Abstract
Oncolytic adenoviruses (OAds) are among the most promising oncolytic viruses. Almost all oncolytic adenoviruses are composed of human adenovirus serotype 5 (Ad5) (OAd5). However, expression of the primary infection receptor for Ad5, coxsackievirus-adenovirus receptor (CAR), often declines on malignant tumor cells, resulting in inefficient infection in CAR-negative tumor cells. In addition, at least 80% of adults have neutralizing antibodies against Ad5. In this study, we developed a novel OAd fully composed of OAd35. OAd35 recognizes CD46, which is ubiquitously expressed on almost all human cells and is often upregulated on malignant tumor cells, as an infection receptor. Moreover, 20% or fewer adults have neutralizing antibodies against Ad35. OAd35 mediated efficient cell lysis activities at levels similar to OAd5 in CAR-positive tumor cells, while OAd35 showed higher levels of cell lysis activities than OAd5 in CAR-negative tumor cells. Anti-Ad5 serum significantly inhibited in vitro tumor cell lysis activities of OAd5, whereas OAd35 exhibited comparable levels of in vitro tumor cell lysis activities in the presence of anti-Ad5 and naive serum. OAd35 significantly suppressed growth of the subcutaneous CAR-positive and CAR-negative tumors following intratumoral administration. These results indicated that OAd35 is a promising alternative oncolytic virus for OAd5.
Keywords: oncolytic virus, adenovirus serotype 35, CD46, CAR, neutralizing antibody
Graphical Abstract
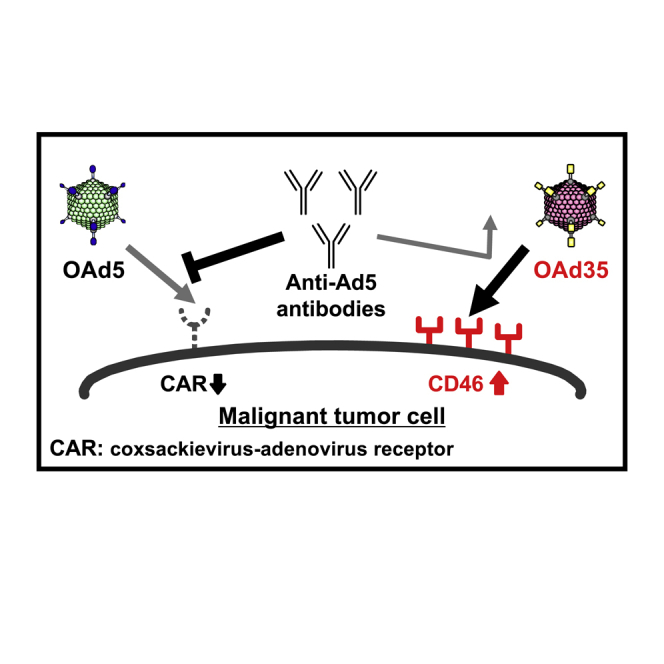
A conventional oncolytic adenovirus (OAd) based on adenovirus serotype 5 (Ad5) is a promising anticancer agent. However, more than 80% of adults have neutralizing anti-Ad5 antibodies. OAd serotype 5 inefficiently infects coxsackievirus-adenovirus receptor-negative tumors. In order to overcome these drawbacks, we developed a novel OAd composed of Ad35.
Introduction
Oncolytic viruses are attracting much attention as a novel cancer therapeutic agent. Oncolytic viruses specifically replicate in and kill tumor cells without apparent toxicity to normal cells. Currently, more than 10 types of oncolytic viruses have been tested in clinical trials against various types of tumors and have shown promising results.1,2 Among these oncolytic viruses, the oncolytic adenoviruses (OAds) are one of the most promising. Almost all OAds are composed of human adenovirus (Ad) serotype 5 (Ad5), which belongs to species C. Ad5 has various advantages as a framework for oncolytic viruses. For example, Ad5 can be grown to high titers, and efficiently infects a variety of cells. In addition, transgenes of relatively large size can be incorporated into the Ad5 genome by genetic recombination technology.
However, the OAd composed of Ad5 (OAd5) recognizes coxsackievirus-adenovirus receptor (CAR) as an infection receptor. Ad5 efficiently infects CAR-positive cells but does not readily infect CAR-negative cells. CAR expression is often reduced on malignant tumor cells, leading to inefficient infection with OAd5.3, 4, 5 In addition, more than 80% of adults have neutralizing antibodies against Ad5 due to natural infection with Ad5 during childhood.6, 7, 8, 9 Neutralizing anti-Ad5 antibodies might inhibit antitumor effects of an OAd5, especially when high titers of neutralizing anti-Ad5 antibody titers were produced.
In this study, in order to overcome these drawbacks of OAd5, we developed a novel OAd35, which belongs to species B2. Ad35 has superior properties as a framework for an oncolytic virus. First, Ad35 recognizes human CD46 as an infection receptor.10,11 CD46, which is a complement regulatory protein, is ubiquitously expressed on all human cells except erythrocytes.12, 13, 14 Moreover, CD46 is often upregulated on malignant tumor cells.15,16 Second, 20% or fewer adults have neutralizing antibodies against Ad35.6,17 Several groups, including us, developed a replication-incompetent Ad35 vector and demonstrated that an Ad35 vector showed efficient transduction, especially in CAR-negative human cells.17, 18, 19 In addition, a previous study demonstrated the efficient tumor-killing activities of wild-type Ad35 on several human tumor cell lines.20 These findings led us to hypothesize that an OAd35 became a promising oncolytic agent. We demonstrated that OAd35 efficiently killed not only CAR-positive but also CAR-negative tumor cells. Intratumoral administration of OAd35 resulted in significant growth suppression of the subcutaneous CAR-positive and CAR-negative tumors.
Results
Construction of an OAd fully composed of an Ad35
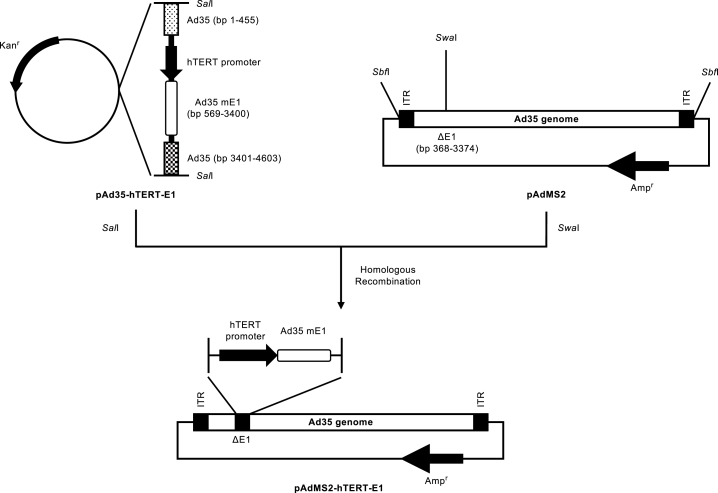
In order to produce an OAd35, an OAd35 plasmid, pAdMS2-hTERT-E1, was created using the Ad35 vector plasmid pAdMS2.21 pAdMS2-hTERT-E1 contains an expression cassette of the mutated Ad35 E1 gene (Ad35 genome, bp 569-3400, referred to GenBank 56160914) under the control of the human telomerase reverse transcriptase (hTERT) promoter (Figure 1). The Ad35 E1B gene (Ad35 genome, bp 1611-3400) was controlled by the native viral promoter in the Ad35 genome. OAd35 exhibited a cytopathic effect (CPE) in HEK293 cells when administered by a conventional method using a recombinant Ad vector preparation within 21 days after the transfection of pAdMS2-hTERT-E1. Propagation of OAd35 in HEK293 cells was lower than that of OAd5 in H1299 cells. The physical titers of OAd35 were approximately 10-fold lower than those of OAd5 (Table 1). The ratio of biological titers to physical titers of OAd35 was 1:8.5, which was comparable to that for OAd5.
Figure 1.
Construction strategy for an OAd35 plasmid, pAdMS2-hTERT-E1
pAd35-hTERT-E1 and pAdMS2 were digested by SalI and SwaI, respectively. pAdMS2-hTERT-E1 was produced via homologous recombination in Escherichia coli BJ5183 using these fragments. mE1, mutated E1 gene; ITR, inverted terminal repeat.
Table 1.
Virus titers
| Virus | Physical titer (VP/mL) | Biological titer (PFU/mL) | VP/PFU ratio |
|---|---|---|---|
| OAd5 | 1.95 × 1012 | 3.0 × 1011 | 6.5 |
| OAd35 | 2.89 × 1011 | 3.4 × 1010 | 8.5 |
PFU, plaque forming unit.
CAR and CD46 expression on human tumor cells
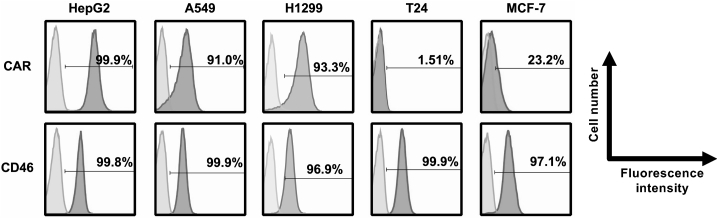
In order to examine the expression levels of the OAd infection receptors, CAR and CD46, on the five human tumor cell lines, flow cytometric analysis was performed (Figure 2). More than 90% of HepG2 cells, A549 cells, and H1299 cells expressed CAR, whereas 23.2% of MCF-7 cells and only 1.51% of T24 cells expressed CAR. Despite these differing levels of CAR expression among the cell lines, CD46 was highly expressed on all tumor cell lines. Indeed, almost 100% of the cells were CD46 positive. Therefore, in the following experiments, HepG2, A549, and H1299 cells were used as CAR-positive tumor cells, and T24 and MCF-7 cells were used as CAR-negative tumor cells.
Figure 2.
Flow cytometric analysis of CAR and CD46 expression on human tumor cells
Cells were labeled with mouse anti-CAR monoclonal antibody and PE-conjugated goat anti-mouse IgG secondary antibody or PE-conjugated mouse anti-CD46 monoclonal antibody. The data were analyzed by using FlowJo flow cytometry data analysis software. Light gray histogram, isotype control; gray histogram, anti-CAR or anti-CD46 antibody.
Tumor cell lysis activities and safety profiles of OAd35
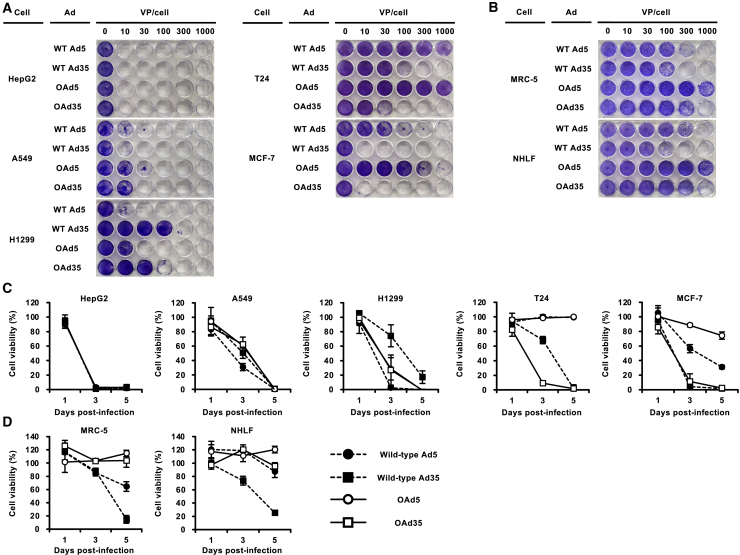
In order to examine the tumor cell lysis activities of OAds, crystal violet staining was performed following a 5-day incubation after virus infection (Figure 3A). Both OAds showed efficient and comparable tumor cell lysis activities on HepG2 and A549 cells, both of which were CAR-positive. OAd35 killed H1299 cells less efficiently than OAd5. OAd35 achieved 100% lysis of H1299 cells at 300 virus particle (VP)/cell, whereas 100% lysis of H1299 cells was observed at 100 VP/cell of OAd5. On the other hand, in T24 and MCF-7 cells, which are CAR-negative, OAd5 did not show apparent tumor cell lysis activities at less than 1,000 VP/cell, whereas OAd35 mediated efficient tumor cell lysis activities in these CAR-negative tumor cells. Approximately 100% tumor cell lysis was achieved by 100 and 10 VP/cell of OAd35 in T24 and MCF-7 cells, respectively. When we compared the tumor cell lysis activities of OAds to those of wild-type Ads, OAd5 mediated less efficient tumor cell lysis activities than wild-type Ad5 in A549, H1299, and MCF-7 cells. On the other hand, OAd35 showed comparable or higher levels of tumor cell lysis activities in all tumor cells except for A549 cells when compared to wild-type Ad35. These data indicated that the use of the hTERT promoter for the E1A gene expression reduced the tumor cell lysis activities of OAd5, while the tumor cell lysis activities of OAd35 were enhanced by insertion of the hTERT promoter.
Figure 3.
Tumor cell lysis activities and safety profiles of OAd35
(A and B) Viabilities of (A) human tumor cell lines and (B) normal cells were assessed by crystal violet staining assay. Cells were infected with OAds and wild-type Ads at the indicated VP/cell. Cells were stained with crystal violet following a 5-day incubation after infection. The representative images from at least 2 independent experiments were shown. (C and D) Viabilities of (C) human tumor cells and (D) normal cells were also evaluated by WST-8 assay. Cell lines were infected with OAds and wild-type Ads at 300 VP/cell. At the indicated time points, cell viabilities were determined by WST-8 assay. The viability in the mock-infected group was normalized to 100%. These data are expressed as the means ± SD (n = 4).
We also examined the safety profiles of OAd5 and OAd35 on normal human cells by crystal violet staining assay. Both OAd5 and OAd35 induced cell lysis in NHLF and MRC5 cells less efficiently than the corresponding wild-type Ads (Figure 3B), indicating that insertion of the hTERT promoter significantly enhanced the safety profiles of OAd5 and OAd35.
Next, the tumor cell lysis activities of OAds were evaluated by water soluble tetrazolium salts (WST) -8 assay (Fig 3C). In CAR-positive tumor cells, efficient OAd35- and OAd5-mediated tumor cell lysis (less than 50% of cells remaining viable) was observed at 3 and 5 days after virus infection. In CAR-negative cells, almost 100% of the cells survived after a 5-day incubation with OAd5, while more than 90% of CAR-negative cells were killed after a 5-day incubation with OAd35. As shown in the crystal violet staining assay, the viabilities of MRC-5 cells and NHLF following infection with OAd5 and OAd35 were comparable to or higher than those of wild-type Ad5 and Ad35 in the WST-8 assay (Figure 3D). These data of crystal violet staining and WST-8 assay indicated that OAd35 exhibited efficient tumor cell lysis activities in a wide variety of tumor cells, irrespective of the CAR expression levels, with superior safety profiles in normal human cells.
E1A gene expression by OAd5 and OAd35
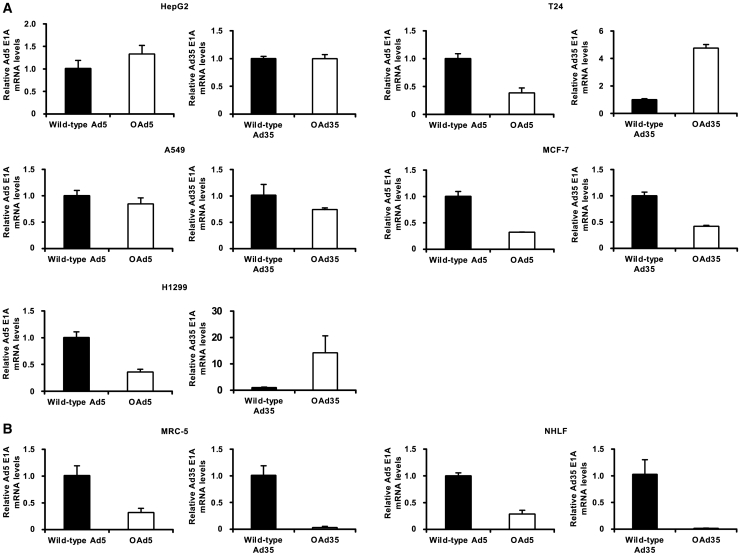
Next, in order to examine the E1A gene expression levels of OAds and wild-type Ads, real-time RT-PCR analysis was performed (Figures 4A and 4B). The results showed that OAd5-mediated E1A gene expression was comparable to or lower than that mediated by wild-type Ad5 in both tumor cells and normal cells. In particular, the E1A gene expressions by OAd5 in MRC-5 cells and NHLF were 3-fold lower than those by the wild-type Ad5. On the other hand, the OAd35-mediated E1A gene-expression levels differed among the tumor cells. OAd35 mediated comparable or lower levels of the E1A gene expression than wild-type Ad35 in HepG2, A549, and MCF-7 cells, while the E1A gene expressions by OAd35 in T24 and H1299 cells were 4- and 15-fold higher than that by wild-type Ad35, respectively. The finding that OAd35 mediated higher levels of E1A gene expression in T24 and H1299 cells than wild-type Ad35 agreed with the result that the tumor cell lysis activities by OAd35 were higher than those by the wild-type Ad35 in these tumor cells. Similar to OAd5, OAd35 induced more than 10-fold lower levels of E1A gene expression than wild-type Ad35 in the normal cells. These results indicated that the hTERT promoter-regulated expression of the E1A gene led to the tumor cell-specific lysis activities of OAd5 and OAd35.
Figure 4.
The E1A gene expression following infection with OAds and wild-type Ads
(A and B) Human tumor (A) and normal (B) cells were infected with OAds and wild-type Ads at 100 VP/cell. Total RNA was recovered at 72 h after infection, followed by real-time RT-PCR analysis of the E1A genes. The values were normalized by the mRNA levels of a housekeeping gene, human GAPDH. The E1A gene expression levels of wild-type Ads were normalized to 1. These data are expressed as the means ± SD (n = 4).
Virus genome replication of OAd35 in tumor cells
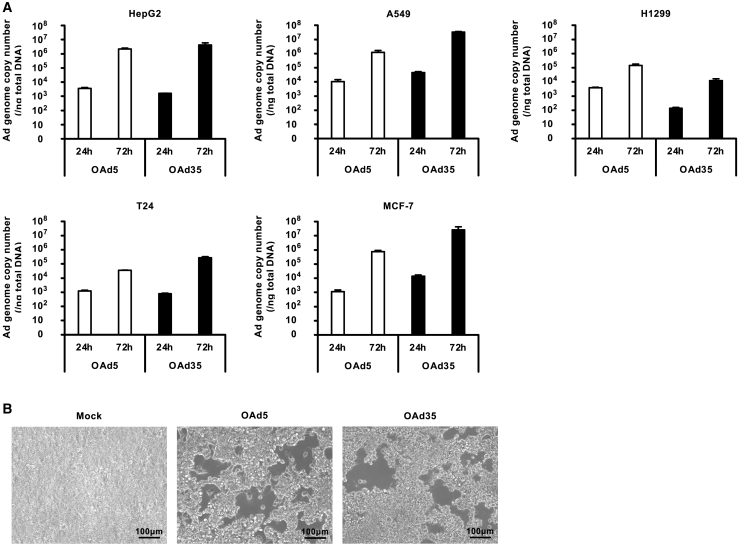
In order to examine whether the OAd35 genome efficiently replicated in tumor cells, we measured the Ad genome copy numbers in the tumor cells 24 and 72 h after virus infection (Figure 5A). The OAd35 genome copy numbers were similar to or higher than the OAd5 genome copy numbers in CAR-positive HepG2 cells and A549 cells, respectively. At 72 h after infection, the genome copy numbers of OAd35 were 2-fold and 27-fold higher than those of OAd5 in HepG2 and A549 cells, respectively, whereas in H1299 cells the genome copy numbers of OAd35 were 11-fold lower than those of OAd5. On the other hand, the OAd35 genome more efficiently replicated in the CAR-negative cells than the OAd5 genome. In T24 cells, the OAd5 genome copy numbers increased by approximately 30-fold from 24 h to 72 h, whereas the OAd35 genome exhibited a more than 300-fold increase in replication efficiency over this period. In MCF-7 cells, the OAd5 genome copy numbers increased by approximately 670-fold, while the OAd35 genome copy numbers increased by more than 1,800-fold.
Figure 5.
OAd35 genome copy numbers in tumor cells
(A) Human tumor cell lines were infected with OAds at 100 VP/cell. Total DNA was recovered at the indicated time points, followed by real-time PCR analysis of viral genome copy numbers. These data are expressed as the means ± SD (n = 3). (B) HepG2 cells were infected with OAds at 0.001 VP/cell. Following an 8-day incubation after infection, phase-contrast photomicrographs were obtained. Scale bar, 100 μm.
In order to examine the virus-spread-based oncolytic activities of OAds, plaque-forming assay was performed following infection of HepG2 cells at 0.001 VP/cell (Figure 5B). Both OAds formed similar sizes of plaques in HepG2 cells after an 8-day incubation. These data indicated that the OAd35 genome efficiently replicated and produced progeny virus in tumor cells and showed tumor cell killing activities by spread of infection to adjacent tumor cells.
Tumor cell lysis activities of OAd35 in the presence of anti-Ad5 neutralizing antibodies
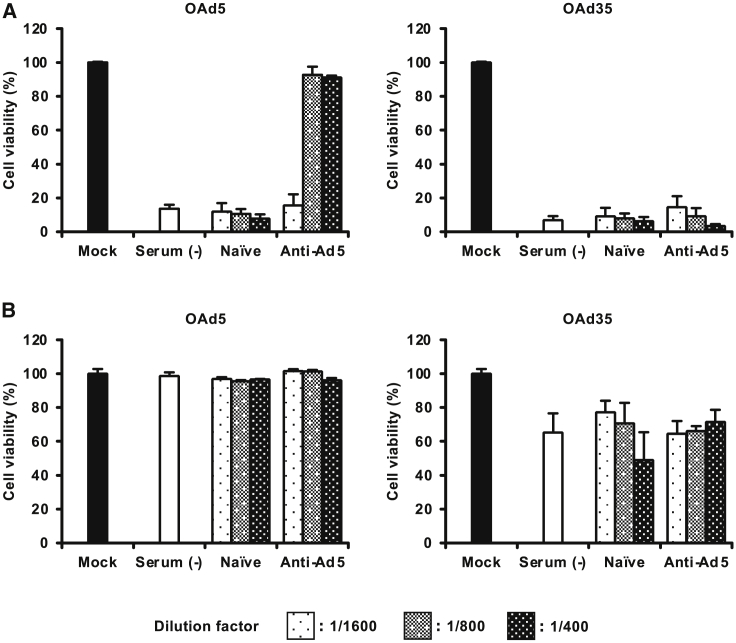
Next, in order to examine the in vitro tumor cell lysis activities of OAds in the presence of anti-Ad5 neutralizing antibodies, OAds were pre-incubated with anti-Ad5 serum recovered from the pre-immunized mice and were added to the tumor cells. OAd5-mediated lysis of HepG2 cells was significantly inhibited in the presence of 400- and 800-fold diluted anti-Ad5 serum (Figure 6A). The cell viabilities following OAd5 infection in the presence of naive serum were less than 12%, while approximately 90% of HepG2 cells were alive following OAd5 infection in the presence of 400- and 800-fold diluted anti-Ad5 serum. In contrast, OAd35-mediated tumor cell lysis activities were not inhibited by anti-Ad5 serum. The viabilities of HepG2 cells following OAd35 infection were 5% or less under all the anti-Ad5 serum concentrations used. OAd35 also exhibited similar levels of cell lysis activities in T24 cells in the presence of naive and anti-Ad5 serum (Figure 6B). These results indicated that OAd35 efficiently killed human tumor cells in vitro in the presence of anti-Ad5 neutralizing antibodies.
Figure 6.
Tumor cell lysis activities of OAd35 in the presence of anti-Ad5 serum
(A and B) HepG2 (A) and T24 (B) cells were infected with OAds at 300 VP/cell in the presence or absence of mouse anti-Ad5 serum. As a control, serum collected from naive mice was used. Cell viabilities were determined by WST-8 assay following a 5-day incubation. The viability in the mock-infected group was normalized to 100%. These data are expressed as the means ± SD (n = 4).
OAd35-mediated growth suppression of subcutaneous tumors following intratumoral administration
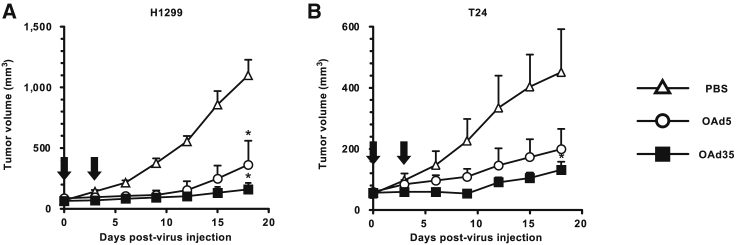
In order to examine the in vivo antitumor effects of OAd35, OAds were intratumorally administered in the mice bearing subcutaneous H1299 and T24 tumors (Figures 7A and 7B). H1299 and T24 cells were CAR-positive and -negative, respectively (Figure 2). Growth of the subcutaneous H1299 tumors was significantly suppressed following intratumoral administration of OAd5 and OAd35 (Figure 7A). Although the in vitro tumor cell lysis activities of OAd5 were higher than those of OAd35 in H1299 cells (Figure 3A), there were no statistically significant differences in the tumor growth-suppression effects of OAd5 and OAd35. OAd35 mediated significant growth inhibition of the CAR-negative T24 tumors (Figure 7B). Although OAd5 tended to inhibit the growth of T24 tumors, statistically significant differences between the tumor growth of PBS-treated and OAd5-treated T24 tumors were not found. These results indicated that OAd35 mediated efficient in vivo antitumor effects on both CAR-positive and -negative tumors following intratumoral administration.
Figure 7.
Tumor growth following intratumoral administration of OAd35
(A and B) OAds were intratumorally injected into (A) H1299 and (B) T24 tumor-bearing mice at a dose of 2.4 × 109 VP/mouse. Arrows indicate the number of days after virus injection (days 0 and 3). Tumor volume is expressed as the mean tumor volume ± SE. ∗p < 0.05 (versus PBS; H1299 tumor; n = 7, T24 tumor; n = 6).
Discussion
OAd5 has shown significant antitumor effects in not only preclinical studies but also clinical trials; however, low infection efficiencies in CAR-negative tumor cells have been reported.3, 4, 5, 6, 7, 8, 9 Furthermore, neutralizing anti-Ad5 antibodies might inhibit antitumor effects of OAd5. In order to overcome these problems, we developed OAd35 in this study. OAd35 recognizes CD46 as an infection receptor. CD46 is ubiquitously expressed on all human cells except erythrocytes. CD46 is a complement regulatory protein and plays a role in protecting cells from cell damage caused by the complement system. Malignant tumor cells often express high levels of CD46 to evade the immune system,16,22 suggesting that CD46 is a suitable target for virotherapy against tumors. A previous study demonstrated that the tumor cell lysis efficiencies of oncolytic measles virus, which infects via CD46, were correlated with CD46 expression levels on the tumor cells.23,24 CD46 expression levels on tumor cells would be a predictive biomarker of antitumor effects of OAd35. Moreover, our group reported that Ad35 downregulated CD46 expression from the cell surface following infection.25 This finding suggested that OAd35 also downregulated CD46 expression on tumor cells following infection, leading to additional antitumor effects by activation of complement-mediated antitumor immunity.
The physical titers of OAd35 were approximately 10-fold lower than those of OAd5 (Table 1), although OAd35 efficiently replicated in all the tumor cell lines used in this study at levels similar to OAd5 (Figure 5A). A replication-incompetent Ad35 vector has been reported to have a lower titer than an Ad5 vector.26 The Ad5 genome encodes adenovirus death protein (ADP), which is located in the E3 region. ADP is known to play an important role in virus growth, cell lysis, and the spread of Ad.27,28 However, the Ad35 genome does not encode ADP. The absence of ADP in OAd35 might have been partly responsible for the finding that the titers of OAd35 were lower than those of OAd5. Alternatively, HEK293 cells might thus be less suitable as a packaging cell line for OAd35. Since OAd35 efficiently replicates in various types of tumor cell lines, other cell lines can be used as packaging cells. Optimization of virus production, including the packaging cells, will be a crucial issue in the clinical development of OAd35.
We found approximately 10- to 100-fold lower levels of the OAd5 genome in T24 cells than in the CAR-positive tumor cells, indicating that OAd5 less efficiently infected T24 cells due to the lack of CAR expression on T24 cells. On the other hand, the levels of OAd5 genome copy numbers in MCF-7 cells were similar to those in CAR-positive tumor cells, although the OAd5-mediated cytotoxicity levels in MCF-7 cells were much lower than those in CAR-positive cells. The discrepancy in MCF-7 cells remained unclear. MCF-7 cells might be more resistant to OAd5-induced cell death than the CAR-positive tumor cells used in this study.
It is controversial whether anti-Ad5 antibodies inhibit the antitumor effects of OAd5. The inhibitory effects of anti-Ad5 antibodies on the antitumor effects of OAd5 have differed substantially among studies due to the different experimental conditions used, including the virus doses, titers of anti-Ad5 antibodies, and administration routes of OAd5.29, 30, 31, 32, 33 There is a possibility that the antitumor effects of OAd5 are inhibited by neutralizing anti-Ad5 antibodies, especially when the neutralizing anti-Ad5 antibody titers are high or when the antibodies can easily access the OAd5 before attachment to tumor cells. OAd35 can circumvent neutralizing anti-Ad5 antibodies even under such conditions, which is an advantage of OAd35.
Several studies have reported that various fiber modifications, including replacement of the Ad5 fibers with the fiber proteins of other serotypes and insertion of the Arg-Gly-Asp (RGD) peptide motif or polylysine into fibers, significantly improved the potent antitumor effects of OAds, especially on CAR-negative tumor cells.34, 35, 36 However, such fiber modifications cannot evade the anti-Ad5 neutralizing antibodies that recognize the hexon, although it is controversial whether antitumor effects of OAd5 are significantly inhibited by neutralizing anti-Ad5 antibodies.29, 30, 31, 32, 33 Anti-Ad5 neutralizing antibodies mainly recognized the hexon proteins,37,38 which are major outer capsid proteins of Ad, leading to inhibition of Ad5 infection. Anti-Ad5 neutralizing antibodies are one of the concerns for OAd5-mediated virotherapy, especially when neutralizing anti-Ad5 antibodies easily access to OAd5, as discussed above. A previous study from our group demonstrated that when an Ad5 vector was intratumorally injected into mice pre-immunized with an Ad5 vector, the transduction efficiencies in the tumors of pre-immunized mice were significantly lower than those in the tumors of non-immunized mice.39 Vaccination effects of intramuscularly injected Ad5 vector were inhibited by anti-Ad5 neutralizing antibodies.40 Hence, avoiding anti-Ad5 neutralizing antibodies might enhance the antitumor effects of OAds. For this purpose, several groups developed OAds fully composed of serotypes other than Ad5, such as Ad3 or Ad6.41,42 These OAds exhibited high-level anti-tumor effects in the presence of anti-Ad5 neutralizing antibodies. However, the seroprevalences of Ad3 and Ad6 in adults were higher than that of Ad35,43 indicating that infections with OAds composed of Ad3 or Ad6 are more likely to be inhibited by pre-existing neutralizing antibodies, compared with infection with OAd35. Moreover, a previous study has reported that titers of anti-Ad35 neutralizing antibodies remained lower after repeated administration of an Ad35 vector, than after repeated administration of an Ad5 vector,44 suggesting that OAd35 mediates efficient antitumor effects following repeated administration. An OAd based on Ad11, which belongs to species B2,45,46 also uses CD46 as an infection receptor. However, the transduction efficiencies of a fiber-substituted Ad5 vector bearing the fiber protein of Ad35 were higher than those of a fiber-substituted Ad5 vector bearing the fiber protein of Ad11, suggesting that the affinity of Ad35 fiber protein to CD46 would be higher than that of Ad11 fiber protein.47,48 These findings suggest that OAd35 might show a higher level of antitumor effects than OAd11.
In conclusion, we developed a novel OAd fully composed of Ad35 in this study. OAd35 showed high tumor cell lysis activities in not only CAR-positive but also CAR-negative tumor cells without apparent toxicity to normal cells. The in vitro tumor cell lysis activities of OAd35 were not inhibited by anti-Ad5 antibodies. Moreover, OAd35 showed significant antitumor effects on both CAR-positive and -negative tumors following intratumoral administration. These results indicated that OAd35 can become a promising alternative oncolytic virus.
Materials and methods
Cells
HEK293 cells (a transformed human embryonic kidney cell line) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM L-glucose, 100 μg/mL streptomycin, and 100 U/mL penicillin. A549 (a human lung epithelial cell line), HepG2 (a human hepatocellular carcinoma cell line), T24 (a human urinary bladder carcinoma cell line), normal human lung fibroblast (NHLF; Lonza, CC-2512), and MRC-5 (a normal fetal human diploid lung fibroblast) cells were cultured in DMEM supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. H1299 (a non-small cell lung carcinoma cell line) and MCF-7 (a human breast carcinoma cell line) cells were cultured in RPMI1640 supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin.
Plasmids
An OAd5 plasmid, pAdHM3-hTERT-E1, was constructed as follows. pHM5-hTERT-E1, which includes the hTERT promoter and the Ad5 E1 gene (Ad5 genome, bp 560-3509), and an E1-deleted Ad5 vector plasmid, pAdHM3,49 were digested with I-CeuI/PI-SceI and then ligated, resulting in pAdHM3-hTERT-E1. An OAd35 plasmid, pAdMS2-hTERT-E1, was constructed as follows. pAd35-hTERT-E1, which includes the hTERT promoter, the mutant Ad35 E1 gene (Ad35 genome, bp 569-3400), and the homology arm (Ad35 genome, bp 1-455, bp 3401-4603), was digested with SalI. The sequence of 5′-TTGCACTGCTATGAA-3′ (Ad35 genome, bp 921-935) in the Ad35 E1 gene was changed to 5′-GTGCACTCCTATGAT-3′ by reference to the previous studies to circumvent the binding between the E1A protein and STING (stimulation of interferon genes), which are involved in innate immune responses.50, 51, 52, 53 The binding between the E1A protein and STING caused competitive inhibition of STING, resulting in suppression of anti-tumor immunity. pAdMS2-hTERT-E1 was produced by homologous recombination of the SalI-digested pAd35-hTERT-E1 with the SwaI-digested E1-deleted Ad35 vector genome plasmid, pAdMS2,21 in Escherichia coli BJ5183. Further details on the construction methods are available upon request.
Viruses
PacI-digested pAdHM3-hTERT-E1 and SbfI-digested pAdMS2-hTERT-E1 were transfected into HEK293 cells using Lipofectamine 2000 (Thermo Fisher Scientific, San Jose, CA, USA), producing OAd5 and OAd35, respectively. OAd5 was propagated in H1299 cells, while OAd35 was propagated in HEK293 cells. These OAds were purified by two rounds of cesium chloride gradient ultracentrifugation, dialyzed, and stored at −80°C. The determination of VP titers was accomplished according to Maizel et al.54 Biological titers of OAds were determined by a 50% tissue culture infectious dose (TCID50) assay using HEK293 cells.
Flow cytometric analysis
For the measurement of human CAR expression on tumor cells, cells (5 × 105 cells) were labeled with mouse anti-CAR monoclonal antibody (RmcB; Merck Millipore, Darmstadt, Germany) and then incubated with phycoerythrin (PE)-conjugated goat anti-mouse immunoglobulin G (IgG) secondary antibody (BD PharMingen, San Diego, CA, USA). For the measurement of human CD46 expression on tumor cells, cells were labeled with PE-conjugated mouse anti-CD46 monoclonal antibody (8E2; Thermo Fisher Scientific). Mouse IgG1, kappa isotype control antibody (BD PharMingen) was used as a negative control. Flow cytometric analysis was performed using a MACS Quant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). The data were analyzed using FlowJo flow cytometry data analysis software (TreeStar, San Carlos, CA, USA).
Evaluation of cell lysis activities of OAds
In the crystal violet staining assay, cells were seeded on a 24-well plate at a density of 2–5 × 104 cells/well. On the following day, cells were infected with OAds and wild-type Ads at the indicated multiplicities of infection (MOIs). After a 5-day incubation, the medium was removed. Cells were then fixed with 4% paraformaldehyde phosphate buffer solution (FUJIFILM Wako Pure Chemical, Osaka, Japan) for 1 h at room temperature and then treated with 1 mL of 2% crystal violet in 100% methanol. The plates were washed, dried, and observed.
In the WST-8 assay, cells were seeded on a 96-well plate at a density of 0.5–1 × 104 cells/well. On the following day, cells were infected with OAds and wild-type Ads at 300 VP/cell. Cell viabilities were determined using a cell counting kit-8 (Dojindo Laboratories, Kumamoto, Japan) on the indicated days.
Real-time RT-PCR analysis
Cells were seeded on a 12-well plate at a density of 0.4–1 × 105 cells/well. On the following day, cells were infected with OAds and wild-type Ads at 100 VP/cell. Total RNA was then recovered using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions at 72 h after infection. cDNA was synthesized using a Superscript VILO cDNA synthesis kit (Thermo Fisher Scientific). Real-time RT-PCR was performed using a StepOnePlus System (Thermo Fisher Scientific) and THUNDERBIRD SYBR qPCR Mix reagents (TOYOBO, Osaka, Japan). The values were normalized by the mRNA levels of a housekeeping gene, human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of the primers are described in Table S1.
Determination of Ad genome copy numbers
Cells were seeded on a 12-well plate at a density of 0.5–1 × 105 cells/well. On the following day, cells were infected with OAds at 100 VP/cell. Total DNA, including Ad genomic DNA, was isolated by using DNAzol (Molecular Research Center, Cincinnati, OH, USA) at 24 and 72 h after infection. The Ad genome copy numbers were quantified by real-time PCR analysis using the primers for the Ad5 E4 and the Ad35 E1A genes, a StepOnePlus System (Thermo Fisher Scientific), and THUNDERBIRD SYBR qPCR Mix reagents (TOYOBO). The sequences of the primers are described in Table S1.
Plaque-forming assay
HepG2 cells were seeded on a collagen-coated 12-well plate at a density of 1 × 105 cells/well. On the following day, cells were infected with OAds at 0.001 VP/cell. After 2 h from infection, culture medium containing virus was removed, followed by addition of Temin’s Modified Eagle Medium (MEM) supplemented with 10% FBS, 0.5% agarose, 100 μg/mL streptomycin, and 100 U/mL penicillin to the wells. After an 8-day incubation, plaques were observed by phase-contrast microscopy (BIOREVO BZ-9000, KEYENCE, Osaka, Japan)
Immunization with an Ad5 vector
An E1-deleted replication-incompetent Ad5 vector, Ad-L2,49 was intravenously administered to C57BL/6 mice at a dose of 1.0 × 1010 VP/mouse. Blood samples were collected via retro-orbital bleeding 19 days after administration. Blood samples were allowed to clot for 30 min at room temperature. After incubation overnight at 4°C, samples were centrifuged at 3,000 rpm for 5 min. The supernatants were collected as anti-Ad5 serum.
In vivo animal experiments
H1299 cells (3 × 106 cells per mouse) and T24 cells (approximately 1–2 mm3 pieces of tumor tissue) were subcutaneously injected into the right flank of 5-week-old female BALB/c nu/nu mice (Nippon SLC, Hamamatsu, Japan) with 50% Matrigel (Corning, Corning, NY, USA). T24 tumor tissues were obtained by subcutaneous transplantation of T24 cells (5 × 106 cells per mouse), which were normally cultured in vitro, into nude mice. When the tumors grew to approximately 5 to 6 mm in diameter, mice were randomly assigned into three groups. PBS, OAd5, and OAd35 were intratumorally injected to each group at a dose of 2.4 × 109 VP/mouse, followed by reinjection 3 days after the first administration. The tumors were measured every 3 days using vernier calipers. Tumor volume was calculated by the following formula: tumor volume (mm3) = 1/2 × a × b2, where a is the longest dimension and b is the shortest.55 These experiments were approved by the Animal Experiment Committee of Osaka University.
Statistical analyses
Student’s t test and one way-ANOVA with Dunnett’s post hoc test were used for statistical analyses. These analyses were done with Graph Pad Prism (GraphPad Software, San Diego, CA, USA). Data are presented as means ± SD or SE.
Acknowledgments
We thank Sena Ikemoto, Kazuo Takayama, Eiko Sakai (Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan), and Toshiyoshi Fujiwara (Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan) for their support. This study was supported by grants-in-aid for Scientific Research (A) (20H00664) from the Ministry of Education, Culture, Sports, Science and Technology(MEXT) of Japan and the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from the Japanese Agency for Medical Research and Development (AMED) under grant numbers JP20am0101084 and JP20am0101123, and Grant from Oncolys Biopharma, Inc.
Author contributions
R.O., K.T., F.S., and H.M. designed the experiments. R.O., K.T., and F.S. performed the experiments and analyzed the data. R.O. and F.S. wrote the manuscript. H.M. supervised the project and revised the manuscript.
Declaration of interests
F.S. and H.M. have the potential to receive patent royalities from Oncolys BioPharm, Inc. The other authors declare no conflicts of interest.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2021.01.015.
Contributor Information
Fuminori Sakurai, Email: sakurai@phs.osaka-u.ac.jp.
Hiroyuki Mizuguchi, Email: mizuguch@phs.osaka-u.ac.jp.
Supplemental information
References
- 1.Zheng M., Huang J., Tong A., Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington K., Freeman D.J., Kelly B., Harper J., Soria J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 3.Rauen K.A., Sudilovsky D., Le J.L., Chew K.L., Hann B., Weinberg V., Schmitt L.D., McCormick F. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res. 2002;62:3812–3818. [PubMed] [Google Scholar]
- 4.Huang K.C., Altinoz M., Wosik K., Larochelle N., Koty Z., Zhu L., Holland P.C., Nalbantoglu J. Impact of the coxsackie and adenovirus receptor (CAR) on glioma cell growth and invasion: requirement for the C-terminal domain. Int. J. Cancer. 2005;113:738–745. doi: 10.1002/ijc.20623. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y.Y., Wang X.J., Han Y., Li G., Wang H.J., Wang S.B., Chen X.Y., Liu F.L., He X.L., Tong X.M., Mou X.Z. Loss of coxsackie and adenovirus receptor expression in human colorectal cancer: A potential impact on the efficacy of adenovirus-mediated gene therapy in Chinese Han population. Mol. Med. Rep. 2016;14:2541–2547. doi: 10.3892/mmr.2016.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbink P., Lemckert A.A.C., Ewald B.A., Lynch D.M., Denholtz M., Smits S., Holterman L., Damen I., Vogels R., Thorner A.R. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker A.L., Waddington S.N., Buckley S.M.K., Custers J., Havenga M.J.E., van Rooijen N., Goudsmit J., McVey J.H., Nicklin S.A., Baker A.H. Effect of neutralizing sera on factor x-mediated adenovirus serotype 5 gene transfer. J. Virol. 2009;83:479–483. doi: 10.1128/JVI.01878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun C., Zhang Y., Feng L., Pan W., Zhang M., Hong Z., Ma X., Chen X., Chen L. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine. 2011;29:3837–3841. doi: 10.1016/j.vaccine.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Barouch D.H., Kik S.V., Weverling G.J., Dilan R., King S.L., Maxfield L.F., Clark S., Ng’ang’a D., Brandariz K.L., Abbink P. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaggar A., Shayakhmetov D.M., Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 11.Marttila M., Persson D., Gustafsson D., Liszewski M.K., Atkinson J.P., Wadell G., Arnberg N. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 2005;79:14429–14436. doi: 10.1128/JVI.79.22.14429-14436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liszewski M.K., Post T.W., Atkinson J.P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 13.McNearney T., Ballard L., Seya T., Atkinson J.P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J. Clin. Invest. 1989;84:538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J. Immunol. 1990;145:238–245. [PubMed] [Google Scholar]
- 15.Buettner R., Huang M., Gritsko T., Karras J., Enkemann S., Mesa T., Nam S., Yu H., Jove R. Activated signal transducers and activators of transcription 3 signaling induces CD46 expression and protects human cancer cells from complement-dependent cytotoxicity. Mol. Cancer Res. 2007;5:823–832. doi: 10.1158/1541-7786.MCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 16.Fishelson Z., Donin N., Zell S., Schultz S., Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 17.Vogels R., Zuijdgeest D., van Rijnsoever R., Hartkoorn E., Damen I., de Béthune M.P., Kostense S., Penders G., Helmus N., Koudstaal W. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai F., Mizuguchi H., Yamaguchi T., Hayakawa T. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol. Ther. 2003;8:813–821. doi: 10.1016/s1525-0016(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 19.Seshidhar Reddy P., Ganesh S., Limbach M.P., Brann T., Pinkstaff A., Kaloss M., Kaleko M., Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 20.Shashkova E.V., May S.M., Barry M.A. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009;394:311–320. doi: 10.1016/j.virol.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai F., Kawabata K., Yamaguchi T., Hayakawa T., Mizuguchi H. Optimization of adenovirus serotype 35 vectors for efficient transduction in human hematopoietic progenitors: comparison of promoter activities. Gene Ther. 2005;12:1424–1433. doi: 10.1038/sj.gt.3302562. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer E., Havenga M.J., Ophorst O., de Leeuw B., Gijsbers L., Gillissen G., Hoeben R.C., ter Horst M., Nanda D., Dirven C. Human adenovirus type 35 vector for gene therapy of brain cancer: improved transduction and bypass of pre-existing anti-vector immunity in cancer patients. Cancer Gene Ther. 2007;14:211–219. doi: 10.1038/sj.cgt.7701010. [DOI] [PubMed] [Google Scholar]
- 23.Anderson B.D., Nakamura T., Russell S.J., Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 24.Ong H.T., Timm M.M., Greipp P.R., Witzig T.E., Dispenzieri A., Russell S.J., Peng K.W. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 2006;34:713–720. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai F., Akitomo K., Kawabata K., Hayakawa T., Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai F., Mizuguchi H., Hayakawa T. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 2003;10:1041–1048. doi: 10.1038/sj.gt.3301959. [DOI] [PubMed] [Google Scholar]
- 27.Tollefson A.E., Scaria A., Hermiston T.W., Ryerse J.S., Wold L.J., Wold W.S. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tollefson A.E., Ryerse J.S., Scaria A., Hermiston T.W., Wold W.S. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 29.Dhar D., Spencer J.F., Toth K., Wold W.S.M. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J. Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa N., Abei M., Yokoyama K.K., Fukuda K., Seo E., Kawashima R., Nakano Y., Yamada T., Nakade K., Hamada H. Cyclophosphamide enhances antitumor efficacy of oncolytic adenovirus expressing uracil phosphoribosyltransferase (UPRT) in immunocompetent Syrian hamsters. Int. J. Cancer. 2013;133:1479–1488. doi: 10.1002/ijc.28132. [DOI] [PubMed] [Google Scholar]
- 31.Thomas M.A., Spencer J.F., Toth K., Sagartz J.E., Phillips N.J., Wold W.S. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the syrian hamster model. Mol. Ther. 2012;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Yu D.C., Charlton D., Henderson D.R. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- 33.Tsai V., Johnson D.E., Rahman A., Wen S.F., LaFace D., Philopena J., Nery J., Zepeda M., Maneval D.C., Demers G.W., Ralston R. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin. Cancer Res. 2004;10:7199–7206. doi: 10.1158/1078-0432.CCR-04-0765. [DOI] [PubMed] [Google Scholar]
- 34.Kanerva A., Hemminki A. Modified adenoviruses for cancer gene therapy. Int. J. Cancer. 2004;110:475–480. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- 35.Rivera A.A., Davydova J., Schierer S., Wang M., Krasnykh V., Yamamoto M., Curiel D.T., Nettelbeck D.M. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K., Fueyo J., Krasnykh V., Reynolds P.N., Curiel D.T., Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 37.Bradley R.R., Lynch D.M., Iampietro M.J., Borducchi E.N., Barouch D.H. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J. Virol. 2012;86:625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumida S.M., Truitt D.M., Lemckert A.A., Vogels R., Custers J.H., Addo M.M., Lockman S., Peter T., Peyerl F.W., Kishko M.G. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 39.Tomita K., Sakurai F., Tachibana M., Mizuguchi H. Correlation between adenovirus-neutralizing antibody titer and adenovirus vector-mediated transduction efficiency following intratumoral injection. Anticancer Res. 2012;32:1145–1152. [PubMed] [Google Scholar]
- 40.Roberts D.M., Nanda A., Havenga M.J.E., Abbink P., Lynch D.M., Ewald B.A., Liu J., Thorner A.R., Swanson P.E., Gorgone D.A. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 41.Hemminki O., Bauerschmitz G., Hemmi S., Lavilla-Alonso S., Diaconu I., Guse K., Koski A., Desmond R.A., Lappalainen M., Kanerva A. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 2011;18:288–296. doi: 10.1038/cgt.2010.79. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.V., Crosby C.M., Heller G.J., Mendel Z.I., Barry M.E., Barry M.A. Oncolytic adenovirus Ad657 for systemic virotherapy against prostate cancer. Oncolytic Virother. 2018;7:43–51. doi: 10.2147/OV.S155946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 2009;19:165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 44.Keefer M.C., Gilmour J., Hayes P., Gill D., Kopycinski J., Cheeseman H., Cashin-Cox M., Naarding M., Clark L., Fernandez N. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS ONE. 2012;7:e41936. doi: 10.1371/journal.pone.0041936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandberg L., Papareddy P., Silver J., Bergh A., Mei Y.F. Replication-competent Ad11p vector (RCAd11p) efficiently transduces and replicates in hormone-refractory metastatic prostate cancer cells. Hum. Gene Ther. 2009;20:361–373. doi: 10.1089/hum.2007.124. [DOI] [PubMed] [Google Scholar]
- 46.Wong H.H., Jiang G., Gangeswaran R., Wang P., Wang J., Yuan M., Wang H., Bhakta V., Müller H., Lemoine N.R., Wang Y. Modification of the early gene enhancer-promoter improves the oncolytic potency of adenovirus 11. Mol. Ther. 2012;20:306–316. doi: 10.1038/mt.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L., Takenobu H., Shimozato O., Kawamura K., Nimura Y., Seki N., Uzawa K., Tanzawa H., Shimada H., Ochiai T., Tagawa M. Increased infectivity of adenovirus type 5 bearing type 11 or type 35 fibers to human esophageal and oral carcinoma cells. Oncol. Rep. 2005;14:831–835. [PubMed] [Google Scholar]
- 48.Yu L., Shimozato O., Li Q., Kawamura K., Ma G., Namba M., Ogawa T., Kaiho I., Tagawa M. Adenovirus type 5 substituted with type 11 or 35 fiber structure increases its infectivity to human cells enabling dual gene transfer in CD46-dependent and -independent manners. Anticancer Res. 2007;27(4B):2311–2316. [PubMed] [Google Scholar]
- 49.Mizuguchi H., Kay M.A. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- 50.Heise C., Hermiston T., Johnson L., Brooks G., Sampson-Johannes A., Williams A., Hawkins L., Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 51.Demaria O., De Gassart A., Coso S., Gestermann N., Di Domizio J., Flatz L., Gaide O., Michielin O., Hwu P., Petrova T.V. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lau L., Gray E.E., Brunette R.L., Stetson D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350:568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 53.Anghelina D., Lam E., Falck-Pedersen E. Diminished innate antiviral response to adenovirus vectors in cGAS/STING-deficient mice minimally impacts adaptive immunity. J. Virol. 2016;90:5915–5927. doi: 10.1128/JVI.00500-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maizel J.V., Jr., White D.O., Scharff M.D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 55.Ware J.L., DeLong E.R. Influence of tumour size on human prostate tumour metastasis in athymic nude mice. Br. J. Cancer. 1985;51:419–423. doi: 10.1038/bjc.1985.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.