Abstract
Introduction
Chronic kidney disease (CKD) is associated with impaired muscle strength. Patients with cystinosis have an increased risk for impaired muscle strength because of early development of CKD and cystinosis-induced myopathy. This study assesses muscle strength in patients with cystinosis and investigates risk factors of decreased muscle strength.
Methods
Adult and pediatric patients were recruited from Cystinosis Research Network conferences and a large pediatric nephrology clinic between 2017 and 2019. Patients and caregivers completed questionnaires on demographic characteristics, disease course, daily physical activity, and neuromuscular symptoms. Grip strength was assessed using a dynameter and calculated z-scores for age and sex were assessed for associations with patient characteristics.
Results
We included 76 patients with a mean grip strength z-score of −2.1 (SD, 1.1), which was lower than seen in patients with CKD without cystinosis. Male sex and delayed cysteamine initiation were independently associated with impaired grip strength. Among adults, a low level of physical activity was associated with lower grip strength z score, but no association was found in children. A third of the patients reported neuromuscular symptoms, with swallowing issues associated with lower grip strength. There was no significant correlation between eGFR and grip strength z-score.
Conclusion
Patients with cystinosis have impaired muscle strength compared with healthy control subjects and patients with CKD. This impairment is greater in male patients and in patients with late initiation of cysteamine therapy and is associated with lower physical activity. Further studies investigating the effect of different types of physical activities, optimizing cysteamine therapy, and other interventions are needed.
Keywords: chronic kidney disease, cystinosis, grip strength, muscle strength
Graphical abstract
Cystinosis is a systemic lysosomal storage disease caused by pathogenic variants in CTNS, the gene encoding cystinosin, which is the lysosomal transporter of cystine.1 Although cystinosis affects multiple organs, the clinical focus has long been on the renal manifestations given the early development of Fanconi syndrome and kidney failure. However, progression to end-stage kidney disease can be delayed with cysteamine, which removes cystine from the lysosomes.2 Since the discovery of cysteamine and the improvement in kidney transplantation outcomes, the survival of patients with cystinosis has greatly improved,3 allowing the development of additional systemic manifestations of the disease.4,5 Among these, muscle weakness has emerged as a critically important complication.6, 7, 8 Muscle weakness prevents patients from completing basic tasks of daily living, negatively affecting patients’ quality of life. Moreover, muscle weakness can lead to life-threatening complications, such as respiratory muscle weakness, causing impaired lung function and sleep apnea,9,10 and oral, pharyngeal, and esophageal muscle dysfunction, causing swallowing difficulties and an increased risk of aspiration.11
Chronic kidney disease (CKD) is also associated with impaired muscle mass and strength independent of its etiology.12,13 Many adult studies have investigated the role of CKD complications (e.g., metabolic acidosis and inflammation) and treatments (dialysis) in the loss of muscle mass.14 Among children, a recent study reported a lower grip strength in patients with CKD compared with healthy control subjects independent of growth retardation and body mass index.15 Exposure to CKD at a young age and for a prolonged period of time was a risk factor for impaired muscle strength.
Despite advances in the treatment of cystinosis, patients still develop early Fanconi syndrome and CKD, with half of the patients reaching end-stage kidney disease by 15 years of age.2 Therefore, patients with cystinosis have a high risk of muscle loss and weakness given the combination of CKD and the direct effect of cystine accumulation in the muscle. There are limited data on muscle strength in patients with cystinosis. Sadjadi et al.6 reported the results of muscular evaluation in 20 adults, mostly transplanted patients with cystinosis with clinical symptoms of muscular weakness. However, the contribution of CKD versus cystine accumulation in the development of muscle weakness remains to be investigated.
There is no known specific treatment for muscle weakness in cystinosis, although clinicians have prescribed carnitine and other therapies classically used in patients with mitochondrial disorders.16,17 It is believed that the regular use of cysteamine may prevent or slow the development of muscle weakness.2,18 Sonies et al.11 reported that among patients with cystinosis with swallowing difficulties, the prevalence of confirmed dysfunction (barium swallow test) increased over time without cysteamine treatment, but this association became nonsignificant after adjustment for patients’ age; therefore, data are lacking to support the effect of cysteamine on preventing muscle weakness. In this study, we assess muscle strength using hand grip strength in adults and children with cystinosis and investigate risk factors of decreased muscle strength.
Methods
Study Population and Variables
Adult and pediatric patients with cystinosis were recruited at the Cystinosis Research Network conference held in Salt Lake City, Utah (July 2017) and Philadelphia, PA (July 2019). Additional pediatric patients were recruited at the large pediatric nephrology clinic at Children’s Healthcare of Atlanta. All patients ≥6 years of age with a diagnosis of cystinosis were eligible. Clinical and demographic data were collected via questionnaires from patients and/or caregivers and included date of birth, date of dialysis initiation, date of kidney transplant, race, ethnicity, age of cystinosis diagnosis, age at cysteamine therapy initiation, current preparation and dose of cysteamine, self-reported adherence to cysteamine treatment since diagnosis (defined as <25%, 25%–50%, 50%–75%, and >75%), and current weight, height, and creatinine level. Glomerular filtration rate (GFR) was estimated using the bedside Schwartz formula in children19 and the Chronic Kidney Disease Epidemiology Collaboration equation in adults.20 Current muscular symptoms (limitation on daily activity or physical exercise because of muscle weakness, respiratory muscle weakness, and difficulty swallowing) were collected by self-report. Patients or parents completed a standardized questionnaire describing the patient’s current level of exercise (Physical Activity Questionnaire for Older Children [PAQ-C] in children, PAQ for Adolescents [PAQ-A] in adolescents, and the Rapid Assessment of Physical Activity for both aerobic and strength exercises in adults). Each of the 8 (PAQ-A) or 9 (PAQ-C) questionnaire items is scored between 1 (low) and 5 (high physical activity), and a mean score of all items constitutes the overall PAQ score. The Rapid Assessment of Physical Activity questionnaire includes 7 items on aerobic activity and 2 items of strength and flexibility.21,22
Grip strength was assessed using a Jamar dynamometer (Preston, Jackson, MI). The best of 3 measurements on each hand was recorded for each patient. The sum of the best measurements from both hands (referred to as combined grip strength) was were normalized for sex and age based on norms derived from the National Health and Nutrition Examination Survey, expressed as standard deviation scores (SDSs).23
This study was approved the institutional review boards at Emory University and Children’s Healthcare at Atlanta. Consent was obtained from patients ≥18 years of age or guardians of patients <18 years of age; assent was obtained from all patients <18 years of age.
Statistical Analysis
Data are presented as median and interquartile ranges (IQRs) for continuous variables and counts and percentages for categorical variables.
We compared the distributions of grip strength z-score by CKD stage (defined according to the Kidney Disease: Improving Global Outcomes guidelines24) using a Kruskal-Wallis test. We used univariable and multivariable linear regression models to assess the association between patient characteristics and grip strength z-score. Explanatory variables with a P < 0.05 were included in the multivariable analysis and GFR was added because of potential clinical relevance. Similarly, linear regression models were used to assess the association between patient physical activity level (continuous PAQ score) and grip strength z-score in children and Wilcoxon rank test were used to compare median grip strength z-score by level of physical activity in adults.
The χ2 and Wilcoxon rank tests were used to compare characteristics between patients with and without self-reported neuromuscular symptoms for categorical and continuous variables, respectively.
Missing data are reported in the tables and multiple imputation was used to account for missingness in the multivariate analysis. Statistical analyses were performed using SAS software (version 9.4; SAS Inc., Cary, NC) and P < 0.05 was considered statistically significant.
Results
Study Population
We included 76 patients, including 28 children and 48 adults (Table 1). The mean age was 26.0 years (IQR, 15.0–32.0) and 38 were male (50%). Forty-eight (65%) previously received a kidney transplant and the mean estimated GFR (eGFR) at grip strength assessment was 55 ml/min per 1.73 m2. Only 29 patients (39%) had a history of dialysis and none were currently on dialysis.
Table 1.
Characteristics of patients with cystinosis at time of grip strength measurement
| Characteristics | Median (IQR) or n (%) |
|---|---|
| Age at diagnosis, mo | 17 (12–24) |
| Age at cysteamine initiation, mo | 18 (13–30) |
| Male sex | 38 (50) |
| History of dialysis | 30 (39) |
| History of kidney transplantation | 50 (66) |
| Age at inclusion, yr | 26.0 (15.0–32.0) |
| Pediatric patients (vs. adults) | 28 (37) |
| Type of treatment at inclusion | |
| Immediate release cysteaminea | 25 (33) |
| Delayed release cysteamineb | 50 (66) |
| Self-reported adherence, % | |
| <50 | 2 |
| 50–75 | 7 |
| >75 | 64 |
| eGFR at inclusion, ml/min per 1.73 m2 | 55 (37–74) |
| CKD stage | |
| 1 | 4 (7) |
| 2 | 21 (36) |
| 3A | 12 (21) |
| 3B | 13 (22) |
| 4 | 8 (14) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Missing: age at diagnosis (n = 1), age at cysteamine initiation (n = 3), age at inclusion (n = 1), self-reported adherence (n = 3), and eGFR (n = 18).
Immediate release cysteamine (Cystagon; Mylan Pharmaceuticals, Canonsburg, PA).
Delayed release cysteamine (Procysbi; Horizon Therapeutics, Dublin, Ireland).
Grip Strength Among Patients With Cystinosis
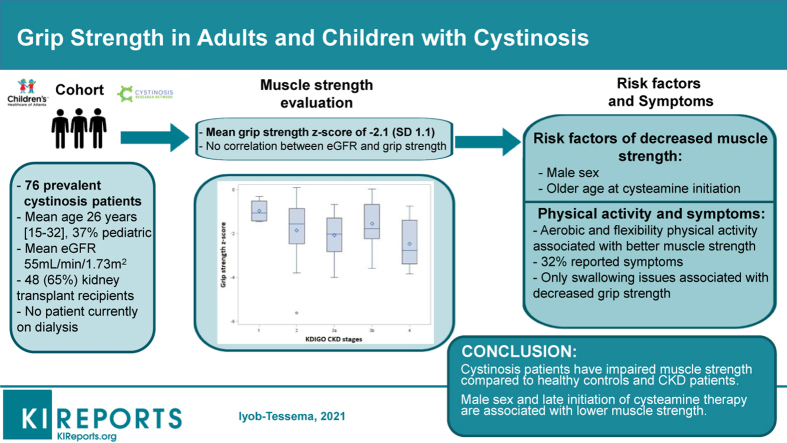
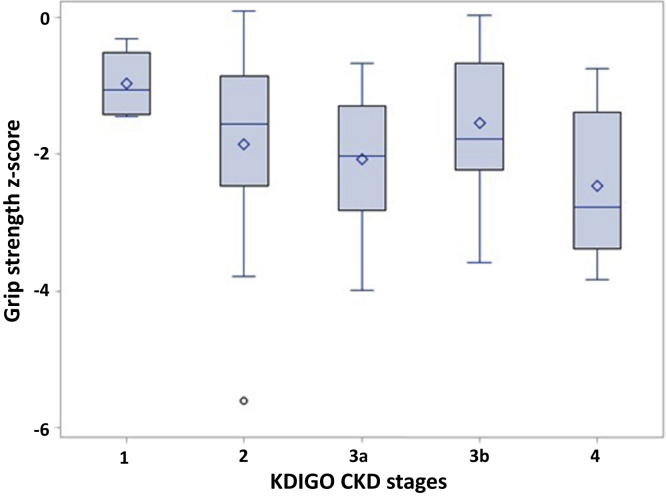
The median best hand grip strength was 26.8 kg (IQR, 20.6–31.6 kg) in adults and 14.2 kg (IQR, 9.7–21.6 kg) in children. The median combined hand grip strength was 51.8 kg (IQR, 38.9–60.3 kg) in adults and 27.7 kg (IQR, 18.3–39.7 kg) in children. Overall, muscle strength was impaired, with a mean grip strength z-score of −2.1 (SD 1.1). The correlation between eGFR and grip strength z-score was weak and not statistically significant (P = 0.56). Figure 1 presents the distribution of grip strength z-score by CKD stages and shows a nonsignificant trend toward a lower grip strength in patients with decreased eGFR (CKD stage 2–4) compared with patients with preserved eGFR (stage 1; P = 0.18).
Figure 1.
Association between chronic kidney disease (CKD) stage and muscle strength among patients with cystinosis. KDIGO, Kidney Disease: Improving Global Outcomes.
Table 2 presents the unadjusted associations between patients’ characteristics and grip strength z-score. Overall, older age at cysteamine treatment initiation, male sex, use of delayed release cysteamine bitartrate treatment at the time of inclusion, and lower self-reported adherence were associated with lower grip strength z-score. After adjustment, only age at cysteamine treatment initiation and male sex remained significantly associated with grip strength z-score (Table 3).
Table 2.
Unadjusted association between patient characteristics and grip strength z-score (bivariate analysis)
| Patient characteristics | Difference in mean grip strength z-score | P value |
|---|---|---|
| Age at cysteamine initiation, months | −0.006 | 0.008 |
| Age at inclusion, yr | −0.002 | 0.86 |
| Male sex | −0.9 | 0.0006 |
| History of dialysis | −0.1 | 0.73 |
| History of kidney transplantation | 0.12 | 0.69 |
| Pediatric patients (vs. adults) | 0.24 | 0.68 |
| Type of treatment at inclusion | 0.03 | |
| Immediate release cysteaminea | Reference | |
| Delayed release cysteamineb | −0.64 | |
| Self-reported adherence, % | 0.006 | |
| ≤75 | Reference | |
| >75 | 1.05 | |
| eGFR at inclusion | 0.004 | 0.56 |
eGFR, estimated glomerular filtration rate.
Immediate release cysteamine (Cystagon; Mylan Pharmaceuticals, Canonsburg, PA).
Delayed release cysteamine (Procysbi; Horizon Therapeutics, Dublin, Ireland).
Table 3.
Adjusted associations between patient characteristics and grip strength z-score (multivariate analysis)
| Patient characteristics | Difference in mean grip strength z-score (95% CI) | P value |
|---|---|---|
| Intercept | −1.68 (–2.80 to −0.56) | 0.003 |
| Age at cysteamine initiation, mo | −0.006 (–0.01 to −0.001) | 0.02 |
| Male sex | −0.70 (−1.19 to −0.21) | 0.005 |
| Type of treatment at inclusion | ||
| Immediate release cysteaminea | Reference | 0.13 |
| Delayed release cysteamineb | −0.38 (−0.88 to 0.11) | |
| Self-reported adherence, % | 0.55 | |
| ≤75 | Reference | |
| >75 | 0.29 (−0.67 to 1.25) | |
| eGFR at inclusion | 0.006 (−0.006 to 0.02) | 0.35 |
CI, confidence interval; eGFR, estimated glomerular filtration rate.
Immediate release cysteamine (Cystagon; Mylan Pharmaceuticals, Canonsburg, PA).
Delayed release cysteamine (Procysbi; Horizon Therapeutics, Dublin, Ireland).
Clinical Manifestation of Impaired Grip Strength in Patients With Cystinosis
Physical activity data were available for 46 (96%) adult and 26 (93%) pediatric patients. Among adults, a lower grip strength z-score was associated with lower level of aerobic physical activity (mean grip strength z-score, −1.60 SD [IQR, −2.24 to −0.83 SD] in active patients vs. −2.31 SD [IQR, −3.65 to −0.03 SD] in underactive patients; P = 0.02). Practicing flexibility activities was associated with a higher grip strength (mean grip strength z-score, −1.22 SD [IQR −2.24 to −0.74 SD] in practicing patients vs. −2.03 SD [IQR, −3.06 to −1.40 SD] in nonpracticing patients; P = 0.02). No difference was found based on the practice of strength activities (P = 0.44). The median PAQ score among young children and adolescents was 2.3 (IQR, 1.8–2.7). There was no statistically significant association between PAQ score and grip strength z-score in children (average increase of 0.18 SD [95% confidence interval, −0.50 to 0.86] in grip strength per additional unit of PAQ score).
Overall, 24 (32.4%) patients in our cohort reported neuromuscular symptoms. Sixteen patients reported limitation in their daily activities; 7 and 6 patients reported respiratory and swallowing issues, respectively. There was no significant difference in patients’ characteristics between symptomatic and asymptomatic patients; however, symptomatic patients tended to have a lower eGFR (Table 4). Swallowing issues were associated with a lower grip strength z-score (mean grip strength z-score, −3.13 SD [IQR, −3.99 to −1.68 SD] in patients with swallowing issues vs. −1.84 SD [IQR, −2.49 to −0.93 SD] in patients without such issues; P = 0.02). There was no significant association between grip strength z-score and reported limitation in daily activities or respiratory issues.
Table 4.
Comparison of patients’ characteristics between patients with and without neuromuscular symptoms
| Patient characteristics | Patients with neuromuscular symptoms, n = 24 | Patients without neuromuscular symptoms, n = 76 | P value |
|---|---|---|---|
| Age at diagnosis, mo, median (IQR) | 16 (12–23) | 18 (12–24) | 0.5 |
| Age at cysteamine initiation, mo, median (IQR) | 18 (12–30) | 18 (13–30) | 0.74 |
| Male sex, n (%) | 13 (54) | 24 (48) | 0.62 |
| History of dialysis, n (%) | 8 (33) | 21 (42) | 0.47 |
| History of kidney transplantation, n (%) | 16 (67) | 32 (64) | 0.82 |
| Age at inclusion, yr, median (IQR) | 25.0 (12.0–33.0) | 26.0 (15.0–32.0) | 0.67 |
| Pediatric patients (vs. adults), n (%) | 10 (42) | 16 (32) | 0.41 |
| Type of treatment at inclusion, n (%) | 0.78 | ||
| Immediate release cysteaminea | 8 (33) | 17 (34) | |
| Delayed release cysteamineb | 16 (67) | 32 (64) | |
| eGFR at inclusion, ml/min per 1.73 m2, median (IQR) | 48 (32–62) | 60 (37–79) | 0.28 |
| CKD stage, n (%) | |||
| 1 | 1 (6) | 3 (8) | 0.12 |
| 2 | 3 (19) | 17 (44) | |
| 3A | 6 (38) | 5 (13) | |
| 3B | 2 (13) | 10 (26) | |
| 4 | 4 (25) | 4 (10) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Immediate release cysteamine (Cystagon; Mylan Pharmaceuticals, Canonsburg, PA).
Delayed release cysteamine (Procysbi; Horizon Therapeutics, Dublin, Ireland).
Discussion
In this study, we showed that both children and adults with cystinosis have impaired muscle strength and that grip strength was only weakly and nonsignificantly associated with eGFR. Male patients and patients with late initiation of cysteamine therapy had a lower grip strength. Conversely, physical activity was associated with a better grip strength in adults, but we were not able to demonstrate a similar association in children.
Along with decreased grip strength when compared with age- and sex-matched healthy control subjects, the comparison of our results with previous reports among patients with CKD suggest that patients with cystinosis have decreased grip strength when compared with noncystinosis patients with CKD. Indeed, grip strength reported in adult patients with CKD stage 3 to 5 by Zhou et al.25 was higher than those found in our adult patients. In children, when comparing patients at the same CKD stage, patients with cystinosis have a grip strength that is >1 SD lower compared with CKD control subjects, who had a mean grip strength z-score of −0.72.15 These results suggest that the direct effect of cystinosis on muscle strength is additive to the effect of CKD.
There was no significant relationship between eGFR and grip strength in patients with cystinosis. This is similar to a previous study investigating the relationship between grip strength and GFR in children with CKD that did not find a significant effect of CKD stages 2 to 5 on grip strength, although it did report a significantly higher grip strength in children with CKD stage 1 compared with all other stages. In the study of children with CKD, risk factors for decreased grip strength included longer duration of CKD, prepubertal status, delayed puberty, neuropsychiatric comorbidities, need for feeding support, need for alkali therapy, and hemoglobin level.15 We speculate that, similar to noncystinosis patients with CKD, the effect of CKD stage is less important compared with other factors that affect grip strength in patients with cystinosis.
The 2 factors associated with poor grip strength in this population were age at cysteamine treatment initiation and male sex. In our study, although statistically significant, the effect of age at treatment initiation was weak with a mean decrease of −0.1 SD per year of delay. This is likely because the great majority of our patients began therapy early. However, our result is consistent with previous studies including patients with delayed treatment initiation and showing a higher incidence of neuromuscular disorders in patients initiating cysteamine therapy after 5 years of age.2 In our study, male patients had greater impairment of grip strength compared with females. This may be related to the hypogonadism observed in approximately 70% of male patients with cystinosis. Indeed, previous studies have shown that the mean testosterone level in male patients with cystinosis was 50% of control patients.26 Therefore, it can be hypothesized that male patients with cystinosis experience an additional burden with respect to muscle strength because of the hypogonadism induced by cystinosis. This finding deserves further exploration and raises the question of the potential benefit of testosterone supplementation in male patients with cystinosis to prevent muscle weakness.
Finally, our study found an association between physical activity and grip strength in adults. Although the design of our study does not allow us to conclude whether a low level of physical activity is a cause or a consequence of impaired muscle strength, it is interesting to note that strength exercises, which are usually thought to be beneficial for increasing or preserving muscle strength, were not associated with better grip strength. However, previous reports demonstrated the ability of resistance training to increase muscle strength. Therefore, the absence of association in our study might suggest that this type of exercise may be recommended to patients with evidence of muscle weakness, creating an indication bias precluding our ability to assess the potential benefit of this type of physical activity. On the contrary, aerobic and flexibility activities were associated with better grip strength and deserve to be investigated in future interventional trials. Indeed, studies in adults with end-stage kidney disease have demonstrated that an exercise training program improves muscle strength,27, 28, 29 but there are currently no data available in children with CKD or any patients with cystinosis. The absence of association between physical activity and grip strength in children may be explained by the smaller sample size for pediatric patients and by the progressive development of muscle weakness that increases with age.
Preserving muscle strength in patients with cystinosis is important because impaired muscle strength is associated with lower quality of life in both pediatric15 and adult patients with CKD.27 Our study demonstrates the high prevalence of neuromuscular symptoms in patients with cystinosis. However, it is important to note that only swallowing difficulties were associated with grip strength. The lack of other associations may be related to the relatively small number of patients and the possibility that other factors may cause problems with activities of daily living or sleep disturbances.
This study is one of the larger studies to date assessing muscle strength and its risk factors in patients with cystinosis, and it provides novel information regarding the increased impairment in male patients and the association with different types of physical activities. However, this study also has some limitations. First, we only focused on grip strength given the known specific impairment of muscle strength in patients with cystinosis; however, a broader assessment of patients’ frailty would be of interest. The retrospective nature of this study limited the granularity of data available, and we cannot rule out the potential effect of unmeasured confounders on the associations reported in this study. Indeed, several potential risk factors are only assessed at the time of grip strength measurement while patients’ cumulative exposure to these factors would be more likely to significantly impact grip strength (e.g., type of cysteamine treatment and self-reported adherence). Moreover, more than half of the patients previously received a kidney transplantation. Therefore, although a history of kidney transplantation was not associated with grip strength measurement, we cannot rule out that the association between eGFR and grip strength was confounded by the presence of transplanted patients in our cohort and by the use of eGFR at the time of evaluation that may not capture the real exposure to CKD. Further studies stratified by transplant status, with longitudinal grip strength measurements coupled with GFR measurements and with data on patients’ physical activities or concurrent treatments are needed to assess the respective impact of cystinosis, CKD and other modifiable risk factors on grip strength loss.
Conclusion
Patients with cystinosis have impaired muscle strength compared with both healthy control subjects and patients with CKD at similar CKD stage without cystinosis. This impairment is greater in male patients and in patients with late initiation of cysteamine therapy and may be improved by physical exercise. Further studies should focus on the benefits of long-term cysteamine treatment, different types of physical activity, and other interventions, such as the use of testosterone in male patients.
Disclosure
All the authors declared no competing interests.
Acknowledgment
We thank the patients and families who participated in the study. We would also like to thank the Cystinosis Research Network (CRN) for their support in allowing us to enroll patients during 2 of their conferences. We would like to thank Christy Greely, CRN Executive Director and CRN Vice President of Research.
References
- 1.Town M., Jean G., Cherqui S. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 2.Brodin-Sartorius A., Tête M.J., Niaudet P. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 3.Van Stralen K.J., Emma F., Jager K.J. Improvement in the renal prognosis in nephropathic cystinosis. Clin J Am Soc Nephrol. 2011;6:2485–2491. doi: 10.2215/CJN.02000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geelen J.M., Monnens L.A.H., Levtchenko E.N. Follow-up and treatment of adults with cystinosis in the Netherlands. Nephrol Dial Transplant. 2002;17:1766–1770. doi: 10.1093/ndt/17.10.1766. [DOI] [PubMed] [Google Scholar]
- 5.Thoene J.G. Introduction to “extra-renal complications of cystinosis. J Pediatr. 2017;183S:S1. doi: 10.1016/j.jpeds.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Sadjadi R., Sullivan S., Grant N. Clinical myopathy in patients with nephropathic cystinosis. Muscle Nerve. 2020;61:74–80. doi: 10.1002/mus.26726. [DOI] [PubMed] [Google Scholar]
- 7.Gahl W.A., Dalakas M.C., Charnas L. Myopathy and cystine storage in muscles in a patient with nephropathic cystinosis. N Engl J Med. 1988;319:1461–1464. doi: 10.1056/NEJM198812013192206. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera-Serrano M., Junckerstorff R.C., Alisheri A. Cystinosis distal myopathy, novel clinical, pathological and genetic features. Neuromuscul Disord. 2017;27:873–878. doi: 10.1016/j.nmd.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Anikster Y., Lacbawan F., Brantly M. Pulmonary dysfunction in adults with nephropathic cystinosis. Chest. 2001;119:394–401. doi: 10.1378/chest.119.2.394. [DOI] [PubMed] [Google Scholar]
- 10.Simon R.H. Pulmonary complications of cystinosis. J Pediatr. 2017;183S:S9–S14. doi: 10.1016/j.jpeds.2016.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Sonies B.C., Almajid P., Kleta R., Bernardini I., Gahl W.A. Swallowing dysfunction in 101 patients with nephropathic cystinosis: benefit of long-term cysteamine therapy. Medicine (Baltimore) 2005;84:137–146. doi: 10.1097/01.md.0000164204.00159.d4. [DOI] [PubMed] [Google Scholar]
- 12.Beddhu S., Pappas L.M., Ramkumar N., Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 13.Souza VA de, Oliveira D., Barbosa S.R. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PloS One. 2017;12 doi: 10.1371/journal.pone.0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X.H., Mitch W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–516. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan J., Schneider M.F., Pai R. Grip strength in children with chronic kidney disease. Pediatr Nephrol. 2020;35:891–899. doi: 10.1007/s00467-019-04461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gahl W.A., Bernardini I., Dalakas M. Oral carnitine therapy in children with cystinosis and renal Fanconi syndrome. J Clin Invest. 1988;81:549–560. doi: 10.1172/JCI113353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahl W.A., Bernardini I.M., Dalakas M.C., Markello T.C., Krasnewich D.M., Charnas L.R. Muscle carnitine repletion by long-term carnitine supplementation in nephropathic cystinosis. Pediatr Res. 1993;34:115–119. doi: 10.1203/00006450-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Gahl W.A., Balog J.Z., Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147:242–250. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz G.J., Muñoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalski K.C., Crocker R.E., Donen R.M. The physical activity questionnaire for older children (PAQ-C) and adolescents (PAQ-A) manual. https://www.researchgate.net/publication/228441462_The_Physical_Activity_Questionnaire_for_Older_Children_PAQ-C_and_Adolescents_PAQ-A_Manual Available at:
- 22.Topolski T.D., LoGerfo J., Patrick D.L., Williams B., Walwick J., Patrick M.B. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention website National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Examination&CycleBeginYear=2013 Available at:
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 25.Zhou Y., Hellberg M., Svensson P., Höglund P., Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2018;33:342–348. doi: 10.1093/ndt/gfw466. [DOI] [PubMed] [Google Scholar]
- 26.Chik C.L., Friedman A., Merriam G.R., Gahl W.A. Pituitary-testicular function in nephropathic cystinosis. Ann Intern Med. 1993;119(7 pt 1):568–575. doi: 10.7326/0003-4819-119-7_part_1-199310010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Cheema B., Abas H., Smith B. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 28.Chen J.L.T., Godfrey S., Ng T.T. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25:1936–1943. doi: 10.1093/ndt/gfp739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen K.L., Painter P.L., Sakkas G.K., Gordon P., Doyle J., Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]