Abstract
Head and neck squamous cell carcinomas (HNSCCs) are a type of common malignant tumor, mainly manifesting as oropharyngeal, oral cavity, laryngopharyngeal, hypopharyngeal, and laryngeal cancers. These highly aggressive malignant tumors reportedly affect more than 830,000 patients worldwide every year. Currently, the main treatments for HNSCC include surgery, radiotherapy, chemotherapy, and immunotherapy, as well as combination therapy. However, the overall 5-year survival rate of HNSCC has remained 50%, and it has not significantly improved in the past 10 years. Previous studies have shown that the tumor microenvironment (TME) plays a crucial role in the recurrence, metastasis, and drug resistance of patients with HNSCC. In this review, we summarize the role of anti-tumor and pro-tumor immune cells, as well as extracellular components in the TME of HNSCC. We also discuss classical HNSCC immunotherapy and highlight examples of clinical trials using CTLA-4 inhibitors and programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1)-related combination therapies. We also outline some molecules in the TME known to regulate immunosuppressive cells. Furthermore, the role and underlying mechanism of radiation therapy on the TME, immune cells, and immune response are discussed.
Keywords: head and neck squamous cell carcinoma, tumor microenvironment, immunotherapy, PD-1, PD-L1, CTLA-4
Graphical Abstract
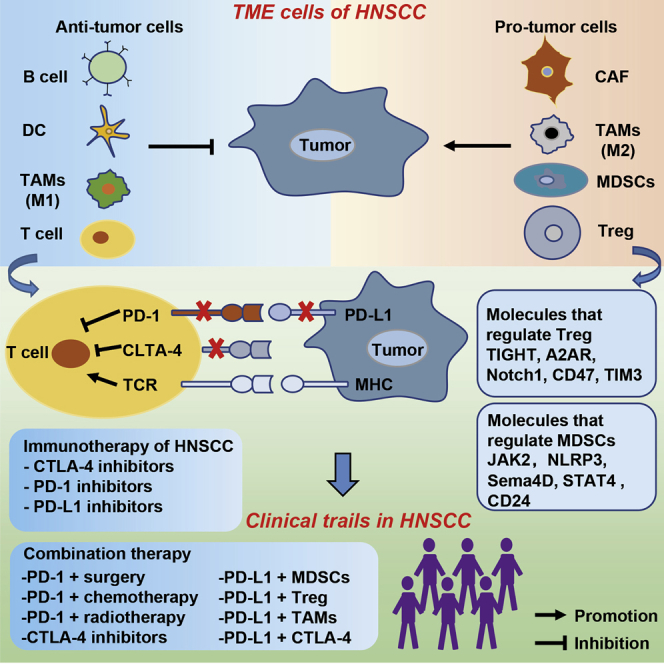
The tumor microenvironment (TME) plays a critical role in the recurrence, metastasis, and resistance of cancers. Wu and colleagues present an overview of the TME and immunotherapy in head and neck squamous cell carcinoma (HNSCC), together with a discussion of the effects and prospects of radiotherapy on the TME and HNSCC treatment.
Main text
About 90% of head and neck cancers occur as head and neck squamous cell carcinoma (HNSCC). According to the global cancer statistics of 2018,1 more than 830,000 new HNSCC cases and 430,000 related deaths occur worldwide each year. HNSCC incidence and mortality are very high, with the condition reportedly exacerbated by human papillomavirus infection, alcohol consumption, and tobacco smoking. Approaches for managing HNSCC, such as surgery, radiotherapy, chemotherapy, new immunotherapy, and combination therapies, have been applied, although tumor recurrence still occurs in 50% of the patients. In addition, surgical removal of the tumor will reduce the patient’s postoperative physical function, but many patients still have recurrence and metastasis.2,3 Consequently, the 5-year overall survival rate of HNSCC still has not improved.1,4
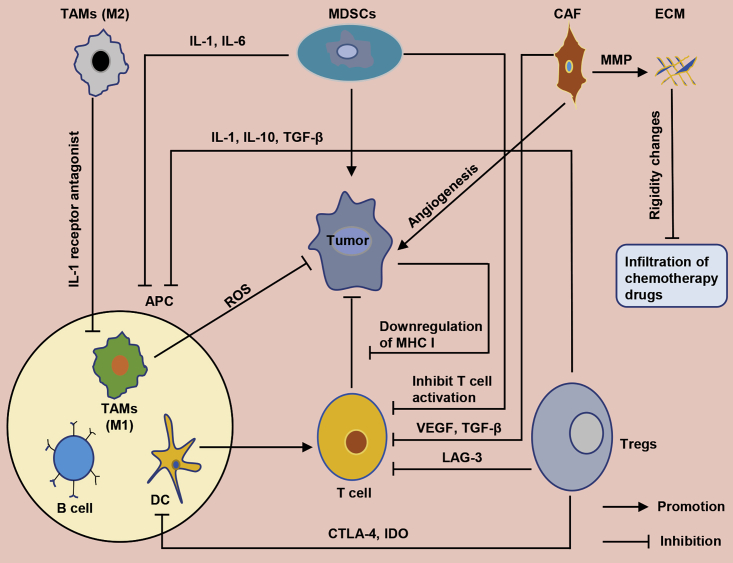
The tumor microenvironment (TME) comprises immune and non-immune cells, as well as extracellular components, that play a very important role in tumor recurrence and metastasis. Specifically, immune cells include myeloid-derived suppressor cells (MDSCs), regulatory T (Treg) cells, tumor-associated macrophages (TAMs), natural killer (NK) cells, and dendritic cells (DCs), whereas non-immune cells are mainly made up of cancer-associated fibroblasts (CAFs). Alternatively, extracellular components comprise cytokines, growth factors, extracellular matrix (ECM), and exosomes, among others. Generally, the TME of HNSCC harbors some unique aspects that cause a decline in anti-tumor immune function. Although our body’s immune system can recognize and eliminate tumor cells in a timely manner,5 HNSCC may hijack immune cells in the TME and use them to activate immune suppression and avoid recognition.5 Previous studies have shown that downregulating expression of human leukocyte antigen (HLA) not only achieves immune evasion, but it also reduces recognition of cancer cells by T cells.6 In addition, the TME of HNSCC has been found to also destroy tumor-infiltrating lymphocytes (TILs) and NK cells,7 whereas some important immune cell subpopulation, such as MDSCs, reportedly play a crucial role in tumor growth and metastasis. A summary of mechanisms underlying the interaction between immune cells and tumor cells in the TME of HNSCC is shown in Figure 1. The tumor immune microenvironment plays an important regulatory role in tumorigenesis and development, with numerous studies implicating it in the occurrence, metastasis, diagnosis, and treatment of HNSCC.8, 9, 10, 11, 12
Figure 1.
Schematic diagram represents the interaction between the tumor microenvironment and the tumor cells
The tumor microenvironment includes immune cells (MDSCs, Treg cells, TAMs, DCs, and B cells), non-immune cells (CAFs), and extracellular matrix (ECM).
In this review, we focus on the role of anti-tumor and pro-tumor immune cells, as well as extracellular components in the TME of HNSCC. We highlight classical TME cells in HNSCC and provide examples of clinical trials using CTLA-4 inhibitors and programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1), as well as combination therapies. Finally, we outline molecules that regulate immunosuppressive cells in the TME.
Immunosuppressive cells
MDSCs
MDSCs promote angiogenesis and metastasis via multiple mechanisms.13 Functionally, they regulate immune escape and have a negative association with overall survival rates of patients. Previous studies have shown that MDSCs not only inhibit activated T cells, but they also produce reactive oxygen species (ROS), which interact to catalyze nitrification of T cell receptors, thereby inducing T cell tolerance.14 MDSCs present in the TME promote immunosuppression via various mechanisms, including T cell suppression and innate immune regulation.15 In the TME, vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), and other factors have been shown to induce MDSC aggregation.16 In HNSCC, elevated MDSC levels reportedly upregulate inflammatory mediators, such as IL-1 and IL-6, making the environment unconducive for maturation of antigen-presenting cells (APCs), thereby indirectly promoting growth of tumor cells. Moreover, MDSCs can also induce development of Treg cells.17
Treg cells
The normal function of Treg cells is to suppress excessive immune responses and ensure that an immune balance in the body is maintained,18 whereas in the tumor immune microenvironment, they regulate tumor progression by lowering anti-tumor immunity.19 Treg cells are T cell subsets involved in the HNSCC immunosuppressive TME,20 and their aggregation is regulated by chemokines and related receptors, such as CCR4-CCL17/22, CCR8-CCL1, CCR10-CCL28, and CXCR3-CCL10. In the TME, Treg cells such as effector Treg (eTreg) cells are associated with poor prognosis, just as Foxp3 has been reported in other cancers to be associated with poor prognosis.21
Treg cells have been shown to use multiple mechanisms and generate immunosuppressive effects. The first mechanism entails overexpression of IL-2, the production of inhibitory cytokines, such as transforming growth factor (TGF)-β, IL-10, and IL-35, as well as perforin and granzyme B, which directly kill effector cells or APCs.22 The second mechanism involves Treg cell-mediated inhibition of effector T cells through major histocompatibility complex class II (MHC class II) by the ligand LAG-3,23 whereas the third immunosuppressive mechanism involves controlling indoleamine 2,3-dioxygenase (IDO) in DCs to reduce tryptophan. Consequently, T cells are inhibited due to depletion of key substances.24 In addition, adenosine produced by ATP metabolism, and regulated by CD39 and CD73 in activated Treg cells, causes inhibition of T cells through induction of negative signals to effector T cells and APCs. The last mechanism entails DC inhibition, via CTLA-4, which subsequently downregulates CD80 expression by combining with CTLA-4 produced by stimulated eTreg cells. Consequently, this leads to APC maturation and functional impairment.25 Additionally, CTLA-4, PD-1, and PD-L1 regulate immunization of a variety of tumors to suppress the microenvironment.26 Therefore, treatment approaches for HNSCC have used antibody therapy against these molecules.
TAMs
TAMs have two phenotypes, namely M1 and M2. The resulting phenotypes have different shapes and functions, with the M1 phenotype found to promote the T helper 1 (Th1) response and exhibit anti-tumor properties, whereas the M2 phenotype reportedly promotes the Th2 response, which is associated with tumor growth and migration.27 TAMs occupy the main part of the TME.28 Functionally, TAMs promote development of tumors into malignancies, and this has been associated with poor prognosis.29 M1 macrophages can kill microorganisms and tumor cells by producing ROS. In addition, chemokines, such as CXCL9 and CXCL10, as well as proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-1, IL-6, and IL-12, have been shown to cause M1-polarized macrophages to absorb new Th1 cells.30 Moreover, tumor M1 infiltration is positively correlated with prognosis of certain cancers.31
Alternatively, M2 macrophages initiate immunosuppression through Th2, with the M2 phenotype shown to be induced by cytokines, such as IL-4 and IL-10.32 M2 macrophages secrete a large number of chemokines, including CCL24, IL-10, and IL-12, among others.33,34 Previous studies have shown that activated M2 macrophages downregulate M1 by secreting anti-inflammatory cytokines, including IL-1 receptor antagonists, thereby inhibiting anti-tumor immunity.32 When they lose the ability to present antigens, M2 macrophages have been found to regulate tissue remodeling, debris removal, and immune regulation,35 and they have also been associated with poor prognosis of nasopharyngeal carcinoma.36
CAFs
CAFs are very active cellular components of the TME that play a vital role in tumor angiogenesis and invasion, as well as metastasis.37, 38, 39 In the TME, fibroblasts are transformed into CAFs through the TGF-β and IL-1β signaling pathways.40,41 According to other cancer reports, CAFs account for the largest tumor volume in the TME.42 Functionally, CAFs work synergistically with tumor cells to build an immunosuppressive network, which promotes tumor escape from immune killing. In HNSCC, CAFs reportedly inhibit T cell proliferation, via VEGF and TGF-β, and subsequently induce immune suppression by inducing Treg cells.38 In addition, CAFs have been shown to help tumor cells escape the body’s immune killing effect by gathering M2 macrophages and MDSCs.43,44 Since production of matrix metalloproteinases (MMPs) depends on CAFs, CAFs control the microenvironment by regulating remodeling and degradation of ECM, which causes increased cancer cell invasiveness.45,46
Anti-tumor immune cells
NK cells
NK cells are one of the most significant cells in anti-tumor immune cells. Although cancer cells and their TME inhibit NK cell activity,47 NK cells have been shown to eliminate cancer cells through secretion of immunomodulatory cytokines.48 Consequently, NK cell-based immunotherapies are on the rise,49,50 and they seek to restore anti-tumor immunity by regulating immune checkpoints that regulate NK cell activity.51 Several mechanisms, including binding tumor ligands, promoting NK cell infiltration, and targeting multiple activated NK cell receptors, have been shown to release NK cells against tumors.51
DCs
DCs, an outpost of the immune system, play a crucial role as a bridge between innate and adaptive immune responses.52 Functionally, CD4+ T cells support the CD8+ T cell response through DCs.53 DCs have been described as a strong APC, due to their role in activation of antigen-specific CD4 and CD8 T cells by processing and presenting antigens to initiate an adaptive immune response.54 Several factors in the TME, such as IL-6, macrophage colony-stimulating factor (M-CSF), and IL-10,55 have been implicated in the downregulation of DC function, which subsequently inhibits T cell activation and impairs immune checkpoint blocking therapy.56
CD8+ T cells
CD8+ T cells can fight tumors by producing cytokines and killing effects.57,58 Some substances in the TME have been shown to induce CD8+ T cell exhaustion,59 although these cells are continuously stimulated in some cases, such as chronic inflammation and cancer, which leads to exhaustion of their function.57 An early indication of this exhaustion entails significant reduction of IL-2 secretion,60 which subsequently lowers production of cytokines such as TNF. In this state, T cell apoptosis may also occur, resulting in a significant decrease in virus-specific T cells.61,62 There have been reviews comprehensively describing how to reverse T cell failure.63
Extracellular components
Pro-tumor effect of ECM
Massive remodeling of ECM causes changes in its density and rigidity, which are related to the malignant phenotype.64 In fact, ECM rigidity can prevent drugs from reaching tumor cells to promote migration and growth of cancer cells, through epithelial-mesenchymal transition (EMT) and angiogenesis.65 Despite its degradation being closely associated with tumor metastasis,66 ECM has been implicated in tumor-related immunosuppression due to its role in regulating proliferation, localization, and function of myeloid cells.67
Anti-tumor effect of ECM
Under normal circumstances, drug transport in the tumor stroma mainly depends on diffusion. The ECM component reportedly acts as an effective barrier to the spread of tumor cells,68,69 which generally increase ECM rigidity, and it blocks chemotherapeutic drugs from entering and making contact with tumor cells.70 To enhance drug penetration, it is imperative to normalize ECM during treatment.71,72
Tumor-promoting cytokines
In most HNSCC cases, epidermal growth factor receptor (EGFR) has been shown to increase immature DCs in the TME, thereby causing abnormal T cell function.73 IL-10, which is produced by Th2 cells,74 can induce recruitment of M2 macrophages and increase the number of Treg cells, subsequently inhibiting DC function. All cytokines listed in Table 1, except those that induce the M1 phenotype, can also promote suppressive immune cells.
Table 1.
Immunosuppressive cells and their functions in the TME of HNSCC
| Immunosuppressive cells | Substance that causes its aggregation or production | Function and mechanism |
|---|---|---|
| MDSCs | VEGF, IL-6, GM-CSF | induction of T cell tolerance; inhibition of activated T cells; downregulation of anti-tumor immunity through innate immune regulation |
| Treg cells | CCR4-CCL17/22, CCR8-CCL1, CCR10-CCL28, and CXCR3-CCL10 | destruction of APCs by expressing IL-2, TGF-β, and IL-10; inhibition of effector T cells through the MHC class II by ligand LAG-3; inhibition of T cells by regulating IDO in DCs; inhibition of DCs through CTLA-4, impairs APC maturation |
| TAMs | (1) CXCL9, CXCL10, TNF-α, IL-1, IL-6, and IL-12 can induce the M1 phenotype; (2) IL-4, IL-10, and IL-13 can induce the M2 phenotype | M1 macrophages kill microorganisms and tumor cells by ROS; M2 macrophages initiate anti-tumor immunity through Th2; M2 macrophages lose the ability to present antigen; activated M2 macrophages downregulate M1 by secreting anti-inflammatory cytokines; M2 macrophages downregulate anti-tumor immunity by expressing IL-1β, IL-10, MMPs, and TGF-β |
| CAFs | fibroblasts are transformed into CAFs through TGF-β and IL-1β signaling pathways | inhibition of T cell proliferation through VEGF and TGF-β; induction of immune suppression by inducing Treg cells; combine with tumor cells to establish an immunosuppressive network; help tumor cells escape the body’s immune killing effect by gathering M2 macrophages and MDSCs; regulation of the microenvironment by activating ECM remodeling and degradation |
GM-CSF, granulocyte-macrophage colony-stimulating factor.
Anti-tumor cytokines
Type I interferons (IFNs) induce expression of MHC class I molecules in tumor cells, promote DC maturation, and increase anti-tumor immunity.75 Alternatively, IL-12 and IL-18 stimulate Th1 immune response to initiate anti-tumor immunity.74 In addition, CXCL9, CXCL10, TNF-α, IL-1, IL-6, and IL-12 can induce the M1 phenotype of TAMs. Some cytokines have two sides in promoting tumor or anti-tumor factors, such as IL-6. Functionally, IL-6 makes DCs immature, thereby inhibiting activation of neutrophils, macrophages, NK cells, and T cells,76 and it is closely related to HNSCC prognosis.77 However, IL-6 can also induce production of M1 macrophages for an anti-tumor immune response. The function of cytokines in tumor immunotherapy has been extensively reviewed.75
“Messenger” in TME: exosomes
Communication among cancer cells, as well as between cancer and other cells in the TME, occurs through direct contact or indirectly via secretion of chemokines/cytokines. Alternatively, a special way in which extracellular vesicles act as messengers among cancer cells or between cancer and normal cells has also been described.78 One type of these vesicles, called exosomes and produced by CAFs, increases tumor cell growth and drug resistance.79 Cancer cells convert fibroblasts into CAFs by producing exosomes.80 Exosomes produced by HNSCC inhibit immune cell function by promoting CD8+ T cell apoptosis.81 Future research should explore the potential for restoring the body’s anti-tumor immunity via anti-exosomes.
Immune-related therapy of HNSCC
The existing therapies for HNSCC include surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy (small molecule inhibitors or antibodies), and combination therapy. Generally, either surgery or radiotherapy may be selected as the treatment of choice when HNSCC is diagnosed early. However, HNSCC patients may decline surgery, owing to the serious impacts on body functions (pronunciation, swallowing, among others) as well as tumor recurrence. The genetic heterogeneity of NHSCC presents a big challenge for specific targeted therapies. Consequently, the associated drug resistance and recurrence problems mean that the current HNSCC treatment strategies are far from enough. To solve this problem, there is a need to explore additional novel treatment strategies and identify potential treatment targets that can generate effective treatment options for HNSCC patients to improve outcomes and overall survival rates. To date, numerous studies have reviewed the use of different approaches to treat HNSCC, including the combination of surgery and immunotherapy,82 radioimmunotherapy,83 and a combination of chemotherapy and immunotherapy.84 In this review, we introduce classical immunotherapy and PD-1/PD-L1-related combination therapy, as well as molecules that regulate immunosuppressive cells.
Anti-PD-1/PD-L1 therapy
Under normal circumstances, the inhibitory receptor PD-1 is expressed on the surface of T cells and acts to protect normal cells in the body.85 Functionally, this receptor binds to its ligand, PD-L1, and transmits information to T cells via downstream signaling pathways, thereby inhibiting T cell activation and proliferation.85,86 However, tumor cells use this mechanism to escape immunity by expressing a large number of PD-L1 ligands on the surface. Currently, the US Food and Drug Administration (FDA) have approved two immunotherapeutic agents, pembrolizumab86,87 and nivolumab,88 for treatment of recurrent and metastatic HNSCC. In 2019, pembrolizumab was approved for treatment of unresectable recurrent/metastatic (R/M) HNSCC patients.89 However, PD-1/PD-L1 blocking alone showed low safety and efficiency in HNSCC,90,91 indicating that a combination with other treatments is needed (Table 2).
Table 2.
Clinical trials of PD-1/PD-L1-related therapy in HNSCC
| PD-1/PD-L1-related combination therapy | Status | Phase | NCT no.: ClinicalTrials.gov |
|---|---|---|---|
| PD-1 + surgery | recruiting | 2 | NCT03355560 |
| recruiting | 2 | NCT03565783 | |
| recruiting | 2 | NCT04126460 | |
| not yet recruiting | 1/2 | NCT04340258 | |
| PD-1 + chemotherapy (only phase 3 and phase 4) | not yet recruiting | 2/3 | NCT04129320 |
| active, not recruiting | 3 | NCT02358031 | |
| recruiting | 3 | NCT03855384 | |
| recruiting | 3 | NCT04428333 | |
| PD-1 + radiotherapy (only phase 3 and phase 4) | recruiting | 3 | NCT03765918 |
| active, not recruiting | 3 | NCT03040999 | |
| PD-1 + CTLA-4 | recruiting | 1/2 | NCT03019003 |
| recruiting | 2 | NCT 04080804 | |
| recruiting | 1 | NCT04140526 | |
| recruiting | 2 | NCT04326257 | |
| recruiting | 2/3 | NCT03755739 | |
| PD-L1 + MDSCs | not yet recruiting | 2 | NCT 04262388 |
| PD-L1 + Treg cells | recruiting | 1/2 | NCT 03844763 |
| not yet recruiting | 2 | NCT04262388 | |
| PD-L1 + TAMs | recruiting | 2 | NCT 02554812 |
For more information, refer to https://clinicaltrials.gov/. NCT, National Clinical Trial.
CTLA-4 inhibitors
Under normal circumstances, CTLA-4 plays a crucial role in maintaining a normal immune balance.92 Tumor cells take advantage of CTLA-4’s negative immune regulation, and they generate signals that inhibit T cell activation through CTLA-4.93,94 Overall, CTLA-4 inhibitors represent an important immune checkpoint inhibitor. In the TME of HNSCC, Treg cells have been shown to suppress anti-tumor immunity by regulating CTLA-4 expression on the cell surface.95 Therefore, CTLA-4 inhibitors can effectively reverse Treg cell-induced suppressive immunity. Some clinical trials evaluating CTLA-4 blockers in HNSCC are listed in Table 3.
Table 3.
Clinical trials evaluating CTLA-4 inhibitors for HNSCC treatment
| NCT no.: ClinicalTrials.gov | Status | Intervention/treatment | Phase |
|---|---|---|---|
| NCT02812524 | recruiting | Ipilimumab | 1 |
| NCT02919683 | active, not recruiting | Nivolumab | 2 |
| Ipilimumab | |||
| NCT02741570 | active, not recruiting | nivolumab, ipilimumab | 3 |
| cetuximab/Erbitux, cisplatin, carboplatin, fluorouracil | |||
| NCT02823574 | active, not recruiting | Nivolumab | 2 |
| Ipilimumab | |||
| NCT04080804 | recruiting | nivolumab, relatlimab, ipilimumab | 2 |
| NCT03690986 | recruiting | VX15/2503, ipilimumab, nivolumab | 1 |
| NCT03700905 | recruiting | surgery + radiotherapy and chemotherapy | 3 |
| neoadjuvant nivolumab, adjuvant nivolumab, ipilimumab | |||
| NCT03162731 | active, not recruiting | nivolumab, ipilimumab | 1 |
| NCT01935921 | active, not recruiting | cetuximab, ipilimumab + radiotherapy | 1 |
| NCT03003637 | recruiting | nivolumab | 2 |
| ipilimumab | |||
| NCT03098160 | recruiting | Evofosfamide | 1 |
| ipilimumab | |||
| NCT04290546 | recruiting | IL-15 superagonist (N-803) | 1 |
| CIML NK cell infusion | |||
| Ipilimumab | |||
| NCT04326257 | recruiting | nivolumab + relatlimab | 2 |
| nivolumab + ipilimumab | |||
| NCT03620123 | recruiting | nivolumab and ipilimumab | 2 |
| docetaxel | |||
| NCT03058289 | recruiting | anti-CTLA-4 antibody | 1 |
| 2 |
For more information, refer to https://clinicaltrials.gov/.
Anti-EGFR
EGFR promotes cell proliferation, anti-apoptosis, and angiogenesis by activating downstream pathways such as phosphatidylinositol 3-kinase (PI3K)-AKT. Previous studies have reported a high rate of EGFR expression (90%) in HNSCC,96 which is inversely proportional to patient survival rates.97,98 Currently, the FDA has approved use of cetuximab, a monoclonal antibody with a specific molecular target, for HNSCC treatment. However, this drug’s efficacy in treating HNSCC is only 13%.99
Potential therapeutic molecules targeting immunosuppressive cells
Molecules that regulate MDSCs
Liu et al.100 revealed that JAK2/STAT3 suppression could reduce angiogenesis in tumor cells and reduce MDSCs in the HNSCC mouse model. In HNSCC, Younis et al.101 found that knockdown of SEMA4D disables MDSCs, while Anderson et al.102 found that STAT4 is a vital bridge for HNSCC tumor metastasis. In fact, its mechanism is related to T cell immunosuppression, enhanced MDSC activity, precancerous inflammation, and decreased cytotoxic antitumor lymphocyte activity. Moreover, Fugle et al.103 demonstrated that low expression of CD24 in oral cancer was associated with poor prognosis. Specifically, their animal models showed that CD24 negatively regulates the number and functional characteristics of MDSCs and protects mice from oral cancer.
Molecules that regulate Treg cells
Wu et al.104 found that anti-TIGIT treatment significantly delays growth of tumors in transgenic HNSCC mice, and it inhibits tumor growth by activating CD8+ T cell effector functions and reducing the number of Treg cells. Mao et al.105 demonstrated that targeting the Notch1 signal reduces the number of MDSCs and Treg cells, thereby downregulating inhibitory immune checkpoint molecules, such as PD-1 and CTLA-4. Results from survival analysis in their work revealed overexpression of CD47 in HNSCC. In addition, CD47 upregulates expression of inhibitory markers PD-1 and PD-L1, thereby accumulating Treg cells and MDSCs. Blocking CD47 was found to effectively stimulate cytotoxic T cells, reduce immunosuppressive cells, and improve the immunosuppressive environment.106 Apart from these, Ma et al.107 found that A2AR is positively correlated with HIF-1α, CD73, and Foxp3. Blocking A2AR resulted in significant reduction in the number of CD4+Foxp3+ Treg cells and upregulated the killing of CD8+ T cells in tumor cells. Alternatively, Liu et al.108 showed that blocking TIM3 in HNSCC significantly enhanced the anti-tumor immune response by alleviating tumor cell toxicity and reducing the number of Treg cells (Table 4). All of these molecules are constrained by various factors, including the need to identify target molecules that must only be expressed in tumor cells and marked side effects associated with the treatment. Consequently, there is a need for comprehensive studies to validate these targets for effective clinical conversion.
Table 4.
Potential therapeutic molecules regulating immunosuppressive cells in TME of HNSCC
| Potential target and mechanism | Effect | Reference |
|---|---|---|
| Inhibition of JAK2/STAT3 | reducing tumor-induced angiogenesis and MDSCs | 100 |
| Blocking activation of NLRP3 | significant decrease in production of IL-1β and MDSCs | 109 |
| Blocking COX-2 | reduces induction and function of MDSCs and inhibits tumor growth | 110 |
| Downregulation of Sema4D | reduces tumor-promoting cytokines produced by MDSCs | 101 |
| Activating the STAT4 pathway | decreases T cell immunosuppression and MDSC activity | 102 |
| Upregulation of CD24 | decreases the number and function of MDSCs | 103 |
| Anti-TIGIT treatment | enhances anti-tumor immune response by activating the effector function of CD8+ T cells and reducing the number of Treg cells | 104 |
| Targeting Notch1 | reduces the number of MDSCs and Treg cells, as well as expression of inhibitory immune checkpoint molecules, such as PD-1 and CTLA-4 | 105 |
| Anti-CD47 treatment | stimulates cytotoxic T cells and reduces MDSCs | 106 |
| Blocking A2AR | reduces the number of CD4+Foxp3+ Treg cells and enhances the anti-tumor response of CD8+ T cells | 107 |
| Blocking TIM3 | enhances anti-tumor immune response by reducing tumor cytotoxicity | 108 |
| Blocking CD73 | downregulates total expression of PD-1 and CTLA-4 on T cells | 111 |
Conclusions and perspectives
Previous studies have focused on changes in gene expression, as well as abnormal genetic and epigenetic mutations in tumor cells. In recent years, numerous studies have focused on the tumor-promoting functions of cellular components in the HNSCC tumor microenvironment, and these are expected to better our understanding of the immunosuppressive mechanism underlying tumor recurrence and drug resistance. Overall, these studies are expected to reveal novel targets to guide future treatment options.
In this review, we mainly described the role of anti-tumor and pro-tumor immune cells as well as extracellular components in the TME of HNSCC. We also outlined classical HNSCC immunotherapies, with focus on clinical trials using CTLA-4 inhibitors and a PD-1/PD-L1-related combination. Finally, we listed some molecules that target immunosuppressive cells. In addition, we reviewed literature on the FDA’s approved anti-EGFR therapy (cetuximab) and anti-PD-1 therapy (pembrolizumab and nivolumab) for immunotherapeutic management of HNSCC, and further listed some potential treatments that are not yet approved by the FDA but are under clinical trials. Results from these clinical trials and potential therapeutic targets are expected to guide development of effective therapies to improve overall survival rates of R/M HNSCC patients.
Radiation therapy (also known as radiotherapy) is one of the primary treatment methods for patients with HNSCC, particularly for nasopharyngeal carcinoma.90 Recent studies revealed that radiotherapy plays an important role in the TME and a tumor’s response to immunotherapy. Increasing evidence has implicated TME as an essential mediator of radiation responses both locally and systemically, and radiotherapy functions as an immunomodulatory tool that facilitates recruitment and activation of the immune system to fight tumors.112 The main mechanisms of radiotherapy’s effect on antitumoral immunity are: (1) increasing the release of tumor antigens and their availability for APCs;113,114 (2) enhancing activation of T cells and destroying the immune inhibitory TME;115,116 (3) promoting T cell homing into the tumor bed through modulating chemokine expression levels, macrophage polarization, and expression of adhesion molecules on tumor vasculature;117,118 and (4) regulating the expression levels of immune checkpoint molecules on the surfaces of both cancer cells and immune cells.119,120 Further work is required to understand the effects and exact mechanisms of radiotherapy on the TME, immune cells, and immune response in HNSCC. Moreover, it is necessary to optimize combinations and timing of radiotherapy with immunotherapy for HNSCC. We think that the combination of immunotherapy with radiotherapy is a promising strategy for the treatment of HNSCC in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 81872210 and 81802948); The Excellent Talent Science and Technology Innovation Project of Shanxi Province (nos. 201605D211029, 201705D211018, and 201805D211007); the Shanxi Province Scientific and Technological Achievements Transformation Guidance Foundation (no. 201804D131043); the Shanxi Province Science Foundation for Excellent Young Scholars (no. 201901D211486); the Youth Foundation of The First Hospital Affiliated with Shanxi Medical University (no. YQ1503); and by the Fund of Shanxi “1331” Project.
Author contributions
B.W., Y.W., and W.G. designed the review and contributed to manuscript preparation. Y.Q. and X.Z. wrote the manuscript. Y.W. and W.G. performed technical and administrative support. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Wei Gao, Email: gaoweisxent@sxent.org.
Binquan Wang, Email: wbq_xy@sxent.org.
Yongyan Wu, Email: wuyongyan@sxent.org.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Gao W., Zhang Y., Luo H., Niu M., Zheng X., Hu W., Cui J., Xue X., Bo Y., Dai F. Targeting SKA3 suppresses the proliferation and chemoresistance of laryngeal squamous cell carcinoma via impairing PLK1-AKT axis-mediated glycolysis. Cell Death Dis. 2020;11:919. doi: 10.1038/s41419-020-03104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y., Zhang Y., Zheng X., Dai F., Lu Y., Dai L., Niu M., Guo H., Li W., Xue X. Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol. Cancer. 2020;19:99. doi: 10.1186/s12943-020-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Canning M., Guo G., Yu M., Myint C., Groves M.W., Byrd J.K., Cui Y. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front. Cell Dev. Biol. 2019;7:52. doi: 10.3389/fcell.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meissner M., Reichert T.E., Kunkel M., Gooding W., Whiteside T.L., Ferrone S., Seliger B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin. Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 7.Curry J.M., Sprandio J., Cognetti D., Luginbuhl A., Bar-ad V., Pribitkin E., Tuluc M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014;41:217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Horton J.D., Knochelmann H.M., Day T.A., Paulos C.M., Neskey D.M. Immune evasion by head and neck cancer: foundations for combination therapy. Trends Cancer. 2019;5:208–232. doi: 10.1016/j.trecan.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forster M.D., Devlin M.J. Immune checkpoint inhibition in head and neck cancer. Front. Oncol. 2018;8:310. doi: 10.3389/fonc.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markwell S.M., Weed S.A. Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion. Cancers (Basel) 2015;7:382–406. doi: 10.3390/cancers7010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santuray R.T., Johnson D.E., Grandis J.R. New therapies in head and neck cancer. Trends Cancer. 2018;4:385–396. doi: 10.1016/j.trecan.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draghiciu O., Lubbers J., Nijman H.W., Daemen T. Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. OncoImmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S., Cheng X., Zhang L., Lu X., Chaudhary S., Teng R., Frederickson C., Champion M.M., Zhao R., Cheng L. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA. 2018;115:10094–10099. doi: 10.1073/pnas.1800695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinshaw D.C., Shevde L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner M.G., Liebertz D.J., Epstein A.L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang B., Pan P.Y., Li Q., Sato A.I., Levy D.E., Bromberg J., Divino C.M., Chen S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 18.Peltanova B., Raudenska M., Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol. Cancer. 2019;18:63. doi: 10.1186/s12943-019-0983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H.C., Chan L.P., Cho S.F. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front. Oncol. 2019;9:1084. doi: 10.3389/fonc.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T., Nishikawa H., Wada H., Nagano Y., Sugiyama D., Atarashi K., Maeda Y., Hamaguchi M., Ohkura N., Sato E. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi R., Hori S., Takahashi T., Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camisaschi C., Casati C., Rini F., Perego M., De Filippo A., Triebel F., Parmiani G., Belli F., Rivoltini L., Castelli C. LAG-3 expression defines a subset of CD4+CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. J. Immunol. 2010;184:6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- 24.Acovic A., Simovic Markovic B., Gazdic M., Arsenijevic A., Jovicic N., Gajovic N., Jovanovic M., Zdravkovic N., Kanjevac T., Harrell C.R. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap. Adv. Gastroenterol. 2018;11 doi: 10.1177/1756284818793558. 1756284818793558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., Baker J., Jeffery L.E., Kaur S., Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorta-Estremera S., Hegde V.L., Slay R.B., Sun R., Yanamandra A.V., Nicholas C., Nookala S., Sierra G., Curran M.A., Sastry K.J. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer. J. Immunother. Cancer. 2019;7:252. doi: 10.1186/s40425-019-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J., Bae J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quail D.F., Joyce J.A. Molecular pathways: deciphering mechanisms of resistance to macrophage-targeted therapies. Clin. Cancer Res. 2017;23:876–884. doi: 10.1158/1078-0432.CCR-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genard G., Lucas S., Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front. Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quatromoni J.G., Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 32.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 34.Galdiero M.R., Marone G., Mantovani A. Cancer inflammation and cytokines. Cold Spring Harb. Perspect. Biol. 2018;10:a028662. doi: 10.1101/cshperspect.a028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H., Liu X., Zhao F., Lu J., Zhang B., Peng X., Zhang M., Chen X., Li G., Li X. M2-polarized tumour-associated macrophages in stroma correlate with poor prognosis and Epstein-Barr viral infection in nasopharyngeal carcinoma. Acta Otolaryngol. 2017;137:888–894. doi: 10.1080/00016489.2017.1296585. [DOI] [PubMed] [Google Scholar]
- 37.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi H., Sakakura K., Kawabata-Iwakawa R., Rokudai S., Toyoda M., Nishiyama M., Chikamatsu K. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2015;64:1407–1417. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 40.Lewis M.P., Lygoe K.A., Nystrom M.L., Anderson W.P., Speight P.M., Marshall J.F., Thomas G.J. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br. J. Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudás J., Fullár A., Bitsche M., Schartinger V., Kovalszky I., Sprinzl G.M., Riechelmann H. Tumor-produced, active interleukin-1β regulates gene expression in carcinoma-associated fibroblasts. Exp. Cell Res. 2011;317:2222–2229. doi: 10.1016/j.yexcr.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler S.E., Shi H., Lin F., Dasari S., Bednash J., Thorne S., Watkins S., Joshi R., Thomas S.M. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head Neck. 2014;36:385–392. doi: 10.1002/hed.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W., Dang Y., Chu Y., Fan J., He R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 44.Flavell R.A., Sanjabi S., Wrzesinski S.H., Licona-Limón P. The polarization of immune cells in the tumour environment by TGFβ. Nat. Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glentis A., Oertle P., Mariani P., Chikina A., El Marjou F., Attieh Y., Zaccarini F., Lae M., Loew D., Dingli F. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017;8:924. doi: 10.1038/s41467-017-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaggioli C., Hooper S., Hidalgo-Carcedo C., Grosse R., Marshall J.F., Harrington K., Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 47.Terrén I., Orrantia A., Vitallé J., Zenarruzabeitia O., Borrego F. NK cell metabolism and tumor microenvironment. Front. Immunol. 2019;10:2278. doi: 10.3389/fimmu.2019.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 49.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Correa B., Lopez-Sejas N., Duran E., Labella F., Alonso C., Solana R., Tarazona R. Modulation of NK cells with checkpoint inhibitors in the context of cancer immunotherapy. Cancer Immunol. Immunother. 2019;68:861–870. doi: 10.1007/s00262-019-02336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Shmuel A., Biber G., Barda-Saad M. Unleashing natural killer cells in the tumor microenvironment—the next generation of immunotherapy? Front. Immunol. 2020;11:275. doi: 10.3389/fimmu.2020.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 53.Laidlaw B.J., Craft J.E., Kaech S.M. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat. Rev. Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veglia F., Gabrilovich D.I. Dendritic cells in cancer: the role revisited. Curr. Opin. Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang M., Diao J., Cattral M.S. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell. Mol. Life Sci. 2017;74:761–776. doi: 10.1007/s00018-016-2317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vonderheide R.H. The immune revolution: a case for priming, not checkpoint. Cancer Cell. 2018;33:563–569. doi: 10.1016/j.ccell.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurachi M. CD8+ T cell exhaustion. Semin. Immunopathol. 2019;41:327–337. doi: 10.1007/s00281-019-00744-5. [DOI] [PubMed] [Google Scholar]
- 58.Kaech S.M., Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma X., Bi E., Lu Y., Su P., Huang C., Liu L., Wang Q., Yang M., Kalady M.F., Qian J. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuller M.J., Khanolkar A., Tebo A.E., Zajac A.J. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 61.Moskophidis D., Lechner F., Pircher H., Zinkernagel R.M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 62.Wherry E.J., Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saeidi A., Zandi K., Cheok Y.Y., Saeidi H., Wong W.F., Lee C.Y.Q., Cheong H.C., Yong Y.K., Larsson M., Shankar E.M. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front. Immunol. 2018;9:2569. doi: 10.3389/fimmu.2018.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaudhuri O., Koshy S.T., Branco da Cunha C., Shin J.W., Verbeke C.S., Allison K.H., Mooney D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 65.Najafi M., Farhood B., Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019;120:2782–2790. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 66.Erdogan B., Webb D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sangaletti S., Chiodoni C., Tripodo C., Colombo M.P. Common extracellular matrix regulation of myeloid cell activity in the bone marrow and tumor microenvironments. Cancer Immunol. Immunother. 2017;66:1059–1067. doi: 10.1007/s00262-017-2014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paz H., Pathak N., Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2014;33:4193–4202. doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branch K.M., Hoshino D., Weaver A.M. Adhesion rings surround invadopodia and promote maturation. Biol. Open. 2012;1:711–722. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Jain R.K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H.M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 73.Mazorra Z., Lavastida A., Concha-Benavente F., Valdés A., Srivastava R.M., García-Bates T.M., Hechavarría E., González Z., González A., Lugiollo M. Nimotuzumab induces NK cell activation, cytotoxicity, dendritic cell maturation and expansion of EGFR-specific T cells in head and neck cancer patients. Front. Pharmacol. 2017;8:382. doi: 10.3389/fphar.2017.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jebreel A., Mistry D., Loke D., Dunn G., Hough V., Oliver K., Stafford N., Greenman J. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J. Laryngol. Otol. 2007;121:246–252. doi: 10.1017/S0022215106002428. [DOI] [PubMed] [Google Scholar]
- 75.Waldmann T.A. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 2018;10:a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong J.L., Mailliard R.B., Moschos S.J., Edington H., Lotze M.T., Kirkwood J.M., Kalinski P. Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells. J. Immunother. 2011;34:270–278. doi: 10.1097/CJI.0b013e31820b370b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao J., Zhao S., Halstensen T.S. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol. Rep. 2016;35:3265–3274. doi: 10.3892/or.2016.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paggetti J., Haderk F., Seiffert M., Janji B., Distler U., Ammerlaan W., Kim Y.J., Adam J., Lichter P., Solary E. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126:1106–1117. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ludwig S., Floros T., Theodoraki M.N., Hong C.S., Jackson E.K., Lang S., Whiteside T.L. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017;23:4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bryan R.B., Gough M.J., Seung S.K., Jutric Z., Weinberg A.D., Fox B.A., Crittenden M.R., Leidner R.S., Curti B. Cytoreductive surgery for head and neck squamous cell carcinoma in the new age of immunotherapy. Oral Oncol. 2016;61:166–176. doi: 10.1016/j.oraloncology.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 83.Karam S.D., Raben D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019;20:e404–e416. doi: 10.1016/S1470-2045(19)30306-7. [DOI] [PubMed] [Google Scholar]
- 84.Guidi A., Codecà C., Ferrari D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: a systematic review. Med. Oncol. 2018;35:37. doi: 10.1007/s12032-018-1096-5. [DOI] [PubMed] [Google Scholar]
- 85.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 86.Yang B., Liu T., Qu Y., Liu H., Zheng S.G., Cheng B., Sun J. Progresses and perspectives of anti-PD-1/PD-L1 antibody therapy in head and neck cancers. Front. Oncol. 2018;8:563. doi: 10.3389/fonc.2018.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 88.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.T., Lee N.Y., Leidner R., Lewis R.L. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother. Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saleh K., Eid R., Haddad F.G., Khalife-Saleh N., Kourie H.R. New developments in the management of head and neck cancer—impact of pembrolizumab. Ther. Clin. Risk Manag. 2018;14:295–303. doi: 10.2147/TCRM.S125059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H., Mustafa A., Liu S., Liu J., Lv D., Yang H., Zou J. Immune checkpoint inhibitor toxicity in head and neck cancer: from identification to management. Front. Pharmacol. 2019;10:1254. doi: 10.3389/fphar.2019.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krummel M.F., Allison J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matoba T., Imai M., Ohkura N., Kawakita D., Ijichi K., Toyama T., Morita A., Murakami S., Sakaguchi S., Yamazaki S. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int. J. Cancer. 2019;144:2811–2822. doi: 10.1002/ijc.32024. [DOI] [PubMed] [Google Scholar]
- 96.Sim F., Leidner R., Bell R.B. Immunotherapy for head and neck cancer. Oral Maxillofac. Surg. Clin. North Am. 2019;31:85–100. doi: 10.1016/j.coms.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Bossi P., Resteghini C., Paielli N., Licitra L., Pilotti S., Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;7:74362–74379. doi: 10.18632/oncotarget.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalyankrishna S., Grandis J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 99.Vermorken J.B., Trigo J., Hitt R., Koralewski P., Diaz-Rubio E., Rolland F., Knecht R., Amellal N., Schueler A., Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 100.Liu J.F., Deng W.W., Chen L., Li Y.C., Wu L., Ma S.R., Zhang W.F., Bu L.L., Sun Z.J. Inhibition of JAK2/STAT3 reduces tumor-induced angiogenesis and myeloid-derived suppressor cells in head and neck cancer. Mol. Carcinog. 2018;57:429–439. doi: 10.1002/mc.22767. [DOI] [PubMed] [Google Scholar]
- 101.Younis R.H., Han K.L., Webb T.J. Human head and neck squamous cell carcinoma-associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J. Immunol. 2016;196:1419–1429. doi: 10.4049/jimmunol.1501293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson K., Ryan N., Volpedo G., Varikuti S., Satoskar A.R., Oghumu S. Immune suppression mediated by STAT4 deficiency promotes lymphatic metastasis in HNSCC. Front. Immunol. 2020;10:3095. doi: 10.3389/fimmu.2019.03095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fugle C.W., Zhang Y., Hong F., Sun S., Westwater C., Rachidi S., Yu H., Garret-Mayer E., Kirkwood K., Liu B., Li Z. CD24 blunts oral squamous cancer development and dampens the functional expansion of myeloid-derived suppressor cells. OncoImmunology. 2016;5:e1226719. doi: 10.1080/2162402X.2016.1226719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L., Mao L., Liu J.F., Chen L., Yu G.T., Yang L.L., Wu H., Bu L.L., Kulkarni A.B., Zhang W.F., Sun Z.J. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol. Res. 2019;7:1700–1713. doi: 10.1158/2326-6066.CIR-18-0725. [DOI] [PubMed] [Google Scholar]
- 105.Mao L., Zhao Z.L., Yu G.T., Wu L., Deng W.W., Li Y.C., Liu J.F., Bu L.L., Liu B., Kulkarni A.B. γ-Secretase inhibitor reduces immunosuppressive cells and enhances tumour immunity in head and neck squamous cell carcinoma. Int. J. Cancer. 2018;142:999–1009. doi: 10.1002/ijc.31115. [DOI] [PubMed] [Google Scholar]
- 106.Wu L., Yu G.T., Deng W.W., Mao L., Yang L.L., Ma S.R., Bu L.L., Kulkarni A.B., Zhang W.F., Zhang L., Sun Z.J. Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. OncoImmunology. 2018;7:e1397248. doi: 10.1080/2162402X.2017.1397248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma S.R., Deng W.W., Liu J.F., Mao L., Yu G.T., Bu L.L., Kulkarni A.B., Zhang W.F., Sun Z.J. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol. Cancer. 2017;16:99. doi: 10.1186/s12943-017-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J.F., Wu L., Yang L.L., Deng W.W., Mao L., Wu H., Zhang W.F., Sun Z.J. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 2018;37:44. doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L., Huang C.F., Li Y.C., Deng W.W., Mao L., Wu L., Zhang W.F., Zhang L., Sun Z.J. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 2018;75:2045–2058. doi: 10.1007/s00018-017-2720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen W.C., Lai C.H., Chuang H.C., Lin P.Y., Chen M.F. Inflammation-induced myeloid-derived suppressor cells associated with squamous cell carcinoma of the head and neck. Head Neck. 2017;39:347–355. doi: 10.1002/hed.24595. [DOI] [PubMed] [Google Scholar]
- 111.Deng W.W., Li Y.C., Ma S.R., Mao L., Yu G.T., Bu L.L., Kulkarni A.B., Zhang W.F., Sun Z.J. Specific blockade CD73 alters the “exhausted” phenotype of T cells in head and neck squamous cell carcinoma. Int. J. Cancer. 2018;143:1494–1504. doi: 10.1002/ijc.31534. [DOI] [PubMed] [Google Scholar]
- 112.Menon H., Ramapriyan R., Cushman T.R., Verma V., Kim H.H., Schoenhals J.E., Atalar C., Selek U., Chun S.G., Chang J.Y. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front. Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., Camphausen K., Luiten R.M., de Ru A.H., Neijssen J. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Q., Allouch A., Martins I., Brenner C., Modjtahedi N., Deutsch E., Perfettini J.L. Modulating both tumor cell death and innate immunity is essential for improving radiation therapy effectiveness. Front. Immunol. 2017;8:613. doi: 10.3389/fimmu.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Filatenkov A., Baker J., Mueller A.M., Kenkel J., Ahn G.O., Dutt S., Zhang N., Kohrt H., Jensen K., Dejbakhsh-Jones S. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kachikwu E.L., Iwamoto K.S., Liao Y.P., DeMarco J.J., Agazaryan N., Economou J.S., McBride W.H., Schaue D. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R.H., Timke C., Umansky L. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 118.Matsumura S., Wang B., Kawashima N., Braunstein S., Badura M., Cameron T.O., Babb J.S., Schneider R.J., Formenti S.C., Dustin M.L., Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sato H., Niimi A., Yasuhara T., Permata T.B.M., Hagiwara Y., Isono M., Nuryadi E., Sekine R., Oike T., Kakoti S. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]