Abstract
Background
The use of rifamycin antibiotics for TB prevention carries a risk of detrimental drug–drug interactions with concomitantly used ART.
Objectives
To evaluate the interaction of the antiretroviral drug nevirapine in combination with 4 weeks of daily rifapentine and isoniazid for TB prevention in people living with HIV.
Methods
Participants were individuals enrolled in the BRIEF-TB study receiving nevirapine and randomized to the rifapentine/isoniazid arm of the study. Participants provided sparse pharmacokinetic (PK) sampling at baseline and weeks 2 and 4 for trough nevirapine determination. Nevirapine apparent oral clearance (CL/F) was estimated and the geometric mean ratio (GMR) of CL/F prior to and during rifapentine/isoniazid was calculated.
Results
Seventy-eight participants had evaluable PK data: 61 (78%) female, 51 (65%) black non-Hispanic and median (range) age of 40 (13–66) years. Median (IQR) nevirapine trough concentrations were: week 0, 7322 (5266–9302) ng/mL; week 2, 5537 (3552–8462) ng/mL; and week 4, 5388 (3516–8243) ng/mL. Sixty out of 78 participants (77%) had nevirapine concentrations ≥3000 ng/mL at both week 2 and 4. Median (IQR) nevirapine CL/F values were: week 0 pre-rifapentine/isoniazid, 2.03 (1.58–2.58) L/h; and during rifapentine/isoniazid, 2.62 (1.81–3.42) L/h. The GMR (90% CI) for nevirapine CL/F was 1.30 (1.26–1.33).
Conclusions
The CL/F of nevirapine significantly increased with concomitant rifapentine/isoniazid. The decrease in nevirapine trough concentrations during rifapentine/isoniazid therapy suggests induction of nevirapine metabolism, consistent with known rifapentine effects. The magnitude of this drug–drug interaction suggests daily rifapentine/isoniazid for TB prevention should not be co-administered with nevirapine-containing ART.
Introduction
TB is the leading cause of death for people living with HIV (PLWH). In 2018 it is estimated that TB was responsible for 251000 deaths in HIV-infected individuals.1 Current WHO guidelines recommend TB prophylaxis therapy in individuals with HIV regardless of viral load or CD4+ cell count. Regimens for TB prophylaxis in PLWH currently utilize 6–9 months of daily isoniazid therapy or, alternatively, involve the use of daily or weekly rifamycin antibiotics with or without isoniazid to decrease treatment durations to as short as 1 month.2 Recent data from the BRIEF-TB trial showed that 1 month of daily rifapentine+isoniazid (1HP) was non-inferior to 9 months of daily isoniazid for TB prevention in PLWH and that treatment completion rates were significantly increased with the ultra-short-course rifapentine-based regimen.3
Nevirapine is an NNRTI used in the treatment of HIV as part of combination ART. While nevirapine use globally has declined substantially, nevirapine continues to hold a presence in specific patient populations, such as neonates and young children, and for the prevention of maternal to child transmission. The use of nevirapine in combination with rifapentine may be problematic as rifapentine induces cytochrome P450 (CYP) and nevirapine is a CYP substrate, leading to concern that co-administration could result in decreased nevirapine exposure and an increased risk of virological failure. Previous data from de Vries-Sluijs et al.4 found an increased risk of virological failure when nevirapine trough concentrations fell below 3000 ng/mL (relative risk = 5.0, 95% CI = 1.8–13.7). To date there are no published data on the effect of daily rifapentine+isoniazid on nevirapine pharmacokinetics (PK). We evaluated the effect of daily 1HP on nevirapine PK in the first 90 participants who enrolled in BRIEF-TB while on a stable nevirapine-containing ART regimen.
Methods
AIDS Clinical Trials Group (ACTG) Study A5279 (BRIEF-TB, NCT 01404312) was a Phase 3 clinical trial (N = 3000) comparing 4 weeks of daily rifapentine+isoniazid (1HP) with 36 weeks of daily isoniazid for the prevention of active TB in PLWH.3 Ninety participants receiving ART containing nevirapine (200 mg by mouth twice daily) randomized to daily 1HP (weight-based rifapentine ∼10 mg/kg, max 600 mg; isoniazid 300 mg) treatment in A5279 were included in the nevirapine pharmacology substudy. HIV-1 RNA was assessed at baseline and either week 4 or 8 of the study. Trough nevirapine plasma samples were collected at week 0 (pre-1HP) and weeks 2 and 4 during concomitant 1HP treatment. Nevirapine concentrations were determined using a validated, quality-controlled, HPLC assay with an analytical range of 25–10000 ng/mL. The nevirapine analytical assay met all criteria for bioanalytical method validation as defined by the US FDA. Nevirapine apparent oral clearance (CL/F) was modelled using maximum a posteriori probability-Bayesian estimation implemented in ADAPT II (Biomedical Simulations Resource at the University of Southern California, Los Angeles, CA, USA).5 Population mean and SD values for nevirapine CL/F used in the Bayesian analysis were 3.86 ± 0.31 L/h and were taken from published literature.6 Week 2 and 4 nevirapine concentrations were both used to estimate nevirapine CL/F while taking 1HP. The geometric mean ratio (GMR) (90% CI) of the during 1HP to pre-1HP nevirapine CL/F values were calculated in SAS statistical software (SAS Institute, Cary, NC, USA). The protocol specified a priori that nevirapine exposure would be judged acceptable if there was evidence that the proportion of participants with nevirapine trough concentrations ≥3000 ng/mL exceeds >80% at both week 2 and 4. Adherence to nevirapine was collected by patient self-report of previous 3 days dosing of nevirapine; dates and times of nevirapine doses were collected on a study-specific case report form.
Results
Demographic and baseline data for the 78 evaluable participants were: female, 61 (78%); black non-Hispanic, 51 (65%); median (range) age, 40 (13–66) years; median (IQR) weight, 57.9 (47.1–66.8) kg; undetectable HIV-1 RNA at entry, 70 (90%); and median (IQR) CD4+ cell count, 548 (91–1233) cells/mm3 (Table 1). All patients reported adherence to nevirapine medication in the 3 days leading up to nevirapine sample collection. Median (IQR) post-dose nevirapine sampling times were: week 0, 13.2 (12.6–13.5) h; week 2, 13.2 (12.4–13.6) h; and week 4, 13.2 (12.2–13.5) h. Median (IQR) nevirapine trough concentrations were: week 0, 7322 (5266–9302) ng/mL; week 2, 5537 (3552–8462) ng/mL; and week 4, 5388 (3516–8243) ng/mL (Figure 1a). The GMR (95% CI) of the week 4 to week 0 nevirapine trough concentrations was 0.71 (0.63–0.77). Median (IQR) nevirapine CL/F values were: week 0 pre-1HP, 2.03 (1.58–2.58) L/h; and during 1HP, 2.62 (1.81–3.42) L/h (Figure 1b). The GMR (90% CI) for nevirapine CL/F was 1.30 (1.26–1.33). The number (%, 90% CI) of participants with nevirapine concentrations ≥3000 ng/mL was: week 0, 78 (100%); week 2, 64 (82%, 73.2%–88.6%); week 4, 63 (81%, 71.7%–87.6%); both week 2 and 4, 60 (77%, 67.6%–84.3%). Seventy-two of the 78 evaluable participants had either a week 4 or 8 HIV viral load available. HIV-1 RNA was <40 copies/mL in 31 of 33 participants (94%) with week 4 viral load data available and 37 of 39 participants (95%) with week 8 viral load data available. Neither of the two participants with HIV-1 RNA >40 copies/mL at week 4 had a measured week 2 or 4 nevirapine concentration that was <3000 ng/mL. Conversely, both of the participants with HIV-1 RNA >40 copies/mL at week 8 had measured week 4 nevirapine concentrations that were <3000 ng/mL. Resistance testing for HIV NNRTI resistance was not performed for these two individuals.
Table 1.
Baseline demographic and clinical characteristics; N = 78
| Age (years), median (range) | 40 (13–66) |
| Female, n (%) | 61 (78) |
| Race/ethnicity, n (%) | |
| black non-Hispanic | 51 (65) |
| Hispanic (regardless of race) | 2 (3) |
| Asian, Pacific Islander | 25 (32) |
| Country, n (%) | |
| South Africa | 3 (4) |
| Botswana | 7 (9) |
| Zimbabwe | 33 (42) |
| Thailand | 25 (32) |
| Kenya | 5 (6) |
| Malawi | 3 (4) |
| Peru | 1 (1) |
| Brazil | 1 (1) |
| HIV-1 RNAa | |
| log10 copies/mL, median (IQR) | 2.18 (1.72–4.06) |
| log10 copies/mL, min and max | 1.68 and 6.46 |
| undetectable (<40 copies/mL), n (%) | 70 (90) |
| CD4+ cell count (cells/mm3), median (IQR) | 548 (91–1233) |
| Nevirapine duration (weeks), median (IQR) | 410 (303–565) |
Entry HIV-1 RNA was available for 78 participants; median, min and max reported for participants with detectable HIV-1 RNA at entry.
Figure 1.
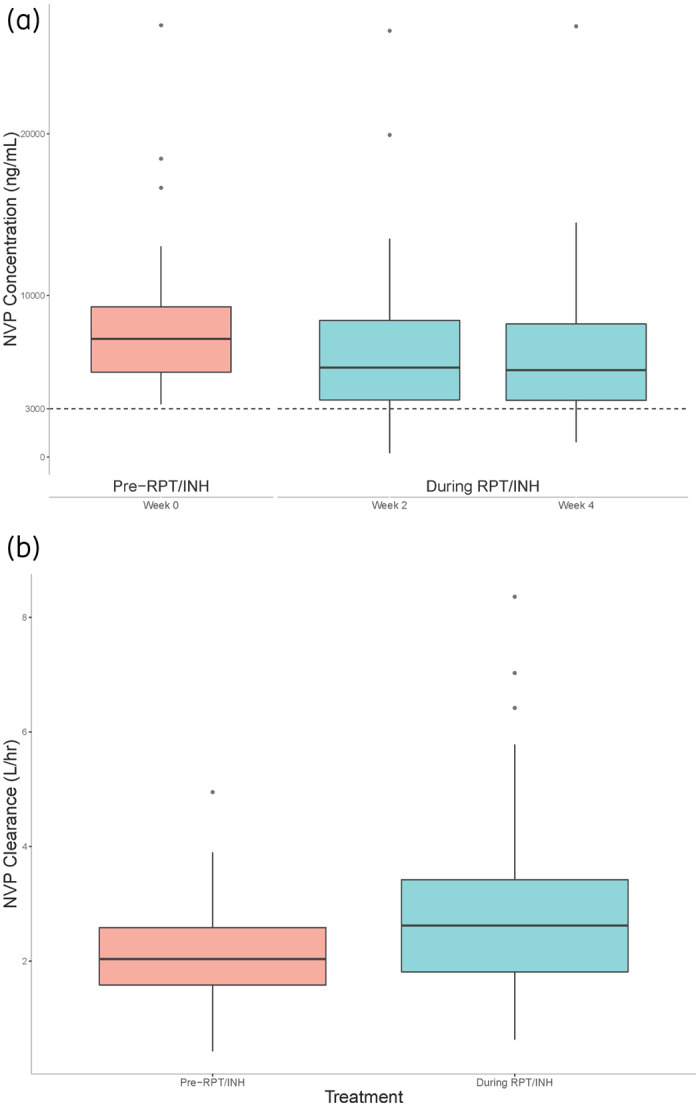
Nevirapine trough concentrations by study week, with line of reference at 3000 ng/mL (a) and nevirapine apparent oral clearance before and during 1HP therapy (b). Boxes represent medians and 25th and 75th percentiles. NVP, nevirapine; RPT, rifapentine; INH, isoniazid. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
The CL/F of nevirapine significantly increased, by a mean of 30%, with concomitant 1HP, as judged by the GMR and 90% CI. Furthermore, the proportion of participants with nevirapine trough concentrations ≥3000 ng/mL at weeks 2 and 4 crossed below the pre-specified threshold of >80%. Collectively, the increased CL/F, decreased percentage of participants with nevirapine trough concentrations ≥3000 ng/mL and decrease in overall nevirapine trough concentrations during 1HP therapy suggests induction of nevirapine clearance, consistent with the known effects of rifapentine.
Nevirapine metabolism is largely hepatically driven via oxidative metabolism through CYPP450 enzymes. CYP3A4 and CYP2B6 are the two major isoenzymes responsible for biotransformation of nevirapine to hydroxylated metabolites. In vitro studies of rifapentine in primary human hepatocytes have suggested rifapentine induces CYP3A4 expression in a dose-dependent manner, although not to the extent of rifampicin on an equimolar basis.7,8 Clinically, studies utilizing midazolam, a prototypical CYP3A4 probe drug, indicated that the ability of rifapentine to induce CYP3A4 may be greater than that of rifampicin. Rifampicin decreased the area under the concentration–time curve (AUC) of midazolam by 75%, whereas rifapentine decreased midazolam AUC by 92%, when rifapentine and rifampicin were each administered at a dose of 10 mg/kg.9
In the present study, nevirapine trough concentrations were reduced approximately 29% from baseline to week 4. One possible explanation for the difference in magnitude of reduction between the Dooley et al.9 study of rifapentine+midazolam and the present study could be the addition of isoniazid. Previous studies of isoniazid have found it is a mechanistic inhibitor of CYP enzymes, such as CYP3A.10 Isoniazid, given concomitantly with rifapentine, therefore might blunt or reduce the magnitude of rifapentine enzyme induction. Pharmacogenomic evaluations from the BRIEF-TB study have shown that slow acetylators, as determined by NAT2 status, have increased isoniazid concentrations in participants taking 1HP, potentially magnifying effects of isoniazid enzyme inhibition.11 Additionally, alternative accessory metabolic pathways, such as CYP2D6, have been reported for nevirapine metabolism. It is possible the moderate reduction we observed in nevirapine concentrations reflects a shift in metabolism to the accessory pathways.12 Finally, it must be noted that nevirapine auto induces its own metabolism primarily via the CYP3A4 pathway.12 The reduction of nevirapine trough concentrations observed in the present study may reflect a cumulative effect of enzyme induction between nevirapine and rifapentine, with an inhibitory effect of isoniazid.
Unfortunately, week 4 viral load data are incomplete for the 72 participants included in the PK study. While there are data on 33 of the participants at week 4, and there was a general trend toward increased viral suppression during the study, it must be noted that only 42% of participants with PK results had week 4 HIV-1 RNA data available. All four of the individuals with either week 4 or 8 HIV-1 RNA >40 copies/mL had a detectable viral load at entry. Three of these four individuals had a decrease in HIV-1 RNA from entry to week 4 or 8, while one of the participants had an increase in viral load from entry to week 8; this participant also had a week 4 nevirapine concentration <3000 ng/mL. Previous studies with larger sample sizes found a 5-fold higher relative risk of virological failure in individuals with steady-state nevirapine concentrations below 3000 ng/mL.4 This increased risk of virological failure formed the basis for the a priori PK threshold in our present study.
Finally, diurnal variation has previously been shown to effect nevirapine PK with a peak amplification in nevirapine CL/F observed at 12 noon.13 In the present study, sample collection for nevirapine PK determination was to occur before the morning dose of nevirapine was taken and thus was approximately 12 h after the evening dose, with a window of 10–14 h deemed acceptable. It is uncertain what effect, if any, diurnal variation may have on nevirapine CL/F in the present study design.
In summary, these combined PK and viral load data suggest 1HP should not be co-administered with nevirapine-containing ART as the risk of the loss of virological suppression is increased when utilizing the two therapies together. Persons currently receiving nevirapine-based ART with the need for TB prophylaxis should either utilize a non-rifamycin-based approach for TB prophylaxis, such as daily isoniazid, or, alternatively, switch to an ART regimen that has been shown to be safe and effective with 1HP-based therapy, such as the use of efavirenz as an anchor drug.3 Previous studies of efavirenz combined with 1HP suggest only minimal changes in efavirenz PK.14 Other alternatives are more modern ART regimens, such as integrase-inhibitor-anchored regimens, which have been shown to be acceptable from a drug–drug interaction standpoint with a 12 week course of once weekly rifapentine+isoniazid (3HP).15 A study of daily dolutegravir-based ART in combination with 1HP is ongoing (NCT 04272242) and will provide evidence as to whether once- or twice-daily dolutegravir is required with 1HP. Lastly, our study included individuals down to 13 years of age, which aligned with the lower age limit in the BRIEF-TB study of the 1HP regimen. Whether or not these findings can be extended to individuals <13 years of age remains to be studied.
Acknowledgements
We thank the BRIEF-TB study participants who agreed to participate in this PK substudy of nevirapine. We thank Sanofi for generous supply of rifapentine and isoniazid for the BRIEF-TB study. We also thank members of the Antiviral Pharmacology Laboratory at the University of Nebraska Medical Center who assisted in the analysis of the nevirapine plasma concentrations. We thank the other members of the AIDS Clinical Trials Group A5279 Team (see below). Finally, we thank Michelle Pham for her assistance with figure production for the manuscript.
Other members of the AIDS Clinical Trials Group A5279 Team
Peter Kim, Daniel Johnson, Laura Moran, Janet Andersen, Yajing Bao, Shirley Wu, Christina Blanchard-Horan, Ann Walawander, Katherine Shin, Ruth Ebiasah, David Holland, Marc Antoine Jean Juste, Eric Nuermberger, Sandy Pillay, Ian Sanne, Janet Nicotera, David Shugarts, Amina Shali, Jimi Tutko, Brigitte Demers, Marilyn Maroni, Jorge L. Sanchez, David Iglesias, Javier Lama, Mitch Matoga, Guilherme do Amaral Calvet, Ronald Kibet Tonui, Taolo Modise, Margaret Kasaro, Kogieleum Naidoo, Deelip Kadam and William Burman.
Funding
This work was supported by grants 1K23-AI134307 (to A.T.P.) and UM1-AI068634, UM1-AI106701 and UM1-AI068636 from the National Institute of Allergy and Infectious Diseases.
Transparency declarations
R.E.C. reports an honorarium from Sanofi. S.S. reports research grants to her institution from ViiV Healthcare. All other authors: none to declare.
Author contributions
Wrote original manuscript: A.T.P., S.S. and C.V.F. Designed research: A.T.P., A.G., C.A.B., R.E.C., S.S. and C.V.F. Performed research: A.T.P., J.L.-C., J.H., K.S., A.O.-O., D.L., N.W., C.K., A.G., C.A.B., R.E.C., S.S. and C.V.F. Analysed data: A.T.P., J.L.-C., A.G., C.A.B., R.E.C., S.S. and C.V.F. Contributed clinical site study conduct: J.H., K.S., A.O.-O., D.L., N.W. and C.K.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Contributor Information
the AIDS Clinical Trials Group A5279 Team:
Peter Kim, Daniel Johnson, Laura Moran, Janet Andersen, Yajing Bao, Shirley Wu, Christina Blanchard-Horan, Ann Walawander, Katherine Shin, Ruth Ebiasah, David Holland, Marc Antoine JeanJuste, Eric Nuermberger, Sandy Pillay, Ian Sanne, Janet Nicotera, David Shugarts, Amina Shali, Jimi Tutko, Brigitte Demers, Marilyn Maroni, Jorge L Sanchez, David Iglesias, Javier Lama, Mitch Matoga, Guilherme do Amaral Calvet, Ronald Kibet Tonui, Taolo Modise, Margaret Kasaro, Kogieleum Naidoo, Deelip Kadam, and William Burman
References
- 1. Glaziou P, Dodd PJ, Dean A. et al. Methods Used by WHO to Estimate the Global Burden of TB Disease. WHO, 2019. [Google Scholar]
- 2. Rapid Communication on Forthcoming Changes to the Programmatic Management of Tuberculosis Preventive Treatment. WHO/UCN/TB/2020.4. WHO, 2020. [Google Scholar]
- 3. Swindells S, Ramchandani R, Gupta A. et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380: 1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Vries-Sluijs TE, Dieleman JP, Arts D. et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet 2003; 42: 599–605. [DOI] [PubMed] [Google Scholar]
- 5. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource, 2009. [Google Scholar]
- 6. Zhou X-J, Sheiner LB, Richard T. et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 1999; 43: 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson B, Dooley KE, Zhang Y. et al. Induction of influx and efflux transporters and cytochrome P450 3A4 in primary human hepatocytes by rifampin, rifabutin, and rifapentine. Antimicrob Agents Chemother 2013; 57: 6366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dyavar SR, Mykris TM, Winchester LC. et al. Hepatocytic transcriptional signatures predict comparative drug interaction potential of rifamycin antibiotics. Sci Rep 2020; 10: 12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dooley KE, Bliven‐Sizemore E, Weiner M. et al. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther 2012; 91: 881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desta Z, Soukhova NV, Flockhart DA.. Inhibition of cytochrome P450 (CYP450) isoforms by isoniazid: potent inhibition of CYP2C19 and CYP3A. Antimicrob Agents Chemother 2001; 45: 382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas DW, Podany AT, Bao Y. et al. Pharmacogenetic interactions of rifapentine plus isoniazid with efavirenz or nevirapine. Pharmacogenet Genomics 2020; doi:10.1097/FPC.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riska P, Lamson M, MacGregor T. et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos 1999; 27: 895–901. [PubMed] [Google Scholar]
- 13. Bienczak A, Cook A, Wiesner L. et al. Effect of diurnal variation, CYP2B6 genotype and age on the pharmacokinetics of nevirapine in African children. J Antimicrob Chemother 2017; 72: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Podany AT, Bao Y, Swindells S. et al. Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving rifapentine and isoniazid for tuberculosis prevention. Clin Infect Dis 2015; 61: 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dooley KE, Savic R, Gupte A. et al. Once-weekly rifapentine and isoniazid for tuberculosis prevention in patients with HIV taking dolutegravir-based antiretroviral therapy: a phase 1/2 trial. Lancet HIV 2020; 7: E401–9. [DOI] [PubMed] [Google Scholar]


