Abstract
Background
There is no clear consensus on the recommended second-line treatment for patients with metastatic pancreatic cancer who have disease progression following gemcitabine-based therapy. We retrospectively evaluated the clinical outcomes of liposomal irinotecan (nal-IRI) plus fluorouracil/leucovorin (FL) and FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) in patients who had failed on the first-line gemcitabine-based therapy.
Patients and methods
From January 2015 to August 2019, 378 patients with MPC who had received nal-IRI/FL (n = 104) or FOLFIRINOX (n = 274) as second-line treatment across 11 institutions were included in this retrospective study.
Results
There were no significant differences in baseline characteristics between groups, except age and first-line regimens. With a median follow-up of 6 months, the median progression-free survival (PFS) was 3.7 months with nal-IRI/FL versus 4.6 months with FOLFIRINOX (P = 0.44). Median overall survival (OS) was 7.7 months with nal-IRI/FL versus 9.7 months with FOLFRINOX (P = 0.13). There was no significant difference in PFS and OS between the two regimens in the univariate and multivariate analyses. The subgroup analysis revealed that younger age (<70 years) was associated with better OS with FOLFIRINOX. In contrast, older age (≥70 years) was associated with better survival outcomes with nal-IRI/FL. Adverse events were manageable with both regimens; however, the incidence of grade 3 or higher neutropenia and peripheral neuropathy was higher in patients treated with FOLFIRINOX than with nal-IRI/FL.
Conclusions
Second-line nal-IRI/FL and FOLFIRINOX showed similar effectiveness outcomes after progression following first-line gemcitabine-based therapy. Age could be the determining factor for choosing the appropriate second-line therapy.
Key words: pancreatic cancer, second-line treatment, liposomal irinotecan, FOLFIRINOX
Highlights
-
•
This multicenter retrospective study investigated nal-IRI/FL and FOLFIRINOX outcomes after gemcitabine-based therapy.
-
•
We found no significant differences in outcome between nal-IRI/FL and FOLFIRINOX treatment.
-
•
Both regimens were well tolerated; however, neutropenia and peripheral neuropathy were more frequent with FOLFIRINOX.
-
•
Age (cut-off, 70 years) showed differential efficacy between chemotherapy regimens.
Introduction
Despite recent advances in diagnostic technology and anti-cancer drugs, pancreatic cancer continues to have a poor prognosis worldwide. It is the fourth leading cause of cancer-related death in the United States1 and the fifth leading cause of cancer-related death in South Korea.2 Approximately 80%-90% of patients with pancreatic cancer are diagnosed at an advanced stage and overall the 5-year survival rate is only 3%.1,2
The MPACT study has reported that a first-line therapy of albumin-bounded paclitaxel (nab-paclitaxel) with gemcitabine significantly improve the survival of patients with metastatic pancreatic cancer compared with gemcitabine monotherapy.3 Gemcitabine monotherapy remains a valid treatment option for patients with poor performance status.4 Previous studies have assessed the role of 5-fluorouracil (5-FU)-based therapies in patients who progressed following gemcitabine-based therapy5, 6, 7, 8, 9; however, there is no clear consensus on the best second-line treatment for patients with metastatic pancreatic cancer (MPC) after progression following gemcitabine-based chemotherapy.
The randomized, phase III NAPOLI-1 study has revealed that liposomal irinotecan (nal-IRI) plus fluorouracil/leucovorin (FL) has shown significant survival benefit when compared with FL monotherapy in patients with MPC who had been previously treated with gemcitabine-based therapy.10 FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), one of the standard first-line chemotherapy regimens for patients with MPC,11 shows clinically acceptable outcomes as the second-line treatment after progression following gemcitabine-based chemotherapy; however, no randomized trial data have been published.12, 13, 14 Both nal-IRI/FL and FOLFIRINOX are effective combination second-line chemotherapies for medically fit patients; however, no prospective or retrospective comparative study of these regimens has been undertaken.
In this study, we carried out a multicenter retrospective analysis to compare the efficacy and safety profiles of nal-IRI/FL and FOLFIRINOX as the second-line treatment for patients with MPC after progression following gemcitabine-based therapy.
Patients and methods
Patients
This retrospective, multicenter (11 tertiary referral centers) study was conducted by the hepatobiliary and pancreatic cancer division of the Korean Cancer Study Group (KCSG). Patients with histologically or cytologically confirmed MPC who received either nal-IRI/FL or FOLFIRINOX as the second-line treatment after progression on first-line gemcitabine-based therapy between January 2015 and August 2019 were identified and their medical records were reviewed. Patients who had recurrence within 6 months after the completion of adjuvant gemcitabine-based chemotherapy were regarded as failed on palliative first-line therapy. This study was approved by the institutional review board of each institution as required. The requirement of informed consent was waived due to the retrospective nature of this study.
Treatment and assessment
Nal-IRI/FL consisted of intravenous infusion of nal-IRI 70 mg/m2 followed by leucovorin 400 mg/m2 with a continuous fluorouracil 2400 mg/m2 every 2 weeks, as described in the NAPOLI-1 trial.10 FOLFIRINOX consisted of intravenous infusion of oxaliplatin 85 mg/m2 followed by irinotecan 180 mg/m2, leucovorin 400 mg/m2, and a bolus of fluorouracil 400 mg/m2 with a continuous infusion of fluorouracil 2400 mg/m2 every 2 weeks, as described in the PRODIGE 4/ACCORD 11 trial.11 Dose modification was allowed at the discretion of the attending physician in patient subgroups, such as older age patients or those with a poor performance status. Treatment was continued until disease progression or intolerable toxicity. Imaging studies, including computed tomography or magnetic resonance imaging, were carried out every 6 or 8 weeks. Adverse events were monitored at every clinic visit. Tumor response and adverse events were graded by Response Evaluation Criteria In Solid Tumor (RECIST) version 1.1 and National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, respectively.
Statistical analysis
Progression-free survival (PFS) was defined as the duration from treatment initiation to disease progression or any cause of death, whichever occurred first. Overall survival (OS) was defined as the duration from treatment initiation to any cause of death. Pearson's chi-square and Fisher's exact tests were used to compare discrete data. Survival outcomes were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses were carried out using the Cox proportional hazard model. A two-sided P value <0.05 was considered statistically significant. All statistical analyses were carried out using Statistical Package for the Social Sciences, version 24.0 for Windows (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 378 patients were included in this study who received nal-IRI/FL (n = 104) or FOLFIRINOX (n = 274). Patient characteristics are shown in Table 1. All baseline patient characteristics, except age and prior gemcitabine-based chemotherapy regimens, were not significantly different between groups. Median age was higher in patients treated with nal-IRI/FL than those treated with FOLFIRINOX (64 versus 61 years, P = 0.013). The gemcitabine/nab-paclitaxel combination was used more frequently in patients treated with nal-IFI/FL than those with FOLFIRINOX (85.6% versus 51.5%). Gemcitabine monotherapy or an older gemcitabine-based combination using capecitabine, erlotinib, or cisplatin were more commonly used in patients with FOLFIRINOX (48.5% versus 14.4%). There was no difference in the history of prior surgery between nal-IRI plus FL and FOLFIRINOX groups (36.5% versus 40.7%, P = 0.464); however, more patients who had a recurrence within 6 months after the completion of adjuvant gemcitabine monotherapy or gemcitabine-capecitabine combination therapy were treated with FOLFIRINOX than with nal-IRI/FL [28.9% (n = 74) versus 10.6% (n = 11), P < 0.001]. Third-line chemotherapy was administered to 153 patients (40.5%) following progression after second-line nal-IRI/FL or FOLFIRINOX; there was no difference between groups (P = 0.227).
Table 1.
Baseline patient characteristics
| nal-IRI/FL (n = 104) |
FOLFIRINOX (n = 274) |
P value | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | |||||
| Median (range) | 64 | (35-78) | 61 | (34-81) | 0.013 |
| <65 | 53 | 51.0 | 170 | 62.0 | 0.05 |
| ≥65 | 51 | 49.0 | 104 | 38.0 | |
| <70 | 76 | 73.1 | 215 | 78.5 | 0.266 |
| ≥70 | 28 | 26.9 | 59 | 21.5 | |
| Sex | |||||
| Male | 61 | 58.7 | 152 | 55.5 | 0.578 |
| Female | 43 | 41.3 | 122 | 44.5 | |
| ECOG | |||||
| 0-1 | 86 | 82.7 | 223 | 88.5 | 0.142 |
| 2 | 18 | 17.3 | 29 | 11.5 | |
| Histology | |||||
| Adenocarcinoma | 103 | 99.0 | 261 | 95.3 | 0.083 |
| Othersa | 1 | 1.0 | 13 | 4.7 | |
| Location | |||||
| Head | 46 | 44.2 | 146 | 53.7 | 0.1 |
| Body | 26 | 25.0 | 46 | 16.9 | |
| Tail | 30 | 28.8 | 66 | 24.3 | |
| Multicentric | 2 | 2.0 | 14 | 5.1 | |
| Prior surgery | |||||
| No | 66 | 63.5 | 162 | 59.3 | 0.464 |
| Yes | 38 | 36.5 | 111 | 40.7 | |
| Metastatic site | |||||
| Liver | |||||
| No | 33 | 31.7 | 97 | 35.4 | 0.502 |
| Yes | 71 | 68.3 | 177 | 64.6 | |
| Lung | |||||
| No | 81 | 77.9 | 217 | 79.2 | 0.78 |
| Yes | 23 | 22.1 | 57 | 20.8 | |
| Peritoneum | |||||
| No | 70 | 67.3 | 168 | 61.3 | 0.281 |
| Yes | 34 | 32.7 | 106 | 38.7 | |
| Bone | |||||
| No | 95 | 91.3 | 253 | 92.3 | 0.751 |
| Yes | 9 | 8.7 | 21 | 7.7 | |
| Number of metastatic sites | |||||
| <2 | 69 | 66.3 | 179 | 65.3 | 0.852 |
| ≥2 | 35 | 33.7 | 95 | 34.7 | |
| Laboratory values (median, range) | |||||
| Albumin (g/dl) | 3.7 | (1.6-4.7) | 3.8 | (2.1-4.9) | 0.038 |
| NLR (ratio) | 2.4 | (0.6-14.6) | 2.8 | (0.4-74.4) | 0.537 |
| CA19-9 (U/ml) | 292.3 | (1.2-91 765) | 259 | (0-69 938) | 0.83 |
| Prior first-line therapy | |||||
| Gemcitabine monotherapy | 6 | 5.8 | 67 | 24.4 | <0.001 |
| Gemcitabine/nab-paclitaxel | 89 | 85.6 | 141 | 51.5 | |
| Gemcitabine/othersb | 9 | 8.6 | 66 | 24.1 | |
| Subsequent chemotherapy | |||||
| No | 64 | 61.5 | 142 | 51.8 | 0.227 |
| Yes | 35 | 33.6 | 118 | 43.1 | |
| Unknown | 5 | 4.8 | 14 | 5.1 | |
CA19-9, carbohydrate antigen 19-9; ECOG, European Eastern Oncology Group; nal-IRI, liposomal irinotecan; NLR, neutrophil-lymphocyte ratio; No, number.
Intraductal neoplasm with invasive carcinoma, and acinar cell carcinoma.
Capecitabine, cisplatin, erlotinib, or investigational product.
Effectiveness outcomes
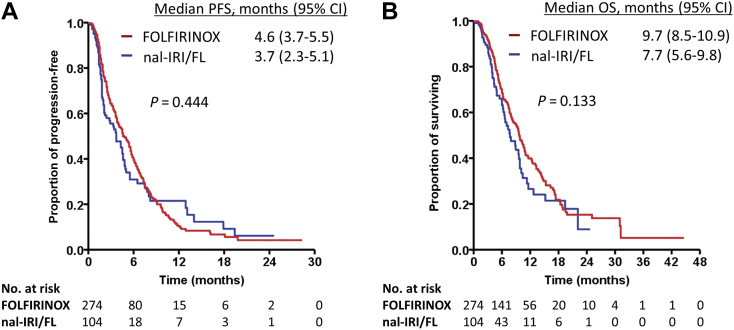
The median follow-up durations for all patients, patients treated with nal-IRI/FL, and patients treated with FOLFIRINOX were 6 (range, 0.2-44.4), 4.9 (range, 0.2-24.5), and 6.2 (range, 0.5-44.4) months, respectively. The effectiveness outcomes are summarized in Table 2. There was no difference in median PFS between groups: 3.7 [95% confidence interval (CI): 2.3-5.1] and 4.6 (95% CI: 3.7-5.5) months for patients treated with nal-IRI/FL and FOLFIRINOX, respectively (P = 0.444; Figure 1A). Six-month PFS rates were 30.9% and 39.5% in nal-IRI/FL and FOLFIRINOX groups, respectively. There was no difference in median OS between groups: 7.7 (95% CI: 5.6-9.8) and 9.7 (95% CI: 8.5-10.9) months for patients treated with nal-IRI/FL and FOLFIRINOX, respectively (P = 0.133; Figure 1B). One-year survival rates were 26.5% and 39.9% in nal-IRI/FL and FOLFIRINOX groups, respectively. Objective response rates were 11.5% and 14.2% with nal-IRI/FL and FOLFIRINOX, respectively (P = 0.493). The disease control rate was higher in patients treated with FOLFIRINOX compared with nal-IRI plus FL (63.1% versus 47.1%, P = 0.005).
Table 2.
Effectiveness outcomes
| Variables | nal-IRI/FL (n = 104) | FOLFIRINOX (n = 274) | P value | ||
|---|---|---|---|---|---|
| PFS, months, median (95% CI) | 3.7 | 2.3-5.1 | 4.6 | 3.7-5.5 | 0.444 |
| PFS rate at 6 months, % (95% CI) | 30.9 | 20.6-41.2 | 39.5 | 33.2-45.8 | |
| PFS rate at 12 months, % (95% CI) | 21.5 | 11.6-31.5 | 10.6 | 6.0-15.1 | |
| OS, months, median (95% CI) | 7.7 | 5.6-9.8 | 9.7 | 8.5-10.9 | 0.133 |
| OS rate at 6 months, % (95% CI) | 63.1 | 52.7-73.5 | 68.6 | 62.7-74.4 | |
| OS rate at 12 months, % (95% CI) | 26.5 | 14.7-38.3 | 39.9 | 32.8-47.0 | |
| Objective response rate, No. (%) | 12 | 11.5 | 39 | 14.2 | 0.493 |
| Disease control rate, No. (%) | 49 | 47.1 | 173 | 63.1 | 0.005 |
| Best overall response, No. (%) | 0.063 | ||||
| CR | 1 | 1 | 2 | 0.7 | |
| PR | 11 | 10.6 | 37 | 13.5 | |
| SD | 37 | 35.6 | 134 | 48.9 | |
| PD | 37 | 35.6 | 70 | 25.5 | |
| Not evaluable | 18 | 17.3 | 31 | 11.3 | |
CI, confidence intervals; CR, complete response; nal-IRI, liposomal irinotecan; No, number; OS, overall survival; PFS, progression-free survival; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Kaplan–Meier curves according to chemotherapy regimens.
(A) Progression-free survival, (B) overall survival.
CI, confidence interval; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin; nal-IRI/FL, liposomal irinotecan plus fluorouracil/leucovorin; No, number; OS, overall survival; PFS, progression-free survival.
The multivariate analysis identified ECOG status (0 or 1 versus 2), liver metastasis, albumin (<3.5 versus ≥3.5 g/dl), and CA19-9 (<40 versus ≥40 U/ml) as independent prognostic factors for PFS (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100049) while liver metastasis, peritoneal metastasis, albumin (<3.5 versus ≥3.5 g/dl), and CA19-9 (<40 versus ≥0 U/ml) were independent prognostic factors for OS (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100049). Interestingly, chemotherapy regimen (nal-IRI/FL versus FOLFIRINOX) was not retained in the final multivariate models for PFS and OS.
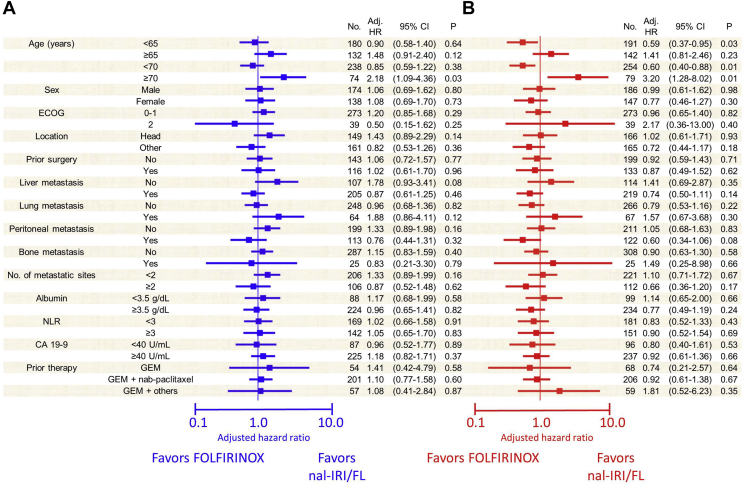
Subgroup analyses revealed no difference in median PFS between patients aged <70 years [adjusted hazard ratio (HR) = 0.85, 95% CI: 0.59-1.22, P = 0.382]; however, treatment with nal-IRI/FL showed a better PFS than with FOLFIRINOX in patients aged ≥70 years (adjusted HR for PFS = 2.18, 95% CI: 1.09-4.36, P = 0.027; Figure 2A). The FOLFIRINOX group showed better OS than with the nal-IRI/FL group in patients aged <70 years [median OS, 9.8 months (95% CI: 8.2-11.4) versus 6.6 months (95% CI: 5.4-7.9); adjusted HR = 0.60, 95% CI: 0.40-0.88, P = 0.01; Figure 2B]. In contrast, the nal-IRI/FL group showed better OS than the FOLFIRINOX group [10.4 (95% CI: 0.0-24.0) versus 9.5 months (95% CI: 7.3-11.8); adjusted HR = 3.20, 95% CI: 1.28-8.02, P = 0.013].
Figure 2.
Forest plots showing the survival outcomes of patient subgroups.
(A) Progression-free survival, (B) overall survival.
Adj, adjusted; CA19-9, carbohydrate antigen 19-9; CI, confidence interval; ECOG, European Eastern Oncology Group; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin; GEM, gemcitabine; HR, hazard ratio; nal-IRI/FL, liposomal irinotecan plus fluorouracil/leucovorin; NLR, neutrophil-lymphocyte ratio; No, number; OS, overall survival; PFS, progression-free survival.
Safety profiles
Dose modification at first cycle was more commonly done for patients treated with FOLFIRINOX than those with nal-IRI/FL (78.5% versus 33.7%). The proportions of patients with a dose intensity at first cycle ≥80% of the original dose were 91.3% and 59.5% in the FOLFIRINOX and nal-IRI/FL groups, respectively; those with ≥70% of the original dose were 100% and 93.4%, respectively.
The treatment-emergent adverse events of grade 3 or higher are listed in Table 3. Grade 3-4 neutropenia was reported more often in patients treated with FOLFIRINOX than in those with nal-IRI/FL (47.2% versus 35%, P = 0.033). Grade 3-4 peripheral neuropathy was more common in the FOLFIRINOX group than in the nal-IRI/FL group (5.9% versus 1.0%, P = 0.049). There were no other significant differences in toxicity profiles. The treatment-emergent adverse events of all grades are listed in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100049. Any grade of neutropenia and thrombocytopenia were more common in the FOLFIRINOX group than in the nal-IRI/FL group (neutropenia: 65.3% versus 46.6%, P = 0.001; thrombocytopenia: 17.3% versus 6.8%, P = 0.01). Any grade of peripheral neuropathy was more frequently observed in the FOLFIRINOX group than in the nal-IRI/FL group (34.7% versus 20.4%, P = 0.007).
Table 3.
Treatment-emergent adverse events of grade 3 or higher
| Event | nal-IRI/FL (n = 104) |
FOLFIRINOX (n = 274) |
P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Hematologic | |||||
| Neutropenia | 36 | 35 | 128 | 47.2 | 0.033 |
| Febrile neutropenia | 7 | 6.8 | 28 | 10.3 | 0.294 |
| Thrombocytopenia | 2 | 1.9 | 11 | 4.1 | 0.528 |
| Anemia | 8 | 7.8 | 32 | 11.8 | 0.259 |
| Non-hematologic | |||||
| Fatigue | 9 | 8.7 | 15 | 5.5 | 0.259 |
| Mucositis | 1 | 1.0 | 4 | 1.5 | 1.000 |
| Nausea | 7 | 6.8 | 26 | 9.6 | 0.394 |
| Vomiting | 6 | 5.8 | 16 | 5.9 | 0.977 |
| Diarrhea | 2 | 1.9 | 4 | 1.5 | 0.669 |
| Peripheral neuropathy | 1 | 1.0 | 16 | 5.9 | 0.049 |
| AST/ALT elevation | 2 | 1.9 | 9 | 3.3 | 0.734 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin; nal-IRI, liposomal irinotecan; No, number.
Discussion
This multicenter retrospective study represents that nal-IRI/FL and FOLFIRINOX showed clinically meaningful effectiveness as the second-line chemotherapy for patients with MPC after progression following first-line gemcitabine-based chemotherapy. Severe neutropenia and peripheral neuropathy were more common in the FOLFIRINOX group; however, adverse events were well manageable in both treatment groups. Interestingly, the subgroup analysis revealed that FOLFIRINOX was associated with better OS compared with nal-IRI/FL in patients aged <70 years, while nal-IRI/FL showed better PFS and OS compared with FOLFIRINOX in patients aged ≥70 years.
Gemcitabine has been used as the standard therapy for patients with MPC.15 First-line nab-paclitaxel plus gemcitabine has shown significant survival benefits when compared with gemcitabine monotherapy.3 Oxaliplatin-5-FU doublet combination chemotherapy has shown conflicting results after progression following first-line gemcitabine therapy among phase II and III trials5, 6, 7; however, NAPOLI-1, international multicenter phase III trial, has reported that nal-IRI/FL improves clinical outcomes when compared with FL in patients with gemcitabine-refractory MPC.10 In the current study, patients who were treated with nal-IRI/FL showed 3.7 months of median PFS and 7.7 months of median OS, which were in line with the results of the NAPOLI-1 trial and previous real-world retrospective studies.10,16, 17, 18 Therefore, our study reaffirms the clinical value of nal-IRI/FL in the management of gemcitabine-refractory MPC.
FOLFIRINOX has been investigated as second-line therapy after failure of gemcitabine-based therapy because of its proven clinical benefit as a first-line treatment.11 Prior single-arm studies have reported median PFS and OS of 3-6 and 9-10 months, respectively.13,14,19 This could be regarded as clinically meaningful because of the numerically better survival outcomes than from historical data on 5-FU-based monotherapy or 5-FU-oxaliplatin/conventional irinotecan doublets.5, 6, 7, 8, 9 The median PFS and OS results found in this study are in line with previous studies.13,14,19 Reduced doses of FOLFIRINOX were administered to 78.5% of patients that were included in our retrospective analysis. However, modified version of FOLFIRINOX showed similar effectiveness with improved safety profiles when compared with the original FOLFIRINOX dose reported in previous studies.20, 21, 22, 23 This dose modification does not exclude the possibility of reduced effectiveness outcomes compared with the original FOLFIRINOX study; however, 93.4% of patients were administered with a dose intensity ≥70% of the original dose, which has been suggested as the cut-off for preserving effectiveness in a prior retrospective study.23
Multiple chemotherapeutic regimens, including nal-IRI/FL, FOLFIRINOX, 5-FU-oxaliplatin doublets (OFF, FOLFOX or XELOX) or 5-FU-based monotherapy (FL, capecitabine or S-1), are listed in guidelines as appropriate therapies after progression following gemcitabine-based therapy24; therefore, determining the optimal sequence of chemotherapy remains challenging in the daily management of medically fit patients with MPC. This is primarily due to the lack of randomized trials comparing combination chemotherapy regimens. To date, no data has been published comparing the clinical outcomes between second-line treatment with nal-IRI/FL and FOLFIRINOX.
This multicenter retrospective analysis found no significant difference in terms of objective response rate, PFS, and OS between second-line nal-IRI/FL and FOLFIRINOX after progression following first-line gemcitabine-based therapy. Effectiveness outcomes were numerically better with FOLFIRINOX than nal-IRI plus FL/LV; however, this did not reach significance in univariate and multivariate analyses. Safety profiles were acceptable and consistent with prior studies using both regimens. Neutropenia and peripheral neuropathy were more common in FOLFIRINOX when compared with nal-IRI/FL, despite reduced starting doses in most patients. Our results should be cautiously interpreted because of potential biases; chemotherapy selection was carried out by attending physicians in a daily practice setting and this decision is typically dependent on the patient's age, performance status, co-morbidities, effectiveness/toxicity profiles on prior gemcitabine, and the physicians' own clinical experiences with second-line chemotherapy regimens.
Interestingly, our study found that age may be a significant factor when choosing between nal-IRI/FL and FOLFIRINOX as the second-line treatment. Patients treated with FOLFIRINOX showed better OS if <70 years old, while patients treated with nal-IRI/FL showed better PFS and OS if ≥70 years old. These results were obtained from subgroup analysis; therefore, further adjustments of other prognostic factors could not be carried out. This suggests cautious interpretation is required. Further investigation should be carried out to define the indicators that should be used to determine the most appropriate second-line chemotherapy for patients who show progression following gemcitabine-based chemotherapy.
This study has several limitations. Primarily, this is a retrospective study. Potential prognostic factors, such as age, prior gemcitabine-based regimen, dose modification at initial cycle, and recurrence within six months after the completion of adjuvant gemcitabine-based therapy in patients with prior surgery were imbalanced between groups. Differences in the first-line gemcitabine-based regimens might be attributed to the different approval time points of FOLFIRINOX (2013), nab-paclitaxel (2016), and nal-IRI (2017) during the study period (since 2015). All our analyses were carried out by adjusting the potential impact of these factors; however, the interpretation of our study should be at the level of hypothesis-generation. Finally, adverse events might be underestimated considering the retrospective nature of study design.
Conclusion
Both nal-IRI/FL and FOLFIRINOX showed clinically meaningful effectiveness outcomes as the second-line therapy after progression following gemcitabine-based therapy. Safety profiles with these chemotherapy regimens were well manageable and in line with previous studies. Interestingly, age (cut-off, 70 years) interacted with these chemotherapy regimens to show differential outcome measures.
Acknowledgments
Funding
None declared.
Disclosure
CY received research grants and honoraria from Servier, Ipsen, Celgene, and Boryung Pharmaceutical; honoraria from Sanofi. All other authors have declared no conflicts of interest.
Data sharing
Anonymous individual data could be requested from the corresponding author upon reasonable request.
Contributor Information
C. Yoo, Email: yooc@amc.seoul.kr.
H.J. Choi, Email: choihj@yuhs.ac.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Jung K.W., Won Y.J., Hong S. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020;52:351–358. doi: 10.4143/crt.2020.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prager G.W., Oehler L., Gerger A. Comparison of nab-paclitaxel plus gemcitabine in elderly versus younger patients with metastatic pancreatic cancer: analysis of a multicentre, prospective, non-interventional study. Eur J Cancer. 2020;143:101–112. doi: 10.1016/j.ejca.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H., Riess H., Stieler J.M. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–2429. doi: 10.1200/JCO.2013.53.6995. [DOI] [PubMed] [Google Scholar]
- 6.Gill S., Ko Y.J., Cripps C. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol. 2016;34:3914–3920. doi: 10.1200/JCO.2016.68.5776. [DOI] [PubMed] [Google Scholar]
- 7.Ohkawa S., Okusaka T., Isayama H. Randomised phase II trial of S-1 plus oxaliplatin vs S-1 in patients with gemcitabine-refractory pancreatic cancer. Br J Cancer. 2015;112:1428–1434. doi: 10.1038/bjc.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K., Bang K., Yoo C. Clinical outcomes of second-line chemotherapy after progression on nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic adenocarcinoma. Cancer Res Treat. 2020;52:254–262. doi: 10.4143/crt.2019.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo C., Hwang J.Y., Kim J.E. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658–1663. doi: 10.1038/sj.bjc.6605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang-Gillam A., Li C.P., Bodoky G. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 11.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 12.Chiorean E.G., Von Hoff D.D., Tabernero J. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer. 2016;115:188–194. doi: 10.1038/bjc.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assaf E., Verlinde-Carvalho M., Delbaldo C. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301–306. doi: 10.1159/000329803. [DOI] [PubMed] [Google Scholar]
- 14.Chung M.J., Kang H., Kim H.G. Multicenter phase II trial of modified FOLFIRINOX in gemcitabine-refractory pancreatic cancer. World J Gastrointest Oncol. 2018;10:505–515. doi: 10.4251/wjgo.v10.i12.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burris H.A., 3rd, Moore M.J., Andersen J. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 16.Yoo C., Im H.S., Kim K.P. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y.Y., Chiang N.J., Tsai H.J. The impact of liposomal irinotecan on the treatment of advanced pancreatic adenocarcinoma: real-world experience in a Taiwanese Cohort. Sci Rep. 2020;10:7420. doi: 10.1038/s41598-020-64421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieler M., Unseld M., Bianconi D. A real-world analysis of second-line treatment options in pancreatic cancer: liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919853196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi N., Shimamura T., Tokuhisa M. Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine (Baltimore) 2017;96:e6769. doi: 10.1097/MD.0000000000006769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J., Hwang I., Yoo C. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018;36:732–741. doi: 10.1007/s10637-018-0598-5. [DOI] [PubMed] [Google Scholar]
- 21.Yoo C., Lee S.S., Song K.B. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a phase 2 study for clinical and biomarker analysis. Br J Cancer. 2020;123:362–368. doi: 10.1038/s41416-020-0867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conroy T., Hammel P., Hebbar M. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.C., Kim J.W., Ahn S. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer. 2017;76:125–133. doi: 10.1016/j.ejca.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Tempero M.A., Malafa M.P., Chiorean E.G. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw. 2019;17:202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.