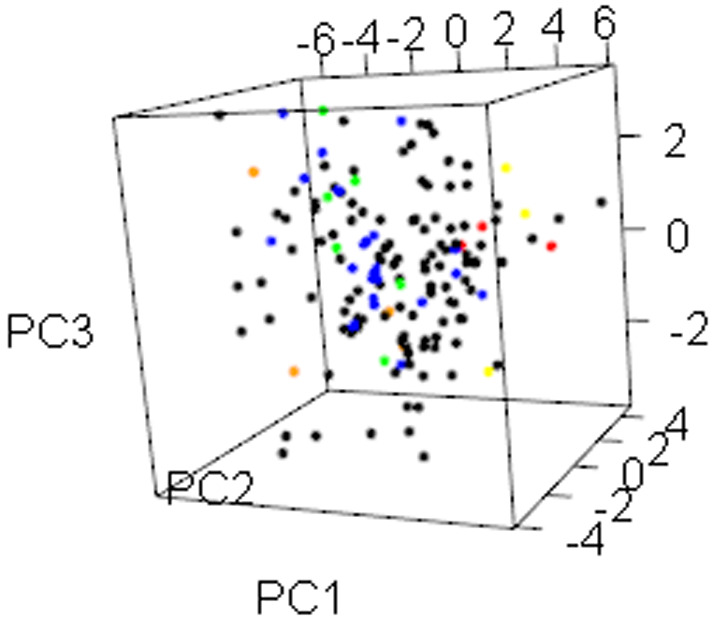
Principal component analysis of 150 C. oleifera germplasms in three-dimensional space based on the quantitative traits. Colors of points reflects the origin sources of individuals. Black – Hunan province, blue – Jiangxi province, green – Hubei province, yellow – Guangxi province, orange – Anhui province, red – Fujian province.
Detection of polymorphisms
By using 9 pairs of SSR primer among 150 C. oleifera germplasms, as shown in Table 4, a total of 145 alleles (100% polymorphic alleles) were detected, ranging 5 to 28 per locus with an average of 16.11 (Table 4). The highest NA (number of alleles) was observed at NJFUC243 and NJFUC251. Alleles with low frequencies (<5%) accounts for 66.21% (96/145), and the specific alleles (only observed in one accession) account for 8.97% (13/145). The PIC (polymorphism information content) values varied from 0.588 to 0.937 with an average of 0.749, the highest PIC value was observed at NJFUC243 and the lowest was observed at NJFUC273 and NJFUC601.
Genetic diversity and cluster analysis
Based on the Dice genetic similarity coefficient between two different samples, a clustering map of genetic relationships among C. oleifera germplasms was constructed using UPGMA method. The Dice genetic similarity coefficients of 150 germplasm materials ranged from 0.05 to 0.91, which performed the rich genetic diversity.
A very complicated phylogenetic tree based on the SSR cluster analysis results of 150 germplasms was showed in Fig. 5. When the genetic similarity coefficient was 0.31, 150 C. oleifera germplasms could be divided into five groups. Cluster I consists of only one germplasm, namely ‘ChaL75-1’ (short for ‘Chaling No. 75-1’), from Hunan Province. Its average genetic similarity with other germplasms was 0.24, indicated that there was a significant genetic difference between the identified germplasm material and other materials. Cluster II consists of four germplasms, namely ‘XL108’, ‘ChaL77-6’ and two ancient germplasms, all from Hunan province. Cluster III consists of four germplasms which were ‘XL3’, ‘XL210’, and ‘ChaL75-3’ from Hunan province while ‘EY1’ from Hubei province. Cluster IV consists of four germplasms which were ‘XL141’ and ‘XL197’ from Hunan province while ‘G6’ and ‘DBS3’ from Jiangxi and Anhui province, respectively. The rest germplasms constituted Cluster V which divided into five subcluster. Cluster Va consists of five germplasms which were ‘XL134’, ‘XL376’, ‘JY7805’ and ‘Zao7730’ from Hunan province while ‘EY2’ from Hubei province. Cluster Vb consists of six germplasms which were ‘XL26’, ‘XL109’, ‘XL192’, ‘ChaL74-14’ and ‘Zao7722’ from Hunan province while ‘CL53’ from Jiangxi province. Cluster Vc consists of eight germplasms which were six ancient germplasms from Hunan province while ‘CL40’ and ‘HB361’ from Jiangxi and Hubei province. Cluster Vd consists of nine germplasms which were ‘XL5’, ‘XL12’, ‘XL32’, ‘XL81’, ‘DP7101’ and two ancient germplasms from Hunan province while ‘GS84-8’ and ‘GW83-4’ from Jiangxi province. Cluster Ve consists of the rest 109 germplasms with an average genetic similarity of 0.40.
Fig. 5.
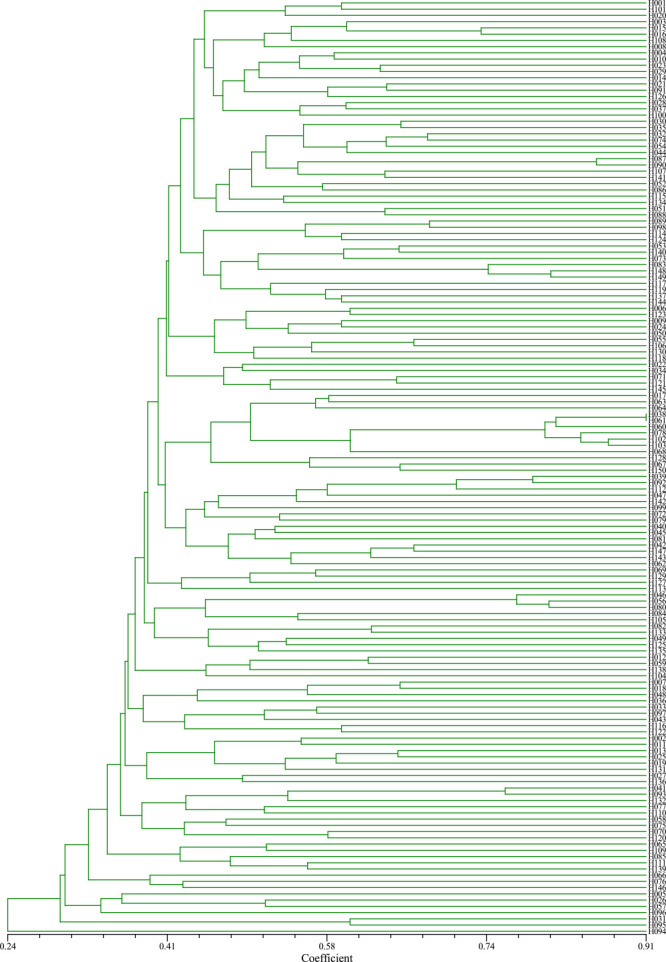
Phylogenetic tree of 150 C. oleifera germplasms based on SSR data.
Discussion
Interactions of genetic diversity and environmental background resulted in phenotypic diversity (Backouchi et al. 2015). The variations of quantitative traits were mainly under regulation of multiple genes, and they also differentiated by adapting to different environmental conditions (Zhou et al. 2015). Camellia oleifera is a perennial economic tree species that has a long history of cultivation for more than 2,000 years. Since its characteristics of cross-pollinating, the phenotypic variations of C. oleifera germplasm were highly enriched under the environmental stress and genetic selection (Zhang et al. 2019). Although DNA molecular markers had been applied to study the genetic diversity of C. oleifera germplasm for a long time, it was still reliable to carry out phenotypic studies on the genetic diversity of the same species of C. oleifera with the same habitat and age. In this study, phenotypic variations were analyzed within 29 ancient C. oleifera trees and 121 unique C. oleifera seedlings that bred by human. The coefficient of variation (CV) can evaluate the degree of dispersion of quantitative traits to a certain extent. The germplasm materials applied to this study exhibit significant genetic variation for all the seventeen morphological and economic traits, since the CV of each quantitative traits was higher than 12.20%. The heatmap showed that the germplasm materials cluster into two groups. Cluster I consisted with 55 C. oleifera germplasms with relatively high economic traits, whose fresh seed rate, dry seed rate, dry kernel rate, dry kernel oil content, fresh fruit oil production rate were 17.26%, 36.00%, 49.23%, 13.93% and 69.11% higher than those of Cluster II, respectively. In contrast, the leaf area, fruit height, fruit diameter, fruit weight and peel thickness of Cluster II were 12.80%, 13.17%, 14.43%, 44.52% and 31.12% higher than those of Cluster I, respectively. Together with the result of correlation analysis of 17 quantitative traits, negative correlations between economic traits and morphological traits were existed, which provided a new strategy for C. oleifera breeding of high oil content.
Previously, various DNA markers, including simple sequence repeat (SSR), inter-simple sequence repeat (ISSR), random amplified polymorphic DNA (RAPD) and sequence-related amplified polymorphism (SRAP) have been used in the studies of the genetic diversity of C. oleifera (Chen et al. 2019, Jiang et al. 2018, Zhai 2018, Zhang et al. 2018). In this study, 29 ancient C. oleifera trees living for over three hundred years collected from different areas in Hunan province and 121 human bred varieties from the main cultivation areas were used for the analysis of their genetic relationship and origins. As shown in Fig. 2 and Fig. 5, it was showed that all the C. oleifera materials are divided into different groups in molecular level, respectively. These estimates of genetic diversity could be biased by the choice of phenotypic or molecular data (Vinu et al. 2013). Thus, both phenotypic traits and molecular markers should be considered to achieve unbiased genetic diversity estimation. The genetic similarity coefficients ranged from 0.05 to 0.91, which indicated that these SSR primers used in this study could distinguish all the germplasm materials completely. This result might due partly to the long history of cultivation and characteristics of cross-pollination. With the help of cross-pollination characteristics, the genetic resources of C. oleifera constantly exchanged with each other during the long-term cultivation, resulted in a high level of genetic diversity of germplasm materials. C. oleifera germplasms from six provinces were analyzed, 112 of them were from Hunan province while 22, 6, 3, 4 and 3 were from Jiangxi, Hubei, Guangxi, Anhui and Fujian province, respectively. The uneven distribution of materials might affects the analysis of genetic diversity, but it was showed that some of the germplasm materials with the same geographic origin or similar genetic background can be clustered in the same group, while the other germplasm materials with geographical origin and inconsistent genetic background also were clustered in the same group.
Generally, C. oleifera is a hexaploid specie with 90 chromosome (Ye et al. 2020), studies using SSR molecular markers to evaluate the genetic diversity of C. oleifera could hardly cover whole genome. Furthermore, C. oleifera has no reference genome data, SSR molecular markers used in the studies of C. oleifera had been made were selected from the prediction of transcriptomic data. In this experiment, 9 pairs of SSR primers were applied under the consideration of the purpose of genetic relationship evaluation of germplasm resources and the practical feasibility of completing the analysis process. In the result, the genetic similarity coefficients between C. oleifera germplasm materials ranged from 0.05 to 0.91, indicated that these SSR primers used in this study could distinguish all the germplasm materials completely. Among these 150 accessions, there were 29 ancient C. oleifera trees which lives for at least three hundred years those were the special precious germplasm resources used in this study, they were collected from different district in Hunan Province by the authorship due to their remaining abilities of normal flowering and fruiting. They were chosen in this study helping explore the genetic origin of artificial bred cultivar. For the rest 121 accessions, there were superior cultivars recommended by the National Forestry and Grassland Administration of China, and candidate breeding germplasm resources with great potential in improving production of C. oleifera. Firstly, Since these SSR molecular markers can distinguish all germplasm materials, these SSR molecular markers can be used to construct fingerprints of superior cultivars. Secondly, the genetic relationship between candidate breeding resources provide the study on affinity optimization of hybridization combination for fundamentals. Thirdly, the assessment of genetic relationship between the ancient C. oleifera trees and candidate breeding resources provide evidences for the studies on the origin of germplasm materials. There are still a numerous of candidate C. oleifera breeding resources which are left need to been analyzed in our future project to establish the phenotype and genetic characteristics database of C. oleifera core breeding population. During the process, more SSR and maybe some other molecular markers would be applied to meet its need. Altogether, the results of this study indicated that phenotypic traits and SSR markers can be efficiently integrated in the exploration of their genetic diversity for improvement and germplasm utilization.
Author Contribution Statement
Software, CL; validation, ZH and CL; investigation, RW and XW; data curation, ZH; writing—original draft preparation, ZH; writing—review and editing, ZH; supervision, YT; project administration, YC; funding acquisition, YC. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was funded by Science and Technology Major Program of Hunan Province, China (Grant No. 2018NK1030), the National Key Research and Development Project (Grant No. 2018YFD1000603), and the Natural Science Foundation of Hunan Province, China (Grant No. 2019JJ50303).
Literature Cited
- Backouchi, I.Z., Aouida M., Khemiri N. and Jebara M. (2015) Genetic diversity in Tunisian populations of faba bean (Vicia faba L.) based on morphological traits and molecular markers. Genet. Mol. Res. 14: 7587–7596. [DOI] [PubMed] [Google Scholar]
- Chen, X., Yun Y., Wu Y., Qi H., Yang L., Chen J. and Zheng D. (2019) Genetic diversity analysis of Camellia oleifera resources based on SRAP markers in Hainan Island. Journal of Tropical and Subtropical Botany 27: 659–668 (in Chinese). [Google Scholar]
- Chen, Y., Wang B., Chen J., Wang X., Wang R., Peng S., Chen L., Ma L. and Luo J. (2015) Identification of Rubisco rbcL and rbcS in Camellia oleifera and their potential as molecular markers for selection of high tea oil cultivars. Front. Plant Sci. 6: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Dai X., Hou J., Guan H., Wang Y., Li Y. and Yin T. (2016) DNA fingerprinting of oil camellia cultivars with SSR markers. Tree Genet. Genomes 12: 7. [Google Scholar]
- Costa, J.C., Fracetto G.G.M., Fracetto F.J.C., Santos M.V.F. and Lira Júnior M.A. (2017) Genetic diversity of Desmanthus sp accessions using ISSR markers and morphological traits. Genet. Mol. Res. 16. [DOI] [PubMed] [Google Scholar]
- Huang, G., Guan T., Zhao Y., Chen M. and Liu H. (2018) A rapid and efficient method for extracting DNA from Camellia oleifera leaves. Mol. Plant Breed. 16: 4350–4354 (in Chinese). [Google Scholar]
- Huang, Y. (2013) Population genetic structure and interspecific introgressive hybridization between Camellia meiocarpa and C. oleifera. Chinese Journal of Applied Ecology 24: 2345–2352 (in Chinese). [PubMed] [Google Scholar]
- Jeffreys, A.J., Wilson V. and Thein S.L. (1985) Individual-specific ‘fingerprints’ of human DNA. Nature 316: 76–79. [DOI] [PubMed] [Google Scholar]
- Jiang, D., Fang Y., Xiao X., Cheng H., Zhang X., Cheng J. and Li L. (2018) Identifying Camellia oleifera germplasm accessions with inter simple sequence repeat markers. Hubei Agricultural Science 57: 119–125 (in Chinese). [Google Scholar]
- Liu, B., Jin L., Cao C., Wang Q., Zhou M., Wu S., Shu Q. and Zhang L. (2011) Establishment and optimization of SSR-PCR reaction system for oil tea (Camellia oleifera Abel.). Journal of Anhui Agricultural University 38: 858–862 (in Chinese). [Google Scholar]
- Liu, C., Chen L., Tang W., Peng S., Li M., Deng N. and Chen Y. (2018) Predicting potential distribution and evaluating suitable soil condition of oil tea Camellia in China. Forests 9: 487. [Google Scholar]
- Rohlf, F. (2000) Ntsys-pc numerical taxonomy and multivariate analysis system version 2.1. owner manual. [Google Scholar]
- Verma, H., Borah J.L. and Sarma R.N. (2019) Variability assessment for root and drought tolerance traits and genetic diversity analysis of rice germplasm using SSR markers. Sci. Rep. 9: 16513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinu, V., Singh N., Vasudev S., Yadava D.K., Kumar S., Naresh S., Bhat S.R. and Prabhu K.V. (2013) Assessment of genetic diversity in Brassica juncea (Brassicaceae) genotypes using phenotypic differences and SSR markers. Rev. Biol. Trop. 61: 1919–1934. [PubMed] [Google Scholar]
- Ye, T., Y. Li, J. Zhang, Q. Gong, D. Yuan and S. Xiao (2020) Optimization of chromosome mounting technique and karyotype analysis of Camellia oleifera. Journal of Nanjing Forestry University (Natural Sciences Edition). DOI: 10.3969/j.issn.1000-2006.202003022 (in Chinese). [Google Scholar]
- Zhai, Q. (2018) Genetic variation of Camellia oleifera in Anhui province based on AFLPs. Dissertation, Anhui Agricultural University (in Chinese). [Google Scholar]
- Zhang, G., Zhong W., Wu Y., Tan X. and Du T. (2007) Identification of oil tea (Camellia oleifera) superior clones by ISSR molecular marker. Forest Research 20: 278–282 (in Chinese). [Google Scholar]
- Zhang, M., Zhang H., Cai X., Wang H., Ye Y. and Wu Y. (2018) SSR analysis of genetic relationship between Camellia germplasm resources. Nonwood Forest Research 36: 130–134 (in Chinese). [Google Scholar]
- Zhang, T., Liu S., Mei H. and Dong Y. (2011) SRAP analysis of genetic diversity of Camellia oleifera resources in Hubei province. Journal of Huazhong Normal University (Nat. Sci.) 45: 477–479 (in Chinese). [Google Scholar]
- Zhang, W., Zhao Y., Yang G., Peng J., Chen S. and Xu Z. (2019) Determination of the evolutionary pressure on Camellia oleifera on Hainan Island using the complete chloroplast genome sequence. PeerJ 7: e7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. (2003) Analysis of genetic diversity and genetic characters by RAPD markers on oil Camellia. Dissertation, Fujian Normal University (in Chinese). [Google Scholar]
- Zhou, R., Wu Z., Cao X. and Jiang F.L. (2015) Genetic diversity of cultivated and wild tomatoes revealed by morphological traits and SSR markers. Genet. Mol. Res. 14: 13868–13879. [DOI] [PubMed] [Google Scholar]
- Zhou, W., Qiang W., Yang J., Wang J., Xv L. and Xv L. (2017) Establishment of DNA fingerprints and cluster analysis for Oil Camellia cultivars based on SSR markers. Mol. Plant Breed. 15: 238–249 (in Chinese). [Google Scholar]
- Zhu, G., Liu H., Xie Y., Liao Q., Lin Y., Liu Y., Liu Y., Xiao H., Gao Z. and Hu S. (2020) Postharvest processing and storage methods for Camellia oleifera seeds. Food Rev. Int. 36: 319–339. [Google Scholar]


