Abstract
Background
Inflammation can facilitate development of coronavirus disease 2019 (COVID-19) and cardiac injury is associated with worse clinical outcomes. However, data are relatively scarce on the association between hyper-inflammatory response and cardiac injury among COVID-19 patients.
Methods
The study was designed based on severe and critically ill patients with COVID-19. Information on clinical characteristics and laboratory examinations was collected from the electronic medical records and analyzed.
Results
There were 32.4% (n = 107) of patients with cardiac injury. The median age was 67 years, and 48.8% (n = 161) of patients were men. Hypertension was the most common in 161 (48.8%) patients, followed by diabetes (16.7%, n = 55) and coronary heart disease (13.3%, n = 44). Compared to cases without cardiac injury, those with cardiac injury were older, had higher proportions of coronary heart disease, and leukocyte counts, significantly elevated concentrations of N-terminal pro-B-Type natriuretic peptide, high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)-α, interleukin-2 receptor (IL-2R), IL-6, and IL-8, but lower lymphocyte counts. A significant positive correlation was observed between high-sensitivity troponin I and inflammatory cytokines. Logistic regression analysis showed that hs-CRP, TNF-α and IL-6 were independent risk factors for cardiac injury.
Conclusions
Cardiac injury was associated with elevated levels of inflammatory cytokines among severe and critically ill patients with COVID-19, suggesting that hyper-inflammatory response may involve in cardiac injury.
Key Indexing Terms: Coronavirus, Inflammation, Troponin, Cardiac injury, COVID-19
Introduction
Coronavirus disease 2019 (COVID-19), a new type of highly contagious illness in human, has caused an emerging serious outbreak. The causative agent is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an enveloped, single-stranded, positive-sense RNA virus, producing a respiratory and systemic illness and progressing to a severe pneumonia.1 According to updated statistics reported by the World Health Organization (WHO), COVID-19 has already affected over 3 900 000 people throughout the world, carrying a fatality of approximately 6.9% by May 8, 2020. It has been reported that cardiac injury can lead to adverse cardiac events in severe and critically ill cases and is associated with worse clinical outcomes.2 , 3 Cytokines have been thought to play a pivotal role in regulating inflammatory response during virus infections.4 , 5 Accumulating evidences have shown that increased amounts of inflammatory cytokines in serum are related to multiorgan involvement and facilitate the disease development.2 , 3 , 6 To our knowledge, data are relatively scarce on the correlation between inflammation cytokines and cardiac injury. Therefore, the present study retrospectively analyzed the data from a single center in Wuhan, China, to elucidate the potential association between inflammatory cytokines and cardiac injury for guiding therapies and reducing fatality of severe and critically ill patients with COVID-19.
Materials and methods
Study design and participants
This single-center, retrospective, observational study was designed based on severe and critically ill patients with COVID-19 admitted to hospitalization at Tongji Hospital of Huazhong University of Science and Technology, located in Wuhan, China. The hospital was assigned responsibility for treatment of patients with severe and critically ill COVID-19 by the Wuhan government from February 1, 2020, to March 10, 2020. All patients were tested positive for SARS-CoV-2 by use of real-time reverse-transcriptase polymerase-chain reaction (RT-PCR) on throat swab specimens. The severe and critically illness of COVID-19 was identified according to the Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance published by the National Health Commission of China.7 Those who met following criteria were defined as severe illness: (1) Respiratory distress with respiratory rate over 30 times per minute; (2) Oxygen saturation ≤ 93% in the resting state; (3) Arterial blood oxygen partial pressure / fraction of inspired oxygen ≤ 300 mm Hg. Critically ill patients were defined as those admitted to the hospital who had either one of the following criteria: (1) Respiratory failure in need of mechanical ventilation; (2) Shock; (3) Other organ dysfunction. Patients were excluded when they had hospitalization length-of stay of < 24 hours; lacked medical records; were not laboratory-confirmed up to March 24, 2020; had history of renal insufficiency; lacked a serum high-sensitivity troponin I (hs-cTnI) measurement. A total of 330 patients aged > 42 years enrolled in our study were categorized into the cardiac injury group and the non-cardiac injury group. Cardiac injury was defined as the serum level of hs-cTnI above the 99th percentile upper reference limit (26.2 pg/mL).2
The study was conducted in accordance with the principles of the declaration of the National Health Commission of China and approved by the Research Ethics Commission of Tongji Hospital of Huazhong University of Science and Technology (Approval number WDRY2020-K061) and written informed consent was waived by the Ethics Commission of the designated hospital for patients due to the rapid emergence of this infectious disease.
Data collection
Data from the electronic medical records were obtained from February 1, 2020, to March 10, 2020. We collected information on clinical characteristics including age, gender, current smoker, heart rate, systolic blood pressure, diastolic blood pressure, cardiovascular comorbidities. We also collected chest computed χ-ray tomography (CT) images and the laboratory data on hs-cTnI, N-terminal pro-B-Type natriuretic peptide (NT-proBNP), routine blood examinations including leukocyte counts, lymphocyte counts, haemoglobin, albumin, alanine aminotransferase, aspartate aminotransferas, urea nitrogen, serum creatinine and inflammatory cytokines including high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)-α, interleukin-2 receptor (IL-2R), IL-1β, IL-6, IL-8, IL-10 on admission.
Detection procedures of RT-PCR for SARS-CoV-2
Throat swab specimens were extracted and tested for SARS-CoV-2 by real-time RT-PCR. The commercial kit (Biogerm, Shanghai, China) was used to extract total RNA. Each 200μL respiratory sample was added into Trizol in the Biosafety Level 3 laboratory. Total RNA was extracted according to the manufacturer's instructions and 40μL of cell lysates was obtained for each sample and transferred into a tube followed by vortex for 10 s. 8μL RNA was used for real-time RT-PCR according to the manufacturer′s protocol (Bio-germ Medical Technology company, Shanghai, China), which targeted the ORF1ab gene and the N gene. The reaction conditions as following: incubation at 50°C for 15 min and 95°C for 5 min, 45 cycles of amplification at 95°C for 10 s, and extending at 55°C for 45 s. A cycle threshold value less than 37 was defined as a positive result, and a cycle threshold value of 40 or more was defined as a negative test. The positive confirmatory case of SARS-CoV-2 infection was defined as those with positive results of both the ORF1ab gene and the N gene tests from laboratory in accordance with the WHO guidance.
Statistical analysis
Continuous data were presented as median with interquartile range (IQR), and categorical variables were summarized using numbers with percentages. We assessed differences between the cardiac injury group and the non-cardiac injury group using independent group t test for continuous variables that were normally distributed, or Mann-Whitney U test for continuous variables that were not normally distributed; Chi-square test for the other categorical variables. The Spearman correlation coefficient was calculated to disclose relationship of hs-cTnI with inflammatory cytokines. To explore the risk factors for cardiac injury, logistic regression analysis was used. All statistical analyses were performed using SPSS software (Statistical Package for the Social Sciences) version 26.0 (SPSS Inc.). Statistical graphs were applied to GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Tests were two-sided with statistical significance set at P value of less than 0.05.
Results
Clinical characteristics
We included 330 cases, which were clinically diagnosed as severe and critically ill COVID-19. The median age was 67 years, ranging from 42 to 92 years, and 48.8% (n = 161) of patients were men. We found that 32.4% (n = 107) of patients presented cardiac injury. Among cardiovascular comorbidities, hypertension was the most common in (48.8%, n = 161) patients, followed by diabetes (16.7%, n = 55) and coronary heart disease (CHD) (13.3%, n = 44). The serum concentrations of hs-cTnI in patients with cardiac injury significantly increased compared with those without cardiac injury [51.4 (32.4-91.8) pg/mL vs 15.0 (9.5-20.8) pg/mL, P < 0.001; Figure 1 ]. In the further analysis, we found that the patients in the cardiac injury group were significantly older than those in the non-cardiac injury group. Meanwhile, there was significant difference in the proportions of CHD between the two groups (Table 1 ).
Figure 1.
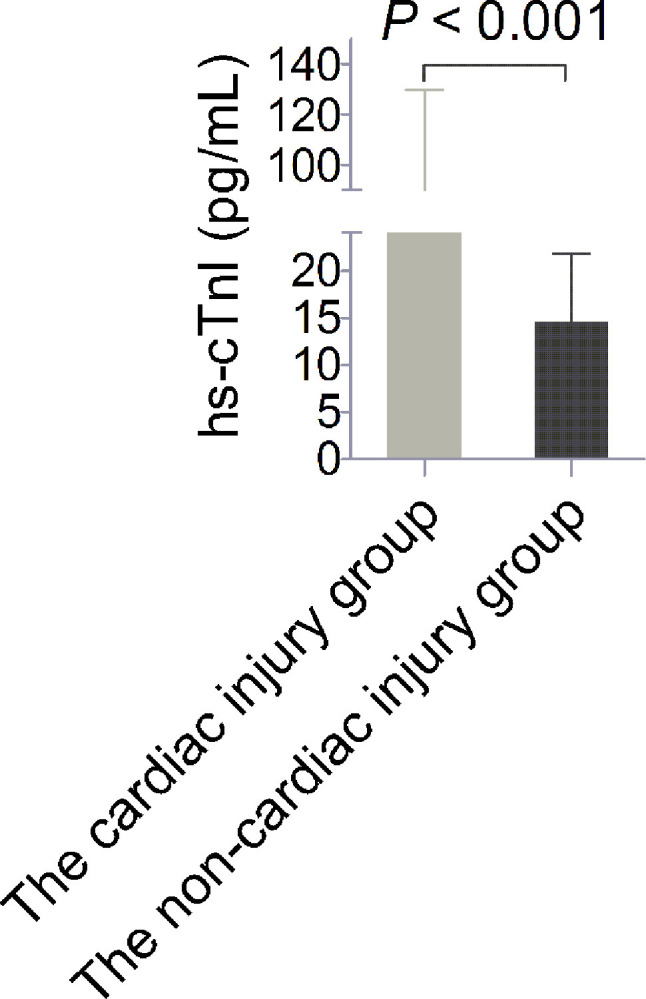
Serum concentrations of high-sensitivity troponin I (hs-cTnI). The levels of hs-cTnI in the cardiac injury group significantly increased compared with those in the non-cardiac injury group.
TABLE 1.
Clinical characteristics.
| Parameters | Total (n = 330) | Cardiac injury (n = 107) | Non-cardiac injury (n = 223) | P value |
|---|---|---|---|---|
| Age, years | 67 (59-75) | 68 (62-77) | 66 (57-73) | 0.021 |
| Male gender, n (%) | 161 (48.8) | 50 (46.7) | 111 (49.8) | 0.604 |
| Current smokers, n (%) | 67 (20.3) | 21 (19.6) | 46 (20.6) | 0.832 |
| Heart rate, beats per min | 88 (78-103) | 89 (80-107) | 88 (78-100) | 0.197 |
| SBp, mm Hg | 135 (122-146) | 137 (124-146) | 134 (122-147) | 0.952 |
| DBp, mm Hg | 78 (73-88) | 78 (75-88) | 78 (73-88) | 0.597 |
| Hypertension, n (%) | 161 (48.8) | 53 (49.5) | 108 (48.4) | 0.851 |
| CHD, n (%) | 44 (13.3) | 20 (18.6) | 24 (10.7) | 0.047 |
| Diabetes, n (%) | 55 (16.7) | 17 (15.9) | 38 (17.0) | 0.793 |
Abbreviations: SBp, systolic blood pressure; DBp, diastolic blood pressure; CHD, coronary heart disease. Continuous and categorical data were expressed as median with interquartile rang and numbers with percentages, respectively.
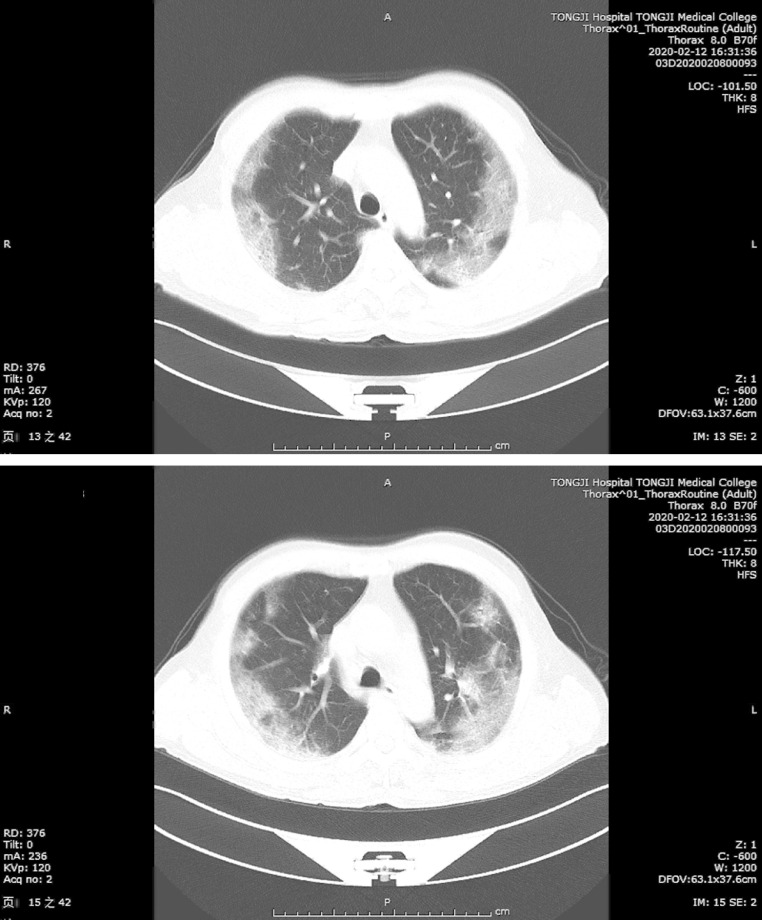
CT examination of the chest could provide great help in diagnosis of COVID-19 with typical images of chest. As shown in Figure 2 , the most common CT manifestation was multiple mottling and ground-glass opacity along the outer bands of both lungs of a men aged 70 years with severe COVID-19.
Figure 2.
Typical images of chest computed χ-ray tomography of COVID-19. Chest CT images from a severely ill man aged 70 years with COVID-19 showed multiple mottling and ground glass opacity along the outer bands of both lungs on February 12, 2020 (A, B).
Laboratory Findings Analysis
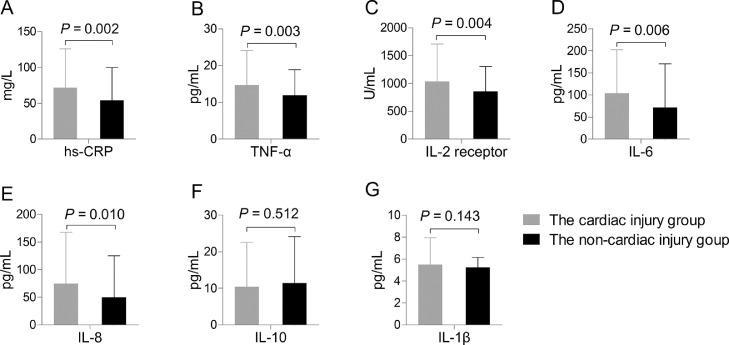
Of 330 severe and critically ill cases with COVID-19 who underwent laboratory examination on admission, the patients had elevated leukocyte counts and concentrations of NT-proBNP, and inflammatory cytokines above upper limit of normal (hs-CRP > 10.0 mg/L, TNF-α > 8.1 pg/mL, IL-2R > 710 U/mL, IL-6 > 7.0 pg/mL, IL-8 > 62 pg/mL, IL-10 > 9.1 pg/mL, and IL-1β > 5.0 pg/mL) but decreased lymphocyte counts (Table 2 ). Compared with patients in the non-cardiac injury group, those in the cardiac injury group had significantly increased levels of hs-CRP [67.5 (26.8-105.5) mg/L vs 46.4 (12.5-82.6) mg/L, P = 0.002], TNF-α [11.9 (8.3-19.9) pg/mL vs 10.2 (7.4-14.6) pg/mL, P = 0.003], IL-2R [858 (556-1305) U/mL vs 781 (530-1084) U/mL, P = 0.004], IL-6 [72.2 (31.7-150.7) pg/mL vs 33.6 (10.0-82.7) pg/mL, P = 0.006], IL-8 [42.0 (20.2-86.1) pg/mL vs 19.6 (8.8-52.5) pg/mL, P = 0.010] (Figure 3 ). However, no significant difference was found in levels of IL-10, IL-1β or other routine blood biomarkers between the cardiac injury group and the non-cardiac injury group.
TABLE 2.
Laboratory findings.
| Parameters | Total (n = 330) | Cardiac injury (n = 107) | Non-cardiac injury (n = 223) | P value |
|---|---|---|---|---|
| Leukocyte counts, cells × 109/L | 7.34 (5.37-10.45) | 9.16 (5.73-12.26) | 6.73 (5.08-10.00) | 0.004 |
| Lymphocyte counts, cells × 109/L | 0.70 (0.52-1.06) | 0.65 (0.50-1.01) | 0.75 (0.53-1.09) | 0.039 |
| Haemoglobin, g/L | 126 (110-138) | 126 (109-142) | 125 (110-136) | 0.922 |
| Albumin, g/L | 31.9 (29.6-34.7) | 31.8 (29.5-34.6) | 32.0 (29.8-35.0) | 0.395 |
| Alanine aminotransferase, U/L | 26 (17-39) | 28 (17-39) | 25 (16-40) | 0.270 |
| Aspartate aminotransferas, U/L | 34 (24-50) | 35 (25-55) | 33 (23-49) | 0.670 |
| Urea nitrogen, mmol/L | 6.6 (4.6-8.8) | 7.5 (5.3-9.2) | 6.2 (4.1-8.6) | 0.052 |
| Serum creatinine,μmol/L | 78 (58-94) | 85 (64-106) | 75 (57-93) | 0.105 |
| NT-proBNP, pg/mL | 831 (235-1949) | 969 (349-2643) | 718 (207-1785) | 0.027 |
Abbreviations: NT-proBNP, N-terminal pro-B-Type natriuretic peptide. Continuous and categorical data were expressed as median with interquartile rang and numbers with percentages, respectively.
Figure 3.
Concentrations of inflammatory cytokines. Patients in the cardiac injury group had significantly increased levels of high-sensitivity C-reactive protein (hs-CRP) (A), tumor necrosis factor (TNF)-α (B), interleukin-2 receptor (IL-2R) (C), IL-6 (D), and IL-8 (E), but no significant difference was found in levels of IL-10 (F) and IL-1β (G) between the two groups.
Correlates of hs-cTnI with inflammatory cytokines
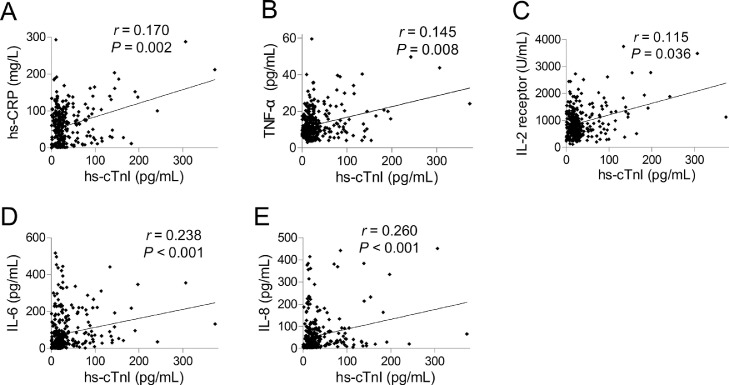
To investigate the relationship of hs-cTnI and inflammatory cytokines, we We excluded variables from the univariable analysis if their between-group differences were not significant. We discovered a significant positive correlation of hs-cTnI with hs-CRP (r = 0.170, P = 0.002), TNF-α (r = 0.145, P = 0.008), IL-2R (r = 0.115, P = 0.036), IL-6 (r = 0.238, P < 0.001), and IL-8 (r = 0.260, P < 0.001) (Figure 4 ).
Figure 4.
Correlation of high-sensitivity troponin I (hs-cTnI) with inflammatory cytokines. Significant positive correlation was analyzed between hs-cTnI and high-sensitivity C-reactive protein (hs-CRP) (A), tumor necrosis factor (TNF)-α (B), interleukin (IL)-2 receptor (C), IL-6 (D) and IL-8 (E).
Risk factors for cardiac injury
Logistic regression analysis were employed to predict the risk factors for cardiac injury. In order to avoid overfitting in the model, we fitted logistic regression models with the variables with a P value of < 0.05 identified from the univariate analysis. In stepwise logistic regression analysis, the result showed that hs-CRP (OR 1.006, 95% CI 1.001-1.012, P = 0.014), TNF-α (OR 1.039, 95% CI 1.006-1.072, P = 0.020), IL-6 (OR 1.003, 95% CI 1.001-1.005, P = 0.042) were independent risk factors for cardiac injury.
Discussion
COVID-19 infected by SARS-CoV-2, can lead to critical illness with acute respiratory distress and multi-organ damage due to the pathophysiology of inflammation.8 , 9 To the best of our knowledge, inflammatory response is considered to help protect against infection and a self-limiting process as once the infection has been cleared by immune system. However, disruption or poor regulation of this normal pattern could lead to excessive release of inflammatory cytokines, resulting in a high inflammatory burden known as hyper-inflammatory response.10
In this study, we found that 32.4% of patients suffered from cardiac injury. Therefore, our study highlighted that cardiac injury was commonly observed in the patients with severe and critically ill COVID-19. Moreover, similar evidence is available to support our findings that older cases or patients with underlying CHD were more susceptible to cardiac injury.11 Similarly, concentrations of NT-proBNP were significantly elevated in the cardiac injury group, suggesting severity of inflammatory response and ventricular dysfunction. In the univariate analysis, our data showed that leukocyte and lymphocyte counts, the levels of hs-CRP, TNF-α, IL-2R, IL-6, and IL-8 were significantly different between patients with and without cardiac injury. Moreover, our study showed that the serum levels of hs-cTnI positively correlated with increased inflammatory cytokines levels. Taken together, higher leukocyte counts, and elevated levels of inflammatory cytokines, including hs-CRP, TNF-α, IL-2R, IL-6, IL-8, were common among severe and critically ill patients, more evident in those with cardiac injury. Therefore, it is reasonable to hypothesize that the excess production of particularly, inflammatory cytokines could result enhance inflammatory responses.9 Accumulating evidences suggested that hyper-inflammatory response, a cytokines storm syndrome, reflected by elevated inflammatory cytokines, is an important contributor to cardiac injury and damage of other organs.11
In the further analysis, we found that hs-CRP, TNF-α, and IL-6 were independent risk factors for cardiac injury in severe and critically ill patients with COVID-19 using logistic regression model. The findings were in consistent with previous reports that the patients had a heightened risk of developing cardiac injury with increased levels of inflammatory cytokines.12 Based on these data, SARS-CoV-2 might aggravate inflammatory response and result in upsurge of inflammatory cytokines, which promoted hyper-inflammatory response and the development of cardiac injury.
Up to this day, the pleiotropic properties of the pro-inflammatory cytokines and their multifunctional effects involved in cardiac injury are not completely known in COVID-19. Corovirus-induced cytopathic effects and viral evasion of host inflammatory responses have been thought to play a pivotal role in the pathogenesis of organ damage.4 , 5 Although there is no direct evidence for the involvement of inflammatory cytokines in the cardiac pathology during COVID-19, the change of inflammatory cytokines in infected patients were correlated with myocardial injury. With an increase in differential evidences, hyper-inflammatory response can lead to myocardial ischemia in the presence of preexisting cardiovascular comorbidities as inflammatory cytokines are mediators of atherosclerosis directly contributing to destabilizing atherosclerotic plaques of coronary through excess inflammation.13 , 14 Inflammation also causes endothelial dysfunction and increases the procoagulant activity of the blood, which can contribute to induction of procoagulant factors and haemodynamic changes, and formation of an occlusive thrombus over a ruptured coronary artery plaque, which predispose to worsen microvascular pathophysiology.15 , 16 Based on these lines of evidences, we postulated that hyper-inflammatory response may precipitate cardiac injury in severe and critically ill patients with COVID-19.
In contrast, a previous study found that cardiac injury appeared to be common in acute SARS infection, even among those without underlying cardiovascular disease.17 On the basis of recent studies, angiotensin-converting enzyme 2, the receptor with a strong binding affinity to the spike protein of SARS-CoV-2, is highly expressed on myocytes and vascular endothelial cells, thus there is at least theoretical potential possibility of direct cardiac involvement by the coronavirus.18 , 19 However, a recent study suggested that SARS-CoV-2 might damage myocardium through inflammation rather than not directly impair the heart, and the coronavirus in the acute phase might be attributable to the cytokine storm syndrome.20 The hallmark of cytokine storm syndrome is an uncontrolled and dysfunctional inflammatory response involving the continuous activation and proliferation of lymphocytes and macrophages. Furthermore, the excess production of cytokines could lead to a deficiency in control of viral replication and more prolonged hyper-inflammatory response, inducing endothelial cell adhesion molecule synthesis and activate leukocyte integrins, mediating strong adhesive interactions that ultimately lead to extravasation of inflammatory cells into the myocardium, potentially leading to myocardial damage.21
Our study had some limitations. The retrospective, single-center, small sample study were obvious limitations. Owing to not all inflammatory biomarkers were tested in all patients, interpretation of our findings might be limited by lack of integrative functional data. Furthermore, this study was to identify broad systemic inflammation linked to cardiac injury. In addition, there were also several other clinical tools such as electrovardiogram, chocardiogram, which were unavailable, but could potentially better explain myocardial injury and warranted further research. Last but not least, due to the exploratory nature of the study, standardized data for a larger cohort would be better to identify cardiac injury. Additional research should also be planned to clarify the contributory mechanisms underlying the hyper-inflammatory response involves in cardiac injury, the findings presented here are urgently needed to consider anti-inflammatory treatment and adjunctive cardioprotective therapies in COVID-19 management for reducing fatality among severe and critically ill patients with COVID-19.
Conclusions
In summary, the data of our study explained novel information on the association between hyper-inflammatory response and myocardial damage, which indicated that hyper-inflammatory response may highly involve in cardiac injury. It is hence reasonable to consider that initial measurement of inflammatory cytokines immediately after hospitalization for COVID-19 may help screen a subset of patients with possible cardiac injury and thereby predict the progression of COVID-19 towards a worse clinical picture.
Acknowledgments
Acknowledgements
We thank all patients involved in the study.
Footnotes
Conflict of Interest: We declare no competing interests.
Funding: The authors received no specific funding for this work.
References
- 1.WHO-China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), (2020).
- 2.Shi SB, Qin M, Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CL, Wang YM, Li XG. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min CK, Cheon S, Ha NY. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Channapanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokines storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C, Zhou LQ, Hu ZW. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance. Available at: http://www.nhc.gov.cn/yzygi/s7652m/202002/41c3142b38b84ec4a748e60773cf9d4f.shtml
- 8.Yang Y, Tang H. Aberrant coagulation cause a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol. 2016;13(4):432–442. doi: 10.1038/cmi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. Proinflammatory cytokines. Chest. 2000;111(2):503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 11.Inciardi RM, Lupi L, Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madjid M, Safavi-Naeini P, Solomon SD. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 13.Madjid M, Vela D, Khalili-Tabrizi H. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Ins J. 2007;34(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- 14.Corrales-Medina VF, Musher DM, Shachkina S. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 15.Davidson JA, Warren-Gash C. Cardiovascular complications of acute respiratory infections: current research and future directions. Expert Rev Anti Infect Ther. 2019;17(12):939–942. doi: 10.1080/14787210.2019.1689817. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson J. Atherosclerotic plaque vulnerability in the stain era. Eur Heart J. 2017;38(21):1638–1644. doi: 10.1093/eurheartj/ehx143. [DOI] [PubMed] [Google Scholar]
- 17.Li SS, Cheng CW, Fu CL. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108(15):1798–1803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 18.Xin Z, Ke C, Zou J. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrapp D, Wang N, Corbett KS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Scuence. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng YY, Ma YT, Zhang JY. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(suppl 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]