Abstract
Introduction
Genomic testing is becoming widely available as a diagnostic tool, although widespread implementation is not yet established in nephrology.
Methods
An anonymous electronic survey was administered to investigate experience and confidence with genomic tests, perceived clinical utility of genomic services, preferences for service delivery models, and readiness for implementation among nephrologists. Questions were guided by a comprehensive literature review and published tools, including a validated theoretical framework for implementation of genomic medicine: Consolidated Framework for Implementation Research (CFIR).
Results
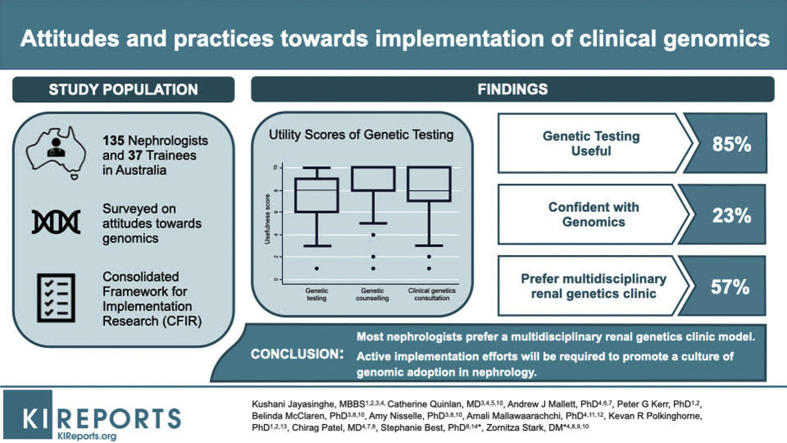
Responses were received from 224 clinicians, of which 172 were eligible for analysis. Most clinicians (132 [76%]) had referred at least one patient to a genetics clinic. Despite most clinicians (136 [85%]) indicating that they believed genetic testing would be useful, only 39 (23%) indicated they felt confident to use results of genomic testing, with pediatric clinicians feeling more confident compared with adult clinicians (12 of 20 [60%] vs. 27 of 149 [18%]), P < 0.01, Fisher exact). A multidisciplinary renal genetics clinic was the preferred model among clinicians surveyed (98 of 172 [57%]). A key implementation barrier highlighted related to the hospital or organizational culture and/or environment. Specific barriers noted in quantitative and qualitative responses included inadequate staffing, learning resources, and funding.
Conclusions
Our findings suggest support for genomic testing among nephrologists, with a strong preference for a multidisciplinary model (involving a nephrologist, clinical geneticist, and genetic counselor). Broad-ranging interventions are urgently required to shift the current culture and ensure successful implementation of genomics in nephrology, including reducing knowledge gaps, increased funding and resources, disease-specific guidelines, and streamlining of testing processes.
Keywords: genetic kidney disease, genomic implementation, implementation science
Graphical abstract
See Commentary on Page 243
Genomic testing is becoming widely available as a diagnostic tool in nephrology,1 and evidence for clinical usefulness in the care of individuals with kidney disease is beginning to emerge,2, 3, 4 with diagnostic yields ranging from 10% to 60% depending on patient selection strategies used. Funding for genetic and especially genomic testing is limited in many health care settings, and access to services such as genetic counseling and clinical genetics consultation is highly variable across many specialties, including nephrology.5, 6, 7 Given the complexity of genetic kidney disease, in addition to the practical challenges associated with patient and test selection, result interpretation, and counseling,6 the nephrology workforce needs to be prepared and supported for diagnostic genomics to be effectively implemented.
A number of diverse approaches have been proposed to meet the challenges of integrating genomics into mainstream health care systems.8 Australia has a national health system, with shared state and federal responsibility for funding of specialist services and testing. In addition, although many Australians choose to have health insurance (which is also supported by the federal government), this does not cover the cost of genetic testing. To improve implementation within nephrology, multidisciplinary renal genetics clinics have been established throughout the country (Supplementary Figure S1), driven through a national nephrology genetics collaborative, KidGen.6 This has occurred in the context of substantial investments in state and national initiatives, such as Australian Genomics and Melbourne Genomics Health Alliances, aimed at accelerating the integration of genomics into mainstream health care.9,10 These have facilitated large-scale genomic testing in selected patient groups, including kidney disease.
There is a paucity of data surrounding nephrologists’ practices relating to clinical genomic testing, with current evidence focussing on genetic predictors of chronic kidney disease risk progression and pharmacogenomic testing in broad chronic kidney disease populations, rather than those with suspected monogenic conditions.11,12 Furthermore, the readiness for implementation of genomics among the nephrology workforce is unknown. Studies of other specialists and primary care providers suggest that physicians feel underprepared to incorporate genomics in their clinical practice.13,14
We sought to determine the preparedness of nephrologists in implementing genomics into practice for those with suspected monogenic kidney disease and explore this based on level of experience. Through an electronic survey, we investigated the current attitudes and practices of Australian adult and pediatric nephrologists in genomics, and the perceived barriers and facilitators to widespread implementation. We report the findings from this survey, which may inform the future planning and development of nephrology genetics services.
Methods
Survey Design and Content
We undertook a mixed methods approach in the form of an anonymous survey with free-text boxes to allow additional insight around survey responses. Questions were guided by a comprehensive literature review15 and published tools, including a theoretical framework commonly used in genomic medicine: the Consolidated Framework for Implementation Research (CFIR).16,17 The CFIR is a validated conceptual framework used to facilitate the design, evaluation, and implementation of health care interventions. Because of the paucity of data on nephrologists’ engagement in genomics, the literature review was expanded to include studies that investigated other specialty physician practices.
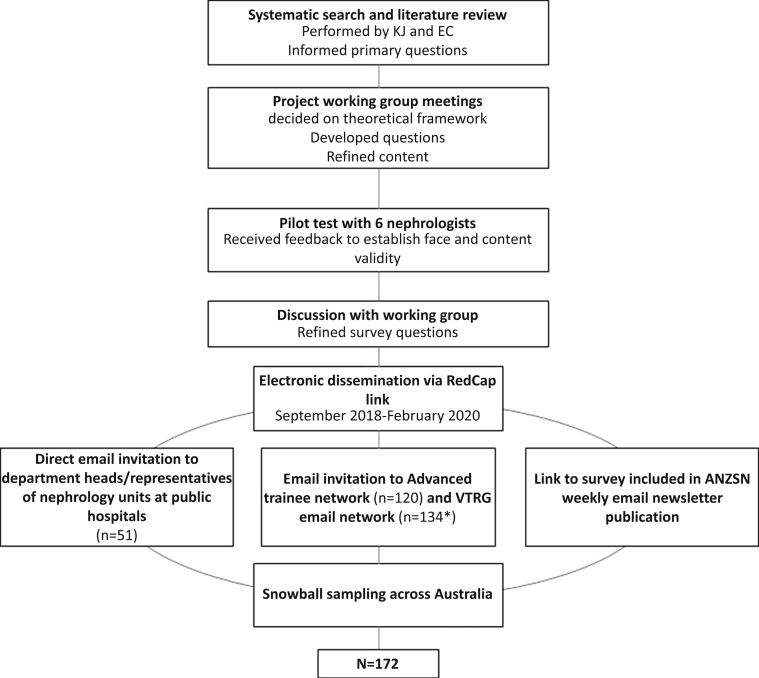
A working group of 6 experts in renal genetics implementation: 2 nephrologists (KJ and CQ), a clinical geneticist (ZS), an implementation scientist (SB), and genomic education and evaluation experts (BM and AN), developed and iteratively refined the survey questions, adapted from previous work by the authors.18,19 KJ made the final decision regarding wording amendments. There were 12 items in the survey, including 7 free-text boxes, and 1 final “overall comments” box at the end of the survey. The survey was piloted with 6 nephrologists to improve content and face validity before final refinements were made by the working group. The final survey can be found in the Supplementary Final Survey Distributed to Nephrologists. Figure 1 summarizes the survey development process.
Figure 1.
Flowchart of survey development and dissemination. ANZSN, Australia and New Zealand Society of Nephrology; VTRG, Victoria and Tasmanian Renal Group. Note: There is overlap between VTRG and Advanced trainee members.
Demographic information was collected, including qualification (nephrologist vs. trainee), years of practice, and location. Respondents were asked to report the number of patients referred to a genetics clinic for genomic testing within the past 12 months and select barriers to incorporating genomics in practice. Nephrologists were also asked about perceived clinical utility of genomic testing, and the preferred model for genomics service delivery for patients with kidney disease. Readiness for implementation was measured using an adapted provider questionnaire from the “Implementing Genomics in Practice (IGNITE)” resource hub,13 which contains 14 5-point Likert statements mapped against constructs from the CFIR.17 The study was approved by The Human Research Ethics Committee at The University of Melbourne (HREC 1646785). The survey had a plain language information statement, and consent was indicated by checking a box on the first page of the survey. Although the term “genetic” is usually used to describe single gene tests, and “genomic” describes tests referencing the whole genome, exome, or a panel of genes, these terms may have caused confusion for some nephrologists. Therefore, we provided a statement at the beginning of the survey to specify that although the term “genetic testing” was used throughout the survey, this included all types of genetic and genomic tests (Supplementary Final Survey Distributed to Nephrologists).
Dissemination Strategy and Study Sample
The survey was hosted in REDCap20 online software from September 2018 to February 2020, accessible by public link, with an estimated time to completion of <10 minutes. All nephrologists and trainees who had formally entered nephrology advanced training before 2019, and who currently practiced in Australia, either in pediatric or adult medicine, were eligible to participate. We included advanced trainees, as they represent the future nephrology workforce and therefore interventions to guide genomics implementation also should consider this group.
The recruitment strategy was multipronged (Figure 1). A convenience sample was obtained by advertising the survey in the Australian and New Zealand Society of Nephrology member newsletter and through voluntary distribution by the department head/administration of nephrology units at tertiary hospitals. An advanced trainee and educational network mailing list were used to target nephrology trainees, and nephrology providers’ practicing in Victoria and Tasmania, respectively. The newsletter and all e-mails included information related to study aims, the expected time to complete the survey, and instructions to complete the survey only once. Recipients and survey respondents were encouraged to forward the survey information to colleagues, at their discretion, resulting in snowball sampling. A presentation describing the study and providing the survey link also was given at the Australian and New Zealand Society of Nephrology annual scientific meeting. An automatic end-survey was initiated at the first page if respondents selected that they had commenced nephrology training after 2018.
Data Analysis
Descriptive statistics were used to summarize demographics, baseline characteristics, and frequency of other survey responses. The utility scores of genetics services were expressed as medians, given the data were nonparametric. Chi-squared, the χ2 test for trend, or Fisher exact tests were performed to compare survey responses across provider type (advanced trainee vs. consultant, pediatric vs. adult clinician), years of experience, and professional development in genomics within the past 12 months (yes vs. no). Given that we hypothesized that genomic technology would not be as widely used by clinicians graduating before 1995, ≤25 versus >25 years of experience was compared. Advanced trainees also are less likely to have hands-on experience with genomics but may have had more exposure to genomics in their undergraduate/postgraduate training. Therefore, we also compared responses between advanced trainees and specialists. Responses from Likert scales were analyzed as ordinal data and were combined where appropriate (for example, strongly agree/agree) to achieve reasonable numbers for analysis and visualization. To analyze the qualitative data generated from the free-text comments section, SB and KJ systematically reviewed all comments independently, and generated coding themes. Data were categorized according to these themes, initially independently and then compared, with differences of opinion discussed. Any unresolved or ambiguous free-text comments were resolved through discussion with BM. Nonidentifying demographic details are presented together with qualitative comments, including whether the clinician had ordered a test in their career (ordered = Y/N).
Results
Participant Characteristics
A total of 224 eligible participants responded to the survey, of whom 52 (23%) chose not to proceed beyond giving demographic information, and therefore were excluded. This resulted in 172 responses suitable for analysis, including 135 nephrologists and 37 nephrology trainees, of whom 141 (83%) fully completed the questionnaire. The remainder of respondents completed ≥90% of questions and were included in the analysis of the questions they answered. Because of the broad dissemination methods used, we were unable to calculate an accurate response rate. However, in 2018, the estimated number of practicing nephrologists and advanced trainees in Australia was 450 and 120, respectively (Australian and New Zealand Society of Nephrology, personal communication, 2018), hence the survey represented at least 30% of practicing nephrologists and advanced trainees in Australia.
Detailed demographic characteristics of the 172 survey participants are summarized in Table 1. The location of practice among clinicians was representative of the distribution of nephrologists in Australia, according to data provided by the Royal Australasian College of Physicians.21 No respondents indicated they had formal genetics subspecialist qualifications.
Table 1.
Baseline data of survey respondents
| Type of provider | n | % | % Representationa |
|---|---|---|---|
| Adult nephrologist | 119 | 69.2 | 78.4 |
| Pediatric nephrologist | 16 | 9.3 | 1.7 |
| Adult trainee | 33 | 19.2 | 18.1 |
| Pediatric trainee | 4 | 2.3 | 1.8 |
| Years of practice | |||
| ≤10 | 58 | 33.7 | |
| 11–15 | 34 | 19.8 | |
| 16–20 | 19 | 11.1 | |
| 21–25 | 17 | 9.9 | |
| 26–30 | 17 | 9.9 | |
| >30 | 27 | 15.7 | |
| Location of practice | |||
| New South Wales | 50 | 29.1 | 31.8 |
| Victoria | 65 | 37.8 | 31.1 |
| South Australia | 7 | 4.1 | 5.8 |
| Northern Territory | 6 | 3.5 | 2.7 |
| Queensland | 22 | 12.8 | 17.3 |
| Tasmania | 3 | 1.7 | 1.6 |
| Western Australia | 7 | 4.1 | 7.4 |
| Australian Capital Territory | 0 | 0 | 2.3 |
| Missing | 12 | 7 | N/A |
| Number of patients referred for genetic/genomic testing (via clinic or directly) over the past 12 mo | |||
| 0 | 80 | 46.5 | |
| 1–4 | 66 | 38.4 | |
| 5–9 | 15 | 8.7 | |
| ≥10 | 11 | 6.4 | |
N/A, not available.
Supplementary Table S6 is a detailed table broken down by provider type.
% representation by location based on data from correspondence from the Royal Australian College of Physicians (https://www.racp.edu.au); % representation by distribution by provider type based on data from Report of the Workforce Review Committee of the Australian and New Zealand Society of Nephrology 2017.
Current Practice and Beliefs
Most clinicians (132 [77%]) had referred at least one patient to a genetics clinic, with a higher proportion of nephrologists referring patients to genetics clinics compared with nephrology trainees (110 of 133, 83% vs. 22 of 37, 59%, P = 0.003). More pediatric clinicians had referred at least one patient for genetic testing compared with adult clinicians (19 of 20 [95%] vs. 113 of 150 [75%], P = 0.047). In addition, there was an increasing trend in number of tests ordered in the past year among pediatric (P < 0.001, Supplementary Table S1) compared with adult clinicians.
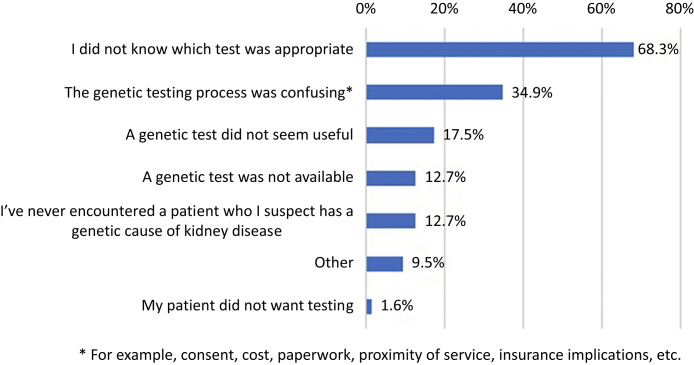
Although 109 respondents (63%) had ordered a genomic test at least once, 28 of 109 (26%) of them stated they had “never” been actively involved with results disclosure, and 58 of 109 (53%) indicated they had “sometimes” been involved. Sixty-three respondents (37%) had never ordered a genomic test, with reasons outlined in Figure 2. Of the 92 clinicians who had ordered a test within the past 12 months, 66 (72%) had ordered a test for 1 to 4 patients over this period (demographics of these respondents are provided in Supplementary Table S2).Clinicians were also asked to select from a list, the most challenging aspects of genomic testing. The most challenging aspects reported were: “selecting the right test”, followed by "interpreting the test result" and “identifying which patient[s] to test” (Table 2). One nephrologist gave further details as to why they had not ordered a test: “the different services available are not clearly delineated or easy to discern. I have no idea where I should refer patients onto. I would be happy to refer onwards if this information were readily available” (adult nephrology trainee, ordered = N, R#192). Another nephrologist commented at the conclusion of the survey that support was needed for results disclosure: “There is definitely a need for a multidisciplinary team specializing in renal genetics to assist with counseling/interpretation of results” (adult nephrologist, R#131).
Figure 2.
Reasons for never ordering a genomic test b (n = 63). Note: Respondents were able to select more than 1 reason.
Table 2.
Most challenging aspects in the management of patients with suspected genetic kidney disease
| Listed options | Scorea |
|---|---|
| Selecting the right test | 215 |
| Interpreting the test result | 188 |
| Identifying which patient(s) to test | 161 |
| Follow-up genetic counseling of family | 96 |
| Discussing results with patient and/or family | 95 |
| Integrating result into clinical care | 83 |
| Ordering the test | 78 |
| Consenting for the genetic test | 60 |
| Other: please specifyb | 16 |
Respondents were asked to rank the top 3 aspects: first most challenging was given score of 3, second most challenging was given score of 2, third most challenging was given score of 1; totals of these scores for each option are displayed. The top 3 challenges were the same in trainees and consultants.
Listed in Supplementary Table S8.
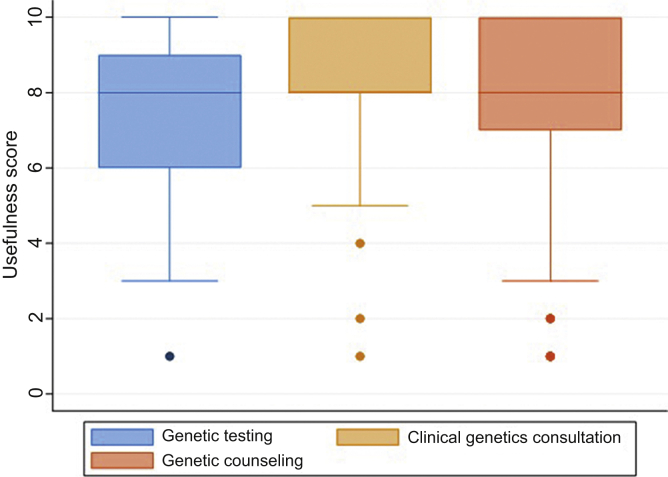
Most providers (136 [85%]) indicated that they believed genetic testing would be useful in the management of patients with suspected genetic kidney disease (6 respondents selected unsure). Median utility scores of genetic testing, clinical genetics consultation, and genetic counseling are displayed in Figure 3. Clinicians also believed that of patients who underwent genetic testing, a high proportion would have a result that would affect patient care (median 70% of patients tested, range 0%–100%). Free-text comments were received from 38 (22%) respondents. Some nephrologists commented at the conclusion of the survey that genetic testing will become more important in the future: “I have referred many patients to a renal genetics clinic and on rare occasions I get something useful. However, that is not the point. This is a new and growing area which needs to be encouraged, and sending in members of interesting kindreds, or conditions of interest to the clinic, will enable these groups to develop a database of matching genes banks, clinical histories and family trees. The relevance and benefit of renal genetics will inevitably be significant, and the more we support it the quicker that will be” (adult nephrologist with >30 years of experience, ordered = Y, R#186).
Figure 3.
Utility scores of genetic testing, clinical genetics consultation, and genetic counseling services. Boxes show median and interquartile range. Whiskers show upper and lower extremes. Outliers are plotted. Note: Clinicians were asked to rank the usefulness of these services from 1 = “Not at all useful” to 10 = “Very useful”; useful is defined as score of ≥6.
Preferences for Model of Service Delivery
Most clinicians (98 [57%]) preferred to refer patients to a multidisciplinary renal genetics clinics (Table 3). The second most popular model was that the nephrologist orders the test themselves (38 [22%]), followed by referring to a clinical genetics service (25 [15%]). Compared with senior nephrologists (>25 years), junior nephrologists were more likely to prefer a multidisciplinary model of service delivery (64% vs. 39%, P = 0.004, Table 3). There was no association between respondents’ self-perceived confidence levels with genomic testing and the preference for a multidisciplinary model (P = 0.87). When considering practicalities of attending a genetics service, the median percentage of patients whom nephrologists considered unable to attend was 10% (range 0–90). Several free-text responses reflected views on the preferred clinic model (Supplementary Table S3), with some nephrologists recognizing the clinic model may evolve: “I think for common genetic conditions being able to order test and counsel patients should be possible for a clinical nephrologist, with backup counseling if needed. For less common conditions, where there is uncertainty (vesicoureteral reflux, etc.) and where the genetic test isn't obvious, then initial genetics referral may be better. I think this is a field that is in its infancy in adult nephrology and time and experience will likely see a reasoned response – somewhere between limited genetic services being swamped with referrals (I'm told the local waiting time is 6 months) and much of the initial testing being ordered and actioned by the treating nephrologist. Obviously, the profession will need to upskill in this area and quality training resources and investigation and management algorithms will be helpful” (adult nephrologist with 21–25 years of clinical experience, ordered = Y, R#205).
Table 3.
Preferred model of service delivery, by level of experience
| Preference for model of service, frequency | ≤25 years of experience (n = 127) | >25 years of experience (n = 44) | Total cohort (n = 171) |
|---|---|---|---|
| Nephrologist refers to multidisciplinary renal genetics clinic | 81 (63.8) | 17 (38.6) | 98 (57.3) |
| Nephrologist orders test and returns result with clinical genetics support as needed | 27 (21.3) | 11 (25.0) | 38 (22.2) |
| Nephrologist orders test and discloses result | 1 (0.8) | 4 (9.1) | 5 (2.9) |
| Nephrologist refers to clinical genetics | 15 (11.8) | 10 (22.7) | 25 (14.6) |
| Other | 3 (2.4) | 2 (4.5) | 5 (2.9) |
Note: there was no difference between preference of model type between trainee and consultant and preference for multidisciplinary clinic versus other (χ2 = 2.71, P = 0.61, and χ2 = 1.10, P = 0.29, respectively). Please refer to Supplementary Table S9 for table broken down by trainee versus nephrologist, and Supplementary Table S10 for details on “other” preferred models.
Data are n (%).
Perceptions of Readiness for Implementation
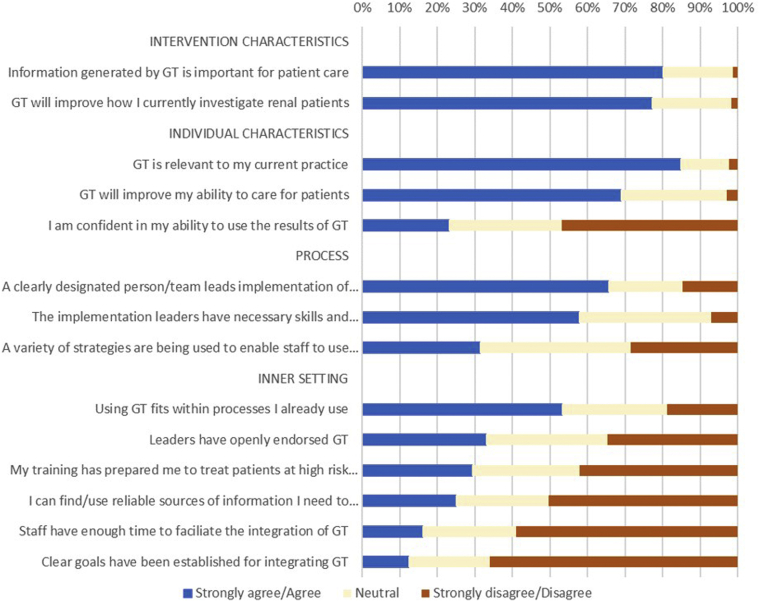
A total of 169 clinicians completed the adapted IGNITE pre-implementation provider questionnaire coded by the CFIR (see Supplementary Tables S4 and S5 for responses and details of constructs).16 The constructs that scored the lowest related to the “Inner Setting.” This construct includes participants’ views on the implementation climate (including staff, processes, learning resources) and indicates concern over the organization’s readiness for implementing genomic sequencing. The highest scoring constructs related to the “intervention characteristics,” with most (136 = 80%) clinicians agreeing that the information generated by genomic testing is important for patient care. Despite this, only 39 (23.1%) indicated they felt confident to use results of genomic testing. Nephrology trainees felt less confident compared with nephrologists (1 of 37, 3% vs. 38 of 132, 29%, P = 0.001); however, there was no significant difference in confidence levels between junior and senior nephrologists (>25 years’ experience, P = 0.23). A greater proportion of pediatric providers selected that they felt confident compared with adult providers (12 of 20, 60% vs. 27 of 149, 18%, P < 0.001). Similarly, more pediatric providers agreed that their training had prepared them to treat patients with genetic kidney disease (13 of 20 [65%]) compared with adult nephrologists (37 of 151, 25%, P < 0.001). Responses of the overall cohort are summarized in Figure 4, and further details including comparisons between pediatric and adult nephrology clinicians are provided in Supplementary Tables S6 and S7.
Figure 4.
Provider responses to the adapted IGNITE pre-implementation provider questionnaire, coded by Consolidation Framework for Implementation Science. GT, genomic testing.
Genomic Education and Training
Fewer than half (77 [45%]) of respondents had attended professional development activities in genomics, with no difference in attendance by years of professional experience (P = 0.98). The most common preferences for modes of professional development were intradisciplinary workshops (69%) and conferences (65%), rather than specific genomics workshops or conferences (Table 4). When asked about current practices in learning about genomics, the most common response was reading journals (55%), then specialty seminars and conferences (49%). Group reflections, such as multidisciplinary meetings, were also favored for both learning about genomics (32%) and regular professional development activities (32%). A significantly higher proportion of providers who attended professional development selected they felt confident to use results of genetic testing (27 of 76, 36% vs. 12 of 92, 13%, P = 0.001), and referred patients for genomic testing (55 of 77 [71%] vs. 52 of 93 [56%] who had not attended professional education, P = 0.037). Providers who had attended education in genomics also preferred a multidisciplinary clinical model compared with those who had not (53 of 77 [69%] vs. 44 of 93 [47%], P = 0.005).
Table 4.
Preferences for genomic education
| Preferences | n | % |
|---|---|---|
| Which of the following do you currently access to keep up to date with, or learn new skills in, genomic medicine?a | ||
| Reading specialty texts (journals, papers, etc.) | 94 | 54.7 |
| Internal specialty seminars, conferences, etc. | 85 | 49.4 |
| External specialty seminars, conferences, etc. | 76 | 44.2 |
| Participating in multidisciplinary meetings | 55 | 32.0 |
| CPD/CME activities | 46 | 26.7 |
| External genetic or genomic seminars, conferences, etc. | 29 | 16.9 |
| Online webinars, courses, MOOCs, etc. | 28 | 16.3 |
| Internal genetic or genomic seminars, conferences, etc. | 21 | 12.2 |
| Certification/fellowship activities | 13 | 7.6 |
| Study days at place of employment | 12 | 7.0 |
| Social media (e.g., Twitter) | 10 | 5.8 |
| Other | 5 | 2.9 |
| Which professional development method/s do you find are most effective for you?b | n | |
| Workshop | 119 | 69.2 |
| Conference | 111 | 64.5 |
| Group discussion/reflection | 60 | 34.9 |
| Hands-on learning | 57 | 33.1 |
| Self-directed | 50 | 29.1 |
| Lecture-style | 49 | 28.5 |
| Preparing and giving a presentation/poster/paper, etc. | 45 | 26.2 |
| Online | 44 | 25.6 |
| One-on-one discussion/reflection | 30 | 17.4 |
| Other | 2 | 1.2 |
CPD/CME, continuing professional development/continuing medical education; MOOCs, massive open online course.
Note: respondents were able to select multiple responses; 172 respondents answered both questions.
Regarding current education use, the only choices that resulted in a statistical difference between trainees and consultants were “external specialty seminars/conferences” (69 of 135 [51%] consultants selected this vs. 7 of 35 [19%] or trainees, P < 0.01) and “certification/fellowship activities” (7 of 135 [5%] consultants selected this vs. 6 of 37 [16%] of trainees, P = 0.03).
Regarding the most effective professional development method, the only choice that resulted in a statistical difference between trainee and consultant was “workshop” (96 of 135 [71%] consultants selected this vs. 15 of 37 [41%] of trainees, P < 0.01). Responses of trainees versus nephrologists can be found in Supplementary Table S8.
Qualitative Responses
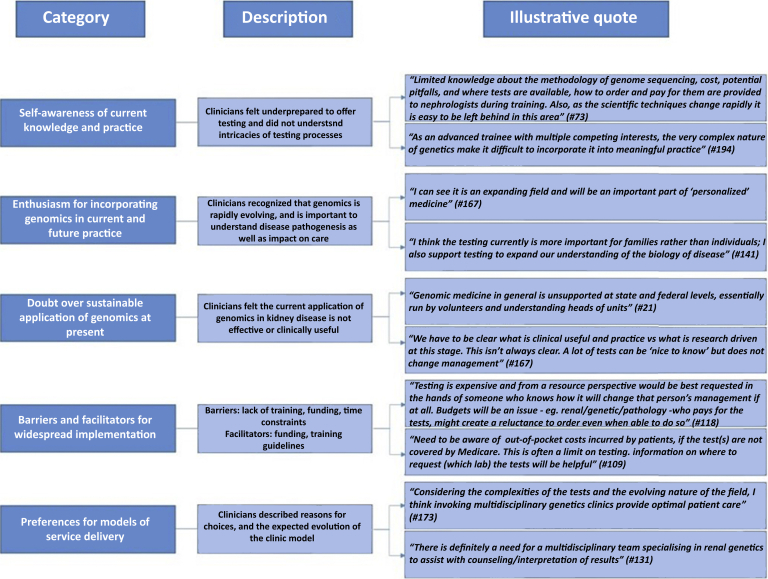
Optional free-text comments were collected at the conclusion of the survey from 38 respondents. Given the broad nature of responses, the authors were unable to categorize response domains using a theoretical framework. Therefore, based on the final comments from respondents, we identified 5 categories that reflected views on genomics implementation in nephrology:
-
•
self-awareness of current knowledge and practice of genomics
-
•
enthusiasm for incorporation of genomics in current and future practice
-
•
doubt over sustainable application at present
-
•
perceived barriers and facilitators to the implementation of genomics
-
•
preferences for model of service delivery
A description of these categories and supporting quotations are provided Figure 5. Detailed responses can be found in Supplementary Table S3.
Figure 5.
Summary of free-text comments from 38 respondents.
Discussion
Our study identified low rates of genomic testing, coupled with low confidence levels among nephrologists and trainees practicing in Australia. Pediatric providers reported higher confidence and experience, as well as increased training in genomics compared with adult providers, which may reflect increased attention to core genomics knowledge in the pediatric nephrology curriculum in Australia22 and/or higher exposure to patients with genetic disease in the pediatric population, giving opportunity for experiential learning.23 Among clinicians surveyed, attendance at genomics professional development activities was associated with increased confidence and referral of patients for genetic testing. However, it was not possible to determine from this survey the reasons for attending education. It is possible that those clinicians interested in genomics are more likely to attend education, and therefore feel more confident and refer patients for genetic testing. The most common barriers to using genomic testing among nephrologists surveyed were the test selection and ordering process, which may reflect lack of both clinical and process knowledge among this cohort. Similarly, the most challenging aspects of genomics for respondents in this survey were patient and test selection, followed by interpreting test results. The development of guidelines for genomic testing may help improve understanding in these areas and testing practices.
Despite low levels of confidence among respondents and limited utilization of genomics services in nephrology, there was enthusiasm among providers about the benefits of genomic testing in current and future practice. Clinicians also recognized the need to invest time and resources into genomics to reap these future benefits, through the generation of evidence, and through building data repositories. Overall, clinicians indicated that genomics has the potential to improve the management of nephrology patients, indicating that a median of 70% of patients who underwent genomic testing would have an impact on their clinical care. This appears to be consistent with current evidence, which reports varying diagnostic utility as high as 60% in carefully selected populations.4 There are limited data on the clinical utility of genomic testing, but a recent study reported changes to management in 59% of patients following a genomic diagnosis.24
Respondents indicated a strong preference for a multidisciplinary model approach to genomic testing, which involves a clinical geneticist and genetic counselor in the clinical consultation. Although one may attribute a preference for this model to lack of confidence with genomic testing, results from this survey suggest otherwise, given that there was no association between self-reported confidence of genomic testing and preference for model of service delivery. Moreover, those who had genomic education were more likely to prefer the multidisciplinary model, suggesting that recognizing the complexities that accompany genomic testing may inform clinician choices. Most respondents indicated minimal involvement with counseling and result disclosure, in keeping with subspecialist surveys that highlight a strong desire for additional genetics support, especially around result return.25,26 Increasing support for multidisciplinary clinics should be a priority for policymakers to help nephrologists better serve individuals with genetic kidney disease. It is also evident from many qualitative responses that as this area evolves, and genomic testing becomes more standardized, nephrologists may be more equipped to adequately assess and counsel these patients. Therefore, the current model involving a nephrologist, clinical geneticist, and genetic counselor may be required only in the short term, whereas knowledge transition is occurring. In the future, if nephrologists feel comfortable with using genomics in routine care, the renal genetics clinics may involve a nephrologist with genetics expertise together with a genetic counselor, with review by a clinical geneticist being reserved for complex cases. This model would provide a more feasible approach, particularly in the context of shortage of genomics professionals in Australia and internationally.27, 28, 29
One of the main implementation barriers highlighted in this survey related to CFIR domain of the “inner setting”; that is, the hospital or working culture and/or environment, in keeping with a recent survey of attitudes toward rapid genomic testing in pediatric critical care, which highlighted the “inner setting” as the lowest scoring construct identified by clinical genetics professionals, and found similar high-scoring constructs to our survey.18 Specifically, inadequate staffing, learning resources, and funding for genomics were highlighted in quantitative and qualitative responses. Currently, there is a paucity of data surrounding the effectiveness of any specific intervention to increase physician preparedness.30 Possible interventions in nephrology that hold the potential to increase uptake of genomics in nephrology include the development of guidelines, streamlining of test practices, and increased funding for tests. Changes in the nephrology learning curriculum may help improve clinician knowledge in the first instance, as results of this study indicate that most nephrologists felt that their training had not prepared them to integrate genomics into their practice, which echoes results of previous studies.31 Our survey suggests that the most useful means of dissemination are through internal and external nephrology seminars and workshops. This is consistent with a recent study on education needs of medical specialists that found that clinicians prefer genomics education through experiential learning opportunities that are tailored to their specialty. Furthermore, clinicians look to experts (i.e., “genomics champions”) within their own specialty.23 Therefore, it may be effective for nephrologists who have experience with genomics to lead these in-house workshops and seminars in collaboration with genomic experts, to allow specific and contextualized support for practicing genomics within the speciality.
This is the first study to investigate nephrologists’ attitudes to and practices of genomics implementation in clinical practice. Previous studies of nephrologists’ attitudes focused on pharmacogenomics11 and APOL1 risk recurrence in living kidney donors,12 neither of which are widely applicable in the Australian health care system. A recent multisite survey13 investigated physician-reported barriers to implementing genomic medicine; however, that study focused on primary care providers rather than nephrologists, and combined a number of speciality disease areas, including pharmacogenomics, diabetes, and chronic kidney disease. We adapted a tool used in the study design by Owusu Obeng et al.13 on perceptions of readiness for implementation and found similar levels of confidence and training among nephrologists to those providers at disease sites reported in this paper. Studies published on other medical specialists focus on clinician knowledge and beliefs, with little consideration to organizational constraints or other factors that influence clinician behavior.30,32 A major limitation emphasized by recent systematic reviews was that almost all reports failed to incorporate theoretical frameworks in their methodology,14,30,33 thereby limiting comparison of findings between various studies and/or specialties. Comparison of our findings of “readiness for implementation” and surveys of other specialties that adopted similar frameworks suggest that barriers and facilitators in implementing genomics may be similar across specialties.13,18 The importance of a multifaceted approach to implementation has been highlighted extensively in the literature.32 By using a theoretical framework to inform our survey design, we were able to capture multiple domains that may influence implementation. Another strength of our study is that we gathered a large and representative sample of the nephrology workforce in Australia, by experience and location.
We recognize that our study has limitations. Although all practicing nephrologists were invited to participate in the survey, our main sampling strategy focused on public sector service provision. We also cannot rule out selection bias in the responses received. For example, advanced trainees may have less practical experience with genomics, and therefore influence results. However, in most cases, apart from the referral patterns for genomic testing, there was no significant difference in responses between trainees and specialists. In addition, trainees represented 22% of the total sample, which is a close representation of the nephrology workforce in Australia (trainees make up 21% of the workforce, Table 1). Furthermore, given the broad range of providers included in this survey, and the representative distribution across location and experience levels, selection bias is less likely. In addition, cross-sectional surveys may introduce response bias, and given several questions regarding practice patterns relied on self-reporting, they were subject to recall bias. Because of our broad and inclusive dissemination technique, we cannot estimate a response rate, as our total sample population was unknown. However, even if all nephrologists in Australia were invited to participate, this would achieve a minimum response rate of 30%. The response rate is higher, and our sampling strategy is more inclusive, compared with previously published surveys of nephrologists and other physicians in developed countries.30,34, 35, 36, 37, 38 Finally, our survey results, including the enthusiasm for genomics implementation by nephrologists in Australia, must be considered alongside the current implementation climate available to nephrologists who were surveyed. Over the past 3 years, more than AUD $125M (USD $87M) have been invested into supporting research and infrastructure to accelerate clinical genomics implementation through Australian genomics, and state-funded genomics programs.10 One of the research programs specifically focuses on determining the workforce training and education needs and evaluating genomic education interventions, whereas another is evaluating genomic testing in suspected monogenic renal disorders.39 Such initiatives have a fundamental role in promoting genomics implementation in the workforce and ultimately will enable effective translation of genomics from research to clinical practice.10
Conclusions
Our study identifies that despite low uptake and confidence in genomic medicine by nephrology specialists, most believed genomics to be useful in clinical care. There was strong support of a multidisciplinary service model, which will help the transition of genomic testing to the nephrology workforce. We highlight a broad range of barriers that span multiple implementation domains. Interventions aimed at addressing the working culture include increased funding and resources, disease-specific guidelines, and streamlining of testing processes to ensure successful implementation of genomics within nephrology. Future research should focus on exploring barriers to implementation in detail, as well as identifying and testing interventions to increase clinician preparedness.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The results presented in this paper have not been published previously in whole or part, except in abstract format.
This work was supported by the Victorian Government’s Operational Infrastructure Support Program and a grant from the Australian National Health & Medical Research Council (GNT1113531); the contents are solely the responsibility of the individual authors and do not reflect the views of the NHMRC.
The authors acknowledge Erin Crellin for her contribution to the systematic search used to conduct the literature review.
Footnotes
Supplementary Final Survey Distributed to Nephrologists.
Figure S1. Map of current multidisciplinary renal genetics clinics in Australia.
Table S1. Number of patients referred for testing by provider type, for the 109 clinicians who selected they had ordered at least 1 test in their career.
Table S2. Demographic details based on referring at least 1 patient for genetic testing in the past 12 months
Table S3. Details of all qualitative responses provided at the conclusion of the survey.
Table S4. IGNITE pre-implementation provider questionnaire responses: comparison of adult and pediatric clinicians.
Table S5. IGNITE pre-implementation provider questionnaire statements mapped to constructs.
Table S6. Baseline data of survey respondents, by provider type.
Table S7. Preferences for genomic education.
Table S8. Details “other” responses for “Most challenging aspects of patients with suspected genetic kidney disease.”
Table S9. Preferred model of service delivery, by trainee versus nephrologist.
Table S10. Qualitative information detailing “other” preferred model of renal genetics service.
STROBE Statement.
Supplementary Material
References
- 1.Groopman E.E., Marasa M., Cameron-Christie S. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142–151. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lata S., Marasa M., Li Y. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann Intern Med. 2018;168:100–109. doi: 10.7326/M17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connaughton D.M., Kennedy C., Shril S. Monogenic causes of chronic kidney disease in adults. Kidney Int. 2019;95:914–928. doi: 10.1016/j.kint.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas C.P., Freese M.E., Ounda A. Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet Med. 2020;22:1025–1035. doi: 10.1038/s41436-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Government Department of Health . Australian Government; Canberra, Australia: 2020. Medicare Benefits Schedule Book Operating from 1 March 2020. [Google Scholar]
- 6.Jayasinghe K., Quinlan C., Stark Z. Renal genetics in Australia: kidney medicine in the genomic age. Nephrology (Carlton) 2019;24:279–286. doi: 10.1111/nep.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea L., Newschaffer C.J., Xie M. Genetic testing and genetic counseling among Medicaid-enrolled children with autism spectrum disorder in 2001 and 2007. Hum Genet. 2014;133:111–116. doi: 10.1007/s00439-013-1362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark Z., Dolman L., Manolio T.A. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet. 2019;104:13–20. doi: 10.1016/j.ajhg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaff C.L., Winship I M., Forrest S M. Preparing for genomic medicine: a real world demonstration of health system change. NPJ Genom Med. 2017;2:16. doi: 10.1038/s41525-017-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark Z., Boughtwood T., Phillips P. Australian genomics: a federated model for integrating genomics into healthcare. Am J Hum Genet. 2019;105:7–14. doi: 10.1016/j.ajhg.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiech K.M., Tripathy P.R., Woodcock A.M. Implementation of a renal precision medicine program: clinician attitudes and acceptance. Life (Basel) 2020;10:32. doi: 10.3390/life10040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh T., Mohan S., Sawinski D. Variation of ApoL1 testing practices for living kidney donors. Prog Transplant. 2020;30:22–28. doi: 10.1177/1526924819892917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owusu Obeng A., Fei K., Levy K.D. Physician-reported benefits and barriers to clinical implementation of genomic medicine: a multi-site IGNITE-Network Survey. J Pers Med. 2018;8:24. doi: 10.3390/jpm8030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med. 202022:1149–1155. [DOI] [PubMed]
- 15.Jayasinghe K., Stark Z., Mallett A.J. Implementing genomics into nephrology services - A review of the literature and study protocol. Nephrology. 2018;23(Supplement 3):64. [Google Scholar]
- 16.Orlando L.A., Sperber N.R., Voils C. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network's Common Measures Working Group. Genet Med. 2018;20:655–663. doi: 10.1038/gim.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damschroder L.J., Aron D.C., Keith R.E. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark Z., Nisselle A., McClaren B. Attitudes of Australian health professionals towards rapid genomic testing in neonatal and paediatric intensive care. Eur J Hum Genet. 2019;27:1493–1501. doi: 10.1038/s41431-019-0429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClaren B., King E., Crellin E. Development of an evidence-based, theory-informed national survey of physician preparedness for genomic medicine and preferences for genomics continuing education. Front Genet. 2020;11:59. doi: 10.3389/fgene.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Royal Australasian College of Physicians Home page. 2020. https://www.racp.edu.au Available at:
- 22.Royal Australasian College of Physicians . RACP; Sydney, Australia: December 2013. Nephrology Advanced Training Curriculum. [Google Scholar]
- 23.McClaren B.J., Crellin E., Janinski M. Preparing medical specialists for genomic medicine: continuing education should include opportunities for experiential learning. Front Genet. 2020;11:151. doi: 10.3389/fgene.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasinghe K., Stark Z., Kerr P.G. Clinical impact of genomic testing in patients with suspected monogenic kidney disease. Genet Med. 2021;23:183–191. doi: 10.1038/s41436-020-00963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson L.-M., Valdez J.M., Quinn E.A. Integrating next-generation sequencing into pediatric oncology practice: an assessment of physician confidence and understanding of clinical genomics. Cancer. 2017;123:2352–2359. doi: 10.1002/cncr.30581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weipert C.M., Ryan K.A., Everett J.N. Physician experiences and understanding of genomic sequencing in oncology. J Genet Counsel. 2018;27:187–196. doi: 10.1007/s10897-017-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penon-Portmann M., Chang J., Cheng M., Shieh J.T. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22:227–231. doi: 10.1038/s41436-019-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragojlovic N., Borle K., Kopac N. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet Med. 2020;22:1437–1449. doi: 10.1038/s41436-020-0825-2. [DOI] [PubMed] [Google Scholar]
- 29.Nisselle A., Macciocca I., McKenzie F. Readiness of clinical genetic healthcare professionals to provide genomic medicine: an Australian census. J Genet Couns. 2019;28:367–377. doi: 10.1002/jgc4.1101. [DOI] [PubMed] [Google Scholar]
- 30.Paul J.L., Leslie H., Trainer A.H., Gaff C. A theory-informed systematic review of clinicians' genetic testing practices. Eur J Hum Genet. 2018;26:1401–1416. doi: 10.1038/s41431-018-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berns J.S. A survey-based evaluation of self-perceived competency after nephrology fellowship training. Clin J Am Soc Nephrol. 2010;5:490–496. doi: 10.2215/CJN.08461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crellin E., McClaren B., Nisselle A. Preparing medical specialists to practice genomic medicine: education an essential part of a broader strategy. Front Genet. 2019;10:789. doi: 10.3389/fgene.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts M.C., Kennedy A.E., Chambers D.A., Khoury M.J. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19:858–863. doi: 10.1038/gim.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koil C.E., Everett J.N., Hoechstetter L. Differences in physician referral practices and attitudes regarding hereditary breast cancer by clinical practice location. Genet Med. 2003;5:364–369. doi: 10.1097/01.gim.0000086477.00766.c9. [DOI] [PubMed] [Google Scholar]
- 35.Waller K.M.J., Wyburn K.R., Shackel N.A. Hepatitis Transmission Risk in Kidney Transplantation (the HINT study): a cross-sectional survey of transplant clinicians in Australia and New Zealand. Transplantation. 2018;102:146–153. doi: 10.1097/TP.0000000000001885. [DOI] [PubMed] [Google Scholar]
- 36.Hendren E.M., Reynolds M.L., Mariani L.H. Confidence in women's health: a cross border survey of adult nephrologists. J Clin Med. 2019;8:176. doi: 10.3390/jcm8020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haga S.B., Burke W., Ginsburg G.S. Primary care physicians' knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82:388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaitovich Groisman I., Hurlimann T., Shoham A., Godard B. Practices and views of neurologists regarding the use of whole-genome sequencing in clinical settings: a web-based survey. Eur J Hum Genet. 2017;25:801–808. doi: 10.1038/ejhg.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayasinghe K., Stark Z., Patel C. Comprehensive evaluation of a prospective Australian patient cohort with suspected genetic kidney disease undergoing clinical genomic testing: a study protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.