Abstract
Introduction
Black men are over-represented in the end stage kidney disease population and are at disproportionate risk of unfavorable outcomes. There is a paucity of investigation to elucidate the mediators of this risk. This study attempts to identify residential community attributes as a possible contributor.
Methods
A post-hoc analysis of prospectively collected data from a cohort of Black men enrolled in the US Dialysis Outcomes and Practice Patterns Study (DOPPS), 2010-–2015, linked to the American Community Survey, by dialysis facility zip codes was undertaken. The exposure variable was the dialysis facility community composition as defined by percent Black residents. Negative binomial regression was used to estimate incidence rate ratio (IRR) of hospitalization (first outcome) for Black men in crude and adjusted models. Similarly, Cox proportional hazards modeling was used to estimate mortality (second outcome) for Black men by type of community.
Results
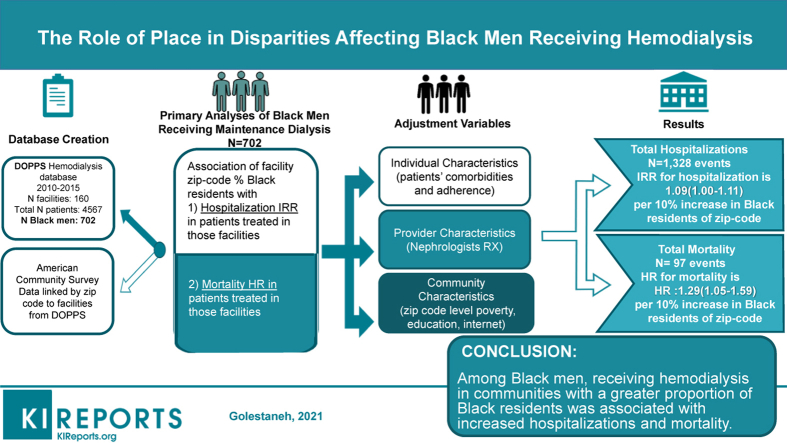
A total of 702 Black men receiving chronic hemodialysis were included in the study. Black men receiving hemodialysis in communities with greater proportions of Black residents had lower Charlson scores and fewer comorbidities, but a higher rate of hypertension. They had equivalent adherence to dialysis treatments, but a lower rate of arteriovenous fistula use and fewer dialysis minutes prescribed. Black men receiving dialysis in communities with a greater proportion of Black residents (per 10% increase) had higher adjusted hospitalization rates (IRR 1.09, 95% confidence interval [CI] 1.00–1.19) and mortality (hazard ratio [HR] 1.29, 95% CI 1.05–1.59).
Conclusions
This study supports the unique role of residential community as a risk factor for Black men with end stage kidney disease, showing higher hospitalization and mortality in those treating in Black versus non-Black communities, despite equivalent adherence and fewer comorbidities.
Keywords: Black men, community, health disparity, hemodialysis
Graphical abstract
See Commentary on Page 252
The burden of kidney disease in the United States is unequally distributed across race and sex with Black men experiencing poorer outcomes than any other race and gender group.1 End-stage kidney disease (ESKD) prevalence is 4-fold higher in Black men than White men and more than 10-fold higher in young Black men age 20 to 44 years old compared with their White peers.2,3 New ESKD cases are highest in Black men as compared to other racial/ethnic groups, including Black women, according to a prospective analysis of the National Health and Nutrition Examination Survey data as well as the US Renal Data System annual report.4,5 Blood pressure control with chronic kidney disease is least successfully attained in Black men compared with Black women and White individuals of both sexes.6, 7, 8 Explanations for these disparities include increased prevalence of APOL1 risk polymorphisms, shortfalls in early and preventive intervention for chronic kidney disease, lack of adequate insurance, inadequate comorbidity management, as well as late referral to kidney transplantation and dialysis access placement.2,9, 10, 11 However, none of them entirely explain the earlier onset, accelerated progression of chronic kidney disease, and premature mortality observed in Black men. It has been suggested that community-level factors that reflect inequities in the social determinants of health are important considerations; however, it is unclear whether place, personal factors, or the combined effect of place and individual matters play the more prominent role in these outcomes.3,9,12
The evidence linking place and health status is mixed, and there are conflicting theories with regard to the role of community on outcomes.13 For example, it has been suggested that communities with a higher percentage of Black residents may have larger social networks and lower exposure to discrimination that lead to improved engagement with health services.14 Although Black men may be among the most affected by structural racism, its effect may be mitigated by strong adaptable community social networks.15,16 By contrast, communities with a high proportion of racial and ethnic minorities are often socially and economically disadvantaged,17 and are burdened by many challenges that are known to adversely impact health such as limited access to high-quality schools, substandard housing, high crime and incarceration rates, and inadequate access to healthcare due to longstanding and persisting structural racism.18 Black men living in communities with sizable Black populations may experience restricted access to health care resources and have poorer health outcomes.19,20 They may also face implicit discrimination from clinicians.13,16,21 Community characteristics have been linked to select adverse outcomes in the ESKD population; however, detailed associations between community risk factors and health outcomes of key populations, such as Black men, have not been described.22,23 Previous studies considering the association between place and health outcomes did not adequately adjust for comorbidities and adherence behaviors, both of which are cited reasons for worse outcomes among Black men.24,25 A recent paper discussed the positive association of residential community racial composition (% Black) with hospitalization rate in a national sample of patients receiving maintenance hemodialysis, with Hispanic and White patients exhibiting the strongest association.26 This study did not examine the persistence of this association in various subgroups.
The purpose of this study was to examine whether community racial composition (% Black) was associated with risk of hospitalization and mortality in a cohort of Black men receiving maintenance hemodialysis and enrolled in the DOPPS. By focusing on Black men, we aim to provide insights into an often-overlooked population that has one of the most horrific health profiles in the US, gain better understanding of health at the intersection of race and sex, and examine the hypothesis that Black men receiving dialysis therapy in communities with higher proportions of Black residents will have lower hospitalization and mortality rates.
Methods
Data Sources
After obtaining institutional review board approval for this study from the Albert Einstein College of Medicine, and in compliance with the Declaration of Helsinki, we analyzed a sample of Black men enrolled in the de-identified prospectively collected patient database of the US DOPPS, between 2010 and 2015, linked by facility zip code to the same period American Community Survey, which is an ongoing survey by the US Census Bureau that captures information such as ancestry, citizenship, educational attainment, income, language proficiency, employment, and housing characteristics. The DOPPS sample consists of randomly selected patients from a stratified random sample of dialysis facilities across the United States.27
Outcome Variable
The outcome variables of interest were hospitalization and death. Follow-up data on hospitalization, death, and disenrollment was available in the DOPPS database through June 2015. Hospitalizations and date of death or dropout were recorded prospectively by DOPPS study coordinators at dialysis facilities during study follow-up visits.
Exposure Variable
The exposure variable was the dialysis facility residential community characterized by percentage of Black residents. For descriptive and bivariate analyses, we dichotomized the exposure variable in the following manner: after limiting the database to Black men only, we dichotomized based on the median of % Black residential communities in which Black men received hemodialysis (<, ≥30%). In multivariate analyses, the exposure variable was parameterized by 10% (for every 10% increase in Black residents of dialysis facility zip code defined community) to allow for the most power. In sensitivity analyses, we used the dichotomized variable and examined its association with the main outcomes. The association of each outcome with communities with <10% Black residents versus those with >50% Black residents was further tested in additional sensitivity analyses (total n = 283).
Other Variables
Individual-level variables were abstracted from medical records at DOPPS enrollment, including comorbidities, etiology of ESKD, and case mix variables, such as laboratory, dialysis treatment adherence, and medication data. We used a modified Charlson comorbidity score previously validated in DOPPS research.28 We extracted community-level data (Table 1) from the American Community Survey and linked it to the DOPPS database by dialysis facility zip code.
Table 1.
Characteristics of Black men in non-Black and Black residential communities
| Total = 702 Black males enrolled in DOPPS phase 4–5 |
Percent Black residents <30% “non-Black” (total N = 389) |
Percent Black residents ≥30% “Black” (total N = 313) |
P value |
|---|---|---|---|
| Demographic and clinical variables | |||
| Age, yr, mean (SD) | 58.5 (13.9) | 56.8 (14.4) | 0.12 |
| Vintage, yr, median (IQR) | 4.13 (1.74–7.01) | 3.85 (1.76–6.81) | 0.76 |
| Vascular access: AVF, n (%) | 238 (61.2) | 165 (52.7) | 0.02 |
| Psychiatric illness yes, n (%) | 55 (14.1) | 38 (12.1) | 0.44 |
| Coronary artery disease (n = 671) yes, n (%) | 137 (38.2) | 88 (28.2) | 0.006 |
| Heart failure (n = 669) yes, n (%) | 131 (36.6) | 112 (36.0) | 0.88 |
| Diabetes mellitus yes, n (%) | 211 (54.2) | 153 (48.9) | 0.16 |
| Hypertension (n = 671) yes, n (%) | 300 (83.6) | 293 (93.9) | <0.001 |
| Charlson score (n = 630), median (IQR) | 5 (3–6) | 4 (3–5) | 0.002 |
| HTN as etiology of kidney disease, n (%) | 170 (43.7) | 157 (50.2) | 0.09 |
| BMI (n = 671), median (IQR) | 27.1 (23.3–31.7) | 26.5 (23.0–30.6) | 0.08 |
| Phosphorus (mg/dl) (n 692), mean (SD) | 5.31 (1.52) | 5.39 (1.69) | 0.51 |
| Hemoglobin (g/l) (n = 690), mean (SD) | 116 (13) | 112 (12) | <0.001 |
| Albumin (mg/dl) (n = 690), mean (SD) | 3.91 (0.40) | 3.92 (0.42) | 0.65 |
| Physician/prescriber behavior variables | |||
| URR (n = 643), mean (SD) | 71.6 (7.5) | 71.2 (7.5) | 0.44 |
| Number of renal meds, median (IQR) (n = 671) | 3 (2–1)_ | 3 (2–3) | 0.41 |
| Prescriptions in past 4 months: | |||
| ESA, n (%) (n = 668) | 296 (78.1) | 241 (83.4) | 0.09 |
| Calcimimetic, n (%) (n = 624) | 128 (35.0) | 65 (25.2) | 0.01 |
| Phosphorus binders, n (%) (n = 627) | 327 (89.3) | 231 (88.5) | 0.74 |
| Dialysis minutes prescribed per wk, (n = 678), mean (SD) | 693.7 (96.5) | 657 (90.3) | <0.001 |
| Patient behavior variables | |||
| Number of missed dialysis treatments per mo (n = 556) | |||
| 0 | 245 (88.1) | 227 (84.7) | 0.24 |
| ≥1 | 33 (11.9) | 41 (15.3) | |
| Number of shortened dialysis treatments (by ≥ 30 min) per mo (n = 692) | |||
| 0 | 250 (65.8) | 191 (61.2) | 0.21 |
| ≥1 | 130 (34.2) | 121 (38.8) | |
| Fluid removed (% dry weight) (n = 676) Mean (SD) |
3.09 (1.50) | 3.15 (1.62) | 0.64 |
| Facility variables | |||
| Profit status: for-profit, n (%) | 344 (88.4) | 295 (94.2) | 0.007 |
| Facility size, median number patients (IQR) | 82 (64–110) | 65 (39–254) | 0.29 |
| Community variables | |||
| Primary payer, n (%) | |||
| Medicare | 305 (78.4) | 234 (74.8) | |
| Medicaid | 18 (4.6) | 20 (6.4) | 0.44 |
| Other | 66 (17.0) | 59 (18.8) | |
| Rurality, %, median (IQR) | 8.85 (0–47.5) | 0 (0–62.2) | <0.001 |
| Poverty, %, median (IQR) | 11.8 (8.4–14.9) | 22.1 (17.7–22.5) | <0.001 |
| Household married, %, median (IQR) | 42.5 (37.2–49.7) | 36.0 (36.0–43.8) | <0.001 |
| Head of household: single female, %, median (IQR) | 11.7 (8.6–15.9) | 22.7 (15.6–23.8) | <0.001 |
| Household: 1 member bachelor’s degree or higher, %, median (IQR) | 29.6 (10.9–49.8) | 19.7 (13.6–30.5) | <0.001 |
| Household: active Internet, %, median (IQR) | 78.3 (77.6–83.4) | 67.9 (54.1–78.6) | <0.001 |
AVF, arteriovenous fistula; BMI, body mass index; DOPPS, Dialysis Outcomes and Practice Patterns Study; ESA, erythropoietin stimulating agent; HTN, hypertension; IQR, interquartile range; Rx, prescription; URR, urea reduction ratio.
Statistically significant values are in bold.
Statistical Analysis
We used STATA version 15.0 (StataCorp, College Station, TX) for all analyses. We executed bivariate comparisons, using the dichotomous exposure variable for community type, across patient (demographic and clinical) level, dialysis facility level, and community-level characteristics. Comparisons were made among all Black male patients using t-test, Kruskal Wallis, and χ2 or Fisher exact tests.
To estimate the hospitalization IRR for every 10% increase in Black residents, we used negative binomial regression adjusted to duration of enrollment in DOPPS. To estimate the mortality HR for every 10% increase in Black residents, we used Cox proportional hazards modeling and confirmed proportional hazards by examination of log-log plots and observed versus expected plots. We also performed sensitivity (or additional) analyses using the median % Black residents (<, ≥30%) to characterize the exposure. We followed up with a sensitivity analysis in which we categorized % Black residents into <10% and >50% to test the association of this newly categorized variable with the outcomes. All models accounted for facility clustering using a sandwich estimator.29
Models were built strategically based on an a priori plan to examine the association of categories of potentially explanatory variables. Variables were included as model adjustment categories, if they were moderately associated with the exposure in bivariate analyses (P < 0.1) or were shown to be associated with outcomes in previous research, and were grouped according to the following categories: (i) demographic, (ii) clinical, (iii) dialysis provision specific (nephrologist prescriptions and facility characteristics), (iv) patient behavior, and (v) community related.22,30 The final model (Model 6) for both outcome analyses included all variables from models 1 to 5. All tests were 2-sided and P values <0.05 were considered statistically significant in the multivariable models.
Missing Data
The proportion of missing data was ≤5% for most variables with the exception of the following: number of missed dialysis sessions within the prior 30 days (18%), Charlson score (12.2%), and urea reduction ratio (7.8%). We used a multiple imputation technique with chained predictive analytics (10 imputations) to account for randomly occurring missing data.31 The models were built using this imputed database. We performed additional sensitivity analyses limiting the study sample to those with complete data and compared it with the imputed database.
Results
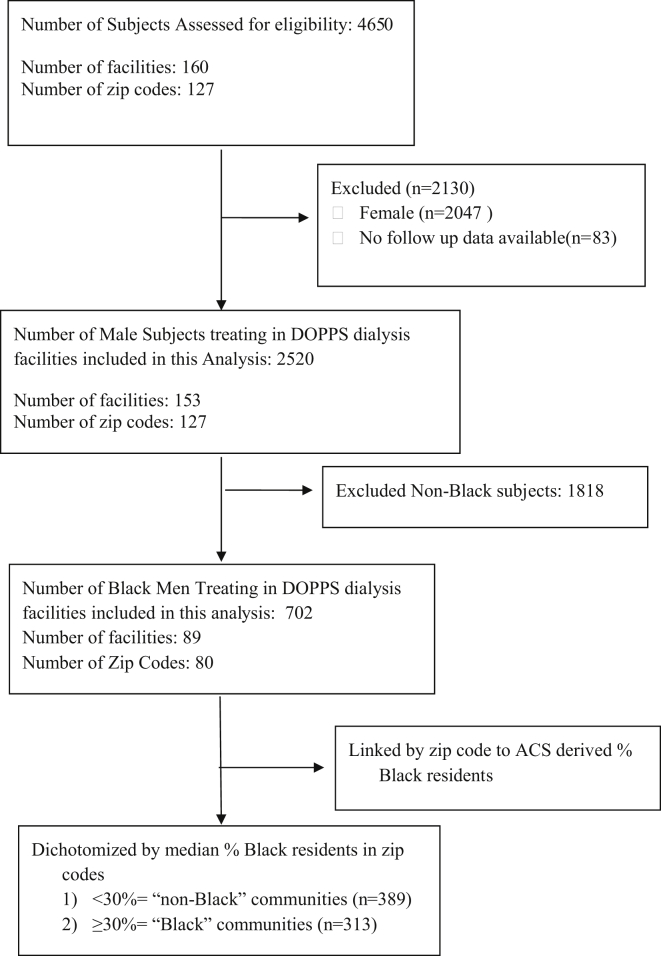
The overall cohort included 4567 patients receiving hemodialysis enrolled in US DOPPS phases 4 and 5, and our analyses included 702 Black male patients (Figure 1). Forty-five percent of the Black men in our study received hemodialysis in communities with ≥30% Black residents (Table 1). Black men receiving hemodialysis in places with ≥30% Black residents versus those <30%, had fewer comorbidities, with lower Charlson scores (4 vs. 5; P = 0.002) and prevalence of coronary disease (28.2 vs. 38.2%; P < 0.01), but higher prevalence of hypertension and hypertensive kidney disease (93.9% vs. 83.6%, P < 0.001; 50.2% vs. 43.7%, P = 0.1). They also had a lower arteriovenous fistula use (52.7% vs. 61.2%, P = 0.02), fewer prescriptions for calcimimetics (25.2% vs. 35.0%, P = 0.01), and shorter prescribed dialysis duration (657 vs. 693 minutes per week, P < 0.001). They had slightly lower hemoglobin levels (113 g/l vs. 116 g/l, P < 0.001), but there was similar erythropoietin use between the 2 community profiles. Facilities were more commonly for-profit and were in more urban settings with significantly lower socioeconomic means (households below federal poverty level: 22.1% vs. 11.8%, P = 0.001) in communities with greater proportion of Black residents. There was no significant difference in the rates of substance use (4.2% vs. 5.5%) and alcohol use disorder (2.8% vs. 1.9%) among patients dialyzing in the communities with ≥30% Black residents versus <30%. Communities with a greater proportion of Black residents had higher percentage of households with single women as primary provider, lower educational attainment, and lower rates of active Internet subscriptions (Table 1).
Figure 1.
The study cohort. ACS, American Community Survey; DOPPS, Dialysis Outcomes and Practice Patterns Study.
Over the 5-year follow-up period, there were a total of 1328 hospitalizations and 97 deaths. Crude hospitalization and mortality rates were higher in communities with more Black residents (Supplementary Figure 1). We observed an association between community racial composition and hospitalization; the IRR (95% CI) per 10% greater Black residents was 1.05 (95% CI 1.00–1.11) in the unadjusted model and 1.09 (95% CI 1.00–1.19) in the fully adjusted model (Table 2). Similarly, the mortality rate was significantly higher in Black men for each 10% increase in Black residents in both unadjusted (HR 1.09 [1.01–1.17]) and fully adjusted models (HR 1.29 [95% CI 1.05–1.59]) (Table 3). Adding clinical variables, such as comorbidities, albumin, and hemoglobin levels did not significantly alter the association between community racial composition and (i) IRR for hospitalization (1.07 [1.01–1.14] vs. 1.05 [1.00–1.11] in adjusted and unadjusted models, respectively) and (ii) HR for mortality (1.11 [1.03–1.19] vs. 1.09 [1.01–1.17] in adjusted and unadjusted models, respectively). Nor did addition of community-level attributes. Adjustment for additional community-level variables did not significantly alter the point estimate of the association of % Black residents and number of hospitalizations (1.05 [0.95–1.16] vs. 1.05 [1.00–1.11]) or mortality (1.26 [1.04–1.53] vs. 1.09 [1.01–1.17]). In the latter case, addition of community-level variables seemed to have slightly strengthened the positive association of community racial composition with mortality (Table 3).
Table 2.
Association of racial composition of community of residence with hospitalization rate in Black men receiving hemodialysis
| Association of hospitalization rate and community racial composition in Black male patients (by race) during enrollment in DOPPS N = 702 Total number of hospitalizations: 1328 |
Exposure variable as continuous with IRR for every 10% increase in % Black residents | P value |
|---|---|---|
| Unadjusted model | ||
| (IRR for hospitalization) | 1.05 (1.00–1.11) | 0.07 |
| Model 1-demographic | ||
| + Age and vintage and DOPPS phase | 1.06 (1.00–1.12) | 0.07 |
| Model 2 | ||
| Model 1 + clinical variables (psychiatric illness, Charlson, CAD, DM, HTN, albumin, BMI, hemoglobin) | 1.07 (1.01–1.14) | 0.03 |
| Model 3 | ||
| Model 1+ dialysis provision (dialysis minutes per wk prescribed, URR, cinacalcet, AVF) | 1.05 (0.98–1.13) | 0.14 |
| Model 4 | ||
| Model 1 + patient behavior (substance use, shortened and missed treatments,a fluid removed) | 1.05 (1.00–1.12) | 0.07 |
| Model 5 | ||
| Model 1 + Community/Facility characteristics (profit status, size, poverty, insurance, % married, higher education, Internet) | 1.05 (0.95–1.16) | 0.35 |
| Model 6 | ||
| Adjustment for all variables used in Models 1–5 (imputed data) | 1.09 (1.003–1.19) | 0.04 |
AVF, arteriovenous fistula; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; DOPPS, Dialysis Outcomes and Practice Patterns Study; HTN, hypertension; IRR, incidence rate ratio; URR, urea reduction ratio.
Number of missed or shortened (by ≥30 minutes) per month.
Table 3.
Association of racial composition of community of residence with mortality in Black men receiving hemodialysis
| Association of mortality and community racial composition in Black male patients (by race) during enrollment in DOPPS N = 702 Total number of deaths n = 97 (13.8%) |
HR for death for every 10% in percent Black residents | P value |
|---|---|---|
| Unadjusted Model | 1.09 (1.01–1.17) | 0.02 |
| Model 1-Demographic | ||
| + Age and vintage and DOPPS phase | 1.10 (1.03–1.18) | 0.004 |
| Model 2 | ||
| Model 1 + clinical variables (psychiatric illness, Charlson, CAD, DM, HTN, albumin, BMI, hemoglobin) | 1.14 (1.04–1.24) | 0.004 |
| Model 3 | ||
| Model 1+ dialysis provision (dialysis minutes per wk prescribed, URR, cinacalcet, AVF) | 1.11 (1.03–1.19) | 0.005 |
| Model 4 | ||
| Model 1 + patient behavior (substance use, shortened and missed treatments, fluid removed) | 1.09 (1.02–1.18) | 0.02 |
| Model 5 | ||
| Model 1 + community/facility characteristics (profit status, size, poverty, insurance, % married, higher education, Internet) | 1.26 (1.04–1.53) | 0.02 |
| Model 6 | ||
| Adjustment for all variables used in Models 1–5 (imputed data) | 1.29 (1.05–1.59) | 0.02 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; DOPPS, Dialysis Outcomes and Practice Patterns Study; DOPPS Phase, DOPPS 2010–2015; ESA, erythropoietin stimulating agent; HR, hazard ratio; HTN, hypertension; IRR, incidence rate ratio; Rx, prescription; URR, urea reduction ratio.
Dichotomizing the exposure variable using median % Black residents as the cutoff point (<30%, >30%), also suggested a higher hospitalization rate (IRR 1.05; 95% CI 0.77–1.42) and mortality (HR 1.27; 95% CI 0.82–1.97) in Black men receiving dialysis in communities with a higher proportion of Black residents, although not achieving statistical significance (Supplementary Tables S1 and S2). Community level variables, when tested individually, did not show a positive associations with outcomes (Supplementary Table S3). Categorizing the exposure variable into >50% and <10% Black residents in a community revealed a significant difference in adjusted IRR for hospitalization 1.62 (1.05–2.45) and HR for mortality 2.14 (1.14–4.03) (Supplementary Table S4). Sensitivity analysis also showed similar point estimates between nonimputed and imputed data for all analyses.
Discussion
Our results contradicted our hypothesis that communities with a large number of Black residents would be protective against adverse outcomes for Black men receiving hemodialysis. We showed that Black men receiving dialysis in communities with greater minority representation were in fact more likely to be hospitalized and to have a higher risk of all-cause mortality despite younger age, fewer comorbidities and equivalent adherence to dialysis. Those Black men treating in Black versus non-Black communities were poorer, less commonly married, had a lower rate of arteriovenous fistula use and had fewer minutes of dialysis prescribed to them. The latter dialysis quality indicators could theoretically contribute to the poorer outcomes observed but when added as adjustment variables they did not attenuate the observed positive association of community and outcomes. Thus certain community attributes, unaccounted for in this study, mediate the risk of adverse outcomes in Black men.
A recent paper by our group without a focus on pre-specified demographic subgroups such as men or race/ethnicity found community racial composition was associated with a higher risk of hospitalization across the entire cohort, but in stratified secondary analyses noted the association was driven by White and Hispanic patients with no association found in Black patients.26 By contrast, in the current study we focused our analyses on Black men to explore if there were within group differences in clinical outcomes. Because of the paucity of scientific investigation focused on minority men's health in kidney disease, we wanted to elucidate risks unique to this group. The significant association found between percent of Black residents in the community and higher risk for mortality in Black male patients receiving hemodialysis was not assessed in our previous report. Based on our prior stratified analyses of hospitalization finding no association with Black dialysis patients and community racial mix we were reticent to predict an association in this study.26 Our findings, however, highlight the unique vulnerabilities to residential community experienced by Black men in particular.
There is support for the notion that minorities cared for in minority communities may feel more supported by established local social support systems, higher social capital (from religious institutions, immigrant networks, and community centers), with better emotional well-being and with fewer perceived barriers to care.14,24,32 While the findings from our study did not support this premise, it should be noted our study did not adjust for individual socioeconomic status but did provide detailed community-level socioeconomic information. The benefits of higher individual SES can differ by race as high SES Blacks are less able to translate income or educational advantage into better housing or neighbourhood conditions,33 consistent with the broad impact of structural racism. Strikingly, at every level of education Black men earn lower levels of income than White men and these racial differences among men are larger than racial differences among women.33,34 Even when exposed to equivalent social conditions in early life, Black men in the US fare far worse than their White counterparts throughout their lifetime.35 Black men have one of, if not the highest, rates of mortality and overall poor health status of any race-gender group in the US.36 Black men living in communities with sizable Black populations were shown to have lower access to pre-ESKD nephrology care and experience lower quality of dialysis treatments, both of which were thought to mediate their worse outcomes.37,38 Black men have also been shown to suffer from multiple structural inequities that amplify the adverse impact of economic disadvantage on their health outcomes, and thus may not be able to overcome this disadvantage by relying solely on the higher social capital and support they may receive within communities with substantial proportions of Black residents.16,24 Thus, those living in communities with sizable Black populations appear especially vulnerable to socioeconomic attributes of their communities with respect to health outcomes. Black men receiving dialysis in places with substantial proportions of Black residents were also noted to have a higher prevalence of hypertension and ESKD attributed to hypertension compared to those receiving dialysis in places with fewer Black residents. This higher rate of hypertension has been described as a potential reaction to degree of residential segregation and associated factors such as under-employment, lower educational attainment, increased exposure to environmental toxins, violence, noise, and unsafe outdoor recreational space, decreased access to health food and more.39 The increased risk of hypertensive complications may play a role in the disproportionate risk of ESKD in Black men living in Black communities, potentially contributing to the higher risk of hospitalization and mortality observed in our cohort, although the association persisted even after adjusting for hypertension. The diagnosis of hypertension in those receiving care in Black communities may also be associated with early testing and lower clinical scrutiny for other diagnoses leading to less aggressive care as well as reduced adherence to care due to medical mistrust, compared to those receiving care elsewehere.12,24,40,41 Our finding of lower rate of cardiovascular disease and lower comorbidity scores among men treated in communities with substantial proportions of Black resdients could represent a difference in disease severity, but could also represent administrative under-coding or a shortfall in diagnostic vigilance for those cared for by healthcare systems serving these communities.42,43 The role of residential segregation, a phenomenon established by historical redlining and housing policies and perpetuated by governmental institutions and corporate practices in driving healthcare inequities as well established and a recognized contributing factor to health disparities like the ones described here.18,44, 45, 46 The lingering impact of institutional discrimination is evident in our data and consistent with other studies using census track level community data, indicating that areas with sizable Black populations had higher levels of poverty and family disruption as well as lower overall educational attainment.15,44,47,48 Despite legally mandated hospital desegregation after the Civil Rights Act of 1964, the impact of historical and existing patterns of segregation are still evident in today's health outcomes.15,18,44 For example, health care systems that serve clinically complex patients in communities with sizable Black populations more often experience financial shortfalls, and describe higher workforce turnover of primary care and subspecialty providers.44 Specific to Black men, relevant community attributes also involve fragmented or reduced social support, high rates of violent crime and police brutality and fixed barriers to economic mobility.35,49,50
Our study has several limitations. Communities with substantial proportions of Black residents were in mostly urban settings, with additional unaccounted barriers linked to living in the inner city. For example, the impact of poverty extends beyond low income, and includes increased exposure to violent crime, discrimination, unstable housing, unhealthy foods, environment pollutants, noise, and more.51 We adjusted for several community-level variables, but acknowledge that were not able to account for detailed characterization of these variables for individuals, as well as several missing medical-related variables such as smoking. Dialysis facility linked zip codes may not accurately represent a patient’s community of residence, although nearly 50% live within 15 minutes of their facility.52 As this is an observational study, causation cannot be wholly established. Our study has many notable strengths. We included a large random sample of patients receiving hemodialysis from across the United States, enhancing generalizability of our findings. Furthermore, leveraging available data permitted the analysis of community-level data with detailed individual demographic and clinical information.
Conclusions
Black men receiving hemodialysis treatment in communities with a sizable number of Black residents had higher adjusted rates of hospitalization and mortality compared with those receiving dialysis in communities with fewer Black residents, despite being younger, healthier and exhibiting equivalent adherence. To effectuate meaningful health care policy future research should focus on those community level factors most detrimental to the health of Black men, while avoiding the unintended consequence of implementing recommendations that may further disadvantage those populations that are most vulnerable.
Disclosure
LG receives salary support from the Montefiore Care Management Organization, and is a member of the Clinical Events Committee for the Spyral Pivotal Hypertension On-Medications and Spyral Pivotal Hypertension Off-Medications sponsored by Medtronic. DaVita Inc. pays the Montefiore Renal Division a fee for LG’s services as medical director of its home dialysis facility. AK is an employee of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Programs is provided by a consortium of funders listed in the provided URL without restriction on publications. See https://www.dopps.org/AboutUs/Support.aspx for more information. MLM receives an honorarium from the American Board of Internal Medicine for serving on the Nephrology Examination Committee. All the other authors declared no competing interests.
Acknowledgments
This study was supported by a grant from the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), through CTSA grant number UL1TR002556-01 awarded to LG. LG, MLM, and KC are supported by R18 DK118471-01. RJT receives support from the National Institute on Aging (K02AG059140), and the National Institute on Minority Health and Health Disparities (U54MD000214). KCN receives support from NIH grants P30AG021684 and UL1TR001881.
We acknowledge the help of Dr. Colin Rehm for obtaining American Community Survey data and the help of Mr. Brian Bieber for his help with obtaining the DOPPS database.
Author Contributions
All authors contributed to this work. LG, AK, and KCN conceptualized the design of the study and the hypothesis. LG and AK obtained the DOPPS database and LG linked it to the American Community Survey. DMG, EMU, KC, TSJ, MLM, MAB, and RJT contributed to the intellectual content, to the writing and to the revisions of the manuscript. All authors contributed to the final version of the manuscript
Footnotes
Table S1. Association of hospitalization with percent Black residents dichotomized at 30.
Table S2. Association of mortality with percent Black residents dichotomized at 30.
Table S3. Unadjusted association of each community variable with outcome.
Table S4. Reexamination of main analyses with exposure variable categorized as <10% Black residents and >50% Black residents.
Figure S1. (A) Crude hospitalization rates by decile increase in Black residents. (B) Crude mortality rates by decile increase in Black residents.
Supplementary Material
References
- 1.Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley B.D., Stith A.Y., Nelson A.R., editors. National Academies Press (US); Washington (DC): 2003. [PubMed] [Google Scholar]
- 2.Klag M.J., Whelton P.K., Randall B.L., Neaton J.D., Brancati F.L., Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277(16):1293–1298. [PubMed] [Google Scholar]
- 3.Norton J.M., Moxey-Mims M.M., Eggers P.W. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu C.Y., Lin F., Vittinghoff E., Shlipak M.G. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 5.Report UAD ESRD in the United States. https://www.usrds.org/media/1736/v2_c01_incprev_18_usrds.pdf Available at: 2018;2:301-306.
- 6.Duru O.K., Li S., Jurkovitz C. Race and sex differences in hypertension control in CKD: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;51(2):192–198. doi: 10.1053/j.ajkd.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantinga L.C., Miller E.R., 3rd, Stevens L.A. Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension. 2009;54(1):47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klag M.J., Whelton P.K., Randall B.L. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 9.Norris K.C., Williams S.F., Rhee C.M. Hemodialysis disparities in African Americans: the deeply integrated concept of race in the social fabric of our society. Semin Dial. 2017;30(3):213–223. doi: 10.1111/sdi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris K.C., Agodoa L.Y. Race and kidney disease: the scope of the problem. J Natl Med Assoc. 2002;94(8 Suppl):1S–6S. [PMC free article] [PubMed] [Google Scholar]
- 11.Norris K.C., Agodoa L.Y. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 12.Hammond W.P., Matthews D., Mohottige D., Agyemang A., Corbie-Smith G. Masculinity, medical mistrust, and preventive health services delays among community-dwelling African-American men. J Gen Intern Med. 2010;25(12):1300–1308. doi: 10.1007/s11606-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaskin D.J., Dinwiddie G.Y., Chan K.S., McCleary R. Residential segregation and disparities in health care services utilization. Med Care Res Rev. 2012;69(2):158–175. doi: 10.1177/1077558711420263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt Yuan A.S. Racial composition of neighborhood and emotional well-being. Sociol Spectr. 2007;28(1):105–129. [Google Scholar]
- 15.Hall Y.N. Social determinants of health: addressing unmet needs in nephrology. Am J Kidney Dis. 2018;72(4):582–591. doi: 10.1053/j.ajkd.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Fenton A.T., Burkhart Q., Weech-Maldonado R. Geographic context of black-white disparities in Medicare CAHPS patient experience measures. Health Services Research. 2019;54(S1):275–286. doi: 10.1111/1475-6773.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do D.P., Frank R., Iceland J. Black-white metropolitan segregation and self-rated health: investigating the role of neighborhood poverty. Soc Sci Med. 2017;187:85–92. doi: 10.1016/j.socscimed.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Bailey Z.D., Krieger N., Agenor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 19.Airhihenbuwa C.O., Liburd L. Eliminating health disparities in the African American population: the interface of culture, gender, and power. Health Educ Behav. 2006;33(4):488–501. doi: 10.1177/1090198106287731. [DOI] [PubMed] [Google Scholar]
- 20.LaVeist T.A. Joint Center for Political and Economic Studies; Washington, DC: 2011. Segregated spaces, risky places: The effects of racial segregation on health inequalities. [Google Scholar]
- 21.Williams D.R., Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez R.A., Sen S., Mehta K., Moody-Ayers S., Bacchetti P., O'Hare A.M. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146(7):493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 23.Diez Roux A.V., Merkin S.S., Arnett D. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 24.Haas J.S., Phillips K.A., Sonneborn D. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual's county of residence. Med Care. 2004;42(7):707–714. doi: 10.1097/01.mlr.0000129906.95881.83. [DOI] [PubMed] [Google Scholar]
- 25.Eisenstein E.L., Sun J.L., Anstrom K.J. Do income level and race influence survival in patients receiving hemodialysis? Am J Med. 2009;122(2):170–180. doi: 10.1016/j.amjmed.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Golestaneh L., Cavanaugh K.L., Lo Y. Community racial composition and hospitalization among patients receiving in-center hemodialysis. Am J Kidney Dis. 2020;76(6):754–764. doi: 10.1053/j.ajkd.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson B., Fuller D., Zinsser D. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis. 2011;57(6):822–831. doi: 10.1053/j.ajkd.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikbov B., Bieber B., Andrusev A. Hemodialysis practice patterns in the Russia Dialysis Outcomes and Practice Patterns Study (DOPPS), with international comparisons. Hemodial Int. 2017;21(3):393–408. doi: 10.1111/hdi.12503. [DOI] [PubMed] [Google Scholar]
- 29.Becketi S. Regression standard errors in clustered samples. Stata Technical Bulleting. 1993;STB-13:1–32. [Google Scholar]
- 30.Yan G., Cheung A.K., Ma J.Z. The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol. 2013;8(4):610–618. doi: 10.2215/CJN.07780812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsen J.C., Gluud C., Wetterslev J., Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson R.N., Putt M.A., Dean L.T., Long J.A., Montagnet C.A., Armstrong K. Neighborhood racial composition, social capital and black all-cause mortality in Philadelphia. Soc Sci Med. 2009;68(10):1859–1865. doi: 10.1016/j.socscimed.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams D.R. The health of men: structured inequalities and opportunities. Am J Public Health. 2008;98(9 Suppl):S150–S157. doi: 10.2105/ajph.98.supplement_1.s150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams D.R., Mohammed S.A., Leavell J., Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emily Badger Extensive Data Shows Punishing Reach of Racism for Black Boys. New York Times. 2018. https://www.nytimes.com/interactive/2018/03/19/upshot/race-class-white-and-black-men.html The Upshot, Available at:
- 36.Ravenell J.E., Johnson Jr WE, Whitaker E.E. African-American men's perceptions of health: a focus group study. J Nat Med Assoc. 2006;98(4):544. [PMC free article] [PubMed] [Google Scholar]
- 37.Golestaneh L., Farzami A., Madu C., Johns T., Melamed M.L., Norris K.C. The association of neighborhood racial mix and ED visit count in a cohort of patients on hemodialysis. BMC Nephrol. 2019;20(1):343. doi: 10.1186/s12882-019-1520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golestaneh L., Bellin E., Neugarten J., Lo Y. Avoidable visits to the emergency department(ED) and their association with sex, age and race in a cohort of low socio-economic status patients on hemodialysis in the Bronx. PLoS One. 2018;13(8):e0202697. doi: 10.1371/journal.pone.0202697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kershaw K.N., Diez Roux A.V., Burgard S.A., Lisabeth L.D., Mujahid M.S., Schulz A.J. Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol. 2011;174(5):537–545. doi: 10.1093/aje/kwr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hostetter M. In Focus: Reducing Racial Disparities in Health Care by Confronting Racism. The Commonwealth Fund Newsletter. 2018. https://www.commonwealthfund.org/publications/newsletter-article/2018/sep/focus-reducing-racial-disparities-health-care-confronting Available at:
- 41.Schlessinger S.D., Tankersley M.R., Curtis J.J. Clinical documentation of end-stage renal disease due to hypertension. Am J Kidney Dis. 1994;23(5):655–660. doi: 10.1016/s0272-6386(12)70275-5. [DOI] [PubMed] [Google Scholar]
- 42.Arora S., Stouffer G.A., Kucharska-Newton A. Fifteen-Year trends in management and outcomes of non–st-segment–elevation myocardial infarction among black and white patients: The ARIC Community Surveillance Study, 2000–2014. J Am Heart Assoc. 2018;7(19):e010203. doi: 10.1161/JAHA.118.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nee R., Yan G., Yuan C.M., Agodoa L.Y., Norris K.C. Use of percutaneous coronary intervention among black and white patients with end-stage renal disease in the United States. J Am Heart Assoc. 2019;8(15):e012101. doi: 10.1161/JAHA.119.012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White K., Haas J.S., Williams D.R. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. 2012;47(3 Pt 2):1278–1299. doi: 10.1111/j.1475-6773.2012.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do D.P., Finch B.K., Basurto-Davila R., Bird C., Escarce J., Lurie N. Does place explain racial health disparities? Quantifying the contribution of residential context to the Black/white health gap in the United States. Soc Sci Med. 2008;67(8):1258–1268. doi: 10.1016/j.socscimed.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorpe J.R., Norris K.C., Beech B.M., Bruce M.A. Racism Across the Life Course. In: Ford C.L., Griffith D.M., Bruce M.A., Gilbert K., editors. Racism: Science & Tools for the Public Health Professional. APHA; Washington, DC: 2019. [Google Scholar]
- 47.White K., Borrell L.N. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place. 2011;17(2):438–448. doi: 10.1016/j.healthplace.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall Y.N., Jolly S.E., Xu P., Abrass C.K., Buchwald D., Himmelfarb J. Regional differences in dialysis care and mortality among American Indians and Alaska Natives. J Am Soc Nephrol. 2011;22(12):2287–2295. doi: 10.1681/ASN.2011010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas P., Duffrin M., Duffrin C., Mazurek K., Clay S.L., Hodges T. Community violence and African American male health outcomes: An integrative review of literature. Health Soc Care Community. 2020;28(6):1884–1897. doi: 10.1111/hsc.13065. [DOI] [PubMed] [Google Scholar]
- 50.Reeves R. The Century Gap: Low Economic Mobility for Black Men, 150 Years After the Civil War. Brookings Institute: Social Mobility Papers Web site. 2017. https://www.brookings.edu/research/the-century-gap-low-economic-mobility-for-black-men-150-years-after-the-civil-war/ Available at:
- 51.Payne R. Aha! Process; Highlands, Tx: 2005. A Framework for Understanding Poverty. [Google Scholar]
- 52.Moist L.M., Bragg-Gresham J.L., Pisoni R.L. Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;51(4):641–650. doi: 10.1053/j.ajkd.2007.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.