Abstract
Background
Better treatments for glioblastoma (GBM) patients, in particular in the recurrent setting, are urgently needed. Clinical trials performed in Brazil indicated that intranasal delivery of perillyl alcohol (POH) might be effective in this patient group. NEO100, a highly purified version of POH, was current good manufacturing practice (cGMP) manufactured to evaluate the safety and efficacy of this novel approach in a Phase I/IIa clinical trial in the United States.
Methods
A total of 12 patients with recurrent GBM were enrolled into Phase I of this trial. NEO100 was administered by intranasal delivery using a nebulizer and nasal mask. Dosing was 4 times a day, every day. Four cohorts of 3 patients received the following dosages: 96 mg/dose (384 mg/day), 144 mg/dose (576 mg/day), 192 mg/dose (768 mg/day), and 288 mg/dose (1152 mg/day). Completion of 28 days of treatment was recorded as 1 cycle. Adverse events were documented, and radiographic response via Response Assessment in Neuro-Oncology (RANO) criteria was evaluated every 2 months. Progression-free and overall survival were determined after 6 and 12 months, respectively (progression-free survival-6 [PFS-6], overall survival-12 [OS-12]).
Results
Intranasal NEO100 was well tolerated at all dose levels and no severe adverse events were reported. PFS-6 was 33%, OS-12 was 55%, and median OS was 15 months. Four patients (33%), all of them with isocitrate dehydrogenase 1 (IDH1)-mutant tumors, survived >24 months.
Conclusion
Intranasal glioma therapy with NEO100 was well tolerated. It correlated with improved survival when compared to historical controls, pointing to the possibility that this novel intranasal approach could become useful for the treatment of recurrent GBM.
Keywords: intranasal, isocitrate dehydrogenase 1, O6-methylguanine-DNA methyltransferase, perillyl alcohol, recurrent glioblastoma
Key Points.
Intranasal delivery of NEO100 to recurrent glioblastoma patients was safe.
Progression-free and overall survival was greater in patients with IDH1-mutant tumors.
Intranasal NEO100 has the potential to improve the outcome for recurrent glioblastoma.
Importance of the Study.
The prognosis of patients with recurrent glioblastoma remains dismal and better treatment options are urgently needed. Our Phase I study evaluated intranasal administration of NEO100, a highly purified version of the natural limonene-related compound perillyl alcohol, as a potential novel treatment for this patient group. Patients with recurrent glioblastoma self-administered NEO100 daily via nebulizer 4 times a day. The safety profile of NEO100 was excellent and there was suggestive evidence of activity, in particular in isocitrate dehydrogenase 1 (IDH1)-mutant patients. Intranasal NEO100 represents a novel approach to brain cancer therapy and has the potential to become clinically useful to improve treatment outcomes for recurrent glioblastoma patients.
Glioblastoma (GBM, WHO grade IV glioma) is the most common primary malignant brain tumor among adults. Regardless of the treatment regimen, the vast majority of patients relapse and are faced with limited treatment options. The aggressive infiltration of GBM throughout the brain typically limits the efficacy of repeat surgical resection, and tumor cells frequently acquire resistance to further cytotoxic therapy. Therefore, recurrent GBM does not respond well to repeat surgery, re-irradiation, and additional rounds of chemotherapy; while these interventions may moderately increase overall survival, the prognosis for these patients remains exceptionally poor.1
In the U.S. and Canada, the angiogenesis inhibitor bevacizumab has received market approval for the treatment of recurrent GBM.2 It is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) and thus represents a targeted therapy. It can be used alone or in combination with cytotoxic chemotherapy. However, the duration of benefits is short-lived and its impact on overall survival remains limited and unimpressive, which represents a major reason it was not approved by European authorities.3
In view of the persistent medical need for improved treatments, we are investigating a novel type of intervention, intranasal delivery of perillyl alcohol (POH, NEO100), for patients with recurrent GBM. POH (also called p-metha 1,7-diene-6-ol) is a monoterpene isolated from the essential oils of lavender, citrus fruits, peppermint, and several other plants, which synthesize it through the mevalonate pathway.4 Extensive preclinical studies provided strong evidence of this natural compound's anticancer potential. The exact mechanism of POH's anticancer effect is unclear, but most likely results from pleiotropic effects that include cell cycle arrest, endoplasmic reticulum stress, and induction of apoptosis.5 Because POH was shown to inhibit the enzymatic activity of farnesyl-protein transferase (FPT) of the mevalonate pathway, it was hypothesized that POH might cause inhibition of the oncogenic activity of Ras protein, which requires posttranslational farnesylation for plasma membrane anchoring and mitogenic activity.6 However, several studies in this context yielded ambiguous results. Most likely, any impact on Ras activity represents only one of several mechanisms by which POH exerts its anticancer effects (see detailed in ref.5).
Despite the consistent anticancer activity in a variety of preclinical models, numerous Phase I and II trials in the late 1990s in patients with different solid tumors were unable to demonstrate convincing therapeutic activity. In these studies, POH was formulated in gelatin capsules and given orally in rather large doses of several grams 3–4 times daily. Gastrointestinal toxicity proved dose-limiting, and some patients quit the trials due to unrelenting, chronic malaise (fatigue, nausea, belching, reflux, diarrhea, or constipation).7–9 As a result, oral POH was abandoned and did not enter clinical practice.
Nasal delivery of chemotherapy is envisioned as a novel, paradigm-shifting platform to deliver therapeutics to the brain while minimizing systemic toxicity and first-pass metabolism.10–12 Effective nose-to-brain delivery has been demonstrated in a variety of noncancer conditions, such as migraine, stroke, and other neurological conditions.9,13 For example, intranasal insulin was shown to improve cognition in early Alzheimer's disease.14,15 Although not yet fully characterized, the presumed mechanism of brain drug uptake is thought to involve the olfactory and trigeminal nerves, and the nasal mucosa. Combined, these elements facilitate direct access and quick absorption of drugs, thereby providing for greater bioavailability and rapid onset of drug responses.13,16,17 However, despite these distinct benefits, nasal delivery of cancer therapeutics is not established in clinical practice.
Phase II studies in Brazil, undertaken with recurrent malignant glioma patients, pioneered intranasal delivery of POH as a novel paradigm of cancer therapy. Commercial-grade POH was self-administered four times daily. Several reports published from these studies indicated that this alternative mode of drug delivery harbors the potential to achieve an activity in this patient group.18–20 As well, there was good tolerance, without long-term CNS or systemic severe adverse events, and patient compliance reportedly was very high (>95%).20 Radiographic regression was reported.19,20
Inspired by the promising reports from Brazil, we set out to further explore intranasal delivery as a noninvasive means for GBM therapy. In our preclinical studies, we were able to demonstrate that intranasal delivery of POH showed promising activity against temozolomide-resistant GBM cells implanted into the brains of mice,21 setting the stage to initiate a clinical trial here in the United States. We now report on the completed Phase I part of a Phase I/IIa trial to assess the clinical safety and activity of intranasal NEO100, a highly purified form of POH produced under current good manufacturing practice (cGMP) conditions, in patients with recurrent GBM.
Patients and Methods
Phase I Trial
The ongoing interventional clinical trial entitled “An Open-Label, Phase I/IIA Dose Escalation Study of Safety and Efficacy of NEO100 in Recurrent Grade IV Glioma” [ClinicalTrials.gov Identifier: NCT02704858] is a multi-center study. Participant institutions are Cleveland Clinic, University of Washington/Seattle, University of Wisconsin, and the University of Southern California. It is sponsored by NeOnc Technologies, Inc. (Los Angeles, CA) with ClinDatrix, Inc. (Irvine, CA), as the Clinical Data Management CRO (Contract Research Organization). The patients were enrolled under institutional review board (IRB)-approved protocols and after signing appropriate IRB-approved informed consent forms. For the Phase I portion of this trial, the first patient was enrolled in April of 2017, and the 12th patient entered in June of 2019. The primary objectives of Phase I were: (1) to determine the safety and tolerability of intranasal administration of NEO100, and (2) to identify the maximum tolerated dose of NEO100.
NEO100 Administration
NEO100 is highly purified (>99%) perillyl alcohol that was manufactured under cGMP conditions at Norac Pharma (Azusa, CA). It is delivered 4 times a day (QID) by intranasal administration using a nebulizer and nasal mask. After the initial demonstration and instructions by a nurse in the clinic, patients self-administer each dose. NEO100 is provided to each patient formulated as a 10% stock solution in ethanol:glycerol (50:50, v/v). Prior to each use, the stock solution is diluted with water and filled into the nebulizer.
QID dosing frequency was chosen based on (1) the success of this regimen in the Brazilian trials that used intranasal POH,18–20 (2) the known short half-life of POH,22 and (3) several Phase I/II studies that used oral POH and studied different dosing regimens, including QID.5 In the Brazilian trials, 440 and 534 mg/day was well tolerated.19 Based on discussions with and recommendations from the FDA, we used 384 mg/day (96 mg/dose) as the starting (lowest) amount for Cohort 1. Cohort 2 was escalated 1.5-fold to 576 mg/day (144 mg/dose). Cohort 3 was double the Cohort 1 dosing, that is, 768 mg/day (192 mg/dose), and Cohort 4 was 3 times the Cohort 1 dosing, that is, 1152 mg/day (288 mg/dose). Patient adherence to the protocol was assessed by 2 factors: (1) measurement of POH and perillic acid (PA) levels in patients' blood at the time of enrollment and after patient training for inhalation, and (2) patient log that was self-recorded and captured by the CRO.
Main Inclusion Criteria
Among the inclusion criteria are the following. (1) Radiographically confirmed progression or recurrent grade IV glioma, and on a stable dose of steroid for at least 5 days. (2) Patients must have failed previous radiation and temozolomide treatment. (3) Age ≥18 years. (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, or Karnofsky's index of performance status (KPS) ≥60. (5) Expected survival of at least 3 months. (6) Baseline magnetic resonance imaging (MRI) with gadolinium within two weeks of entry into the trial. (7) Seizures were controlled on a stable dose of anti-epileptics for 2 weeks prior to enrollment. Furthermore, patients were screened with MR perfusion scan if there was a possibility that progression seen on MRI scan represented pseudoprogression.
Response Assessment
Patients underwent gadolinium-enhanced brain MRI as part of standard care. Baseline tumor measurement was performed within 2 weeks of registration and assessed by RANO criteria (Response Assessment in Neuro-Oncology). MRIs were repeated after every even 28-day cycle (ie, cycles 2, 4, and 6) and whenever disease progression was suspected based on clinical symptoms. Tumor response was assessed using both the MacDonald and the RANO response criteria for high-grade gliomas, which considers radiologic imaging, neurological status, and steroid dosing. Safety was evaluated throughout the trial by the incidence of adverse events (AEs), physical examination findings, vital signs, and clinical laboratory test results. AEs were graded for severity using NCI Common Terminology Criteria for Adverse Events v.4.0.23
Results
Presented here are results from the completed Phase I part of an ongoing Phase I/IIa study of intranasally administered NEO100 in patients with recurrent GBM after the failure of standard chemoradiation with temozolomide. Twelve patients were enrolled (demographics and baseline characteristics are shown in Table 1). Successive cohorts of 3 patients each received intranasal NEO100 at escalating dosages of 384 mg/d, 576 mg/d, 768 mg/d, and 1152 mg/d. Patients self-administered these amounts, which were divided into 4 equal doses approximately 5–6 h apart throughout each day.
Table 1.
Patient Demographics and Baseline Characteristics
| Patient ID | Gender | Age (Years) | Ethnic Group | MGMT Status | IDH1 Status | Time from Dx to NEO100 (Months)a | KPS | Tumor Location |
|---|---|---|---|---|---|---|---|---|
| 104 | M | 70 | Caucasian | Unmethylated | Wild type | 8.4 | 90 | Left occipital |
| 105 | M | 62 | Asian | Methylated | Mutated | 6.9 | 90 | Left temporal |
| 106 | F | 51 | Caucasian | Methylated | Mutated | 17.4 | 90 | Right frontal |
| 202 | F | 44 | Hispanic | Unmethylated | Mutated | 84.0 | 80 | Left frontal |
| 203 | F | 58 | Caucasian | Unmethylated | Wild type | 6.2 | 80 | Left frontal |
| 204 | M | 54 | Caucasian | Unmethylated | Wild type | 11.7 | 90 | Left superior temporal |
| 301 | F | 39 | Caucasian | Unknown | Mutated | 11.6 | 90 | Right frontal |
| 302 | F | 62 | Asian | Unknown | Mutated | 44.3 | 90 | Left parietal |
| 303 | F | 42 | Hispanic | Unmethylated | Wild type | 15.3 | 70 | Midline |
| 401 | M | 69 | Caucasian | Unmethylated | Wild type | 7.6 | 80 | Right temporal |
| 402 | F | 53 | Hispanic | Methylated | Wild type | 10.6 | 90 | Right parietal |
| 403 | M | 70 | Hispanic | Methylated | Wild type | 33.8 | 90 | Right parietal |
aTime from initial diagnosis of the primary tumor until the beginning of the first cycle of NEO100 treatment.
No severe (grade 3 or 4) adverse effects were noted in any of the cohorts during any of the monthly cycles. Other adverse effects (grade 1) consisted of nasal soreness or itching, runny nose, skin irritation around the nose, or headache. Repeated grade 2 leukopenia was noted in one patient of Cohort 2, but causality to NEO100 treatment was unclear (Table 2).
Table 2.
Adverse Events Attributable to NEO100 Administration
| Number of Events, According to Body System and Grade | NEO100 Dose Level (mg/day) | ||||
|---|---|---|---|---|---|
| 384 | 576 | 768 | 1152 | Causality | |
| General disorder or administration-site condition: | |||||
| Fatigue, grade 1 | 1 | – | – | – | Possibly related |
| Nervous system disorder: | |||||
| Headache, grade 1 | 1 | – | – | – | Probably related |
| Skin and subcutaneous tissue disorders: | |||||
| Piloerection, grade 1 | 1 | – | – | – | Possibly related |
| Skin irritation around nose, grade 1 | – | – | 1 | – | Definitely related |
| Respiratory, thoracic and mediastinal disorders: | |||||
| Rhinorrhea, grade 1 | 2 | – | – | 1 | Definitely related |
| Nasal dryness, grade 1 | 1 | – | 1 | – | Probably related |
| Nasal pruritus, grade 1 | 1 | – | – | – | Probably related |
| Nasal discomfort, grade 1 | 1 | 1 | – | – | Probably related |
| Cough, grade 1 | – | – | – | 1 | Definitely related |
| Blood and lymphatic system disorders: | |||||
| Leukopenia, grade 2 | – | 2 | – | – | Possibly related |
| Total no. of patients with an event: | 3 | 2 | 1 | 1 | |
Initially, NEO100 treatment was scheduled for a continuous 6-month treatment. Patients who had stable disease at 6 months were allowed to continue treatment on an extended use protocol, whereas patients who progressed early discontinued the treatment. These latter patients were taken off the protocol but remained under the care of their respective physician who provided individualized care as deemed appropriate. Progression-free survival during the first 6 months is summarized in Figure 1 and Table 3. As shown, patients in Cohort 1 (lowest dose) only completed 2 cycles (ie, 2 months) of NEO100 treatment, due to progressive disease at the end of these cycles. In Cohort 2, 2 patients also experienced progressive disease early on (after 1 and 2 cycles), while the third patient (ID 202) had stable disease at 6 months and since then has continued to administer NEO100 for a total of 33 cycles at this time. Her tumor has shrunk by greater than 75% as measured via MRI. In Cohort 3, only 1 patient terminated treatment early due to progressive disease, whereas the other 2 patients were stable at 6 months and therefore continued treatment. One of these 2 patients (ID 302) completed 11 cycles, followed by another 16 months without NEO100 treatment, and is still alive. The other (ID 301) has been continuing treatment for a total of 24 cycles and is still alive. This patient also had a complete radiographic remission, which has continued to this date. In Cohort 4, 2 patients did not complete the full 6-month treatment due to progression at 2 and 4 months, respectively. One of these patients (ID 402) survived for another 13 months after discontinuation of NEO100. Another (ID 401) was lost to follow-up right after completion of 4 cycles and his current status is unknown. The third patient in this cohort (ID 403) presented with stable disease at 6 months, but thereafter rapidly worsened and died 3 months later.
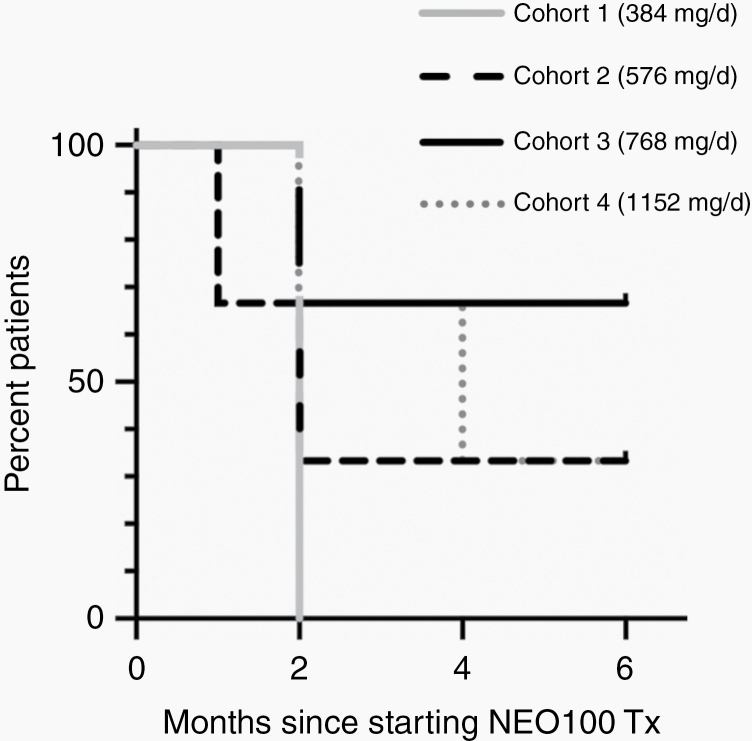
Figure 1.
Progression-free survival of different cohorts. Shown is the progression-free survival of patients within the first 6 months (PFS-6) after initiation of NEO100 treatment, separated into the 4 cohorts with n = 3 patients each.
Table 3.
Cohorts, Dosages, and Results
| Patient ID | Cohort | Dosage (mg/day) | Completed Number of Cyclesa | RANOb | Survival After Start of NEO100 (Months) | IDH1 Status | Current Status | NEO100 Tx Ongoing |
|---|---|---|---|---|---|---|---|---|
| 104 | 1 | 384 | 2 | PD | 18 | Wild type | Deceased | N/A |
| 105 | 1 | 384 | 2 | PD | 9 | Mutated | Deceased | N/A |
| 106 | 1 | 384 | 2 | PD | 33 | Mutated | Deceased | N/A |
| 202 | 2 | 576 | 33 | SD | 33 | Mutated | Alive | Yes |
| 203 | 2 | 576 | 2 | PD | 11 | Wild type | Deceased | N/A |
| 204 | 2 | 576 | 1 | N/A | 2 | Wild type | Deceased | N/A |
| 301 | 3 | 768 | 24 | SD | 24 | Mutated | Alive | Yes |
| 302 | 3 | 768 | 11 | SD | 27 | Mutated | Alive | No |
| 303 | 3 | 768 | 2 | PD | 10 | Wild type | Deceased | N/A |
| 401 | 4 | 1152 | 4 | PD | >4 | Wild type | Unknown | No |
| 402 | 4 | 1152 | 2 | PD | 15 | Wild type | Deceased | N/A |
| 403 | 4 | 1152 | 8 | SD | 9 | Wild type | Deceased | N/A |
aEach cycle is 28 days.
bPerformed at end of even-numbered cycles and 6 month final.
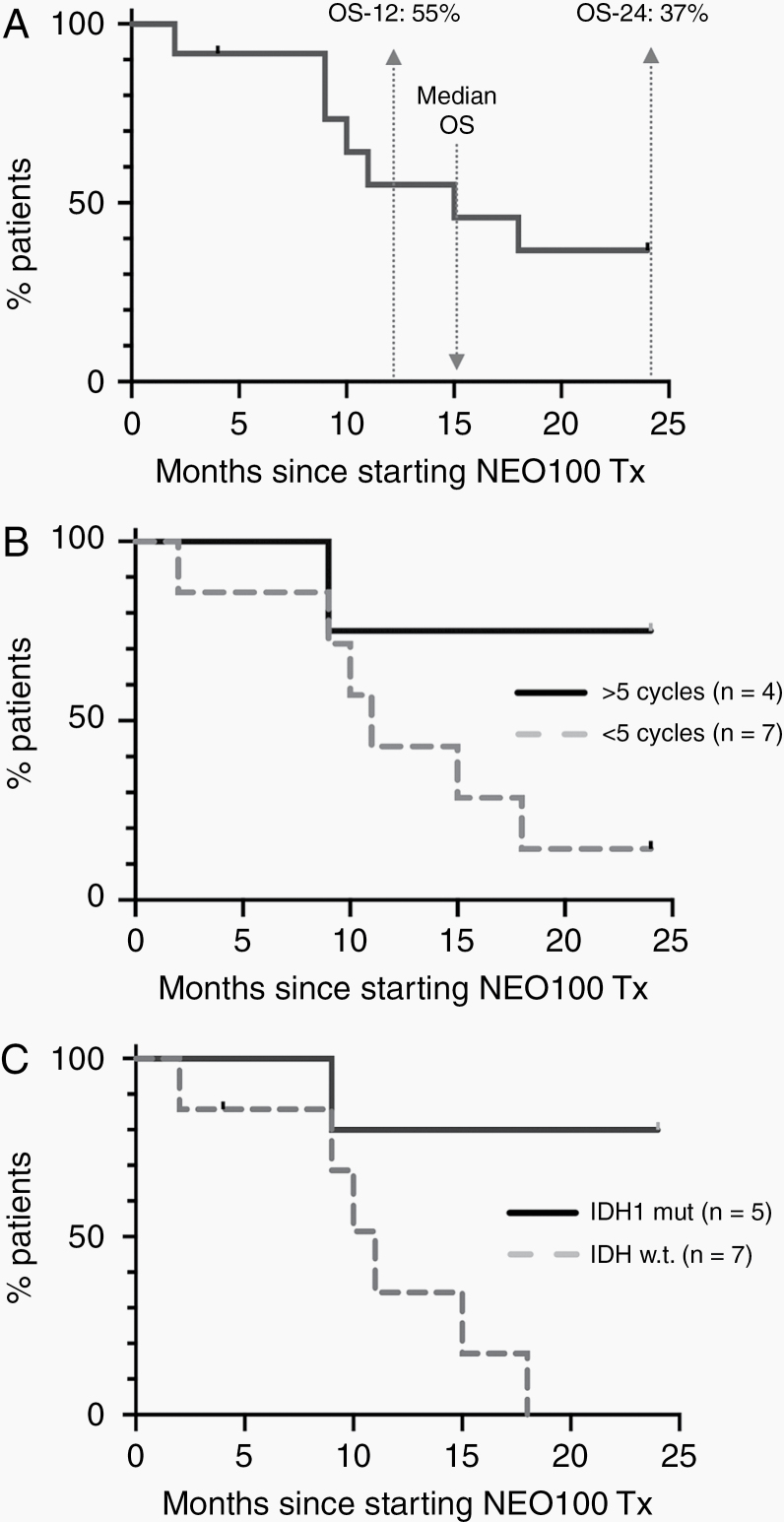
In all, PFS-6 was 33% among the entire group of patients (n = 12) enrolled in this Phase I, with Cohort 1 having the lowest (0%) and Cohort 3 having the highest (67%) PFS-6 (Figure 1). Examples of radiographic responses are presented in Figure 2, showing a partial response after 10 months and a complete response after 12 months of NEO100 treatment. Overall survival at 12 months (OS-12) was 55%, at 24 months (OS-24) it was 37%, and median OS was 15 months (Figure 3A). In all, there were several patients with notably long survival: 4 patients survived at least 24 months, and 3 of these are still alive (Table 3). Thus, despite only 33% PFS-6, a median OS of 15 months emerged as an encouraging result for this recurrent glioblastoma population.
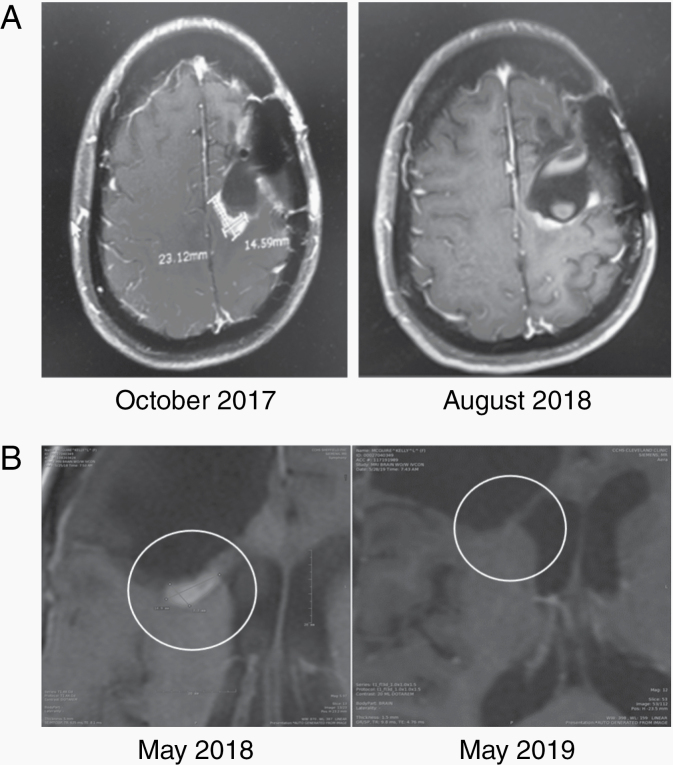
Figure 2.
Examples of radiographic responses. (A) MRI scans of Patient 202 before treatment and after 10 months of NEO100 show partial response. (B) MRI scans of Patient 301 before and after 12 months of NEO100, showing a lack of recurrence during NEO100 treatment.
Figure 3.
Overall survival. (A) Shown is the survival of all patients (n = 12) within the first 24 months after initiation of NEO100 treatment, irrespective of the number of treatment cycles that were completed. Overall survival at 12 months (OS-12) and 24 months (OS-24) is indicated. Median OS is shown at 15 months. Note that one patient (ID 401) was censored at 4 months (tick mark) because he was lost to follow-up. (B) Shown is the survival of patients within the first 24 months after initiation of NEO100 treatment, separated into groups of patients who completed at least 6 cycles (n = 4; indicated as >5 cycles) and those who completed fewer than 5 cycles (n = 7; <5 cycles). The status of Patient 401 was lost to follow-up after completion of 4 cycles and progressive disease, and therefore he was not included in this comparison. (C) Shown is the survival of patients within the first 24 months after initiation of NEO100 treatment, separated as per IDH1 status in their tumor tissues. Four of 5 patients (80%) with mutated IDH1 survived at least 24 months. Six patients with wild type IDH1 had succumbed to their disease by 18 months. P =.018 (log-rank test). Patient 401 (IDH1 wild type) was censored at 4 months (tick mark).
For further analysis, we separated all patients into 2 groups: those that had completed at least 6 cycles (n = 4) of NEO100, and those that had not (n = 7). The latter group included one patient with 1 cycle, 6 patients with 2 cycles, and 1 patient with 4 cycles (who was lost to follow-up immediately after completing 4 cycles and therefore was omitted from the comparison). Intriguingly, there was a noticeable difference in longer-term survival between these 2 groups, although it did not reach statistical significance. As shown in Figure 3B, for those 4 patients who completed at least 6 cycles, OS-24 was 75%. In comparison, for the evaluable 6 patients who completed only 1 or 2 cycles, OS-24 was 14%. However, despite the poorer outcome of this second group as compared to the first group, the median OS was a notable 11 months, again demonstrating that despite early progression the longer-term survival was quite encouraging.
We also analyzed overall survival based on the status of the IDH1 gene. Mutations in this gene are known to confer a survival advantage for newly diagnosed glioma patients.24 As shown in Figure 3C, there was significantly longer (P =.018) overall survival for patients with IDH1 mutant tumors, with 4 of 5 patients (80%) surviving at least 24 months. In comparison, none of the patients with wild type IDH1 survived beyond 18 months, although the median OS still was a notable 11 months.
The presence of PA was determined in plasma obtained from all patients at different time points after administration of the first daily dose of intranasal NEO100. These blood draws were done on Day 1 and 8 of the first 28-day cycle, and repeated on the first day of the second cycle. PA is a major metabolite of perillyl alcohol and is more stable, making it a convenient, easy to detect marker of POH exposure. As shown in Supplementary Figure 1, plasma concentrations of PA were readily quantifiable and present at maximum concentrations at 5 min after NEO100 administration, with an initial half-life of approximately 20 min. Maximum plasma PA concentrations on average were higher in patients administering the higher dosages. As well, within each cohort, these concentrations were noticeably higher during the 2 later days, as compared to the measurements from the very first dose administration (Day 1 of Cycle 1). Despite noticeable interpatient variability in absolute values, Cmax was reduced by >90% in most patients within 2 h after intranasal delivery. In all, these data indicated rapid drug entry into the systemic circulation that was followed by first-order kinetics of elimination and lack of accumulation.
Discussion
The present study provides evidence that intranasal NEO100, when delivered 4 times a day, is safe and potentially effective in recurrent GBM patients. The treatment was very well tolerated at all dose levels and no severe adverse events were reported. At the highest dosage used, 1152 mg/day divided into 4 equal doses of 288 mg, maximum tolerated dose (MTD) was not reached. These results are consistent with those obtained in Phase I/II studies in Brazil that used commercial-grade POH in patients with recurrent GBM, grade III anaplastic astrocytoma, and anaplastic oligodendroglioma, although at lower dosages of 133 mg QID (534 mg/day).18–20 In those studies, adherence to the protocol was high (>95%) and occasionally caused nose soreness but no severe adverse effects, even after several years of continuous application.20
Despite the small patient number in our current study, the initial analysis of the efficacy of intranasal NEO100 for recurrent GBM patients appears promising. PFS-6 was 33%, OS-6 was 92%, OS-12 was 58%, and 4 patients (33%) survived >24 months. This compares very favorably to prior single-agent studies with recurrent GBM patients, several of which are summarized in Supplementary Table 1. For instance, Wong et al. reviewed 8 Phase II studies with various treatments performed during the pretemozolomide era, which averaged 21% OS-12 and 5.7 months median OS.25 Several newer studies, completed over the past 8 years mostly with patients that had failed standard chemoradiation with temozolomide (ie the Stupp protocol26), yielded mixed results and achieved only incremental improvements in survival. For example, alternating electric fields (tumor treatment fields [TTFs], NovoTTF-100A) emerged as a conceptually novel approach a decade ago, but it did not show improved outcomes in the recurrent setting27 as compared to historical controls or conventional chemotherapy, such as lomustine28 or fotemustine.29 Bevacizumab was granted accelerated approval for the treatment of recurrent GBM in the United States, although its impact on OS-12 and median OS remained muted.30–32 A very recent trial with nivolumab, a fully human monoclonal antibody targeting the programmed death-1 (PD-1) immune checkpoint receptor, also did not yield substantial improvements, and survival results were comparable to those achieved with conventional chemotherapy or bevacizumab.33
Two very recent trials reported outcomes that pushed the median OS beyond the 1-year mark (Supplementary Table 1). One study used Toca-511 (vocimagene amiretrorepvec), a nonlytic retroviral replicating vector that delivers yeast cytosine deaminase, which converts separately administered Toca FC (extended-release 5-fluorocytosine) into the antimetabolite 5-fluorouracil.34 This trial achieved an OS-12 of 55% and a median OS of 13.6 months. Similar results were obtained with direct intratumoral delivery of PVSRIPO, a recombinant polio-rhinovirus chimera that recognizes the poliovirus receptor CD155, which is commonly expressed on the surface of tumor cells.35 This trial achieved an OS-12 of 54% and median OS of 12.5 months. Results from our current study on intranasal NEO100 compare very favorably to these improved outcomes, as we achieved an OS-12 of 55% and median OS of 15 months.
An important advantage of our study lies in its very low toxicity, noninvasiveness, and lack of serious adverse events, emphasizing that this treatment approach does not lead to deterioration of quality of life for the patients. In comparison, many other treatments mentioned above have a less than optimal safety profile. For example, nitrosoureas are known for their bone marrow suppression, liver/renal toxicity, or interstitial lung disease, and bevacizumab may cause hemorrhage and hypertension. Direct administration via convection-enhanced delivery, as is practiced in the case of PVSRIPO,35 is invasive and includes all risks associated with surgical catheter placement and removal. In general, combination regimens do not produce evidence for superior activity, but commonly produce more toxicity.36
We further made the intriguing observation that even those patients who progressed before completion of the planned 6 months of treatment with NEO100 lived longer than expected. Upon progression, these patients were switched to a mixture of the best standard of care as per their neurooncologist. Although it is too early for firm conclusions, it is tempting to speculate that NEO100 perhaps exerted beneficial effects that lingered beyond its discontinuation, and that despite early progression and treatment termination there was an advantage for longer survival. Moreover, there may have been pseudoprogression on MRI scan, leading to premature stoppage of NEO100. It will be important to pay particular attention to these unresolved issues in the Phase IIa part of this study.
Another intriguing result was our observation that patients with IDH1 mutation appeared to have a survival advantage. IDH1 gene mutation is a known predictor of better overall survival in malignant glioma.24 However, while this link has been firmly established in the case of newly-diagnosed patients, it is not clear whether it also applies to the recurrent setting, as inconsistent outcomes (on small numbers of patients) have been reported. For instance, Mandel et al.37 reported that the IDH1 mutation might have a positive influence on survival, although only at first recurrence. However, another report by Tabei et al.38 was unable to confirm a positive correlation of IDH1 mutation and survival after the first progression. Among the 12 patients of our study, some of those with IDH1 mutant status tended to have the longest time intervals from the initial diagnosis to enrollment in our study (Table 1), consistent with the expectation that these patients generally have longer survival times after initial diagnosis. It is further noteworthy that the majority of these patients (3 of 5; 60%) completed the scheduled minimum of 6 cycles of NEO100, whereas among the group of IDH1 wild type patients only 1 of 7 patients (14%) completed 6 cycles (Table 3). This differential complicates a straightforward interpretation of the contribution of IDH1 status, because longer survival of IDH1 mutant patients in our study could be the result of their IDH1 status, or rather be derived from longer exposure to NEO100 treatment. However, it is remarkable that among those patients who completed 6 cycles, those 3 with IDH1 mutant status still went on to survive substantially longer (24, 27, 33 months since enrollment and all still alive) than would have been expected based solely on their IDH1 status. In comparison, the one IDH1 wild type patient who completed 6 cycles of NEO100 succumbed to disease only 9 months after enrollment (Table 3). Based on these considerations, we conjecture that IDH1 mutant status perhaps might provide a suitable genetic background for NEO100 to unfold its increased therapeutic benefit (although several of the IDH1 wild type patients also survived longer than expected, despite early termination of their NEO100 cycles). Naturally, interpretation of these effects is limited by small patient numbers, and further studies on this topic are warranted. As planned, the MTD that was determined in our Phase I study (1152 mg/day) has become the selected dosage for Phase IIa with anticipated 28 patients to be enrolled. However, in view of the arisen issue concerning IDH1 status, we have performed a new statistical analysis and are resending this new recruitment criterion to the FDA, so that the upcoming Phase IIa may focus on IDH1 mutant recurrent glioblastoma patients.
In conclusion, intranasal glioma therapy with NEO100 was well tolerated. It correlated with improved survival when compared to historical controls, pointing to the possibility that this novel conceptual approach could become useful for the treatment of recurrent GBM. Due to its very low toxicity profile, it might offer the possibility of combining this regimen with other, more taxing approaches without increasing adverse events. As well, based on the facile administration process and continued quality of life, patients who progress on intranasal NEO100 might be more inclined to pursue further lines of therapy. Although resistance mechanisms against NEO100 have not yet been identified and characterized, one might surmise that standard postprogression treatments and approaches presented in Supplementary Table 1 could still unfold significant activity and benefit for such patients.
Supplementary Material
Funding
This study was supported by funding provided by NeOnc Technologies, Inc. (Los Angeles, CA).
Conflict of interest statement. T.C.C. is founder and stakeholder of NeOnc Technologies, Inc., Los Angeles, California, USA. V.F.S. is Chief Regulatory Officer of NeOnc Technologies. C.O. da F. is a member of the Board of Directors of NeOnc Technologies.
Authorship Statement. D.M.P., N.W., S.P.H., L.P.T., A.J.M., K.M.H., and V.F.S. contributed to running the clinical trial. T.C.C., R.L., V.F.S., and C.O. da F. conceived of the study and designed the trial. A.H.S. drafted the manuscript. A.H.S., V.F.S., F.C., and T.C.C. analyzed the data. All authors read and approved the final version of the manuscript.
References
- 1. Mehta M, Wen P, Nishikawa R, Reardon D, Peters K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit Rev Oncol Hematol. 2017;111:60–65. [DOI] [PubMed] [Google Scholar]
- 2. Ghiaseddin A, Peters KB. Use of bevacizumab in recurrent glioblastoma. CNS Oncol. 2015;4(3):157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lemos ML, Markarian A, Chan E, Schaff K, Walisser S. Clinical effectiveness of bevacizumab in patients with recurrent brain tumours: a population-based evaluation. J Oncol Pharm Pract. 2018;24(1):33–36. [DOI] [PubMed] [Google Scholar]
- 4. Crowell PL, Elson CE. Isoprenoids, health and disease. In: Wildman REC, ed. Nutraceuticals and Functional Foods. Boca Raton, FL: CRC Press; 2001:31–54. [Google Scholar]
- 5. Chen TC, Fonseca CO, Schönthal AH. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am J Cancer Res. 2015;5(5):1580–1593. [PMC free article] [PubMed] [Google Scholar]
- 6. Gelb MH, Tamanoi F, Yokoyama K, Ghomashchi F, Esson K, Gould MN. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995;91(2):169–175. [DOI] [PubMed] [Google Scholar]
- 7. Bailey HH, Levy D, Harris LS, et al. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern Cooperative Oncology Group Study E2E96. Gynecol Oncol. 2002;85(3):464–468. [DOI] [PubMed] [Google Scholar]
- 8. Liu G, Oettel K, Bailey H, et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Invest New Drugs. 2003;21(3):367–372. [DOI] [PubMed] [Google Scholar]
- 9. Meadows SM, Mulkerin D, Berlin J, et al. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. Int J Gastrointest Cancer. 2002;32(2-3):125–128. [DOI] [PubMed] [Google Scholar]
- 10. Bitter C, Suter-Zimmermann K, Surber C. Nasal drug delivery in humans. Curr Probl Dermatol. 2011;40:20–35. [DOI] [PubMed] [Google Scholar]
- 11. Dhuria SV, Hanson LR, Frey WH II. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. [DOI] [PubMed] [Google Scholar]
- 12. Peterson A, Bansal A, Hofman F, Chen TC, Zada G. A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option. J Neurooncol. 2014;116(3):437–446. [DOI] [PubMed] [Google Scholar]
- 13. Gizurarson S Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. 2012;9(6):566–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman CD, Schiöth HB, Grillo CA, Benedict C. Intranasal insulin in Alzheimer's disease: food for thought. Neuropharmacology. 2018;136(Pt B):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–448. [DOI] [PubMed] [Google Scholar]
- 16. Illum L Nasal drug delivery - recent developments and future prospects. J Control Release. 2012;161(2):254–263. [DOI] [PubMed] [Google Scholar]
- 17. Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57(11):1640–1665. [DOI] [PubMed] [Google Scholar]
- 18. da Fonseca CO, Schwartsmann G, Fischer J, et al. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol. 2008;70(3):259–266. [DOI] [PubMed] [Google Scholar]
- 19. da Fonseca CO, Simão M, Lins IR, Caetano RO, Futuro D, Quirico-Santos T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol. 2011;137(2):287–293. [DOI] [PubMed] [Google Scholar]
- 20. da Fonseca CO, Teixeira RM, Silva JC, et al. Long-term outcome in patients with recurrent malignant glioma treated with perillyl alcohol inhalation. Anticancer Res. 2013;33(12):5625–5631. [PubMed] [Google Scholar]
- 21. Cho HY, Wang W, Jhaveri N, et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol Cancer Ther. 2012;11(11):2462–2472. [DOI] [PubMed] [Google Scholar]
- 22. Ripple GH, Gould MN, Arzoomanian RZ, et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin Cancer Res. 2000;6(2):390–396. [PubMed] [Google Scholar]
- 23. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Version 4.0. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed August 30, 2017.
- 24. Huang LE Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis. 2019;40(11):1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 26. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 27. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 28. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brandes AA, Finocchiaro G, Zagonel V, et al. AVAREG: a phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol. 2016;18(9):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Field KM, Simes J, Nowak AK, et al. ; CABARET/COGNO investigators Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A. Progression-free and overall survival in patients with recurrent glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol. 2016;126(3):567–575. [DOI] [PubMed] [Google Scholar]
- 32. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 33. Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Desjardins A, Gromeier M, Herndon JE II, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mandel JJ, Cachia D, Liu D, et al. Impact of IDH1 mutation status on outcome in clinical trials for recurrent glioblastoma. J Neurooncol. 2016;129(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tabei Y, Kobayashi K, Saito K, et al. Survival in patients with glioblastoma at a first progression does not correlate with isocitrate dehydrogenase (IDH)1 gene mutation status. Jpn J Clin Oncol. 2020;51(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.