Abstract
Human xanthine oxidoreductase (XOR) is a multiple-level regulated enzyme, resulting from a complicated evolutionary process that assigned it many physiological roles. The main XOR activities are: (i) xanthine dehydrogenase (XDH) activity that performs the last two steps of purine catabolism, from hypoxanthine to uric acid; (ii) xanthine oxidase (XO) activity that, besides purine catabolism, produces reactive oxygen species (ROS); (iii) nitrite reductase activity that generates nitric oxide, contributing to vasodilation and regulation of blood pressure; (iv) NADH oxidase activity that produces ROS. All these XOR activities contribute also to metabolize various endogenous and exogenous compounds, including some drugs. About XOR products, it should be considered that (i) uric acid is not only a proinflammatory agent, but also a fundamental antioxidant molecule in serum and (ii) XOR-derived ROS are essential to the inflammatory defensive response. Although XOR has been the object of a large number of studies, most of them were focused on the pathological consequences of its activity and there is not a clear and schematic picture of XOR physiological roles. In this review, we try to fill this gap, reporting and graphically schematizing the main roles of XOR and its products.
Keywords: Nitric oxide, Reactive oxygen species, Uric acid, Xanthine oxidoreductase
1. Introduction
Xanthine oxidoreductase (XOR) is a member of a highly conserved family of molybdo-flavoenzymes that are widely distributed from prokaryotic to eukaryotic organisms and are hypothesized to derive from a common ancestral progenitor [1]. In most living beings, the catabolism of hypoxanthine and xanthine to uric acid is ensured by the xanthine dehydrogenase activity (XDH, EC 1.17.1.4), but only mammals possess the xanthine oxidase (XO, EC 1.17.3.2), whose activity in milk was already described at the end of the 19th century [2,3].
Mammalian XOR protein is a homodimer of approximately 300 kDa [4] in which each subunit has three domains whose characteristics and functions are detailed in Fig. 1. The largest domain contains the substrate pocket for XDH, XO and nitrite reductase activity of XOR, while the NADH oxidase activity takes place in the FAD domain. XOR shows low substrate specificity and highly versatile activity, which allow it to oxidize and reduce a number of endogenous and exogenous products, thus acting as a detoxifying and drug-metabolizing enzyme [5]. At the post-translational level, XOR may be produced in a form that is deficient of the molybdenum or sulfur, thus resulting in enzymatic inactivity at the Moco site [6].
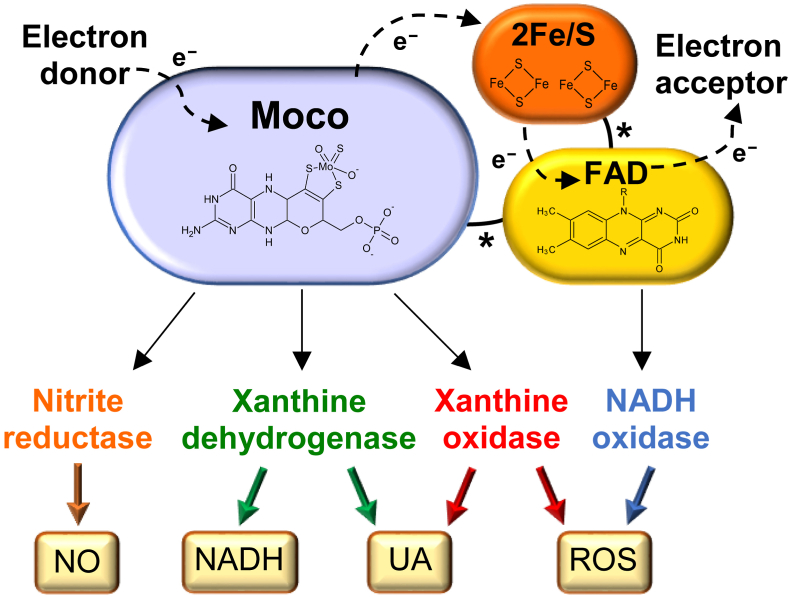
Fig. 1.
Mammalian xanthine oxidoreductase (XOR): structure and functions.
XOR has two identical subunits, each composed of three domains connected by unstructured hinge regions (indicated by asterisks): the 20-kDa N-terminal domain (orange) has two non-identical iron-sulfur clusters (2Fe/S), the 40-kDa intermediate domain (yellow) has a flavin adenine dinucleotide (FAD) cofactor and the 85-kDa C-terminal domain (lilac) has a molybdopterin cofactor containing a molybdenum atom (Moco). The electron (e−) flux moves from the Moco site, where oxidation occurs, through the two iron-sulfur redox centers towards the FAD site, where the electron acceptor is reduced. The products of XOR activities are: uric acid (UA) and reduced nicotinamide adenine dinucleotide (NADH) from xanthine dehydrogenase (XDH), UA, superoxide ion and hydrogen peroxide (ROS) from xanthine oxidase (XO), nitric oxide (NO) from nitrate and nitrite reductase and ROS from NADH oxidase [30].
Moreover, mammalian XOR is constitutively an NAD+ dependent dehydrogenase, which can be transformed in oxidase in a reversible way through the oxidation of two cysteine residues or irreversibly through a partial proteolysis of the fragment containing such cysteine groups. The transition from XDH to XO includes an intermediate XOR form with both dehydrogenase and oxidase activities, depending on the oxidation of only one of the two crucial sulfhydryl groups [7]. Therefore, XO has been referred as an isoform of XDH [8], although it is the result of post-translational modifications and not of genetic differences [9]. The conversion from XDH to XO physiologically occurs through oxidation of the sulfhydryl groups when the enzyme is released from the cell into gastrointestinal lumen and urinary tract, as well as in biological fluids, such as milk and serum [6]. XDH/XO conversion was observed to occur through oxidation (reversible) in a variety of hypoxic/ischemic and other pathological conditions; but a proteolytic conversion (irreversible) has been also described in some prolonged ischemic conditions [6,10,11]. Low oxygen tension induced by sickle cell disease (SCD) can result in significant release of XDH from the liver to circulation, thus greatly increasing the amount of serum XO, as reported in SCD patients as well as in knockout transgenic SCD mice [12].
The XO form can catalyze the monovalent and divalent electron transfer to O2, which generate superoxide ion (O2•−) and hydrogen peroxide (H2O2), respectively, with a variable proportion between these two reactive oxygen species (ROS). Acidic pH, low oxygen tension and purine concentration slow down the electron flux rate, thus favoring the divalent transfer that generates H2O2 [13]. Depending on the same parameters, XDH can also produce these ROS at the FAD site by acting as a NADH oxidase. This activity is retained when the molybdenum or sulfur atoms are missing in the molybdopterin cofactor, as well as when XOR is inhibited by competing or non-competing drugs, which block the functionality of the Moco site [6]. In addition, XOR can act as a nitrate reductase at the Moco site by reducing nitrates to nitrites and as a nitrite reductase reducing nitrites to nitric oxide (NO) [13]. NO can further react with O2•−generating peroxynitrite (ONOO−). In the presence of transition metals, O2•− and H2O2 give rise to the hydroxyl radical (OH•) which, together with ONOO−, contributes to the cytocidal activity of the inflammatory reaction and to the defense mechanism against bacteria in innate immunity [6]. The XOR activities are schematized in Fig. 1.
In humans and other higher primates, XOR catalyzes the last two steps of purine catabolism, because of the lack of uricase that has been lost during evolution; for this reason, uricemia is higher in these primates than in ureotelic mammals [14]. XOR has the rate-limiting function of generating irreversible products, xanthine and uric acid, that preclude the salvage pathway of purine nucleotides [15]. The purine catabolism and the physiological roles of uric acid are reported in Fig. 2.
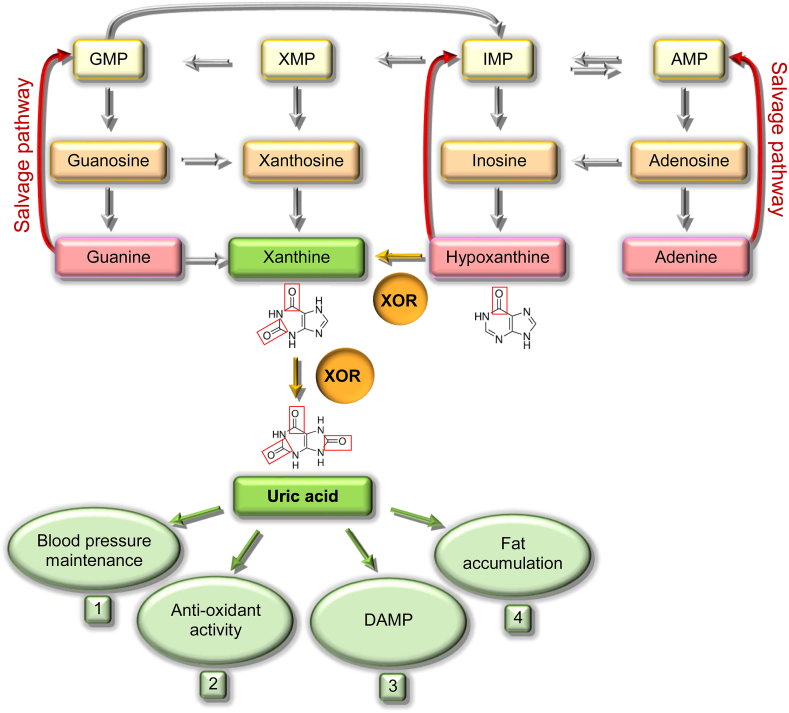
Fig. 2.
Catabolism and salvage pathway of purine nucleotides and physiologic roles of uric acid.
AMP, GMP, IMP and XMP are the monophosphate nucleotides of adenine, guanine, hypoxanthine and xanthine, respectively. The purine salvage pathways are indicated with red arrows. The reactions catalyzed by xanthine oxidoreductase (XOR) are indicated with yellow arrows and the boxes corresponding to irreversible products are colored in green [6]. The physiologic functions of uric acid are reported: 1. Uric acid supports blood pressure, even in the absence of an adequate dietary intake of salt, counteracting the nitric oxide-induced vasodilation, increasing cyclooxygenase-2 expression and activating the renin-angiotensin system [31]. 2. Uric acid is a free radical scavenger that takes part in the non-enzymatic defense system against oxidative stress in biological fluids. The antioxidant activity of uric acid plays an essential function in preventing cancer, cardiovascular diseases [32] and other pathological conditions [33]. Indeed, a correlation has been proposed in different species between levels of uricemia and their life expectancy [30]. 3. During tissue injury, intracellular uric acid is released from dead cells, stimulating macrophages and inducing the inflammatory response by acting as a damage-associated molecular pattern (DAMP) [34]. 4. Uric acid promotes fat accumulation, which can help to overcome periods of starvation, as well as hepatic gluconeogenesis, thus elevating blood sugar [26,35].
The normal subcellular localization of XOR is the cytosol, but it was also found in peroxisomes [16]. In addition, XOR can be found in extracellular compartments, such as blood and milk. Serum XOR mainly derives from the physiological hepatic cell turnover, which induces the release of liver enzymes from dead cells into circulation. The level of serum XOR strongly increases as a consequence of several liver pathologies that cause tissue damage [6]. The abundant presence of XOR in milk results from the apocrine secretion of lipid globules from breast cells during lactation. Butyrophilin1A1-bound XOR is clustered in the apical membrane, which is secreted with the cytoplasmic lipid droplet [17].
The production of human XOR protein is strictly regulated at both transcriptional and post-translational levels. The basal activity of the human XOR promoter is minimal when compared with other mammals; this is probably due to repressor elements identified in non-coding regions of XOR [18]. The expression of human XOR gene (hXOR) is usually subjected to down-regulation in almost every tissue with the exception of the epithelial cells of lactating breast, gastrointestinal tract, kidney and liver [19]. Genetic mutations causing the loss of XOR activity are responsible of xanthinuria I and II that may be asymptomatic in primates, although they can promote urolithiasis [20]. A mice model of XOR gene knockout, XOR(−/−), showed crystals and triglyceride accumulation in the renal tubules and increased interstitial fibrosis, ultimately resulting in renal failure with early death within several months [21,22]. Higher hXOR expression and enzyme activity can be induced by several factors, as low oxygen tension, cytokines, growth factors and various hormones, through both the upregulation of XOR transcription and post-translational activation [6,23,24]. This explains the increased XOR activity during hypoxic/ischemic conditions and inflammation, as well as the high presence of XOR in mammary cells and consequently in milk. The specific functions carried out by XOR in high expressing-tissues are depicted in Fig. 3.
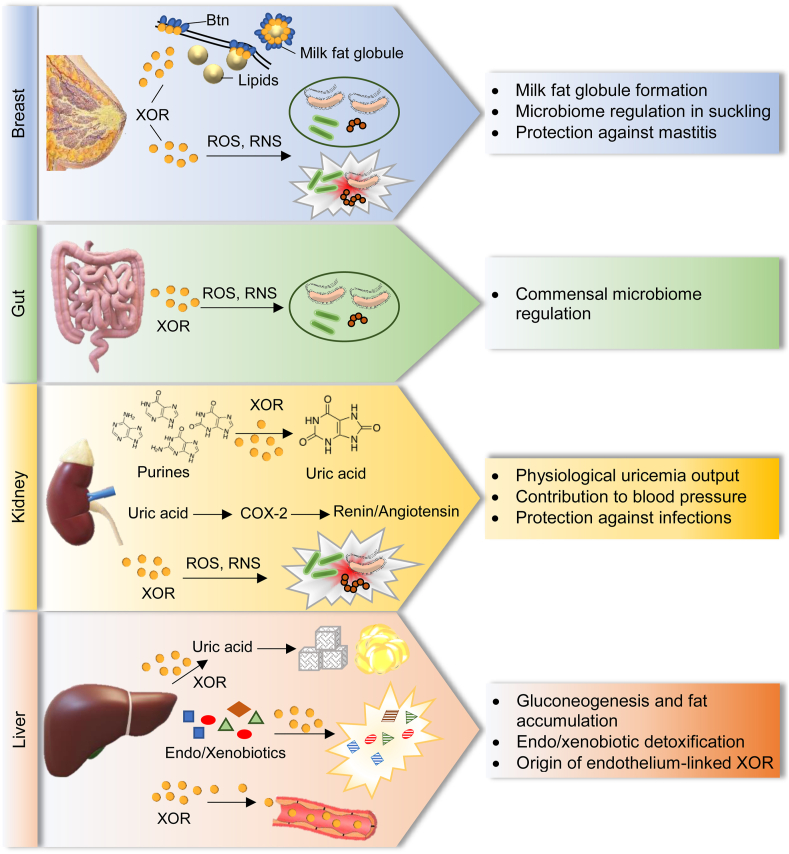
Fig. 3.
Human tissues expressing high level of xanthine oxidoreductase (XOR) and local function of XOR activities and products.
In breast, milk-fat globule secretion during lactation is mediated by the clustering of the transmembrane protein, butyrophilin1A1 (Btn). XOR contributes to apocrine lipid secretion by inducing apical membrane reorganization, thus allowing the Btn clustering and the membrane docking of milk‐fat droplets [17]. In milk, XOR produces hydrogen peroxide (H2O2) and nitric oxide (NO) that lactoperoxidase utilizes to form hypothiocyanite and nitrogen dioxide, which counteract the growth of opportunistic bacteria, thus protecting the breast from mastitis. Furthermore, this bactericidal action in milk leaves unharmed the commensal flora of neonatal oral cavity, stomach and intestine, thus regulating the intestinal microbiome. For all these reasons, XOR is essential for the regular growth of the newborn [36]. In gut, the frequent turnover of enterocytes ensures an abundant presence of XOR in the intestinal lumen where XOR-derived reactive oxygen species (ROS) and reactive nitrogen species (RNS) exert a protective activity against opportunistic infections, while sparing the commensal microbiome. In kidney, most purine catabolism occurs; in this district XOR is responsible for uricosuria, and consequently for uricemia levels, which in turn contributes to support blood pressure by up-regulating cyclooxygenase-2 (COX-2) expression and consequently the renin/angiotensin pathway. XOR oxidant products can also contribute to keep sterile the urinary tract. In liver, XOR carries out all its activities and, in addition to purines, metabolizes a lot of endogenous and exogenous substrates, including drugs. The uric acid produced by XOR influences the hepatic metabolism of glucose and lipids and increases gluconeogenesis and fat accumulation [37]. Furthermore, serum XOR mainly derives from the physiological hepatic cell turnover, which induces the release of liver enzymes from dead cells. Circulating XOR can bind endothelial cells, thus promoting endothelial activation during inflammation, modulating vascular tone and consequently contributing to blood pressure regulation [31].
XOR-derived NO, H2O2 and O2•− increase the permeability of vascular lining and modulate local vasodilation, thus having a pro-inflammatory activity [25]. The contribution of XOR activity and products to inflammation is shown in Fig. 4A. XOR upregulates signal transduction molecules promoting cell proliferation, migration and tissue repair [19]. Furthermore, XOR is one of the endothelial enzymes responsible for the control of NO level that is crucial for the arteriolar tone and, consequently, for blood pressure regulation. The interactions between these enzymes is shown in Fig. 4B.
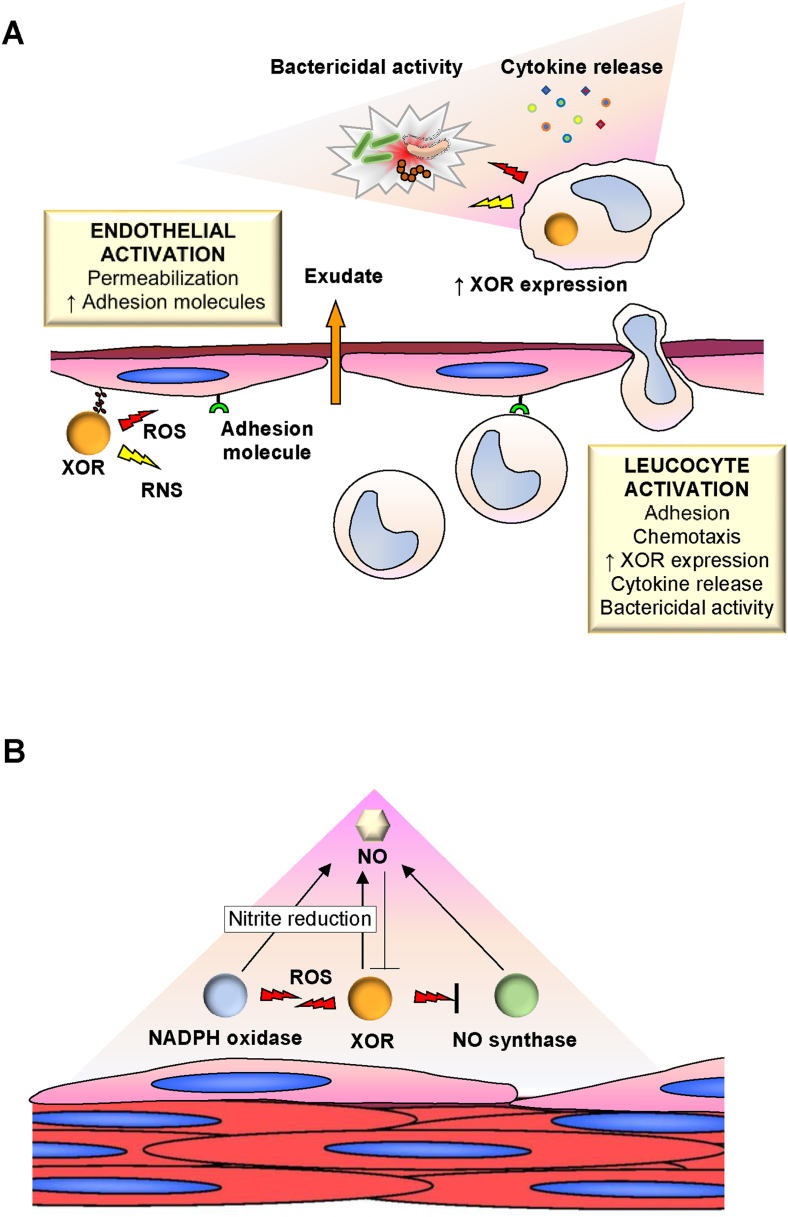
Fig. 4.
Activities of endothelium-linked human xanthine oxidoreductase (XOR) and its products.
When XOR is released from hepatocytes into the circulation, it is converted from the dehydrogenase to the oxidase form. XOR binds with high affinity to the glycosaminoglycans on the surface of the endothelium. The XOR bound to endothelial cells acts as a systemic modulator of redox balance, setting some important endothelial functions. (A): Roles in the inflammatory reaction: Endothelium-linked XOR generates reactive oxygen (ROS) and nitrogen (RNS) species, which activate endothelial cells and contribute to their permeabilization and the formation of phlogistic exudate. XOR-derived oxidants also induce the expression of adhesion molecules on the inner surface of the microcirculation, thus promoting leukocyte activation, diapedesis and migration towards the phlogistic stimulus. Activated phagocytes release cytokines, increase XOR expression and produce cytocidal oxidant products. (B): Regulation of vascular tone: Vascular tone is locally regulated by the level of nitric oxide (NO) produced by the interplay of the endothelial enzymes reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, XOR and NO synthase. NADPH oxidase and XOR activate each other through ROS and generate NO through the reduction of nitrite. XOR and NO synthase inhibit each other through their products: NO synthase is inhibited by ROS and XOR is inhibited by NO or by RNS derived from ROS and NO.
XOR-produced ROS and NO promote adipogenesis as well as the browning of the white fat tissue [26,27]. XOR can also provide physiologically relevant signaling that are involved in metabolic regulation [28] and in the modulation of cellular functions such as proliferation, differentiation, apoptosis, autophagy, adhesion and migration [29], thus contributing to successful aging [30].
2. Conclusions
XOR is present in all cell types, acting mainly as dehydrogenase and in most cases with a low level of activity. During phylogenesis, mammalian XOR has acquired the important regulatory function of producing ROS and NO. ROS influence the redox equilibrium and mediate signal transduction as secondary messengers, while NO has complex interactions with endothelial NADPH oxidase and NO synthase activities. Thus, XOR is implicated in many biological processes, such as inflammation, repair and aging, in physiological pathways, such as cell growth, differentiation and mobility, and also in the regulation of both endothelial function and vascular tone.
On the other side, XOR and its products are involved in many pathological conditions and the therapy with XOR inhibitors is recommended not only in gout, which is caused by an excess of serum uric acid, but also in renal and cardiovascular diseases, even if some controversial data are present in literature. However, it should be considered that XOR inhibition, and the consequent urate lowering, can cause many adverse effects, due to the reduction of XOR physiologic functions.
Acknowledgements
This work was supported by funds for selected research topics from the Alma Mater Studiorum - University of Bologna and by the Pallotti Legacies for Cancer Research.
Contributor Information
Massimo Bortolotti, Email: massimo.bortolotti2@unibo.it.
Letizia Polito, Email: letizia.polito@unibo.it.
Maria Giulia Battelli, Email: mariagiulia.battelli@unibo.it.
Andrea Bolognesi, Email: andrea.bolognesi@unibo.it.
References
- 1.Terao M., Romão M.J., Leimkühler S., Bolis M., Fratelli M., Coelho C., Santos-Silva T., Garattini E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016;90:753–780. doi: 10.1007/s00204-016-1683-1. [DOI] [PubMed] [Google Scholar]
- 2.Massey V., Harris C.M. Milk xanthine oxidoreductase: the first one hundred years. Biochem. Soc. Trans. 1997;25:750–755. doi: 10.1042/bst0250750PMID9388538. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer W. Die Ueberführung von Nucleïnbase in Harnsaüre durch die sauerstoffübertragende Wirkung von Gewebsauszügen. Arch. Ges. Physiol. 1899;76:192–203. [Google Scholar]
- 4.McManaman J.L., Bain D.L. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J. Biol. Chem. 2002;277:21261–21268. doi: 10.1074/jbc.M200828200. [DOI] [PubMed] [Google Scholar]
- 5.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in drug metabolism: beyond a role as a detoxifying enzyme. Curr. Med. Chem. 2016;23:4027–4036. doi: 10.2174/0929867323666160725091915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelli M.G., Bolognesi A., Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta. 2014;1842:1502–1517. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Nishino T., Okamoto K., Kawaguchi Y., Matsumura T., Eger B.T., Pai E.F., Nishino T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015;282:3075–3090. doi: 10.1111/febs.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecerska-Heryć E., Jesionowska A., Klaudyna S., Katarzyna S., Dominika M., Dominika P., Marta U., Dołęgowska B. Xanthine oxidoreductase reference values in platelet-poor plasma and platelets in healthy volunteers. Oxid. Med. Cell. Longev. 2015;2015:341926. doi: 10.1155/2015/341926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terao M., Garattini E., Romão M.J., Leimkühler S. Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes. J. Biol. Chem. 2020;295:5377–5389. doi: 10.1074/jbc.REV119.007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKelvey T.G., Höllwarth M.E., Granger D.N., Engerson T.D., Landler U., Jones H.P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am. J. Physiol. 1988;254:G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- 11.Battelli M.G., Abbondanza A., Stirpe F. Effects of hypoxia and ethanol on xanthine oxidase of isolated rat hepatocytes: conversion from D to O form and leakage from cells. Chem. Biol. Interact. 1992;83:73–84. doi: 10.1016/0009-2797(92)90093-z. [DOI] [PubMed] [Google Scholar]
- 12.Aslan M., Ryan T.M., Adler B., Townes T.M., Parks D.A., Thompson J.A., Tousson A., Gladwin M.T., Patel R.P., Tarpey M.M., Batinic-Haberle I., White C.R., Freeman B.A. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide. 2013;34:19–26. doi: 10.1016/j.niox.2013.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R.J., Lanaspa M.A., Gaucher E.A. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin. Nephrol. 2011;31:394–399. doi: 10.1016/j.semnephrol.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuhashi M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020;319:E827–E834. doi: 10.1152/ajpendo.00378.2020. [DOI] [PubMed] [Google Scholar]
- 16.Angermüller S., Bruder G., Völkl A., Wesch H., Fahimi H.D. Localization of xanthine oxidase in crystalline cores of peroxisomes. A cytochemical and biochemical study. Eur. J. Cell Biol. 1987;45:137–144. [PubMed] [Google Scholar]
- 17.Monks J., Dzieciatkowska M., Bales E.S., Orlicky D.J., Wright R.M., McManaman J.L. Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. J. Physiol. 2016;594:5899–5921. doi: 10.1113/JP272390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P., LaVallee P., Hoidal J.R. Repressed expression of the human xanthine oxidoreductase gene. E-box and TATA-like elements restrict ground state transcriptional activity. J. Biol. Chem. 2000;275:5918–5926. doi: 10.1074/jbc.275.8.5918. [DOI] [PubMed] [Google Scholar]
- 19.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichida K., Amaya Y., Okamoto K., Nishino T. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int. J. Mol. Sci. 2012;13:15475–15495. doi: 10.3390/ijms131115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsubo T., Matsumura K., Sakagami K., Fujii K., Tsuruya K., Noguchi H., Rovira I.I., Finkel T., Iida M. Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension. 2009;54:868–876. doi: 10.1161/HYPERTENSIONAHA.109.135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piret S.E., Esapa C.T., Gorvin C.M., Head R., Loh N.Y., Devuyst O., Thomas G., Brown S.D., Brown M., Croucher P., Cox R., Thakker R.V. A mouse model of early-onset renal failure due to a xanthine dehydrogenase nonsense mutation. PloS One. 2012;7 doi: 10.1371/journal.pone.0045217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J., Xu P., LaVallee P., Hoidal J.R. Identification of proteins binding to E-Box/Ku86 sites and function of the tumor suppressor SAFB1 in transcriptional regulation of the human xanthine oxidoreductase gene. J. Biol. Chem. 2008;283:29681–29689. doi: 10.1074/jbc.M802076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garattini E., Mendel R., Romão M.J., Wright R., Terao M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem. J. 2003;372:15–32. doi: 10.1042/BJ20030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid. Med. Cell. Longev. 2016;2016:3527579. doi: 10.1155/2016/3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battelli M.G., Bortolotti M., Polito L., Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2018;1864:2557–2565. doi: 10.1016/j.bbadis.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Roberts L.D. Does inorganic nitrate say NO to obesity by browning white adipose tissue? Adipocyte. 2015;4:311–314. doi: 10.1080/21623945.2015.1005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy J., Galano J.M., Durand T., Le Guennec J.Y., Lee J.C. Physiological role of reactive oxygen species as promoters of natural defenses. Faseb. J. 2017;31:3729–3745. doi: 10.1096/fj.201700170R. [DOI] [PubMed] [Google Scholar]
- 30.Battelli M.G., Bortolotti M., Bolognesi A., Polito L. Pro-aging effects of xanthine oxidoreductase products. Antioxidants. 2020;9:839. doi: 10.3390/antiox9090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neogi T., George J., Rekhraj S., Struthers A.D., Choi H., Terkeltaub R.A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012;64:327–338. doi: 10.1002/art.33369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battelli M.G., Polito L., Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis. 2014;237:562–567. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Joosten L.A.B., Crişan T.O., Bjornstad P., Johnson R.J. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat. Rev. Rheumatol. 2020;16:75–86. doi: 10.1038/s41584-019-0334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenbacher J.L., Schrezenmeier H., Jahrsdörfer B., Kaltenmeier C., Rojewski M.T., Yildiz T., Beyer T., Erle A., Wiegmann D.S., Grassl S., Hang R., Körper S., Wiesneth M., Lotze M.T., Lotfi R. S100A4 and uric acid promote mesenchymal stromal cell induction of IL-10+/Ido+ lymphocytes. J. Immunol. 2014;192:6102–6110. doi: 10.4049/jimmunol.1303144. [DOI] [PubMed] [Google Scholar]
- 35.Battelli M.G., Bortolotti M., Polito L., Bolognesi A. Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol. 2019;21:101070. doi: 10.1016/j.redox.2018.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Shehri S.S., Duley J.A., Bansal N. Xanthine oxidase-lactoperoxidase system and innate immunity: biochemical actions and physiological roles. Redox Biol. 2020;34:101524. doi: 10.1016/j.redox.2020.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima W.G., Martins-Santos M.E., Chaves V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 2015;116:17–23. doi: 10.1016/j.biochi.2015.06.025. [DOI] [PubMed] [Google Scholar]