Abstract
Background & Aims
Plasma bile acids (BAs) have been extensively studied as pathophysiological actors in non-alcoholic steatohepatitis (NASH). However, results from clinical studies are often complicated by the association of NASH with type 2 diabetes (T2D), obesity, and insulin resistance (IR). Here, we sought to dissect the relationship between NASH, T2D, and plasma BA levels in a large patient cohort.
Methods
Four groups of patients from the Biological Atlas of Severe Obesity (ABOS) cohort (Clinical Trials number NCT01129297) were included based on the presence or absence of histologically evaluated NASH with or without coincident T2D. Patients were matched for BMI, homeostatic model assessment 2 (HOMA2)-assessed IR, glycated haemoglobin, age, and gender. To study the effect of IR and BMI on the association of plasma BA and NASH, patients from the HEPADIP study were included. In both cohorts, fasting plasma BA concentrations were measured.
Results
Plasma BA concentrations were higher in NASH compared with No-NASH patients both in T2D and NoT2D patients from the ABOS cohort. As we previously reported that plasma BA levels were unaltered in NASH patients of the HEPADIP cohort, we assessed the impact of BMI and IR on the association of NASH and BA on the combined BA datasets. Our results revealed that NASH-associated increases in plasma total cholic acid (CA) concentrations depend on the degree of HOMA2-assessed systemic IR, but not on β-cell function nor on BMI.
Conclusions
Plasma BA concentrations are elevated only in those NASH patients exhibiting pronounced IR.
Lay summary
Non-alcoholic steatohepatitis (NASH) is a progressive liver disease that frequently occurs in patients with obesity and type 2 diabetes. Reliable markers for the diagnosis of NASH are needed. Plasma bile acids have been proposed as NASH biomarkers. Herein, we found that plasma bile acids are only elevated in patients with NASH when significant insulin resistance is present, limiting their utility as NASH markers.
Keywords: NASH, NAFLD, Bile acids, Diabetes, Insulin resistance, Obesity, Translational study
Abbreviations: ABOS, Biological Atlas of Severe Obesity; ADA, American Diabetes Association; BA, bile acids; C4, 7alpha-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; FPG, fasting plasma glycaemia; FXR, farnesoid-X-receptor; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GHCA, glycohyocholic acid; GHDCA, glycohyodeoxycholic acid; GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid; HbA1c, glycated haemoglobin; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; HOMA2, homeostatic model assessment 2; IR, insulin resistance; LCA, lithocholic acid; MAFLD, metabolic associated fatty liver disease; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; OGTT, oral glucose tolerance test; T2D, type 2 diabetes; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; THCA, taurohyocholic acid; THDCA, taurohyodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid
Graphical abstract
Highlights
-
•
Bile acids have been studied as pathophysiological actors and biomarkers in NASH.
-
•
Plasma BAs have been reported to be higher in NASH vs. No-NASH patients.
-
•
Plasma BAs are altered in patients with T2D, IR, and obesity, risk factors for NASH.
-
•
Thus, the independent association between plasma BA increases and NASH is unclear.
-
•
NASH-associated increases in plasma BA depend on the degree of insulin sensitivity.
Introduction
Non-alcoholic fatty liver disease (NAFLD) covers a spectrum of hepatic disorders ranging from isolated steatosis (non-alcoholic fatty liver [NAFL]) to non-alcoholic steatohepatitis (NASH).1 Although steatosis is generally considered benign, NASH, which combines steatosis with inflammation and hepatocyte damage (evidenced histologically by ballooning), is a risk factor for progressive fibrosis and, ultimately cirrhosis and hepatocellular carcinoma, as well as for cardiovascular disease, type 2 diabetes (T2D) and other extra-hepatic consequences.2 Given the importance of metabolic alterations in this disease, metabolic associated fatty liver disease (MAFLD) has recently been proposed as a more accurate denomination of fatty liver disease associated with metabolic dysfunction.3 The pathogenesis of NAFLD remains poorly understood, and there is currently an urgent need to identify therapeutic targets.
Bile acids (BAs) are amphipathic molecules that facilitate absorption of dietary fat and lipophilic vitamins in the small intestine. Recently, BAs have attracted growing interest as they are also signalling molecules that control energy expenditure and glucose and lipid metabolism via receptors such as the farnesoid-X-receptor (FXR, NR1H4) and TGR5 (GPBAR1).4,5 BAs are synthesised in the liver as primary BAs (cholic acid [CA], chenodeoxycholic acid [CDCA], hyocholic acid [HCA]), secreted in the bile and the intestine where they are transformed by the gut flora into secondary BAs (deoxycholic acid [DCA], lithocholic acid [LCA], hyodeoxycholic acid [HDCA], ursodeoxycholic acid [UDCA]). Each BA species can be measured as free or conjugated to taurine or glycine. In humans, more than 90% of BAs are reabsorbed in the intestine and returned to the liver via the portal vein in an enterohepatic cycle. Although it is still unclear whether alterations in BA metabolism drive NASH development, BA-activated signalling pathways remain attractive therapeutic targets for its treatment. For example, treating NASH patients with the FXR agonist obeticholic acid improved several key histological features of NAFLD.[6], [7], [8] Thus, BA may play a role in NASH pathophysiology.
Several studies have assessed whether NAFLD patients display changes in plasma BA profiles (for review, see Chávez-Talavera et al.9). Globally, plasma BA concentrations are higher in NASH vs. no-NASH patients, with qualitative differences varying among studies, i.e. increased primary BA10 or conjugated BA.11,12 However, increased plasma BA concentrations are also observed in patients with T2D, insulin resistance (IR), and obesity,9,[13], [14], [15] all major risk factors for NASH.16 Thus, the intricate relationship between NAFLD, obesity, IR, and T2D complicates the establishment of clear and independent associations between plasma BA alterations and these individual clinical features. Indeed, in our previous study, Legry et al.17 reported that plasma BA concentrations positively correlated with glucose homeostasis parameters (homeostatic model assessment [HOMA]-IR, fasting plasma glucose, 2-h oral glucose tolerance test [OGTT] - glucose), but were not affected by the presence of histologically assessed NASH.
In the present study, we aimed to investigate the association of plasma BA changes and NASH depending on T2D status. To do this, we analysed fasting plasma BA profiles in patients with histologically assessed NASH with or without coincident T2D, selected from the Biological Atlas of Severe Obesity (ABOS) cohort,18 enlarged with previously studied patients from the HEPADIP cohort17 for part of the analyses. Groups of NASH and No-NASH patients were carefully matched to control for obesity and IR as potential confounding factors.
Patients and methods
Description of the patients
ABOS study
Patients were selected from the prospective ABOS cohort, which included patients referred to the Lille University Hospital bariatric surgery unit for evaluation. All patients fulfilling the subsequent criteria for bariatric surgery were prospectively included in the cohort before surgery. No specific dietary restrictions were imposed immediately preceding the surgery. Blood collection was performed the morning of the surgery after an overnight fast. Patients were to be 18 years or older at time of evaluation and meet the criteria for bariatric surgery according to French national guidelines: severe obesity with at least 1 comorbidity factor (i.e. arterial hypertension or diabetes mellitus) for at least 5 years and failure of a well-conducted medical treatment during 6–12 months, including lifestyle modification and appropriate drug treatment; absence of medical or psychological contraindications for bariatric surgery; social security insurance coverage; no current significant alcohol consumption (maximum average daily consumption of alcohol of 20 g/day for women and 30 g/day for men), and no past excessive drinking for a period longer than 2 years at any time in the past 20 years; absence of long-term consumption of hepatotoxic drugs; negative screening for chronic liver disease (including, but not limited to viral hepatitis and autoimmune liver diseases). Informed written consent was obtained from all patients and the study was conducted in conformity with the Helsinki Declaration. The Lille University Hospital ethics committee approved the cohort (NCT01129297).
HEPADIP study
Additionally, patients visiting the obesity clinic of the Antwerp University Hospital from the HEPADIP protocol were included as described in our previous study (see patient characteristics in Table 1 of Legry et al.17). Briefly, overweight (BMI between 25 and 30 kg/m2) or obese (BMI ≥30 kg/m2) patients were recruited between October 2006 and May 2014. When NAFLD was suspected based on abnormal blood biochemistry or ultrasound, patients were screened for the presence of NAFLD by liver biopsy upon additional informed consent.
Table 1.
Clinical, biological, and liver histological characteristics of non-diabetic patients (No-T2D) from the ABOS study cohort grouped according to NASH status.
| No-NASH (n = 85) | NASH (n = 17) | p value | |
|---|---|---|---|
| Sex (F/M) | 57/28 | 12/5 | 1 |
| Age (years) | 38.5 ± 12.3 | 38.2 ± 10.5 | 0.91 |
| BMI (kg/m2) | 46.1 ± 7.4 | 46.1 ± 6.3 | 0.99 |
| HOMA2S (%) | 42 ± 24 | 35 ± 16 | 0.27 |
| HOMA2B (%) | 164 ± 56 | 179 ± 48 | 0.26 |
| HbA1c (%) | 5.7 ± 0.3 | 5.6 ± 0.3 | 0.08 |
| Fasting plasma glucose (mmol/L) | 5.5 ± 0.5 | 5.5 ± 0.6 | 0.89 |
| Fasting plasma insulin (mUI/L) | 23.2 ± 10.7 | 27.1 ± 12.3 | 0.24 |
| Steatosis grade (0, 1, 2, 3) | 20/65/0/0 | 0/6/6/5 | <0.001 |
| Ballooning (0, 1, 2) | 85/0/0 | 0/15/2 | <0.001 |
| Lobular inflammation (0, 1, 2, 3) | 85/0/0/0 | 0/12/5/0 | <0.001 |
| Fibrosis stage (0, 1, 2, 3, 4) (n.a.) | 78/2/1/2/0 (2) | 7/4/2/4/0 | <0.001 |
Data are expressed as mean ± standard deviation or number of patients for categorical variables. p values are obtained using the χ2 test for qualitative data and the t test for quantitative data. Values of p <0.05 are considered statistically significant (bold). ABOS, Biological Atlas of Severe Obesity; HbA1c, glycated haemoglobin; HOMA, homeostatic model assessment; n.a., not available; NASH, non-alcoholic steatohepatitis.
Rationale of study group compositions
To study the relation between plasma BA concentrations and NASH according to diabetes status, patients were first classified into 2 clearly distinct groups (‘diabetes status’): non-type 2 diabetes (No-T2D) and T2D. From these two groups, patients were subsequently classified according to their liver histological characteristics in distinct groups of normal or nearly normal liver (No-NASH) (accepting the presence of up to grade 1 [mild] steatosis without any other lesion) or NASH patients.19 Among the No-T2D or T2D patients, No-NASH and NASH groups were propensity score matched, without predefined ranges, for the following parameters: BMI, HOMA2S, HOMA2B, glycated haemoglobin (HbA1c), and insulin/statin treatments (Fig. S1), yielding a study cohort of 219 patients divided into 4 groups depending on NASH and T2D diagnosis (No-T2D No-NASH, n = 85; No-T2D NASH, n = 17; T2D No-NASH, n = 59; and T2D NASH, n = 58). The relatively low proportion of patients with NASH without T2D in the ABOS cohort (only 17 patients) is in agreement with epidemiological observations.20 As expected from the study group selection and matching criteria, the NASH and No-NASH patients showed no significant differences for HOMA2S, HOMA2B, HbA1c, fasting plasma glucose, and insulin within the No-T2D (Table 1) nor the T2D (Table 2) groups.
Table 2.
Clinical, biological, and liver histological characteristics of diabetic patients (T2D) from the ABOS study cohort grouped according to NASH status.
| No-NASH (n = 59) | NASH (n = 58) | p value | |
|---|---|---|---|
| Sex (F/M) | 39/20 | 37/21 | 0.95 |
| Age (years) | 47.1 ± 9.7 | 49.3 ± 7.6 | 0.17 |
| BMI (kg/m2) | 47.0 ± 7.7 | 46.0 ± 8.1 | 0.85 |
| HOMA2S (%) | 57 ± 130 | 39 ± 45 | 0.52 |
| HOMA2B (%) | 105 ± 88 | 100 ± 76 | 0.62 |
| HbA1c (%) | 7.3 ± 1.9 | 8.1 ± 1.9 | 0.053 |
| Fasting plasma glucose (mmol/L) | 8.8 ± 3.4 | 9.3 ± 3.2 | 0.39 |
| Fasting plasma insulin (mUI/L) | 30.8 ± 28.1 | 37.1 ± 44.7 | 0.36 |
| Insulin treatment | 20 | 18 | 0.89 |
| Steatosis grade (0, 1, 2, 3) | 9/50/0/0 | 0/16/18/24 | <0.001 |
| Ballooning (0, 1, 2) | 59/0/0 | 0/43/15 | <0.001 |
| Lobular inflammation (0, 1, 2, 3) | 59/0/0/0 | 0/41/17/0 | <0.001 |
| Fibrosis stage (0, 1, 2, 3, 4) (n.a.) | 40/14/2/1/0 (2) | 8/13/7/23/1 (6) | <0.001 |
Data are expressed as mean ± standard deviation or number of patients for categorical variables. p values are obtained using the χ2 test for qualitative data and the ANOVA test for quantitative data. Values of p <0.05 are considered statistically significant (bold). ABOS, Biological Atlas of Severe Obesity; HbA1c, glycated haemoglobin; HOMA, homeostatic model assessment; n.a., not available; NASH, non-alcoholic steatohepatitis.
There was no significant difference in the proportion of patients taking insulin or anti-diabetic treatments (i.e. sulfonylurea, glinides, glucagon-like peptide 1 receptor agonists, and dipeptidylpeptidase-4 inhibitors) between the T2D No-NASH and T2D NASH patients. Only a slightly higher number of patients were treated with metformin in the NASH group (No-NASH: 47% vs. NASH: 70%, p = 0.02). Similarly, the proportion of patients on statin or fibrate treatment was also similar between T2D No-NASH and T2D NASH patients, as well as between the 2 No-T2D groups (data not shown).
To investigate the interaction between NASH and plasma BA according to IR levels, a patient group covering a large range of HOMA2 was studied by including the 58 patients with histological NASH assessment and accompanying clinical data (26 No-NASH; 32 NASH) from the HEPADIP study.17 As exogenous insulin therapy impacts on HOMA2 values, patients on insulin treatment (n = 38) were excluded, yielding a ‘combined cohort’ of 239 patients.
Biological assays
Fasting plasma glucose, insulin, and HbA1c were measured as previously described.18 The HOMA2 was used to estimate steady state β-cell function (HOMA2B) and insulin sensitivity (HOMA2S) from fasting plasma insulin and glucose using the online calculator.21 Results are expressed as percentages of normal values in a reference population.
Diabetes assessment
All participants were submitted to a standard OGTT (75 g). Diabetes was diagnosed according to American Diabetes Association (ADA) guidelines as defined by fasting glycaemia >1.26 g/L (7.0 mmol/L) or plasma HbA1c >6.5 % (48 mmol/mol) or 2 h glycaemia ≥2 g/L (11.1 mmol/L) during OGTT or use of an anti-diabetic drug.22
Histological assessment on liver biopsies
Liver biopsies were systematically planned during the surgical procedure in the ABOS cohort and analysed as previously described.23 A liver needle biopsy was performed during the first part of the surgical procedure after trocar insertion and abdominal exploration, within 10 min after pneumo-peritoneum installation. The MONOPTY needle biopsy system (16G, ref: 121620; C.R. Bard, Tempe, AZ, USA) was used. Biopsies were routinely stained with H&E, Saffron and Masson’s trichrome, Sirius Red, and Perl’s iron staining. Pathologists were blinded to clinical and biological data and independently graded steatosis (range 0–3), lobular inflammation (range 0–3), and ballooning (range 0–2) according to NASH Clinical Research Network criteria.24 Liver fibrosis was assessed using the Kleiner fibrosis score.24 In the present study, in order to compare 2 groups with clearly distinct liver phenotypes, 2 histological groups were defined: No-NASH was defined as steatosis <2, ballooning = 0 and lobular inflammation = 0, which hence means normal liver or minimal isolated steatosis without any other lesion; NASH was defined by the combined presence of steatosis and ballooning and lobular inflammation of any degree.19,25 Patients with moderate to severe steatosis (grade 2–3) and/or some ballooning or lobular inflammation meeting neither the No-NASH definition as described for our study nor the NASH definition, were excluded. Histological assessment and classification of the HEPADIP cohort was identical, as reported in our previous study, but in this cohort, the majority of the biopsies were taken outside the setting of bariatric surgery.17
Plasma BA and C4 quantification
Plasma concentrations of 21 BA species (Table S1) and 7alpha-hydroxy-4-cholesten-3-one (C4, an intermediate product of the classical BA synthesis pathway and marker of hepatic BA synthesis) were quantified as previously described.17 Briefly, after protein precipitation with iced methanol, BAs were quantified by HPLC (UFLC-XR device; Shimadzu, Kyoto, Japan) coupled to tandem mass spectrometry (MS/MS) (QTRAP 5500 hybrid system, equipped with a Turbo VTM ion source; Sciex, Foster City, CA, USA) using 5 deuterated BAs (D4-CA, D4-glycocholic acid [GCA], D4-taurocholic acid [TCA], D4-CDCA, D4-glycochenodeoxycholic acid [GCDCA]) as internal standards. After isolation using a SPE column, C4 was quantified by LC-MS/MS using a deuterated C4 as internal standard. Plasma BA and C4 concentrations were expressed in nmol/L. Ratio and total values were determined according to formulas presented in Table S1.
Statistical analysis
Values are expressed as mean ± SD, mean ± SEM, or median ± IQR as indicated in the figure legends. Statistical differences in clinical and biological parameters were assessed using the Mann-Whitney U or Student t test for continuous variables or χ2 test for categorical values. Values of p <0.05 were considered statistically significant. Random forest analyses were used to obtain a variable importance plot for discrimination of NASH status for each plasma BA species. A 2-way ANOVA and moderated multiple regression analysis model were used to test interaction between plasma BA, NASH, BMI, HOMA2B, and HOMA2S.
Results
Plasma BA concentrations are elevated both in T2D and No-T2D NASH patients
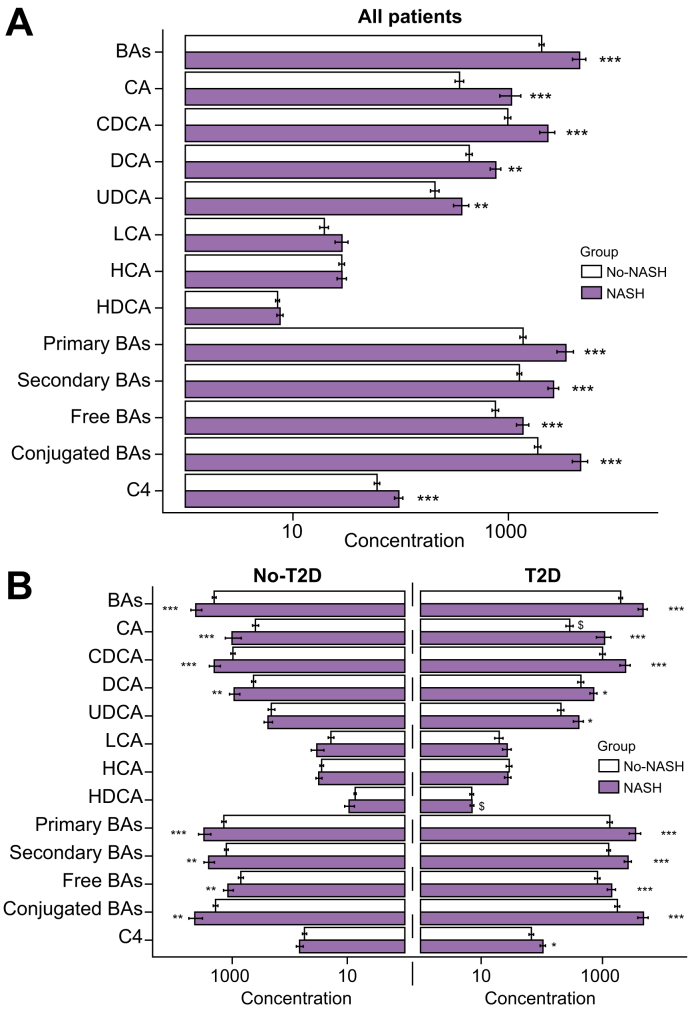
To investigate a potential association between plasma BA and NASH in patients with T2D, fasting plasma BA were measured in patients from the ABOS cohort, composed of both T2D and No-T2D patients with biopsy-assessed NAFLD. In the overall study cohort (n = 219), irrespective of diabetes status, plasma total BA concentrations were elevated in patients with NASH (NASH: 4589 ± 5640 nmol/L; n = 75; vs. No-NASH: 2035 ± 1299 nmol/L; n = 144; mean ± SD, p <0.0001) (Fig. 1A). Among the measured plasma BA species, only total LCA, total HCA, and total HDCA were not significantly increased in NASH patients.
Fig. 1.
Plasma BA concentrations are higher in NASH vs. No-NASH irrespective of T2D status in the ABOS study cohort (n = 219).
(A) Plasma total (free + conjugated) BA and C4 concentrations (log-scale, nmol/L) according to NASH status (grey, No-NASH; purple, NASH). (B) Plasma total (free + conjugated) BA and C4 concentrations (log-scale, nmol/L) according to T2D status (left, No-T2D; right, T2D) and NASH status (grey, No-NASH; purple, NASH). Data are expressed as mean and SEM (standard error of mean). p values from the non-parametric Mann-Whitney U test. To compare No-NASH and NASH groups: ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001. To compare No-T2D and T2D groups: $p <0.05. ABOS, Biological Atlas of Severe Obesity; BAs, bile acids; C4, 7alpha-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes; UDCA, ursodeoxycholic acid.
T2D NASH patients displayed 2-fold higher total plasma BA than T2D No-NASH patients (4671 ± 6100 nmol/L, n = 58; vs. 1996 ± 1078 nmol/L, n = 59; mean ± SD; p <0.0001) (Fig. 1B and Table 3). Surprisingly, total plasma BA concentrations were also approximately 2-fold higher in No-T2D patients with NASH compared with No-T2D No-NASH (NASH: 4311 ± 3806 nmol/L; n = 17; No-NASH: 2063 ± 1438 nmol/L; n = 85; mean ± SD, p <0.001) (Fig. 1B and Table 3). Primary (CA, CDCA, and HCA) and secondary (DCA, LCA, UDCA and HDCA) BA were elevated in NASH patients both in the No-T2D and T2D groups. Similarly, both 12aOH (CA and DCA) and non-12aOH BA (CDCA, HCA, LCA, UDCA, and HDCA) (Table S1) were higher in both groups of NASH patients compared with their respective No-NASH controls, leaving their ratio unchanged (Table 3). Moreover, plasma C4 was higher in NASH vs. No-NASH patients in the overall study cohort (Fig. 1A), this increase being statistically significant only in T2D patients (Fig. 1B and Table 3). Thus, total plasma BA concentrations were higher in NASH patients both with or without T2D, findings which were at first sight conflicting with our previous report.17
Table 3.
Plasma BA and C4 concentrations (nmol/L) according to diabetes and NASH status.
| No-T2D (n = 102) |
T2D (n = 117) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No-NASH (n = 85) | NASH (n = 17) | P | No-NASH (n = 59) | NASH (n = 58) | p | |||||
| Total BA (a, b, c, d) | 2063 | (1438) | 4311 | (3806) | 6E-04 | 1996 | (1078) | 4671 | (6100) | 1.6E-05 |
| Total CA (a, d) | 396 | (434) | 1010 | (1287) | 5E-04 | 289 | (305) | 1085 | (2217) | 2E-06 |
| CA | 141 | (299) | 205 | (327) | 0.03 | 114 | (269) | 266 | (688) | 0.01 |
| GCA | 201 | (211) | 671 | (960) | 3E-04 | 150 | (113) | 641 | (1216) | 3E-07 |
| TCA | 54 | (101) | 134 | (191) | 0.001 | 24 | (27) | 178 | (484) | 2E-07 |
| Total DCA (a, d) | 427 | (321) | 928 | (778) | 0.01 | 441 | (385) | 716 | (733) | 0.05 |
| DCA | 220 | (165) | 417 | (313) | 0.01 | 258 | (234) | 371 | (312) | 0.09 |
| GDCA | 154 | (160) | 405 | (467) | 0.02 | 144 | (173) | 271 | (402) | 0.03 |
| TDCA | 53 | (75) | 106 | (136) | 0.13 | 39 | (48) | 75 | (168) | 0.05 |
| Total CDCA (b, d) | 976 | (772) | 2059 | (1833) | 0.001 | 1004 | (792) | 2401 | (3539) | 7E-04 |
| CDCA | 245 | (286) | 452 | (400) | 0.002 | 334 | (389) | 608 | (887) | 0.04 |
| GCDCA | 611 | (517) | 1382 | (1487) | 0.005 | 598 | (481) | 1497 | (2146) | 8E-04 |
| TCDCA | 120 | (208) | 224 | (277) | 0.02 | 72 | (88) | 296 | (780) | 0.0012 |
| Total UDCA (b, d) | 210 | (257) | 240 | (165) | 0.10 | 206 | (188) | 407 | (575) | 0.05 |
| UDCA | 85 | (152) | 91 | (68) | 0.07 | 98 | (104) | 153 | (187) | 0.09 |
| GUDCA | 114 | (134) | 138 | (120) | 0.12 | 103 | (100) | 236 | (393) | 0.03 |
| TUDCA | 10 | (17) | 11 | (10) | 0.06 | 5 | (7) | 19 | (40) | 0.04 |
| Total LCA (b, d) | 19 | (21) | 34 | (35) | 0.13 | 20 | (24) | 27 | (34) | 0.22 |
| LCA | 9 | (10) | 13 | (12) | 0.2 | 10 | (14) | 14 | (18) | 0.11 |
| GLCA | 8 | (12) | 17 | (20) | 0.1 | 9 | (13) | 11 | (14) | 0.26 |
| TLCA | 1.6 | (2.4) | 3.7 | (5.3) | 0.1 | 1.4 | (2.8) | 2.2 | (4.9) | 0.12 |
| Total HCA (b, c) | 28 | (18) | 31 | (15) | 0.31 | 29 | (24) | 28 | (26) | 0.45 |
| HCA | 8 | (12) | 8 | (7) | 0.52 | 12 | (22) | 7 | (13) | 0.18 |
| GHCA | 18 | (8) | 20 | (9) | 0.25 | 16 | (7) | 18 | (14) | 0.35 |
| THCA | 2.2 | (2.8) | 2.8 | (3.7) | 0.41 | 1.3 | (1.5) | 2.1 | (4.5) | 0.64 |
| Total HDCA (b, c) | 7.3 | (2.5) | 9.3 | (7.4) | 0.47 | 7.0 | (3.8) | 7.1 | (3.5) | 0.87 |
| HDCA | 1.6 | (1.8) | 3.3 | (7.1) | 0.93 | 1.4 | (2.8) | 1.2 | (2.1) | 0.92 |
| GHDCA | 5.4 | (1.4) | 5.6 | (1.3) | 0.37 | 5.3 | (1.4) | 5.5 | (2.0) | 0.70 |
| THDCA | 0.3 | (0.2) | 0.4 | (0.2) | 0.05 | 0.3 | (0.1) | 0.3 | (0.2) | 0.27 |
| Total primary BAs | 1400 | (1133) | 3100 | (3077) | 0.001 | 1322 | (1005) | 3513 | (5672) | 2E-04 |
| Free | 394 | (524) | 665 | (705) | 0.004 | 460 | (588) | 882 | (1537) | 0.04 |
| Conjugated | 1006 | (946) | 2435 | (2860) | 0.002 | 862 | (662) | 2632 | (4470) | 9E-05 |
| Total secondary BAs | 1266 | (834) | 2576 | (2194) | 0.004 | 1264 | (655) | 2644 | (2707) | 5E-05 |
| Free | 316 | (223) | 525 | (349) | 0.02 | 367 | (264) | 538 | (360) | 0.006 |
| Conjugated | 950 | (778) | 2052 | (2084) | 0.008 | 897 | (608) | 2106 | (2598) | 3E-04 |
| Total free BAs | 710 | (647) | 1190 | (951) | 0.003 | 827 | (634) | 1420 | (1673) | 0.007 |
| Total conjugated BAs | 1956 | (1704) | 4486 | (4926) | 0.005 | 1759 | (1246) | 4737 | (6966) | 1E-04 |
| 6aOH BAs | 35 | (18) | 40 | (20) | 0.22 | 36 | (23) | 34 | (26) | 0.49 |
| non-6aOH BAs | 2027 | (1424) | 4270 | (3794) | 7E-04 | 1960 | (1071) | 4635 | (6081) | 2E-04 |
| Ratio 6aOH | 0.02 | (0.01) | 0.01 | (0.007) | 2E-04 | 0.02 | (0.02) | 0.01 | (0.006) | 1E-08 |
| 12aOH BAs | 823 | (635) | 1938 | (1929) | 0.001 | 730 | (419) | 1801 | (2435) | 1E-05 |
| non-12aOH BAs | 1240 | (958) | 2373 | (1937) | 0.002 | 1266 | (913) | 2870 | (3892) | 5E-04 |
| Ratio 12aOH | 0.8 | (0.4) | 0.8 | (0.3) | 0.63 | 0.8 | (0.7) | 0.8 | (0.5) | 0.61 |
| C4 | 56 | (43) | 68 | (38) | 0.14 | 67 | (44) | 105 | (80) | 0.013 |
Data are mean and SD (standard deviation), p values from non-parametric Mann-Whitney U test. p values in bold are statistically significant. a, 12aOH species; b, non-12aOH species; c, 6aOH species; d, non-6aOH species. BA, bile acid; C4, 7alpha-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; GHCA, glycohyocholic acid; GHDCA, glycohyodeoxycholic acid; GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; THCA, taurohyocholic acid; THDCA, taurohyodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid.
Plasma BA concentrations are mainly associated with NASH and not with glucose homeostasis parameters in the ABOS study cohort
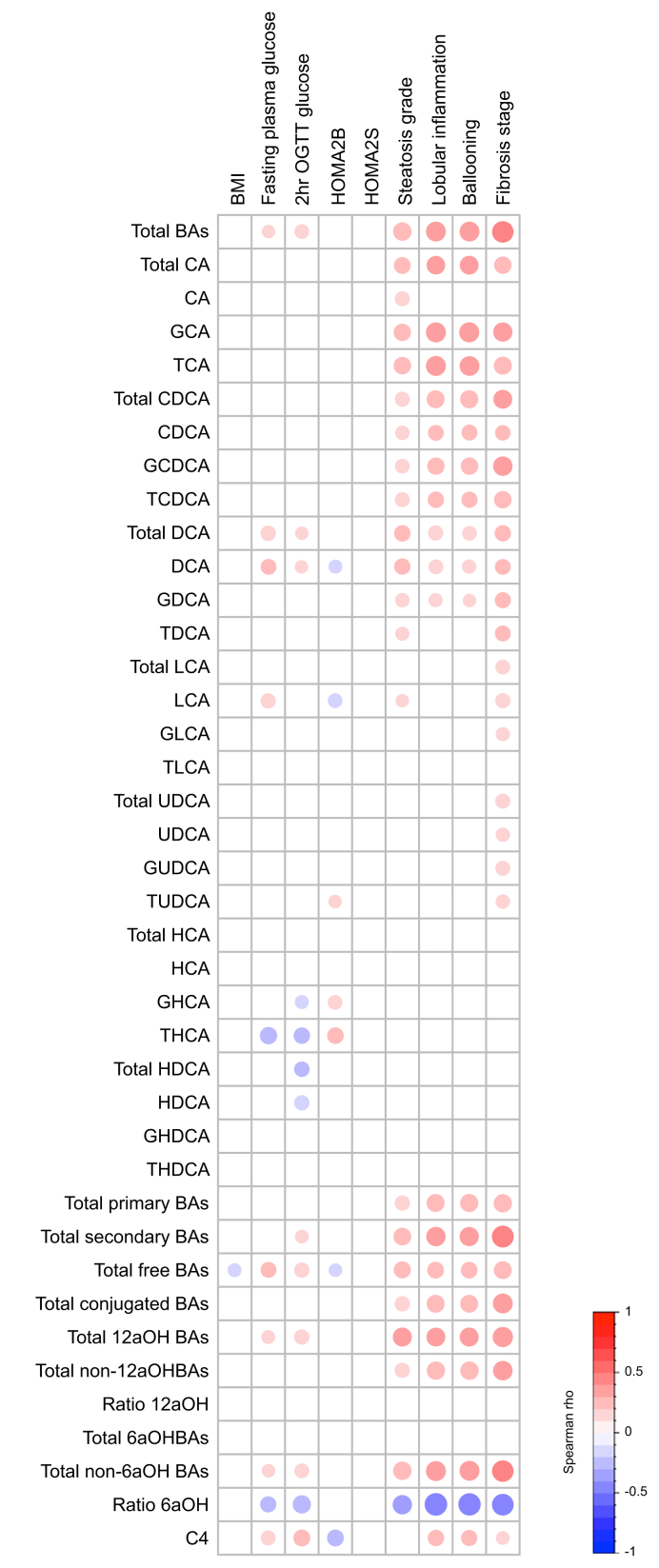
To identify the specific disease features associated with plasma BA changes in NASH, we assessed correlations between plasma BA concentrations and BMI, parameters of glucose homeostasis and NAFLD histological characteristics in the entire ABOS study cohort including both No-T2D and T2D patients (n = 219) (Fig. 2). A modest inverse correlation between the ratio of plasma 6aOH species (HCA and HDCA) to non-6aOH species (CA, CDCA, DCA, LCA, and UDCA), glycohyocholic acid (GHCA), taurohyocholic acid (THCA), total HDCA, free HDCA, and glycaemia at 2 h after the OGTT, as well as a modest positive correlation between plasma free LCA, DCA, total DCA, and C4 and fasting plasma glycaemia (FPG) were observed (Fig. 2). However, the most pronounced correlations were observed with hepatic lesions: plasma total BA, total, free and conjugated CA, CDCA, DCA, and C4 all positively correlated with NAFLD histological characteristics including steatosis, lobular inflammation, ballooning, and also with fibrosis stage (except for free CA) (Fig. 2). Conversely, total 6aOH BA as well as each specific species (i.e. total, free, and conjugated HCA and HDCA) did not correlate with hepatic lesions, but correlated negatively with 2-h OGTT glucose levels. These latter results are in line with our previous findings in a different cohort showing an association of total HCA with parameters of glucose metabolism in patients with prediabetes.26 These results indicate that in this ABOS study cohort, plasma BA alterations are mainly associated with NASH rather than with glucose homeostasis parameters.
Fig. 2.
Plasma BA concentrations are mainly associated with NASH and not with glucose homeostasis parameters in patients of the ABOS study cohort.
Unadjusted Spearman correlations between plasma BA concentrations and BMI, glucose homeostasis parameters and NASH parameters in the ABOS study cohort (n = 219). Colours and area of circles reflect the Spearman rho values (red for positive, blue for inverse correlations). Only rho values with significant p value (p <0.05) were represented. BA species, ratios and total values were determined according to Table S1. ABOS, Biological Atlas of Severe Obesity; BA, bile acid; C4, 7alpha-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GHCA, glycohyocholic acid; GHDCA, glycohyodeoxycholic acid; GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; HOMA, Homeostatic Model Assessment; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis; OGTT, oral glucose tolerance test; T2D, type 2 diabetes; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; THCA, taurohyocholic acid; THDCA, taurohyodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid.
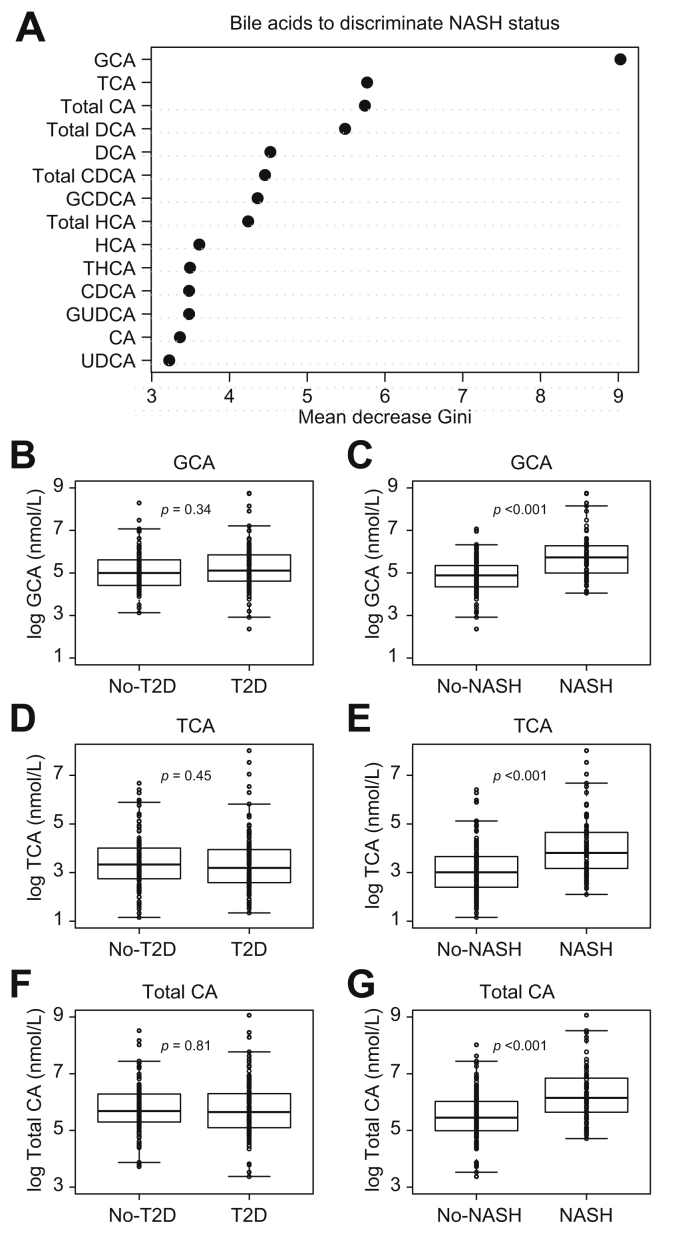
CA species discriminate NASH independently of glucose homeostasis parameters
We next sought to identify the plasma BA which most efficiently discriminate NASH status independently of glucose homeostasis parameters. Interestingly, the top 3 plasma BAs identified by random forest analysis were all CA species (GCA, TCA, and total CA) (Fig. 3A). Although no significant differences were found for GCA, TCA, and total CA between No-T2D and T2D patients (Fig. 3B, D, and F), significantly higher levels of these BAs were found in NASH vs. No-NASH patients (p <0.001) (Fig. 3C, E, and G). Multiple linear regression analysis of NASH status, T2D status, BMI, HOMA2S, HOMA2B, HbA1c, and total CA, GCA, and TCA showed that NASH was the only parameter significantly contributing to total CA, GCA, and TCA (Table 4), suggesting that, in the ABOS cohort, these BAs most efficiently discriminate NASH status, independently of other confounding factors.
Fig. 3.
Identification of specific BA discriminating NASH independently of type 2 diabetes (T2D) in the ABOS study cohort (n = 219).
(A) Variable importance plot of the Random Forest analysis. The variables are ordered top-to-bottom as most-to-least important in classifying between No-NASH and NASH groups. (B–G) Plasma BA concentrations (log-scale, nmol/L) according to T2D status (B, D, F) and NASH status (C, E, G). Data are expressed as median and IQR. p values were from the non-parametric Mann-Whitney U test. ABOS, Biological Atlas of Severe Obesity; BA, bile acids; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; HCA, hyocholic acid; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes; TCA, taurocholic acid; THCA, taurohyocholic acid; UCDA, ursodeoxycholic acid.
Table 4.
Multiple linear regression analysis of plasma BA concentrations potential confounding factors in the ABOS study cohort (n = 219).
| GCA |
TCA |
Total CA |
||||
|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | |
| NASH | 419 | <10-6 | 95 | <0.001 | 570 | <0.0001 |
| T2D | 4 | 0.96 | 1 | 0.96 | -65 | 0.7 |
| BMI | -6 | 0.3 | -1 | 0.32 | -9 | 0.2 |
| HOMA2S | 0.005 | 0.9 | 0.003 | 0.98 | -0.2 | 0.8 |
| HOMA2B | 0.86 | 0.1 | 0.29 | 0.1 | 0.9 | 0.3 |
| HbA1c | -8 | 0.7 | -2 | 0.8 | 8.7 | 0.8 |
Values are β coefficients for bile acids from multiple linear regressions. p values in bold are statistically significant. ABOS, Biological Atlas of Severe Obesity; BA, bile acid; CA, cholic acid; GCA, glycocholic acid; HOMA, homeostatic model assessment; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes; TCA, taurocholic acid.
Insulin sensitivity interacts with NASH status in the determination of plasma BA levels
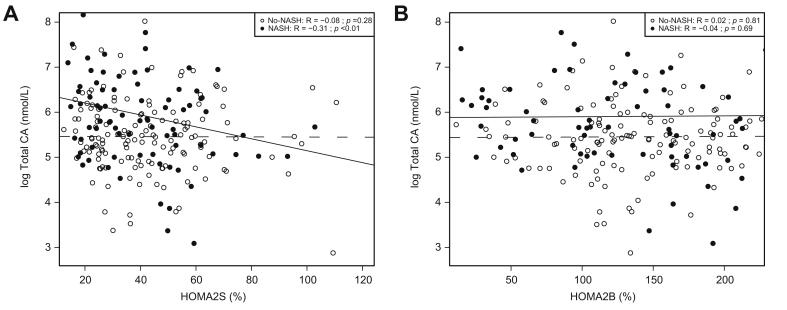
As the results obtained in the ABOS cohort appear in contradiction to our previous study in the HEPADIP cohort (Legry et al.17), we compared the 2 cohorts to try to explain this discrepancy. In the ABOS study, the No-T2D patients (No-NASH and NASH combined) were more obese and insulin resistant compared with the HEPADIP patients (BMI: 40.0 ± 5.8 kg/m2 vs. 46.1 ± 7.2 kg/m2, and HOMA2S: 62 ± 37% vs. 41 ± 23%, for HEPADIP vs. ABOS patients, respectively, mean ± SD). To determine whether differences in metabolic status may alter the correlations between plasma BA and NASH, we assessed whether BMI, HOMA2S, or HOMA2B affect the NASH-associated increase in plasma BA. We focused on total CA, the major plasma BA component most correlated with NASH10,11,27 (Fig. 3A) or IR,17,28 by combining HEPADIP and ABOS cohort patients as described in the Patients and methods section. Two-way ANOVA analysis assessing the influence of glucose parameters and BMI on plasma total CA levels showed an interaction between HOMA2S, but not HOMA2B nor BMI, with NASH status in the determination of plasma total CA levels (Table 5). Identical results were obtained when the interaction was tested by moderated multiple regression analysis (data not shown) and when patients treated with secretagogues (sulfonylureas) were excluded. Interestingly, there was no correlation between total CA and HOMA2S or HOMA2B in No-NASH patients (respectively, R = -0.08, p = 0.28 and R = 0.02, p = 0.81) (Fig. 4A and B). By contrast, a significant negative correlation was found in NASH patients between total CA and HOMA2S (R = -0.31, p <0.01) (Fig. 4A), but not between total CA and HOMA2B (R = -0.04, p = 0.69) (Fig. 4B).
Table 5.
Interaction of plasma BA concentrations with NASH, IR and BMI in the combined cohort (n = 239).
| Total BA |
Total CA |
CDCA |
DCA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p value | F | p value | F | p -value | F | p -value | ||
| BMI | NASH | 14 | <0.001 | 14 | <0.001 | 8 | 0.003 | 8 | 0.005 |
| BMI | 0.14 | 0.71 | 0.63 | 0.42 | 0.02 | 0.87 | 0.6 | 0.43 | |
| NASH-BMI | 0.19 | 0.66 | 0.63 | 0.66 | 0.41 | 0.51 | 0.7 | 0.38 | |
| HOMA2S | NASH | 16 | <0.001 | 13 | <0.001 | 9 | 0.002 | 8 | 0.004 |
| HOMA2S | 1.7 | 0.19 | 0,7 | 0.39 | 2 | 0.15 | 0.5 | 0.47 | |
| NASH-HOMA2S | 18 | <0.001 | 12 | <0.001 | 15 | <0.001 | 12 | <0.001 | |
| HOMA2B | NASH | 14 | <0.001 | 13 | <0.001 | 8 | 0.003 | 8 | 0.005 |
| HOMA2B | 0.02 | 0.86 | 0.7 | 0.37 | 0.4 | 0.49 | 3 | 0.06 | |
| NASH-HOMA2B | 0.07 | 0.78 | 0.3 | 0.54 | 0.5 | 0.46 | 1.5 | 0.21 | |
F-ratios from two-way ANOVA test of main interactive effects of NASH, BMI, HOMA2S and HOMA2B on plasma total BA, total CA, free CDCA and free DCA levels are shown. p values in bold indicate statistical significance. BA, bile acids; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HOMA, homeostatic model assessment; IR, insulin resistance; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes.
Fig. 4.
Insulin sensitivity modulates the NASH-associated increase in plasma BA in the combined cohort (n = 239).
(A) Plasma total CA concentrations (nmol/L) according to HOMA2S (%). (B) Plasma total CA concentrations (nmol/L) according to HOMA2B (%). White circles represent the No-NASH and black circles the NASH patients. Dashed lines: regression curves for No-NASH patients; solid lines: regression curves for NASH patients. Rho coefficients and p values were obtained using the Spearman Rank test. BA, bile acids; CA, cholic acid; HOMA, homeostatic model assessment; NASH, non-alcoholic steatohepatitis.
These results suggest that alterations caused by reduced insulin sensitivity drive the NASH-associated increase in plasma total CA.
Discussion
Previous studies assessed whether NASH is associated with altered BA metabolism either in No-T2D patients or in cohorts not stratified for T2D. However, T2D and obesity are also associated with changes in plasma BA. Therefore, we measured plasma BA concentrations in patients selected from the ABOS cohort, which contains T2D and No-T2D patients with histologically evaluated NASH. Our results showed that plasma BA concentrations are higher in NASH compared with No-NASH patients, irrespective of the T2D status. Additionally, this is the first study showing an interaction between the degree of insulin sensitivity and NASH status in the determination of plasma BA levels. These findings provide an explanation for the previous discrepant reports on plasma BA in NASH.
Our results are in agreement with several previous studies showing increased plasma BA in NASH compared with No-NASH patients.[9], [10], [11] Both fasting primary10 and conjugated BA species were reported to be higher in NASH patients.[10], [11], [12] Moreover, positive correlations between histological lesions of NASH (lobular inflammation, portal inflammation, and ballooning) and plasma BA concentrations (mainly GCA, TCA, and GCDCA) have been reported,11 findings largely in agreement with the present study.
However, in a previous study,17 we found that plasma BA levels were unaffected by NASH when patients were matched by BMI and had relatively low levels of IR. Moreover, BA concentrations were rather associated with IR than with NASH. These findings were in line with previous studies showing strong associations of BA with IR and obesity.13,29,30 Unfortunately, in many published studies, BMI and IR were not always carefully controlled when comparing NASH and healthy liver patients, complicating their interpretation.8 Therefore, the present findings in the ABOS cohort, carefully controlled for IR and BMI, were surprising and prompted us to perform a combined analysis including the HEPADIP cohort dataset to understand this apparent discrepancy. A clear difference between the 2 cohorts was that the patients in the HEPADIP cohort were generally less metabolically compromised. In line, the No-T2D groups in the ABOS cohort contained a high number (78%) of prediabetic subjects (fasting blood glucose between 100 and 125 mg/dl and/or 2-h OGTT glucose between 140 and 199 mg/dl31) with higher BMI and IR (46.1 ± 7.2 and 41 ± 23 % for BMI and HOMA2S, respectively). Comparatively, patients in the HEPADIP cohort have lower BMI and IR, indicated by higher HOMA2S (40.0 ± 5.8 kg/m2 and 62 ± 37% for BMI and HOMA2S, respectively). Therefore, we addressed the hypothesis of an interaction between metabolic status and NASH on BA metabolism by combining the HEPADIP and ABOS cohorts, hence covering a large range of BMI and HOMA2S (Fig. 4A and Fig. S2).
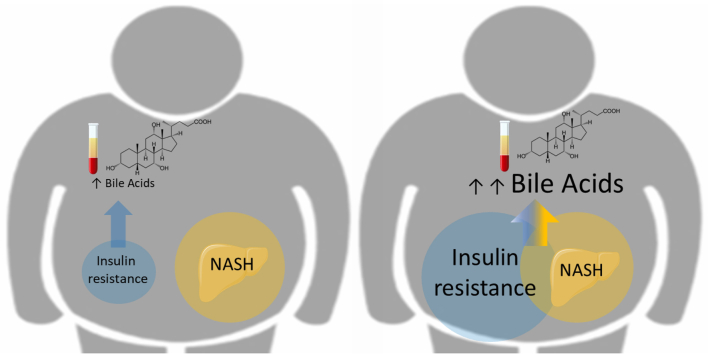
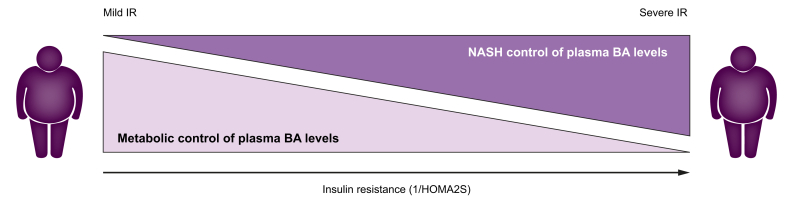
Overall, our findings suggest that in a setting of milder metabolic perturbations (less severe obesity with less pronounced IR), as in the HEPADIP cohort, NASH is not associated with BA profile changes.17 Conversely, in a context of worse insulin-resistance (with lower HOMA2S), BAs are elevated in NASH and correlate better with histological parameters than with measures of glucose homeostasis, suggesting an interaction between NASH pathophysiology and altered glucose metabolism pathways in the control of plasma BA levels (Fig. 5).
Fig. 5.
The impact of NASH on plasma BA concentrations depends on the degree of IR.
In patients with mild IR, NASH does not affect plasma BA which are driven by metabolic homeostasis. In contrast, in patients with severe IR, NASH is associated with increased plasma BA. BA, bile acid; IR, insulin resistance; NASH, non-alcoholic steatohepatitis.
The molecular mechanisms linking BA and glucose homeostasis involve alterations in signalling pathways through BA-activated receptors, such as the membrane receptor TGR5 and the nuclear receptor FXR. For instance, FXR regulates not only genes coding for enzymes involved in BA synthesis, such as cholesterol 7α-hydroxylase (Cyp7a1), the rate limiting enzyme in the classical BA synthesis pathway, and 12α-hydroxylase (Cyp8b1), but also genes involved in glucose and lipid metabolism.5 Moreover, hepatic FXR expression is altered in animal models of diabetes, and is regulated by glucose.32 Furthermore, insulin and glucose regulate Cyp7a1 gene transcription in human hepatocytes, hence reciprocally impacting on BA metabolism.33 Moreover, mice deficient in Cyp8B1 display improved glucose tolerance, insulin sensitivity, and β-cell function.34 Therefore, severe IR may precipitate NASH induced alterations of hepatic BA metabolism. In addition, hepatic BA clearance and/or intestinal BA metabolism and uptake may also be differently affected in NASH patients exhibiting severe IR. Unfortunately, detailed studies on the involved mechanisms are difficult to perform in humans.
Importantly, this study has a number of limitations. First, the cohorts consisted mainly of overweight and obese patients (BMI >27 kg/m2) who were generally younger than in other NASH cohorts. Furthermore, we have not explored how genetic and lean forms of NAFLD may also affect plasma BA homeostasis.10,35 Second, many diabetic patients have pharmacological treatments which may impact BA metabolism. To avoid these potential confounding effects, patients were matched for their treatments, and patients treated with insulin were excluded from the combined analysis. Finally, IR was not evaluated by the gold standard hyperinsulinemic-euglycemic clamp technique but estimated by HOMA2 calculation, and the duration since diagnosis of either T2D or NASH are not considered. This may have led to underestimation of the severity of IR in some patients.
In conclusion, our findings confirm the high inter-regulation of BA and glucose metabolism and show that insulin sensitivity interacts with NASH in the regulation of plasma BA metabolism.
Financial support
BS is a recipient of an ERC Advanced Grant (694717). This work benefits from State grant managed by the National Research Agency under the program ‘Investissement d’Avenir’ with the reference ANR-16-RHUS-0006_PreciNASH. This study was also supported by funding from the Société Francophone du Diabète (AAP2014 - VL). This work was supported by grants from the European Genomic Institute for Diabetes (E.G.I.D. ANR-10-LABX-46). This work was supported by grants from the European Commission: HEPADIP (Contract LSHM-CT-2005-018734) and RESOLVE (Contract FP7-305707).
Authors’ contributions
Concept and design: GG, OCT, RP, DT, SF, FP, JH, AT, BS
Experiments and procedures: GG, OCT, AD, MK, JFG, AV, SF, RC, LVG, HV, VL, DT, BL, JH, BS, AT
Writing and approval of manuscript: all authors
Data availability
The data sets generated during and/or analysed during the current study are not publicly available, as they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure data privacy of the study participants. However, they can be applied for through an individual project agreement with the principal investigator of the University Hospital of Lille, France. The study protocol and the methods (NCT01129297) have been published as well as the cohort profile and are unrestrictedly available.36
Conflict of interest
The authors declare that they have no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Paul-Emile Hecquet for analytical support.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found at https://doi.org/10.1016/j.jhepr.2020.100222.
Supplementary data
References
- 1.Haas J.T., Francque S., Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181–205. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N., Häring H.-U., Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 3.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers F., Bloks V.W., Groen A.K. Beyond intestinal soap – bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 5.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694. doi: 10.1053/j.gastro.2017.01.055. e3. [DOI] [PubMed] [Google Scholar]
- 6.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H., Kipnes M. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–852. doi: 10.1053/j.gastro.2013.05.042. e1. [DOI] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri P.B.A., Loomba R., Sanyal P.A.J., Lavine P.J.E., Natta M.L.V., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 9.Chávez-Talavera O., Haas J., Grzych G., Tailleux A., Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tell? Curr Opin Lipidol. 2019;30:244–254. doi: 10.1097/MOL.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 10.Puri P., Daita K., Joyce A., Mirshahi F., Santhekadur P.K., Cazanave S. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalhan S.C., Guo L., Edmison J., Dasarathy S., McCullough A.J., Hanson R.W. Plasma metabolomic profile in non-alcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferslew B.C., Xie G., Johnston C.K., Su M., Stewart P.W., Jia W. Altered bile acid metabolome in patients with non-alcoholic steatohepatitis. Dig Dis Sci. 2015;60:3318. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun W., Zhang D., Wang Z., Sun J., Xu B., Chen Y. Insulin resistance is associated with total bile acid level in type 2 diabetic and nondiabetic population. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prinz P., Hofmann T., Ahnis A., Elbelt U., Goebel-Stengel M., Klapp B.F. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front Neurosci. 2015;9:199. doi: 10.3389/fnins.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brufau G., Bahr M.J., Staels B., Claudel T., Ockenga J., Böker K.H. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab. 2010;7:73. doi: 10.1186/1743-7075-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younossi Z.M., Golabi P., Avila L de, Paik J.M., Srishord M., Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Legry V., Francque S., Haas J.T., Verrijken A., Caron S., Chávez-Talavera O. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J Clin Endocrinol Metab. 2017;102:3783–3794. doi: 10.1210/jc.2017-01397. [DOI] [PubMed] [Google Scholar]
- 18.Margerie D., Lefebvre P., Raverdy V., Schwahn U., Ruetten H., Larsen P. Hepatic transcriptomic signatures of statin treatment are associated with impaired glucose homeostasis in severely obese patients. BMC Med Genomics. 2019;12:80. doi: 10.1186/s12920-019-0536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Younossi Z.M., Stepanova M., Younossi Y., Golabi P., Mishra A., Rafiq N. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2019;69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 21.Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 23.Caiazzo R., Lassailly G., Leteurtre E., Baud G., Verkindt H., Raverdy V. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893–899. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 26.Chávez-Talavera O., Wargny M., Pichelin M., Descat A., Vallez E., Kouach M. Bile acids associate with glucose metabolism, but do not predict conversion from impaired fasting glucose to diabetes. Metabolism. 2020;103:154042. doi: 10.1016/j.metabol.2019.154042. [DOI] [PubMed] [Google Scholar]
- 27.Jiao N., Baker S.S., Chapa-Rodriguez A., Liu W., Nugent C.A., Tsompana M. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.-G., Lee Y., Choi E., Cho Y., Kim J.-H. Fasting serum bile acids concentration is associated with insulin resistance independently of diabetes status. Clin Chem Lab Med. 2019;57:1218–1228. doi: 10.1515/cclm-2018-0741. [DOI] [PubMed] [Google Scholar]
- 29.Sonne D.P., van Nierop F.S., Kulik W., Soeters M.R., Vilsbøll T., Knop F.K. Postprandial plasma concentrations of individual bile acids and FGF-19 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101:3002–3009. doi: 10.1210/jc.2016-1607. [DOI] [PubMed] [Google Scholar]
- 30.Vincent R.P., Omar S., Ghozlan S., Taylor D.R., Cross G., Sherwood R.A. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50:360–364. doi: 10.1177/0004563212473450. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duran-Sandoval D., Mautino G., Martin G., Percevault F., Barbier O., Fruchart J.-C. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–898. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 33.Li T., Chanda D., Zhang Y., Choi H.-S., Chiang J.Y.L. Glucose stimulates cholesterol 7α-hydroxylase gene transcription in human hepatocytes. J Lipid Res. 2010;51:832–842. doi: 10.1194/jlr.M002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur A., Patankar J.V., Haan W de, Ruddle P., Wijesekara N., Groen A.K. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64:1168–1179. doi: 10.2337/db14-0716. [DOI] [PubMed] [Google Scholar]
- 35.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically-characterised cohort. J Hepatol. 2020;73:505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Raverdy V., Baud G., Pigeyre M., Verkindt H., Torres F., Preda C. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg. 2016;264:878–885. doi: 10.1097/SLA.0000000000001915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analysed during the current study are not publicly available, as they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure data privacy of the study participants. However, they can be applied for through an individual project agreement with the principal investigator of the University Hospital of Lille, France. The study protocol and the methods (NCT01129297) have been published as well as the cohort profile and are unrestrictedly available.36