See Clinical Research on Page 342
Hyponatremia as Marker of Poor Outcome in Hemodialysis Patients
Predialytic hyponatremia is a marker that has been associated with poor outcome in dialysis patients in several recent studies.1,S1 In brief, hyponatremia is a proxy of a hypotonic extracellular condition reflecting relative free water excess and intracellular hyperhydration. Even though, Edelman discovered that serum sodium concentration does not depend only on total body sodium and total body water, but on the ratio of total body solutes (e.g., total body sodium and total body potassium) to total body water.S2
In this context, Fujisaki and colleagues2 bring a new light on this issue with this retrospective study using the Japanese dialysis national registry that has explored clinical outcomes associated with predialytic hyponatremia as well as plasma sodium changes occurring during hemodialysis session (post to predialysis plasma sodium concentration). For this purpose, they performed a cross-sectional analysis in 178,114 prevalent hemodialysis patients across the year 2008 that were then followed for the next 2 years. Predialysis plasma sodium concentrations were categorized in quintiles considering threshold values for the lowest as ≤136 mM/l and the highest as >141 mM/l. Traditional Cox regression and survival analyses were performed to calculate hazard ratio for mortality (all-cause, cardiovascular, cerebrovascular, infection) according to clusters of predialysis natremia (pNa) and plasma sodium (ΔpNa post-to-pre) changes. Dialysate sodium prescription was relatively homogeneous across the cohort relying on a sodium concentration of 140 mmol/l in 85.9% of patients (>140 in 4.5%; <140 in 8.9%, unknown 0.7%). In brief, the main findings of the study are the following. First, the category with the lowest predialysis plasma sodium concentrations is then further increased by the magnitude of the intradialytic plasma sodium concentration changes. Second, cause-specific mortality indicated that hyponatremia was associated with both an increase in cardiovascular as well as infection-related mortality. Third, the patient profile landing into the lowest hyponatremic quintile is significantly different (older, higher prevalence of past history of cardiovascular disease, higher interdialytic weight gain). Furthermore, penalized spline regression analyses were used to explore the relationship between mortality and plasma sodium concentration disorders (pNa and ΔpNa) as continuous variables. The hazard ratio for mortality and pNa followed a negative sigmoidal relationship with an inflexing point at 140. The hazard ratio for death and ΔpNa followed also a sigmoidal relationship presenting with an inflexing point at zero corresponding to isonatremic condition. An intradialytic increase in pNa above zero was associated with an increased risk of death and a decline appeared to be protective. Interestingly, low pNa increased the risk for ischemic stroke, limb amputation, and hip fracture but not for myocardial infarction
Put New Findings in Perspective
This study confirmed hyponatremia as a marker of poor outcome in hemodialysis patients, even in view of the low crude annual mortality rate (4.75%) reported in this Japanese dialysis cohort. Low pNa levels were also associated with an increased risk of relevant morbidity. Furthermore, correction of hyponatremia during a dialysis treatment has no protective effect. Although, given that a dialysate Na concentration of 140 mmol/l was used in the great majority of cases, the magnitude of ΔpNa is likely mainly related to predialytic natremia, the combination of both a low pNa and a large change in post- to predialysis pNa tended to aggravate mortality risk. The deleterious effect of intradialytic ΔpNa may reflect cyclic changes of brain structure (swelling and shrinking) following osmotic changes induced by dialysis.
Another striking finding is that hyponatremia (defined as pNa ≤136 mmol/l) was highly prevalent, affecting 19% of patients in this large hemodialysis cohort. Even though this figure tends to be at the upper range, it is still in agreement with the prevalence reported (median value 12%, min-max [6–29%]) in a recent review by Rhee et al.3 The association of low pNa with risk factors such as low serum albumin and high C-reactive protein levels suggests that hyponatremia may reflect the presence of an underlying disease or a severe comorbid condition, rather than a primary fluid or osmotic disorder due to inadequate dialysis treatment. In this report, fluid status of patients presenting with hyponatremia was not measured, meaning that it is impossible to elucidate further on the root cause of this problem. However, interdialytic weight gain/loss expressed in percentage of body weight is significantly higher in the hyponatremic group, suggesting that these patients were fluid overloaded. Now, the major question is to understand why predialytic hypotonic hyponatremia is associated with poor outcome in hemodialysis patients.
How to Interpret Hyponatremia in Hemodialysis Patients
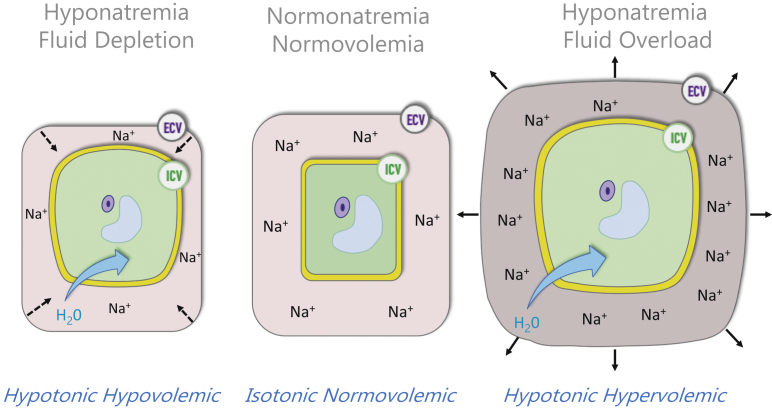
In the absence of circulating osmotic substances (i.e., glucose, fructose, sucrose, ethanol, methanol, glycol), hyponatremia is indicative of hypotonic condition reflecting free water excess and intracellular hyperhydration, as depicted in Figure 1. However, hypotonic hyponatremia in hemodialysis patients should be interpreted in this specific context with a more mechanistic approach.4 This is summarized in Table 1.
Figure 1.
Clinical meaning of hyponatremia with the most likely link with hypotonic hypervolemia in hemodialysis patients. ECV, extracellular volume; ICV, intracellular volume.
Table 1.
Interpreting hyponatremia in hemodialysis (HD) patients from a mechanical perspective
| No. | HD characteristics | Pathophysiologic explanations | |
|---|---|---|---|
| 1 | Hemodialysis patient represents a closed system with 2 main compartments (intracellular volume/extracellular volume) | Law of mass conservation applies to fluid and sodium imbalance (in/out) in the patient-HD system | Input: diet intake, endogenous production Output: hemodialysis system (Role of residual kidney function is quite limited and most likely absent) |
| 2 | Lack of kidney function (anuric) | Exclude all causes referring to decrease of free water excretion (vasopressin, cortisol, tubular defect) | Orients toward free water excess intake or compartmental translocation |
| 3 | Free water excess is quite unlikely in HD patient | Except in case of excessive thirst (i.e., hyperglycemia, angiotensin II) or potomania (mental disorders) | Combined fluid disorders (extracellular fluid [ECF] and intracellular fluid [ICF] excess) are most likely to be present |
| 4 | Fixed dialysate sodium concentration is used in general practice | Dialysate Na prescription ranges between 135 and 142 mM/l | Hyponatremia is unlikely reflecting low dialysate sodium concentration except with technical failure or human error |
| 5 | Interdialytic weight gain is a marker of fluid and sodium accumulation | Hyponatremia may be used to quantify free water excess (hypotonic fluid imbalance) and reflect combined ECF and ICF excess | Normonatremia suggests isotonic accumulation of fluid with predominant ECF expansion |
In brief, predialysis hypotonic hyponatremia should be best considered by clinicians as a marker of excess of free water globally associated with sodium and potassium mass imbalance. Although hyponatremia may occur independently from extracellular fluid overload, the association with interdialytic weight gain suggests that hypervolemic hyponatremia may have been present in a significant percentage of patients.5
How to Manage Dialysis Patients Presenting With Hyponatremia
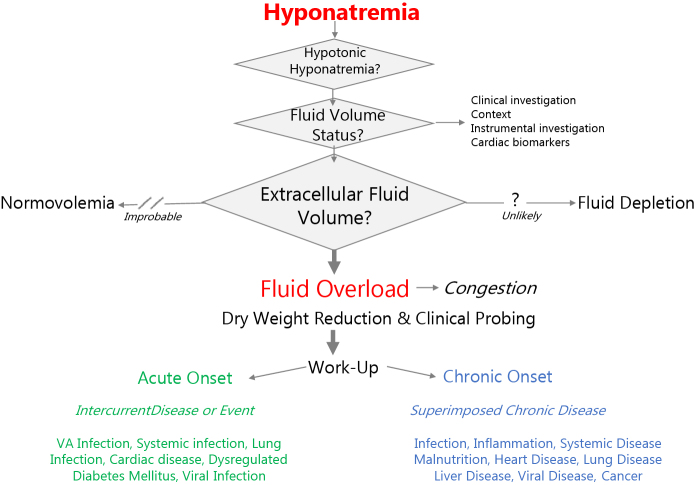
Occurrence of hypotonic hyponatremia should be perceived not only as free water excess in hemodialysis, but as a clinical indicator that deserves clinical attention and precise management as presented schematically in the algorithm of Figure 2. In this section, we briefly review the different steps that should be performed to address this issue.
Figure 2.
Clinical algorithm proposed for managing hyponatremia in hemodialysis patients. VA, vascular access.
As a first step, a precise assessment of extracellular fluid volume status should be performed to quantify degree of fluid overload.6 This relies on a stepwise approach consisting of searching for clinical symptoms (i.e., dyspnea, edema, hypertension, lung crackles) that may be complemented by instrumental measures (e.g., vascular refilling capacity and ultrafiltration tolerance, multifrequency bioimpedance, lung ultrasound, inferior vena cava diameter) and dosing cardiac biomarkers (i.e., brain natriuretic protein, N-terminal pro-brain natriuretic protein).
As a second step, based on the assessment described in the previous paragraph, fluid volume control by dry weight reduction might be planned by adjusting hemodialysis treatment schedule and conditions (i.e., extended or extra dialysis session, additional isolated ultrafiltration).7 Stepdown dry weight adjustment can be probed over a period of a few weeks with periodic reassessment for tolerance and efficacy.S3
As a third step, the search for an underlying cause is indicated. Clinical algorithm relies on the nature of onset of hyponatremia (acute, chronic), severity (moderate, severe), and cause as frequentist probability will be useful in this approach. Acute onset of hyponatremia may occur due to an acute intercurrent event (i.e., systemic infection, pulmonary disease, cardiac disease, or dysregulated diabetes mellitus).8 Chronic onset of hyponatremia should prompt cardiac assessment to exclude chronic heart failure. Malnutrition and inflammation are other frequent causes of hyponatremia that should be investigated, and their origin identified. Chronic diseases also may be encountered in hemodialysis patients revealed by chronic hyponatremia, such as liver disease, lung disease (chronic obstructive pulmonary disease, fibrosis), severe protein-energy wasting (cachexia), or even cancer. According to patient complaints and associated symptoms, a more specific investigation could then be launched
The Take-Home Message
As discussed in this study commentary, hyponatremia is a reliable indicator of poor cardiovascular and infectious outcome in hemodialysis patients that should be carefully monitored on a regular basis. Hypotonic hyponatremia acts may be associated with fluid overload, but also with inflammation, malnutrition, or underlying diseases. Regarding the highly relevant clinical value of this biomarker, one may consider monitoring predialysis natremia more frequently in the future. As recently reported, natremia may be monitored on every session based on an automated embedded sodium control module embedded in the hemodialysis machine.9 Validation of this concept deserves further long-term clinical studies. Given that hyponatremia may be associated with extracellular fluid overload corresponding to a hypervolemic hyponatremia state, hyponatremia should encourage clinicians to assess and manage more precisely extracellular fluid volume status and then subsequently to work up on the root cause search of this disorder rather than trying to correct by adjusting dialysate sodium prescription.
Disclosures
BC is part time employee of FMC. FVDS and JK declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Ye X., Kooman J.P., van der Sande F.M. Increased mortality associated with higher pre-dialysis serum sodium variability: results of the international MONitoring Dialysis Outcome Initiative. Am J Nephrol. 2019;49:1–10. doi: 10.1159/000495354. [DOI] [PubMed] [Google Scholar]
- 2.Fujisaki K., Joki N., Tanaka S. Pre-dialysis hyponatremia and change in serum sodium concentration during a dialysis session are significant predictors of mortality in patients undergoing hemodialysis. Kidney Int Rep. 2021;6:342–350. doi: 10.1016/j.ekir.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C.M., Ayus J.C., Kalantar-Zadeh K. Hyponatremia in the dialysis population. Kidney Int Rep. 2019;4:769–780. doi: 10.1016/j.ekir.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S.R., Wystrychowski G., Zhu F. Fluid dynamics during hemodialysis in relationship to sodium gradient between dialysate and plasma. ASAIO J. 2007;53:339–342. doi: 10.1097/MAT.0b013e318033cba7. [DOI] [PubMed] [Google Scholar]
- 5.Dekker M.J., Marcelli D., Canaud B. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr. 2016;70:779–784. doi: 10.1038/ejcn.2016.49. [DOI] [PubMed] [Google Scholar]
- 6.Canaud B., Chazot C., Koomans J., Collins A. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol. 2019;41:550–559. doi: 10.1590/2175-8239-JBN-2019-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charra B., Laurent G., Chazot C. Clinical assessment of dry weight. Nephrol Dial Transplant. 1996;11(Suppl 2):16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 8.Dekker M.J.E., van der Sande F.M., van den Berghe F. Fluid overload and inflammation axis. Blood Purif. 2018;45:159–165. doi: 10.1159/000485153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harlos J., Maierhofer A., Canaud B. Conductivity based online monitoring of predialytic hyponatreamia as diagnostic tool in hemodialyis. Nephrol Dial Transplant. 2020;35(Suppl 3) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.