Abstract
Background
Serious neurological complications of SARS-CoV-2 are increasingly being recognized.
Case
We report a novel case of HHV6 myelitis with parainfectious MOG-IgG in the setting of COVID-19-induced lymphopenia and hypogammaglobulinemia. The patient experienced complete neurological recovery with gancyclovir, high dose corticosteroids, and plasma exchange. To our knowledge, this is the first case of HHV6 reactivation in the central nervous system in the setting of COVID19 infection and the first case of MOG-IgG myelitis in the setting of SARS-CoV-2 and HHV6 coinfection.
Conclusion
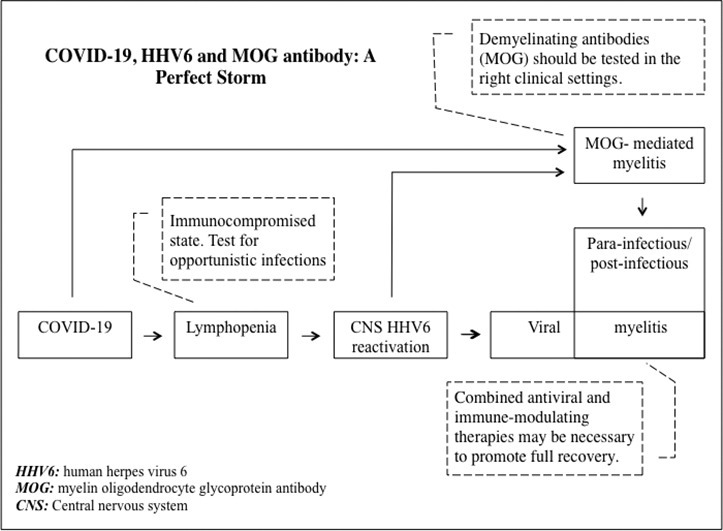
Patients with neurological manifestations in the setting of COVID19-related immunodeficiency should be tested for opportunistic infections including HHV6. Viral infection is a known trigger for MOG-IgG and therefore this antibody should be checked in patients with SARS-CoV-2 associated demyelination.
Keywords: COVID-19, SARS-CoV-2, HHV6, MOG, Transverse myelitis, Demyelination
Graphical abstract
1. Introduction
COVID-19 infection predominantly presents with fever and lower respiratory tract involvement (Needham et al., 2020). Lymphopenia is a cardinal laboratory finding (Terpos et al., 2020) that creates an acquired immunodeficiency state. Neurological presentations are increasingly being recognized and include headache, anosmia, ageusia, cerebrovascular accidents, Guillain-Barré syndrome, encephalopathy, acute encephalitis, and acute transverse myelitis (Chakraborty et al., 2020). We report a novel case of human herpesvirus-6 (HHV6) myelitis with concomitant myelin oligodendrocyte glycoprotein antibody (MOG-IgG) mediated parainfectious myelitis in a patient with COVID-19-induced immunodeficiency.
2. Case presentation
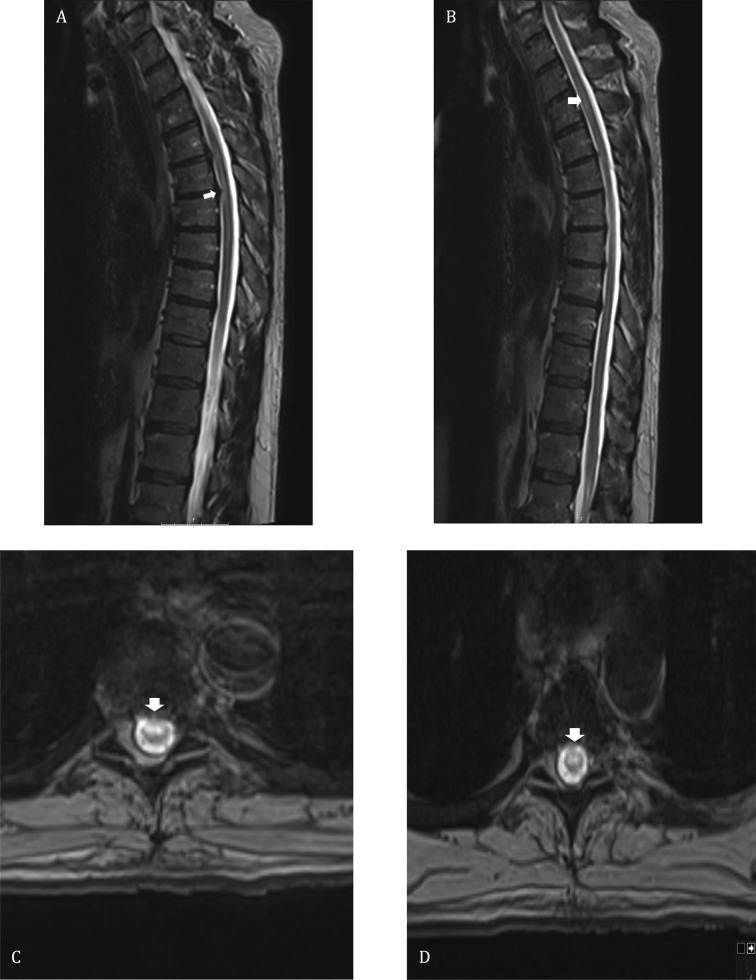
A 61-year-old man with no significant medical history presented with acute urine retention after one week of fever, chills, arthralgia, and ribcage pain. He had been recently exposed to his son who had confirmed COVID-19 infection. A Foley catheter was placed in the ER and nasopharyngeal polymerase chain reaction (PCR) tested negative for SARS-CoV-2. Over the following day, he developed progressive bilateral lower extremity weakness and decreased sensation below the ribcage. His examination was remarkable for flaccid paraplegia with a T7 sensory level. Spine MRI showed non-enhancing T2 hyperintense lesions of variable length in the mid-thoracic spinal cord, while brain MRI was unremarkable (Fig. 1 ). He had elevated CRP (3.08 mg/dL), ESR (45 mm/h), and ferritin (881 μg/L), with low absolute lymphocyte count (ALC) of 790 cells/mm3, low immunoglobulin-G level of 193 mg/dL (normal range 700–166), low immunoglobulin-M level of 12 mg/dL (normal range 40–230), and negative HIV. Both serum SARS-CoV-2 IgG and repeat nasopharyngeal PCR were positive, confirming COVID-19 infection albeit without respiratory symptoms. Interestingly, plasma human herpes virus 6 (HHV6) PCR was positive (14,500 copies/mL). Cerebrospinal fluid (CSF) analysis showed lymphocyte-predominant pleocytosis (279 cells/μL), hyperproteinorrachia (106 mg/dL), elevated myelin basic protein (16.7 mcq/L), negative oligoclonal bands, and normal IgG index and synthesis rate. Although SARS-CoV-2 PCR was negative in the CSF, CSF HHV6 PCR was significantly positive (67,000 copies/mL). Viral CSF PCR was negative for herpes simplex 1 and 2, varicella zoster, cytomegalovirus, enterovirus, and human parechovirus. The preliminary diagnosis was HHV6 myelitis in the setting of an immunocompromised state caused by COVID-19 infection. He was started on a 2-week-course of ganciclovir along with high dose intravenous methylprednisolone for presumed concomitant parainfectious inflammatory myelitis. He had mild clinical improvement with this regimen and was subsequently started on 7 sessions of plasma exchange (PLEX). Parainfectious inflammatory myelitis was later confirmed when his serum demyelinating panel tested positive for high titer (1:1000) myelin oligodendrocyte glycoprotein antibody (MOG-IgG) and negative aquaporin-4 (AQP4-IgG) antibody. MOG-IgG was tested commercially at the Mayo Clinic via live cell fluorescence-activated cell sorting assay. The patient improved significantly with PLEX and ganciclovir, and was discharged on a prednisone taper. Repeat blood tests after improvement and recovery from COVID19 showed normalization of ALC and immunoglobulin levels, declining titer of MOG-IgG, and reduction of HHV6 viral copies in plasma (2100 copies/mL). Post-treatment serum HHV6-IgM was negative and HHV6-IgG was positive. Repeat CSF sampling was not pursued due to the remarkable clinical improvement. At his outpatient appointment five weeks after discharge, his paraplegia had completely resolved with minimal residual sensory and bladder symptoms.
Fig. 1.
Spinal cord magnetic resonance imaging of the patient: (A, B) T2 weighted sagittal images of the thoracic spine shows multifocal hyperintese lesions (white arrows) throughout the thoracic spinal cord. (C, D) Axial GRE T2 weighted images demonstrate ventral and central cord hyperintensity (white arrows) at the level of T6 and T5 respectively.
3. Discussion
This case illustrates an unusual, albeit plausible, sequence of events that led to a severe, yet reversible, neurological presentation. COVID-19 infection is characterized by acquired lymphopenia, which is a predictor of disease severity (Terpos et al., 2020). This, in addition to the hypogammaglobulinemia seen in a subset of patients (Dupont et al., 2020), creates an acquired immunocompromised state during COVID-19 infection as evidenced by an increased susceptibility to secondary bacterial infections and viral reactivation (Li et al., 2020; Drago et al., 2020; Abadías-Granado et al., 2021). HHV6 myelitis is a rare disorder that is almost exclusively seen in immunocompromised patients such as post bone marrow transplantation and in the setting of HIV infection (Shiroshita et al., 2020). Given that our patient had no prior history of immunodeficiency and tested negative for HIV, the most likely trigger of his HHV6 reactivation was COVID-19-induced immunodeficiency. Most cases of true viral myelitis are thought to have a concomitant post-viral or parainfectious immune-mediated response that contributes to spinal cord inflammation, justifying the use of corticosteroids with or without anti-viral agents. This, in addition to the growing literature on post-COVID-19 immune-mediated neurological complications is what prompted us to initiate immunotherapy early on. The decision to add PLEX after the limited initial response to corticosteroids was based on literature suggesting improved outcomes of transverse myelitis with combined therapy (Greenberg et al., 2007). In this case, the combined SARS-CoV-2 and HHV6 infection likely triggered immune-mediated myelitis. This was confirmed by the positivity of MOG-IgG, an antibody linked to monophasic or recurrent demyelinating disorders including acute disseminated encephalomyelitis, optic neuritis, and transverse myelitis (Cobo-Calvo et al., 2020). MOG-IgG-related demyelination can be post-viral or idiopathic.
Several cases of post-COVID-19 myelitis have been reported often with negative SARS-CoV-2 PCR in the CSF and good response to corticosteroids, suggesting an immune-mediated etiology (Table 1 ). An increased incidence of HHV6-related diseases such as Kawasaki disease and pityriasis rosea has been reported during the COVID-19 pandemic, suggesting a link between COVID-19 immune dysfunction and reactivation of HHV6 (Dursun, 2020; Drago et al., 2020, Abadías-Granado et al., 2021). Interestingly, the HHV6 and SARS-CoV-2 viruses have each been separately linked to MOG-IgG positive transverse myelitis (Zhou et al., 2020 and Vieira et al., 2017). To our knowledge, this is the first report of possible HHV6 reactivation in the central nervous system in the setting of COVID-19 immune dysfunction, and the first with parainfectious MOG-IgG myelitis in the setting of SARS-CoV-2/HHV6 co-infection.
Table 1.
Reported cases of SARS-CoV-2-related transverse myelitis.
| Case report | Size-location | SARS-CO-V2 | HHV6 | Other viruses | Oligoclonal bands | MOG-IgG | AQP4-IgG | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Munz et al., 2020 | Short segment – thoracic | Positive pharyngeal PCR, Negative CSF PCR | Negative CSF PCR | Negative HSV CSF PCR | Negative | Negative | Negative | Steroids | Full recovery |
| Zachariadis et al., 2020 | Short segment – thoracic | Negative nasopharyngeal PCR, positive IgG and IgM on serology | Negative in serum, not reported in CSF | Negative | Negative | Negative | Negative | IVIG, steroids | Partial recovery |
| Sotoca and Rodríguez-Álvarez, 2020 | Longitudinally extensive from the medulla to the thoracic cord | Positive oropharyngeal PCR, Negative CSF PCR | Negative serum and CSF PCR | Negative | Negative | Negative | Negative | Plasma exchange, steroids | Partial recovery |
| Sarma and Bilello, 2020 | Longitudinally extensive from the medulla to the conus | Positive oropharyngeal PCR, Negative CSF PCR | Not reported | Not reported | Not reported | Not reported | Not reported | Plasma exchange and steroids | Partial recovery |
| Valiuddin et al., 2020 | Longitudinally extensive – cervical | Positive nasopharyngeal PCR, Negative CSF PCR | Not reported | Not reported | Negative | Negative in CSF | Not reported | Steroids and plasma exchange | Partial recovery (little improvement) |
| Alketbi et al., 2020 | Longitudinally extensive from the cervical cord to the conus | Positive nasopharyngeal PCR, CSF not reported | Not reported | Negative - Serum HSV 1 and 2, adenovirus, EBV, CMV and HIV | Not reported | Not reported | Not reported | Steroids | Partial recovery |
| Baghbanian and Namazi, 2020 | Longitudinally extensive thoracic | Positive nasopharyngeal PCR, Negative CSF PCR | Not reported | Negative -CSF PCR for CMV and HSV | Negative | Negative | Negative | Plasma exchange | Partial recovery |
| Chow et al., 2020 | Longitudinally extensive – thoracic | Positive nasopharyngeal PCR, Negative CSF PCR | Not reported | Negative - Serology for EBV, CMV, HIV, hepatitis B and C | Negative | Negative | Negative | steroids | Full recovery |
| Chakraborty et al., 2020 | Short segment - thoracic | Positive nasopharyngeal PCR, negative CSF PCR | Not reported | Negative - serology for hepatitis B, hepatitis C, HIV I and II | Not reported | Not reported | Not reported | Steroids | N/A – died of respiratory failure 1 day after treatment |
| Abdelhady et al., 2020 | Longitudinally extensive – thoracic | Positive nasopharyngeal PCR, Negative CSF PCR | Not reported | Negative - hepatitis B and C, herpes simplex viruses | Not reported | Not reported | Not reported | Steroids+ acyclovir | N/A – died 2 days after MRI from cardiac arrest |
| Saberi et al., 2020 | Longitudinally extensive- cervical | Negative nasopharyngeal PCR, “suspicious” CSF PCR | Not reported | Not reported | Not reported | Negative | Negative | Steroids | No improvement |
| Lisnic et al., 2020 |
Longitudinally extensive – cervical and thoracic |
Positive pharyngeal PCR, CSF not reported | Not reported | Negative - Serum and CSF HSV 1,2,6, CMV, EBV, | Negative | Negative | Negative | Steroids and Plasma Exchange | Full recovery |
| Zhao et al., 2020 | Uncertain – sensory level at T10 with bowel and bladder incontinence and LE paralysis but no spinal MRI was done | Positive nasopharyngeal PCR, CSF not reported | Not reported | Negative - EBV IgM, influenza B virus IgM, adenovirus IgM, coxsackievirus IgM, influenza A virus IgM, parainfluenza virus IgM, CMV IgM, RSV IgM | Not reported | Not reported | Not reported | Steroids, IVIG, antivirals, antibiotics | Partial Recovery |
| Zhou et al., 2020 | Longitudinally extensive – cervical and thoracic | Positive nasopharyngeal PCR, Negative CSF PCR | Not reported | Negative | Positive | Positive | Negative | Steroids | Full recovery |
Short segment less than three spinal levels (e.g. C5-C6).
Longitudinally extensive more than three spinal levels (e.g. from C2 to T12).
One important consideration relevant to our case is the possibility of chromosomally-integrated HHV6. This is an inherited condition in which the HHV6 genome is transmitted from a parent germ cell and becomes integrated with the individual's cellular genome in all tissues (Aimola et al., 2020). This can give false positive HHV6 PCR results when tested in cell-containing samples like whole blood albeit fewer viral copies can also be found in plasma secondary to cell lysis. Testing HHV6 PCR in hair follicles can differentiate this condition from true HHV6 infection/reactivation. Since we did not perform this test in our patient, it is possible that the patient could have had chromosomally-integrated HHV6. However, chromosomally-integrated HHV6 is very rare (less than 1% of the population) and individuals with this rare condition are still at risk for HHV6 reactivation and CNS infection when immunocompromised (Aimola et al., 2020). The fact that our patient tested positive for serum HHV6-IgG argues against chromosomally-integrated HHV6 since these patients often develop immune tolerance to viral proteins and usually test negative for HHV6 antibodies (Tanaka-Taya et al., 2004). The decline in viral copies after treatment with ganciclovir also supports HHV6 reactivation. Second to encephalitis, HHV6 myelitis is a relatively common manifestation of HHV6 reactivation in immunocompromised patients (Shiroshita et al., 2020). Therefore, the occurrence of this typical presentation in our immunodeficient patient with positive HHV6 PCR in CSF, CSF pleocytosis in the typical viral range, and good clinical and serological response to ganciclovir is less likely to be a rare coincidence. Moreover, HHV6 infection/reactivation has been shown to trigger demyelination so the positivity to the demyelinating antibody in our patient is in itself supportive of HHV6 infection (Dunn et al., 2020).
In conclusion, patients with neurological manifestations in the setting of COVID-19 lymphopenia/hypogammaglobulinemia may need to be tested for opportunistic infections including HHV6 in the correct clinical setting. Combined anti-viral and immunomodulating therapies may be necessary to promote recovery in these special cases. Demyelinating antibodies like MOG-IgG and AQP4-IgG should be tested in the setting of a suspicious clinical picture, such as longitudinally extensive myelitis or severe optic neuritis. MOG-IgG should then be repeated as persistent positivity may predict recurrent disease while transient positivity usually indicates a monophasic course (Cobo-Calvo et al., 2020). This case expands the spectrum of autoimmune and infectious neurological complications of COVID-19.
Financial disclosures
S. Gunzler receives research support from NIH, Impax, Biogen, and the Parkinson Study Group; H. Abboud is a consultant for Biogen, Genentech, Sanofi-Genzyme, Celgene, Alexion, and Viela Bio. He receives research support from Novartis, Celgene, and Genentech. The other authors report no disclosures.
Appendix A. Appendix
| Name | Location | Contribution |
|---|---|---|
| Muruj Jumah, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Writing manuscript first draft, acquisition and interpretation of data, literature review, constructing figure and graphical abstract. |
| Farah Rahman | Case Western Reserve University, Cleveland, OH | Co-writing manuscript first draft, acquisition and interpretation of data, literature review, constructing table |
| Mark Figgie, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Acquisition and interpretation of data. |
| Ankita Prasad, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Acquisition and interpretation of data. |
| Anthony Zampino, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Acquisition and interpretation of data. |
| Ali Fadhil, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Acquisition and interpretation of data. |
| Kaitlin Palmer, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Acquisition and interpretation of data. |
| Robin Arthur Buerki, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Review, critique, and manuscript revision. |
| Steven Gunzler, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Review, critique, and manuscript revision. |
| Praveen Gundelly, MD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Review, critique, and manuscript revision. |
| Hesham Abboud, MD, PHD | University Hospitals of Cleveland/Case Western Reserve University, Cleveland | Study concept and design, data acquisition, literature review, revised the manuscript for intellectual content. |
References
- Abadías-Granado I., Navarro-Bielsa A., Morales-Callaghan A.M., Roc L., Suso-Estívalez C.C., Povar-Echeverría M., Gilaberte Y. COVID-19-associated cutaneous manifestations: does HHV-6 play an etiological role? Br. J. Dermatol. 2021 doi: 10.1111/bjd.19806. Jan 8. (Epub ahead of print. PMID: 33420720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhady M., Elsotouhy A., Vattoth S., et al. Acute flaccid myelitis in COVID-19: a case report. BJR Case Rep. 2020;6(3) doi: 10.1259/bjrcr.20200098. 20200098. Jul24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimola G., Beythien G., Aswad A., Kaufer B.B. Current understanding of human herpesvirus 6 (HHV-6) chromosomal integration. Antivir. Res. 2020;176:104720. doi: 10.1016/j.antiviral.2020.104720. Apr. (Epub 2020 Feb 7. PMID: 32044155) [DOI] [PubMed] [Google Scholar]
- Alketbi R., Alnuaimi D., Almulla M., Altalai N., Samir M., Kumar N., et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol. Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbanian S., Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)–a case report. Acta Neurol. Belg. 2020 doi: 10.1007/s13760-020-01497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., Chandra A., Ray A.K., et al. COVID-19 – associated transverse myelitis: a rare entity. BMJ Case. Rep. 2020;13 doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.C.N., Magnussen J., Ip J., et al. Acute transverse myelitis in COVID-19 infection: a case report. BMJ Case. Rep. 2020;13 doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo-Calvo A., Ruiz A., Rollot F., Arrambide G., Deschamps R., Maillart E., Papeix C., Audoin B., Lépine A.F., Maurey H., Zephir H., Biotti D., Ciron J., Durand-Dubief F., Collongues N., Ayrignac X., Labauge P., Pierre M., Thouvenot E., Bourre B., Montcuquet A., Cohen M., Horellou P., Tintoré M., De Seze J., Vukusic S., Deiva K., Marignier R., NOMADMUS, KidBioSEP and OFSEP study groups Clinical features and risk of relapses in children and adults with MOGAD. Ann. Neurol. 2020 doi: 10.1002/ana.25909. Sep 21. (Epub ahead of print. PMID: 32959427) [DOI] [PubMed] [Google Scholar]
- Drago F., Ciccarese G., Rebora A., Parodi A. Human herpesvirus-6, -7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26549. Sep 24. (Epub ahead of print. PMID: 32970319; PMCID: PMC7537064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N., Kharlamova N., Fogdell-Hahn A. The role of herpesvirus 6A and 6B in multiple sclerosis and epilepsy. Scand. J. Immunol. 2020;92(6) doi: 10.1111/sji.12984. e12984. Dec. (Epub 2020 Oct 23. PMID: 33037649; PMCID: PMC7757173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont T., Caillat-Zucman S., Fremeaux-Bacchi V., Morin F., Lengliné E., Darmon M., Peffault de Latour R., Zafrani L., Azoulay E., Dumas G. Identification of distinct immunophenotypes in critically Ill coronavirus disease 2019 patients. Chest. 2020 doi: 10.1016/j.chest.2020.11.049. Dec 11:S0012-3692(20)35351-4. (Epub ahead of print. PMID: 33316234; PMCID: PMC7831685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun R. The clinics of HHV-6 infection in COVID-19 pandemic: Pityriasis rosea and Kawasaki disease. Dermatol. Ther. 2020 doi: 10.1111/dth.13730. (May 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B.M., Thomas K.P., Krishnan C., Kaplin A.I., Calabresi P.A., Kerr D.A. Idiopathic transverse myelitis: corticosteroids, plasma exchange, or cyclophosphamide. Neurology. 2007;68(19):1614–1617. doi: 10.1212/01.wnl.0000260970.63493.c8. May 8. (PMID: 17485649) [DOI] [PubMed] [Google Scholar]
- Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z., et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. May. (Epub 2020 Apr 3. PMID: 32251805; PMCID: PMC7128884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisnic V., Nemtan V., Hacina E., Topciu G., Manole E., Thurnher M.M., et al. Acute transverse myelitis in a HIV-positive patient with COVID-19. Moldovan Med. J. 2020;63(5):51–53. doi: 10.21203/rs.3.rs-50901/v1. Oct2. [DOI] [Google Scholar]
- Munz M., Wessendorf S., Koretsis G., et al. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020;267:2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham E.J., Chou S.H.Y., Coles A.J., et al. Neurological implications of COVID-19 infections. Neurocrit. Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi A., Ghayeghran A., Hatamian H., Hosseini-Nejad M., Eghbali B.B., et al. COVID-19-associated myelitis, para/post infectious or infectious myelitis: a case report from the North of Iran. Casp. J. Neurol. Sci. 2020;6(2):132–138. doi: 10.32598/CJNS.6.21.1. Apr10. [DOI] [Google Scholar]
- Sarma D., Bilello L.A. Case report of acute transverse myelitis following novel coronavirus infection. Clin. Pract. Case Emerg. Med. 2020;4(3):321–323. doi: 10.5811/cpcem.2020.5.47937. May12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroshita K., Mori T., Kato J., Sakurai M., Koda Y., Abe R., Murakami K., Sumiya C., Fujita S., Yamaguchi K., Yamazaki R., Nakayama H., Suzuki S., Nakahara J., Okamoto S. Clinical characteristics of human herpesvirus-6 myelitis after allogeneic hematopoietic stem cell transplantation and its favorable outcome by early intervention. Bone Marrow Transplant. 2020;55(5):939–945. doi: 10.1038/s41409-019-0755-2. May. (Epub 2019 Nov 21. PMID: 31754252) [DOI] [PubMed] [Google Scholar]
- Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000803. e803. Jun 10. (PMID: 32522767; PMCID: PMC73095210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Taya K., Sashihara J., Kurahashi H., Amo K., Miyagawa H., Kondo K., Okada S., Yamanishi K. Human herpesvirus 6 (HHV-6) is transmitted from parent to child in an integrated form and characterization of cases with chromosomally integrated HHV-6 DNA. J. Med. Virol. 2004;73(3):465–473. doi: 10.1002/jmv.20113. Jul. 15170644. [DOI] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. Jul. (Epub 2020 May 23. PMID: 32282949; PMCID: PMC7262337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiuddin H., Skwirsk B., Paz-Arabo P., et al. Acute transverse myelitis associated with SARS-CoV-2: a case-report. Brain Behav. Immun. Health. 2020;5:100091. doi: 10.1016/j.bbih.2020.100091. May. (Epub 2020 Jun 6. PMID: 32835294; PMCID: PMC7275168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J.P., Sequeira J., Brito M.J., et al. Postinfectious anti – myelin oligodendrocyte glycoprotein antibody positive optic neuritis and myelitis. J. Child Neurol. 2017;32(12):996–999. doi: 10.1177/0883073817724927. [DOI] [PubMed] [Google Scholar]
- Zachariadis A., Tulbu A., Strambo D., et al. Transverse myelitis related to COVID-19 infection. J. Neurol. 2020;267:3459–3461. doi: 10.1007/s00415-020-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. 2020 doi: 10.1101/2020.03.16.20035105. 2020.03.16.20035105. [DOI] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R., et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J. Neuroophthalmol. 2020;40(3):398–402. doi: 10.1097/WNO.0000000000001049. Sep. (PMID: 32604245; PMCID: PMC7382408) [DOI] [PMC free article] [PubMed] [Google Scholar]