Abstract
Most reports of Multisystem Inflammatory Syndrome (MIS-C) have come from Europe and North America. The paucity of reports in Africa is in contrast with the demographics of the series in New York, Paris and UK with children of African ancestry accounting for over 40% of all cases of MIS-C. With the global trend of higher prevalence of MIS-C in children of African ancestry, enhanced surveillance and awareness for this syndrome in children with COVID-19 in Africa are therefore important. A case report of a 12-year-old Nigerian girl with MIS-C is presented in line with the WHO global surveillance especially in areas were MIS-C is considered a rarity. This case report stimulates a call for vigilance and expanded effort at surveillance to promote early recognition and diagnosis of MIS-C in Nigeria and Africa. The favourable outcome and experience from this case will create awareness, expand knowledge, and support clinicians in Nigeria and the African continent in their approach to other potential cases.
Keywords: SARS-COV2, COVID-19, MIS-C, Kawasaki, Africa
Introduction
At the onset of the COVID-19 pandemic, it was observed that infection was less prevalent in children, with a 2% prevalence for people aged ≤19 years among a Chinese cohort of 72314 cases. (Wu, MacGoogan, 2020) Children were also reported to have a lower risk for severe COVID-19 disease requiring critical care in North America. (Shekerdemian et al., 2020) As the pandemic progressed, reports from European and North American countries highlighted the occurrence of a hyper, multisystem inflammatory process in children that had features like Kawasaki disease (KD) (WHO, 2020).
Verdoni et al. (2020) in Italy reported a 30-fold increase in the incidence of Kawasaki-like disease with evidence of immune response to SARS COV-2 over 30 days; Toubiana et al. (2020) from Paris also reported a KD-like syndrome in a cohort of children with COVID-19. Dufort et al. (2020) in New York also reported 95 confirmed cases with the multisystem inflammatory syndrome in children (MIS-C). The KD-like syndrome associated with COVID-19 in children has been described as atypical KD and a possible distinct clinical entity (Kam et al., 2020). These distinctions in the clinical manifestations have thus prompted the development of case definitions and diagnostic criteria by the World Health Organization (WHO), Centers for Disease Control (CDC) and the Royal College of Paediatric and Child Health (RCPCH) (Simpson and Newburger, 2020). MIS-C has also been selected as an urgent reportable condition by the WHO emphasizing the importance of surveillance especially in places where it is considered a rarity.
Most reports of MIS-C associated with COVID-19 have come from Europe and North America (WHO, 2020, Son, 2020). The paucity of reports in Africa is in contrast with the demographics of the series in New York (Dufort et al., 2020), Paris (Toubiana et al., 2020) and UK (Riphagen et al., 2020, Whittaker et al., 2020), which reported that children of African ancestry accounted for 40%, 57%, 75%, and 38%, respectively, of all cases of MIS-C. The only report from Africa is from South Africa, with patients of African and black ethnicities comprising 78.3% of the study population. (Webb et al., 2020). It has been previously observed that very few cases of KD have been reported from Nigeria, with four separate reports documenting only 10 cases since 2010 (Animasahun et al., 2017). With the global trend of higher prevalence of MIS-C in children of African ancestry, enhanced surveillance and awareness for this syndrome in children with COVID-19 in Africa are therefore important. This first report of a case of MIS-C in a Nigerian female child is expected to promote awareness of this syndrome and improve the care of children with severe COVID-19 disease in Nigeria and Africa.
Case report
A 12-year-old girl presented on referral to the COVID-19 treatment centre at the University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria, on the 3rd of June 2020, with high-grade intermittent fever for 10 days; progressively worsening breathlessness for 4 days; skin rashes, mucosal excoriations, conjunctivitis and diarrhoea for 3 days, respectively. (Clinical features are presented in Table 1 ). On admission, clinical signs comprised bodyweight of 50 kg (within 75 percentile, (+0.66 SD) for age), a temperature of 39 °C, respiratory rate of 48 per minute, pulse rate of 135 per minute and blood pressure of 90/50 mmHg. Oxygen saturation (SP02) was 76% in room air. The paediatric early warning systems (PEWS) score which was used in monitoring severity and clinical progression was 11, which is indicative of high risk (NICE, 2017), as displayed in Figure 1 and Table 2 . She had received empirical treatment for bacterial pneumonia with a poor response before referral.
Table 1.
Showing clinical presentation and progression of the patient.
| Clinical symptoms | Duration (Days) | Description | Day 5 status | Day 14 status |
|---|---|---|---|---|
| Fever | 10 | High grade and intermittent | ||
| Breathlessness | 4 | Progressive worsening | ||
| Rashes | 4 | Face, upper limbs and trunks, pruritic | ||
| Flaking of lips | 4 | Peeling of the upper lips and mucous membranes of the mouth | ||
| Redness of eyes | 3 | Cream-coloured sticky discharge | ||
| Diarrhoea | 3 | Stools were loose, greenish, non-bloody with 3 to 4 bowel motions per day | ||
| Physical examination signs | Values | |||
| AVPU | Responds to Voice | Alert | Alert | |
| Temperature (OC) | 39 | 37 | 36.5 | |
| Respiratory rate/minute | 48 | 32 | 28 | |
| Pulse/minute | 135 | 90 | 84 | |
| Blood Pressure (mmHg) | 90/50 | 90/50 | 90/60 | |
| SPO2 (room air) % | 76 | 98 | 99 | |
| Capillary refill | 2–4 s | <2 s | <2 s | |
| PEWs score | 11 | 0 | 0 | |
| Eyes | Bilateral conjunctival injection | |||
| Mouth and mucosa | Dried peeled lips and hyperaemic oral mucosa | |||
| Skin | Diffuse maculopapular rashes were found on the face and upper limbs | |||
| Legs | pedal oedema | |||
| Chest | Bibasilar crackles | |||
| Abdomen | Tender hepatomegaly |
| Parameters | Result | Normal ranges | Interpretation |
|---|---|---|---|
| Haemoglobin (g/dl) | 8.95 | 11.5–13.5 | Anaemia |
| White blood count (x109/L) | 21.20 × 109/L | 4.5–13.5 | Leucocytosis |
| Neutrophils % | 85.19 | 40–75 | Neutrophilia |
| Lymphocytes % | 8.7 | 20–45 | Lymphopenia |
| Neutrophils (x109/L) | 18.06 | 1.50–8.50 | Neutrophilia |
| Lymphocyte (x109/L) | 0.85 | 1.00–6.5 × 109/L | Lymphopenia |
| Eosinophil (x109/L) | 0.42 | 0.00–0.40 | Marginal elevation |
| Monocytes (x109/L) | 0.77 | 0.00–1.00 | Normal |
| Platelet (x 109/L) | 84.3 | 150–450 | Thrombocytopenia |
| ESR (mm/h) | 70 mm/h | 0.00–4 | Elevated |
| Sodium (mmol/L) | 135 mmol/L | 135–145 | Normal |
| Potassium (mmol/L) | 3.4 mmol/L | 3.5–5.5 | Hypokalemia |
| Bicarbonate (mmol/L) | 29 mmol/L | 24–30 | Normal |
| Urea (mmol/L) | 2.9 mmol/L | 2.4–6.2 | Normal |
| Creatinine (umol/L) | 130 mmol/L | 60–120 | Azotemia |
| Random blood sugar (mmol/l) | 5.6 | 4.0–10.0 | Normal |
| HIV screening | Seronegative | ||
| Glomerular filtration rate (GFR) (mls/min/BSA) | 70.87 | Evidence of renal impairment (AKI) | |
| RT-PCR for SARS-COV-2 Test date: (3/6/2020) | Positive | RT-PCR for SARS-COV-2 Test date: (24/6/2020) | Negative |
| Chest radiograph | Dense lung fields with bilateral background tiny nodular lesions | Atypical interstitial pneumonia |
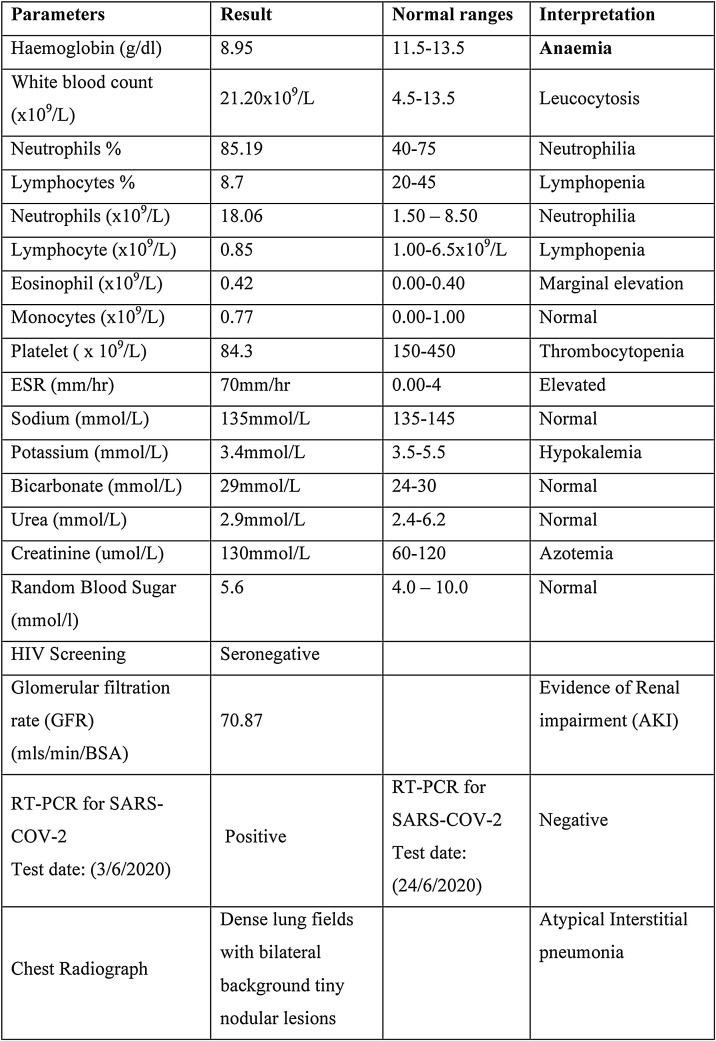
Figure 1.
Showing laboratory parameters of the patient.
Table 2.
Paediatrics early warning system (PEWS) chart for ages 5–12 years (NICE, 2017).
 |
RT-PCR for SARS-COV-2 done on 3rd June 2020 was positive, and other laboratory and ancillary results are shown in Figure 1. She was diagnosed with severe COVID-19 disease in line with the Nigerian case guidelines (NCDC, 2020) with MIS-C following the WHO preliminary case definition (WHO, 2020) and the RCPCH and CDC criteria (Simpson and Newburger, 2020, Whittaker et al., 2020, WHO 2020), as shown in Table 3 .
Table 3.
Criteria for diagnosis of MIS-C based on case definitions for multisystem inflammatory syndrome in children (MIS-C) (Simpson and Newburger, 2020, Whittaker et al, 2020, WHO 2020).
| Royal college of paediatrics and child health | Centres for disease control and prevention | WHO | Features in index case | Remarks | |
|---|---|---|---|---|---|
| 1 | A child presenting with persistent fever AND | An individual age <21 years presenting with fever* AND | Children and adolescents 0–19 years of age with fever >3 days AND two of the following | 12 years, fever >10 days | Meets RCPCH, CDC and WHO criteria |
| 2 | Inflammation, (neutrophilia, elevated C-reactive protein and lymphopenia), and evidence of single organ or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal or neurologic disorder) | Laboratory evidence of inflammation,† and evidence of clinically severe illness requiring hospitalization, with multisystem (≥2) organ involvement (cardiac, renal, respiratory, haematologic, gastrointestinal, dermatologic or neurologic) AND | Rash or bilateral non-purulent conjunctivitis or muco-cutaneous inflammation signs (oral, hands or feet). Hypotension or shock. Features of myocardial dysfunction, pericarditis, valvulitis or coronary abnormalities (including ECHO findings or elevated Troponin/NT-proBNP), d) Evidence of coagulopathy (by PT, PTT, elevated d-Dimers). Acute gastrointestinal problems (diarrhoea, vomiting or abdominal pain) AND |
Rash, bilateral conjunctivitis, Mucocutaneous inflammation and involvement of hands, Diarrhoea, 2 or more organ involvement (GIT, Renal, Respiratory, Dermatology), Neutrophilia and lymphopenia |
Meets RCPCH, CDC and WHO criteria |
| 3 | AND Elevated markers of inflammation such as ESR, C-reactive protein or procalcitonin |
Elevated ESR | Meets WHO criteria | ||
| 4 | With additional clinical, laboratory or imaging and ECG features; children fulfilling full or partial criteria for Kawasaki disease may be included |
Not Reported in Patient | |||
| 5 | Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes and infections associated with myocarditis such as enterovirus |
No alternative plausible diagnoses AND | AND No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes |
No evidence of bacterial infection and non-response to prior treatment before referral | Meets RCPCH, CDC and WHO criteria |
| 6 | SARS-CoV-2 PCR testing positive or negative | Positive for current or recent SARS-CoV-2 infection by RTPCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks before the onset of symptoms |
AND Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patients with COVID-19 |
Positive SARS-CoV-2 PCR | Meets RCPCH, CDC and WHO criteria |
The treatment regimen comprised initial high-flow oxygen at 10 l per minute via a non-rebreather mask, blood transfusion with intravenous (IV) furosemide to prevent fluid overload, 1 g IV ceftriaxone daily for superimposed bacterial infection, 200 mg IV hydrocortisone daily, prophylaxis anticoagulation with 40 mg subcutaneous (SC) enoxaparin daily, 500 mg Azithromycin daily in tablet form, 1 g Paracetamol thrice a day in tablet form, 200 mg Hydroxychloroquine twice a day for 24 h in tablet form then 100 mg twice a day for 4 days, 200,000 iu vitamin A capsules on days 1, 2 and 4 and 500 mg vitamin C tablets once a day. On day 5 of hospitalization, her clinical symptoms improved remarkably with normal vital signs and PEWs score of 0 and 0 respectively, prompting ICU discharge on day 6. The patient was discharged after 14 days of hospitalization following a negative SARS COV-2 test result. She was seen two weeks post-discharge at the clinic with no symptoms, with a long-term follow-up plan and echocardiography request for cardiac evaluation.
Discussion
To the best of our knowledge, this is the first reported case of MIS-C in Nigeria and probably in Sub-Saharan Africa (SSA) since the onset of the COVID-19 pandemic. The diagnosis of MIS-C in this case, which was centred on the clinical presentation and basic laboratory evaluation, also demonstrates the utility of the WHO preliminary case definition criteria as a tool that applies to all settings irrespective of resource capacity.
The diagnostic of MIS-C, in this case, is further strengthened by the observation that the clinical features conform to diagnostic criteria under the WHO, RCPCH and CDC case definitions (Simpson and Newburger, 2020, WHO 2020, Whittaker et al., 2020). These features include age of 0–19 years with fever >3 days; rash or bilateral non-purulent conjunctivitis or mucocutaneous inflammation signs (oral, hands or feet); acute gastrointestinal problems (diarrhoea, vomiting, or abdominal pain); features of involvement of more than 2 organ systems; elevated markers of inflammation such as ESR; evidence of COVID-19 with positive RT-PCR test and the improbability of bacterial sepsis, the rarity of rickettsia diseases in Nigeria and the absence of toxic shock features.
This case also brings to the fore the challenges of diagnosis in settings of low resource where access to investigations like BNP, ferritin, procalcitonin and daily echocardiography are limited. It is however observed that all the criteria and particularly the WHO criteria can easily be applied for diagnosis of MIS-C in low-resource settings if all the options in the criterion are followed. The non-performance and non-reporting of echocardiography is an acknowledged limitation of this report; it limited the ability to evaluate the presence of cardiac involvement or abnormalities in this case. This observation has also been observed in other series of MIS-C where echocardiography was not reported and, in some instances, found to be normal, as shown in a systematic review (Ahmed et al., 2020).
The differentiation between MIS-C and other paediatric inflammatory syndromes such as KD and TSS may be challenging; however, there is evidence that these entities are distinct despite similarities in the presentation (Jiang et al., 2020, WHO, 2020). The older age of onset, being of African or Hispanic descent and the presence of more intense inflammation has been documented as a key distinguishing feature between MIS-C and other inflammatory syndromes (KD and TSS). The results of reviews and case series (Jiang et al., 2020, Ahmed et al., 2020, Whittaker et al., 2020) indicate that presentation at an age above 7 years is more characteristic of MIS-C compared to KD and TSS which are consistent with the age of 12 years for the index case. Besides, MIS-C is more prevalent in children of African ancestry and found to run a less severe clinical course with an average ICU admission duration of 6 days, as observed by Godfred-Cato et al. (2020) in a USA Series. The exclusion of other causes is also a key criterion for the diagnosis of MIS-C. In this index case, there was prior treatment for suspected bacterial sepsis before referral with poor response, in contrast to the response to anti-inflammatory treatment. Further, the features of shock were not prominent despite the evidence of extensive multisystem inflammation (Jiang et al., 2020, Whittaker et al., 2020).
The differentiation between MIS-C and severe COVID-19 is also important. This case was more likely a case of MIS-C rather than severe COVID-19. This assertion is based on key distinguishing features highlighted by Ahmed et al. (2020) in a review of 39 studies. These features are fever over 5 days and presence of diarrhoea and rashes which are more in keeping with MIS-C than severe COVID-19 found in this index case. Further, the presence of more severe leucocytosis, neutrophilia, lymphopenia, thrombocytopenia, higher creatinine and ESR are more in line with MIS-C than severe COVID-19, as shown by Ahmed et al. (2020).
Intravenous steroids with other adjunctive therapy were applied in our patient, as part of our severe COVID-19 treatment protocol, with a favourable outcome and rapid resolution of symptoms. The favourable outcome and ICU stay of 6 days, in this case, is similar to the outcome of other reported series by Riphagen et al. (2020) in the UK, Verdoni et al. (2020) in Italy and Toubiana et al. (2020) in France. Despite the non-use of intravenous immune globulins (IVIG) for our patient, response to treatment was still good. The use of steroids as a treatment option in low-resource settings has also been observed by Ahmed et al., 2020). The outcome of this case also justifies the role of steroids in the management of these cases either alone or in combination with IVIG as implemented by Riphagen et al. (2020) for cases in the UK.
The diagnosis of MIS-C in this Nigerian child, therefore, reinforces the need for physicians and health care systems in Africa to be more vigilant in the assessment of children with severe COVID-19 disease. The observations of higher prevalence of MIS-C in children of African ancestry in Europe and North America may suggest a racial and genetic susceptibility which puts African children at higher risk.
The issues of ethnic disparities associated with COVID-19 have emerged since the onset of the pandemic with some observations suggesting the people of Black, Asian and Minority Ethnic (BAME) backgrounds are at an increased risk of acquiring infection and adverse outcomes (Pan et al., 2020). The reports of MIS-C series around the world have observed a higher prevalence in black and Asian populations. The paucity of reports in SSA is in contrast with the demographics from New York, USA, with 40% of MIS-C patients being of African Ancestry (Dufort et al., 2020) while Godfred-Cato et al. (2020) reported that 40.5% of their patients were (Hispanic) and 33.1% were black. Other studies from the European continent in Paris (Toubiana et al., 2020) and UK (Riphagen et al., 2020, Whittaker et al., 2020) reported that children of African ancestry accounted for 57%, 75% and 38% of all cases of MIS-C, respectively. This pattern of ethnic predilection is also observed on the African continent with a study from South Africa, showing that patients of African and black ethnicity made up 78.3% of MIS-C patients (Webb et al., 2020). It is thought that the higher prevalence of MIS-C among children of African ancestry is linked to a genetic locus that increases the risk of more severe disease in some ethnic groups (Jiang et al., 2020).
It is therefore anticipated that with the increased risk of MIS-C in African children, there may be more cases of MIS-C, especially in SSA with increased surveillance for MIS-C in Africa. However, it may turn out that the incidence of MIS-C may be lower in African than in non-African communities with a similar rate of infection in children, indicating that factors other than race play a role in the increased percentage of children of African ancestry among children with MIS-C.
In conclusion, we report a rare case of MIS-C in Nigerian child with COVID-19 and recommend vigilance among physicians and expanded effort at surveillance to promote early recognition and diagnosis of MIS-C in Africa. All the current case definitions of MIS-C by the CDC, RCPCH and WHO are easy to use with the WHO preliminary case definition particularly promoting the recognition of MIS-C in areas of resource limitation. It is expected that increased surveillance will provide the required evidence for understanding the geographic and racial factors which influence the prevalence of MIS-C and KD. The experience from this case will create awareness, expand knowledge and support clinicians on the African continent in their approach to other potential cases.
Sources of funding
None.
Conflict of interest
None.
Ethical approval and informed consent obtained.
Acknowledgements
Staff of the COVID-19 Response Team, University of Port Harcourt Teaching Hospital, Professor Henry A Ugboma, Professor Princewill Stanley.
References
- Ahmed M., Advani S., Moreira A., Zoretic S., Martinez J., Chorathet K., et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26(September):100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animasahun B.A., Adekunle M.O., Kusimo O.Y., Fadipe C. The diagnosis of Kawasaki disease among Nigerian children: a nightmare for the caregivers and the doctors. J Public Health Emerg. 2017;1(69):6. doi: 10.21037/jphe.2017.06.06. [DOI] [Google Scholar]
- Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome of children in New-York state. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., et al. COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(November (11)):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K.-Q., Ong J.S.M., Lee J.H. Kawasaki disease in the COVID-19 era: a distinct clinical phenotype? The Lancet Child Adolescent Health. 2020;4(9):642–643. doi: 10.1016/S2352-4642(20)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE . 2017. Paediatric early warning scoring tool (PEWS) Available from. http://generalpracticemedicine.org/PEWSSheetApril2017Final.pdf. [Accessed 10 January 2021] [Google Scholar]
- Nigerian Centre for Disease Control (NDCD) 2020. National interim guidelines for clinical management of COVID-19 version 3, June 2020.https://covid19.ncdc.gov.ng/media/files/National_Interim_Guidelines_for_Clinical_Management_of_COVID-19_v3.pdf [Google Scholar]
- Pan D., Szec S., Minhasc J.S., Bangash M.N., Pareek N., Divall P., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;(June) doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian paediatric intensive care units. JAMA Pediatr. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.M., Newburger J.W. Multisystem Inflammatory Syndrome in Children in Association with COVID-19. Circulation. 2020;142:437–440. doi: 10.1161/CIRCULATIONAHA.120.048726. [DOI] [PubMed] [Google Scholar]
- Son M.B.F. Paediatric inflammatory syndrome temporally related to covid-19. BMJ. 2020;369:m2123. doi: 10.1136/bmj.m2123. [DOI] [PubMed] [Google Scholar]
- Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K., Abraham D.R., Faleye A., McCulloch M., Rabie H., Scott C. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. 2020;4(October (10)):e38. doi: 10.1016/S2352-4642(20)30272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., MD Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with sars-cov-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Multisystem inflammatory of children and adolescents with covid-19. WHO- 2019-ncov/sci-brief/multisystem syndrome children/2020.1. [Google Scholar]
- Wu Z., MacGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]