Abstract
An increasing number of primary immunodeficiencies (PIDs) have been identified over the last decade, which are caused by deleterious mutations in genes encoding for proteins involved in actin cytoskeleton regulation. These mutations primarily affect hematopoietic cells and lead to defective function of immune cells, such as impaired motility, signaling, proliferative capacity, and defective antimicrobial host defense. Here, we review several of these immunological “actinopathies” and cover both clinical aspects, as well as cellular mechanisms of these PIDs. We focus in particular on the effect of these mutations on human neutrophil function.
Keywords: Actin cytoskeleton, Primary immunodeficiency, Neutrophils, Immune cells
Introduction
Actin is one of the most highly conserved proteins during the course of evolution and plays a key role in many cellular functions, including cell motility, cell division, and endocytosis. Furthermore, by forming filaments, that is, the polymerization of globular actin (G-actin) into filamentous actin (F-actin), it provides structural support and helps cells to maintain their shape and internal organization [1]. To date, several primary immunodeficiencies (PIDs) have been identified which are caused by mutations in genes which encode for proteins involved in actin regulation, also referred to as “immuno-actinopathies” [2, 3, 4]. PIDs can impact the immune system on different levels, from the innate immune system (phagocyte and complement defects) to the adaptive immune system (T-cell and B-cell defects) [2, 3]. In this review, we focus on PIDs in humans caused by defects in the actin cytoskeleton and the impact on phagocytes, specifically neutrophils. Once released from the bone marrow, granulocytes circulate for some hours in the circulation before extravasation for routine tissue surveillance, for instance of the oropharynx or gut, or they will be more massively recruited into tissues during localized or disseminated infection or inflammation. Neutrophils are capable of eliminating pathogens by several mechanisms, that is, by the intracellular production of reactive oxygen species (ROS) and degranulation of antimicrobial proteases into the so-called phagosomes following uptake or phagocytosis of microbes or release into the extracellular environment of ROS and proteolytic activity from their (azurophilic) granules, as well as localized accumulation of this activity onto net-like DNA-containing structures, released upon cell death in which microbes might be “trapped,” known as neutrophil extracellular traps [5]. As will be illustrated by the severely impaired hematopoietic immune system in patients with dysregulation of their actin cytoskeleton, an efficient host defense is dependent on the rapid reorganization of the actin cytoskeleton.
The Actin Cytoskeleton
The actin cytoskeleton is a highly dynamic network of filamentous proteins, which consists of 4 main components, that is, microtubules, intermediate filaments, septins, and actin filaments [6, 7]. There are 6 mammalian actin isoforms which share nearly identical amino acid sequences. Four of these isoforms are mainly expressed in different types of muscles, namely, α-cardiac, α-skeletal, α-smooth muscle, and γ-smooth muscle actin. The other 2 isoforms are non-muscle actin, also termed β-cytosplasmic and γ-cytosplasmic actin, which are ubiquitously expressed. Although there are similarities between these isoforms (i.e., cytoplasmic actins only differ by 4 amino acids), studies have shown that they have distinct biological roles [8, 9]. Part of the actin is localized in the cytoplasm as monomeric globular or G-actin and part is contributing to filamentous or F-actin which localizes just beneath the plasma membrane, in the cytoplasm to give the cell its basal form (including stress fibers), and in part also in the nucleus in some cells.
As mentioned, these actin filaments are linear polymers of G-actin. F-actin adopts 2 main forms, namely, branched actin networks or linear actin networks (and mixtures of the 2). The formation of either of the 2 major subforms is mediated by distinct actin nucleators. The actin-related proteins-2/3 (ARP2/3) complex, consisting of 7 subunits, mediates the formation of branched actin networks by nucleating a daughter filament to the side of the preexisting actin filaments in an ATP-dependent process [10]. Branched actin networks are needed for the generation of protrusive force that aids in cell adhesion and motility. The ARP2/3 complex is essential in the formation of lamellipodia [11]. Formins form a family of different actin nucleators involved in the formation of linear actin networks but also promote elongation of preexisting filaments [12, 13]. Aside from actin nucleators, actin dynamics are regulated by numerous actin-binding proteins which have diverse functions, for example, stabilizing, capping, bundling, and cross-linking of actin filaments (shown in Fig. 1) [1, 14, 15, 16]. Mutations in genes encoding several of these actin-binding proteins have been described to cause PIDs of the innate immune system, and these will be further discussed in this review (Table 1).
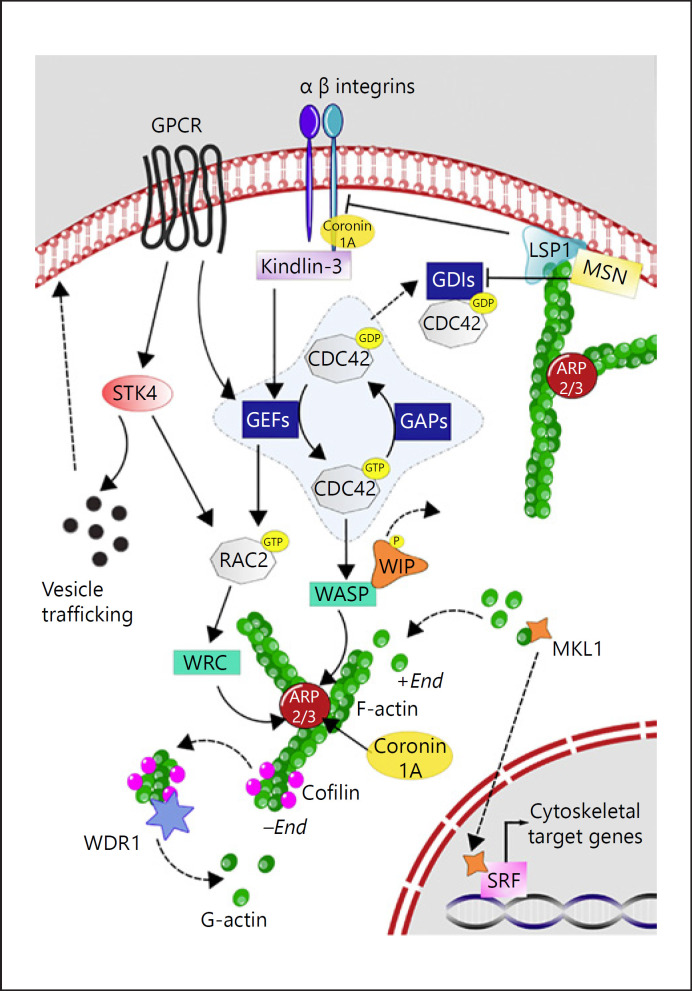
Fig. 1.
Summary of actin cytoskeleton dynamics. The proteins involved in PIDs described in this review are depicted. When a neutrophil gets activated (e.g., by ligand binding to GPCRs) several signaling cascades are initiated. STK4 controls the translocation of vesicles containing laminin-binding integrins and neutrophil elastase to the surface (indicated by mice studies). STK4 can also activate RAC via the kinase PKC-α and the GDP-dissociation inhibitor RHOGDI2. RHO GDIs sequester Rho GTPases family members (e.g., RAC2 and CDC42) in the cytosol to render them inactive. Activated GEFs (e.g., DOCK2) mediate the exchange of GDP to GTP, thereby promoting the binding of Rho GTPase family members to their specific effectors (WRC and WASP, respectively). These effectors in turn activate the actin nucleator complex ARP2/3 (which ARPC1B is part of). Also coronin-1A can directly interact with ARP2/3, as well as with integrin beta-2. Upon activation of WASP, WIP dissociates from WASP. Actin depolymerizes at the “pointed (−) end,” and the severing and disassembly of actin filaments by cofilin is enhanced by WDR1. Monomers of actin (G-actin) can be incorporated in the growing filamentous actin (F-actin) strand (barbed [+] end), which allows for the dissociation of MKL1 from G-actin, thereby allowing translocation of MKL1 to the nucleus, where it can activate SRF-dependent transcription of cytoskeletal target genes. MSN links actin filaments to the plasma membrane and membrane receptors, and it can initiate activation of Rho family members by reducing the activity of RHO GDI. LSP1 is an actin-binding protein which bundles actin filaments. Also, LSP-1 is thought to act as a negative regulator of integrin beta-2-mediated adhesion (mice studies). The most prominently expressed integrin complex on neutrophils is the integrin alpha-M/integrin beta-2 complex. Upon activation, it will first move from a low to a medium avidity state. The high avidity state is induced by kindlin-3, and this allows for an effective binding of integrins to substrates on the endothelium and extracellular matrix. ARP2/3, actin-related proteins-2/3; ARPC1B, actin-related protein complex-1; CDC42, cell division control protein 42 homolog; DOCK2, dedicator of cytokinesis protein 2; F-actin, filamentous actin; GDP, guanosine diphosphate; GEF, guanine nucleotide-exchange factor; GPCR, G protein-coupled receptors; GTP, guanosine triphosphate; LSP-1, leukocyte specific protein I; MKL1, megakaryoblastic leukemia 1; MSN, moesin; PIDs, primary immunodeficiencies; RAC2, Ras-related C3 botulinum toxin substrate 2; RHO GDI, RHO protein guanine nucleotide-dissociation inhibitor; SRF, serum response factor; STK4, Serine/threonine-protein kinase 4; WASP, Wiskott-Aldrich syndrome protein; WRC, WASP-family verprolin homologue protein regulatory complex; WDR1, WD repeat-containing protein 1; WIP, WASP-interacting protein.
Table 1.
List of actinopathies with corresponding protein function, clinical symptoms, and neutrophil defects
| Disease | Protein function | Clinical symptoms | Defects reported in primary neutrophils from patients |
|---|---|---|---|
| ACTB mutations | Non-muscle actin isoform | Recurrent infections and mental disability | Impaired chemotaxis and ROS production, lower membrane potential response |
| MKL1 deficiency | Co-activator of SRF | Severe, recurrent bacterial infections | Reduced actin content and impaired actin polymerization, impaired migration, impaired spreading, enhanced degranulation, and defective endothelial transmigration |
| ARPC1B deficiency | Component of the ARP2/3 complex | Viral and bacterial infections, bleeding tendency, vasculitis, eczema, allergy, thrombocytopenia, and eosinophilia | Impaired actin polymerization, impaired motility, enhanced degranulation, and impaired podosome formation |
| Wiskott-Aldrich syndrome (WAS) | Nucleation factor for the ARP2/3 complex | Recurrent infections, severe bleeding, eczema, autoimmune diseases, lymphoma, thrombocytopenia, and lymphocytopenia | None reported |
| X-linked thrombocytopenia (WAS) | Nucleation factor for the ARP2/3 complex | Mild immunodeficiency, bleeding, and thrombocytopenia | None reported |
| X-linked neutropenia (WAS) | Nucleation factor for the ARP2/3 complex | Severe neutropenia, monocytopenia, thrombocytopenia, late-onset malignancies, and recurrent infections | Enhanced basal F-actin levels, proliferation, and maturation defect |
| WIP deficiency (WIPF1) | Interactor of WASP and keeps WASP stable and inactive | Recurrent infections, eczema, papulovesicular/ulcerative lesions, bloody diarrhea, and thrombocytopenia | None reported |
| HEM1 deficiency | Component of the WRC | Recurrent bacterial and viral infections and atopic and allergic disease | Impaired migration |
| Coronin-1A deficiency | Actin-binding protein, interacts with the ARP2/3 complex, signaling mediator | Recurrent bacterial and viral respiratory infections, skin lesions, chronic warts, and chronic T-lymphopenia | Very short telomere length |
| LLS/WDR1 deficiency | Enhances severing and disassembly of actin filaments by cofilin | Severe stomatitis, recurrent infections and moderate neutropenia (periodic), fever, thrombocytopenia, and intellectual impairment | Migration defect, abnormal spreading, increased basal F-actin levels, enhanced ROS production, and abnormal location of the nucleus |
| RAC2 mutations (activating) | Rho GTPase involved in the respiratory burst and activator of the WRC | Neutropenia, recurrent respiratory infections, and lymphopenia | Impaired chemotaxis, increased basal F-actin levels, increased actin polymerization, and increased ROS production |
| RAC2 mutations (loss-of-function) | Rho GTPase involved in the respiratory burst and activator of the WRC | Neutrophilia, severe bacterial infections, poor wound-healing, lymphopenia, and hypogammaglobulinemia | Impaired chemotactic response, decreased basal F-actin levels, impaired actin polymerization, decreased ROS production, and absent azurophilic granule release |
| DOCK2 deficiency | GEF activating RAC | Severe bacterial and viral infections and T-cell lymphopenia | Impaired actin polymerization and impaired ROS production |
| DOCK8 deficiency | GEF activating CDC42 | Severe recurrent viral, bacterial, and fungal infections; eczema; and allergies | Mild motility defect |
| STK4 deficiency | Kinase for several nuclear proteins and activator of RAC through PKC-α and LyGDI | Recurrent bacterial and viral infections, CD4+ T-lymphopenia, mucocutaneous candidiasis, eczema, molluscum contagiosum, high IgA/E levels, cardiac aberrations, and episodes of neutropenia | Loss of mitochondrial membrane potential and susceptibility to apoptosis |
| MSN deficiency | Links actin filaments to the plasma membrane and signaling mediator for selectins | Recurrent bacterial and viral infections, recurrent molluscum, eczema, and lymphopenia | None reported |
| LAD-I (ITGB2) | Facilitates cell-cell and cell-ECM interactions | Early onset recurrent bacterial or fungal infections, delayed umbilical cord detachment, omphalitis, poor wound healing, non-pyogenic wounds, and severe neutrophilia | Impaired ROS production, reduced killing of Candida albicans, impaired adhesion (static and flow), defective chemotaxis, and defective endothelial transmigration |
| LAD-III (FERMT3) | Key-activator of integrins | Milder immune deficiency compared to LAD-I, but accompanied by a Glanzmann-like bleeding disorder | Impaired ROS production, reduced killing of C. albicans, impaired adhesion (static and flow), defective chemotaxis, and defective endothelial transmigration |
| CalDAG-GEFI deficiency | GEF activating RAP1 | Impaired platelet function and bleeding disorder | Defective integrin activation |
| NAD | Unknown gene | Recurrent fevers, recurrent pulmonary infections, leukocytosis, non-pyogenic infections, hepatosplenomegaly, and thrombocytopenia | Decreased basal F-actin levels, impaired actin polymerization, impaired podosome formation, and increased ROS production |
ACTB, β-actin; ARPC1B, actin-related protein complex 2/3 subunit 1B; ARP2/3 complex, actin-related protein complex 2/3; CalDAG-GEFI, calcium and DAG-regulated guanine nucleotide exchange factor I; CD, marker of cell differentiation; CDC42, cell division control protein 42; DOCK, dedicator of cytokinesis; ECM, extracellular matrix; FERMT3, fermitin family homolog 3; F-actin, filamentous actin; GEF, guanidine exchange factor; Ig, immunoglobin; ITGB2, β-2-integrin; LAD, leukocyte adhesion deficiency; LLS, lazy leukocyte syndrome; LyGDI, lysine GDP-dissociation inhibitor; MKL1, megakaryoblastic leukemia 1; MSN, moesin; NAD, neutrophil actin dysfunction; PKC, protein kinase C; RAC, Ras-related C3 botulinum toxin substrate; RAP1, Ras-related protein 1; Rho GTPase, rho guanidine triphosphosphatase; ROS, reactive oxygen species; SRF, serum response factor; STK4, serine/threonine kinase 4; WAS, Wiskott-Aldrich syndrome; WASP, Wiskott-Aldrich syndrome protein; WAVE, WASP family verprolin-homologous protein; WRC, WAVE regulatory complex; WDR1, WD repeat-containing domain 1; WIP, WASP-interacting protein.
PIDs Caused by Defective Cytoskeletal Regulation
The assembly of G-actin into F-actin is mediated by actin nucleators, including the ARP2/3 complex. This complex is inherently inactive and needs activation by Wiskott-Aldrich syndrome protein (WASP) or the WASP-family verprolin homologous protein (WAVE) regulatory complex (WRC) (shown in Fig. 2). Ena/VASP proteins enhance actin filament elongation and can also bind to the WRC to enhance ARP2/3 complex activity [17]. Furthermore, depolymerization of actin filaments is essential for dynamic actin remodeling and mediated by several regulatory proteins (shown in Fig. 1). We will discuss several PIDs, which impair the assembly and disassembly of actin filaments. To our knowledge, there are no disease-causing mutations described in the genes which encode Ena/VASP proteins (i.e., VASP, ENAH, or EVL) in humans, and therefore, these proteins will not be discussed.
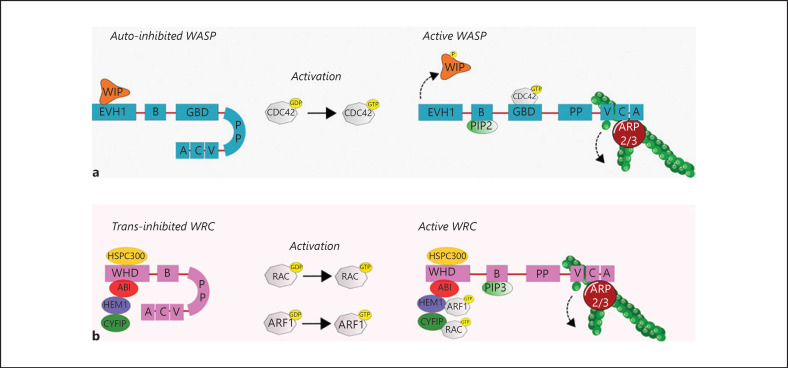
Fig. 2.
Activation of WASP and the WRC. WASP consists of 5 domains (a): EVH1 domain, Basic domain, GBD domain, PP domain, and the VCA region. The protein is kept in an inactive state through auto-inhibition by interaction of the GBD region with the VCA region. The EVH1 domain of WASP can interact with WIP, which stabilizes the inactive state of WASP. Upon activation of WASP with GTP-CDC42 (which binds on the GBD domain), PIP2 (which binds to the B domain), or tyrosine phosphorylation of WIP (which causes dissociation of WIP), the VCA region can interact and activate the ARP2/3 complex, thereby initiating actin polymerization. Furthermore, the PP domain of WASP can bind SH3-domain-containing proteins (e.g., cortactin and WISH) and profilin, which enhances ARP2/3 activation (not depicted). b The WRC is a protein complex consisting of HSPC300, ABI, HEM1, CYFIP, and WAVE. WAVE has a similar protein structure as WASP, with a B domain, PP domain, and VCA region. The WRC is activated by GTP-RAC or GTP-ARF1, which bind to CYFIP or HEM1, respectively. Subsequently, the VCA region of WAVE can bind G-actin and the ARP2/3 complex, thereby initiating actin polymerization. PIP3 binds to the B domain, which is important for the localization of WAVE. Also, the protein IRSp53 binds to the PP domain of WAVE. It can also bind to RAC and enhance the activity of the WRC (not depicted). More WASP and WAVE interacting proteins are reviewed by Takenawa and Suetsugu [223]. ABI, Abelson interactor 1; ARF1, ADP-ribosylation factor 1; ARP2/3, actin-related proteins-2/3; CDC42, cell division cycle 42; CYFIP, cytoplasmic FMR1-interacting protein 1; EVH1, Ena-VASP-homology-1; G-actin, globular actin; GBD, GTPase binding domain; GTP, guanosine triphosphate; HEM1, hematopoietic protein 1; HSPC300, hematopoietic stem/progenitor cell protein 300; PIP2, phosphatidylinositol-(4,5)-biphosphate; PIP3, phosphatidylinositol-(3,4,5)-triphosphate; PP, polyproline; SHR, Src-homology-3; WASP, Wiskott-Aldrich syndrome protein; WAVE, WASP-family verprolin homologue protein; WIP, WASP-interacting protein; WISH, WASP-interacting SH3-domain protein; WRC, WAVE regulatory complex.
ACTB Mutations
Cytoplasmic beta-actin (encoded by ACTB, chromosome 7p22.1), is 1 of the 6 isoforms of actin. Most heterozygous autosomal dominant mutations in ACTB are de novo alterations associated with Baraitser-Winter syndrome (BRWS type 1; OMIM 243310), a rare disorder characterized by distinct facial features, for example, hypertelorism and a broad nasal bridge, with cerebral malformations and intellectual disability [18, 19]. Apart from ACTB, mutations in gamma-actin (ACTG, chromosome 17q25.3), the other non-muscle actin, can also cause BRWS (type 2, OMIM 614583) [20]. While most mutations causing BRWS are postulated to be gain-of-function mutations [20], loss-of-function mutations in ACTB can result in a pleiotropic malformation syndrome with intellectual disability distinct from BRWS [21]. Although susceptibility to infection is not reported as a general phenotype in these patients, infections, including chronic respiratory infections, recurrent/chronic sinusitis, and frequent otitis media, are being observed, suggesting that mutations in ACTB might have an effect on the immune system [21].
To date, the consequence of mutated beta-actin on neutrophil function has been reported in a single patient by Nunoi et al. [22]. This patient suffered from recurrent infections, photosensitivity and mental retardation. A missense mutation in exon 6 of ACTB was identified, leading to the expression of both normal and mutated beta-actin in the patient's leukocytes, platelets, and fibroblasts. Compared to control neutrophils, patient's neutrophils demonstrated an impaired chemotactic response toward N-formyl-met-leu-phe (fMLF) and zymosan-activated serum. This impaired mobilization was confirmed by a skin window test, where a poor influx of leukocytes was observed. These neutrophils also showed a reduced ROS production and impaired membrane potential response when stimulated with fMLF [22]. This mutant actin was shown to have a significantly slower polymerization rate than control actin, explaining the impaired chemotaxis [23].
The patient also suffered from nonimmune thrombocytopenia, a symptom also observed in a separate cohort of 6 BRWS patients (of 4 unrelated families) with mutations in exon 5 and 6 of ACTB without a history of recurrent infections [24]. Two patients had leukocytosis with an increased eosinophil count, while the patient of Nunoi et al. [22] showed leukopenia with a large percentage of band neutrophils. The concomitant decrease in neutrophil count might explain the difference in phenotype between patients.
Megakaryoblastic Leukemia 1 Deficiency
Megakaryoblastic leukemia 1 (MKL1), member of the myocardin-related transcription factor family, is a transcriptional co-regulator of numerous genes involved in actin cytoskeletal dynamics, cell survival, and cell proliferation through activation of serum response factor [25]. In the cell, MKL1 is found in the cytosol (in complex with G-actin) and in the nucleoplasm. Initiation of actin polymerization promotes the dissociation of MKL1 from G-actin, thereby allowing translocation of MKL1 to the nucleus, where it can activate serum response factor-dependent transcription [26].
Loss-of-function mutations in MKL1 result in a PID, first described by Record et al. [27]. More recently, a second family with 2 siblings with a homozygous frameshift mutation in MKL1 was identified [28]. The first identified MKL1-deficient patient suffered from severe bacterial infections, and the second MKL1-deficient patient deceased as an infant from progressive and severe pneumonia by Pseudomonas aeruginosa. The MKL1-deficient sibling of the latter patient underwent a successful hematopoietic stem cell transplantation (HSCT) shortly after birth.
MKL1-deficient neutrophils showed reduced overall actin content and displayed an actin polymerization defect, subsequently leading to a migratory defect. Furthermore, proteomic and transcriptomic analyses of MKL1-deficient primary neutrophils and an HL-60 myeloid leukemia MKL1 knockdown cell line revealed several actin-related proteins and genes to be downregulated, confirming a role of MKL1 as transcriptional co-regulator. No difference in the capacity of production of ROS was observed in MKL1-deficient neutrophils, while degranulation was shown to be enhanced [27, 28]. In contrast to the first report [27], no phagocytosis defect and an intact killing of various microbial pathogens was found in neutrophils of the MKL1-deficient patient described more recently [28]. The susceptibility to (bacterial) infections observed in MKL1 deficiency could thus be explained by the inability of neutrophils to migrate towards the site of infection as opposed to a killing defect.
Both MKL1-deficient pedigrees described a mild nonimmune thrombocytopenia, which can be explained by the role of MKL1 in megakaryocyte maturation [29]. Proliferative capacity of T-cells, B-cells and NK-cells were normal. Their motility has not been formally tested. Non-hematopoietic MKL1-deficient fibroblasts were not affected in their morphology, F-actin content, and migratory capacity, which is most likely due to compensatory mechanisms of MKL2, also member of the myocardin-related transcription factor family [28]. It is reported that MKL1 and MKL2 could have redundant roles. However, in contrast to fibroblasts, MKL2 is not expressed in neutrophils [28].
Characterization of the complete clinical and immunological phenotype of MKL1 deficiency will involve identification and description of new patients. Furthermore, it would be of interest to identify which pathways are solely dependent on MKL1, considering possible redundancy between MKL1 and MKL2.
Actin-Related Protein Complex-1B Deficiency
The ARP2/3 complex is the key regulator in branching of actin filaments and therefore of importance for many cellular processes, including cell migration [10]. One of the 7 subunits of ARP2/3 is actin-related protein complex-1 (ARPC1), which is present in 2 isoforms in humans, namely, ARPC1A and ARPC1B [30]. The latter is predominantly expressed in hematopoietic cells, whereas ARPC1A is mostly expressed in non-hematopoietic tissues [31, 32].
Since 2017, several reports on ARPC1B deficiency have been published [31, 32, 33, 34, 35, 36], herein describing patients suffering from a combined immunodeficiency (CID), including a neutrophil defect. The disorder is caused by autosomal recessive, mostly homozygous mutations in ARPC1B (chromosome 7q22.1; OMIM 617718). A wide range of clinical manifestations has been described; most patients suffer from infections (both bacterial and viral), bleeding tendency, vasculitis, eczema, and allergy. The immunological phenotype is characterized by thrombocytopenia, eosinophilia, high immunoglobulin (Ig)A and IgE levels, an increased number of CD19+ B-cells, and reduced numbers of CD3+CD4+ and CD3+CD8+ T-cells [31, 32, 33, 34, 35, 36].
ARPC1B deficiency has a major impact on neutrophil motility due to an actin polymerization defect. No defect in ROS production or the phagocytosis and killing of Escherichia coli and Staphylococcus aureus was found, whereas the azurophilic granule release was also increased by ARPC1B-deficient neutrophils under suboptimal stimulation, similar to our findings in MKL1 deficiency. Investigation of the patient's primary fibroblasts revealed normal migratory behavior, most likely due to the expression of ARPC1A in these cells [31]. Interestingly, ARPC1A is expressed at a very low level in ARPC1B-deficient hematopoietic cells (i.e., platelets and neutrophils) and more so in activated peripheral blood mononuclear cells (PBMCs), compared to control cells as assessed by Western blot [33]. Apart from neutrophil defects, ARCP1B deficiency causes defects in thrombocytes [35], T-cells [33, 34, 35, 36], B-cells [35], NK-cells [33], and platelets [32], illustrating the crucial function of this ARPC1 isoform in hematopoietic cells. Apparently, the increase of ARPC1A expression is not sufficient to rescue these cells from the actin polymerization defects.
Wiskott-Aldrich Syndrome
Wiskott-Aldrich syndrome (WAS) was first described in 1937, and it was found 57 years later that a defect in the X-chromosomal WAS gene, which was named accordingly, was causative of this disease [37]. The WAS gene (chromosome Xp11.23) encodes for WASP, which is expressed solely in the non-erythroid hematopoietic lineage and is a key regulator of actin polymerization [38]. It acts as a nucleator for the ARP2/3 complex to form branched F-actin networks [39] and is important for adhesion, chemotaxis, and phagocytosis of immune cells [40, 41, 42]. On the protein level, WASP consists of 5 domains: EVH1 domain, Basic domain, GBD domain, Polyproline domain, and the VCA region. The VCA region is important for the interaction and activation of the ARP2/3 complex, and the protein is kept in an inactive state through auto-inhibition by interaction of the GBD region with the VCA region (shown in Fig. 2a) [43].
The total number of mutations found in the WAS gene numbers over 400 and depending on the location of the mutation can result in classic WAS (OMIM 30100), X-linked thrombocytopenia (XLT; OMIM 313900) or neutropenia (XLN; OMIM 300299). Patients suffering from classic WAS have a severe clinical phenotype of immunodeficiency, bleeding, and eczema, and patients who survive infancy have the tendency to develop autoimmune diseases and lymphoid malignancies [44, 45]. In the case of XLT, patients have a similar but milder “bleeding-only” phenotype compared to WAS patients [44]. In contrast to WAS and XLT, patients suffering from XLN have mutations in the GBD domain, which is important for the auto-inhibition of WASP, thus resulting in expression of constitutively active WASP (CA-WASP) [46, 47, 48, 49, 50, 51, 52]. First classified as severe congenital neutropenia [53], individuals are usually admitted to the hospital early in life due to recurring bacterial infections due to severe neutropenia, coinciding with monocytopenia, thrombocytopenia, low NK-cell numbers, and abnormal CD4/CD8 ratio [46, 47, 48, 49, 50, 51, 52]. Late-onset malignancies have been reported in 1 pedigree [46], but not the other 2 [48, 54]. Infections for all these patients are generally less severe and more manageable than for WAS patients. Individuals are generally put on prophylactic antibiotics after discharge and do not require treatment with G-CSF despite the episodes of neutropenia [44, 45, 54]. A common strategy for definitive treatment in case of classical WAS, but not XLT or XLN, consists of HSCT [44]. Recently, gene therapy for diseases caused by mutated WAS has emerged as an alternative treatment option next to HSCT [55, 56]. XLT patients may require platelet transfusions in case of severe bleeding or preoperatively.
Notably, all 3 clinical variants are caused by mutations in the WAS gene, but the clinical phenotype differs between mutations. WAS and XLT are generally caused by mutations which affect WASP expression, where WAS is usually characterized by the absence of protein, while patients with XLT generally have a partial decrease in expression and behaves as a hypomorphic variant [44]. This difference also explains the milder clinical phenotype of XLT [44, 57]. It is unclear whether and − if at all − the downregulation of WASP affects neutrophil function. Most studies do not find any impairment of neutrophil phagocytosis, killing, and migration [58, 59], but 1 study in a single WAS patient showed reduced adhesion under flow and impaired integrin clustering and respiratory burst upon coating on intercellular adhesion molecule-1 (ICAM-1) [40]. Notably, integrin activation was not affected [40]. Additionally, migration seems to be affected toward complement component 5a (C5a) [60], but not to fMLF or interleukin (IL)-8 [58, 61]. Differences between these studies might arise from different mutations, experimental settings, or use of medication. Some of these factors might cause neutrophil dysfunction, while others may not.
In contrast to WAS and XLT, patients suffering from XLN have normal to near-normal protein expression [46, 48]. These patients have mutations in the GBD domain of WAS which is important for the auto-inhibition of WASP, thus resulting in the expression of CA-WASP [43, 46, 47, 48, 49, 50, 51, 52]. The presence of CA-WASP hampers neutrophil proliferation and maturation of patient-derived CD34+ cells into fully differentiated neutrophils [46, 51, 62], which can be attributed to the increased F-actin polymer content [46, 51]. Also, patient bone marrow progenitor cells (CD34+, CD33+, and CD15+ cells) showed increased levels of spontaneous apoptosis [46]. The maturation defect is the most likely cause of the observed neutropenia in these patients due to the gain-of-function mutations in the WASP GBD domain (L270P, S272P, or I294T). Furthermore, FACS data from XLN patient neutrophils indicate that the ratio CD11b+/CD16high (mature neutrophils) to CD11b+/CD16low (metamyelocytes) is lower than healthy controls. The presence of neutrophils in saliva suggests that XLN patient neutrophils have the capacity to migrate to the gingival tissues, thus protecting the oral site from periodontal diseases which could be explained by the faster migratory properties of these cells. Another striking observation is the increased membrane expression of the NADPH oxidase subunit Gp91phox and a strong ROS production upon activation [62], although this may vary among patients [46]. Together with a normal polymorphonuclear morphology, this suggests a reduced bone marrow output of neutrophils with a mature cellular phenotype. Proteomic and transcriptomic analysis would be useful to dissect what causes this distinct phenotype.
WASP-Interacting Protein Deficiency
As the name suggests, the WASP-interacting protein (WIP) interacts with WASP. The EVH1 domain of WASP can interact with WIP, which stabilizes the inactive state of WASP, thus preventing the degradation of WASP [63, 64]. Moreover, WIP is important for correct localization of WASP [65]. Activation of WASP with the small Rho GTPase cell division cycle 42 (CDC42) or tyrosine phosphorylation of WIP causes the dissociation of WIP with WASP (shown in Fig. 2a) [43, 66].
To the best of our knowledge, 3 pedigrees with totally 6 affected individuals have been described with WIP deficiency, caused by autosomal recessive mutations in a gene for WIP family member 1 (WIPF1, chromosome 2q31.3, OMIM 614493) [67, 68, 69]. Most patients were admitted early in life with recurrent infections, eczema, papulovesicular or ulcerative lesions, bloody diarrhea, and thrombocytopenia [67, 68, 69]. The infections are treated depending on the pathogen, and individuals are generally put on prophylactic antibiotics [67, 68]. Malignancies have not been reported to date. HSCT with umbilical cord blood or bone marrow has proven successful as definitive treatment of WIP deficiency [67, 68].
In patients with WIP deficiency, WIP protein expression is found to be absent which also causes WASP expression to be abrogated, thus mimicking WAS [68]. No reports on neutrophil function of WIP-deficient neutrophils have been published, but defects have been described in patient lymphocytes. Reconstitution of WIP in 1 patient's T-cells was found to reconstitute WASP expression [68]. Furthermore, WIP deficiency seems to cause severe abrogation of migration, cell polarity, and morphology of the cell and cytoskeleton in T-cells and B-cells derived from patient material [69].
Hematopoietic Protein 1 Deficiency
Hematopoietic protein 1 (HEM1), encoded by NCKAP1L (chromosome 12q13.1), is a hematopoietic cell-specific member of the WRC [70]. This regulatory complex is intrinsically inactive and can be activated by diverse signals (including Ras-related C3 botulinum toxin substrate (RAC), kinases, and phosphatidylinositols) and thereby controls ARP2/3 complex-mediated actin polymerization (shown in Fig. 2b) [71]. Knockout of NCKAP1L in HL-60 cells has been shown to result in the concomitant degradation of other subunits of the WRC, thereby impairing cell polarity and motility. Specifically, these cells failed to form lamellipod-like protrusions at the leading edge [72]. Furthermore, loss of HEM1 results in aberrant levels and polarization of RAC activity in chemoattractant-stimulated HL-60 cells [72, 73].
Recently, 5 patients have been identified with loss-of-function mutations in NCKAP1L[74]. These bi-allelic missense mutations lead to either a loss of HEM1 protein or abrogated binding of HEM1 to the GTPase ADP-ribosylation factor 1, thereby leading to destabilization of the WRC or disruption of ADP-ribosylation factor 1-WRC binding and activation, respectively. These patients suffered from a severe immunodeficiency with recurrent bacterial and viral skin infections, otitis media, upper respiratory tract infections, as well as immune system hyperreactivity which included atopic and allergic disease [74].
Neutrophils of these patients showed impaired migration in response to fMLF, C5a, and IL-8. More specifically, HEM1-deficient neutrophils could not form a broad leading edge or maintain directional persistence, but instead became dramatically elongated with competing leading edges moving parallel to or against the chemotactic gradient [74]. Impaired migration is also observed in HEM1-deficient mouse neutrophils, as well as impaired actin polymerization [75].
HEM1 deficiency also resulted in T-cell defects, including defective proliferation and activation, despite normal FAS (tumor necrosis factor receptor superfamily member 6) and T-cell receptor-mediated apoptosis. These cells showed reduced CD11a/CD18 inside-out activation, while adhesion to immobilized ICAM-1 was intact. However, T-cells migrated poorly on ICAM-1-coated surfaces and showed a loss of lamellipodia formation and F-actin at the leading edge. Also, HEM1-deficient T-cells displayed decreased levels of F-actin at immune synapses, and there was diminished immunological synapse stability. This defective F-actin accumulation was also observed in the NK-cell-target synapse. Reduction of the cortical actin barrier in HEM1-deficient T-cells resulted in exaggerated degranulation and IL-10, perforin, and granzyme A and B release, which could explain the patients' autoinflammatory phenotype [74]. Increased degranulation has also been observed in ARPC1B-deficient [31] and MKL1-deficient [28] neutrophils. Although degranulation of neutrophils was not investigated in HEM1 deficiency, it is likely that the cortical actin barrier of neutrophils is also affected, resulting in an increased release of granular proteases which could also contribute to autoimmunity.
Coronin-1A Deficiency
Coronin members constitute a family of evolutionarily conserved regulators of the actin cytoskeleton turnover, represented by 7 members in mammals. They have been grouped into 3 classes based on phylogenetic and functional criteria. Class I includes coronins 1, 2, 3, and 6 (also called 1A, 1B, 1C, and 1D) that associate with the actin cytoskeleton, localize at the leading edge of migrating cells, and participate in various signaling processes [76, 77, 78, 79]. The actin-binding protein coronin-1A (encoded by CORO1A, chromosome 16p11.2, OMIM 615401), also known as p57, is predominantly expressed in the hematopoietic lineage. It is classified as a 7 beta-propeller protein, which contains 5 WD40 repeats and has a C-terminal coiled-coil leucine-zipper domain connected via an extension (CE) or linker region [80]. Two actin-binding regions are described within the beta-propeller [80, 81], and 1 within the linker region [82, 83]. Mutation of the C-terminal leucine-zipper results in failure to homo-oligomerize [82, 84, 85]. It has been described that coronin-1A can directly interact with the ARP2/3 complex [86], is present at the leading edge during chemotaxis [78, 87], and plays a role in phagocytosis [87] and signal transduction [88]. Class II includes coronin-4 and coronin-5 (also called 2A and 2B), involved in focal adhesion turnover, reorganization of the cytoskeleton, and cell migration [89, 90]. The class III coronin-7 has an unusual structure and plays a role in Golgi morphology maintenance [91, 92].
Several reports have been published listing 10 different mutations in CORO1A, which describe patients suffering from either autosomal recessive SCID [85, 93, 94, 95] or hemophagocytic lymphohistiocytosis [96, 97], whereas copy number duplication has been linked to autism [98]. Patients diagnosed with SCID suffered from recurrent bacterial and viral infections of the respiratory tracts and lungs, as well as skin lesions and chronic warts [85, 93, 94, 95]. Another characteristic is chronic T-lymphopenia of varying T-cells and to a lesser extent (memory) B-cells and NK-cells, but a normal-sized thymus and normal IgG and specific antibodies with a marginally elevated IgE levels [85, 93, 94, 95]. Some patients with SCID also presented with attention hyperactivity disorder or other cognitive impairments, which relates to expression of coronin-1A in the hematopoietic system and the brain [94, 95]. Three patients carried CORO1A mutations which have developed hemophagocytic lymphohistiocytosis, of which only one had a compound heterozygous mutation in CORO1A (CORO1A p.L235V and p.P277R). Telomere length in patient lymphocytes and granulocytes was very low, indicating they suffer from impaired hematopoiesis in the lymphoid and myeloid compartment, although neutropenia has not been described to date [93].
Regarding the function of coronin-1A deficiency in patient neutrophils, there is 1 report evaluating ROS production in coronin-1A-deficient patients, which was found to be normal [93]. One of the 2 siblings described suffered from tuberculoid leprosy, which is surprising since coronin-1A-deficient macrophages have been described to have increased killing capacity towards mycobacterial pathogens [99, 100, 101].
Several studies on the role of coronin-1A in neutrophils have been conducted. An initial study showed that transducing primary neutrophils with the beta-propeller region of coronin-1A changed the F-actin distribution of the cells and reduced neutrophil adhesion, spreading, chemotaxis and phagocytosis [87]. Co-immunoprecipitation of CD18 and coronin-1A has been found in adherent differentiated HL-60 cells, and intravital microscopy in inflamed cremaster muscle venules revealed that leukocyte adhesion and extravasation was severely impaired in coronin-1a-knockout mice, while the number of rolling leukocytes was unaffected [78]. Overexpression of coronin-1 in PLB-985 cells (which were differentiated towards neutrophils) resulted in less apoptosis-induced mitochondrial depolarization. Also, neutrophils from patients with cystic fibrosis showed increased coronin-1 expression and lower apoptosis rates, indicating a pro-survival role for coronin-1 [102].
Notably, several case reports describing SCID patients with coronin-1A mutations have evaluated patient T-lymphocytes or T-cell lines derived from patient cells [85, 93, 94, 95], but it is not clear if granulocytes are also affected in this type of SCID to date. Additional class I coronins may, however, compensate for the absence of coronin-1A in granulocytes, similar to platelets. Coronin-1A-deficient platelets retain the ability of the ARP2/3 complex to accumulate at the cell cortex or endosomes and phagosomes, as well as enable the formation of lamellipodia and spreading under some conditions in vitro [103]. Further investigation of the relevance of coronins for granulocyte function could elucidate the importance of this cell type in this SCID and would expand fundamental understanding of coronins in neutrophil function.
WD Repeat-Containing Protein 1 Deficiency (Lazy Leukocyte Syndrome)
The lazy leukocyte syndrome (LLS, OMIM 150550), first described in 1971 [104], derives its name from the observation that neutrophils of these patients show a severe migration defect. Almost half a century later, Kuhns et al. [105] identified autosomal recessive loss-of-function mutations in the gene WD repeat-containing protein 1 (WDR1, chromosome 4p16.1). Although the authors mentioned the defect as a possible cause of LLS, this is debatable because consanguinity or the clinical manifestation of severe oral stenosis had not been mentioned in the past. LSS is more descriptive in nature and can have miscellaneous backgrounds representing 1 distinct disorder among many different “actinopathies” in general terms.
WDR1 encodes for actin-interacting protein 1 (AIP1), which plays a major role in enhancing the severing and disassembly of actin filaments by cofilin [106, 107]. Cofilin has been shown to bind to the sides of actin filaments and to destabilize monomer-monomer interactions within the filament, causing actin filaments to break apart. Cofilin activity is regulated by LIM kinase-mediated phosphorylation at Ser3, which disrupts the F-actin-binding domain, resulting in decreased actin depolymerization [108]. The ability to sever actin filaments varies depending on the stoichiometry of cofilin binding (cofilin:actin ratio). When actin filaments are saturated with cofilin, severing is minimal and filaments are stabilized, but in living cells, despite the high concentrations of cofilin, actin filaments undergo rapid severing when bound by coronins, Srv2/cyclase-associated protein, or AIP1 [109, 110, 111]. Several patients with WDR1 deficiency have been described to date, and these patients mainly suffer from severe stomatitis, recurrent infections, and moderate neutropenia (homozygous [p.D26 N], compound heterozygous [p.delK7 and p.V424M], and [p.G121R and p.L286V], respectively) [105].
Mutations in WDR1 are also described to cause an autoinflammatory phenotype with periodic fever, immunodeficiency with increased IL-18 serum levels and thrombocytopenia (PFIT, due to a homozygous missense mutation [c.877C>T] affecting both transcripts of WDR1, that is, transcript 1 exon 8 c.877C>T, L293F and transcript 2 exon 5 c.457C>T, L153F) [112]. Also most of the patients described by Pfajfer et al. [113] (which have homozygous missense mutations [p.H145Q] and [p.D572V] or compound heterozygous mutations [p.D572V and p.G501S]) suffered from fever and/or thrombocytopenia, similar to hypomorphic Wdr1 mutations in mice [114], as well as intellectual impairment. Clearly, not all patients with WDR1 mutations are reported to be neutropenic, in contrast to LLS patients [112, 113, 115], and may even show elevated neutrophil counts instead.
The pathophysiology of LSS phenotype or PFIT may be different. The mildly neutropenic patients studied by Kuhns et al. [105] did not exhibit persistently elevated levels of IL-18 nor did LPS stimulation of patient PBMCs show exaggerated IL-18 production. Also, there were comparable levels of mutated AIP1 protein in patients compared to normal AIP1 protein in control neutrophils [105]. The mutations causing PFIT were associated with near-absent levels of WDR1 expression in PBMCs, and only some variable residual WDR1 expression in expanded T-cells, which may suggest that activation of lymphocytes stimulates or stabilizes expression of the WDR1 variants [113]. The other kindred described with PFIT showed mutated WDR1 expression which formed aggregates that co-localized with pyrin [112].
Functionally, neutrophils of patients with WDR1 deficiency show a severe migration defect and abnormal spreading in response to fMLF. Furthermore, these cells showed an enhanced F-actin content, which is illustrating the defective actin depolymerization [105, 113]. No defect was seen in the killing of S. aureus[105] and phagocytosis of opsonized E. coli [112], as also has been described for LLS [104, 115]. Granule content and release were also reported to be normal, whereas ROS production was, depending on the stimulus used, found to be enhanced [105, 112]. Mitochondrial membrane potential and apoptosis in patient neutrophils was not reported to be affected [113]. Furthermore, around 40–60% of the neutrophils showed abnormal “herniation” of their nuclear lobes being pushed to the perimeter of the cell [105, 113]. Interestingly, depletion of coronin-1a fully restored the adverse effects of WDR1 deficiency (i.e., neutropenia and impaired actin dynamics, motility, and nuclear morphology) in neutrophils of WDR1-deficient zebra fish embryos, which display a similar phenotype as human WDR1-deficient neutrophils [116].
Apart from neutrophils, also defects in monocytes, dendritic cells, and lymphocytes have been reported [112, 113]. These cells also showed increased F-actin content. Monocytes showed an increased spreading capacity, both basally as upon stimulation with LPS, and this was associated with F-actin-rich podosome-like structures [113]. Furthermore, IL-18 production was increased by dendritic cells both basally as upon stimulation, while no difference was observed in IL-1β levels. Increased caspase-1 cleavage was observed in CD14+ PBMCs, indicative of inflammasome activity, which corresponds with the autoinflammatory phenotype observed in these patients and the co-localization of WDR1 mutant protein with pyrin, a protein involved in the classical fever syndrome familial Mediterranean fever [112].
The adaptive immune system is also shown to be affected in WDR1 deficiency. Pfajfer et al. [113] observed abnormalities in the B-cell and T-cell compartment, including profound peripheral B-cell lymphopenia, lack of switched memory B-cells, and reduced clonal diversity. Migration of B-cells was shown to be normal, even though an increased adhesion was observed. Similarly, T-cells showed increased spreading, but unaffected T-cell receptor internalization, migration toward to C-X-C motif chemokine ligand 12 (CXCL12), and killing of target cells by CD8+ T-cells were not affected. Follicular T-helper cells were reduced in all patients described by Pfajfer et al. [113], which can explain the variety of microbial pathogens causing infections in WDR1 deficiency including Streptococcus pneumoniae and Haemophilus influenza, being pathogens more compatible with typical humoral immunodeficiency instead of a neutrophil defect.
When Signaling Goes Wrong: Actin Polymerization Defects due to Defective Downstream Signaling
There are several PIDs known which are caused by mutations in genes which are essential in the induction of actin polymerization. These proteins are found downstream of receptor activation (e.g., G protein-coupled receptors or integrin receptors) and induce actin polymerization by activation of the WRC or WASP. Key components in these signaling pathways are Rho GTPases, which cycle between an (inactive) guanosine diphosphate (GDP)- and (active) guanosine triphosphate (GTP)-bound state [117]. This is coordinated by 3 groups of regulatory molecules, namely, guanine nucleotide-exchange factors (GEFs), GTPase-activating proteins, and guanine nucleotide-dissociation inhibitors (GDIs) [118]. Also, ezrin/radixin/moesin proteins can directly interact with RHO GDI, thereby reducing its activity (shown in Fig. 1) [119]. Defective downstream signaling will result in impaired actin polymerization and thus reduced cell motility, which subsequently results in the increased susceptibility of these patients to infections.
RAC2 Mutations
RAC2 is a member of the Rho GTPases family, which also includes CDC42, which binds to WASP upon cell activation, and Ras homologous family member A which acts upon Rho-associated protein kinase and mammalian diaphanous-related formin 1 [120]. All 3 proteins can function as molecular switches, thereby regulating signal transduction. In their inactive state, they are GDP-bound, while they become active when bound to GTP [117]. RAC2 (encoded by RAC2, chromosome 22q13.1) in particular is shown to be of importance for activating the respiratory burst in neutrophils by interacting with p67phox. RAC2 also plays a major role in actin polymerization by activating cofilin and the ARP2/3 complex through the WRC [121, 122].
RAC2 expression is considered to be restricted to the hematopoietic system. Following the first description of a dominant-negative autosomal dominant mutation in RAC2 as heterozygous de novo RAC2D57N variant [123], subsequent reports have further diversified the clinical spectrum of RAC2 mutations [124, 125, 126, 127, 128, 129]. In neutrophils, RAC2 is the major isoform (95%) with RAC1 being only a minute fraction of total RAC [123].
Mutations in RAC2 leading to immunodeficiency include both loss-of-function (4 patients described) and activating (9 patients described) mutations. Loss-of-function mutations are described to cause neutrophilia, severe bacterial infections, and poor wound healing, as well as T- and B-cell lymphopenia and hypogammaglobulinemia [123, 125, 126]. Recurrent respiratory infections, severe lymphopenia, and (mild) neutropenia have been described in patients with activating gain-of-function mutations in RAC2[127, 128, 129]. The activating variant RAC2E62K retains intrinsic GTP hydrolysis, but the GTPase-activating protein failed to accelerate hydrolysis to switch RAC2 activity off [127]. The RAC2G12R mutation was in the GDP/GTP-binding domain and shows strong impact causing another clinical phenotype of early SCID with neutropenia, known as reticular dysgenesis [129].
Both loss-of-function and activating mutations in RAC2 resulted in a severely impaired chemotactic response by neutrophils [123, 124, 125, 126, 127]. Neutrophils did not polarize and showed less or disorganized F-actin in response to fMLF stimulation in case of loss-of-function mutations [123, 125], while neutrophils of patients with gain-of-function mutations showed increased F-actin levels at basal state, as well as upon fMLF stimulation [127, 128]. Also, the respiratory burst of patients' neutrophils was either decreased (loss-of-function mutations) or increased (gain-of-function mutations) in response to fMLF when compared to controls. Patient neutrophils responded normally to phorbol 12-myristate 13-acetate (PMA), irrespective of the presence of a gain-of-function or loss-of-function mutation in the RAC2 gene, which is indicative of a functional oxidase complex [123, 125, 127, 128]. However, RAC2E62K-transfected COS-7 cells (co-transfected with NADPH oxidase components) did show elevated ROS production both at the basal state as when stimulated with PMA [127]. Neutrophil granular content was shown to be normal in 1 patient with a (dominant negative) loss-of-function mutation and no defect in specific granule release was observed. However, release of azurophilic granules was absent as assessed by myeloperoxidase release [123]. In 1 case with a homozygous loss-of-function mutation, neutrophils showed reduced granule content, as well as altered secondary granule morphology [126].
The T- and B-cell lymphopenia seen in these patients also illustrates a role for RAC2 in lymphocyte development. Functionally, Lougaris et al. [128] showed that both B- and T-cells of patients with activating RAC2 mutations have increased apoptosis rates [128]. It would be of interest to further functionally characterize the different lymphocyte subsets in RAC2 patients.
DOCK2 (and DOCK8) Deficiency
As mentioned previously, Rho GTPases cycle between (inactive) GDP- and (active) GTP-bound states. GEFs mediate the exchange of GDP to GTP, thereby promoting the binding of Rho family proteins to their specific effectors [118, 130]. The GEF dedicator of cytokinesis protein 2 (DOCK2) is an important regulator of the activation of RAC and acts downstream of various mitogenic chemokine receptors [131, 132].
DOCK2 is predominantly expressed in hematopoietic cells [133]. Upon stimulation of neutrophils with fMLF, DOCK2 translocates to the plasma membrane. This process is mediated by the phospholipid phosphatidylinositol 3,4,5-trisphosphate. Another phospholipid, phosphatidic acid, subsequently interacts with DOCK2 and stabilizes the leading edge of the cell, thereby ensuring localized RAC activation during neutrophil chemotaxis [134]. To date, 10 patients from 6 families with autosomal recessive loss-of-function mutations in DOCK2 (chromosome 5q35.1, OMIM 616433) have been identified. These patients suffer from early-onset invasive bacterial infections, severe viral infections (including vaccine-related varicella zoster infections), and T-cell lymphopenia. Some patients show high IgE levels [135, 136].
Neutrophil function of 1 patient with DOCK2 deficiency has been described by Moens et al. [136]. Patient neutrophils showed impaired actin polymerization upon fMLF stimulation, as well as reduced cell protrusions upon fMLF or IL-8 stimulation. The respiratory burst upon PMA stimulation was impaired [136]. It would be of interest to test the effect of other stimuli (e.g., fMLF) on ROS production, as the PMA-induced respiratory burst was also normal in neutrophils of patients with RAC2 mutations [123, 125, 127, 128].
Next to neutrophils, Epstein-Barr virus-transformed B-cells, T-cells, NK-cells, and Simian virus 40-immortalized primary fibroblasts from DOCK2 patients were assessed [135, 136]. These B-cells and T-cells showed impaired actin polymerization and migration in response to CXCL12 or chemokine coiled-coil-ligand 21 (CCL21), respectively. Also, basal F-actin levels were lower in DOCK2-deficient cells compared to control cells [135]. DOCK2-deficient NK-cells showed aberrant degranulation upon stimulation with K562 target cells [135, 136]. Furthermore, these cells displayed lower basal levels of F-actin, as well as impaired actin polymerization, signaling, and interferon-gamma production, which may explain the susceptibility of these patients to viral infections [135]. While DOCK2 is predominantly expressed in hematopoietic cells [133], some DOCK2 expression was found in healthy control fibroblasts, but not in fibroblasts of DOCK2 patients. DOCK2-deficient fibroblasts were found to be more susceptible for virus-induced cell death, although this effect could be rescued by interferon-alfa-2b treatment. Since HSCT resolved the immunodeficiency completely, it seems that this defect in fibroblasts is not contributing to the susceptibility to viral infections when there is a healthy hematopoietic system [135].
Whereas DOCK2 is an important regulator of the activation of RAC, its family member, DOCK8, acts as GEF for CDC42 activation. Mutations in DOCK8 cause the major form of autosomal recessive hyper IgE syndrome (chromosome 9p24.3, OMIM 243700) [137]. DOCK8 is expressed in the hematopoietic system and coordinates the actin cytoskeleton response to mitogenic and chemokine signals through the reversible activation of CDC42. DOCK8 interacts with signal transducer and activator of transcription 3 and regulates signal transducer and activator of transcription 3-dependent T-helper 17 cell differentiation [138], which may largely explain the recurrent and severe bacterial, viral, and fungal infections, as well as the eczema and environmental allergies in DOCK8 deficiency [139]. Our data with DOCK8-deficient patient neutrophils show by and large normal functions, including neutrophil actin polymerization (Sprenkeler, Tool, Kuijpers, unpublished). Also, neutrophil chemotaxis, phagocytosis, and ROS production was assessed in DOCK8-deficient patients, and these functions were mostly found to be normal (2 out of 6 patients showed a mild to moderate chemotaxis defect) [140].
Mutations in the gene encoding CDC42 have been identified to cause severe disease related to early-onset macrophage activation and defective hematopoiesis with an inherited hemophagocytic syndrome [141]. Directed migration toward CXCL12 by bone marrow CD34+-cells and PBMCs was defective; neutrophils were not analyzed.
The absence of major effect of DOCK8 mutations may also explain the observations that the absence of WASP may not affect actin polymerization and motility in human neutrophils. Although neutrophils by and large show normal actin polymerization to chemotactic stimuli, a much more subtle defect in DOCK8-deficient neutrophils has been observed under specific conditions in which neutrophil motility seems affected (Sprenkeler, unpublished). Nonetheless, the signaling unit DOCK8-CDC42-WASP seems not to be the dominant circuitry involved in actin polymerization and motility in human neutrophils.
Serine/Threonine-Protein Kinase 4 Deficiency
Serine/threonine-protein kinase 4 (STK4; also known as mammalian sterile 20-like kinase 1) is a member of the large family of kinases named after the yeast sterile20 kinase. It is ubiquitously expressed throughout the body, with a high expression in bone marrow and lymphoid tissue.
This cytoplasmic kinase is activated upon stress signals, and upon caspase-cleavage, will translocate to the nucleus where it can phosphorylate multiple proteins, including histone H2B, transcription factors forkhead box O1 (FOXO1) and FOXO3, and microtubule-associated proteins 1A/1B light chain 3B (LC3), an important regulator of autophagy [142, 143, 144]. STK4 is a key component of the evolutionary conserved HIPPO pathway, which plays a pivotal role in controlling the balance between cell survival and cell death [145, 146]. Furthermore, upon phagocytosis and TLR signaling, STK4 can activate RAC via the kinase PKC-α and RHOGDI2, the RHO GDI expressed in high levels by hematopoietic cells. Subsequently, activated RAC promotes cytoskeletal rearrangements that recruit mitochondria to phagosomes. Also, mitochondrial- and phagosomal ROS production is promoted by RAC, thereby enhancing the killing of the pathogens [147].
Since 2012, several loss-of-function mutations have been identified in STK4 (chromosome 20q13.12) [148, 149, 150, 151, 152, 153, 154, 155]. The prominent future of STK4 deficiency (OMIM 614868) is CD4+ T-cell lymphopenia. Patients suffer from recurrent bacterial and viral infections, mucocutaneous candidiasis, eczema, molluscum contagiosum, high levels of IgE and IgA, and may have some cardiac aberrations [148, 149, 150, 151, 152, 153, 154, 155].
A number of patients suffer from intermittent neutropenia [148, 149, 154, 155]. Bone marrow evaluation of STK4-deficient patients showed mature neutrophils, indicating that there is no maturation arrest. However, primary neutrophils of patients showed an enhanced loss of mitochondrial membrane potential, and these cells were more susceptible to apoptosis, which could explain the observed neutropenia [148]. This susceptibility to apoptosis is also seen for T-cells, and defective IL-7R signaling, reduced expression of the anti-apoptotic protein Beclin-2, and increased FAS expression/signaling are likely mediating this impaired survival. Also, STK4-deficient T-cells showed an impaired proliferative response [148, 152, 155]. Furthermore, STK4 has been shown to play a role in CD11b/CD18 surface mobilization and localization in lymphocytes [156], and T-cells indeed show impaired migratory capacity upon stimulation with CCL19, CCL21, or CXCL11 [150, 155]. Although neutrophil adhesion in flow chambers coated with E-selectin/ICAM-1/CXCL8 was shown to be normal in 2 patients [157], motility was not tested. Mouse studies have shown that mammalian sterile 20-like kinase 1 is critical for neutrophil extravasation by controlling the translocation of vesicles containing laminin-binding integrins and neutrophil elastase to the surface, suggesting normal adhesion but inappropriate motility [157]. It would be of interest to assess whether chemotaxis and extravasation is also defective in humans.
Moesin Deficiency
Moesin (MSN) is part of the ezrin/radixin/moesin protein family of which members contain so called 4-point-one protein, ezrin, radaxin, moesin (FERM) domains capable of binding to lipids [158]. MSN links actin filaments to the plasma membrane and membrane receptors, which increases cell rigidity and polarity [159, 160]. It has also been reported that MSN is important for signal transduction of selectins [161], and that its N-terminal fragment interacts with RHO GDI (i.e., bovine RHO GDI, which is 97% identical to human RHO GDI on the peptide level [162] and 78% identical to RHOGDI2, the RHO GDI expressed in high levels by hematopoietic cells [163]). This initiates activation of Rho family members, including Ras homologous family member A, RAC1, and CDC42, by reducing the activity of RHO GDI to inhibit GDP/GTP exchange (shown in Fig. 1) [119]. During cytoskeletal reorganization, MSN becomes phosphorylated, causing the N-terminus and C-terminus to interact, which obscures them from interacting with the plasma membrane and cytoskeleton, respectively [164, 165].
To our knowledge, 3 reports which describe mutations in the X-chromosomal MSN gene (Xq12) have been published, 1 missense mutation in the FERM domain and 1 frameshift mutation close to the carboxyl-terminus [166, 167, 168]. The first report introduces the name X-linked MSN-associated immunodeficiency (X-MAID; OMIM 300988) [166]. All patients described having suffered from CID with recurrent bacterial infections of the respiratory, urinary, and/or gastrointestinal tracts and viral infections, including varicella zoster virus infection, recurrent molluscum, and persistent eczema. One case had autoimmune-related thrombotic thrombocytopenic purpura due to anti-ADAMTS13 autoantobodies [166, 167, 168]. Notably, bacterial infections could be treated with either immunoglobulin replacement therapy, antibiotic treatment, or both [166, 167, 168]. Examination of blood cell counts revealed a severely reduced lymphoid compartment, and especially younger patients suffered from episodes with reduced neutrophil counts [166, 167, 168], which was successfully compensated by treatment with granulocyte-colony stimulating factor (G-CSF) in some patients [166, 167]. In addition to lymphopenia, all patients, except 1 [167], were reported to have a normal-sized thymus, which indicates impaired migration of lymphocytes from the thymus [166]. All patients who were studied as children showed a normal cell distribution in the bone marrow which indicates that hematopoietic differentiation was not affected by this genetic disorder [166, 167, 168]. Therefore, the most likely cause for the patient's phenotype would be impaired migration of T-lymphocytes or granulocytes [166, 167, 168].
For unknown reasons, MSN protein expression in patient granulocytes was not affected by the R171W missense mutation or R553X truncation of the MSN protein, whereas expression was reduced in lymphocytes, which seems to relate to the lifetime of lymphocytes in vivo [166, 167]. No functional assays have been performed using granulocytes from these patients. However, deletion of MSN in mice affected the velocity of neutrophil rolling, impaired neutrophil chemotaxis toward invading bacteria, and diminished neutrophil-mediated microbial killing and inflammation [169, 170]. Patient lymphocytes showed impaired proliferation, migration, and adhesion [166, 167]. These results support the hypothesis that defective lymphocyte egress from the thymus causes the observed lymphopenia. Since patients also suffer from episodes of neutropenia, it would be interesting to perform migration and adhesion assays with patient granulocytes. This could elucidate a role of MSN in neutrophil migration from the bone marrow, which might be separate from G-CSF-induced bone marrow egress based on the positive response of patients upon G-CSF treatment.
Adhesion Deficiencies Resulting in Impaired Chemotaxis
Although leukocyte adhesion deficiencies (LADs) do not result in impaired actin polymerization, the defect in adhesion results in defective chemotaxis which is also commonly found in actinopathies. Therefore, these patients also have an increased susceptibility to infections. Whereas LAD-II is caused by the absence of fucosylated ligands for selectins resulting in the inability of neutrophils to bind selectins on the endothelium, both LAD-I and LAD-III are caused by mutations in genes involved in leukocyte integrin signaling. For that reason, the latter two will be discussed. Furthermore, we will discuss shortly calcium and DAG-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) deficiency, which is also described to lead to defective leukocyte integrin activation (but not a chemotaxis defect in neutrophils). Mutations in the ankyrin-spectrin network (these proteins link membrane proteins to the cytoskeleton) are reported to cause human disease, including spherocytosis type 1, cardiac arrhythmia, and neurodevelopmental disorder [171, 172]. In mice, disruption of the ankyrin-binding site of CD44 impaired neutrophil rolling on E-selectin [173]. However, to our knowledge, there are thus far no reports on neutrophil dysfunction caused by mutations in ankyrin or spectrin proteins in humans.
LAD-I and LAD-III
For LAD, 3 different variants are described. Here, we will focus on LAD-I and LAD-III, which are caused by mutations in ITGB2 and FERMT3, respectively. Although LAD-I and LAD-III do not encompass an actin defect, these genes are part of the CD11/CD18 complex which links extracellular components to the cytoskeleton and play a major role in adhesion and chemotaxis, both commonly found defective in actinopathies. The membrane protein CD18 (integrin β2) is found as a heterodimer with 1 of 4 different α-integrins, of which complement receptor 3 (CR3; CD11b/CD18) is the most prominently expressed dimer on neutrophils. These β2-integrins are in a dormant state and are activated in a process termed “inside-out” signaling. Inflammatory stimuli and chemoattractants induce β2-integrins from low avidity to medium avidity by talin-1 [174, 175, 176] and then to their high avidity state by kindlin-3 [177]. After activation by talin-1, kindlin-3 interacts with the CD18 cytoplasmic tail at a membrane-distal NxxY/F motif [177]. Binding of kindlin-3 induces the transition of CD11b/CD18 from the medium avidity state induced by talin-1 toward the high avidity state [178, 179], which allows these β2-integrins to effectively bind to substrates on the endothelium and extracellular matrix. Upon binding of substrates, CD11b/CD18 forms clusters and relays signals into the cell, also known as outside-in signaling [180]. Furthermore, CD11b/CD18 binds actin through Talin-1 and recruits vinculin [181], which binds actin and recruits the actin-binding protein actinin [182]; thus in these 2 ways, CD11b/CD18 anchors the extracellular matrix and actin cytoskeleton. Additionally, CD11b/CD18 is also involved in the recognition of fungal components in neutrophils, and upon recognition causes release of specific granules [183, 184, 185].
There are several commonalities between LAD-I and LAD-III. Patients with either LAD-I or LAD-III are admitted to the hospital early in life or from birth onward with recurrent bacterial and fungal infections, and delayed detachment of the umbilical cord and omphalitis are often early symptoms [186, 187, 188]. Early morbidity is high due to poor wound healing, and these lesions do not show puss formation. Patients also exhibit a severe neutrophilia and often fatal early episodes of sepsis [186, 187, 188]. Patients who survive early infancy often present with chronic periodontitis and gingivitis [186, 187, 188]. Compared to LAD-I patients, LAD-III patients typically show milder immunological defects but suffer from a severe Glanzmann thrombasthenia-like bleeding disorder [189, 190, 191, 192]. Additionally, some LAD-III patients exhibit osteopetrosis [178, 193]. Definitive treatment is possible through HSCT [178, 194, 195]. Otherwise, infections in LAD-I and LAD-III patients are treated with antibiotics and anti-fungal medication, and patients commonly receive prophylactic antibiotics.
Although LAD-I and LAD-III patients suffer from similar symptoms, the underlying mechanisms for these diseases differ. LAD-I is caused by mutations in ITGB2, which encodes CD18 and generally results in the loss of protein expression [187, 188, 196]. Generation of ROS is impaired in patient neutrophils upon stimulation with unopsonized pathogens, and CD18 has been implicated in NADPH oxidase activity [197]. Furthermore, blocking CD11b/CD18 in neutrophils impairs their in vitro killing capacity of Candida albicans[183, 184]. Primary neutrophils from these patients show impaired adhesion in static conditions [188] and under flow, as well as defective chemotaxis [188] and reduced transendothelial migration [198]. The lack of transendothelial migration is the most likely cause of the observed neutrophilia in these patients. This, along with an impaired killing capacity, explains the difficulty of clearing bacterial and fungal infection. Patients suffering from LAD-III have mutations in the FERMT3 gene, which encodes kindlin-3 [178, 179, 199]. Similar results as in LAD-I regarding adhesion, chemotaxis, ROS production, and killing capacity have been found using primary neutrophils from LAD-III patients [177, 178, 183, 184, 200, 201]. Similar to the β2 integrins, kindlin-3 expression is restricted to the hematopoietic lineage and is also important for activation of β1 and β3-integrins, of which the latter explains the observed severe Glanzmann-like bleeding phenotype. To effectively discriminate between LAD-I and LAD-III, adhesion of neutrophils can be tested in the presence of dithiothreitol, which forces CD18 into an open conformation. Patient neutrophils with LAD-III will show normal adhesion, while patients with LAD-I will show severely abrogated adhesion due to the absence of CD11b/CD18 dimers [201]. Collectively, these PIDs illustrate the importance of functional β2-integrins in the myeloid compartment for normal immune response and pathogen clearance through facilitating the interaction between the extracellular matrix and actin cytoskeleton.
CalDAG-GEFI Deficiency
RAS guanyl-releasing protein 2 (RASGRP2), also known as CalDAG-GEFI, is part of the RasGRP family. Initially, mutations in RASGRP2 (chromosome 11q13.1) were proposed to be the cause for LAD-III [202]. The main target of CalDAG-GEFI is Ras-related protein 1 (RAP1), a small GTPase which regulates the activation of integrins in platelets (αIIbβ3 integrin) and neutrophils (β2 integrin) [203, 204, 205].
Several reports on CalDAG-GEFI deficiency (OMIM 615888) have been published, describing patients who suffer from impaired platelet function and bleeding disorder. The main platelet phenotype in these patients was impaired αIIbβ3 integrin activation upon stimulation (except upon PMA stimulation), impaired RAP1 activation, and defective platelet aggregation [206, 207, 208, 209, 210].
Neutrophil function was also investigated in these patients. Some patients showed normal β2 integrin expression, but defective integrin activation as assessed by the ability to bind soluble fibrinogen or the conformation-specific antibody m24 [207, 209], as also described in Rasgrp2 knockout mice [211]. These patients had homozygous mutations in RASGRP2, leading to no or severely reduced expression of CalDAG-GEFI. However, no defects in integrin activation by neutrophils were observed in 2 other pedigrees. The patient described by Kato et al. [208] had compound heterozygous mutations leading to absence of CalDAG-GEFI in platelets, while the pedigree from Canault et al. [206] suffered from homozygous point mutations leading to a mutated version of CalDAG-GEFI with impaired catalytic activity, without any defect in neutrophil ROS production, adhesion, chemotaxis, and transendothelial migration [206]. More importantly, none of the patients suffered from immune defects or susceptibility to bacterial infections associated with neutrophil dysfunction, suggesting that CalDAG-GEFI has an insignificant or redundant role in RAP1 activation/integrin activation in neutrophils.
Undefined “Actinopathies”: Neutrophil Actin Dysfunction
There may still be a group of “actinopathies” which remain unsolved, even though some of the functional and biochemical changes have been reported, such as the early description of a defect called neutrophil actin dysfunction (NAD) at the time. Because the genetic nature of NAD is not known, we will discuss here the function of lymphocyte-specific protein 1 (encoded by LSP1, chromosome 11p15.5) as it was found to be overexpressed in this PID. LSP1 is found in different types of leukocytes [212]. Known as 47-kDa actin-binding protein in NAD, LSP1 has been implicated in actin-mediated cell function of the hematopoietic lineage such as migration and phagocytosis [213]. It has several actin-binding domains in the latter half of the protein, which includes 2 caldesmon-like and villin-headpiece-like domains [214, 215] and on a molecular level it most likely functions by bundling actin filaments [216]. Postmortem samples indicate that LSP1 is upregulated in the epithelium, endothelium, and leukocytes of septic lungs; thus, it seems to play a role during inflammation in these cell types [17].
Two cases are known of NAD, both characterized by the overexpression of a 47-kDa protein [217, 218]. Additionally, a 89-kDa protein was absent from whole cell lysates of the second patient [217]. Both patients were admitted to hospitals early in life due to recurrent fevers and pulmonary infections [218, 219]. The index patient was first suspected of LAD-I because of the marked leukocytosis and non-pyogenic infections. Although CD11b/CD18 expression on neutrophils was found to be downregulated in the index patient, his mother, and sister, an increased CD11b/CD18 expression was present in the second patient [217, 218, 219]. The second patient was part of a large family and had 2 siblings who had died early in life with similar symptoms, suggesting an autosomal recessive genetic defect [219]. In addition to recurrent infections, this patient suffered from hepatosplenomegaly and thrombocytopenia but had normal neutrophil counts. Moreover, morphology of resting neutrophils was normal, but fMLF stimulation induced abnormal hair-like protrusions. Both patients have been successfully treated by HSCT [218, 219].
In the second patient, the 47-kDa protein was identified as LSP1 [217]. It was then inferred that the first patient most likely also exhibited overexpression of LSP1. Unfortunately, the underlying genetic defect for this disease has not been elucidated. Based on the different phenotype and the absence of an 89-kDa protein in the second cases [219], it is more feasible that these defects are not identical and may neither be restricted nor even causally related to LSP1, although this remains unclear until a genetic cause has been elucidated.
Despite the unknown molecular cause for NAD, neutrophils from both patients have been functionally assessed prior to HSCT [217, 218, 219]. Evaluation of actin polymerization in the neutrophils of the index patient revealed that F-actin contents were lower both in the resting state and upon activation. Having a normal morphology in the resting state, these neutrophils produce hair-like protrusions upon stimulation with fMLF and fail to form podosomes or migrate, which seems very similar to neutrophils of ARPC1B-deficient patients [31]. Moreover, the index patient showed an abnormal degranulation [218], while ROS production was increased in neutrophils from the NAD 47/89 patient [219]. Because LSP1 functions as an actin-capping protein, the overexpression of LSP1 probably prevents F-actin formation. The effect of LSP1 overexpression may not be limited to neutrophils but can also affect endothelial and epithelial cells in mouse models [17, 214, 220].
The LLS as well as the 2 NAD cases discussed here indicate that we may well expect additional gene defects related to actin polymerization and motility to be uncovered. Such defects may or not present as a severe neutrophil disorder with an early onset for which HSCT is a potential life-saving cure.
Conclusion
The recent advances in whole-exome sequencing have resulted in the identification of numerous deleterious mutations in genes which are involved in actin cytoskeleton regulation to be causative of PIDs. Not only new PIDs have been described, like MKL1 deficiency and ARPC1B deficiency, but also deficiencies which have been known for decades, now have a known cause. PIDs involving the actin cytoskeleton, or so-called “actinopaties,” have greatly increased our knowledge on the role of the actin cytoskeleton in immune function. However, treatment is often challenging, as patients can display combinations of immunodeficiency, auto-immunity, and auto-inflammation. Currently, the only definitive treatment option remains HSCT, which has its obvious challenges. Recent phase I/II clinical trials using lentiviral gene therapy in PIDs, for example, X-linked severe CID [221], X-linked chronic granulomatous disease [222], and WAS [55] showed promising results and demonstrate that gene therapy can be a valuable treatment option for these diseases.
Conflict of Interest Statement
The authors declare that they have no relevant conflicts of interest.
Funding Sources
The authors were partially funded by the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 668303, Program on Prevention Outcomes Practices Grant PPOP-12-001, the Center of Immunodeficiencies Amsterdam Grant CIDA-2015, and the E-Rare ZonMW grant #90030376506.
Author Contributions
E.G.G.S. and S.W. wrote the manuscript under the supervision of T.W.K.
References
- 1.Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–86. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40((1)):66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. 2020;40((1)):24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa R, Picco P, Gattorno M. The expanding pathways of autoinflammation: a lesson from the first 100 genes related to autoinflammatory manifestations. Adv Protein Chem Struct Biol. 2020;120:1–44. doi: 10.1016/bs.apcsb.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11((3)):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463((7280)):485–92. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13((3)):183–94. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 8.Vedula P, Kashina A. The makings of the ‘actin code’: regulation of actin's biological function at the amino acid and nucleotide level. J Cell Sci. 2018;131((9)):131. doi: 10.1242/jcs.215509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dugina V, Zwaenepoel I, Gabbiani G, Clément S, Chaponnier C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci. 2009;122((Pt 16)):2980–8. doi: 10.1242/jcs.041970. [DOI] [PubMed] [Google Scholar]
- 10.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95((11)):6181–6. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, et al. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. Biochem J. 1997;328((Pt 1)):105–12. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, et al. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297((5581)):612–5. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 13.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol. 2002;4((8)):626–31. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 14.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112((4)):453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 15.Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118((Pt 4)):651–4. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- 16.Pollard TD. Actin and actin-binding proteins. Cold Spring Harb Perspect Biol. 2016;8((8)):a018226. doi: 10.1101/cshperspect.a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le NP, Channabasappa S, Hossain M, Liu L, Singh B. Leukocyte-specific protein 1 regulates neutrophil recruitment in acute lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2015;309:L995–1008. doi: 10.1152/ajplung.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraitser M, Winter RM. Iris coloboma, ptosis, hypertelorism, and mental retardation: a new syndrome. J Med Genet. 1988;25((1)):41–3. doi: 10.1136/jmg.25.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verloes A, Di Donato N, Masliah-Planchon J, Jongmans M, Abdul-Raman OA, Albrecht B, et al. Baraitser-Winter cerebrofrontofacial syndrome: delineation of the spectrum in 42 cases. Eur J Hum Genet. 2015;23((3)):292–301. doi: 10.1038/ejhg.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivière JB, van Bon BW, Hoischen A, Kholmanskikh SS, O'Roak BJ, Gilissen C, et al. De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet. 2012;44((4)):440–2. doi: 10.1038/ng.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuvertino S, Stuart HM, Chandler KE, Roberts NA, Armstrong R, Bernardini L, et al. ACTB loss-of-function mutations result in a pleiotropic developmental disorder. Am J Hum Genet. 2017;101((6)):1021–33. doi: 10.1016/j.ajhg.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunoi H, Yamazaki T, Tsuchiya H, Kato S, Malech HL, Matsuda I, et al. A heterozygous mutation of beta-actin associated with neutrophil dysfunction and recurrent infection. Proc Natl Acad Sci U S A. 1999;96((15)):8693–8. doi: 10.1073/pnas.96.15.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hundt N, Preller M, Swolski O, Ang AM, Mannherz HG, Manstein DJ, et al. Molecular mechanisms of disease-related human β-actin mutations p.R183W and p.E364K. FEBS J. 2014;281((23)):5279–91. doi: 10.1111/febs.13068. [DOI] [PubMed] [Google Scholar]
- 24.Latham SL, Ehmke N, Reinke PYA, Taft MH, Eicke D, Reindl T, et al. Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia. Nat Commun. 2018;9((1)):4250. doi: 10.1038/s41467-018-06713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11((5)):353–65. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gau D, Roy P. SRF'ing and SAP'ing: the role of MRTF proteins in cell migration. J Cell Sci. 2018;131((19)):131. doi: 10.1242/jcs.218222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Record J, Malinova D, Zenner HL, Plagnol V, Nowak K, Syed F, et al. Immunodeficiency and severe susceptibility to bacterial infection associated with a loss-of-function homozygous mutation of MKL1. Blood. 2015;126((13)):1527–35. doi: 10.1182/blood-2014-12-611012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprenkeler EGG, Henriet S, Tool A, Kreft IC, van der Bijl I, Aarts C, et al. MKL1 deficiency results in a severe neutrophil motility defect due to impaired actin polymerization. Blood. 2020;135((24)):2171–81. doi: 10.1182/blood.2019002633. [DOI] [PubMed] [Google Scholar]
- 29.Cheng EC, Luo Q, Bruscia EM, Renda MJ, Troy JA, Massaro SA, et al. Role for MKL1 in megakaryocytic maturation. Blood. 2009;113((12)):2826–34. doi: 10.1182/blood-2008-09-180596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abella JV, Galloni C, Pernier J, Barry DJ, Kjær S, Carlier MF, et al. Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat Cell Biol. 2016;18((1)):76–86. doi: 10.1038/ncb3286. [DOI] [PubMed] [Google Scholar]
- 31.Kuijpers TW, Tool ATJ, van der Bijl I, de Boer M, van Houdt M, de Cuyper IM, et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol. 2017;140((1)):273–7.e10. doi: 10.1016/j.jaci.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 32.Kahr WH, Pluthero FG, Elkadri A, Warner N, Drobac M, Chen CH, et al. Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nat Commun. 2017;8:14816. doi: 10.1038/ncomms14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpi S, Cicalese MP, Tuijnenburg P, Tool ATJ, Cuadrado E, Abu-Halaweh M, et al. A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. J. Allergy Clin. Immunol. 2019;143((6)):2296–2299. doi: 10.1016/j.jaci.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randzavola LO, Strege K, Juzans M, Asano Y, Stinchcombe JC, Gawden-Bone CM, et al. Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. J Clin Invest. 2019;129((12)):5600–14. doi: 10.1172/JCI129388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somech R, Lev A, Lee YN, Simon AJ, Barel O, Schiby G, et al. Disruption of thrombocyte and T lymphocyte development by a mutation in ARPC1B. J Immunol. 2017;199((12)):4036–45. doi: 10.4049/jimmunol.1700460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brigida I, Zoccolillo M, Cicalese MP, Pfajfer L, Barzaghi F, Scala S, et al. T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood. 2018;132((22)):2362–74. doi: 10.1182/blood-2018-07-863431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;79((5)) [PubMed] [Google Scholar]
- 38.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8((25)):1347–56. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 39.Zalevsky J, Lempert L, Kranitz H, Mullins RD. Different WASP family proteins stimulate different Arp2/3 complex-dependent actin-nucleating activities. Curr Biol. 2001;11((24)):1903–13. doi: 10.1016/s0960-9822(01)00603-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, Hu Y, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25((2)):285–95. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones GE, Zicha D, Dunn GA, Blundell M, Thrasher A. Restoration of podosomes and chemotaxis in Wiskott-Aldrich syndrome macrophages following induced expression of WASp. Int J Biochem Cell Biol. 2002;34((7)):806–15. doi: 10.1016/s1357-2725(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 42.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13((7)):376–85. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 43.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404((6774)):151–8. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 44.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, et al. Clinical course of patients with WASP gene mutations. Blood. 2004;103((2)):456–64. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125((6 Pt 1)):876–85. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 46.Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, Kempski H, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108((7)):2182–9. doi: 10.1182/blood-2006-01-010249. [DOI] [PubMed] [Google Scholar]
- 47.Arwani M, Lee D, Haddad A, Mewawalla P. A novel mutation in Wiskott-Aldrich gene manifesting as macrothrombocytopenia and neutropenia. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-225123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van Den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27((3)):313–7. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 49.Imai K, Nonoyama S, Ochs HD. WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype. Curr Opin Allergy Clin Immunol. 2003;3((6)):427–36. doi: 10.1097/00130832-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Yokoyama K, Shimizu E, Yusa N, Ito M, Yamaguchi R, et al. Phenotype-based gene analysis allowed successful diagnosis of X-linked neutropenia associated with a novel WASp mutation. Ann Hematol. 2018;97:367–9. doi: 10.1007/s00277-017-3134-3. [DOI] [PubMed] [Google Scholar]
- 51.Moulding DA, Blundell MP, Spiller DG, White MR, Cory GO, Calle Y, et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J Exp Med. 2007;204((9)):2213–24. doi: 10.1084/jem.20062324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Y, Huang Y, Huang YY, Yang J. [X-linked neutropenia caused by gain-of-function mutation in WAS gene: two cases report and literature review] Zhonghua Er Ke Za Zhi. 2019;57((8)):631–5. doi: 10.3760/cma.j.issn.0578-1310.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Klein C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol. 2011;29:399–413. doi: 10.1146/annurev-immunol-030409-101259. [DOI] [PubMed] [Google Scholar]
- 54.Beel K, Cotter MM, Blatny J, Bond J, Lucas G, Green F, et al. A large kindred with X-linked neutropenia with an I294T mutation of the Wiskott-Aldrich syndrome gene. Br J Haematol. 2009;144((1)):120–6. doi: 10.1111/j.1365-2141.2008.07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrua F, Cicalese MP, Galimberti S, Giannelli S, Dionisio F, Barzaghi F, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6((5)):e239–53. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sereni L, Castiello MC, Di Silvestre D, Della Valle P, Brombin C, Ferrua F, et al. Lentiviral gene therapy corrects platelet phenotype and function in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2019;144((3)):825–38. doi: 10.1016/j.jaci.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shcherbina A, Rosen FS, Remold-O'Donnell E. WASP levels in platelets and lymphocytes of Wiskott-Aldrich syndrome patients correlate with cell dysfunction. J Immunol. 1999;163((11)):6314–20. [PubMed] [Google Scholar]
- 58.Cooper MD, Chae HP, Lowman JT, Krivit W, Good RA. Wiskott-Aldrich syndrome. An immunologic deficiency disease involving the afferent limb of immunity. Am J Med. 1968;44((4)):499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- 59.Thrasher AJ, Jones GE, Kinnon C, Brickell PM, Katz DR. Is Wiskott-Aldrich syndrome a cell trafficking disorder? Immunol Today. 1998;19((12)):537–9. doi: 10.1016/s0167-5699(98)01350-4. [DOI] [PubMed] [Google Scholar]
- 60.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55((2)):243–52. [PubMed] [Google Scholar]
- 61.Zicha D, Allen WE, Brickell PM, Kinnon C, Dunn GA, Jones GE, et al. Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br J Haematol. 1998;101((4)):659–65. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 62.Keszei M, Record J, Kritikou JS, Wurzer H, Geyer C, Thiemann M, et al. Constitutive activation of WASp in X-linked neutropenia renders neutrophils hyperactive. J Clin Invest. 2018;128((9)):4115–31. doi: 10.1172/JCI64772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de la Fuente MA, Sasahara Y, Calamito M, Antón IM, Elkhal A, Gallego MD, et al. WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP) Proc Natl Acad Sci U S A. 2007;104((3)):926–31. doi: 10.1073/pnas.0610275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konno A, Kirby M, Anderson SA, Schwartzberg PL, Candotti F. The expression of Wiskott-Aldrich syndrome protein (WASP) is dependent on WASP-interacting protein (WIP) Int Immunol. 2007;19((2)):185–92. doi: 10.1093/intimm/dxl135. [DOI] [PubMed] [Google Scholar]
- 65.Chou HC, Antón IM, Holt MR, Curcio C, Lanzardo S, Worth A, et al. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16((23)):2337–44. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vijayakumar V, Monypenny J, Chen XJ, Machesky LM, Lilla S, Thrasher AJ, et al. Tyrosine phosphorylation of WIP releases bound WASP and impairs podosome assembly in macrophages. J Cell Sci. 2015;128((2)):251–65. doi: 10.1242/jcs.154880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanzi G, Moratto D, Vairo D, Masneri S, Delmonte O, Paganini T, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med. 2012;209((1)):29–34. doi: 10.1084/jem.20110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Mousa H, Hawwari A, Al-Ghonaium A, Al-Saud B, Al-Dhekri H, Al-Muhsen S, et al. Hematopoietic stem cell transplantation corrects WIP deficiency. J Allergy Clin Immunol. 2017;139((3)):1039–40.e4. doi: 10.1016/j.jaci.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 69.Pfajfer L, Seidel MG, Houmadi R, Rey-Barroso J, Hirschmugl T, Salzer E, et al. WIP deficiency severely affects human lymphocyte architecture during migration and synapse assembly. Blood. 2017;130((17)):1949–53. doi: 10.1182/blood-2017-04-777383. [DOI] [PubMed] [Google Scholar]
- 70.Hromas R, Collins S, Raskind W, Deaven L, Kaushansky K. Hem-1, a potential membrane protein, with expression restricted to blood cells. Biochim Biophys Acta. 1991;1090((2)):241–4. doi: 10.1016/0167-4781(91)90109-y. [DOI] [PubMed] [Google Scholar]
- 71.Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468((7323)):533–8. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graziano BR, Town JP, Sitarska E, Nagy TL, Fošnarič M, Penič S, et al. Cell confinement reveals a branched-actin independent circuit for neutrophil polarity. PLoS Biol. 2019;17((10)):e3000457. doi: 10.1371/journal.pbio.3000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiner OD, Rentel MC, Ott A, Brown GE, Jedrychowski M, Yaffe MB, et al. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4((2)):e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Comrie WA, Poli MC, Cook SA, Similuk M, Oler AJ, Faruqi AJ, et al. Genetic immunodeficiency and autoimmune disease reveal distinct roles of Hem1 in the WAVE2 and mTORC2 complexes. bioRxiv. 2019:692004. [Google Scholar]
- 75.Park H, Staehling-Hampton K, Appleby MW, Brunkow ME, Habib T, Zhang Y, et al. A point mutation in the murine Hem1 gene reveals an essential role for Hematopoietic protein 1 in lymphopoiesis and innate immunity. J Exp Med. 2008;205((12)):2899–913. doi: 10.1084/jem.20080340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128((5)):915–29. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Ip FC, Shi L, Zhang Z, Tang H, Ng YP, et al. Coronin 6 regulates acetylcholine receptor clustering through modulating receptor anchorage to actin cytoskeleton. J Neurosci. 2014;34((7)):2413–21. doi: 10.1523/JNEUROSCI.3226-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pick R, Begandt D, Stocker TJ, Salvermoser M, Thome S, Böttcher RT, et al. Coronin 1A, a novel player in integrin biology, controls neutrophil trafficking in innate immunity. Blood. 2017;130((7)):847–58. doi: 10.1182/blood-2016-11-749622. [DOI] [PubMed] [Google Scholar]
- 79.Tilley FC, Williamson RC, Race PR, Rendall TC, Bass MD. Integration of the Rac1- and actin-binding properties of Coronin-1C. Small GTPases. 2015;6((1)):36–42. doi: 10.4161/21541248.2014.992259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: a regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14((1)):87–96. doi: 10.1016/j.str.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 81.Oku T, Itoh S, Okano M, Suzuki A, Suzuki K, Nakajin S, et al. Two regions responsible for the actin binding of p57, a mammalian coronin family actin-binding protein. Biol Pharm Bull. 2003;26((4)):409–16. doi: 10.1248/bpb.26.409. [DOI] [PubMed] [Google Scholar]
- 82.Gatfield J, Albrecht I, Zanolari B, Steinmetz MO, Pieters J. Association of the leukocyte plasma membrane with the actin cytoskeleton through coiled coil-mediated trimeric coronin 1 molecules. Mol Biol Cell. 2005;16((6)):2786–98. doi: 10.1091/mbc.E05-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oku T, Nakano M, Kaneko Y, Ando Y, Kenmotsu H, Itoh S, et al. Constitutive turnover of phosphorylation at Thr-412 of human p57/coronin-1 regulates the interaction with actin. J Biol Chem. 2012;287((51)):42910–20. doi: 10.1074/jbc.M112.349829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oku T, Itoh S, Ishii R, Suzuki K, Nauseef WM, Toyoshima S, et al. Homotypic dimerization of the actin-binding protein p57/coronin-1 mediated by a leucine zipper motif in the C-terminal region. Biochem J. 2005;387((Pt 2)):325–31. doi: 10.1042/BJ20041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yee CS, Massaad MJ, Bainter W, Ohsumi TK, Föger N, Chan AC, et al. Recurrent viral infections associated with a homozygous CORO1A mutation that disrupts oligomerization and cytoskeletal association. J Allergy Clin Immunol. 2016;137((3)):879–88.e2. doi: 10.1016/j.jaci.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Humphries CL, Balcer HI, D'Agostino JL, Winsor B, Drubin DG, Barnes G, et al. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J Cell Biol. 2002;159((6)):993–1004. doi: 10.1083/jcb.200206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan M, Di Ciano-Oliveira C, Grinstein S, Trimble WS. Coronin function is required for chemotaxis and phagocytosis in human neutrophils. J Immunol. 2007;178((9)):5769–78. doi: 10.4049/jimmunol.178.9.5769. [DOI] [PubMed] [Google Scholar]
- 88.Ojeda V, Robles-Valero J, Barreira M, Bustelo XR. The disease-linked Glu-26-Lys mutant version of Coronin 1A exhibits pleiotropic and pathway-specific signaling defects. Mol Biol Cell. 2015;26((16)):2895–912. doi: 10.1091/mbc.E15-01-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marshall TW, Aloor HL, Bear JE. Coronin 2A regulates a subset of focal-adhesion-turnover events through the cofilin pathway. J Cell Sci. 2009;122((Pt 17)):3061–9. doi: 10.1242/jcs.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rogg M, Yasuda-Yamahara M, Abed A, Dinse P, Helmstädter M, Conzelmann AC, et al. The WD40-domain containing protein CORO2B is specifically enriched in glomerular podocytes and regulates the ventral actin cytoskeleton. Sci Rep. 2017;7((1)):15910. doi: 10.1038/s41598-017-15844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rybakin V. Role of Mammalian coronin 7 in the biosynthetic pathway. Subcell Biochem. 2008;48:110–5. doi: 10.1007/978-0-387-09595-0_10. [DOI] [PubMed] [Google Scholar]
- 92.Rybakin V, Stumpf M, Schulze A, Majoul IV, Noegel AA, Hasse A. Coronin 7, the mammalian POD-1 homologue, localizes to the Golgi apparatus. FEBS Lett. 2004;573((1–3)):161–7. doi: 10.1016/j.febslet.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 93.Stray-Pedersen A, Jouanguy E, Crequer A, Bertuch AA, Brown BS, Jhangiani SN, et al. Compound heterozygous CORO1A mutations in siblings with a mucocutaneous-immunodeficiency syndrome of epidermodysplasia verruciformis-HPV, molluscum contagiosum and granulomatous tuberculoid leprosy. J Clin Immunol. 2014;34((7)):871–90. doi: 10.1007/s10875-014-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol. 2009;131((1)):24–30. doi: 10.1016/j.clim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moshous D, Martin E, Carpentier W, Lim A, Callebaut I, Canioni D, et al. Whole-exome sequencing identifies Coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. J Allergy Clin Immunol. 2013;131((6)):1594–603. doi: 10.1182/blood-2012-07-440339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chi ZH, Wei W, Bu DF, Li HH, Ding F, Zhu P. Targeted high-throughput sequencing technique for the molecular diagnosis of primary immunodeficiency disorders. Medicine. 2018;97((40)):e12695. doi: 10.1097/MD.0000000000012695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao Y, Zhu HY, Qiao C, Xia Y, Kong Y, Zou YX, et al. Pathogenic gene mutations or variants identified by targeted gene sequencing in adults with hemophagocytic lymphohistiocytosis. Front Immunol. 2019;10:395. doi: 10.3389/fimmu.2019.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo R, Sanders SJ, Tian Y, Voineagu I, Huang N, Chu SH, et al. Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am J Hum Genet. 2012;91((1)):38–55. doi: 10.1016/j.ajhg.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130((1)):37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 100.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97((4)):435–47. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 101.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142((1)):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moriceau S, Kantari C, Mocek J, Davezac N, Gabillet J, Guerrera IC, et al. Coronin-1 is associated with neutrophil survival and is cleaved during apoptosis: potential implication in neutrophils from cystic fibrosis patients. J Immunol. 2009;182((11)):7254–63. doi: 10.4049/jimmunol.0803312. [DOI] [PubMed] [Google Scholar]
- 103.Riley DRJ, Khalil JS, Pieters J, Naseem KM, Rivero F. Coronin 1 is required for integrin β2 translocation in platelets. Int J Mol Sci. 2020;21((1)):21. doi: 10.3390/ijms21010356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller ME, Oski FA, Harris MB. Lazy-leucocyte syndrome. A new disorder of neutrophil function. Lancet. 1971;1((7701)):665–9. doi: 10.1016/s0140-6736(71)92679-1. [DOI] [PubMed] [Google Scholar]
- 105.Kuhns DB, Fink DL, Choi U, Sweeney C, Lau K, Priel DL, et al. Cytoskeletal abnormalities and neutrophil dysfunction in WDR1 deficiency. Blood. 2016;128((17)):2135–43. doi: 10.1182/blood-2016-03-706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q, Courtemanche N, Pollard TD. Aip1 promotes actin filament severing by cofilin and regulates constriction of the cytokinetic contractile ring. J Biol Chem. 2015;290((4)):2289–300. doi: 10.1074/jbc.M114.612978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ono S. Functions of actin-interacting protein 1 (AIP1)/WD repeat protein 1 (WDR1) in actin filament dynamics and cytoskeletal regulation. Biochem Biophys Res Commun. 2018;506:315–22. doi: 10.1016/j.bbrc.2017.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393((6687)):809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 109.Kanellos G, Frame MC. Cellular functions of the ADF/cofilin family at a glance. J Cell Sci. 2016;129((17)):3211–8. doi: 10.1242/jcs.187849. [DOI] [PubMed] [Google Scholar]
- 110.Mikati MA, Breitsprecher D, Jansen S, Reisler E, Goode BL. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. J Mol Biol. 2015;427((19)):3137–47. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shekhar S, Chung J, Kondev J, Gelles J, Goode BL. Synergy between Cyclase-associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat Commun. 2019;10((1)):5319. doi: 10.1038/s41467-019-13268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Standing AS, Malinova D, Hong Y, Record J, Moulding D, Blundell MP, et al. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J Exp Med. 2017;214((1)):59–71. doi: 10.1084/jem.20161228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfajfer L, Mair NK, Jiménez-Heredia R, Genel F, Gulez N, Ardeniz Ö, et al. Mutations affecting the actin regulator WD repeat-containing protein 1 lead to aberrant lymphoid immunity. J Allergy Clin Immunol. 2018;142((5)):1589–604.e11. doi: 10.1016/j.jaci.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 114.Kile BT, Panopoulos AD, Stirzaker RA, Hacking DF, Tahtamouni LH, Willson TA, et al. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood. 2007;110((7)):2371–80. doi: 10.1182/blood-2006-10-055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pinkerton PH, Robinson JB, Senn JS. Lazy leucocyte syndrome: disorder of the granulocyte membrane? J Clin Pathol. 1978;31((4)):300–8. doi: 10.1136/jcp.31.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bowes C, Redd M, Yousfi M, Tauzin M, Murayama E, Herbomel P. Coronin 1A depletion restores the nuclear stability and viability of Aip1/Wdr1-deficient neutrophils. J Cell Biol. 2019;218((10)):3258–71. doi: 10.1083/jcb.201901024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9((9)):690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 118.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6((2)):167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272((37)):23371–5. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 120.Gambardella L, Vermeren S. Molecular players in neutrophil chemotaxis: focus on PI3K and small GTPases. J Leukoc Biol. 2013;94((4)):603–12. doi: 10.1189/jlb.1112564. [DOI] [PubMed] [Google Scholar]
- 121.Dorseuil O, Reibel L, Bokoch GM, Camonis J, Gacon G. The Rac target NADPH oxidase p67phox interacts preferentially with Rac2 rather than Rac1. J Biol Chem. 1996;271((1)):83–8. doi: 10.1074/jbc.271.1.83. [DOI] [PubMed] [Google Scholar]
- 122.Sun CX, Magalhães MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179((2)):239–45. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A. 2000;97((9)):4654–9. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96((5)):1646–54. [PubMed] [Google Scholar]
- 125.Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, Surfus J, et al. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol. 2011;127((2)):535–2. doi: 10.1016/j.jaci.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 126.Alkhairy OK, Rezaei N, Graham RR, Abolhassani H, Borte S, Hultenby K, et al. RAC2 loss-of-function mutation in 2 siblings with characteristics of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135((5)):1380–4.e1-5. doi: 10.1016/j.jaci.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hsu AP, Donkó A, Arrington ME, Swamydas M, Fink D, Das A, et al. Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects. Blood. 2019;133((18)):1977–88. doi: 10.1182/blood-2018-11-886028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lougaris V, Chou J, Beano A, Wallace JG, Baronio M, Gazzurelli L, et al. A monoallelic activating mutation in RAC2 resulting in a combined immunodeficiency. J Allergy Clin Immunol. 2019;143((4)):1649–53.e3. doi: 10.1016/j.jaci.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 129.Lagresle-Peyrou C, Olichon A, Sadek H, Roche P, Tardy C, Da Silva C, et al. A gain-of-function RAC2 mutation is associated with bone-marrow hypoplasia and an autosomal dominant form of severe combined immunodeficiency. Haematologica. 2020 doi: 10.3324/haematol.2019.230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laurin M, Côté JF. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev. 2014;28((6)):533–47. doi: 10.1101/gad.236349.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412((6849)):826–31. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 132.Shulman Z, Pasvolsky R, Woolf E, Grabovsky V, Feigelson SW, Erez N, et al. DOCK2 regulates chemokine-triggered lateral lymphocyte motility but not transendothelial migration. Blood. 2006;108((7)):2150–8. doi: 10.1182/blood-2006-04-017608. [DOI] [PubMed] [Google Scholar]
- 133.Nishihara H, Kobayashi S, Hashimoto Y, Ohba F, Mochizuki N, Kurata T, et al. Non-adherent cell-specific expression of DOCK2, a member of the human CDM-family proteins. Biochim Biophys Acta. 1999;1452((2)):179–87. doi: 10.1016/s0167-4889(99)00133-0. [DOI] [PubMed] [Google Scholar]
- 134.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324((5925)):384–7. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dobbs K, Domínguez Conde C, Zhang SY, Parolini S, Audry M, Chou J, et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med. 2015;372((25)):2409–22. doi: 10.1056/NEJMoa1413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moens L, Gouwy M, Bosch B, Pastukhov O, Nieto-Patlàn A, Siler U, et al. Human DOCK2 deficiency: report of a novel mutation and evidence for neutrophil dysfunction. J Clin Immunol. 2019;39((3)):298–308. doi: 10.1007/s10875-019-00603-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124((6)):1289–304.e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keles S, Charbonnier LM, Kabaleeswaran V, Reisli I, Genel F, Gulez N, et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes T H 17 cell differentiation. J Allergy Clin Immunol. 2016;138((5)):1384–94.e2. doi: 10.1016/j.jaci.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options: a review of 136 patients. J Clin Immunol. 2015;35((2)):189–98. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 140.Mandola AB, Levy J, Nahum A, Hadad N, Levy R, Rylova A, et al. Neutrophil functions in immunodeficiency due to DOCK8 deficiency. Immunol Invest. 2019;48((4)):431–9. doi: 10.1080/08820139.2019.1567533. [DOI] [PubMed] [Google Scholar]
- 141.Lam MT, Coppola S, Krumbach OHF, Prencipe G, Insalaco A, Cifaldi C, et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J Exp Med. 2019;216((12)):2778–99. doi: 10.1084/jem.20190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, Chang JT, et al. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol Cell. 2015;57((1)):55–68. doi: 10.1016/j.molcel.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113((4)):507–17. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 144.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125((5)):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 145.Ura S, Masuyama N, Graves JD, Gotoh Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A. 2001;98((18)):10148–53. doi: 10.1073/pnas.181161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ura S, Nishina H, Gotoh Y, Katada T. Activation of the c-Jun N-terminal kinase pathway by MST1 is essential and sufficient for the induction of chromatin condensation during apoptosis. Mol Cell Biol. 2007;27((15)):5514–22. doi: 10.1128/MCB.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;16((11)):1142–52. doi: 10.1038/ni.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schäffer AA, et al. The phenotype of human STK4 deficiency. Blood. 2012;119((15)):3450–7. doi: 10.1182/blood-2011-09-378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Halacli SO, Ayvaz DC, Sun-Tan C, Erman B, Uz E, Yilmaz DY, et al. STK4 (MST1) deficiency in two siblings with autoimmune cytopenias: a novel mutation. Clin Immunol. 2015;161((2)):316–23. doi: 10.1016/j.clim.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 150.Dang TS, Willet JD, Griffin HR, Morgan NV, O'Boyle G, Arkwright PD, et al. Defective leukocyte adhesion and chemotaxis contributes to combined immunodeficiency in humans with autosomal recessive MST1 deficiency. J Clin Immunol. 2016;36((2)):117–22. doi: 10.1007/s10875-016-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sherkat R, Sabri MR, Dehghan B, Bigdelian H, Reisi N, Afsharmoghadam N, et al. EBV lymphoproliferative-associated disease and primary cardiac T-cell lymphoma in a STK4 deficient patient: a case report. Medicine. 2017;96((48)):e8852. doi: 10.1097/MD.0000000000008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schipp C, Schlütermann D, Hönscheid A, Nabhani S, Höll J, Oommen PT, et al. EBV negative lymphoma and autoimmune lymphoproliferative syndrome like phenotype extend the clinical spectrum of primary immunodeficiency caused by STK4 deficiency. Front Immunol. 2018;9:2400. doi: 10.3389/fimmu.2018.02400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moran I, Avery DT, Payne K, Lenthall H, Davies EG, Burns S, et al. B cell-intrinsic requirement for STK4 in humoral immunity in mice and human subjects. J Allergy Clin Immunol. 2019;143((6)):2302–5. doi: 10.1016/j.jaci.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Crequer A, Picard C, Patin E, D'Amico A, Abhyankar A, Munzer M, et al. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS One. 2012;7((8)):e44010. doi: 10.1371/journal.pone.0044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nehme NT, Schmid JP, Debeurme F, André-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;119((15)):3458–68. doi: 10.1182/blood-2011-09-378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7((9)):919–28. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 157.Kurz AR, Pruenster M, Rohwedder I, Ramadass M, Schäfer K, Harrison U, et al. MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane. J Clin Invest. 2016;126((11)):4125–39. doi: 10.1172/JCI87043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998;23((8)):281–2. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- 159.Pestonjamasp K, Amieva MR, Strassel CP, Nauseef WM, Furthmayr H, Luna EJ, et al. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol Biol Cell. 1995;6((3)):247–59. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turunen O, Wahlström T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126((6)):1445–53. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urzainqui A, Serrador JM, Viedma F, Yáñez-Mó M, Rodríguez A, Corbí AL, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17((4)):401–12. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 162.Leffers H, Nielsen MS, Andersen AH, Honoré B, Madsen P, Vandekerckhove J, et al. Identification of two human Rho GDP dissociation inhibitor proteins whose overexpression leads to disruption of the actin cytoskeleton. Exp Cell Res. 1993;209((2)):165–74. doi: 10.1006/excr.1993.1298. [DOI] [PubMed] [Google Scholar]
- 163.Scherle P, Behrens T, Staudt LM. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci U S A. 1993;90((16)):7568–72. doi: 10.1073/pnas.90.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270((52)):31377–85. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- 165.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101((3)):259–70. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 166.Lagresle-Peyrou C, Luce S, Ouchani F, Soheili TS, Sadek H, Chouteau M, et al. X-linked primary immunodeficiency associated with hemizygous mutations in the moesin (MSN) gene. J Allergy Clin Immunol. 2016;138((6)):1681–89.e8. doi: 10.1016/j.jaci.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 167.Bradshaw G, Lualhati RR, Albury CL, Maksemous N, Roos-Araujo D, Smith RA, et al. Exome sequencing diagnoses X-linked moesin-associated immunodeficiency in a primary immunodeficiency case. Front Immunol. 2018;9:420. doi: 10.3389/fimmu.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Delmonte OM, Biggs CM, Hayward A, Comeau AM, Kuehn HS, Rosenzweig SD, et al. First case of X-linked moesin deficiency identified after newborn screening for SCID. J Clin Immunol. 2017;37((4)):336–8. doi: 10.1007/s10875-017-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matsumoto M, Hirata T. Moesin regulates neutrophil rolling velocity in vivo. Cell Immunol. 2016;304–305:59–62. doi: 10.1016/j.cellimm.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 170.Liu X, Yang T, Suzuki K, Tsukita S, Ishii M, Zhou S, et al. Moesin and myosin phosphatase confine neutrophil orientation in a chemotactic gradient. J Exp Med. 2015;212((2)):267–80. doi: 10.1084/jem.20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cunha SR, Mohler PJ. Ankyrin protein networks in membrane formation and stabilization. J Cell Mol Med. 2009;13((11–12)):4364–76. doi: 10.1111/j.1582-4934.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agre P, Orringer EP, Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982;306((19)):1155–61. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- 173.Wang Y, Yago T, Zhang N, Abdisalaam S, Alexandrakis G, Rodgers W, et al. Cytoskeletal regulation of CD44 membrane organization and interactions with E-selectin. J Biol Chem. 2014;289((51)):35159–71. doi: 10.1074/jbc.M114.600767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274((40)):28071–4. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 175.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302((5642)):103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 176.Xing B, Jedsadayanmata A, Lam SC. Localization of an integrin binding site to the C terminus of talin. J Biol Chem. 2001;276((48)):44373–8. doi: 10.1074/jbc.M108587200. [DOI] [PubMed] [Google Scholar]
- 177.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15((3)):300–5. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 178.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15((3)):313–8. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15((3)):306–12. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gaborski TR, Clark A, Jr, Waugh RE, McGrath JL. Membrane mobility of beta2 integrins and rolling associated adhesion molecules in resting neutrophils. Biophys J. 2008;95((10)):4934–47. doi: 10.1529/biophysj.108.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hemmings L, Rees DJ, Ohanian V, Bolton SJ, Gilmore AP, Patel B, et al. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci. 1996;109((Pt 11)):2715–26. doi: 10.1242/jcs.109.11.2715. [DOI] [PubMed] [Google Scholar]
- 182.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381((6582)):531–5. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 183.Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, et al. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol. 2016;196((3)):1272–83. doi: 10.4049/jimmunol.1501811. [DOI] [PubMed] [Google Scholar]
- 184.Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood. 2014;124((4)):590–7. doi: 10.1182/blood-2014-01-551473. [DOI] [PubMed] [Google Scholar]
- 185.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47((2–3)):575–81. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 186.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50–5. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 187.Movahedi M, Entezari N, Pourpak Z, Mamishi S, Chavoshzadeh Z, Gharagozlou M, et al. Clinical and laboratory findings in Iranian patients with leukocyte adhesion deficiency (study of 15 cases) J Clin Immunol. 2007;27((3)):302–7. doi: 10.1007/s10875-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 188.Crowley CA, Curnutte JT, Rosin RE, André-Schwartz J, Gallin JI, Klempner M, et al. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980;302((21)):1163–8. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- 189.Alon R, Aker M, Feigelson S, Sokolovsky-Eisenberg M, Staunton DE, Cinamon G, et al. A novel genetic leukocyte adhesion deficiency in subsecond triggering of integrin avidity by endothelial chemokines results in impaired leukocyte arrest on vascular endothelium under shear flow. Blood. 2003;101((11)):4437–45. doi: 10.1182/blood-2002-11-3427. [DOI] [PubMed] [Google Scholar]
- 190.Etzioni A. Defects in the leukocyte adhesion cascade. Clin Rev Allergy Immunol. 2010;38((1)):54–60. doi: 10.1007/s12016-009-8132-3. [DOI] [PubMed] [Google Scholar]
- 191.Jurk K, Schulz AS, Kehrel BE, Räpple D, Schulze H, Möbest D, et al. Novel integrin-dependent platelet malfunction in siblings with leukocyte adhesion deficiency-III (LAD-III) caused by a point mutation in FERMT3. Thromb Haemost. 2010;103((5)):1053–64. doi: 10.1160/TH09-10-0689. [DOI] [PubMed] [Google Scholar]
- 192.Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, Weening RS, et al. Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest. 1997;100((7)):1725–33. doi: 10.1172/JCI119697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schmidt S, Nakchbandi I, Ruppert R, Kawelke N, Hess MW, Pfaller K, et al. Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J Cell Biol. 2011;192((5)):883–97. doi: 10.1083/jcb.201007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bauer TR, Jr, Creevy KE, Gu YC, Tuschong LM, Donahue RE, Metzger ME, et al. Very low levels of donor CD18+ neutrophils following allogeneic hematopoietic stem cell transplantation reverse the disease phenotype in canine leukocyte adhesion deficiency. Blood. 2004;103((9)):3582–9. doi: 10.1182/blood-2003-11-4008. [DOI] [PubMed] [Google Scholar]
- 195.Horikoshi Y, Umeda K, Imai K, Yabe H, Sasahara Y, Watanabe K, et al. Allogeneic hematopoietic stem cell transplantation for leukocyte adhesion deficiency. J Pediatr Hematol Oncol. 2018;40((2)):137–40. doi: 10.1097/MPH.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 196.van de Vijver E, Maddalena A, Sanal Ö, Holland SM, Uzel G, Madkaikar M, et al. Hematologically important mutations: leukocyte adhesion deficiency (first update) Blood Cells Mol Dis. 2012;48((1)):53–61. doi: 10.1016/j.bcmd.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reumaux D, Kuijpers TW, Hordijk PL, Duthilleul P, Roos D. Involvement of Fcgamma receptors and beta2 integrins in neutrophil activation by anti-proteinase-3 or anti-myeloperoxidase antibodies. Clin Exp Immunol. 2003;134((2)):344–50. doi: 10.1046/j.1365-2249.2003.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.von Andrian UH, Berger EM, Ramezani L, Chambers JD, Ochs HD, Harlan JM, et al. In vivo behavior of neutrophils from two patients with distinct inherited leukocyte adhesion deficiency syndromes. J Clin Invest. 1993;91((6)):2893–7. doi: 10.1172/JCI116535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009;113((19)):4740–6. doi: 10.1182/blood-2008-10-182154. [DOI] [PubMed] [Google Scholar]
- 200.Robert P, Canault M, Farnarier C, Nurden A, Grosdidier C, Barlogis V, et al. A novel leukocyte adhesion deficiency III variant: kindlin-3 deficiency results in integrin- and nonintegrin-related defects in different steps of leukocyte adhesion. J Immunol. 2011;186((9)):5273–83. doi: 10.4049/jimmunol.1003141. [DOI] [PubMed] [Google Scholar]
- 201.van de Vijver E, Tool AT, Sanal Ö, Çetin M, Ünal S, Aytac S, et al. Kindlin-3-independent adhesion of neutrophils from patients with leukocyte adhesion deficiency type III. J Allergy Clin Immunol. 2014;133((4)):1215–8. doi: 10.1016/j.jaci.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 202.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204((7)):1571–82. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stefanini L, Bergmeier W. RAP1-GTPase signaling and platelet function. J Mol Med. 2016;94((1)):13–9. doi: 10.1007/s00109-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.M'Rabet L, Coffer P, Zwartkruis F, Franke B, Segal AW, Koenderman L, et al. Activation of the small GTPase rap1 in human neutrophils. Blood. 1998;92((6)):2133–40. [PubMed] [Google Scholar]
- 205.Lagarrigue F, Kim C, Ginsberg MH. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood. 2016;128((4)):479–87. doi: 10.1182/blood-2015-12-638700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Canault M, Ghalloussi D, Grosdidier C, Guinier M, Perret C, Chelghoum N, et al. Human CalDAG-GEFI gene (RASGRP2) mutation affects platelet function and causes severe bleeding. J Exp Med. 2014;211((7)):1349–62. doi: 10.1084/jem.20130477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lozano ML, Cook A, Bastida JM, Paul DS, Iruin G, Cid AR, et al. Novel mutations in RASGRP2, which encodes CalDAG-GEFI, abrogate Rap1 activation, causing platelet dysfunction. Blood. 2016;128((9)):1282–9. doi: 10.1182/blood-2015-11-683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kato H, Nakazawa Y, Kurokawa Y, Kashiwagi H, Morikawa Y, Morita D, et al. Human CalDAG-GEFI deficiency increases bleeding and delays αIIbβ3 activation. Blood. 2016;128((23)):2729–33. doi: 10.1182/blood-2016-03-704825. [DOI] [PubMed] [Google Scholar]
- 209.Sevivas T, Bastida JM, Paul DS, Caparros E, Palma-Barqueros V, Coucelo M, et al. Identification of two novel mutations in RASGRP2 affecting platelet CalDAG-GEFI expression and function in patients with bleeding diathesis. Platelets. 2018;29:1–4. doi: 10.1080/09537104.2017.1336214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bermejo E, Alberto MF, Paul DS, Cook AA, Nurden P, Sanchez Luceros A, et al. Marked bleeding diathesis in patients with platelet dysfunction due to a novel mutation in RASGRP2, encoding CalDAG-GEFI (p.Gly305Asp) Platelets. 2018;29:1–3. doi: 10.1080/09537104.2017.1332759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, et al. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117((6)):1699–707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li Y, Guerrero A, Howard TH. The actin-binding protein, lymphocyte-specific protein 1, is expressed in human leukocytes and human myeloid and lymphoid cell lines. J Immunol. 1995;155((7)):3563–9. [PubMed] [Google Scholar]
- 213.Howard TH, Hartwig J, Cunningham C. Lymphocyte-specific protein 1 expression in eukaryotic cells reproduces the morphologic and motile abnormality of NAD 47/89 neutrophils. Blood. 1998;91((12)):4786–95. [PubMed] [Google Scholar]
- 214.Cervero P, Wiesner C, Bouissou A, Poincloux R, Linder S. Lymphocyte-specific protein 1 regulates mechanosensory oscillation of podosomes and actin isoform-based actomyosin symmetry breaking. Nat Commun. 2018;9((1)):515. doi: 10.1038/s41467-018-02904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Q, Li Y, Howard TH. Human lymphocyte-specific protein 1, the protein overexpressed in neutrophil actin dysfunction with 47-kDa and 89-kDa protein abnormalities (NAD 47/89), has multiple F-actin binding domains. J Immunol. 2000;165((4)):2052–8. doi: 10.4049/jimmunol.165.4.2052. [DOI] [PubMed] [Google Scholar]
- 216.Demma M, Warren V, Hock R, Dharmawardhane S, Condeelis J. Isolation of an abundant 50,000-dalton actin filament bundling protein from Dictyostelium amoebae. J Biol Chem. 1990;265((4)):2286–91. [PubMed] [Google Scholar]
- 217.Howard T, Li Y, Torres M, Guerrero A, Coates T. The 47-kD protein increased in neutrophil actin dysfunction with 47- and 89-kD protein abnormalities is lymphocyte-specific protein. Blood. 1994;83((1)):231–41. [PubMed] [Google Scholar]
- 218.Boxer LA, Hedley-Whyte ET, Stossel TP. Neutrophil actin dysfunction and abnormal neutrophil behavior. N Engl J Med. 1974;291((21)):1093–9. doi: 10.1056/NEJM197411212912101. [DOI] [PubMed] [Google Scholar]
- 219.Coates TD, Torkildson JC, Torres M, Church JA, Howard TH. An inherited defect of neutrophil motility and microfilamentous cytoskeleton associated with abnormalities in 47-Kd and 89-Kd proteins. Blood. 1991;78((5)):1338–46. [PubMed] [Google Scholar]
- 220.Petri B, Kaur J, Long EM, Li H, Parsons SA, Butz S, et al. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011;117((3)):942–52. doi: 10.1182/blood-2010-02-270561. [DOI] [PubMed] [Google Scholar]
- 221.Mamcarz E, Zhou S, Lockey T, Abdelsamed H, Cross SJ, Kang G, et al. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N Engl J Med. 2019;380((16)):1525–34. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kohn DB, Booth C, Kang EM, Pai SY, Shaw KL, Santilli G, et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat Med. 2020;26((2)):200–6. doi: 10.1038/s41591-019-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8((1)):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]