Abstract
Introduction
Catheter-related infections such as exit site infection (ESI) and tunnel infection (TI) are major causes of peritoneal dialysis (PD) discontinuation. For ESI/TI treatment, catheter diversion procedure (CDP) with exit-site renewal for catheter salvage presents an alternative to catheter removal. Nevertheless, CDP capability of improving PD catheter survival remains unclear.
Methods
We retrospectively reviewed our hospital patients who started PD during 2001–2019 (n=148): 33 treated for ESI/TI by CDP (CDP group) and 115 treated for ESI/TI using conservative therapy or none (non-CDP group). A “virtual discontinuation group” was designated for patients in the CDP group who had received PD catheter removal instead of CDP and who had stopped PD. Kaplan-Meier analysis and log-rank test PD were used for intergroup catheter survival comparison. Associations between clinical factors and PD discontinuation or death were examined using Cox proportional hazards regression analyses.
Results
For patients (76% male, mean age of 61.7±13.0 years), 40 CDP were performed for 33 CDP group patients. Infection-free rates at 30 and 90 days after CDP were, respectively, 90% and 67%. The CDP group PD catheter survival rate was significantly higher than that of virtual discontinuation group (P < .01) and higher than that of the non-CDP group (P = .03). Multivariate analysis revealed independent association of serum albumin concentration (hazard ratio 0.33, 95% confidence interval 0.17–0.67), PD+HD combination therapy (hazard ratio 0.29, 95% confidence interval 0.17–0.49), and CDP (hazard ratio 0.44, 95% confidence interval 0.24–0.80) with PD discontinuation or death.
Conclusion
Results show that CDP may improve PD catheter survival as an effective and less-invasive surgical treatment for ESI/TI to avoid withdrawal of PD.
Keywords: catheter diversion procedure, exit site infection, peritoneal dialysis, tunnel infection
Graphical abstract
Catheter-related infections such as ESI and TI are severe complications of PD. Actually, ESI and TI are known as major predisposing factors for PD-related peritonitis.1,2 Delayed and/or insufficient treatment for ESI/TI might engender refractory peritonitis and interruption of PD. A NEXT-PD study revealed that PD-related infections such as ESI, TI, and peritonitis account for 20% of all causes of PD discontinuation.3 Treatments of ESI and TI include oral antibiotic therapy, topical antibacterial agents, and daily exit-site care.1 Most ESI and TI respond well to common antibiotic therapy. However, refractory ESI and TI require treatment using surgical procedures including catheter removal and reinsertion of a new catheter. Temporary conversion to hemodialysis using vascular access might be necessary after surgery. Therefore, physical burdens to patients are increased greatly. Laparoscopic replacement of PD catheters, which is actually less invasive, has been performed safely in recent years.4 However, restarting PD with the usual dwell volume soon after the surgery presents risk of peritoneal leaks. Medical costs are also a concern of laparoscopic surgery. Laparotomy is often the option used to insert PD catheters at our facility. One method of PD catheter salvage is CDP with exit-site renewal,1 by which the extraperitoneal part of the CAPD catheter is replaced without involving the peritoneum.5 After incision between the superficial and deep cuffs, the CAPD catheter is cut and separated at that site. A new sterile catheter with a cuff is connected to the original one (deep cuff side) via a titanium adaptor, thereby creating a new subcutaneous tunnel and exit site. Finally, the infected part of the original catheter including the superficial cuff is removed, leaving the old exit site opened.5 In fact, CDP is a less invasive surgical method because the peritoneum is not opened and because the patient can resume PD immediately after the operation.
Although CDP is apparently regarded as a useful treatment strategy for ESI/TI in the ISPD guideline, no consensus has been established for which clinical situations under which CDP should be considered. One reason is that few reports describe treatment outcomes of CDP for ESI/TI. Moreover, few studies have specifically examined relations between CDP and long-term outcomes such as PD catheter survival. This study evaluated the effectiveness of CDP for ESI/TI at our hospital. We also investigated the relation between CDP treatment and long-term outcomes such as PD continuation or patient survival.
Materials and Methods
Patients and Data Collection
This study, which was conducted at a single center (University of Tokyo Hospital), was approved by the Institutional Review Board of University of Tokyo (no. 2879). Because this study retrospectively collected data from medical records, written consent was waived.
We retrospectively collected the medical records of 148 patients who started PD during 2001–2019 at our hospital as their first renal replacement therapy. Patients who disconnected PD because of kidney transplantation were excluded. During follow-up at our facility, 33 patients were treated for ESI/TI by CDP in addition to common conservative therapy including exit-site care and antibiotics (CDP group). The remaining 115 patients were treated for ESI/TI using conservative therapy alone or were not treated (non-CDP group). We also defined a “virtual discontinuation group” assuming that patients in the CDP group had received PD catheter removal instead of CDP and therefore stopped PD. The patients’ clinical courses were followed until 3 months after discontinuation of PD or loss of follow-up at our hospital.
As described in the ISPD guidelines, ESI was diagnosed by clinical findings including purulent discharge from the exit site. Also, TI was determined by clinical inflammation or ultrasonographic evidence of fluid collection along the PD catheter tunnel. For ESI/TI, antibiotic treatment was started and continued initially according to ISPD guidelines.1 Treatment strategies including change of antibiotics and indication of CDP were chosen by attending physicians independently from this study. Diagnosis and follow-up of ESI/TI were conducted using physical, ultrasonographic, and bacteriologic examinations. The indication of CDP was decided by the attending physicians according to the following 3 situations: (i) when there were the findings of inflammation reaching the subcutaneous cuff (but not reaching deeper than the superficial cuff) under the appropriate antibiotic treatment, (ii) when 2 weeks of treatment with appropriate antibiotics was ineffective, or (iii) when nontuberculous mycobacteria (NTM) were identified from bacteriologic examinations of purulent discharge from the exit site. CDP was selected because all these situations were thought to be too difficult to be cured using antibiotics alone.6,7 Although all CDPs were not performed by the same surgeon, surgical procedures were identical among the teams.
Clinical and laboratory data (age, gender, cause of end-stage renal disease, CVD before PD initiation, use of automated PD devices, systolic and diastolic blood pressure, body mass index, hemoglobin [Hb], serum albumin [Alb], blood urea nitrogen [BUN], serum creatinine [Cr], corrected calcium [cCa], phosphate [IP], C-reactive protein [CRP], total cholesterol [T.chol], β2-microglobulin [β2MG], triglyceride [TG], renal weekly Kt/v, total weekly Kt/v, daily urine volume, and the ratio of dialysate-to-plasma creatinine concentration at 4 hours [D/P Cr] evaluated by peritoneal equilibration test [PET] using 2.5% glucose solution) were measured at the induction period of PD (2.3±1.3 months after starting PD). Residual GFR (rGFR) was calculated as the average of renal clearance of BUN and Cr.8 All patients had been treated for their PD therapy using biocompatible neutral pH solution (Terumo Corp., Tokyo, Japan).
Outcomes
This study compared PD catheter survival rates for 3 groups: CDP, non-CDP, and virtual discontinuation groups. We estimated PD catheter survival to the point of catheter removal related to various complications such as peritonitis, ESI/TI, and dialysis inadequacy (overhydration, uremia). We used univariate and multivariate analyses to examine whether clinical factors including CDP were associated with PD discontinuation or death after starting PD. Patient deaths until 3 months after discontinuation of PD were examined.
Statistical Analysis
All statistical analyses were conducted using software (JMP 14; SAS Institute Inc., Cary, NC). Continuous data were expressed as mean ± SD or median (interquartile range). Student t tests or Mann-Whitney U tests were used to compare continuous variables. The χ2 test or Fisher exact test was used to compare categorical variables. The Kaplan-Meier method and the log-rank test were used to compare differences in PD catheter survival between groups. Univariate and multivariate Cox proportional hazards regression analyses were used to examine significant factors associated with study outcomes. Competing risk analysis was performed using a Fine-Gray model to investigate independent factors related to PD discontinuation when death is considered as a competing risk. A P value of less than 0.05 was inferred as significant.
Results
We analyzed the data of 148 patients. Their characteristics are presented in Table 1. Median age at PD initiation was 61.7 ± 13.0. Almost three-quarters were male (76%). The median observational period was 39.8 (19.8, 66.4) months. Combination therapy with PD and HD was used in 39% of the study subjects. Eighty-three patients (56%) were confirmed for PD discontinuation within the observation period. The leading cause of stopping PD was peritonitis (45%), followed by dialysis inadequacy (overhydration, uremia) (15.7%), and ESI/TI (8.4%). The overall incidence of peritonitis was 0.21 per patient-year. Twenty-one (14%) patients died. Results of comparison of variables between the CDP and non-CDP group presented that the total observational period was significantly longer in the CDP group than in the non-CDP group (51.8 [32.0, 75.1] vs. 34.6 [18.3, 60.8] months, P < 0.01) (Table 1). The patients in CDP group were younger than in the non-CDP group (57.2 ± 12.0 vs. 63.0 ± 13.0, P = 0.02). Mortality was found to be significantly higher in the non-CDP group than in the CDP group (17% vs. 3%, P = 0.02). No significant difference was found between the 2 groups for the rate of patients with a history of peritonitis (Table 1).
Table 1.
Characteristics of all peritoneal dialysis patients, CDP and non-CDP groups
| All (N = 148) | CDP group (n = 33) | Non-CDP group (n = 115) | P (CDP vs. non-CDP) | |
|---|---|---|---|---|
| Age at PD initiation, yr | 61.7 ± 13.0 | 57.2 ± 12.0 | 63.0 ± 13.0 | 0.02 |
| Male, n (%) | 113 (76) | 22 (67) | 91 (79) | 0.14 |
| Primary kidney disease, n (%) | 0.24 | |||
| Diabetic nephropathy | 50 (34) | 9 (27) | 41 (36) | |
| Chronic glomerulonephritis | 51 (34) | 15 (45) | 36 (31) | |
| Nephrosclerosis | 20 (16) | 2 (6) | 18 (16) | |
| Others | 27 (18) | 7 (21) | 20 (17) | |
| History of CVD, n (%) | 35 (24) | 5 (15) | 30 (26) | 0.18 |
| Use of automated PD devices, n (%) | 128 (86) | 31 (94) | 97 (84) | 0.13 |
| Systolic blood pressure, mm Hg | 133.4 ± 18.2 | 130.7 ± 21.3 | 134.3 ± 17.2 | 0.33 |
| Diastolic blood pressure, mm Hg | 76.4 ± 12.7 | 76.4 ± 15.0 | 76.4 ± 11.9 | 0.99 |
| Body mass index | 22.6 (19.8, 25.0) | 22.6 (20.8, 24.2) | 22.1 (19.8, 25.0) | 0.46 |
| Hb, g/dl | 10.8 ± 1.1 | 11.0 ± 1.0 | 10.8 ± 1.1 | 0.26 |
| Cr, mg/dl | 6.57 (5.14, 7.88) | 6.94 (5.45, 7.36) | 6.47 (5.09, 8.23) | 0.77 |
| BUN, mg/dl | 51.9 (44.9, 61.2) | 53.7 (46.5, 61.8) | 51.4 (42.9, 60.3) | 0.19 |
| cCa, mg/dl | 8.95 (8.58, 9.30) | 8.9 (8.60, 9.40) | 9.00 (8.50, 9.30) | 0.45 |
| IP, mg/dl | 4.60 (3.90, 5.30) | 4.9 (4.20, 5.50) | 4.60 (3.80, 5.25) | 0.14 |
| Alb, g/dl | 3.60 (3.30, 3.80) | 3.60 (3.30, 4.00) | 3.60 (3.25, 3.80) | 0.36 |
| CRP, mg/dl | 0.19 (0.06, 0.30) | 0.15 (0.05, 0.30) | 0.22 (0.07, 0.31) | 0.95 |
| T.chol, mg/dl | 194 (170, 219) | 186 (166, 210) | 194 (170, 221) | 0.74 |
| TG, mg/dl | 143 (101, 186) | 128 (87.3, 170) | 149 (108, 188) | 0.32 |
| β2MG, mg/dl | 17.8 (14.1, 20.7) | 18.9 (14.8, 20.1) | 17.8 (13.9, 21.0) | 0.60 |
| Renal Kt/v | 1.11 (0.85, 1.52) | 1.08 (0.83, 1.51) | 1.17 (0.87, 1.53) | 0.95 |
| Total Kt/v | 2.18 (1.89, 2.50) | 2.21 (1.95, 2.50) | 2.17 (1.87, 2.51) | 0.71 |
| D/P Cr | 0.58 (0.52, 0.66) | 0.55 (0.52, 0.63) | 0.58 (0.52, 0.67) | 0.10 |
| rGFR, ml/min per 1.73 m2 | 5.90 (4.34, 7.91) | 6.71 (4.78, 8.03) | 5.83 (4.28, 7.72) | 0.92 |
| Urine volume, ml/d | 1190 (800, 1600) | 1200 (800, 1700) | 1180 (770, 1600) | 0.63 |
| Observation duration, mo | 39.8 (19.8, 66.4) | 51.8 (32.0, 75.1) | 34.6 (18.3, 60.8) | <0.01 |
| PD+HD combination therapy, n (%) | 57 (39) | 9 (27) | 49 (43) | 0.11 |
| PD discontinuation, n (%) | 83 (56) | 15 (45) | 68 (59) | 0.16 |
| Patient with peritonitis, n (%) | 66 (45) | 14 (42) | 52 (45) | 0.78 |
| Incidence of peritonitis, per patient-year | 0.21 | 0.16 | 0.22 | — |
| mortality, n (%) | 21 (14) | 1 (3) | 20 (17) | 0.02 |
| Patient age at CDP, yr | — | 59.7 ± 12.1 | — | — |
| PD duration at CDP, mo | — | 23.0 (10.0, 42.0) | — | — |
Alb, albumin; β2MG, β2 microglobulin; BUN, blood urea nitrogen; cCa, corrected calcium; CDP, catheter diversion procedure; Cr, creatinine; CRP, C-reactive protein; CVD, cardiovascular disease; D/P Cr, the ratio of dialysate-to-plasma creatinine concentration at 4 hours; Hb, hemoglobin; HD, hemodialysis; IP, phosphate; rGFR, residual glomerular filtration rate.
Continuous data are presented as median (IQR).
As treatment for ESI/TI, 40 CDP operations were performed on 33 patients of the CDP group. Of the 33 patients, 26 underwent CDP once; 7 patients underwent CDP twice during the observation period. At the time of first surgery, the median age and PD duration of the CDP group were respectively 59.7±12.1 years and 23.0 (10.0, 42.0) months. Table 2 shows details of microbiology and data for antibiotic therapies of ESI/TI cases in both the CDP and non-CDP groups. In the non-CDP group, 215 cases of ESI/TI were observed; 42 of 115 (36%) patients had never developed ESI/TI during the observation period. The most common causative organism was Pseudomonas aeruginosa in the CDP group and Staphylococcus aureus in the non-CDP group. Among S aureus infections, MRSA was detected in 2 of 9 (22%) and in 3 of 65 (5%) cases, respectively, in the CDP and non-CDP groups. Pseudomonas aeruginosa (25%) and Mycobacterium spp. (15%) were found more frequently in the CDP group than in the non-CDP group (P < 0.01). Regarding the types of antibiotics, oral antibiotics were used for gram-positive cocci more frequently in the non-CDP group. The treatment duration was significantly shorter in the CDP group (P < 0.01).
Table 2.
Comparison of ESI/TI cases between the CDP and non-CDP groups
| ESI/TI cases | CDP group (n = 40) | Non-CDP group (n = 215) | P |
|---|---|---|---|
| Causative organisms | |||
| Staphylococcus aureus | 9 (23) | 65 (30) | 0.32 |
| CNS | 2 (5) | 33 (15) | 0.08 |
| Pseudomonas aeruginosa | 10 (25) | 16 (7) | <0.01 |
| Mycobacterium spp. | 6 (15) | 2 (1) | <0.01 |
| Others | 7 (18) | 46 (21) | 0.62 |
| No growth | 6 (15) | 53 (25) | 0.18 |
| Antibiotics use (as first therapy) | |||
| Oral antibiotics | 28 (70) | 186 (87) | 0.01 |
| First-generation cephalosporin, amoxicillin, amoxicillin/clavulanate | 7 (18) | 87 (40) | <0.01 |
| Clindamycin, minocycline, doxycycline | 2 (5) | 42 (20) | 0.03 |
| Quinolones | 22 (55) | 119 (55) | 0.97 |
| Others | 2 (5) | 5 (2) | 0.34 |
| I.v. antibiotics | 15 (38) | 86 (40) | 0.77 |
| Cephazolin, vancomycin | 13 (33) | 80 (37) | 0.57 |
| Ceftazidime, amikacin sulfate | 10 (25) | 67 (31) | 0.44 |
| Others | 2 (5) | 3 (1) | 0.13 |
| Duration of antibiotic therapy, da | 11 (4.5, 25) | 14 (7, 19) | <0.01 |
| Infection-free rate after CDP | |||
| 30 d | 90 | — | — |
| 90 d | 67 | — | — |
| 180 d | 52 | — | — |
| Catheter survival rate after CDP | |||
| 12 mo | 88 | — | — |
| 24 mo | 76 | — | — |
| 36 mo | 67 | — | — |
ESI, exit site infection; CDP, catheter diversion procedure; CNS, coagulase-negative streptococci; TI, tunnel infection.
Until CDP (CDP group) or antibiotics discontinuation (non-CDP group).
Our study demonstrated that the infection-free rate within 30 days after CDP was 90%. Infectious events included recurrence of ESI/TI and peritonitis. Only 1 case required catheter removal within 30 days because of peritonitis by NTM, which occurred secondarily from CDP. The number of infectious events within 180 days after CDP was 19 cases, in 5 of which the isolated causative organism was the same as that of the initial ESI. The catheter survival rate after CDP at 12 months after CDP was 88% (Table 2). In almost all cases, PD was able to resume immediately. No dialysate leak occurred following the procedure.
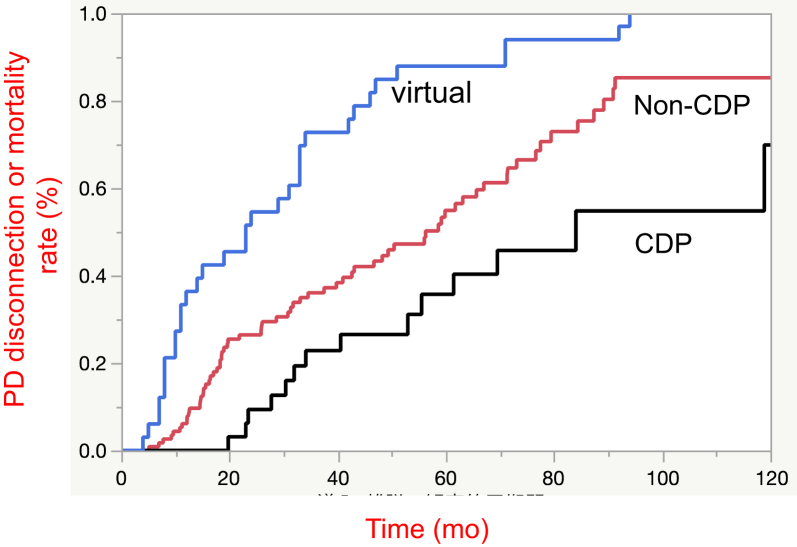
The PD catheter survival rate in the CDP, non-CDP, and virtual discontinuation groups were compared using Kaplan-Meier analysis and log-rank testing (Figure 1). Results show that the PD catheter survival rate in the CDP group was significantly higher than that in the non-CDP group (median survival time [MST], 84.0 vs. 49.0 months, respectively; P = 0.03, Figure 1). For the group in which PD catheter removal was performed instead of CDP (the virtual cessation group), the PD discontinuation or mortality rate in this group was significantly lower than that of the non-CDP group (MST, 23.0 vs. 49.0 months, respectively; P < 0.01, Figure 1). These results demonstrate that CDP can help patients to continue PD through efficient ESI/TI treatment and PD catheter rescue.
Figure 1.
Kaplan-Meier analysis for peritoneal dialysis (PD) catheter survival rate. Results of the Kaplan-Meier survival curve show the PD catheter survival rate in the catheter diversion procedure (CDP) group as significantly higher than that in the non-CDP group (P = 0.03). The PD catheter survival rate in the virtual discontinuation group was significantly lower than in the non-CDP group (P < 0.01).
Cox proportional hazard model analyses were performed to examine independent factors associated with PD discontinuation after PD initiation (Table 3). Age, gender, history of CVD, Cr, Alb, rGFR, D/P Cr, PD+HD combination therapy, history of peritonitis, and CDP were selected as variables and analyzed (Table 4). Age, gender, and CDP were included as a priori. In model 1, the variables with P < 0.01 in univariate regression analysis (Table 3) were selected. In model 2, factors related to PD catheter failure were added to those used in model 1.9 In model 3, the factors related to mortality in PD patients were added to those used in model 2.10,11 Results demonstrated that higher serum albumin level, CDP, and PD+HD combination therapy are significantly associated with risk reduction of PD discontinuation (Table 4). When death was considered as a competing risk, Fine-Gray model results showed CDP as an independent factor associated with reduced risk of PD discontinuation (Table 5). Our additional analysis to simulate the effect of bias because of loss-to-follow-up identified no meaningful difference (see more details in Supplementary Table S1).
Table 3.
Univariate regression analysis for PD discontinuation or death
| Variables | HR (95% CI) | P |
|---|---|---|
| Age | 1.02 (1.00, 1.04) | 0.70 |
| Male | 0.75 (0.46, 1.22) | 0.25 |
| History of CVD | 1.85 (1.15, 2.97) | 0.02 |
| Hb | 0.81 (0.65, 1.00) | 0.054 |
| Cr | 0.87 (0.78, 0.97) | 0.02 |
| BUN | 0.98 (0.97, 1.00) | 0.053 |
| cCa | 1.29 (0.93, 1.79) | 0.13 |
| IP | 0.80 (0.63, 1.00) | 0.051 |
| Alb | 0.29 (0.17, 0.49) | <0.01 |
| CRP | 1.19 (0.94, 1.43) | 0.14 |
| T.chol | 1.00 (0.99, 1.01) | 0.76 |
| TG | 1.00 (1.00, 1.00) | 0.92 |
| β2MG | 1.01 (0.96, 1.04) | 0.83 |
| Renal Kt/v | 0.95 (0.62, 1.42) | 0.82 |
| Total Kt/v | 0.97 (0.61, 1.49) | 0.87 |
| D/P Cr | 15.3 (2.49, 83.8) | <0.01 |
| rGFR | 1.00 (0.92, 1.07) | 0.95 |
| Urine volume | 1.00 (1.00, 1.00) | 0.23 |
| Systolic blood pressure | 1.00 (1.00, 1.02) | 0.53 |
| Diastolic blood pressure | 1.00 (0.97, 1.00) | 0.20 |
| Body mass index | 1.01 (0.96, 1.08) | 0.64 |
| Use of automated PD device, n (%) | 0.77 (0.42, 1.40) | 0.40 |
| PD+HD combination therapy | 0.42 (0.26, 0.68) | <0.01 |
| History of peritonitis | 1.24 (0.79, 1.93) | 0.35 |
| Catheter diversion procedure | 0.54 (0.31, 0.95) | 0.02 |
Alb, albumin; BUN, blood urea nitrogen; cCa, corrected calcium; CI, confidence interval; Cr, creatinine; CRP, C-reactive protein; CVD, cardiovascular disease; D/P Cre, dialysate-to-plasma creatinine concentration ratio; Hb, hemoglobin; HD, hemodialysis; HR, hazard ratio; IP, phosphate; PD, peritoneal dialysis; rGFR, residual glomerular filtration rate; T.chol, total cholesterol; TG, triglyceride.
Table 4.
Results of Cox proportional hazards model for PD discontinuation or death
| Variables | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 0.99 (0.97, 1.01) | 0.54 | 0.99 (0.97, 1.01) | 0.49 | 0.99 (0.98, 1.02) | 0.70 |
| Male | 0.75 (0.45, 1.23) | 0.25 | 0.65 (0.39, 1.10) | 0.11 | 0.55 (0.31, 0.97) | 0.04 |
| Catheter diversion procedure | 0.44 (0.24, 0.79) | <0.01 | 0.44 (0.25, 0.81) | <0.01 | 0.44 (0.24, 0.80) | <0.01 |
| Alb | 0.33 (0.17, 0.62) | <0.01 | 0.35 (0.18, 0.68) | <0.01 | 0.33 (0.17, 0.67) | <0.01 |
| PD+HD combination therapy | 0.32 (0.19, 0.54) | <0.01 | 0.32 (0.19, 0.54) | <0.01 | 0.29 (0.17, 0.49) | <0.01 |
| D/P Cre | 3.97 (0.50, 29.0) | 0.18 | 4.26 (0.58, 29.0) | 0.15 | 3.38 (0.46, 23.3) | 0.22 |
| History of CVD | 1.68 (1.01, 2.78) | 0.05 | 1.65 (0.98, 2.79) | 0.07 | ||
| rGFR | 1.00 (0.93, 1.08) | 0.84 | 1.02 (0.93, 1.11) | 0.66 | ||
| Cr | 1.04 (0.89, 1.21) | 0.66 | ||||
| History of peritonitis | 1.52 (0.93, 2.50) | 0.09 | ||||
Alb, albumin; CI, confidence interval; Cr, creatinine; CVD, cardiovascular disease; D/P Cre, dialysate-to-plasma creatinine concentration ratio; HD, hemodialysis; HR, hazard ratio; PD, peritoneal dialysis; rGFR, residual glomerular filtration rate.
Table 5.
Fine-Gray multivariable regression model for PD discontinuation with death as a competing risk
| Variables | Model 1 |
Model 2 |
Model 3 |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 0.97 (0.95, 0.99) | <0.01 | 0.97 (0.96, 0.99) | <0.01 | 0.98 (0.96, 0.99) | 0.01 |
| Male | 1.01 (0.60, 1.71) | 0.96 | 0.96 (0.57, 1.59) | 0.87 | 0.59 (0.33, 1.07) | 0.08 |
| Catheter diversion procedure | 0.58 (0.35, 0.93) | 0.03 | 0.59 (0.36, 0.98) | 0.04 | 0.55 (0.33, 0.92) | 0.02 |
| Alb | 0.48 (0.28, 0.84) | 0.01 | 0.50 (0.28, 0.90) | 0.02 | 0.45 (0.26, 0.79) | <0.01 |
| PD+HD combination therapy | 0.53 (0.32, 0.87) | 0.01 | 0.53 (0.32, 0.88) | 0.01 | 0.40 (0.23, 0.71) | < 0.01 |
| D/P Cre | 2.07 (0.31, 13.9) | 0.46 | 2.21 (0.37, 13.3) | 0.38 | 2.29 (0.38, 14.0) | 0.37 |
| History of CVD | 1.28 (0.80, 2.07) | 0.30 | 1.43 (0.83, 2.45) | 0.20 | ||
| rGFR | 1.06 (1.00, 1.12) | 0.03 | 1.10 (1.02, 1.18) | 0.01 | ||
| Cr | 1.12 (0.97, 1.30) | 0.13 | ||||
| History of peritonitis | 2.30 (1.35, 3.91) | <0.01 | ||||
Alb, albumin; CI, confidence interval; Cr, creatinine; CVD, cardiovascular disease; D/P Cre, dialysate-to-plasma creatinine concentration ratio; HD, hemodialysis; HR, hazard ratio; PD, peritoneal dialysis; rGFR, residual glomerular filtration rate.
Discussion
For this study, we analyzed 148 PD patients retrospectively to assess the effectiveness of CDP performed for ESI/TI. Our results demonstrated that CDP is associated significantly with higher PD catheter survival rates. Multivariate analysis revealed factors related to reducing PD discontinuation as the serum albumin concentration, CDP, and PD+HD combination therapy.
In PD patients, PD catheter-related infections, ESI and TI, are common complications along with peritonitis. The incidence of ESI/TI has been reported as 0.21 to 0.41 per patient-year, which is not markedly different from that of peritonitis (0.21–0.40 per patient-year).12, 13, 14 Actually, ESI/TI is regarded as a major predisposing factor to PD-related peritonitis, which might result in discontinuation of PD. The proportion of peritonitis with concomitant ESI was 6.2%–20.8%; the risk of peritonitis within 15 days of ESI was especially high with a hazard ratio of 11.1 (4.9–25.1).2,15 Therefore, prompt and proper treatment for ESI/TI is necessary to continue PD while reducing the patient burden. Results show that CDP is both an effective and minimally invasive surgical treatment method for ESI/TI.
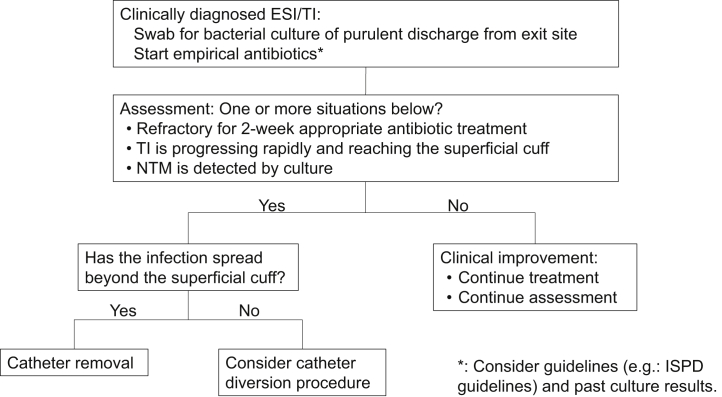
For successful surgical treatment, it is apparently important to perform CDP without delay. In our CDP patients, the median time from the diagnosis of ESI/TI to CDP was 10 days (5.5, 20.8). When clinical findings showed inflammation reaching the subcutaneous cuff, irrespective of the causative organism, CDP was performed without waiting for therapeutic effects of antibiotics. The decision of CDP related to appropriate timing might contribute to lowering the recurrence of infection and improving technique survival. We suggested a new algorithm of ESI/TI therapeutic strategy including CDP (Figure 2).
Figure 2.
Proposed algorithm of exit site infection (ESI)/tunnel infection (TI) therapeutic strategies. NTM, nontuberculous mycobacteria.
In our study, the most identified causative organism of ESI/TI in the CDP patients was Pseudomonas aeruginosa (25%). Following them was NTM (15%), with prevalence higher than in earlier reports. Reportedly, NTM accounts for only 3% of all culture-positive ESI and peritonitis cases.16 Although it remains unclear why NTM was detected frequently in the CDP group in our hospital, several possible reasons can be inferred. We routinely perform microbiological examination of purulent discharge from exit-site not only for general bacteria but also for mycobacterium when ESI/TI is diagnosed clinically. This strategy might enable active identification of NTM as a causative organism of ESI and to decide optimal treatment method at an early stage. However, the indication of CDP might be affected by the type of causative organism. Reportedly, only 18.8% of cases with patients with ESI caused by NTM were resolved by antibiotics alone; 33.3% developed peritonitis.7 Catheter removal and PD withdrawal were required in more than 90% of patients with NTM peritonitis.7 Therefore, to continue PD, early surgical procedures such as CDP should be considered for ESI/TI by NTM before developing peritonitis.
As described in the ISPD guideline, several surgical procedures have been used to treat ESI/TI instead of catheter removal to rescue catheter and avoid withdrawal of PD.1 In addition to CDP, un-roofing and cuff-shaving are typical alternative methods used for catheter removal. The published results of unroofing surgery are generally favorable,17 but it is more likely to develop peritonitis because the subcutaneous tunnel becomes extremely short. Cuff shaving has been adopted as less-invasive surgery. However, it might engender increased risk of peritonitis. Meng et al. reported the probability of catheter survival as 54% at 12 months after cuff shaving, which was much lower than that in our study (54% vs. 88%).18
Regarding CDPs, several studies have yielded favorable results. Cho et al. reported decreased incidence of peritonitis after catheter revision: from 0.26/patient-year at risk to 0.14/patient-year at risk.19 In a study reported by Clouatre et al., the mean catheter survival after catheter diversion was 7.7 months (range 3.5–13 months). No recurrence of ESI was related to the initial infection.5 As described above, our study demonstrated that the infection (including peritonitis and ESI/TI)-free rate within 30 days after CDP was 90%. From this perspective, the short-term clinical result of CDP was regarded as favorable.
Why has CDP been improving PD catheter survival? Little is known about the relation between CDP and the PD catheter survival in past studies. The following explanations have been proposed: (i) CDP prolonged the catheter survival time. CDP allowed patients to resume PD immediately after surgery, which aided in patients’ rapid return to normal life. It is conceivable that this method is generally tolerated with few adverse events, including surgical complications and a burden on patients. (ii) CDP exerted beneficial effects on the treatment of ESI/TI. Infectious parts including tissue and superficial cuff were removed completely. It led to a high infection-free rate within 30 days after CDP. (iii) Patients were able to obtain the opportunities to be educated about exit-site care at the time of hospitalization to receive CDP. Consequently, more careful attention was devoted to the whole PD technique.
The present study had some limitations. First, because this was a retrospective observational study, the severities of ESI/TI between CDP and non-CDP groups might not be equal. The non-CDP group included patients with mild ESI/TI that did not require CDP or without ESI/TI: these patients are expected to have good prognosis. Therefore, it is estimated that the technique survival rate in the CDP group would still be higher than in the non-CDP group even if the analyses were performed after exclusion of those patients in the non-CDP group. Second, we might not have investigated or collected sufficient data of unknown factors affecting technique survival. Third, this retrospective study examined only a few patients from a single institution. A randomized trial with long-term follow-up will be necessary to evaluate CDP efficacy.
In conclusion, CDP with exit-site renewal might be an effective and less-invasive surgical treatment for ESI/TI to avoid withdrawal of PD. CDP can improve the catheter survival of PD patients. Although further study is needed, CDP has been demonstrated as a beneficial therapeutic strategy for the treatment of refractory ESI/TI.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Results of examination for the effect of bias due to loss-to-follow-up.
Supplementary Material
References
- 1.Szeto C.C., Li P.K., Johnson D.W. ISPD Catheter-Related Infection Recommendations: 2017 Update. Perit Dial Int. 2017;37:141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 2.van Diepen A.T., Tomlinson G.A., Jassal S.V. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol. 2012;7:1266–1271. doi: 10.2215/CJN.00980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakayama M., Miyazaki M., Honda K. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit Dial Int. 2014;34:766–774. doi: 10.3747/pdi.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith B.M., Dan A.G. Operative technique for laparoscopic placement of continuous ambulatory peritoneal dialysis catheter. J Laparoendosc Adv Surg Tech A. 2020;30:815–819. doi: 10.1089/lap.2019.0750. [DOI] [PubMed] [Google Scholar]
- 5.Clouâtre Y., Cartier P., Charbonneau R. Outpatient CAPD catheter salvage for persistent exit-site/tunnel infection. Nephrol Dial Transplant. 2000;15:231–234. doi: 10.1093/ndt/15.2.231. [DOI] [PubMed] [Google Scholar]
- 6.Muraoka K., Ishibashi Y., Yamaguchi J. Early partial re-implantation of Tenckhoff catheters to treat intractable exit-site or tunnel infection. Perit Dial Int. 2011;31:350–353. doi: 10.3747/pdi.2010.00181. [DOI] [PubMed] [Google Scholar]
- 7.Washida N., Itoh H. The role of non-tuberculous mycobacteria in peritoneal dialysis-related infections: a literature review. Contrib Nephrol. 2018;196:155–161. doi: 10.1159/000485716. [DOI] [PubMed] [Google Scholar]
- 8.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnyk I., Dekker F.W., Boeschoten E.W. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30:170–177. doi: 10.3747/pdi.2008.00277. [DOI] [PubMed] [Google Scholar]
- 10.Park J., Mehrotra R., Rhee C.M. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant. 2013;28:2146–2155. doi: 10.1093/ndt/gft213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit Dial Int. 2018;38:313. doi: 10.3747/pdi.2018.00030. [DOI] [PubMed] [Google Scholar]
- 12.Masakane I., Hasegawa T., Ogata S. Peritoneal dialysis registry with 2013 survey report. Ther Apher Dial. 2016;20:557–568. doi: 10.1111/1744-9987.12520. [DOI] [PubMed] [Google Scholar]
- 13.Nochaiwong S., Ruengorn C., Noppakun K. Comparative Effectiveness of Local Application of Chlorhexidine Gluconate, Mupirocin Ointment, and Normal Saline for the Prevention of Peritoneal Dialysis-related Infections (COSMO-PD Trial): a multicenter randomized, double-blind, controlled protocol. Trials. 2019;20:754. doi: 10.1186/s13063-019-3953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirmizis D., Bowes E., Ansari B. Exit-site relocation: a novel, straightforward technique for exit-site infections. Perit Dial Int. 2019;39:350–355. doi: 10.3747/pdi.2017.00214. [DOI] [PubMed] [Google Scholar]
- 15.Perl J., Fuller D.S., Bieber B.A. Peritoneal dialysis-related infection rates and outcomes: results From the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) Am J Kidney Dis. 2020;76:42–53. doi: 10.1053/j.ajkd.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura R., Kawanishi M., Fujii S. Peritoneal dialysis-associated infection caused by Mycobacterium abscessus: a case report. BMC Nephrol. 2018;19:341. doi: 10.1186/s12882-018-1148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terawaki H., Nakano H., Ogura M. Unroofing surgery with en bloc resection of the skin and tissues around the peripheral cuff. Perit Dial Int. 2013;33:573–576. doi: 10.3747/pdi.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng C., Beco A., Oliveira A. Peritoneal dialysis cuff-shaving—a salvage therapy for refractory exit-site infections. Perit Dial Int. 2019;39:276–281. doi: 10.3747/pdi.2018.00193. [DOI] [PubMed] [Google Scholar]
- 19.Cho K.H., Do J.Y., Park J.W. Catheter revision for the treatment of intractable exit site infection/tunnel infection in peritoneal dialysis patients: a single centre experience. Nephrology (Carlton) 2012;17:760–766. doi: 10.1111/j.1440-1797.2012.01644.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.