Abstract
Objectives
To provide a basis for clinical management decisions in Paecilomyces variotii infection.
Methods
Unpublished cases of invasive P. variotii infection from the FungiScope® registry and all cases reported in the literature were analysed.
Results
We identified 59 cases with P. variotii infection. Main baseline factors were presence of indwelling devices in 29 cases (49.2%), particularly peritoneal catheters (33.9%) and prosthetic heart valves (10.2%), haematological or oncological diseases in 19 (32.2%), major surgery in 11 (18.6%), and diabetes mellitus in 10 cases (16.9%). The most prevalent infection sites were peritoneum (n = 20, 33.3%) and lungs (n = 16, 27.1%). Pain and fever were frequent (n = 35, 59.3% and n = 33, 55.9%, respectively). Diagnosis was established by culture in 58 cases (98.3%). P. variotii caused breakthrough infection in 8 patients. Systemic antifungals were given in 52 patients (88.1%). Amphotericin B was administered in 39, itraconazole in 15, and posaconazole in 8 patients. Clinical isolates were frequently resistant to voriconazole, whereas the above-mentioned antifungals showed good in vitro activity. Infections of the blood and CNS caused high mortality. Overall mortality was 28.8% and death was attributed to P. variotii in 10 cases.
Conclusions
P. variotii causes life-threatening infections, especially in immunocompromised and critically ill patients with indwelling devices. Patients undergoing peritoneal dialysis are at particular risk. Multidisciplinary management is paramount, including molecular techniques for diagnosis and treatment with efficacious systemic antifungals. Amphotericin B, itraconazole and posaconazole are regarded as treatments of choice. Combination with flucytosine may be considered. Surgical debridement and removal of indwelling devices facilitate favourable outcome.
Introduction
Paecilomyces spp. are filamentous and thermo-tolerant fungi that are ubiquitously found in soil, decomposing organic material, food products and house dust.1,2 They were often considered as contaminants when isolated clinically, but are recently increasingly recognized to cause invasive infections, primarily in immunocompromised patients or those with indwelling material.3 However, immunocompetent individuals can also be affected.3,4
The majority of these infections is caused by the Paecilomyces variotii species complex, comprising P. brunneolus, P. dactylethromorphus, P. divaricatus, P. formosus, and P. variotii sensu stricto.5P. variotii is the asexual state of Byssochlamys spectabilis.1
Until 2011, P. variotii and P. lilacinus were considered the most prevalent agents of human infection in the Paecilomyces genus.4 However, in the last decade, phylogenetic analysis of the 18S ribosomal RNA gene revealed that these species are not related.6Paecilomyces spp. belong to the Family Aspergillaceae (Order Eurotiales), close to Aspergillus, Penicillium and related genera, whereas P. lilacinus has been transferred to the new Family Ophiocordycipitaceae (Order Hypocreales).6 For P. lilacinus, a new genus name, Purpureocillium, has been proposed.5,6 Available in vitro susceptibility data suggest major differences in species-dependent MIC ranges, with voriconazole demonstrating generally good in vitro activity and amphotericin B having poor activity against P. lilacinum.1,7,8 making prompt and accurate species identification vital.
P. variotii can affect various organ systems and has been reported to cause bloodstream infections, CNS infections, osteomyelitis, peritonitis, pneumonia, skin and soft-tissue infections, among others.1,3,8–14 In addition, P. variotii has been associated with severe, often vision-threatening, endophthalmitis.15 Symptoms of infection appear to be non-specific and may be difficult to distinguish from other fungal infections.3 Infections with P. variotii are generally rare.14 However, larger numbers of cases were observed during outbreaks, e.g. after earthquakes.9
Hence, there are several challenges in diagnosing and treating these emerging infections. Owing to the low number of reported cases and the lack of clinical trials, the optimal strategy for disease management has not yet been defined. Therefore, we have conducted a combined analysis of cases registered in FungiScope® and cases reported in the literature, in order to identify baseline factors, establish demographic knowledge and provide a basis for diagnostic and therapeutic decisions for Paecilomyces-associated infections.
Methods
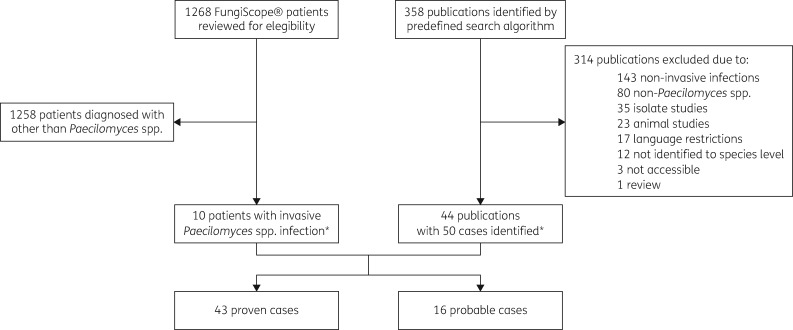
FungiScope® (www.fungiscope.net) is an international web-based registry for rare and emerging invasive fungal infections (IFI) (www.clinicaltrials.gov, NCT 01731353). The methodology has been described elsewhere.16 FungiScope® is approved by the Institutional Review Board and Ethics Committee of the University Hospital Cologne, Germany (Study ID: 05–102). A dataset of Paecilomyces spp. patient cases was extracted from the registry and included in the analysis (Figure 1).
Figure 1.
Enrolment and study flow chart. *One case was reported both in FungiScope® and the literature.
Additionally, a literature search was performed in PubMed® and Web of Science (Clarivate Analytics, USA) for all reported cases of invasive Paecilomyces infections from database inception to 31 July 2020. The predefined search filters ‘(Paecilomyces*) AND ((invasive OR disseminated OR infection) AND (case OR patient OR report))’ yielded 358 results. Publications in English, French, German, Spanish, and Turkish were selected based on title and abstract for further evaluation. Reference lists of articles were screened for other suitable studies and authors were contacted to obtain additional data. Cases with colonization, superficial infections such as keratitis, microbiological studies on isolates and non-human infections were excluded (Figure 1). Small case-series were included to allow complete data reporting.
We excluded cases of P. lilacinus infection, as this fungus was recently transferred to the new genus Purpureocillium.6 For the same reason, we excluded cases of Paecilomyces spp. with identification only to the genus level. Additionally, two cases caused by Metarhizium marquandii (formerly Paecilomyces marquandii) and Cordyceps javanica (formerly Paecilomyces javanicus) were excluded.
Every report was reviewed for patient demographics, underlying conditions and immunosuppression as predisposing factors for IFI, clinical signs and symptoms at diagnosis, site of infection and diagnostic and therapeutic procedures. If available, radiological results suggesting IFI, mycological evidence, susceptibility testing and MIC, antifungals used for prophylaxis and treatment as well as surgical treatment of IFI were documented. Mortality on day 42 and day 90 after diagnosis and mortality attributed to the infection were documented. Follow-up period was defined from day of diagnosis to last patient contact.
Proven or probable IFI were included following the revised 2019 EORTC/MSG criteria.17 Dissemination was defined as either infection at two or more non-contiguous body sites or bloodstream infection with at least one positive blood culture. Breakthrough IFI (BT-IFI) was defined as Paecilomyces infection occurring during exposure to any antifungal agent.18
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) (version 25.0, IBM, USA). Patient characteristics from categorical variables were summarized employing frequencies and percentages, while median and IQR were used for continuous variables. Categorical data were compared using Fisher’s exact test. A P value ≤0.05 was set as statistically significant.
Results
Fifty-nine cases of Paecilomyces infection reported in the FungiScope® registry (n = 9, 15.3%) and the literature (n = 49, 83.1%) were included in our study (Table S1, available as Supplementary data at JAC Online). One case was both published and registered in FungiScope®.19
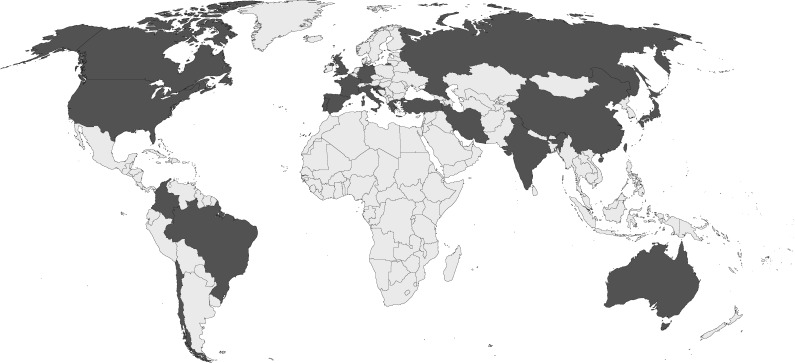
Cases were reported from 22 countries worldwide with the highest number of cases from the United States (n = 15, 25.4%), five cases each (8.5%) from Australia and India, and four each (6.8%) from China and Turkey (Figure 2). Cases were diagnosed between 1967 and 2020. All infections were caused by P. variotii. Coinfections with other fungal pathogens were present in two cases (3.4%) (Table 1). Additional identification of bacterial pathogens was reported in eight cases (13.6%), of which two were detected at other body sites than P. variotii (Table S2), and P. variotii was considered the main causative agent in all cases.
Figure 2.
Countries where Paecilomyces variotii infections have been reported. Fifteen cases were reported from the United States, five cases each from Australia and India, four each from China and Turkey, three each from Chile and Spain, two each from Canada, France, Germany, Greece and Russia, and one each from Brazil, Colombia, Croatia, Iran, Italy, Japan, Portugal, Slovenia, Taiwan and the United Kingdom.
Table 1.
Patient characteristics
| Characteristic | Total (N = 59) |
Deaths in the respective cohort (N, %) |
Mortality (N = 59) |
||
|---|---|---|---|---|---|
| EORTC | |||||
| Proven | 43 | 72.9% | 11 | 25.6% | 18.6% |
| Probable | 16 | 27.1% | 6 | 37.5% | 10.2% |
| Age, years, median (IQR) | 52 (25–61) | ||||
| Sex | |||||
| Female | 26 | 44.1% | 9 | 34.6% | 15.3% |
| Mixed infection | |||||
| Aspergillus fumigatus and A. niger, lung | 1 | 1.7% | 1 | 100.0% | 1.7% |
| Aspergillus spp., lung | 1 | 1.7% | 0 | – | – |
| Underlying conditiona | |||||
| Haematological/oncological diseaseb | 19 | 32.2% | 7 | 36.8% | 11.9% |
| Acute leukaemia | 5 | 8.5% | 2 | 40.0% | 3.4% |
| Breast cancer | 2 | 3.4% | 2 | 100.0% | 3.4% |
| Chronic granulomatous disease | 4 | 6.8% | 0 | – | – |
| Non-Hodgkin lymphoma | 3 | 5.1% | 1 | 33.3% | 1.7% |
| Allogeneic HSCTc | 7 | 11.9% | 3 | 42.9% | 5.1% |
| Autologous HSCTc | 3 | 5.1% | 2 | 66.7% | 3.4% |
| GVHD | 2 | 3.4% | 2 | 100.0% | 3.4% |
| Neutropenia | 9 | 15.3% | 4 | 44.4% | 6.8% |
| Major surgery | 11 | 18.6% | 4 | 36.4% | 6.8% |
| Trauma | 1 | 1.7% | 0 | – | – |
| SOTd | 3 | 5.1% | 1 | 33.3% | 1.7% |
| Diabetes mellitus | 10 | 16.9% | 4 | 40.0% | 6.8% |
| Steroid treatment | 5 | 8.5% | 3 | 60.0% | 5.1% |
| Chronic lung disease | 4 | 6.8% | 4 | 100.0% | 6.8% |
| Chronic renal disease (peritoneal dialysis) | 19 | 32.2% | 3 | 15.8% | 5.1% |
| Indwelling devicesa,e | |||||
| Central venous catheter | 2 | 3.4% | 0 | – | – |
| Peritoneal catheter | 20 | 33.9% | 3 | 15.0% | 5.1% |
| Prosthetic valve | 6 | 10.2% | 4 | 66.7% | 6.8% |
| Other baseline factorsf | 3 | 5.1% | 1 | 33.3% | 1.7% |
| No baseline factor identified | 2 | 3.4% | 0 | – | – |
| Organ involvementa | |||||
| Blood | 6 | 10.2% | 3 | 50.0% | 5.1% |
| Bone and joints | 5 | 8.5% | 0 | – | – |
| CNS | 3 | 5.1% | 3 | 100.0% | 5.1% |
| Deep tissue | 7 | 11.9% | 2 | 28.6% | 3.4% |
| Heart | 6 | 10.2% | 4 | 66.7% | 6.8% |
| Kidney | 5 | 8.5% | 2 | 40.0% | 3.4% |
| Liver | 2 | 3.4% | 2 | 100.0% | 3.4% |
| Lung | 16 | 27.1% | 6 | 37.5% | 10.2% |
| Peritoneum | 20 | 33.9% | 3 | 15.0% | 5.1% |
| Sinuses | 3 | 5.1% | 1 | 33.3% | 1.7% |
| Skin | 3 | 5.1% | 1 | 33.3% | 1.7% |
| Spleen | 4 | 6.8% | 3 | 75.0% | 5.1% |
| Vessels | 2 | 3.4% | 2 | 100.0% | 3.4% |
| Other sitesg | 3 | 5.1% | 2 | 66.6% | 3.4% |
| Dissemination | |||||
| Adjacent organs | 5 | 8.5% | 1 | 20.0% | 1.7% |
| Disseminated | 14 | 23.7% | 5 | 35.7% | 8.5% |
| Not disseminated | 39 | 66.1% | 9 | 23.1% | 15.3% |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; GVHD, graft-versus-host disease; HSCT, haematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; SOT, solid organ transplantation.
Data may be superadditive.
Each one case reported with β-thalassaemia major, chronic leukaemia, hairy cell leukaemia, myelodysplastic syndrome, multiple myeloma.
One case received both autologous and allogenic transplantation.
Lung (n = 2) and liver (n = 1) transplant.
Each one ureteral stent and ventriculoperitoneal shunt.
Other baseline factors include lung resection, multiple episodes of lung and liver abscesses of unknown aetiology, malnutrition (n = 1, each).
Other sites include intestine, ear and pancreas (n = 1, each).
Predisposing factors
The most frequent predisposing factor was presence of indwelling devices (n = 29/59, 49.2%), with 33.9% being peritoneal catheters (n = 20/59), and 10.2% prosthetic heart valve infections (n = 6/59). Haematological and oncological diseases (n = 19/59, 32.2%) were the second most prevalent baseline factor. Major surgery and diabetes mellitus were also frequent with 11 (18.6%) and 10 cases (16.9%), respectively. Only two patients (3.4%) lacked factors predisposing for IFI (Table 1).
Site of infection
The peritoneum was the most prevalent site of infection (n = 20/59, 33.3%), followed by the lungs (n = 16/59, 27.1%). Other frequent sites of infection were deep soft-tissue (n = 7/59, 11.9%), bloodstream (n = 6/59, 10.2%), and heart valve (n = 6/59, 10.2). Disseminated disease occurred in 14 patients (23.7%), adjacent organs were affected in 5 patients (8.5%) (Table 1, Table S2).
Signs and symptoms
Fever at the time of diagnosis of the IFI was frequently reported (n = 33/59, 55.9%). Clinical symptoms were mostly associated with the anatomic region involved, such as pain at the site of infection (n = 35/59, 59.3%), dyspnoea (n = 14/59, 23.7%), gastrointestinal symptoms (n = 11/59, 18.6%), and cough (n = 10/59, 16.9%) (Table 2, Table S3).
Table 2.
Diagnostic procedures and susceptibility testing
| Characteristic | N | Percentage of total |
|---|---|---|
| Signs and symptoms of infectiona | ||
| Bleeding | 2 | 3.4% |
| Cough | 10 | 16.9% |
| Dyspnoea | 14 | 23.7% |
| Erythema | 4 | 6.8% |
| Fever | 33 | 55.9% |
| Gastrointestinal symptoms | 11 | 18.6% |
| Haemoptysis | 2 | 3.4% |
| Neurological signs | 8 | 13.6% |
| Pain | 35 | 59.3% |
| Tachypnoea | 2 | 3.4% |
| Other signs and symptomsb | 14 | 23.7% |
| Imaging proceduresa | ||
| CT head | 1 | 1.7% |
| CT paranasal sinuses | 4 | 6.8% |
| CT thorax | 10 | 16.9% |
| MRI head | 1 | 1.7% |
| Ultrasound heart | 4 | 6.8% |
| X-ray thorax | 5 | 8.5% |
| Mycological evidencea | ||
| Culture | 58 | 98.3% |
| Histology | 5 | 8.5% |
| Microscopy | 4 | 6.8% |
| Susceptibility testing | ||
| CLSI microdilution | 4 | 6.8% |
| Etest | 2 | 3.4% |
| EUCAST microdilution | 3 | 5.1% |
| Macrodilution method | 1 | 1.7% |
| Unknown | 11 | 16.9% |
| MIC (mg/L) by CLSI microdilution, median (IQR) | ||
| Amphotericin B | 1.5 (0.6-2.0) | |
| Caspofungin | 1.0 (1.0-8.0) | |
| Fluconazole | 10.0 (4.0-72.0) | |
| Flucytosine | 0.5 (0.5-0.5) | |
| Itraconazole | 0.8 (0.3-1.5) | |
| Ketoconazole | 2.0 (2.0-2.0) | |
| Miconazole | 32.0 (32.0-32.0) | |
| Posaconazole | 0.1 (0.1-0.1) | |
| Terbinafine | 1.0 (1.0-1.0) | |
| Voriconazole | 1.0 (1.0-1.0) | |
| MIC (mg/L) by concentration gradient diffusion assay, median (IQR) | ||
| Amphotericin B | 0.02 (0.01-0.03) | |
| Caspofungin | 8.5 (1.0-16.0) | |
| Itraconazole | 0.4 (0.1-0.6) | |
| Posaconazole | 0.03 (0.03-0.03) | |
| Voriconazole | 4.8 (1.5-8.0) | |
| MIC (mg/L) by EUCAST microdilution, median (IQR) | ||
| Amphotericin B | 0.1 (0.03-0.5) | |
| Anidulafungin | 0.001 (0.001-0.001) | |
| Caspofungin | 1.1 (0.1-2.0) | |
| Flucytosine | 0.1 (0.1-0.1) | |
| Itraconazole | 0.05 (0.03-0.1) | |
| Posaconazole | 0.03 (0.03-0.03) | |
| Voriconazole | 1.0 (0.3-8.0) | |
MRI, magnetic resonance imaging.
Data may be superadditive.
Other signs and symptoms included haematuria (n = 3), malaise (n = 3), nasal congestion (n = 1), otorrhoea (n = 1), rhinorrhoea (n = 1), signs of heart failure (n = 2), and weight loss (n = 3).
Diagnostics
Imaging procedures supported diagnosis in 25 cases (42.4%). Chest CT (n = 10/59, 16.9%) and chest radiograph (n = 5/59, 8.5%) were predominantly performed, followed by paranasal sinus CT and echocardiography (n = 4/59, 6.8%, each). In chest CT, nodular infiltrates and ground-glass opacifications were common findings. Definitive diagnosis was established via fungal culture in all cases except one, which had an unreported diagnostic procedure (98.3%)(Table 2, Table S4).
Antifungal susceptibility
In vitro antifungal susceptibility was evaluated for 20 clinical isolates by different methods (Table 2). In 11 isolates, the method was not reported.
Amphotericin B demonstrated generally good in vitro activity in susceptibility testing against P. variotii, however, variable results were obtained by CLSI microdilution method (Table 2). Itraconazole and posaconazole showed good in vitro activity by any of the methods used. The same was observed for flucytosine. High MICs were shown for caspofungin and voriconazole, particularly when evaluated with Etest and EUCAST microdilution method. No susceptibility testing was performed for isavuconazole.
Treatment and outcome
Nine patients (15.3%) had received antifungal prophylaxis prior to the diagnosis of invasive Paecilomyces infection, with eight patients developing BT-IFI (13.6%). One case did not fulfil the pharmacokinetic parameters classifying BT-IFI.18 Prophylaxis was administered due to underlying haematological disease or after lung solid organ transplantation. In the majority of patients (n = 52/59, 88.1%), systemic antifungal therapy was administered, mainly with single (n = 23/52, 44.2%) or sequential monotherapy (n = 18/52, 34.6%). Antifungal combination therapies have been described in individual cases (n = 11/52, 21.2%) (Table 3) and with lower mortality rates than with monotherapy (n = 1/11 versus n = 12/41, P = 0.253, Tables S5 and S6).
Table 3.
Antifungal treatment and outcome
| Deaths |
|||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | Proportion of respective cohort | Proportion all cases (n = 59) |
| Prophylactic agent | 9 | 15.3% | 3 | 33.3% | 5.1% |
| Amphotericin B | 5 | 8.5% | 2 | 40.0% | 3.4% |
| Caspofungin | 1 | 1.7% | 1 | 100.0% | 1.7% |
| Fluconazole | 1 | 1.7% | 0 | – | – |
| Itraconazole | 2 | 3.4% | 0 | – | – |
| Voriconazole | 1 | 1.7% | 0 | – | – |
| Breakthrough IFI | 8 | 13.6% | 3 | 37.5% | 5.1% |
| Systemic antifungal therapya | 52 | 88.1% | 12 | 23.1% | 20.3% |
| Amphotericin B | 39 | 66.1% | 11 | 28.2% | 18.6% |
| Triazoles | 31 | 52.5% | 7 | 22.6% | 11.9% |
| Fluconazole | 7 | 11.9% | 1 | 14.3% | 1.7% |
| Itraconazole | 15 | 25.4% | 2 | 13.3% | 3.4% |
| Posaconazole | 8 | 13.6% | 4 | 50.0% | 6.8% |
| Voriconazole | 6 | 10.2% | 1 | 16.7% | 1.7% |
| Echinocandins | 5 | 8.5% | 0 | – | – |
| Anidulafungin | 1 | 1.7% | 0 | – | – |
| Caspofungin | 4 | 6.8% | 0 | – | – |
| Other antifungal agent | 8 | 13.6% | 1 | 12.5% | 1.7% |
| Flucytosine | 3 | 5.1% | 0 | – | – |
| Ketoconazole | 5 | 8.5% | 1 | 20.0% | 1.7% |
| Treatment days, median (IQR) | 35 (28-91) | ||||
| Non-systemic therapy | 2 | 3.4% | 1 | 50.0% | 1.7% |
| Local amphotericin B | 1 | 1.7% | 1 | 100.0% | 1.7% |
| Local fluconazole | 1 | 1.7% | 0 | – | – |
| G-CSF | 3 | 5.1% | 1 | 33.3% | 1.7% |
| Granulocyte infusion | 1 | 1.7% | 0 | – | – |
| Treatment sequence | |||||
| Combination single | 3 | 5.1% | 0 | – | – |
| Combination sequential | 2 | 3.4% | 0 | – | – |
| Monotherapy + combination | 6 | 10.2% | 1 | 16.7% | 1.7% |
| Monotherapy single | 23 | 39.0% | 5 | 21.7% | 8.5% |
| Monotherapy sequential | 18 | 30.5% | 6 | 33.3% | 10.2% |
| No treatment | 5 | 8.5% | 4 | 80.0% | 6.8% |
| Combinations | |||||
| Amphotericin B plus azole | 7 | 11.9% | 1 | 14.3% | 1.7% |
| Amphotericin B plus echinocandin | 3 | 5.1% | 0 | – | – |
| Azoles plus other antifungal agent | 3 | 5.1% | 0 | – | – |
| Antifungal surgery | 15 | 25.4% | 3 | 20.0% | 5.1% |
| Removal of indwelling devices | |||||
| Catheter removal | 21 | 35.6% | 3 | 14.3% | 5.1% |
| Prosthesis replacement | 4 | 6.8% | 2 | 50.0% | 3.4% |
| Removal Double J stent | 1 | 1.7% | 0 | – | – |
| Ventriculoperitoneal shunt removal | 1 | 1.7% | 1 | 100.0% | 1.7% |
| Overall mortality | |||||
| Deaths | 17 | 28.8% | |||
| Unknown | 1 | 1.7% | |||
| Death attributed to IFI | 10 | 58.8% | 16.9% | ||
| Autopsy | 5 | 29.4% | 8.5% | ||
| Death before or on day 42 | 14 | 82.4% | 23.7% | ||
| Death before or on day 90 | 16 | 94.1% | 27.1% | ||
| Date of death unknown | 1 | 5.9% | 1.7% | ||
| Observation time (days), median (IQR) | 58 (19–180) | ||||
G-CSF, granulocyte-colony stimulating factor; IFI, invasive fungal infection.
Data may be superadditive.
Amphotericin B was most frequently used (n = 39/52, 75.0%). Mortality in this group was 28.2%. Azoles were given in 59.6% of cases (n = 31/52) with a 22.6% mortality rate. Echinocandins were used in five cases (9.6%) with no death reported. Flucytosine was combined with fluconazole or itraconazole in three cases (5.8%) with favourable outcome (Table 3).
The systemic administration of a specific antifungal class was not associated with a significant decrease in mortality (amphotericin B, P = 0.475; triazoles, P = 1.000; echinocandins, P = 0.314) (Table S6).
Median duration of systemic antifungal therapy was 35 days (IQR 18–91). Surgical interventions for IFI were performed in 15 patients (25.4%) with good treatment response reported in most (n = 12/15, 80.0%). Removal of vascular or intraperitoneal catheters was performed in all but one patient (n = 21/22, 95.5%; Table 3). In patients where removal of indwelling devices was reported the mortality rate was lower compared with patients with preserved devices (n = 4/25 and n = 2/3, respectively; P = 0.107; Table S6).
All-cause mortality was 28.8% (n = 17/59). One patient was lost to follow-up with unknown outcome. In BT-IFI, the mortality rate did not significantly differ from non-BT-IFI cases (P = 0.690; Table S6). In 10 cases, death was attributed to IFI (58.8%; Table 3).
No deaths were seen in patients with bone or joint infection (n = 0/5). Peritoneal infection also had a comparably low mortality rate of 15.0% (n = 3/20), while CNS and bloodstream infections/endocarditis had a remarkably high mortality (n = 3/3 and n = 7/12, respectively). Mortality in disseminated disease was higher than in cases with single organ involvement or adjacent organs (P = 0.161) (Table 3; Table S6).
Death occurred within 42 days after diagnosis in 13 of 17 cases (76.5%) and within 90 days in 15 cases (88.2%). Autopsy was performed on five patients (29.4%). Median duration from diagnosis to last follow-up day was 58 days (IQR 19–180) (Table 3).
Discussion
Previously considered a contaminant, P. variotii has been identified as the cause of an increasing number of infections in both immunocompromised and immunocompetent hosts. Here, we present the largest analysis addressing management and outcome of invasive P. variotii infections to date by identifying 59 cases in FungiScope® and the literature.
Symptoms of P. variotii infection are often non-specific.3 A multidisciplinary approach seems indispensable to diagnose this infection in at-risk patients. Our analysis shows that imaging techniques have mainly been used to detect pulmonary infections, and to a lesser extent for detection of heart and sinus involvement. Clinical isolation by direct specimen sampling from the affected sites represents the most important diagnostic measure.3
There is potential for misidentification as other fungal pathogens.1 Knowledge of phylogenetic relationships and identification to species level is crucial to allow conclusions on susceptibility and clinical outcome. Additionally, the morphology of other fungi such as Hamigera spp. or Rasamsonia spp. poses a challenge for correct identification.5,20 Molecular diagnostic approaches facilitate definitive identification, e.g. by small subunit ribosomal sequence analysis or proteomic profiling via matrix-assisted laser desorption-ionization mass spectrometry.1,3,5
Notably, we identified no case from the African continent (Figure 2). The geographical distribution of P. variotii infections seems unlikely to be affected by limited diagnostic resources, as most cases were diagnosed by culture. A reporting bias can be suspected, as P. variotii was isolated from non-clinical samples in African countries.21 However, infections caused by Paecilomyces mainly affect patients with severe chronic conditions who are less present in healthcare settings with limited resources.22
In vitro activity is difficult to correlate with clinical efficacy in Paecilomyces spp. infections. Clinical breakpoints have not been established and susceptibility is largely extrapolated from MIC values and breakpoints of related fungi.3 We detected low amphotericin B MICs, as has been reported in other studies.1 Mortality during treatment with amphotericin B was higher than with azoles. However, this is likely to be biased, as amphotericin B may have been used predominantly in critically ill patients. Itraconazole and posaconazole have good in vitro activity against P. variotii isolates. In our analysis, the mortality rate was high in patients treated with posaconazole, however, with a small sample size. Conversely, high MIC values of voriconazole suggest low clinical efficacy.1 Nonetheless, successful treatment with voriconazole was achieved in five patients. Combination therapies yielded high survival rates although infrequently used. Thus, it is not possible to draw firm conclusions on synergistic effects. A combination of active azoles or amphotericin B with flucytosine appears reasonable considering the good in vitro activity of flucytosine and the favourable outcome in cases reporting a combination therapy. However, the adverse effects of flucytosine, particularly bone marrow suppression, must be taken into account in the decision-making process.
Susceptibility data are scant for newer antifungal agents such as isavuconazole and no Paecilomyces-associated infection treated with isavuconazole has been reported to date. Interestingly, the new orally available β-glucan synthase inhibitor ibrexafungerp was shown to be highly active against P. variotii in vitro.23 It is currently being evaluated in clinical trials and may offer new treatment options in the future. Surgery and removal of indwelling devices facilitated favourable outcome in our dataset and should be considered whenever possible.
Unexpectedly, we observed P. variotii as a frequent cause of fungal peritonitis. IFI is a serious complication in peritoneal dialysis, causing 1%–15% of peritonitis episodes.24Candida spp. is most prevalent with approximately 75%, but mould infections, particularly Aspergillus spp., are occasionally reported.25 Reported mortality rates range from 20% to 40%.25,26 Our analysis demonstrates a comparably low mortality of 15%. As for other peritoneal dialysis-associated infections, dialysis catheters should be removed in persistent peritonitis.27 However, catheter preservation and intraperitoneal administration of fluconazole was performed in one case with favourable outcome. The intraperitoneal administration of antifungal drugs, particularly amphotericin B and fluconazole, is sporadically reported in the treatment of peritonitis caused by filamentous fungi and may support cure. The use of intraperitoneal amphotericin B is, however, limited by the occurrence of chemical peritonitis and abdominal pain.28
As with most analyses of rare infections, our study has certain limitations. There is a potential selection bias in case reports and registry data. Patients receiving higher quality of care and successfully treated cases are more likely to be enrolled, implying that the overall mortality in this analysis may be lower than in an unselected population.29 Review of the literature was complicated by frequent microbiological identification only to genus level and recent taxonomic changes of Paecilomyces species. Complete information for analysis was not always retrievable from the original reports and some authors could not provide missing information. Owing to various standards in mycological diagnostics and considering that most of the P. variotii isolates were identified based on culture with subjective observation of morphological characteristics, there may be an unknown rate of misidentification.
In conclusion, P. variotii is an emerging fungal pathogen representing a threat for patients with inherited or acquired immunodeficiency. Patients with indwelling catheters are at particular risk. Infections may present as primary or BT-IFI. High virulence and less predictable susceptibility patterns urge for prompt pathogen identification to the species level. Cultural and molecular techniques are paramount for accurate diagnosis. Treatment is challenging due to resistance to some antifungals and the lack of clinical breakpoints. The dilemma is clearly reflected by the high mortality rates. Amphotericin B, itraconazole and posaconazole show the most favourable in vitro susceptibility patterns and may constitute the best treatment options. This is in line with the guidance for optimal treatment of rare mould infections upcoming by the European Confederation of Medical Mycology (ECMM) Guideline programme (in press) but needs confirmation in a larger and more homogeneous cohort. Broader knowledge on emerging IFI should be acquired, and international registries such as FungiScope® provide a valuable source of comprehensive data, particularly for rare pathogens.
Supplementary Material
Acknowledgements
We thank Susann Bloßfeld (University Hospital Cologne, Germany) for her administrative support.
Funding
FungiScope® is supported by unrestricted grants from Amplyx Pharmaceuticals, Basilea Pharmaceutica, Cidara Therapeutics, F2G Ltd., Matinas BioPharma, Mundipharma, and SCYNEXIS Inc. FungiScope® has been supported in the past by unrestricted grants from Astellas Pharma, Gilead Sciences, MSD Sharp & Dohme GmbH, and Pfizer Inc.
Transparency declarations
R.S., J.S.G., X.M., I.F.R., L.H., L.L.S., M.S. and D.S. declare that they have no conflicts of interest. E.S. reports grants from The Philipp Schwartz Initiative of the Alexander von Humboldt Foundation, during the conduct of the study. N.K. reports personal fees from Astellas, personal fees from Gilead, personal fees from MSD, and grants and personal fees from Pfizer, outside the submitted work. M.H. reports grants from Bayer AG, outside the submitted work. J.M. reports grants from Astellas, Gilead, MSD, Pfizer, outside the submitted work. J. Steinmann received personal fees from Pfizer, outside of the submitted work. O.A.C. is supported by the German Federal Ministry of Research and Education, is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—CECAD, EXC 2030—390661388 and has received research grants from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen, Medicines Company, Melinta, Merck/MSD, Octapharma, Pfizer, Scynexis, is a consultant to Actelion, Allecra, Amplyx, Astellas, Basilea, Biosys, Cidara, Da Volterra, Entasis, F2G, Gilead, Matinas, MedPace, Menarini, Merck/MSD, Mylan, Nabriva, Noxxon, Octapharma, Paratek, Pfizer, PSI, Roche Diagnostics, Scynexis, and Shionogi, and received lecture honoraria from Al-Jazeera Pharmaceuticals, Astellas, Basilea, Gilead, Grupo Biotoscana, Merck/MSD and Pfizer. J. Stemler reports research grants from Basilea Pharmaceutica International Ltd. and travel grants from Meta-Alexander Foundation and from the German Society for Infectious Diseases (DGI).
Author contributions
R.S. conceived the study idea, enrolled cases, performed literature research, analysed and interpreted data, drafted the manuscript, created tables and figures, revised and approved the final manuscript. JSG enrolled cases, performed literature search, analysed and interpreted data, created tables and figures, revised and approved the final manuscript. E.S. and X.M. performed literature research and revised and approved the final manuscript. I.F.R., L.H., M.H., N.K., L.L.S., J.M., J. Steinmann and M.S. contributed cases to the FungiScope® registry and revised and approved the final manuscript. D.S. manages FungiScope®, enrolled cases, interpreted data, revised and approved the final manuscript. O.A.C. conceived and leads FungiScope®, contributed cases to the FungiScope® registry, interpreted data, revised and approved the final manuscript. J. Stemler conceived the study idea, performed literature research, analysed and interpreted data, revised and approved the final manuscript.
Supplementary data
Tables S1 to S6 are available as Supplementary data at JAC Online.
References
- 1. Houbraken J, Verweij PE, Rijs AJ. et al. Identification of Paecilomyces variotii in clinical samples and settings. J Clin Microbiol 2010; 48: 2754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urquhart AS, Mondo SJ, Mäkelä MR. et al. Genomic and genetic insights into a cosmopolitan fungus, Paecilomyces variotii (Eurotiales). Front Microbiol 2018; 9:3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman R, Cockerham L, Buchan BW. et al. Treatment of Paecilomyces variotii pneumonia with posaconazole: case report and literature review. Mycoses 2016; 59: 746–50. [DOI] [PubMed] [Google Scholar]
- 4. Marques DP, Carvalho J, Rocha S. et al. A case of pulmonary mycetoma caused by Paecilomyces variotii. Eur J Case Rep Intern Med 2019; 6: 001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker AP, Horan JL, Slechta ES. et al. Complexities associated with the molecular and proteomic identification of Paecilomyces species in the clinical mycology laboratory. Med Mycol 2014; 52: 537–545. [DOI] [PubMed] [Google Scholar]
- 6. Luangsa-Ard J, Houbraken J, van Doorn T. et al. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett 2011; 321: 141–9. [DOI] [PubMed] [Google Scholar]
- 7. Castelli MV, Alastruey-Izquierdo A, Cuesta I. et al. Susceptibility testing and molecular classification of Paecilomyces spp. Antimicrob Agents Chemother 2008; 52: 2926–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellanger AP, Cervoni JP, Faucher JF. et al. Paecilomyces variotii Fungemia in a Patient with Lymphoma Needing Liver Transplant. Mycopathologia 2017; 182: 761–5. [DOI] [PubMed] [Google Scholar]
- 9. Torres R, Gonzalez M, Sanhueza M. et al. Outbreak of Paecilomyces variotii peritonitis in peritoneal dialysis patients after the 2010 Chilean earthquake. Perit Dial Int 2014; 34: 322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarkkanen A, Raivio V, Anttila V-J. et al. Fungal endophthalmitis caused by Paecilomyces variotii following cataract surgery: a presumed operating room air-conditioning system contamination. Acta Ophthalmol Scand 2004; 82: 232–5. [DOI] [PubMed] [Google Scholar]
- 11. Lee J, Yew WW, Chiu CSW. et al. Delayed sternotomy wound infection due to Paecilomyces variotii in a lung transplant recipient. J Heart Lung Transplant 2002; 21: 1131–4. [DOI] [PubMed] [Google Scholar]
- 12. Eren D, Eroglu E, Ulu Kilic A. et al. Cutaneous ulcerations caused by Paecilomyces variotii in a renal transplant recipient. Transpl Infect Dis 2018; 20: e12871. [DOI] [PubMed] [Google Scholar]
- 13. Cohen-Abbo A, Edwards KM.. Multifocal osteomyelitis caused by Paecilomyces variotii in a patient with chronic granulomatous disease. Infection 1995; 23: 55–7. [DOI] [PubMed] [Google Scholar]
- 14. Salmanton-García J, Koehler P, Kindo A. et al. Needles in a haystack: extremely rare invasive fungal infections reported in FungiScope® - Global Registry for Emerging Fungal Infections. J Infect 2020; 81: 802–15. [DOI] [PubMed] [Google Scholar]
- 15. Lam DSC, Koehler AP, Fan DSP et al. Endogenous fungal endophthalmitis caused by Paecilomyces variotii. Eye 1999; 13: 113–6. [DOI] [PubMed] [Google Scholar]
- 16. Seidel D, Durán Graeff LA, Vehreschild M. et al. FungiScope(™) -Global Emerging Fungal Infection Registry. Mycoses 2017; 60: 508–16. [DOI] [PubMed] [Google Scholar]
- 17. Donnelly JP, Chen SC, Kauffman CA. et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2019; 71: 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cornely OA, Hoenigl M, Lass-Flörl C. et al. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019; 62: 716–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alkorta Gurrutxaga M, Saiz Camín M, Rodríguez Antón L. [Fungal endocarditis in a patient bearing a valve prosthesis]. Enferm Infecc Microbiol Clin 2007; 25: 549–50. [DOI] [PubMed] [Google Scholar]
- 20. Stemler J, Salmanton-García J, Seidel D. et al. Risk factors and mortality in invasive Rasamsonia spp. infection: analysis of cases in the FungiScope(®) registry and from the literature. Mycoses 2020; 63: 265–74. [DOI] [PubMed] [Google Scholar]
- 21. van den Brule T, Punt M, Teertstra W. et al. The most heat-resistant conidia observed to date are formed by distinct strains of Paecilomyces variotii. Environ Microbiol 2020; 22: 986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Essue BM, Laba M, Knaul F. et al. Economic Burden of Chronic Ill Health and Injuries for Households in Low- and Middle-Income Countries In: Jamison DT, Gelband H, Horton S. et al. eds. Disease Control Priorities: Improving Health and Reducing Poverty International Bank for Reconstruction and Development, 2017; 121–43. [PubMed] [Google Scholar]
- 23. Lamoth F, Alexander BD.. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 2015; 59: 4308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prasad N, Gupta A.. Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 2005; 25: 207–22. [PubMed] [Google Scholar]
- 25. Nguyen MH, Muder RR.. Aspergillus peritonitis in a continuous ambulatory peritoneal dialysis patient. Case report and review of the literature. Diagn Microbiol Infect Dis 1994; 20: 99–103. [DOI] [PubMed] [Google Scholar]
- 26. Goldie SJ, Kiernan-Tridle L, Torres C. et al. Fungal peritonitis in a large chronic peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis 1996; 28: 86–91. [DOI] [PubMed] [Google Scholar]
- 27. Ballinger AE, Palmer SC, Wiggins KJ. et al. Treatment for peritoneal dialysis-associated peritonitis. Cochrane Database Syst Rev2014: Cd005284. [DOI] [PMC free article] [PubMed]
- 28. Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int 2009; 29 Suppl 2: S161–165. [PubMed] [Google Scholar]
- 29. Krumholz HM. Registries and selection bias: the need for accountability. Circ Cardiovasc Qual Outcomes 2009; 2: 517–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.