Abstract
BACKGROUND:
In 2010, health care facilities in the United States began voluntary enrollment in the National Healthcare Safety Network (NHSN) Hemovigilance Module. Participants report transfusion practices; red blood cell, platelet (PLT), plasma, and cryoprecipitate units transfused; and transfusion-related adverse reactions and process errors to the Centers for Disease Control and Prevention through a secure, Internet-accessible surveillance application available to transfusing facilities.
STUDY DESIGN AND METHODS:
Facilities submitting at least 1 month of transfused components data and adverse reactions from January 1, 2010, to December 31, 2012, were included in this analysis. Adverse reaction rates for transfused components, stratified by component type and collection and modification methods, were calculated.
RESULTS:
In 2010 to 2012, a total of 77 facilities reported 5136 adverse reactions among 2,144,723 components transfused (239.5/100,000). Allergic (46.8%) and febrile nonhemolytic (36.1%) reactions were most frequent; 7.2% of all reactions were severe or life-threatening and 0.1% were fatal. PLT transfusions (421.7/100,000) had the highest adverse reaction rate.
CONCLUSION:
Adverse transfusion reaction rates from the NHSN Hemovigilance Module in the United States are comparable to early hemovigilance reporting from other countries. Although severe reactions are infrequent, the numbers of transfusion reactions in US hospitals suggest that interventions to prevent these reactions are important for patient safety. Further investigation is needed to understand the apparent increased risk of reactions from apheresis-derived blood components. Comprehensive evaluation, including data validation, is important to continued refinement of the module.
Surveillance designed to monitor and detect adverse transfusion-related outcomes, as part of hemovigilance systems, has been implemented globally. Hemovigilance systems have variable methodologies due to differences in health care infrastructure and regulatory requirements within each country.1–8 In the United States, an estimated 20,933,000 units of whole blood and blood components were transfused in 2011 by more than 4200 health care facilities, and approximately 51,000 adverse transfusion-related reactions were estimated among recipients as a result.9 Some transfusion reactions are serious, contributing to significant patient morbidity and mortality, and may be preventable. However, without national surveillance, the number and severity of reactions are not well quantified or characterized.
Many organizations, both public and private, are involved in the collection and analysis of transfusion-related adverse event data in the United States. Transfusion-related fatalities must be reported to the US Food and Drug Administration; adverse events suspected to be related to the blood product must be reported to the blood supplier; health care facilities may report transfusion-related adverse events to accrediting organizations as part of patient safety initiatives; and suspected transfusion transmission of infectious pathogens, including nationally notifiable diseases such as human immunodeficiency virus and hepatitis B and C infections, are reportable to public health agencies. In addition to notifiable disease requirements, reporting transfusion-related adverse events to public health departments is mandatory in some states, although specific requirements differ.10–14 This fragmentation in monitoring and reporting complicates the national estimation of transfusion-related adverse events.
In 2007, the US Centers for Disease Control and Prevention (CDC), in collaboration with transfusion medicine experts convened by AABB, designed data specifications for a surveillance system to monitor transfusion-related adverse events nationally. The Hemovigilance Module began operation in 2010 as part of the National Healthcare Safety Network (NHSN), an Internet-accessible surveillance system used by more than 12,000 US health care facilities for the primary purpose of describing the epidemiology and risk factors of health care–associated infections. The surveillance system has since evolved to provide a reporting framework for patient safety data to federal agencies, state and local public health departments, and quality improvement organizations and includes modules for antimicrobial use and resistance monitoring, health care personnel vaccination coverage, and adherence to central line insertion practice guidelines.15
The NHSN Hemovigilance Module is available for use by all transfusing health care facilities in the United States. Data reported to the module are aggregated by CDC to quantify the burden of transfusion-related adverse reactions and transfusion process errors (i.e., incidents) and to identify threats to the blood supply to improve patient safety. This system may also be used to satisfy state-mandated reporting requirements or to participate in patient safety initiatives through a unique data sharing mechanism within NHSN that allows facilities to voluntarily direct their data to external partners.15
This report presents a summary of the adverse transfusion reactions reported to the Hemovigilance Module in the United States since initial release in 2010 through 2012. Incident data are not included in this report.
MATERIALS AND METHODS
Ethical considerations
Data for this report were collected for surveillance and program evaluation purposes and determined to not require institutional review board review by the CDC office of the Associate Director for Science. Individual and institutional identifiers are confidentially held and not disclosed by CDC without consent of the participating facility.
Data collection
In 2010 to 2012, facilities participating in recipient hemovigilance were expected to conduct comprehensive monitoring from the time the blood component was received from the blood supplier until the component was transfused, including transfusion recipient monitoring for adverse reactions. Facilities initiate participation by submitting responses to an annual survey that includes health care facility ownership, community setting, and trauma level designation; estimated annual surgery and transfusion volumes; and the number of beds served by the transfusion service. Transfusion service information such as the number of transfusion service staff members employed, the type of transfusion service data management systems in use, and blood bank and transfusion service operations such as component preparation, specimen handling, and sample testing practices were also included.
Facilities reported the total numbers of red blood cell (RBC), platelet (PLT), plasma, and cryoprecipitate units transfused monthly. Transfused components are classified by collection method (apheresis or whole blood derived), by whether the components were irradiated or leukoreduced, and by the numbers of components transfused as full units or as aliquots.
Facilities were instructed to report each occurrence of 12 adverse reactions—allergic, febrile nonhemolytic, acute and delayed hemolytic, delayed serologic, hypotension, circulatory overload, acute lung injury, dyspnea, graft-versus-host disease (GVHD), posttransfusion purpura, and transfusion-transmitted infection—that were temporally associated with a transfusion performed in or by the facility once a clinical investigation was completed by the transfusion service and standardized case definition criteria described in the surveillance protocol were met. Adverse reactions are self-reported using standardized case definitions that were developed in collaboration with transfusion medicine expert working groups convened by AABB. Reactions were categorized as definite, probable, or possible based on the presence of clinical signs and symptoms, laboratory findings, and/or radiologic evidence as specified in their respective case definitions. They were also assigned an imputability designation (definite, probable, possible, doubtful, ruled out, or not determined), which is the likelihood that the reaction was attributable to the transfusion based on the temporal relationship to the transfusion and the presence or absence of alternative etiologies that may also account for the clinical signs and symptoms. Severity grades (nonsevere, severe, life-threatening, fatal, and not determined) were assigned based on the extent of symptoms, clinical course, and medical intervention(s) necessary to treat the reaction.
Facilities were required to report all reactions that met defined case criteria and where imputability met definite, probable, or possible criteria. Facilities were allowed to report adverse reactions that do not meet specified case criteria as “other” or “unknown” reactions or suspected reactions that met imputability criteria of doubtful, ruled out, or not determined into the module for their own use, but these data are not included in aggregate surveillance analyses by CDC.16
Statistical analysis
Data from all facilities submitting at least 1 month of denominator data (total transfused components) from January 1, 2010, to December 31, 2012, were included in this analysis. Adverse reactions associated with RBCs, plasma, PLTs, or cryoprecipitate that met definite or probable case definition criteria and definite, probable, or possible imputability criteria for any of the 12 defined adverse were included.
Data from each included facility’s most recently submitted annual survey were used for descriptive analysis. The facilities were categorized by community setting (urban, suburban, rural, not reported), number of facility beds served by the transfusion service (≤249, 250-499, 500-749, ≥750), number of transfusion service staff members employed (<5, 5-<10, 10-<20, ≥20), whether a dedicated staff member is assigned to investigate transfusion-related adverse reaction reports, and who provides the transfusion service (the facility, a separate facility, blood center or centralized transfusion service).
The total numbers of transfused blood components reported by component type (RBCs, PLTs, plasma, cryoprecipitate), collection method (apheresis or whole blood derived), component modification (irradiation and leukoreduction), and unit size (full unit or aliquot) were calculated for each reporting year and over the 3 years in total. The total numbers of each component, by the respective subgroup, for all 3 reporting years were used as denominators for adverse reaction rate calculations.
The numbers of each of the 12 defined adverse reactions meeting surveillance criteria were calculated in total and by imputability and severity. Acute lung injury and hypotensive reactions were reclassified using updated case definitions implemented in June 2011 and included in analysis. From January 2010 to June 2011, clinical criteria were used to classify hypotensive and acute lung injury reactions as possible for case definition determination. These criteria included the presence of alternative etiologies or underlying risk factors due to the patients’ medical condition that could potentially result in symptoms being classified as an adverse reaction. From July 2011 onward, these criteria were applied toward the possible imputability designation rather than the possible case definition designation. For this analysis, all hypotensive and acute lung injury reactions reported from January 2010 through June 2011 were reclassified according to the case definition and imputability criteria adopted in July 2011 to maintain consistent classification over the analysis period.
The following adverse reaction rates were calculated: 1) total and individual reactions occurring per 100,000 components transfused; 2) total and individual reactions graded as severe, life-threatening, or fatal occurring per 100,000 components transfused; and 3) total and individual reactions occurring per 100,000 components were stratified by component type and stratified by collection method, irradiation, and leukoreduction where this information was reported. All adverse reactions that met the minimum inclusion criteria stated above were included in the numerator for total adverse reaction rate calculations. Whole blood and granulocyte units, although rarely transfused by facilities, are not reported in monthly reports of full unit or aliquot component transfusions and are subsequently excluded from rate calculations. All included adverse reactions graded as severe, life-threatening, or fatal were included in the numerator when calculating the rate of severe reactions occurring among all components. For these estimates, the denominators consisted of the collective number of full units and aliquots of all transfused component types (RBCs, plasma, PLTs, and cryoprecipitate) reported in 2010 to 2012. For rate calculations per specific component type, the numerator included only the adverse reactions that were associated with the respective component type. The denominator included the collective number of full units and aliquots of each respective component type as reported by facilities in 2010 to 2012. Reactions for which a specific transfused blood product was not implicated are included in overall adverse reaction rate calculations but are excluded from component-specific rate calculations.
All analyses were performed using computer software (SAS, Version 9.3, SAS Institute, Inc., Cary, NC). As participating hospitals were not selected using a representative sampling method, statistical comparisons between rates to allow for inference to all transfusion facilities in the United States were not performed.
RESULTS
From January 1, 2010, to December 31, 2012, a total of 164 health care facilities enrolled in the NHSN hemovigilance module. Of these, 112 facilities initiated data reporting by entering an annual facility survey (Fig. 1). Of those entering an annual survey, 77 (68%) entered at least one monthly summary of total transfused components (i.e., denominators). A total of 7147 adverse reaction reports were entered by these 77 facilities during the surveillance period. A total of 1962 (27.5%) of these reactions did not meet case definition and imputability criteria for required reporting and thus were excluded from analysis, leaving 5185 adverse reactions from a total of 1354 facility-months of reporting. Of these 5185 adverse reactions, 43 were excluded because they were reported in months for which the facility did not report denominators, and six other reactions associated with granulocyte or whole blood units were excluded, leaving 5136 adverse reactions remaining for rate calculations.
Fig. 1.
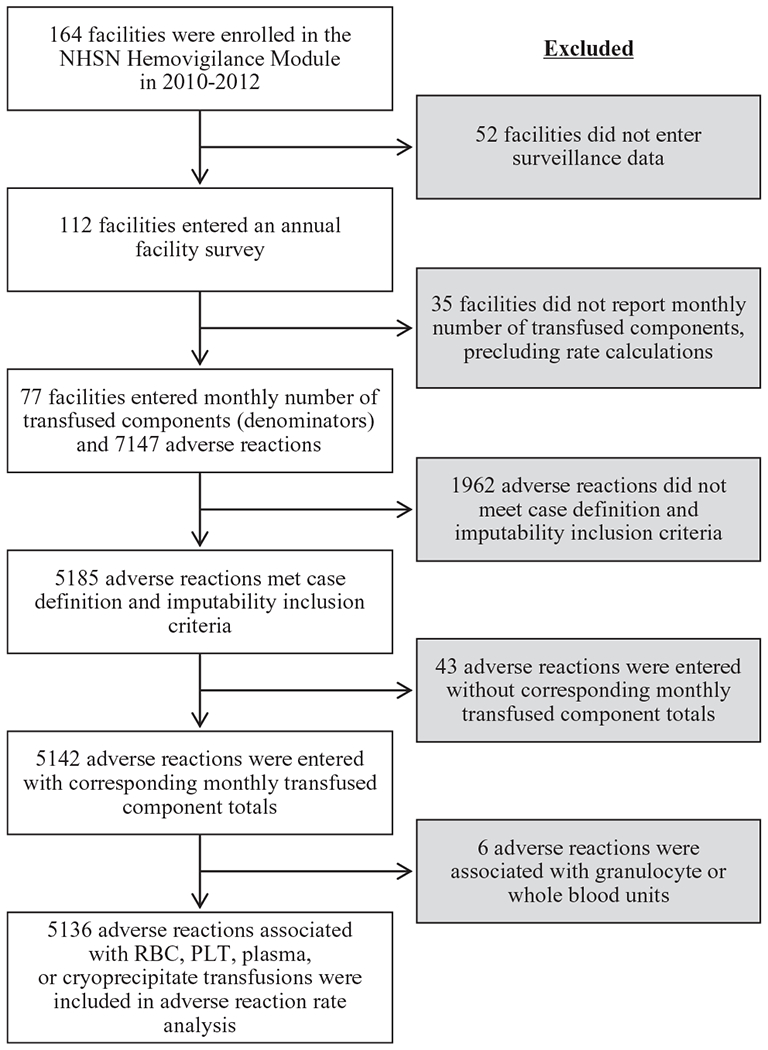
Flow diagram of facility participation and data inclusion for analysis of transfusion-related adverse reactions reported to the NHSN Hemovigilance Module, United States, 2010 to 2012.
Of the 77 included facilities, 49 (63.6%) were located in urban areas and 23 (29.9%) were located in suburban regions (Table 1). Facility size ranged from 10 to 1100 beds (median, 384 beds) served by the transfusion service, with the largest proportion being those with 250 to 499 beds (n = 34 facilities, 44.2%). The number of full-time transfusion service staff, including physicians, technologists, and technicians, ranged from 0 to 115 persons (median, 8.9 persons), with 26 of 77 (33.8%) facilities reporting five to fewer than 10 staff members (reported as full-time equivalents). Fewer than half (33/77, 42.8%) of the included facilities employ a designated staff member in a quality or patient safety role to investigate transfusion-related adverse reaction reports, such as a transfusion safety officer. More than half (42, 54.5%) of the participation health care facilities provide their own transfusion service while 19 of 77 (24.7%) contract some or all of their transfusion service to a blood collection center or centralized transfusion service.
TABLE 1.
Community setting, facility size, number of transfusion service staff, employment of a quality assurance staff member to investigate transfusion-related adverse reactions, and transfusion service provider for health care facilities reporting to the NHSN Hemovigilance Module, United States, 2010 to 2012*
| Health care facility characteristics | Facilities | |
|---|---|---|
| Community setting of health care facility | ||
| Urban | 49 | (63.6) |
| Suburban | 23 | (29.9) |
| Rural | 3 | (3.9) |
| Not reported | 2 | (2.6) |
| Total | 77 | (100) |
| Number of health care facility beds served by the transfusion service | ||
| ≤249 | 16 | (20.8) |
| 250-499 | 34 | (44.2) |
| 500-749 | 18 | (23.4) |
| ≥750 | 9 | (11.7) |
| Total | 77 | (100) |
| Number of transfusion service staff members† | ||
| <5 | 15 | (19.5) |
| 5-<10 | 26 | (33.8) |
| 10-<20 | 19 | (24.7) |
| ≥20 | 17 | (22.1) |
| Total | 77 | (100) |
| Health care facility employs a full-time quality assurance staff member to investigate transfusion-related adverse reaction reports | ||
| Yes | 33 | (42.9) |
| No | 44 | (57.1) |
| Total | 77 | (100) |
| Health care facility’s transfusion service provided by | ||
| Health care facility | 42 | (54.5) |
| Separate health care facility | 7 | (9.1) |
| Blood collection center or centralized transfusion service | 19 | (24.7) |
| Not reported | 9 | (11.7) |
| Total | 77 | (100) |
Data are reported as number (%).
Staff size reported as full-time equivalents.
Over the 3-year period, 77 total facilities reported 2,144,723 blood components transfused including 1,225,496 (57.1%) RBCs, 393,375 (18.3%) PLT components, 400,213 (18.7%) plasma units, and 125,639 (5.9%) cryoprecipitate units (Table 2). Of all RBC units transfused during the reporting period, 1,121,751 (92.5%) were whole blood derived, 1,164,669 (95.0%) were leukoreduced, and 304,629 (24.9%) were irradiated before transfusion. Of all PLT units, 96.9% (381,324) were leukoreduced, 73.1% were obtained by apheresis (287,752), and 76.9% were irradiated (302,472). Similar to RBCs, plasma units were most often whole blood derived (370,013 units, 92.5%).
TABLE 2.
Number of blood components transfused by component type, collection method, irradiation, leukoreduction, and unit size (full unit or aliquot) as reported to the NHSN Hemovigilance Module, United States, 2010 to 2012*
| 2010 |
2011 |
2012 |
2010-2012 |
|||||
|---|---|---|---|---|---|---|---|---|
| Blood component | 26 facilities | 56 facilities | 63 facilities | 77 facilities | ||||
| All components | 432,514 | (100) | 784,866 | (100) | 927,343 | (100) | 2,144,723 | (100) |
| RBCs | 245,293 | (56.7) | 445,682 | (56.8) | 534,521 | (57.6) | 1,225,496 | (57.1) |
| Collection method | ||||||||
| Apheresis | 18,637 | (7.6) | 36,876 | (8.3) | 48,232 | (9.0) | 103,745 | (8.5) |
| Whole blood derived | 226,656 | (92.4) | 408,806 | (91.7) | 486,289 | (91.0) | 1,121,751 | (91.5) |
| Irradiation | ||||||||
| Irradiated | 70,239 | (28.6) | 100,949 | (22.7) | 133,441 | (25.0) | 304,629 | (24.9) |
| Not irradiated | 175,054 | (71.4) | 344,733 | (77.3) | 401,080 | (75.0) | 920,867 | (75.1) |
| Leukoreduction | ||||||||
| Leukoreduced | 233,346 | (95.1) | 419,253 | (94.1) | 512,070 | (95.8) | 1,164,669 | (95.0) |
| Not leukoreduced | 11,947 | (4.9) | 26,429 | (5.9) | 22,451 | (4.2) | 60,827 | (5.0) |
| Unit size | ||||||||
| Full unit | 236,079 | (96.2) | 429,166 | (96.3) | 507,644 | (95.0) | 1,172,889 | (95.7) |
| Aliquot | 9,214 | (3.8) | 16,516 | (3.7) | 26,877 | (5.0) | 52,607 | (4.3) |
| PLTs | 87,252 | (20.2) | 140,647 | (17.9) | 165,476 | (17.8) | 393,375 | (18.3) |
| Collection method | ||||||||
| Apheresis | 57,160 | (65.5) | 99,976 | (71.1) | 130,616 | (78.9) | 287,752 | (73.1) |
| Whole blood derived† | 30,092 | (34.5) | 40,671 | (28.9) | 34,860 | (21.1) | 105,623 | (26.9) |
| Irradiation | ||||||||
| Irradiated | 73,538 | (84.3) | 102,933 | (73.2) | 126,001 | (76.1) | 302,472 | (76.9) |
| Not irradiated | 13,714 | (15.7) | 37,714 | (26.8) | 39,475 | (23.9) | 90,903 | (23.1) |
| Leukoreduction | ||||||||
| Leukoreduced | 84,460 | (96.8) | 135,166 | (96.1) | 161,698 | (97.7) | 381,324 | (96.9) |
| Not leukoreduced | 2,792 | (3.2) | 5,481 | (3.9) | 3,778 | (2.3) | 12,051 | (3.1) |
| Unit size | ||||||||
| Full unit | 82,737 | (94.8) | 131,072 | (93.2) | 149,376 | (90.3) | 363,185 | (92.3) |
| Aliquot | 4,515 | (5.2) | 9,575 | (6.8) | 16,100 | (9.7) | 30,190 | (7.7) |
| Plasma | 76,543 | (17.7) | 153,867 | (19.6) | 169,803 | (18.3) | 400,213 | (18.7) |
| Collection method | ||||||||
| Apheresis | 4,008 | (5.2) | 12,664 | (8.2) | 13,528 | (8.0) | 30,200 | (7.5) |
| Whole blood derived | 72,535 | (94.8) | 141,203 | (91.8) | 156,275 | (92.0) | 370,013 | (92.5) |
| Unit size | ||||||||
| Full unit | 75,131 | (98.2) | 151,487 | (98.5) | 164,515 | (96.9) | 391,133 | (97.7) |
| Aliquot | 1,412 | (1.8) | 2,380 | (1.5) | 5,288 | (3.1) | 9,080 | (2.3) |
| Cryoprecipitate | 23,426 | (5.4) | 44,670 | (5.7) | 57,543 | (6.2) | 125,639 | (5.9) |
Data are reported as number (%).
Whole blood–derived PLTs are also referred to as pooled PLTs in clinical practice.
During the 3-year surveillance period, 5136 adverse reactions that met definite or probable case definition and definite, probable, or possible imputability criteria were reported by 77 health care facilities (Table 3). More than half (2686, 52.3%) of the reactions were of definite imputability and 1522 (29.6%) were probable. Nearly half (2406, 46.8%) of the 5136 reported adverse reactions were allergic reactions. Of these, 172 (7.1%) were severe or life-threatening. The next most frequently reported adverse reactions were febrile nonhemolytic (1853, 36.1%), of which the majority were nonsevere (1829, 99.0%). The most severe reactions were reported least frequently. Acute lung injury was reported 26 times, although 20 were of possible imputability. Nearly two-thirds (17/26, 65.4%) of reported acute lung injury reactions were severe or life-threatening, and three of 26 (11.5%) were reported as either probably or possibly contributing to the patients’ deaths. Of 24 acute hemolytic reactions reported, half (12/24) were severe or life-threatening. Acute hemolytic reactions were most often attributed to anti-A (seven of 24, 29.2%). Transfusion-transmitted infections were infrequently reported and primarily attributed to PLT transfusions (seven of 12, 58.3%) and RBC transfusions (four of 12, 33.3%). Eight of the 12 infections were attributed to Staphylococcus species, one was attributed to a Corynebacterium species, one to Babesia microti, and one to a coinfection of Acinetobacter and Achromobacter species. One of the Staphylococcus transmissions was reported to have resulted in patient death with definite imputability. These least commonly reported adverse reactions—acute lung injury (26, 0.5%), acute hemolytic reactions (24, 0.5%), and transfusion-associated infections (12, 0.2%)—were most often reported as severe, life-threatening, or fatal (84.6, 50.0, and 75.0%, respectively). There were no reports of GVHD, posttransfusion purpura, or viral blood-borne pathogen transmissions in 2010 to 2012.
TABLE 3.
Imputability and severity designations of transfusion-related adverse reactions reported to the NHSN Hemovigilance Module, United States, 2010 to 2012
| Adverse reaction | Number | (%) | Imputability (n = 5136) |
Severity (n = 5136) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Definite | Probable | Possible | Nonsevere | Severe | Life-threatening | Fatal | Not determined | |||
| Allergic | 2406 | (46.8) | 1481 | 735 | 190 | 2232 | 160 | 12 | 0 | 2 |
| FNHTR | 1853 | (36.1) | 670 | 598 | 585 | 1829 | 18 | 0 | 0 | 6 |
| DSTR | 321 | (6.3) | 321 | 302 | 3 | 0 | 0 | 16 | ||
| TACO | 196 | (3.8) | 67 | 85 | 44 | 123 | 59 | 13 | 0 | 1 |
| Hypotensive | 136 | (2.6) | 32 | 55 | 49 | 102 | 28 | 6 | 0 | 0 |
| DHTR | 90 | (1.8) | 67 | 16 | 7 | 69 | 17 | 0 | 0 | 4 |
| TAD | 72 | (1.4) | 21 | 24 | 27 | 54 | 11 | 6 | 0 | 1 |
| TRALI | 26 | (0.5) | 6 | 20 | 4 | 15 | 4 | 3 | 0 | |
| AHTR | 24 | (0.5) | 15 | 7 | 2 | 12 | 10 | 2 | 0 | 0 |
| TTI | 12 | (0.2) | 6 | 2 | 4 | 3 | 8 | 0 | 1 | 0 |
| Total (%) | 5136 | (100) | 2686 (52.3) | 1522 (29.6) | 928 (18.1) | 4730 (92.1) | 329 (6.4) | 43 (0.8) | 4 (0.1) | 30 (0.6) |
AHTR = acute hemolytic transfusion reaction; DHTR = delayed hemolytic transfusion reaction; DSTR = delayed serologic transfusion reaction; FNHTR = febrile nonhemolytic transfusion reaction; TACO = transfusion-associated circulatory overload; TAD = transfusion-associated dyspnea; TRALI = transfusion-related acute lung injury; TTI = transfusion-transmitted infection.
For the 5136 adverse reactions included in analysis, the overall adverse reaction rate was 239.5 per 100,000 total RBC, PLT, plasma, and cryoprecipitate units (full or aliquot) transfused (Table 4). Allergic reactions occurred at the highest overall rate (112.2/100,000) followed by febrile nonhemolytic reactions (86.4/100,000). The rate of severe, life-threatening, or fatal adverse reactions was 17.5 per 100,000 total blood components transfused. The highest rate of adverse reactions reported by component type was among PLT units (421.7 per 100,000) followed by RBCs (205.5 per 100,000), plasma (127.7 per 100,000), and cryoprecipitate (5.6 per 100,000).
TABLE 4.
Rates of transfusion-related adverse reactions per 100,000 total units (full and aliquot) transfused,* by component type, collection method, irradiation, and leukoreduction as reported to the NHSN Hemovigilance Module, United States, 2010 to 2012†
| Transfused blood components | Total adverse reactions‡§ | Allergic | FNHTR | DSTR | TACO‖ | Hypotensive | DHTR | TAD | TRALI | AHTR | TTI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All components | ||||||||||||||||||||||
| All adverse reactions | 5136 | (239.5) | 2406 | (112.2) | 1853 | (86.4) | 321 | (15.0) | 196 | (9.1) | 136 | (6.3) | 90 | (4.2) | 72 | (3.4) | 26 | (1.2) | 24 | (1.1) | 12 | (0.6) |
| Severe, life-threatening, and fatal adverse reactions | 376 | (17.5) | 172 | (8.0) | 18 | (0.8) | 3 | (0.1) | 72 | (3.4) | 34 | (1.6) | 17 | (0.8) | 17 | (0.8) | 22 | (1.0) | 12 | (0.6) | 9 | (0.4) |
| RBCs | ||||||||||||||||||||||
| All units | 2519 | (205.5) | 657 | (53.6) | 1303 | (106.3) | 309 | (25.2) | 75 | (6.1) | 85 | (6.9) | 49 | (4.0) | 18 | (1.5) | 19 | (1.6) | 4 | (0.3) | ||
| Collection method | ||||||||||||||||||||||
| Apheresis | 253 | (243.9) | 63 | (60.7) | 130 | (125.3) | 28 | (27.0) | 11 | (10.6) | 17 | (16.4) | 4 | (3.9) | 0 | (0) | 0 | (0) | 0 | (0) | ||
| Whole blood derived | 2236 | (199.3) | 590 | (52.6) | 1167 | (104.0) | 268 | (23.9) | 64 | (5.7) | 65 | (5.8) | 45 | (4.0) | 16 | (1.4) | 18 | (1.6) | 3 | (0.3) | ||
| Irradiation | ||||||||||||||||||||||
| Irradiated | 610 | (200.2) | 184 | (60.4) | 339 | (111.3) | 41 | (13.5) | 18 | (5.9) | 10 | (3.3) | 10 | (3.3) | 3 | (1.0) | 3 | (1.0) | 2 | (0.7) | ||
| Not irradiated | 1902 | (206.5) | 471 | (51.1) | 964 | (104.7) | 264 | (28.7) | 57 | (6.2) | 74 | (8.0) | 39 | (4.2) | 15 | (1.6) | 16 | (1.7) | 2 | (0.2) | ||
| Leukoreduction | ||||||||||||||||||||||
| Leukoreduced | 2389 | (205.1) | 638 | (54.8) | 1225 | (105.2) | 296 | (25.4) | 71 | (6.1) | 72 | (6.2) | 47 | (4.0) | 18 | (1.5) | 18 | (1.5) | 4 | (0.3) | ||
| Not leukoreduced | 127 | (208.8) | 19 | (31.2) | 78 | (128.2) | 12 | (19.7) | 4 | (6.6) | 11 | (18.1) | 2 | (3.3) | 0 | (0) | 1 | (1.6) | 0 | (0) | ||
| PLTs | ||||||||||||||||||||||
| All units | 1659 | (421.7) | 1188 | (302.0) | 404 | (102.7) | 4 | (1.0) | 28 | (7.1) | 3 | (0.8) | 16 | (4.1) | 4 | (1.0) | 5 | (1.3) | 7 | (1.8) | ||
| Collection method | ||||||||||||||||||||||
| Apheresis | 1547 | (537.6) | 1134 | (394.1) | 358 | (124.4) | 3 | (1.0) | 25 | (8.7) | 3 | (1.0) | 12 | (4.2) | 3 | (1.0) | 4 | (1.4) | 5 | (1.7) | ||
| Whole blood derived | 107 | (101.3) | 52 | (49.2) | 44 | (41.7) | 0 | (0) | 3 | (2.8) | 0 | (0) | 4 | (3.8) | 1 | (0.9) | 1 | (0.9) | 2 | (1.9) | ||
| Irradiation | ||||||||||||||||||||||
| Irradiated | 876 | (289.6) | 604 | (199.7) | 231 | (76.4) | 3 | (1.0) | 15 | (5.0) | 0 | (0) | 14 | (4.6) | 3 | (1.0) | 2 | (0.7) | 4 | (1.3) | ||
| Not irradiated | 782 | (860.3) | 584 | (642.4) | 172 | (189.2) | 1 | (1.1) | 13 | (14.3) | 3 | (3.3) | 2 | (2.2) | 1 | (1.1) | 3 | (3.3) | 3 | (3.3) | ||
| Leukoreduction | ||||||||||||||||||||||
| Leukoreduced | 1643 | (430.9) | 1179 | (309.2) | 398 | (104.4) | 4 | (1.0) | 28 | (7.3) | 2 | (0.5) | 16 | (4.2) | 4 | (1.0) | 5 | (1.3) | 7 | (1.8) | ||
| Not leukoreduced | 15 | (124.5) | 9 | (74.7) | 5 | (41.5) | 0 | (0) | 0 | (0) | 1 | (8.3) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | ||
| Plasma | ||||||||||||||||||||||
| All units | 511 | (127.7) | 423 | (105.7) | 58 | (14.5) | 0 | (0) | 23 | (5.7) | 0 | (0) | 4 | (1.0) | 3 | (0.7) | 0 | (0) | 0 | (0) | ||
| Collection method | ||||||||||||||||||||||
| Apheresis | 44 | (145.7) | 36 | (119.2) | 4 | (13.2) | 0 | (0) | 4 | (13.2) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | ||
| Whole blood derived | 457 | (123.5) | 379 | (102.4) | 54 | (14.6) | 0 | (0) | 18 | (4.9) | 0 | (0) | 3 | (0.8) | 3 | (0.8) | 0 | (0) | 0 | (0) | ||
| Cryoprecipitate | ||||||||||||||||||||||
| All units | 7 | (5.6) | 6 | (4.8) | 1 | (0.8) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | ||
Rates are based on total components transfused as reported by included facilities for 2010-2012.
Data are reported as number (rate).
Adverse reactions that met definite or probable case definition and definite, probable, or possible imputability criteria as reported by 77 health care facilities included in this analysis.
Adverse reactions for which a specific component type was not reported (244 reactions) are included in rate calculations for all components.
Circulatory overload is not reported as due to a specific component type per NHSN Hemovigilance Module protocol.
AHTR = acute hemolytic transfusion reaction; DHTR = delayed hemolytic transfusion reaction; DSTR = delayed serologic transfusion reaction; FNHTR = febrile nonhemolytic transfusion reaction; TACO = transfusion-associated circulatory overload; TAD = transfusion-associated dyspnea; TRALI = transfusion-related acute lung injury; TTI = transfusion-transmitted infection.
When comparing by collection and product modification methods, adverse reaction rates were higher for apheresis RBCs (243.9/100,000) compared to whole blood-derived RBCs (199.3/100,000) but similar between irradiated (200.2/100,000) and not irradiated (206.5/100,000) and leukoreduced (205.1/100,000) and not leukoreduced (208.8/100,000) RBC units. Adverse reaction rates among PLTs varied by collection method (apheresis—537.6/100,000 vs. whole blood derived—101.3/100,000), irradiation (irradiated—289.6/100,000 vs. not irradiated—860.3/100,000), and leukoreduction (leukoreduced—430.9/100,000 vs. nonleukoreduced—124.5/100,000). Reaction rates were slightly higher among apheresis (145.7/100,000) versus whole blood–derived (123.5/100,00) plasma units.
DISCUSSION
In the United States, blood transfusion is one of the most common medical procedures performed during hospitalization;17 the NHSN Hemovigilance Module is the only national, facility-based surveillance program for adverse transfusion-related events. Among facilities participating in recipient hemovigilance from 2010 to 2012, allergic reactions and febrile nonhemolytic reactions occurred most frequently, although these were usually not severe. Acute hemolytic reactions, acute lung injury, and transfusion-transmitted infections carried the greatest morbidity and mortality among recipients and should continue to be targeted for future interventions. Consistent with reports from outside the United States, PLTs were associated with the highest rate of adverse reactions in this report.6,8 However, some preventable reactions, including hemolytic and serologic reactions, occurred most frequently in association with RBC transfusions.
In these data, overall RBC adverse reaction rates did not substantially differ with irradiation or leukoreduction, but higher reaction rates were observed for apheresis in comparison with whole blood–derived RBC units. More striking are the greater reaction rates in apheresis compared with whole blood–derived PLTs and were particularly noted in allergic, febrile nonhemolytic, and hypotensive reactions. Similar observations have been reported from the French hemovigilance system.18,19 Reasons for higher rates among apheresis RBC units and PLT units are unclear but may include production methods, immunologic mechanisms, or the effect of additive solutions.20–22 Additionally, some of the differences in adverse reaction rates observed between apheresis and whole blood–derived PLTs may be attributable to variations in denominator reporting, particularly in the way whole blood–derived PLT units, which are often pooled before transfusion, are counted and reported by facilities. Further study is needed to better understand the observed difference in rates of adverse reactions among apheresis and whole blood–derived components.
Although hemovigilance reporting practices vary by country, the rates of adverse reactions presented here are comparable to those reported in other countries.3,5,6,8,23 Some of the differences may be due to greater recognition and more consistent reporting of adverse reactions in countries with reporting that is mandated by regulation, consistent involvement by a transfusion safety officer, or other nationally standardized investigation processes. For example, overall adverse reaction rates reported from Quebec are higher (348.0/100,000) than those reported to the Hemovigilance Module participant facilities.6 In Quebec, however, awareness and participation in hemovigilance are very high and include involvement of a transfusion safety officer in the investigation of suspected reactions. Overall adverse reaction rates reported in Norway are also slightly higher if minor allergic reactions are disregarded (US—135.4/100,000 vs. Norway—147/100,000). In Norway, participation in hemovigilance is mandatory and each suspected report is investigated and verified by a national hemovigilance task group.23 Meanwhile the hemovigilance requirements in the United Kingdom Serious Hazards of Transfusion (SHOT) system do not include mandatory reporting for nonsevere reactions but the rates of severe acute lung injury, acute hemolytic reactions, and infections are mandatory and are consistent with those reported here.5 These similarities are encouraging, suggesting that the US recipient hemovigilance effort is capable of capturing comparable information upon which national estimates can be established so as to inform and evaluate future preventive measures.
Adverse reaction rates presented from the first 3 years of NHSN hemovigilance data are consistently lower than active, facility-focused transfusion safety surveillance efforts elsewhere in the United States.24–27 These differences may be due to variations in reporting practices within facilities and to differences in how cases are defined or categorized between these different systems. However, the differences do support the need for standardized national estimates in the United States as in other countries. The 2011 National Blood Collection and Utilization Survey estimates that more than 20 million blood components are transfused annually in the United States.9 Since implementation, total transfused components under surveillance in the Hemovigilance Module has grown from 432,514 in 2010 to 927,343 in 2012 (from approx. 2.1% to 4.5% of components transfused nationally). While this growth is encouraging, the number of enrolled facilities still constitutes a small proportion of all eligible health care facilities performing transfusions in the United States.
One reason for the relatively low proportion of participation may be the lack of a reporting mandate in the United States, as is present in many other countries.28 Previous studies have described that reduction of reporting requirements, simplifying case criteria, integrating electronic data capture, and maximizing access to and utility of submitted data can enhance and increase participation in surveillance systems.29–33
Modifications to reporting requirements were implemented in the Hemovigilance Module protocol in January 2013. These include designating reporting of nonsevere allergic reactions as optional rather than mandatory and reducing required reporting of incidents to only those related to patient reactions instead of all errors and accidents occurring throughout the transfusion process. These two changes have reduced the required reporting burden by approximately 50%. In addition to these changes, further system modifications are under way to make the system more useful to participating facilities. These include analysis and reporting functions within the module that allows facilities and groups to calculate adverse reaction and error rates within their institutions and enabling an option allowing electronic data imports from hospital blood inventory management systems into the module for easier denominator data reporting. Another planned function will allow a facility to internally compare its adverse reaction rate with an aggregate rate from all participating facilities for benchmarking purposes.
The findings of this surveillance report are subject to three limitations. First, data entered into the module by facilities and available for this analysis are self-reported and not independently verified. The accuracy of reporting therefore relies on recognition and communication of transfusion reactions within facilities, the availability of relevant patient data, and the reporters’ proficiency in applying the case definition, imputability, and severity criteria. Comprehensive evaluations of other NHSN modules, including validation efforts, have been undertaken after those systems were established and operational. These studies have suggested that inconsistent interpretation of case definitions and underreporting of cases and denominators occur, which can be rectified through simplification of case definitions and improved training for reporters.34–36 Second, inconsistencies in self-reporting by facilities, particularly related to whether PLT and cryoprecipitate components were reported as individual or therapeutic units, may have impacted rate calculations resulting in under- or overestimates of reactions for these components. The impact on the rates reported here is unknown and cannot be quantified. Third, relatively small numbers of hospitals participated in the module and were not a representative sample of all transfusion facilities in the United States. Therefore, statistical comparisons between rates to allow for inference to all transfusion facilities in the country were not performed and findings may not be generalizable. However, they do serve as a starting point for future national surveillance estimates of adverse transfusion reactions.
In summary, although blood transfusion is a common medical procedure that is often lifesaving, it can also pose substantial risk. Early participants in national recipient hemovigilance reported 239.5 adverse reactions per 100,000 transfused blood components, approximately 8% of which were severe, life-threatening, or fatal. Some of these transfusion risks may be preventable or otherwise mitigated. Further analyses are needed to understand the higher rates of adverse reactions reported in apheresis components, the impact of modifications such as leukoreduction and irradiation, and new interventions to reduce the most common causes of severe reactions and death due to transfusion. A national hemovigilance system is essential to appropriately monitor the safety of transfusion practices, to identify areas for enhanced patient safety, and to assess the impact of future interventions.
ACKNOWLEDGMENTS
The authors thank the NHSN facility participants for their efforts to monitor transfusion practices and improve patient safety, the AABB Biovigilance Interorganizational Task Force for their role in development of national hemovigilance, and the NHSN Hemovigilance Module stakeholder and subject matter expert groups for their input to improve the Hemovigilance Module.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
ABBREVIATION:
- NHSN
National Healthcare Safety Network
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.de Vries RR, Faber JC, Strengers PF; Board of the International Haemovigilance Network. Haemovigilance: an effective tool for improving transfusion practice. Vox Sang 2011; 100:60–7. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MF, Stanworth SJ, Yazer M. Transfusion practice and safety: current status and possibilities for improvement. Vox Sang 2011;100:46–59. [DOI] [PubMed] [Google Scholar]
- 3.Keller-Stanislawski B, Lohmann A, Gunay S, et al. The German Haemovigilance System—reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med 2009;19:340–9. [DOI] [PubMed] [Google Scholar]
- 4.Giampaolo A, Piccinini V, Catalano L, et al. The first data from the haemovigilance system in Italy. Blood Transfus 2007;5:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stainsby D, Jones H, Asher D, et al. ; SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 2006;20:273–82. [DOI] [PubMed] [Google Scholar]
- 6.Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfus Apher Sci 2004;31:111–22. [DOI] [PubMed] [Google Scholar]
- 7.Rebibo D, Hauser L, Slimani A, et al. The French Haemovigilance System: organization and results for 2003. Transfus Apher Sci 2004;31:145–53. [DOI] [PubMed] [Google Scholar]
- 8.Andreu G, Morel P, Forestier F, et al. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion 2002;42:1356–64. [DOI] [PubMed] [Google Scholar]
- 9.US. Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: Department of Health and Human Services; 2013. [Google Scholar]
- 10.US. Food and Drug Administration. Vaccines, Blood & Biologics. Transfusion/donation fatalities. 2013. [cited 2013 Dec 20]. Available from: http://www.fda.gov/biologicsbloodvaccines/safetyavailability/reportaproblem/transfusiondonationfatalities/default.htm
- 11.Eder AF, Dy BA, Barton J, et al. The American Red Cross Hemovigilance Program: advancing the safety of blood donation and transfusion. Immunohematology 2009;25: 179–85. [PubMed] [Google Scholar]
- 12.The Joint Commission. Sentinel event policy and procedures. 2013. [cited 2013 Dec 20]. Available from: http://www.jointcommission.org/Sentinel_Event_Policy_and_Procedures/
- 13.US. Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Division of Health Informatics and Surveillance (DHIS), Centers for Disease Control and Prevention; 2013. [cited 2013 Dec 20]. Available from: http://wwwn.cdc.gov/nndss/default.aspx
- 14.Commonwealth of Massachusetts Department of Public Health Clinical Laboratory Program. Blood bank monthly activity report. 2013. [cited 2013 Dec 20]. Available from: http://www.mass.gov/eohhs/gov/departments/dph/programs/hcq/labs/public-health-clinical-lab-blood-banks.html
- 15.US. Centers for Disease Control and Prevention. National Healthcare Safety Network. 2013. [cited 2013 Dec 20]. Available from: http://www.cdc.gov/nhsn/
- 16.US. Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) manual: biovigilance component v1.3. Atlanta (GA): Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, 2011:1–30. [Google Scholar]
- 17.Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. hospitals, 2011: Statistical Brief #165 Healthcare Cost and Utilization Project (HCUP). Rockville (MD): Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 18.Willaert B, Vo Mai MP, Caldani C. French haemovigilance data on platelet transfusion. Transfus Med Hemother 2008; 35:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncharmont P, Meyer F. [Allergic adverse transfusion reactions in paediatrics, a 3-year study]. Transfus Clin Biol 2013;20:455–7. [DOI] [PubMed] [Google Scholar]
- 20.Hamzeh-Cognasse H, Damien P, Nguyen KA, et al. Immune-reactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion 2014;54: 613–25. [DOI] [PubMed] [Google Scholar]
- 21.Nogawa M, Naito Y, Chatani M, et al. Parallel comparison of apheresis-collected platelet concentrates stored in four different additive solutions. Vox Sang 2013;105:305–12. [DOI] [PubMed] [Google Scholar]
- 22.Semple E, Bowes-Schmidt A, Yi QL, et al. Transfusion reactions: a comparative observational study of blood components produced before and after implementation of semiautomated production from whole blood. Transfusion 2012;52:2683–91. [DOI] [PubMed] [Google Scholar]
- 23.Steinsvag CT, Espinosa A, Flesland O. Eight years with haemovigilance in Norway. What have we learnt? Transfus Apher Sci 2013;49:548–52. [DOI] [PubMed] [Google Scholar]
- 24.Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med 2007;131:708–18. [DOI] [PubMed] [Google Scholar]
- 25.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 2012;52:160–5. [DOI] [PubMed] [Google Scholar]
- 26.Cohn CS, Stubbs J, Schwartz J, et al. A comparison of adverse reaction rates for PAS C versus plasma platelet units. Transfusion 2014;54:1927–34. [DOI] [PubMed] [Google Scholar]
- 27.Tobian AA, Fuller AK, Uglik K, et al. The impact of platelet additive solution apheresis platelets on allergic transfusion reactions and corrected count increment (CME). Transfusion 2014;54:1523–9; quiz 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faber JC. The European Blood Directive: a new era of blood regulation has begun. Transfus Med 2004;14:257–73. [DOI] [PubMed] [Google Scholar]
- 29.Sickbert-Bennett EE, Weber DJ, Poole C, et al. Completeness of communicable disease reporting, North Carolina, USA, 1995–1997 and 2000–2006. Emerg Infect Dis 2011;17: 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukwago L, Nanyunja M, Ndayimirije N, et al. The implementation of Integrated Disease Surveillance and Response in Uganda: a review of progress and challenges between 2001 and 2007. Health Policy Plan 2013;28:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin MY, Hota B, Khan YM, et al. ; CDC Prevention Epicenter Program. Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA 2010;304:2035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007;17:643–53. [DOI] [PubMed] [Google Scholar]
- 33.Tokars JI, Richards C, Andrus M, et al. The changing face of surveillance for health care-associated infections. Clin Infect Dis 2004;39:1347–52. [DOI] [PubMed] [Google Scholar]
- 34.Backman LA, Melchreit R, Rodriguez R. Validation of the surveillance and reporting of central line-associated bloodstream infection data to a state health department. Am J Infect Control 2010;38:832–8. [DOI] [PubMed] [Google Scholar]
- 35.Backman LA, Nobert G, Melchreit R, et al. Validation of the surveillance and reporting of central line-associated bloodstream infection denominator data. Am J Infect Control 2014;42:28–33. [DOI] [PubMed] [Google Scholar]
- 36.Dembek ZF, Kellerman SE, Ganley L, et al. Reporting of vancomycin-resistant enterococci in Connecticut: implementation and validation of a state-based surveillance system. Infect Control Hosp Epidemiol 1999;20:671–5. [DOI] [PubMed] [Google Scholar]


