Introduction
Acute kidney injury is frequently present in severe acute respiratory syndrome coronavirus 2 infection (coronavirus disease 2019 [COVID-19]).1 In addition, many patients positive for COVID-19 also develop proteinuria and collapsing glomerulopathy.2, 3, 4 We recently encountered a kidney biopsy specimen showing an immune complex-glomerulonephritis (GN) after a recent COVID-19 infection. To the best of our knowledge, an immune-complex GN has not been described in the setting of COVID-19 infection.
Case Presentation
Clinical History and Laboratory Data at Presentation
A 25-year-old White woman with a history of genetic focal segmental glomerulosclerosis secondary to a SMARCAL1 gene mutation and baseline serum creatinine of 0.98 mg/dl, serum albumin 3.5 g/dl, proteinuria of 3.7g/24 h, and a creatinine clearance of 83 ml/min/1.73 m2, was admitted on 7 November 2020 to our hospital with diarrhea secondary to Shiga toxin-producing Escherichia coli O157:H7, associated hemolytic uremic syndrome (HUS), seizures, encephalopathy, and acute kidney injury requiring hemodialysis from admission to 16 November 2020. On admission, she was also found to be positive for SARS CoV-2 (COVID-19). Laboratory results are presented in Table 1.
Table 1.
Clinical characteristics at specified time points
| Laboratory evaluation (normal range and units) | Initial presentation 3 Nov 2020 |
Kidney biopsy 12 Dec 2020 |
Last follow-up 6 Jan 2021 |
|---|---|---|---|
| Hematology | |||
| White blood cells (3.4–9.6 × 109/l) | 9.9 | 4.7 | 7.0 |
| Hemoglobin (11.6–15.0 g/dl) | 9.5 | 9.1 | 10.5 |
| Platelets (157–371 × 109/l) | 67 | 110 | 242 |
| Reticulocytes (30.4–110.9 × 109/l) | 61.7 | ||
| Peripheral smear | Slight schistocytes | Normal | Normal |
| Coagulation | |||
| INR/prothrombin (0.9–1.1) | 1.2 | 1.0 | 0.9 |
| Fibrinogen (200–393 mg/dl) | 598 | N/A | N/A |
| D-dimer (<500 fibrinogen equivalent units) | 24,746 | 5054 | N/A |
| ADAMSTS-13 activity assay (>70%), % | 67 | N/A | N/A |
| Serum chemistry | |||
| Serum creatinine (0.6–1.0 mg/dl) | 9.0 | 2.5 | 3.2 |
| Estimated GFR by MDRD (ml/min/BSA) | 5 | 28 | 19 |
| Albumin (3.5–5.0 g/dl) | 2.1 | 2.5 | 2.3 |
| Lactate dehydrogenase (122–222 U/l) | 500 | 285 | 269 |
| Haptoglobin (30–200 mg/dl) | <14 (undetectable) | 15 | 102 |
| Infectious workup | |||
| COVID-19 polymerase chain reaction | Detected | Undetected | N/A |
| COVID-19 total antibodies | Negative | N/A | N/A |
| Shiga-like toxin-producing E coli and E coli 0157:H7 | Detected | N/A | N/A |
| Blood cultures | Negative | N/A | N/A |
| Urine cultures | Negative | N/A | N/A |
| HIV antigen and antibody screen | Negative | Negative | N/A |
| Hepatitis B surface antigen | Negative | Negative | N/A |
| Hepatitis B surface antibody | Negative | Negative | N/A |
| Hepatitis C antibody | Negative | Negative | N/A |
| Cytomegalovirus DNA | Negative | N/A | N/A |
| Epstein-Barr virus screen | IgG positive, IgM negative | N/A | N/A |
| Immunology | |||
| Total complement (30-75 U/ml) | 45 | 6 | N/A |
| C3 (75-175 mg/dl) | 47 | 98 | 79 |
| C4 (14-40 mg/dl) | 7 | 20 | 12 |
| Antinuclear antibody (<1.0 U) | 0.3 | 0.2 | N/A |
| Antinuclear antibody, Hep-2 substrate (<1:80) | N/A | <1:80 | N/A |
| Double-stranded DNA antibody (<30.0 IU/ml) | N/A | <12.3 | N/A |
| Extranuclear antibody evaluationa | N/A | Negative | N/A |
| Phospholipid antibody (IgG and IgM) | N/A | Negative | N/A |
| PLA2R receptor antibody (<0.2 RU/ml) | N/A | <0.2 RU/mL | N/A |
| Cryoglobulins | Negative | ||
| Urine studies | |||
| Urinalysis | >100 RBC, nondysmorphic, no visible casts | 11-20 RBC, nondysmorphic, free fat and oval fat bodies and renal epithelial cells | 3-10 RBC, nondysmorphic, free fat and oval fat bodies and renal epithelial cells |
| 24-hour urine total protein (<250 mg/24 h) | 3740 | 12,960 | 7205 |
| Genetic testing | |||
| Atypical HUS/thrombotic microangiopathy genetic panelb | Negative | N/A | N/A |
BSA, body surface area; COVID-19, coronavirus disease 2019; E coli, Escherichia coli; GFR, glomerular filtration rate; HUS, hemolytic–uremic syndrome; INR, international normalized ratio; MDRD, Modification of Diet in Renal Disease; N/A, not available; RBC, red blood cells; RU, relative unit.
Negative antibody testing for SS-A/Rho, SS-B, Sm Ab, RNP, Scl70, and Jo 1.
ADAMTS13, C3, CD46, CFB, CFD, CFH, CFHR1, CFHR3, CFHR5, CFI, DGKE, PLG, and THBD.
Because of the severity of her HUS, she received 2 doses of eculizumab 900 mg i.v., 1 week apart. She was discharged on 4 December 2020 and seen in the clinic on 9 December 2020. Her creatinine had decreased to 2.4 mg/dl, but serum albumin had decreased to 2.5 g/dl and proteinuria had increased to 13 g/24 h. Owing to the unusual course of her renal disease, a kidney biopsy was performed.
Kidney Biopsy Specimen
The kidney biopsy specimen showed a membranoproliferative GN (MPGN) pattern of injury. There were 14 glomeruli present, of which 6 (43%) were globally sclerosed. The glomeruli showed both mesangial and endocapillary hypercellularity, with lobular accentuation of the glomerular capillary tufts. The capillary walls were thickened and showed double contour formation. In some of the loops, large wire loop-type subendothelial deposits and intraluminal hyaline immune microthrombi were present. There was no evidence of crescents, fibrinoid necrosis, or thrombosis. The tubulointerstitium showed acute tubular injury and moderate interstitial inflammation, with moderate (30%–40%) tubular atrophy and interstitial fibrosis. Arteries and arterioles were unremarkable.
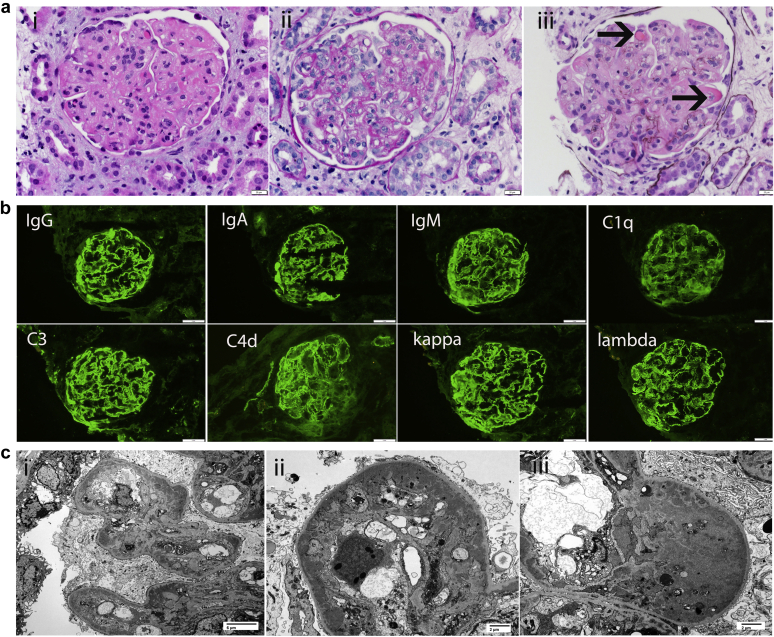
Immunofluorescence microscopy showed bright staining for IgG (3+), IgA (3+), IgM (3+), C1q (3+), C3 (3+), C4d (3+), and kappa (3+) and lambda (3+) light chains. There was no glomerular staining for fibrinogen. Electron microscopy showed numerous large subendothelial and mesangial electron-dense deposits. Glomerular basement membrane double contour formation was present with the subendothelial deposits. In addition, a few loops contained deposits completely filling the capillary lumen, and no substructures were evident. Tubuloreticular inclusions were not present in endothelial cells, and no viral particles were identified. Podocytes displayed diffuse foot process effacement. No deposits were present along the tubular basement membranes. Kidney biopsy specimen findings are shown in Figure 1.
Figure 1.
(a) Light microscopy shows a proliferative glomerulonephritis. (i-iii) Glomeruli show endocapillary and mesangial hypercellularity, lobular accentuation of the glomerular capillary tufts, double contour formation along the capillary walls, and wire loop-type and immune microthrombi (arrows) (hematoxylin and eosin, original magnification ×40). (Immunofluorescence microscopy shows bright staining for all immunoglobulins and complement proteins. Immunofluorescence studies showing bright 3+ granular staining for IgG, IgA, IgM, C1q, C3, C4d, and kappa and lambda light chains (periodic acid–Schiff, original magnification ×40). (c) Electron microscopy shows numerous mesangial and capillary wall electron dense deposits (silver methenamine). (i) Mesangial and subendothelial electron dense deposits are both present (original magnification × 3000). (ii–iii) Large subendothelial electron-dense deposits line the capillary walls (original magnification ×6000).
Diagnosis: Immune-Complex “Full House” GN
The findings are consistent with an immune complex-mediated MPGN.5 Immunofluorescence microscopy showed the “full house” pattern that suggested an autoimmune disease such as systemic lupus erythematosus. However, results of an evaluation for systemic lupus erythematosus was negative, including tests for antinuclear antibodies, double-stranded DNA, SS-A/Rho, SS-B, Smith antibody, ribonucleoprotein , Scl70, Jo 1, and cryoglobulins. Interestingly, the biopsy specimen continued to show the focal segmental glomerulosclerosis that was consistent with the prior focal segmental glomerulosclerosis diagnosis.
The differential diagnosis of the immune-complex MPGN also includes an infection-related GN. However, the COVID-19 infection had resolved at the time of the biopsy, and typically, infection-related glomerulonephritis does not show a full house pattern on immunofluorescence or large subendothelial deposits on electron microscopy. In addition, subepithelial hump-like deposits were not present on electron microscopy. Features of thrombotic microangiopathy were also not present given the history of HUS.
Clinical Follow-up
The patient was treated with methylprednisolone 750 mg i.v., followed by oral prednisone 50 mg/d, and oral mycophenolate mofetil 500 mg twice daily. However, when seen in the clinic on 6 January 2021, her serum creatinine had increased to 3.2 mg/dl, serum albumin was 2.3 g/dl, and proteinuria was 7.2 g/24 h (Table 1). The short term follow-up does not allow us to fully ascertain the response to immunosuppression therapy.
Discussion
Most studies of kidney biopsy findings in COVID-19 infection describe tubulointerstitial lesions of acute tubular necrosis and glomerular lesions of collapsing glomerulopathy.4,6, 7, 8 To the best of our knowledge, an immune complex-mediated MPGN has not been described in the setting of a recent COVID-19 infection. The glomerular findings are reminiscent of HIV-associated immune-complex GN that occurs more commonly in White patients, often presents with nephrotic syndrome, and shows lupus-like features on the kidney biopsy specimen in the absence of systemic lupus erythematosus.9 Although the etiology is unknown, polyclonal B-cell activation and hypergammaglobulinemia has been proposed as a mechanism for HIV-associated immune-complex GN. HIV-associated immune-complex GN may coexist with HIV-associated collapsing glomerulopathy. Our patient developed COVID-19–associated immune-complex GN likely superimposed on the preexisting genetic focal segmental glomerulosclerosis.
The differential diagnosis of immune-complex GN includes cryoglobulinemic GN particularly due to low C3 and C4 levels and the finding of immune-type microthrombi on the kidney biopsy specimen. However, evaluation for cryoglobulins was negative, and the deposits showed no substructure on electron microscopy. In addition, results of the evaluation of the underlying etiologies of cryoglobulins, such as autoimmune disease, hepatitis B and C infections, and monoclonal gammopathy, were negative (Table 2). The recent history of HUS also raises the possibility of entrapped deposits in capillary walls with double contours. However, widespread Ig deposits with full house pattern on immunofluorescence and numerous capillary wall and mesangial electron dense deposits are not seen in HUS. Finally, it is difficult to determine whether the preexisting focal segmental glomerulosclerosis related to SMARCAL1 gene mutation may have contributed to immune complex-GN.
Table 2.
Teaching points
|
|
|
|
COVID-19, coronavirus disease 2019. GN, glomerulonephritis.
To summarize, we describe a unique full house immune-complex GN that developed after a recent COVID-19 infection. The entity should be added to lesions associated with COVID-19 infection.
Patient Consent
Consent was provided by the patient for publication of this case.
Disclosure
All the authors declared no competing interests.
References
- 1.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoriello D., Khairallah P., Bomback A.S. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velez J.C.Q., Caza T., Larsen C.P. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16:565–567. doi: 10.1038/s41581-020-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasr S.H., Alexander M.P., Cornell L.D. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis. 2021;77:465–468. doi: 10.1053/j.ajkd.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis: a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 6.Golmai P., Larsen C.P., DeVita M.V. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31:1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P., Uppal N.N., Wanchoo R. COVID-19–associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas M., Kaul S., Eustace J.A. HIV-associated immune complex glomerulonephritis with “lupus-like” features: a clinicopathologic study of 14 cases. Kidney Int. 2005;67:1381–1390. doi: 10.1111/j.1523-1755.2005.00215.x. [DOI] [PubMed] [Google Scholar]