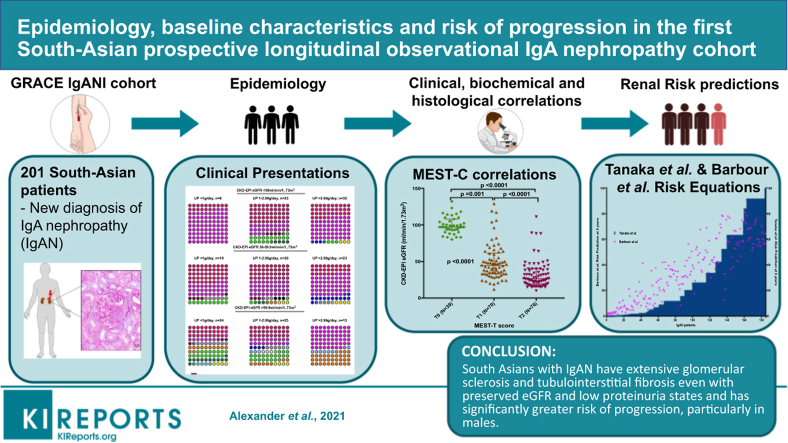
Abstract
Introduction
Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians (GRACE-IgANI) is the first prospective South Asian IgAN cohort with protocolized follow-up and extensive biosample collection. Here we report the baseline clinical, biochemical, and histopathologic characteristics of GRACE IgANI and calculate baseline risk of progression for the cohort.
Methods
201 incident adults with kidney biopsy–proven primary IgAN were recruited into GRACE-IgANI between March 2015 and September 2017. As of April 30, 2020, the cohort had completed a median follow-up of 30 months (interquartile range [IQR] 16-39).
Results
The commonest clinical presentation in GRACE IgANI was hypertension, with or without proteinuria, and nephrotic-range proteinuria was present in 34%, despite <10 months of lead time to kidney biopsy. The GRACE-IgANI kidney biopsy data demonstrated a disproportionate absence of active glomerular lesions and overrepresentation of segmental sclerosing lesions and tubulointerstitial fibrosis at presentation, often coexistent with relatively well-preserved estimated glomerular filtration rate (eGFR) and low levels of proteinuria, especially in males. Baseline risk of progression was calculated for each evaluable patient using 2 different risk prediction tools. The median 5-year absolute risk of end-stage kidney disease (ESKD) was 19.8% (IQR 2.7–57.4) and median 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD was 35.5% using the 2 tools.
Conclusions
The predicted risk of progression in this cohort was considerable. Over the next 5 years, we will dissect the pathogenic pathways that underlie this severe South Asian IgAN phenotype.
Keywords: chronic kidney disease, glomerulonephritis, IgA nephropathy, immune-mediated kidney disease, renal risk score, South Asia
Graphical abstract
IgA nephropathy (IgAN) is the most common glomerulonephritis in the world and is a leading cause of ESKD but has varied manifestations and risks of progression in different ethnicities.1, 2, 3, 4, 5 The incidence of glomerular diseases in the tropics is much higher than in temperate countries. Nephrotic syndrome is 60 to 100 times more common in India than in countries like the United Kingdom and the United States.6 There is no national glomerulonephritis registry in India and, therefore, most reports on the epidemiology of glomerular disease are based on single- or multicenter retrospective studies of kidney biopsy registries. IgAN is reported in 10% to 15% of all kidney biopsies in India, with a high proportion of nephrotic syndrome and renal dysfunction at presentation.7, 8, 9, 10, 11, 12 Indian patients also seem to manifest the disease a decade earlier than Caucasian and East Asian patients.13,14 A retrospective cohort study from our center reported hypertension in 52% and renal impairment in 32% of patients at presentation and only a 35% mean renal survival at 11 years, which is considerably less than that reported in studies of IgAN in Caucasian cohorts.4,5,15,16 It is unclear whether these differences in epidemiology and risk of progression of IgAN in India are due to fundamental differences in the pathogenesis of IgAN or are more a reflection of differences in health care provision in different parts of the world.
The Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians (GRACE-IgANI) longitudinal prospective cohort study was designed to specifically address this question. Consecutive patients diagnosed with IgAN who met the eligibility criteria were consented to enter GRACE-IgANI, with recruitment commencing in March 2015 and completed in September 2017. All patients were recruited from the Department of Nephrology, Christian Medical College, Vellore, which is the largest private not-for-profit tertiary referral hospital in South India. 201 incident adult patients with IgAN were recruited. The full GRACE-IgANI protocol has been published separately.17 In brief, this prospective study was specifically designed to enable collection of a full complement of biosamples (including DNA, serum, plasma, urine, faeces, kidney tissue) for in depth biomarker analysis to elucidate pathogenic pathways operating in the cohort and directly link these to clinical phenotype and kidney disease progression during follow-up.
Here we report the baseline clinical, biochemical, and histopathologic characteristics of this incident cohort and calculate baseline risk of progression for the cohort.
Materials and Methods
The study protocol has previously been published17 and is registered with WHO trial id: ISRCTN36834159. In brief, all patients with a diagnosis of primary IgAN based on a kidney biopsy performed at Christian Medical College, Vellore, and who satisfied the eligibility criteria (Supplementary Table S1) were consecutively and prospectively recruited into GRACE-IgANI after informed consent. All prior medical records for each patient were carefully examined for earliest reported abnormalities and the timelines noted in the case report form. Renal dysfunction prior to presentation to our center was defined as ≥1.3 mg/dl in women and ≥1.5mg/dl in men. In-center follow-up was for a minimum of 2 scheduled visits in the first 2 years and then 1–2 per year depending on the clinical status of the patient. Patients were contacted at least once every 3 to 6 months to ensure medication compliance, optimization of blood pressure medications using self-reported/clinic blood pressures, monitoring of medication side effects, and test results.
Urine red bood cells were manually quantitated on fresh urine samples, and urine protein creatinine ratio was calculated from a 24-hour urine collection. eGFR was calculated using both Modification of Diet in Renal Disease (MDRD) study18 and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)19 equations for adults, and CKD-EPI eGFR was used for stratification and risk assessment. The renal biopsies in our center are sectioned by trained technicians (>10 years’ experience) with specialized automatic Leica microtomes (3-μm thickness). All kidney biopsies were evaluated independently by 2 experienced nephropathologists. A total of 185 biopsies met the criteria for scoring using the revised Oxford MEST-C Classification.20 Sixteen biopsies did not contain adequate (<8) glomeruli for evaluation and were excluded from the analysis. For each case, an Oxford Classification MEST-C score was generated by joint consensus, with discrepant cases being rereviewed using a multiheaded microscope and a final score agreed. All 201 slides were stained for immunoglobulins, complement C3 and C4, kappa and lambda by immunofluorescence in the same laboratory and similarly evaluated. Electron microscopy was not performed as this is not routinely available at our center.
Statistical Analysis
All demographic, clinical, laboratory, and histology variables were stored in a secure database. Categorical and continuous variables are expressed as proportions and means ± standard deviation for normally distributed variables and as median with interquartile range for non-normally distributed variables. The normality of the distribution was assessed using the Kolmogorov-Smirnov test. Correlations were reported using Pearson or Spearman coefficients for parametric and nonparametric variables, respectively. The χ2 test was used to compare categorical variables (Fisher exact test if not applicable). The Student t test or the Mann-Whitney U test was used to compare continuous variables with or without normal distributions, respectively. One-way analysis of variance was used for comparisons between 3 or more groups and the t test with Fisher least significant difference was used to test significance between groups. Table 1 and Supplementary Table S1 are historical data prior to presentation to our center and are descriptive. There are also a small number (≤3/201) of missing laboratory values for the cohort; we do believe the absence of this data has not adversely affected the analyses performed. All analyses were performed using IBM SPSS Statistics for Windows, version 21, and graphs were produced using GraphPad Prism version 7.0e for Macintosh. All P values were 2-sided, and values <0.05 were considered significant.
Table 1.
Demographic and baseline clinical characteristics of the GRACE-IgANI cohort
| Gender, male-to-female n (ratio) | 142:59 (2.4:1) |
| Age, y, mean±SD) | 36±10.02 |
| BMI, mean±SD) | 24.7±4.05 |
| Hypertension, yes/evaluable patients (%) | 169/201 (84.1) |
| Time to renal biopsy from onset of hypertension, mo, median (IQR) (n) | 10 (2–36) (167) |
| Systolic blood pressure, mm Hg (mean±SD | 138±20.33 |
| Diastolic blood pressure, mm Hg, mean±SD | 86.69±12.56 |
| Mean arterial pressure, mm Hg, mean±SD | 103.95±14.34 |
| Blood pressure ≥140/90mm Hg, yes/evaluable patients (%) | 110/201 (55%) |
| Synpharyngitic illness prior to presentation, yes/evaluable patients (%) | 8/200 (4) |
| Pedal edema prior to presentation, yes/evaluable patients (%) | 93/200 (46.5) |
| Time to renal biopsy from onset of pedal edema, mo, median (IQR) (n) | 4 (2–11.5) (93) |
| Visible hematuria prior to presentation , yes/evaluable patients (%) | 20/200 (10) |
| Time to renal biopsy from onset of visible hematuria, mo, median (IQR) (n) | 4 (2–63) (19) |
| Renal dysfunction prior to presentation, yes/evaluable patients (%) | 149/188 (79.3) |
| Time to renal biopsy from onset of renal dysfunction, mo, median (IQR) (n) | 3 (1–7) (184) |
| Proteinuria prior to presentation , yes/evaluable patients (%) | 157/160 (98.1) |
| Time to renal biopsy from onset of proteinuria, mo, median (IQR) (n) | 2 (1–8) (160) |
| Nonvisible hematuria prior to presentation , yes/evaluable patients (%) | 90/124 (72.6) |
| Time to renal biopsy from onset of nonvisible hematuria, mo, median (IQR) (n) | 2 (1–7.75) (120) |
| Family history of CKD, yes/evaluable patients (%) | 11/201 (5.5) |
| On RASB prior to biopsy, yes/evaluable patients (%) | 74/201 (37) |
| Prior exposure to immunosuppression, yes/evaluable patients (%) | 0/201 (0) |
These data were collected from the patients’ medical records prior to inclusion in the GRACE-IgANI cohort. For some patients, these data were not available.
BMI, body mass index; CKD, chronic kidney disease; GRACE-IgANI, Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians; IQR, interquartile range; RASB, renin-angiotensin system blockers; SD, standard deviation.
Results
Baseline Clinical, Biochemical, and Histopathologic Features
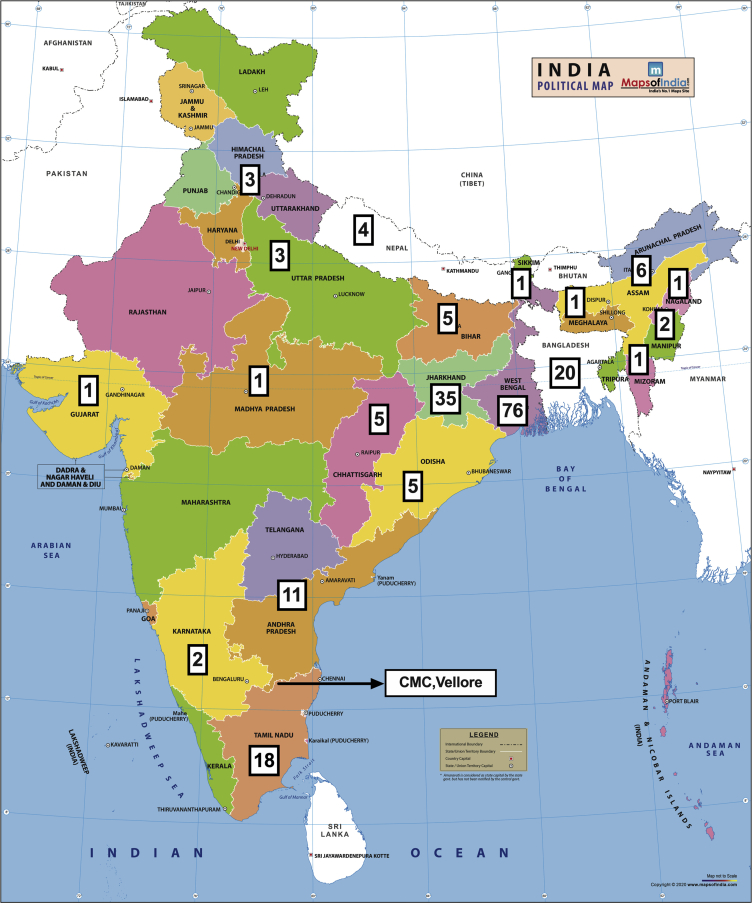
The geographic distribution of the 201 adult IgAN patients recruited into the GRACE-IgANI cohort is shown in Figure 1. As of April 30, 2020, the cohort had completed a median follow-up of 30 (IQR 16–39) months. Baseline clinical, biochemical, and kidney histopathologic characteristics for the cohort are described in Table 1, Table 2, Table 3. Similar to studies in Caucasians, but distinct from East Asians, males predominated (male-female ratio = 2.4:1) in the GRACE-IgANI cohort. The mean age at presentation was 36 years, with more than 75% of incident patients having established CKD and hypertension at the time of kidney biopsy. Female IgAN patients tended to be younger and had significantly lower mean arterial pressure (MAP), hemoglobin, serum albumin, uric acid, and creatinine (Supplementary Tables S2 and S3). However, there was no difference in proteinuria or eGFR and in the majority of histopathologic features between females and males (Supplementary Tables S2–S4).
Figure 1.
Geographic distribution of patients enrolled in the Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians (GRACE-IgANI) cohort. The country and state of origin of the 201 IgAN patients consecutively recruited into the GRACE-IgANI cohort.
Table 2.
Baseline laboratory parameters in the GRACE-IgANI cohort
| Hemoglobin, g/dl, mean±SD (evaluable patients) | 12.12±2.06 (201) |
| Serum total protein, g/dl, mean±SD (evaluable patients) | 6.85±0.7 (198) |
| Serum albumin, g/dl, mean±SD (evaluable patients) | 4±0.56 (198) |
| 24-h urine protein, g/d, median (IQR) (evaluable patients) | 1.9 (1–3.75) (200) |
| Serum total cholesterol, mg/dl, mean±SD (evaluable patients) | 176.88±57.12 (198) |
| Serum uric acid, mg/dl, mean±SD (evaluable patients)) | 7.02±1.88 (199) |
| Serum creatinine, mg/dl, mean±SD (evaluable patients) | 2.1±1.06 (201) |
| eGFR MDRD, ml/min per 1.73 m2, median (IQR) (evaluable patients) | 36 (24–60.5) (201) |
| eGFR CKD-EPI, ml/min per 1.73 m2, median (IQR) (evaluable patients) | 36 (26–67.5) (201) |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; GRACE-IgANI, Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians; MDRD, Modification of Diet in Renal Disease; SD, standard deviation.
Table 3.
Histopathologic parameters in the GRACE-IgANI cohort
| Oxford MEST-C Score (n=185) | |
| M1/M0 (M1 %) | 21/164 (11.4) |
| E1/E0 (E1 %) | 81/104 (43.8) |
| S1/S0 (S1 %) | 148/37 (80) |
| T2/T1/T0 (T2 %/T1 %) | 76/70/39 (41.1/37.8) |
| C2/C1/C0 (C2 %/C1 %) | 4/12/169 (2.2/6.4) |
| Global glomerulosclerosis (GS) (n=185), (GS / total glomeruli) × 100%, median (IQR) | 32.05 (12.5–46.66) |
| Immunofluorescence staining (n=201), n (%) | |
| IgA (+++) | 148 (73.6) |
| IgG (++ & +++) | 11 (5.5) |
| IgM (++ & +++) | 4 (2) |
| C3 (++ & +++) | 74 (36.8) |
GRACE-IgANI, Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians.
Total number of glomeruli per biopsy (median [IQR]) = 9 (7–13).
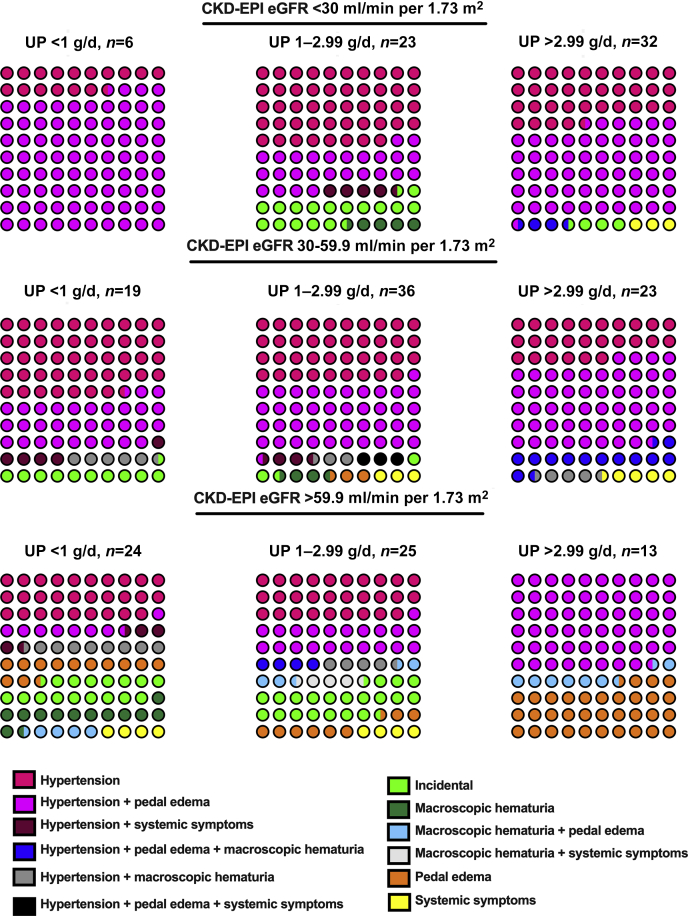
The pattern of clinical presentation according to CKD-EPI eGFR (Chronic Kidney Disease– Epidemiology Collaboration estimated glomerular filtration rate) and proteinuria tertiles are illustrated in Figure 2. Nonvisible hematuria was not included as it was an almost universal feature at presentation of IgAN. The commonest clinical presentation among patients with eGFR <60 ml/min per 1.73 m2 was hypertension (114/139, 82%) with or without pedal edema. Pedal edema without accompanying hypertension was seen predominantly in those patients with an eGFR ≥60 ml/min per 1.73 m2 (13/61, 21%) and heavy proteinuria (Figure 2).
Figure 2.
Patterns of clinical presentation of the Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians (GRACE-IgANI) cohort. Each circle represents 1% of the selected population. Percentage shown for each clinical presentation is stratified by CKD-EPI eGFR and proteinuria. eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; UP, urine protein.
Systemic symptoms (fatigue, arthralgia, low-grade fever) were present in a minority of patients (11/200 [5.5%]). Visible hematuria (20/200 [10%] with 15/20 [75%] associated with a synpharyngitic illness was uncommon. IgAN was incidentally diagnosed in asymptomatic individuals in only 17/200 (8.5%) during health checkups for insurance applications, job recruitment, or among army recruits and had milder clinical manifestations (MAP = 98±14 mm Hg, eGFR 63 ml/min per 1.73 m2 [IQR 24–98], proteinuria 1.6 g/d [IQR 0.7–2.6]). A not infrequent presentation was visual disturbance secondary to a hypertensive urgency (MAP 120.4 ± 18 mm Hg, 12/201 [6%]). Most cases were in young men (10/12 [83%]; mean age 29.5±8 vs. 36±10 years [whole cohort], P = 0.02). All cases had significant kidney damage (eGFR = 28±12 ml/min per 1.73 m2) and heavy proteinuria (4 g/d; IQR 1–6) at presentation along with high S1 and T2 scores (11/12 [91%] and 10/12 [83%]) and a high proportion of globally sclerosed glomeruli (59%, IQR 36%–74%).
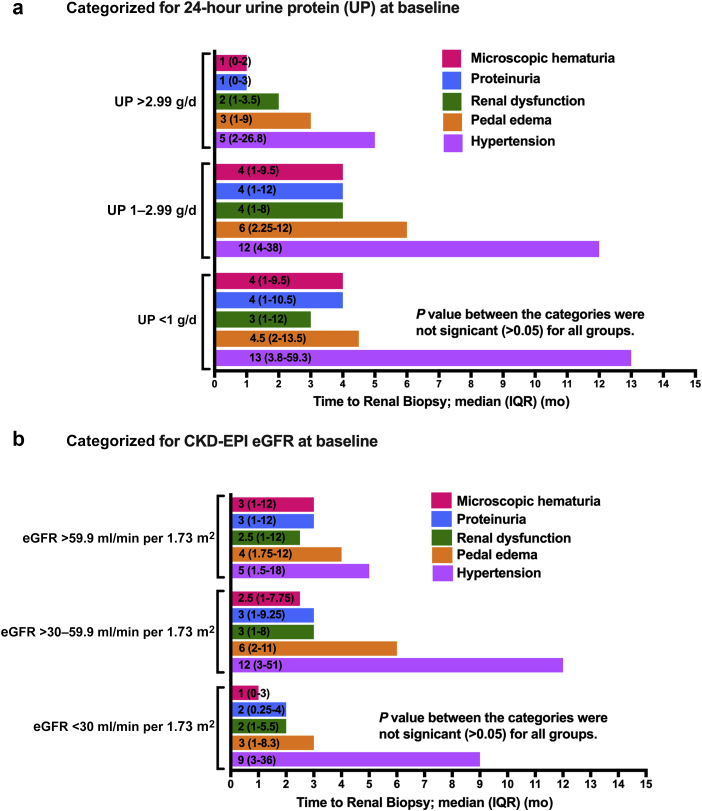
The median lead time to kidney biopsy from detection of hypertension was 10 months (IQR 2–36) (Table 1 and Figure 3). The lead time to kidney biopsy for all other symptoms or from detection of abnormal laboratory parameters was less than 6 months for each tertile of baseline proteinuria (Figure 3a) and for each tertile of CKD-EPI eGFR (Figure 3b). At baseline, higher MAP (>104 mm Hg) was associated with significantly higher proteinuria and lower eGFR (Table 4). The associations of MAP, eGFR, and proteinuria categories with other clinical variables are reported in Table 4. It is noteworthy that those patients presenting with a higher eGFR (≥60 ml/min per 1.73 m2) were significantly younger (31±8 vs 38±10 years, P < 0.001), and patients presenting with higher levels of proteinuria (>1 g/ 24 h) were more likely to be females.
Figure 3.
Time to renal biopsy from onset of symptoms or detection of abnormal laboratory parameters. (a) Categorized for 24-hour urine protein (UP) at baseline; (b) categorized for CKD-EPI eGFR at baseline.
Table 4.
Relationship between MAP, eGFR, proteinuria, and other clinical variables
| Characteristic | MAP (mm Hg) No. of evaluable patients (%) |
P value | eGFR (ml/min per 1.73 m2) No. of evaluable patients (%) |
P value | Proteinuria (g/d) No. of evaluable patients (%) |
P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <104 (111/201 (55.2)) | ≥104 (90/201 (44.8)) | >59.9 (62/201 (30.8)) | 30-59.9 (78/201 (38.8)) | <30 (61/201 (30.3)) | <1 (49/200 (24.5%)) | 1-2.99 (84/200 (42%)) | >3 (67/200 (33.5%)) | ||||
| Age, yr, mean±SD | 35.97±11 | 36.04±8.7 | ns | 31.29±8.23 | 37.91±9.76 | 38.36±10.53 | <0.001a,b | 34.08±11.35 | 36.45±10 | 37.04±8.86 | ns |
| Gender, male-female, n (ratio) | 73:38 (1.9:1) | 69:21 (3.3:1) | 0.06 | 40:22 (1.8:1) | 58:20 (2.9:1) | 44:17 (2.58:1) | ns | 27:22 (1.2:1) | 65:19 (3.4:1) | 50:17 (2.9:1) | 0.017 |
| BMI, mean±SD | 24.62±3.7 | 24.99±4.5 | ns | 25.71±4.4 | 24.5±4.3 | 24.22±3.1 | ns | 24.85±3.73 | 24.58±3.83 | 25.07±4.55 | ns |
| Systolic BP, mm Hg, mean±SD | 125.68±12.7 | 154.27±16.6 | <0.001 | 132±17.0 | 138.67±18.28 | 144.84±23.86 | 0.04a<0.001b | 130.69±18.18 | 137±19.14 | 146.16±21.03 | <0.001b, 0.006c |
| Diastolic BP, mm Hg, mean±SD | 77.94±6 | 97.48±9.8 | <0.001 | 84.48±11.3 | 87.47±11 | 88±15.3 | ns | 82.55±11.53 | 85.29±11.17 | 91.57±13.57 | <0.001b, 0.002c |
| MAP, mm Hg, mean±SD | 93.85±7.1 | 116.41±10.8 | <0.001 | 100.32±12.51 | 104.54±12.6 | 106.89±17.3 | 0.01b | 98.6±13.16 | 102.52±13.06 | 109.77±14.94 | <0.001b, 0.002c |
| BP ≥140/90 mm Hg, n yes (%) | 22 (20) | 88 (97.8) | <0.001 | 26 (41.9) | 44 (56.4) | 40 (65.6) | 0.037 | 16 (32.7) | 43 (51.2) | 51 (76.1) | <0.001 |
| Hemoglobin, g/dl, mean±SD | 12±2.1 | 12.3±2.1 | ns | 13.04±1.9 | 12.3±1.8 | 10.9±2 | 0.01a, <0.001b,c | 12.6±2.24 | 12.18±1.98 | 11.69±1.99 | 0.02b |
| Serum total protein, g/dl, mean±SD, n | 6.86±0.7 | 6.83±0.7 | ns | 7.05±0.84 (60) | 6.97±0.62 (76) | 6.6±0.57 (62) | 0.001b,c | 7.32±0.52 (48) | 6.96±0.62 (82) | 6.4±0.65 (67) |
0.001a <0.001b,c |
| Serum albumin, g/dl, mean±SD, n | 3.94±0.6 | 3.98±0.5 | ns | 3.99±0.7 (60) | 4.06±0.48 (76) | 3.79±0.45 (62) | 0.04b, 0.007c | 4.35±0.36 (48) | 4.06±0.44 (82) | 3.57±0.54 (67) |
0.001a <0.001b,c |
| 24-hour urine protein, g/d, median (IQR) | 1.55 (0.8–2.8) | 2.54 (1.4–4.4) | 0.001 | 1.2 (0.6–2.54) | 1.79 (1–3.5) | 3.1 (1.7–4.8) | <0.001b, 0.002c | 0.55 (0.30–0.82) | 1.74 (1.36–2.28) | 4.6 (3.7–5.9) | <0.001a,b,c |
| Serum total cholesterol, mg/dl, mean±SD | 171.64±62.4 | 183.29±49.5 | ns | 185.53±72.86 (60) | 182.86±50.22 (76) | 160.37±42.37 (62) | 0.01b,c | 151.38±37.12 (48) | 171.11±46.56 (83) | 200.88±69.5 (48) | <0.001b, 0.001c |
| Serum uric acid, mg/dl, mean±SD | 6.62±1.8 | 7.51±1.8 | 0.001 | 6.2±1.48 (60) | 7.52±1.9 (78) | 7.23±1.94 (61) | <0.001a, 0.003b | 6.5±1.98 (49) | 7.05±1.9 (82) | 7.39±1.7 (67) | 0.02b |
| Serum creatinine, mg/dl, mean±SD | 1.9±1 | 2.4±1.1 | 0.003 | 1.02±0.29 | 1.96±0.36 | 3.39±0.76 | <0.001a,b,c | 1.53±0.82 | 2.15±1.06 | 2.50±1.05 |
0.001a, <0.001b 0.04c |
| eGFR, ml/min per 1.73 m2, median (IQR) | 44 (29–80) | 36 (24–54.3) | 0.009 | 89.5 (72.8–114.5) | 40 (33–47) | 21 (16–24) | <0.001a,b,c | 59 (36–107.5) | 43 (28.25–64) | 30 (22–42) | <0.001a,b |
eGFR, estimated glomerular filtration rate (calculated using the CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] formula); BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; SD, standard deviation.
One-way analysis of variance was used for comparisons between 3 or more groups and the t Test with Fisher Least Significant Difference was used to test significance between groups. P value was significant at 0.05 between acolumns 1 and 2, bcolumns 1 and 3, and ccolumns 2 and 3 columns.
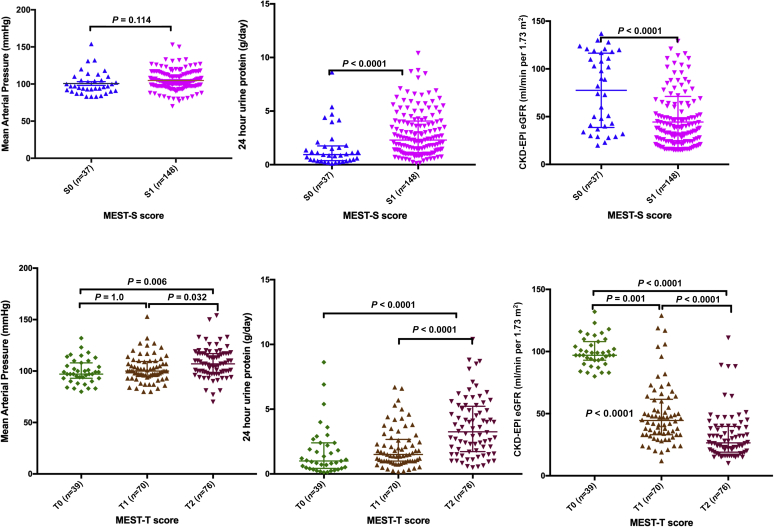
We were able to assess the MEST-C score in 185 of 201 (92.5%) patients and evaluate immunofluorescence staining for IgA, IgG, IgM, and C3 in all kidney biopsies (Table 3). In our cohort, a high proportion of kidneys already showed significant evidence of fibrosis with T1/T2 lesions (79%) at baseline (Table 3). As expected, these sclerosing lesions (S1, T1, and T2) were associated with lower eGFR, higher MAP (expect S1), and higher urine protein excretion at presentation (Table 5 and Figure 4). Similarly, global glomerulosclerosis (GS) was present in a significant number of cases and associated with lower eGFR and higher urine protein excretion. None of the renal biopsies showed evidence of an acute/active or chronic thrombotic microangiopathy. It was interesting to note that the traditionally benign manifestation of visible hematuria with synpharyngitic illness was frequently accompanied by hypertension (15/20, 75%) and the presence of fibrotic lesions (S1 [13/18, 70.2%] and T1/T2 [11/18, 61.1%]). Even incidentally diagnosed clinically “mild” IgAN was associated with a significant number of fibrotic lesions (S1 [11/16, 68.8%] and T1/T2 [10/16, 62.5%]).
Table 5.
Relationship between histopathologic and clinical variables
| Characteristic (no. of valuable biopsies) | MAP (mm Hg) | P value | eGFR (ml/min/per 1.73 m2) | P value | Proteinuria (g/d) | P value |
|---|---|---|---|---|---|---|
| M0 (164/185) | 104.05±14.0 | 0.76 | 40 (27–72.75) | 0.82 | 2.1 (1–4) | 0.10 |
| M1 (21/185) | 103.08±12.9 | 38 (23.5–64.5) | 1.5 (0.96–2.35) | |||
| E0 (104/185) | 102.82±14.9 | 0.21 | 40.5 (28.25–73.75) | 0.49 | 1.43 (0.82–2.7) | <0.001 |
| E1 (81/185) | 105.39±12.4 | 39 (24–64) | 2.85 (1.5–4.83) | |||
| S0 (37/185) | 100.71±15.3 | 0.11 | 82 (38.5–117.5) | <0.001 | 0.94 (0.38–1.75) | <0.001 |
| S1 (148/185) | 104.75±13.4 | 37.5 (24–52.75) | 2.3 (1.1–4.1) | |||
| T0 (39/185) | 99.56±11.8 | 98 (74–116) | 0.97 (0.43–2.25) | |||
| T1 (70/185) | 102.11±13.06 | 0.01a | 44.5 (33–61.5) | <0.001 | 1.5 (1–2.67) | <0.001a |
| T2 (76/185) | 107.89±14.7 | 0.002b | 26.5 (19–39.75) | <0.001 | 3.3 (1.73–5.23) | <0.001b |
| C0 (69/185) | 104.17±14.1 | 40 (26–67) | 1.9 (1–3.8) | |||
| C1 (12/185) | 101.4±12.9 | 51 (31.3–75) | 2.4 (0.9–4) | |||
| C2 (4/185) | 102±4.4 | 0.773 | 36 (22.5–67.5) | 0.49 | 3.2 (1.5–7.7) | 0.78 |
| (GS/total glomeruli)∗100 | ||||||
| <33% (92/184) | 102.33±11.3 | 64.5 (39.3–102) | 1.5 (0.9–3) | |||
| ≥33% (92/184) | 105.42±16 | 0.13 | 31 (20–40) | <0.001 | 2.5 (1.2–4.2) | 0.007 |
| IgA | ||||||
| + & ++ (53/201) | 107.54±17.09 | 0.06 | 31 (24-50) | 0.005 | 1.9 (0.9-4) | 0.71 |
| +++ (148/201) | 102.67±13.04 | 43.5 (28.25-81.5) | 1.9 (1-3.5) | |||
| IgG | ||||||
| – & + (190/201) | 104.2±14 | 0.3 | 39 (25.75-65.25) | 0.031 | 2 (1-3.78) | 0.08 |
| ++ & +++ (11/201) | 99.61±19.6 | 67 (33-118) | 1 (0.7-2.4) | |||
| IgM | ||||||
| - & + (196/201) | 103.9±14.3 | 0.7 | 39 (25.75-65.25) | 0.011 | 1.9 (1-3.79) | 0.13 |
| ++ & +++ (4/201) | 106.67±16.7 | 67 (33-118) | 1.0 (0.34-2.1) | |||
| C3 | ||||||
| – & + (127/201) | 104.8±15 | 0.27 | 38 (26-61) | 0.26 | 1.81 (1-3.8) | 0.89 |
| ++ & +++ (74/201) | 102.5±13.1 | 47 (24-81.25) | 2.2 (0.87-3.63) |
ANOVA, analysis of variance; MAP, mean arterial pressure; eGFR, estimated glomerular filtration rate (calculated using the CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] formula).
One-way ANOVA for significant effect of T score on MAP was F(2, 182) = 5.92, P = 0.003, post hoc comparisons using the t test with Fisher least significant difference was significant for (T1 vs. T2)a and (T0 vs. T2)b; 1-way ANOVA for significant effect of T score on eGFR was F(2, 182) = 73.84, P < 0.001, post hoc comparisons using the t test with Fisher least significant difference was significant for (T1 vs. T2), (T0 vs. T1), and (T0 vs. T2); 1-way ANOVA for significant effect of T score on proteinuria was F(2, 181) = 69.58, P < 0.001, post hoc comparisons using the t test with Fisher least significant difference was significant for (T1 vs. T2)a and (T0 vs. T2).b
Figure 4.
Relationship of the presence of the S and T lesions of the Oxford Classification with key clinical variables. There were significant associations between the presence of segmental glomerular sclerosis (S1) and 24-hour urine protein excretion and eGFR (CKD-EPI) at the time of diagnosis, but not mean arterial blood pressure. Similarly, there were significant associations between the extent of tubulointerstitial inflammation and fibrosis (T1 and T2) and 24-hour urine protein excretion and eGFR (CKD-EPI) at the time of kidney biopsy, as well as mean arterial blood pressure. eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
What is perhaps most surprising when reviewing the kidney histology is the marked infrequency of crescents (8.6%). Despite the absence of crescents, endocapillary hypercellularity (E1) was seen in 44% of kidneys and was associated with the extent of proteinuria but not eGFR at presentation. Mesangial hypercellularity (M1) was also uncommon (11.4%) and did not have any clinical associations in our cohort (Tables 3 and 5). Co-deposition of IgG (>grade 1+ = 10.5%) and/or IgM (>grade 1+ = 22.4%) were seen in one-third of cases, with evidence of complement activation in more than two-thirds of cases (C3 >grade 1+ = 78.6%).
Patients With Proteinuria <1 g/d at Kidney Biopsy
Proteinuria less than 1 g/d is generally regarded as a good prognostic feature in IgAN and was seen in 25% (49/201) of the cohort. Clinical associations are reported in Table 4. Despite the low level of proteinuria, there were already signs of significant kidney damage in this group, with 21% (9/44) having E1 lesions, 57% (25/44) S1 lesions, 57% (25/44) T1/T2 lesions, and 20% GS (IQR 0–42). Even in the 24 patients with eGFR ≥60 ml/min per 1.73 m2, there was still a significant burden of established kidney damage (24% [5/21] E1 lesions; 38% [8/21] S1 lesions; 19% [25/44] T1/T2 lesions; 0% GS). Immunofluorescence (IF) staining showed IgA grade 3+ in 35/49 (71%) and C3 staining in 43/49 (88%) biopsies.
Patients With Proteinuria ≥3 g/d at Kidney Biopsy
Presentation with nephrotic range proteinuria is unusual in Caucasian IgAN cohorts; however, this was observed in 34% (67/201) of the GRACE-IgANI cohort. Clinical correlates are reported in Table 4. As expected, this group had evidence of extensive kidney damage at presentation, with 9.7% (6/62) having E1 lesions, 90% (56/62) S1 lesions, 92% (57/62) T1 and T2 lesions, and 38% GS (IQR 16.7–53.1). Strikingly, crescents were rare in this group. Even among the 12 patients with eGFR ≥60 ml/min per 1.73 m2, kidney damage was already advanced (55% [6/11] E1 lesions; 72% [8/11] S1 lesions; 64% [7/11] T1 and T2 lesions; 11% GS [IQR 0–25]). Immunofluorescence staining showed similar patterns to that seen in the <1 g/d group (IgA grade 3+: 64% [43/67]; C3 staining: 67% [45/67]).
Baseline Risk of Progression in the GRACE-IgANI Cohort Using 2 Different Risk Prediction Scores
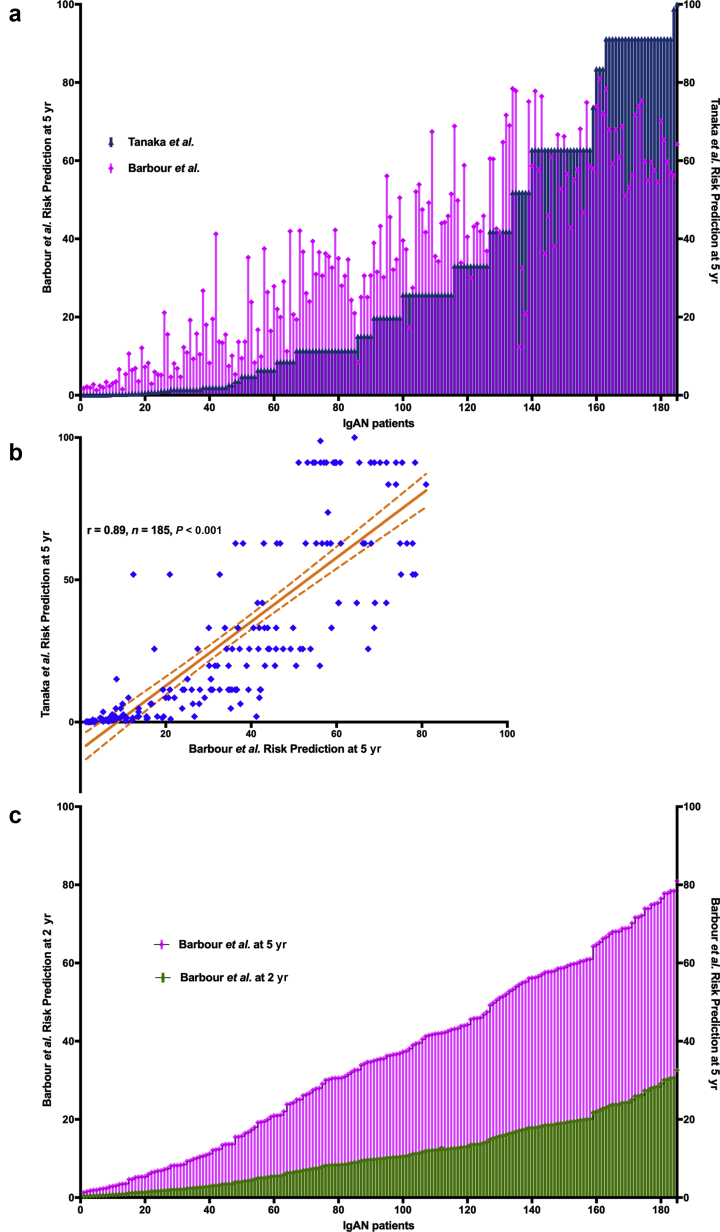
At the time of recruitment to the GRACE-IgANI cohort, the most comprehensive risk prediction score for IgAN was that described by Tanaka et al.,21 and this was used to risk stratify 185 IgAN patients with complete MEST-C scores for longitudinal follow-up.17 This risk prediction tool uses 5 parameters: proteinuria, eGFR, and the M, S, and T scores of the Oxford classification to give a total risk score (TRS) between 0 and 34, which translates to a 5-year absolute risk of ESKD. In 2019, a new IgAN risk prediction tool was published by Barbour et al.22 on behalf of the International IgA Nephropathy Network (IIGANN). This tool incorporates 7 additional parameters and allows calculation of individual risk of progression to a combined endpoint of 50% decline in eGFR or ESKD up to 5 years from diagnosis. We calculated the 5-year risk of ESKD (Tanaka et al.) and the 5-year risk of progression to a 50% decline in eGFR or ESKD (Barbour et al.) for each patient in the GRACE-IgANI cohort (Figure 5a). The median TRS using the renal risk equation developed by Tanaka et al.21 was 19 (IQR 13–24), which equates to a 5-year absolute risk of ESKD of 19.8% (IQR 2.7–57.4). The median 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD (Barbour et al.) was 35.5% (IQR 13.7–56.2). Not surprisingly, for most patients there was good concordance (Figure 5a) and a reasonable correlation (Figure 5b; r = 0.89, n = 185, P < 0.001) between the 2 risk scores. However, there was a divergence in calculated risk between the 2 risk prediction tools in patients at greatest risk of progression. To confirm the high-risk profile of the GRACE-IgANI cohort, we also calculated the corresponding 2-year risk of progression for each patient using the Barbour et al. tool (Figure 5c).
Figure 5.
Baseline risk of progression in the Glomerular Research And Clinical Experiments–IgA Nephropathy in Indians (GRACE-IgANI) cohort using 2 different risk prediction scores. (a) We calculated the 5-year risk of a 50% decline in eGFR or ESKD using the International IgA Nephropathy Network (IIGANN) risk prediction tool (Barbour et al.) and the 5-year risk of ESKD using the renal risk equation developed by Tanaka et al. for the 185 IgAN patients with complete MEST-C scores in the GRACE-IgANI cohort. Each patient is represented in a separate column and ordered according to increasing 5 year risk of ESKD using the renal risk equation developed by Tanaka et al. (b) There was a clear correlation between each patients 5-year risk of a 50% decline in eGFR or ESKD using the International IgA Nephropathy Network (IIGANN) risk prediction tool (Barbour et al.) and the 5-year risk of ESKD using the renal risk equation developed by Tanaka et al. for the 185 IgAN patients with complete MEST-C scores in the GRACE-IgANI cohort. As would be expected a number of patients with no risk of ESKD at 5 years using the Tanaka et al. prediction tool had appreciable risk of progression using the IIGANN tool which likely represents those at risk of a 50% decline in eGFR rather than ESKD within 5 years. (c) We also calculated the corresponding 2 year risk of a 50% decline in eGFR or ESKD using the International IgA Nephropathy Network (IIGANN) risk prediction tool (Barbour et al.) in the 185 IgAN patients with complete MEST-C scores in the GRACE-IgANI cohort. Short term risk of progression in this incident cohort was high.
The GRACE-IgANI cohort was divided into lower-risk (LR) and higher-risk (HR) groups based on these median 5-year risks according to the Taneka et al. tool (LR = TRS <19 [n = 91]; HR = TRS ≥19 [n = 94]) and Barbour et al. tool (LR = IIGANN risk <35% [n=90]; HR = IIGANN risk ≥35% [n=95]). Thirteen patients in the LR group as defined by the Taneka et al. tool would have been reclassified as HR using the Barbour et al. tool and 12 patients in the HR group reclassified as LR. The clinical, biochemical, and kidney histopathologic characteristics for each group cohort are reported in Supplementary Tables S5 to S10. When comparing HR with LR as defined by the TRS of Tanaka et al., 4 of the 5 included parameters were significantly different between the groups apart from the M score. In addition, there were significant differences in blood pressure, hemoglobin, serum total protein, serum albumin, serum uric acid, and frequency of E1 and GS lesions between the risk groups. Both blood pressure and E1 lesions are in fact included in the Barbour et al. risk prediction tool. Age at diagnosis—another parameter included in the more recent tool—was not significantly different between groups.
Discussion
This is the first South Asian IgAN cohort with prospective longitudinal design and protocolized follow-up. Over the next 5 years, GRACE-IgANI will provide unrivalled potential to study biochemical pathways important in both development of IgAN and disease progression in South Asians. We will use the generated bioresource to perform discovery studies for novel biomarkers that could refine prognostication in South Asians and inform treatment decisions in IgAN. A fundamental foundation for this work is a detailed characterization of the GRACE-IgANI cohort.
Its noteworthy that young adults (29.5±8 vs. 36±10.02 years [whole cohort]) had significantly better eGFR and lower proteinuria, signifying that IgAN smolders subclinically for more than a decade before it clinically manifests. Younger patients having better prognosis has been reported in various studies.23,24 Consistent with European studies, male patients predominated (male-female, 2.4:1) in GRACE-IgANI, which is distinct from East Asian cohorts that report either equal incidence in males and females or even an increased frequency in females.25, 26, 27, 28 Similar to studies reported from China, at presentation males had more advanced disease with lower eGFR, more frequent hypertension, and more fibrosis on kidney biopsy.26 A meta-analysis of 25 studies in IgAN reported that male gender was associated with a worse outcome.29 GRACE-IgANI will allow us to determine if this is true of South Asian males with longitudinal follow-up data.
Globally, asymptomatic urine abnormalities and recurrent visible hematuria are the commonest presentations of IgAN.3 This is very different in GRACE-IgANI. Visible hematuria and asymptomatic presentation following routine screening were far less common than reported in other cohorts1,2,30 and, distinct from reports in Caucasians, were not benign manifestations, being commonly associated with hypertension and a significant burden of glomerular and tubulointerstitial fibrosis on kidney biopsy. We believe that asymptomatic presentations may increase with universal targeted screening. The majority of South Asian patients presented with hypertension, with or without pedal edema. Over recent years, it has become recognized that there has been a shift in the countries with the highest blood pressure levels, moving from high- to low-income countries, particularly countries in South Asia and sub-Saharan Africa.31,32 The average age of hypertensives in India is 32±11.1 years in men and 30±9.8 years in women,33 and this has been attributed to excessive daily salt consumption.34, 35, 36 This is strikingly similar to our cohort though there was ethnic preponderance from the south, central-east and north-east parts of the country. It is sobering to acknowledge that the vast majority of young hypertensives in South Asia are not screened for underlying CKD. Introduction of a simple screening test such as a urine dipstick for hematuria and proteinuria in this population is likely to be a cost-effective public health strategy.
It is reassuring that the lead time to kidney biopsy from first presentation in GRACE-IgANI was short. This is possible because of our center’s open referral policy, short wait-times, and easy to access kidney biopsy service. We believe that this streamlined service has reduced the impact of lead-time bias in evaluation of outcomes and improved the precision of description of the natural history of IgAN before kidney biopsy in GRACE-IgANI.37,38 Furthermore, evaluation of all kidney biopsies in the same center, by the same 2 nephropathologists has ensured consistency in reporting of histomorphometry and immunostaining. A limitation of GRACE-IgANI is that electron microscopy is not routinely performed in our center.
The overwhelming impression from reviewing the GRACE-IgANI kidney biopsy data is a disproportionate absence of active glomerular lesions and overrepresentation of segmental sclerosing lesions and tubulointerstitial fibrosis at presentation, often coexistent with relatively well-preserved eGFR and low levels of proteinuria. This is in stark contrast to studies in East Asian populations where the predominant features at presentation are active glomerular lesions with little background scarring.39,40 Previous kidney biopsy series from India have also reported advanced scarring in the majority of IgAN patients at presentation but, unlike these retrospective registry studies that were confounded by differing referral and biopsy policies and inclusion of patients with ESKD, GRACE-IgANI recruited a large number of patients with preserved kidney function and low-grade proteinuria.16,41 It is important to acknowledge that the original cohorts from which the MEST and MEST-C scores were derived, and the subsequent large validation studies, had very few patients of South Asian ethnicity. The validity of each component of the MEST-C score has, therefore, not formally been assessed in a South Asian population. Although GRACE-IgANI was not designed as a validation study, it is possible to draw comparisons with other validation studies.
Two of the 3 glomerular lesions most associated with active inflammation (mesangial hypercellularity and crescents) were notably rare in GRACE-IgANI. The possibilities for a M1 lesion in IgA-dominant glomerulonephritis could also be due to a concomitant glomerulonephritis such as (1) IgA-dominant infection-related glomerulonephritis/resolving or healed postinfectious glomerulonephritis, (2) proliferative glomerulonephritis with monoclonal IgA deposits, or (3) secondary IgA nephropathy. Our study by detailed clinical correlation and serial close follow-up clearly excluded the presence of an infectious etiology, monoclonal gammopathy, or secondary coexistent systemic disorders. A significant tubulointerstitial scarring could alter the MEST-C M score due to glomerular tuft atrophy/capillary wrinkling secondary to pronounced low-flow ischemic changes. However, all our scoring were made on unaffected/fully inflated glomeruli, which did not show any signs of a vascular injury. Markedly atrophic/ischemic glomeruli were discounted from scoring in order to achieve accurate MEST-C M and S scores. Crescentic IgAN (>50% crescents) does exist,42 but most did not meet the inclusion criteria of this cohort because of low eGFR (Supplementary Table S1). Consistent with studies from Germany,43 France,44 and North America,45 we did not observe any association between the M lesion and baseline clinical characteristics in GRACE-IgANI and may reflect the advanced chronic tubulointerstitial damage and low frequency of M1 in this cohort. By contrast, in the VALIGA cohort, a strong association between the M1 lesion and proteinuria, MAP, and eGFR46 was reported, which was replicated for proteinuria and eGFR in a large Chinese and a smaller Korean cohort.39,40 The only inflammatory glomerular lesion seen in a significant proportion of GRACE-IgANI was the E1 lesion, seen in 44% of evaluable biopsies. Consistent with studies in Europe and China, E1 was associated with the extent of proteinuria.39,43 Why the E lesion is so common and crescents and mesangial cell proliferation so uncommon in South Asians is currently unclear. Future biochemical analysis of blood, urine, and stored peripheral blood mononuclear cells alongside more detailed molecular analysis of the kidney biopsies will hopefully elucidate the underlying mechanisms responsible.
Segmental glomerulosclerosis, global glomerulosclerosis, and tubulointerstitial fibrosis were very common in GRACE-IgANI. As in previous validation studies, the S1 lesion correlated with proteinuria and eGFR but not with MAP at presentation.39,43,46 We noted a higher prevalence of nephrotic-range proteinuria than the reported 5% to 10% in other studies.30,47 This was associated with significant segmental and global glomerulosclerosis compared to the non-nephrotic group, even at a higher eGFR. It has been suggested there may be a podocytopathic variant of IgAN marked by predominant podocyte injury, segmental, and then global glomerulosclerosis and associated with high levels of proteinuria.48, 49, 50, 51 Podocyte hypertrophy and tip variants have been described in IgAN and associated with greater baseline proteinuria.52 The lack of electron microscopy in GRACE-IgANI does limit podocyte evaluation, but work is currently ongoing to determine if podocyte injury is more common in South Asians by scoring the pattern of podocyte injury in line with the recent report by Bellur et al.52
Perhaps most striking was that 79% of GRACE-IgANI had T1/T2 lesions compared to only 21% in VALIGA, 41% in the German, and 27% in the large Chinese validation cohort.39,43,46,53 T2 lesions were rare in VALIGA (3%), German (4%), and the Chinese cohort (3.3%) but seen in 41% in GRACE-IgANI.39,43,46 As previously reported, T lesions were closely associated with eGFR, MAP, and proteinuria in GRACE-IgANI.39,46,54 It is unclear why South Asians should present with such advanced fibrotic lesions apparently so early in their clinical course, and with relatively preserved eGFR and low levels of proteinuria. This may be a reflection of the postulated low nephron number in South Asians, which results in an increased risk of both hypertension and CKD due to glomerular hyperfiltration and hypertrophy, with intraglomerular hypertension resulting in further nephron loss and reduced sodium excretory capacity. Alternatively, there may be a specific fibrotic response to mesangial IgA deposition in South Asians; our laboratory studies will explore this possibility.
Baseline risk of progression was calculated for each evaluable patient using 2 different risk prediction scores. The predicted risk of progression in this South Asian cohort was considerable and far greater than reported in Caucasian55,56 and East Asian cohorts,57,58 with a 5-year absolute risk of ESKD of 19.8% (IQR 2.7–57.4) using the Tanaka et al.21 model and a median 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD of 35.5% using the Barbour et al.22 model. It is worth noting that South Asian patients were not included in the development or validation of either model. There was reasonable concordance between the models at low to medium risk; however, the models appeared to diverge in patients with the highest risk of progression, with the Barbour et al.22 model predicting a much lower risk of progression than the Tanaka et al.21 model. Although the GRACE-IgANI was not designed to formally validate either risk prediction score, there is likely to be sufficient events (50% decline in eGFR or ESKD) in this cohort to establish which score is more suitable to predict outcome in South Asians and potentially identify additional South Asian–specific variables that will improve prognostication in our patients.
Conclusions
This is the first prospective South Asian IgAN cohort with protocolized follow-up and extensive biosample collection. Baseline characteristics of the GRACE-IgANI cohort confirm that South Asians with IgAN have a more severe clinicopathologic presentation than both Caucasians and East Asians. Unlike IgAN in East Asian populations, IgAN is characterized by a very low frequency of inflammatory glomerular lesions but extensive glomerular segmental and global sclerosis and tubulointerstitial fibrosis at diagnosis. The risk of progression in South Asians is significantly higher than that reported in East Asian and Caucasian populations. Over the next 5 years, we will dissect the pathogenic pathways that underlie this severe South Asian IgAN phenotype.
Disclosure
All the authors declared no competing interests.
Acknowledgements
This work was supported by the DBT/Wellcome Trust India Alliance Fellowship (grant number IA/CPHE/14/1/501501) awarded to SA. Initial small funding was by a major fluid research internal grant (8962 [Other] dated July 23, 2014) from Christian Medical College, Vellore, India.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The published work is a part of a PhD thesis submitted to the Tamil Nadu Dr. M.G.R. Medical University.
We acknowledge Prof. Gagandeep Kang (fellowship sponsor for grant number IA/CPHE/14/1/501501 awarded to SA); Laisa Arul Gladis (associate research officers in the project). This trial has been registered with WHO trial ID: ISRCTN36834159.
Footnotes
Supplementary Table S1. Inclusion and exclusion criteria for the GRACE-IgANI cohort.
Supplementary Table S2. Impact of gender on demographic and baseline clinical characteristics of the GRACE-IgANI cohort.
Supplementary Table S3. Impact of gender on baseline laboratory parameters in the GRACE-IgANI cohort.
Supplementary Table S4. Impact of gender on histopathologic parameters in the GRACE-IgANI cohort.
Supplementary Table S5. Baseline clinical characteristics of the GRACE-IgANI cohort stratified by Total Risk Score.
Supplementary Table S6. Baseline laboratory parameters of the GRACE-IgANI cohort stratified by Total Risk Score.
Supplementary Table S7. Histopathologic parameters in the GRACE-IgANI cohort stratified by Total Risk Score.
Supplementary Table S8. Baseline clinical characteristics of the GRACE-IgANI cohort stratified by the 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD using the IIGANN risk calculator.
Supplementary Table S9. Baseline laboratory parameters of the GRACE-IgANI cohort stratified by the 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD using the IIGANN risk calculator.
Supplementary Table S10. Histopathologic parameters in the GRACE-IgANI cohort stratified by the 5-year risk of progression to the combined endpoint of 50% decline in eGFR or ESKD using the IIGANN risk calculator.
Supplementary Material
References
- 1.Wyatt R.J., Julian B.A. IgA Nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis Off J Natl Kidney Found. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 3.Yeo S.C., Goh S.M., Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology. 2019;24:885–895. doi: 10.1111/nep.13592. [DOI] [PubMed] [Google Scholar]
- 4.Bartosik L.P., Lajoie G., Sugar L. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–735. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 5.Bailey R.R., Lynn K.L., Robson R.A. Long term follow up of patients with IgA nephropathy. N Z Med J. 1994;107:142–144. [PubMed] [Google Scholar]
- 6.Chugh K.S., Sakhuja V. Glomerular diseases in the tropics. Am J Nephrol. 1990;10:437–450. doi: 10.1159/000168167. [DOI] [PubMed] [Google Scholar]
- 7.Narasimhan B., Chacko B., John G.T. Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol. 2006;19:205–210. [PubMed] [Google Scholar]
- 8.Das U., Dakshinamurty K.V., Prayaga A. Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21:250–257. doi: 10.4103/0971-4065.85482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhuyan U.N., Dash S.C., Srivastava R.N. IgA-associated glomerulonephritis. J Assoc Physicians India. 1992;40:310–313. [PubMed] [Google Scholar]
- 10.Siddappa S., Kowsalya R., Mythri K.M. IgA nephropathy in a tertiary care center from south India. Indian J Nephrol. 2011;21:230–234. doi: 10.4103/0971-4065.82635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal N., Joshi K., Rane S. Primary IgA nephropathy in north India: Is it different? Postgrad Med J. 2012;88:15–20. doi: 10.1136/postgradmedj-2011-130077. [DOI] [PubMed] [Google Scholar]
- 12.Muthukumar T., Fernando M.E., Jayakumar M. Prognostic factors in immunoglobulin-A nephropathy. J Assoc Physicians India. 2002;50:1354–1359. [PubMed] [Google Scholar]
- 13.Prakash S., Kanjanabuch T., Austin P.C. Continental variations in IgA nephropathy among Asians. Clin Nephrol. 2008;70:377–384. doi: 10.5414/cnp70377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthoux F.C., Mohey H., Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28:4–9. doi: 10.1016/j.semnephrol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.George J., Ninan V.T., Thomas P.P. Primary IgA nephropathy in adults. J Assoc Physicians India. 1993;41:489–491. [PubMed] [Google Scholar]
- 16.Chacko B., John G.T., Neelakantan N. Presentation, prognosis and outcome of IgA nephropathy in Indian adults. Nephrol Carlton Vic. 2005;10:496–503. doi: 10.1111/j.1440-1797.2005.00445.x. [DOI] [PubMed] [Google Scholar]
- 17.Alexander S., John G.T., Korula A. Protocol and rationale for the first South Asian 5-year prospective longitudinal observational cohort study and biomarker evaluation investigating the clinical course and risk profile of IgA nephropathy: GRACE IgANI cohort. Wellcome Open Res. 2018;3:91. doi: 10.12688/wellcomeopenres.14644.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyman H.A., Dowling T.C., Hudson J.Q. Comparative Evaluation of the Cockcroft-Gault Equation and the Modification of Diet in Renal Disease (MDRD) study equation for drug dosing: an opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2011;31:1130–1144. doi: 10.1592/phco.31.11.1130. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis Off J Natl Kidney Found. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimarchi H., Barratt J., Cattran D.C. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S., Ninomiya T., Katafuchi R. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol CJASN. 2013;8:2082–2090. doi: 10.2215/CJN.03480413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbour S.J., Coppo R., Zhang H. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusumoto Y., Takebayashi S., Taguchi T. Long-term prognosis and prognostic indices of IgA nephropathy in juvenile and in adult Japanese. Clin Nephrol. 1987;28:118–124. [PubMed] [Google Scholar]
- 24.Coppo R., Lofaro D., Camilla R.R. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. 2017;32:139–150. doi: 10.1007/s00467-016-3469-3. [DOI] [PubMed] [Google Scholar]
- 25.Li P.K.T., Ho K.K.L., Szeto C.C. Prognostic indicators of IgA nephropathy in the Chinese—clinical and pathological perspectives. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2002;17:64–69. doi: 10.1093/ndt/17.1.64. [DOI] [PubMed] [Google Scholar]
- 26.Deng W., Tan X., Zhou Q. Gender-related differences in clinicopathological characteristics and renal outcomes of Chinese patients with IgA nephropathy. BMC Nephrol. 2018;19:31. doi: 10.1186/s12882-018-0829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriyama T., Tanaka K., Iwasaki C. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoop T., Vikse B.E., Svarstad E. Mortality in patients with IgA nephropathy. Am J Kidney Dis. 2013;62:883–890. doi: 10.1053/j.ajkd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Neugarten J., Acharya A., Silbiger S.R. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol JASN. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 30.Donadio J.V., Grande J.P. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 31.Zhou B., Bentham J., Di Cesare M. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prenissl J., Manne-Goehler J., Jaacks L.M. Hypertension screening, awareness, treatment, and control in India: a nationally representative cross-sectional study among individuals aged 15 to 49 years. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain F.B., Adhikary G., Chowdhury A.B. Association between body mass index (BMI) and hypertension in south Asian population: evidence from nationally-representative surveys. Clin Hypertens. 2019;25:28. doi: 10.1186/s40885-019-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson C., Praveen D., Pope A. Mean population salt consumption in India: a systematic review. J Hypertens. 2017;35:3–9. doi: 10.1097/HJH.0000000000001141. [DOI] [PubMed] [Google Scholar]
- 35.Radhika G., Sathya R.M., Sudha V. Dietary salt intake and hypertension in an urban south Indian population--[CURES - 53] J Assoc Physicians India. 2007;55:405–411. [PubMed] [Google Scholar]
- 36.de Brito-Ashurst I., Perry L., Sanders T.A.B. The role of salt intake and salt sensitivity in the management of hypertension in South Asian people with chronic kidney disease: a randomised controlled trial. Heart. 2013;99:1256–1260. doi: 10.1136/heartjnl-2013-303688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues J.C., Haas M., Reich H.N. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12:677–686. doi: 10.2215/CJN.07420716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H., Hwang J.H., Paik J.H. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol. 2014;15:94. doi: 10.1186/1471-2369-15-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng C.H., Le W., Ni Z. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult chinese patients. Am J Kidney Dis Off J Natl Kidney Found. 2012;60:812–820. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Kang S.H., Choi S.R., Park H.S. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant. 2012;27:252–258. doi: 10.1093/ndt/gfr295. [DOI] [PubMed] [Google Scholar]
- 41.Gowrishankar S., Gupta Y., Vankalakunti M. Correlation of Oxford MEST-C scores with clinical variables for IgA nephropathy in South India. Kidney Int Rep. 2019;4:1485–1490. doi: 10.1016/j.ekir.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander S., Yusuf S., Rajan G. Crescentic glomerulonephritis: what’s different in South Asia? A single center observational cohort study [version 1; peer review: 2 approved] Wellcome Open Res. 2020;5:164. doi: 10.12688/wellcomeopenres.16071.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schimpf J.I., Klein T., Fitzner C. Renal outcomes of STOP-IgAN trial patients in relation to baseline histology (MEST-C scores) BMC Nephrol. 2018;19:328. doi: 10.1186/s12882-018-1128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alamartine E., Sauron C., Laurent B. The use of the Oxford Classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol. 2011;6:2384–2388. doi: 10.2215/CJN.01170211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herzenberg A.M., Fogo A.B., Reich H.N. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80:310–317. doi: 10.1038/ki.2011.126. [DOI] [PubMed] [Google Scholar]
- 46.Coppo R., Troyanov S., Bellur S. Validation of the Oxford Classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delclaux C., Jacquot C., Callard P. Acute reversible renal failure with macroscopic haematuria in IgA nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 1993;8:195–199. [PubMed] [Google Scholar]
- 48.Herlitz L.C., Bomback A.S., Stokes M.B. IgA nephropathy with minimal change disease. Clin J Am Soc Nephrol. 2014;9:1033–1039. doi: 10.2215/CJN.11951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill G.S., Karoui K.E., Karras A. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. I. Immunohistochemical studies. Kidney Int. 2011;79:635–642. doi: 10.1038/ki.2010.466. [DOI] [PubMed] [Google Scholar]
- 50.El Karoui K., Hill G.S., Karras A. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 2011;79:643–654. doi: 10.1038/ki.2010.460. [DOI] [PubMed] [Google Scholar]
- 51.Cook H.T. Focal segmental glomerulosclerosis in IgA nephropathy: a result of primary podocyte injury? Kidney Int. 2011;79:581–583. doi: 10.1038/ki.2010.521. [DOI] [PubMed] [Google Scholar]
- 52.Bellur S.S., Lepeytre F., Vorobyeva O. Evidence from the Oxford Classification cohort supports the clinical value of subclassification of focal segmental glomerulosclerosis in IgA nephropathy. Kidney Int. 2017;91:235–243. doi: 10.1016/j.kint.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran D.C., Coppo R. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 54.Vleming L.J., de Fijter J.W., Westendorp R.G. Histomorphometric correlates of renal failure in IgA nephropathy. Clin Nephrol. 1998;49:337–344. [PubMed] [Google Scholar]
- 55.Reich H.N., Troyanov S., Scholey J.W. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol JASN. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 56.Geddes C.C. A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541–1548. doi: 10.1093/ndt/gfg207. [DOI] [PubMed] [Google Scholar]
- 57.Le W., Liang S., Hu Y. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2012;27:1479–1485. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 58.Koyama A., Igarashi M., Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis. 1997;29:526–532. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.