Abstract
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal solid tumors with an overall five-year survival rate of that has only just reached 10%. The tumor microenvironment of PDAC is characterized by desmoplasia, which consist of dense stroma of fibroblasts and inflammatory cells, resulting in a hypoxic environment due to limited oxygen diffusion through the tumor. Hypoxia contributes to the aggressive tumor biology by promoting tumor progression, malignancy, and promoting resistance to conventional and targeted therapeutic agents. In depth research in the area has identified that hypoxia modulates the tumor biology through hypoxia inducible factors (HIFs), which not only are the key determinant of pancreatic malignancy but also an important target for therapy. In this review, we summarize the recent advances in understanding hypoxia driven phenotypes, which are responsible for the highly aggressive and metastatic characteristics of pancreatic cancer, and how hypoxia can be exploited as a target for drug delivery.
1. Introduction
Pancreatic cancer, with a mortality rate close to its incidence rate, is one of the most lethal cancer. The overall incidence of pancreatic cancer is predicted to be around ~420,000 by the year 2020, with an associated mortality of around 410,000 [1]. The 5-year survival rate just reported by the American Cancer Society is 10% [2]. With surgery, which is the only curative treatment due to metastatic spread and chances of recurrence, the survival rate may increase to 35% [3]. By 2030 [4], pancreatic cancer is expected to be the second deadliest cancer, second only to lung cancer. Pancreatic ductal adenocarcinoma (PDAC), is the most frequent subtype of pancreatic cancer. The appalling numbers for PDAC, are a result of early spread, development of micro-metastasis, and resistance to radio/chemo-therapies [4]. In-depth analysis of genetic drivers of PDAC show frequent mutations including activated KRAS and inactivating mutations in the tumor suppressor genes TP53, SMAD4 and CDKN2A [5,6]. These mutations cause modifications to ductal cells which lead to pancreas reorganization (Fig. 1). Despite substantial progress in identification of the genetics drivers, we have not uncovered mutations which explain the improved biology in some patients which survive compared to the majority of patients with PDAC [7,8]. The exome sequencing of a cohort of very long-term survivors showed no difference of mutations in comparison to short term survivors, suggesting that examining somatic mutations alone may not be sufficient to understand the biological and clinical differences in PDAC tumors [9]. The complexity of PDAC is intensified by the peculiar tumor microenvironment with the abundant stroma which is mainly cancer-associated fibroblast (CAF), primarily derived from pancreatic stellate cells (PSC) and inflammatory cells (Fig. 1). One of the most important characteristics of PDAC is the stroma, which creates a barrier not only for the chemotherapy but also for perfusion of nutrients and oxygen resulting in appearance of hypoxic zones. Lack of oxygen creates a drastic environment where tumor cells have to adapt and rewire their metabolism to survive. The cells that survive this harsh condition contribute to the aggressiveness of PDAC. The identification of two stromal specific and two tumor specific gene signatures that were independent predictors of progressions highlights the importance of studying the stroma compartment with both tumor-promoting and tumor-inhibiting effects [10]. This article aims to review the role of hypoxia on the complex and heterogeneous molecular characteristics of PDAC and to discuss novel promising therapeutic agents and delivery platforms exploiting it.
Fig. 1.
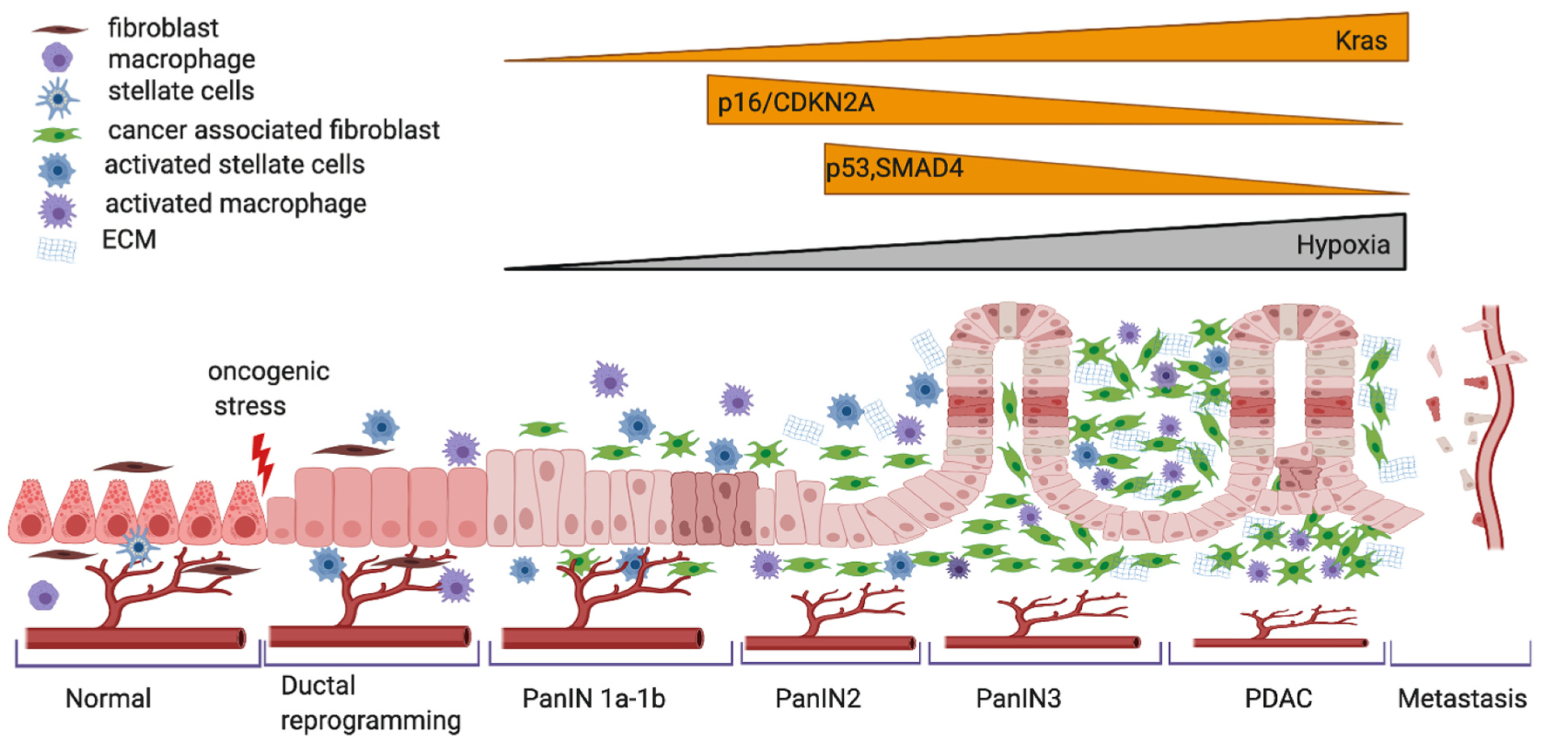
Schematic representation of progression of PDAC. Most PDAC arise from normal acinar cells (epithelial cells) that undergo metaplasia to duct-like cells, initially forming low-grade pancreatic intra-epithelial neoplasia (PanIN) in response to an oncogenic stress, most commonly KRAS mutation, which progress on to high-grade PanIN (2–3) accompanied by accumulating genetic alterations such as loss of p16/CDKN2A, p53 and SMAD4. Recent evidence also suggests a role of hypoxia in the progression of pancreatic ductal adenocarcinoma (PDAC) along with decreased accessibility of the vasculature due to excess ECM development. From left to right: a normal differentiated acinar cell, de-differentiates to ductal state that progress through increasing hyperplastic PanIN grades and on to PDAC and eventually progresses to invasive and metastatic PDAC. Figure created using BioRender.com.
2. Hypoxia and PDAC
Hypoxia represents a lower oxygenation level below 1.5% compared to normoxia which corresponds to atmospheric oxygen pressure or 20% oxygenation in cell culture [11]. Both these terms are poorly defined, as 20% oxygen (160 mmHg) in the tissue culture is significantly higher than the 14.5% (110 mmHg) oxygen level in the lung alveoli or 5% (38 mmHg) oxygen level in peripheral tissues [12]. Thus, tumor size and its density represent various levels of oxygenation, with the lowest levels of oxygen seen within the core of the tumor. To classify hypoxic regions, researchers have classified 2% oxygen or 15 mmHg) as physiological hypoxia, a level expressed by most tumor tissues, and 1% oxygen (8 mmHg) as pathological hypoxia, where the poor oxygenation disrupts normal homeostasis. Pancreatic cancer is considered to be severely hypoxic, expressing a median oxygen level of less than 0.7% (0–5.3 mmHg) compared to adjacent tissue 1.2–12.3% (9.3–92.7 mmHg) [12, 13].
Tumor cells maintain their oxygen homeostasis by numerous changes in gene expression. The pancreatic cells adapt to the hypoxic conditions by activating transcription factors such as hypoxia-inducible factors (HIFs), which in turn stimulate the expression of related genes involved in angiogenesis and glycolysis [14]. HIFs are heterodimeric transcription factors composed of an oxygen regulated α subunit (HIFα) and a constitutively expressed β subunit (HIF1β). There are three isoforms of HIFα (HIF1α, HIF2α, HIF3α) and each can heterodimerize with HIF1β. In normoxia, HIF1/2α protein subunits are rapidly degraded by proteasomes and have a short half-life (<5 min). Under hypoxia, HIFα is stabilized and translocates into the nucleus to bind with HIF1β, where the heterodimer can bind to the regulatory regions of its target genes and adjusts their transcription [15]. HIF1α is a widely used marker of hypoxia [16].
The clinical role of HIF1α in PDAC now ranges from being a predictor for a poor patient outcome to defining early stages of the disease. For example, in a meta-analysis of eight clinical studies the expression of HIF1α [16]correlated with lymph node metastasis and advanced tumor stages resulting in poor survival [17–19]. Similarly, HIF1α mRNA had a sensitivity of 87% in predicting short-term versus long-term survival for PDAC patients [20]. Likewise, G1790A single nucleotide polymorphism in the HIF1α gene is significantly higher in patients than in healthy volunteers [21]. HIF2α has been shown to express in early pancreatic ductal lesions of both human and mouse models of pancreatic cancer and has been implicated in increased progression of low-grade pancreatic intraepithelial neoplasia (mPanIN) lesions to high-grade mPanINs, with a loss of β-catenin and SMAD4 [22]. These studies along with extensive research in the field have established the role for HIF1α and HIF2α as poor predictors for patient outcome in PDAC. However, a recent study has shown that HIF3α expression is stimulated to a greater extent than either HIF1/2α under hypoxic conditions in pancreatic cancer cells [23]. In vitro and in vivo data demonstrated that HIF3α promotes invasion and metastasis by transcriptionally activating the RhoC–ROCK1 signaling pathway [23]. Given the clinical importance of hypoxia and its effect on several cellular pathways, we will discuss the role of hypoxia on metastasis (invasion and migration), stroma and extracellular remodeling, metabolic reprogramming, immune system, and chemoresistance (Fig. 2).
Fig. 2.
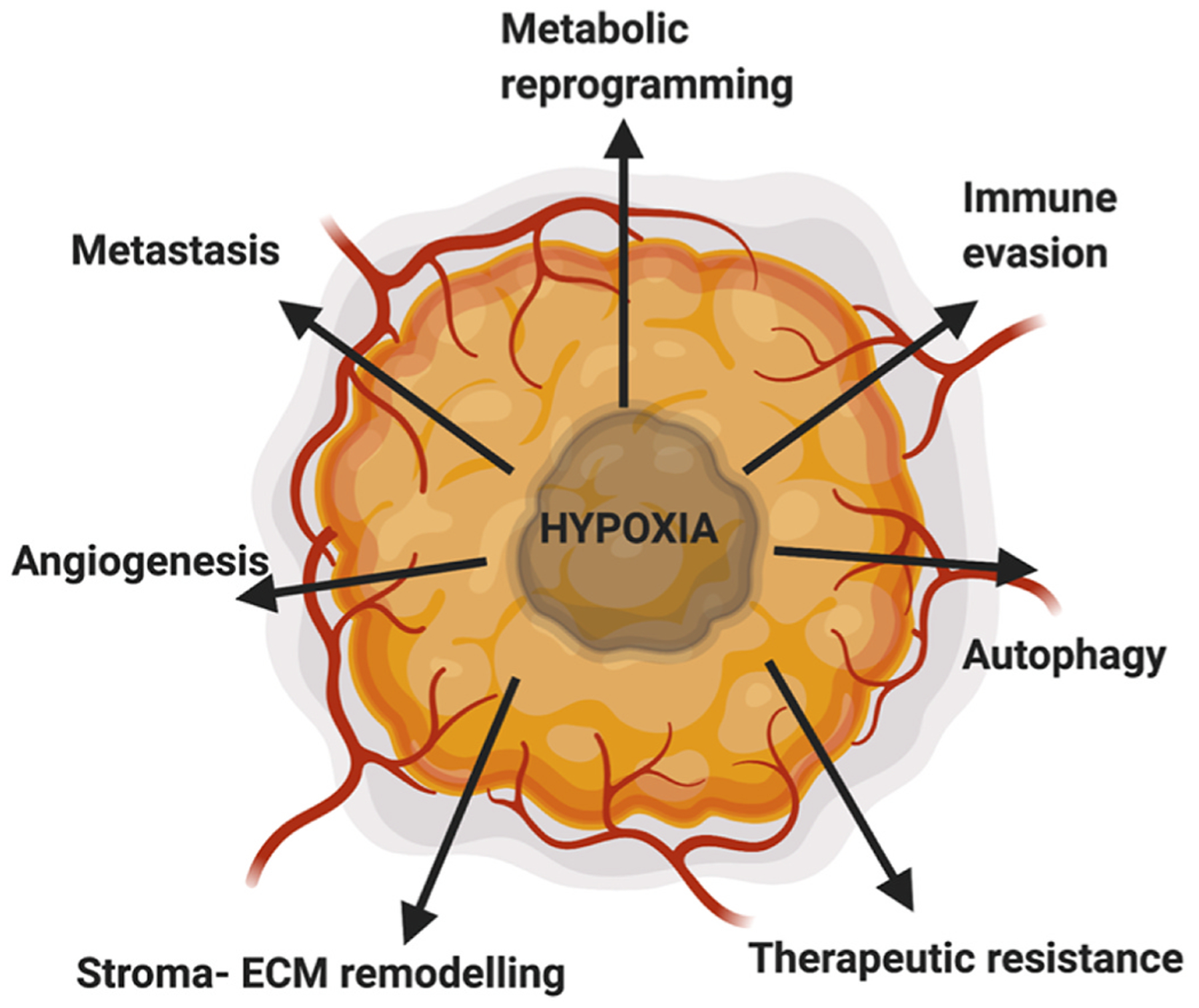
Hypoxia in PDAC drives metastasis, metabolic reprogramming, therapeutic resistance, immune evasion, angiogenesis, autophagy and stroma-ECM remodeling. Figure created using BioRender.com.
2.1. Hypoxia and metastasis
Metastasis is one of the main reasons behind the poor prognosis of PDAC. Hypoxia plays a major role in causing the metastatic phenotype by induction of the metastatic cascade: cancer cell invasion, including epithelial to mesenchymal transition (EMT) and invasive migration through the remodeled extracellular matrix, survival in blood, extravasation, colonization and finally growth of micrometastatic to macrometastatic disease [24]. To acquire properties of migration and invasion, the pancreatic cancer cells undergo a cellular program where they exchange their epithelial characteristics, such as apical-basal polarity and cell-cell adhesion, for mesenchymal traits via a cellular program known as EMT [25]. Following induction of EMT, the mesenchymal phenotype not only has enhanced migratory capacity, and invasiveness but also increased resistance to apoptosis and elevated production of extracellular matrix (ECM) components [26]. EMT increases the survival of cancer cells in blood and metastatic sites by induction of stem-cell like features and creating ECM tracks that other cancer cells can exploit [27–29]. Several studies have revealed that hypoxia induces epithelial plasticity and migratory phenotypes through direct and indirect regulation of hedgehog, nuclear factor kappa light chain enhancer of activated B cells and reactive oxidative species [30–32]. Tumor hypoxia has been correlated well with metastatic burden in both orthotopic xenografts of human pancreatic cell lines and patient derived xenografts [33, 34]. Moreover, only a small portion of tumor cells have cancer stem cell (CSCs) features with the enhanced tumor initiating potential due to their self-renewal property and ability to differentiate to multiple cell types. Accumulating evidence has shown that hypoxia selects for CSC phenotypes and supports their generation and maintenance through regulation of HIF-dependent genes such as c-MYC, NANOG, and SOX2 [35,36]. Recently, it was shown that sustained and intermittent hypoxia induces CSC markers, and drives EMT in both CSC-like cells and CSCs in PDAC [32]. The cells with high CSC-like phenotype showed higher migratory capacity under hypoxia compared to cells with low CSC-like phenotype. Whereas, CSC-high cells displayed migratory capacity in normoxia due to expression of EMT markers like high vimentin, which gets augmented in hypoxia compared to CSC-low cells that are slowly transformed to mesenchymal phenotype when exposed to hypoxia [32]. Therefore, hypoxia induced cellular pathways which trigger migratory, invasiveness and CSC phenotypes contribute to the metastatic potential of PDAC.
2.2. Hypoxia and stroma-ECM
Stroma of PDAC is often hypoxic as confirmed by an increase in HIF1α expression in the stromal cells of human tumors and in mice PDAC tumor xenografts by immunohistochemical analysis approach using pimonidazole, a 2-nitroimidazole molecule that is reductively activated specifically in hypoxic cells [33,37–39]. The stroma consist of ECM produced by activated PSCs, which play a significant role in patient survival for patients which have activated PSC-rich vs activated PSC-poor stroma [40]. In-vitro studies have shown that activated PSCs showed increased expression of HIF1α, α-smooth muscle actin, and increased ECM deposition as a result of synthesis and secretion of collagen, periostin, and fibronectin [41]. Hypoxia also simulates the ligand for sonic hedgehog pathway on the cancer cells which in a paracrine fashion binds to patched-1 receptor on fibroblasts and further promotes secretion of collagen [42,43]. The stroma has decreased blood supply making it hypoxic and the hypoxia stimulated PSCs increase endostatin production by pancreatic cancer cells which further reduces blood flow [41]. Endostatin is a 20-kDa endogenous angiogenesis inhibitor that has recently been shown to inhibit the expression of vascular endothelial growth factor (VEGF), an angiogenic growth factor. Thus, a positive feedback loop is established due to increased activity of PSCs as an effect of low oxygen conditions [43]. Taken together, there is a complex relationship between hypoxia and stellate cell induced fibrotic stroma, which contribute to the aggressiveness of PDAC.
2.3. Hypoxia and metabolic reprogramming
Hypoxia results from insufficient blood supply and high oxygen demand as a consequence of rapidly growing cells which typically triggers angiogenesis. However, in PDAC with its unique stroma it causes less vascularization resulting in desmoplastic reaction and activation of a metabolic switch. The switch results in activation of glycolysis instead of oxidative phosphorylation and release of lactate in the cancer cells, which is used in their proliferation and metabolism of glutamine to glucose that helps cells survive in low oxygen levels [44]. Lactate is taken up by adjacent normoxic cells and promotes tumor symbiosis between hypoxic and normoxic cells in which it is recycled as a fuel for growth [45]. Moreover, due to continuous fluctuation of oxygen levels hypoxic cells may become normoxic at times causing the warburg effect or aerobic glycolysis [46]. Due to the Warburg effect, hepatic gluconeogenesis increases to recycle tumor-produced lactate to glucose, which when released to the blood is processed by tumor cells for glycolysis again. In PDAC patients, the glucose-lactate loop cannot meet the demand for glucose may cause skeletal muscle and adipose tissues to undergo catabolic metabolisms to release more glucose contributing to cancer cachexia. HIF1 α is shown to be essential for survival under prolonged hypoxia as it sustains ATP production by switching to glycolysis from oxidative phosphorylation [47]. HIF also decreases the production of reactive oxygen species (ROS) by shunting glucose away from mitochondria into lactate synthesis [47,48]. Cancer cells, by favoring glycolysis, spare pyruvate for building carbon skeletons needed for cellular growth [48]. Thus, if the Warburg effect decreases following an inhibition of cancer-induced HIF1α, cancer cachexia may be reversed [49]. Although, these metabolic pathways in PDAC contribute towards aggressiveness of the disease, they highlight the gap between the normal and pancreatic cell metabolism, which could be exploited and eliminating the hypoxic areas could in turn limit PDAC progression and metastasis.
2.4. Hypoxia and immune system
PDAC has a unique tumor microenvironment composed of myofibroblasts and immune cells that often outnumber carcinoma cells resulting in a very hypoxic tumor [50]. Hypoxia induces changes in the tumor microenvironment that encourage immunosuppression, which may play a role in diminishing the efficacy of immunotherapy [51]. In PDAC, the cytokines like IL-6, IL-1, TNF-α, TGF-β activates PSCs leading to produce ECM molecules resulting in fibrosis. Increased fibrosis not only produces increased CXCL12 but also interferes with chemokines used in T cell homing causing them not to reach the tumor cells [41,52]. In PDAC cell lines, HIF2α increases survivin production which provides resistance to apoptosis by tumor necrosis factor related apoptosis inducing ligand (TRAIL) [53]. Hypoxia also upregulates carbonic anhydrase and GLUT1 and GLUT3 transporters in PSCs which further contribute to immunosuppressive microenvironment [41,54]. Moreover, HIF1α knockout in mouse pancreas showed an increase in the number of B cells in the tumor microenvironment. HIF1α knockout PDAC mice model showed enhanced PanIN progression at early stages, but depletion of B cells via anti-CD20 antibody reduced their commencement in the early phase of life, prior to tumor appearance by increasing the T cell infiltration into the tumor [55]. M2 macrophages are higher and show immunosuppressive properties in hypoxic regions of PDAC [56]. HIF1α stabilization has been correlated to increased PD-L1 expression on both macrophage and dendritic cell surfaces, along with increased production of MMP-7, thus protecting neighboring cells from cell mediated apoptosis [56]. Hypoxia was shown to decrease circulating dendritic cells with corresponding increase in TNF-α and IL-6, and the absence of HIF1α showed less CD278 expression (expressed on activated T cells), and decreased production of granzyme B mRNA by T cells in culture because of PI3K/Akt pathways [57,58]. Overall, there are conflicting reports on the effects of hypoxia on the functioning of immature and mature dendritic cells within hypoxic tumor microenvironment. HIF1α also promotes differentiation of FOXP3+ cells via increased gene transcription and in presences of TGF-β, they become Tregs [59]. In hypoxic conditions, T cell extravasation from blood vessels is reduced and ROS dependent T cell apoptosis is increased [60]. Therefore, there are multiple mechanisms by which hypoxic PDAC tumors inhibit antitumor immune responses.
The complex interplay of immune effectors is one of the great challenges in establishing effective immunotherapies, which have so far failed in PDAC. Checkpoint blockade therapies targeting PD-1/PDL1 and CTLA-4 have shown remarkable clinical success in other malignancies but showed no activity in PDAC [61]. Their combination with chemotherapy has also showed no significant activity compared to chemotherapy alone. Other single agent therapies such as TGFβ inhibitors, miRNA inhibitors, and Treg depletion, that are promising in other solid tumors have shown little success in PDAC [61]. Research has now shifted to evaluating these therapies as combination therapies with chemotherapy and radiation emphasizing the fact that the immune barrier in PDAC is different compared to other tumors. Further, research has been directed towards vaccine strategies to prime tumor specific T cells such as GVAX, an irradiated granulocyte–macrophage colony stimulating factor (GM–CSF)–secreting allogeneic whole-tumor vaccine, which showed immunomodulation in PDAC by stimulating an increase in T cell infiltrates and myeloid cell activation [62]. The concept was further evaluated using CRS-207, a live attenuated bacterium expressing mesothelin and its combination with cyclophosphamide and GVAX resulted in expansion of a repertoire of mesothelin-specific T cells in the peripheral blood in some patients [63]. Overall, the effects of vaccine therapies for PDAC are mild, suggesting we still need to explore alternatives to reverse immunosuppressive environment of PDAC [64].
2.5. Hypoxia and chemoresistance
Hypoxia can mediate chemotherapy resistance through extrinsic resistance resulting from fibrosis due to CAFs and dense ECM components. Increased tissue tension and intratumoral pressure causes a decrease in perfusion of chemotherapeutic agents and therapeutic response [37]. Hypoxia also regulates drug efflux as HIF1 is able to stimulate the expression of the multidrug resistance 1 (MDR1) gene in response to hypoxia, a gene which can actively transport a number of chemotherapeutic drugs [65]. Additionally, hypoxia influences metabolic reprogramming as it induces up regulation of asparagine synthetase in response to hypoglycemia mediated apoptosis which is also a method for cisplatin resistance [66]. BNIP3, a gene involved in hypoxia-mediated cell induced apoptosis is downregulated in pancreatic cell lines. Loss of BNIP3 has been associated with resistance to gemcitabine and 5-fluorouracil [67]. Hypoxia modulates multiple signaling pathways which play an important role in chemoresistance, for example; resistance to gemcitabine is increased in hypoxia via the PI3K/Akt/NF-κB pathways that increases anti-apoptotic proteins such as Bcl-XL and FLIP. HIF1 functions as a strong suppressor of apoptosis, it can modify programmed cell death upon treatment with chemotherapeutic agents in PDAC. Also, HIF1 induces stemness, if CSCs are not eliminated during chemoradiotherapy, tumor recurrence and subsequent clinical progression may manifest due to the drug resistance of CSCs. Overall, tumor cell survival adapts in hypoxia to minimize apoptosis and increase resistance to chemotherapy. Hypoxia also promotes resistance to radiotherapy through decreased production of DNA free radicals and increased DNA repair enzymes.
3. Targeting hypoxia in PDAC
Given the role and molecular characterization of hypoxia, it is a rational approach to target HIF pathways for treatment of PDAC (Fig. 3). Efforts were directed to create anti-hypoxic therapy to simulate oxygen levels by promoting oxygen dissociation from hemoglobin by OXY111A, a synthetic allosteric effector of hemoglobin 4 [68], with a goal to increase outcomes by combination of anti-hypoxic therapy with the standard cytotoxic treatment in patients with PDAC. HIF, being the master regulator of hypoxia, an antisense oligodeoxynucleotide that specifically targets HIF1α was tested in a pilot clinical trial, two out of six patient’s biopsies showed a significant decrease in HIF1α protein levels but none of them showed a decrease in HIF1α target gene expression [69]. The results led to interest in decreasing HIF1α expression by using compounds that inhibited HIF1α synthesis in PDAC. PX-478, melphalan derivative showed reduced tumor growth in preclinical studies by inhibition of HIF1α expression and digoxin, a cardiac glycoside was shown to inhibit HIF synthesis [70,71]. Minnelide, a water-soluble prodrug of triptolide, a natural compound, was shown to decrease HIF1α as well as CSC features of pancreatic cancer. It is currently being tested in advanced gastrointestinal cancers [72]. Considering the effect of HIF1α on metabolic pathways, glycolytic inhibitors such as 3-bromopyruvarte have been studied. To circumvent its toxicity, it was complexed with β cyclodextrin, which demonstrated strong antitumor effect with no toxicity in an orthotopic pancreatic tumor model [73].
Fig. 3.
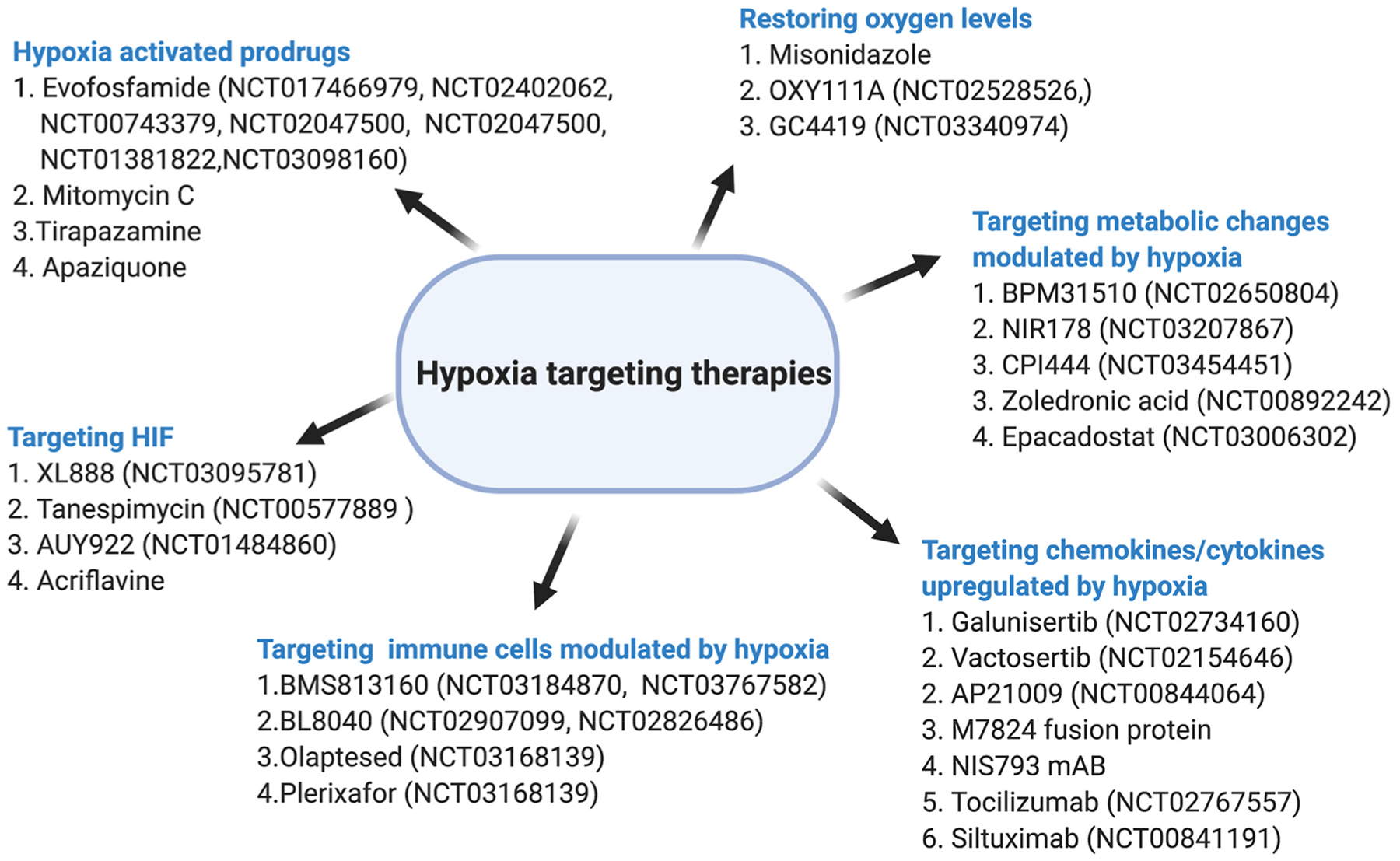
Schematic of therapies targeting hypoxia in PDAC.
Another approach was selectively targeting hypoxic cells by compounds that get activated under hypoxic conditions. Hypoxia activated prodrugs (HAPs), constitute a class of molecules which are inactive in cells with normal oxygen levels but in hypoxic cells undergo chemical reduction to the active compound. HAPs can be classified into chemical groups that can be modified to serve as the as their backbones such as nitro groups, aromatic and aliphatic N-oxides, quinones, and transition metals [74]. They can also be classified based on the hypoxic threshold required for their activation into class I and class II. Class I, comprise of compounds such as benzotriazine, N-oxide, SN30000, and tirapazamine that can be activated under relatively mild hypoxia and class II comprise of PR-104 A and TH-302, which are maximally activated under extreme hypoxia [75]. Tirapazamine, was the first HAP studied to show promising results in preclinical studies but failed to show clinical benefit in large randomized clinical trials when combined with chemotherapy and radiation [76]. Thus, class II HAPs have more potential to be used for targeting hypoxia due to the ability to be activated under extreme hypoxic conditions, which would increase the therapeutic window. Evofosfamide (TH-302), a 2-nitroimidazole-triggered HAP releases bromoisophosphoramide mustard, a DNA crosslinker in hypoxic cells [77]. It is activated through the release of a prodrug radical by reduction in hypoxic cells, which gets oxidized back to the prodrug in oxygenated cells [76]. Promising preclinical trials/models for PDAC led to phase 2 clinical trials of TH-302 alone or in combination of gemcitabine in advanced PDAC. The results showed a significantly improved survival of 5.1 months with gemcitabine + TH-302 compared to 3.4 months with gemcitabine alone [76]. Unfortunately, in the phase III trial that included untreated patients with advanced and metastatic PDAC randomized to TH-302 in combination with gemcitabine versus gemcitabine and placebo, no survival benefit was revealed. This could be due to inclusion of both metastatic and locally advanced disease patients in the study and also the comparison to gemcitabine as a single agent for advanced disease when combination chemotherapy standard of care has changed to FOLFIRINOX and gemcitabine/nab-paclitaxel for patients [76]. In addition, predicting the sensitivity of individual tumors to HAPs is also a concern behind the lack of success of these agents. Research now is focused on screening hypoxic PDAC patients for clinical trials to increase the outcome of the studies. Continued research in this area of HAPs involves developing novel effective therapies in the future, such as tarloxotinib bromide, an EGFR tyrosine kinase inhibitor that is activated in a hypoxic environment along with other HAP’s such as vinblastine N-oxide prodrug developed by Cascade Prodrug Inc, Oregon [78,79].
Direct inhibition of HIF using drug acriflavine, which inhibits dimerization and transcription of HIF by directly binding to HIF1α and or HIF2α, has still not been evaluated in PDAC but indirect inhibition of HIF1α by its increased degradation has been studied in PDAC by inhibition of heat shock protein HSP 90 [80,81]. Other transcriptional targets of HIF1 include, pH-regulating enzymes carbonic anhydrases - CA9. Its expression is a promising therapeutic target in cancer as it is primarily driven by HIF1 activity, and is not detected in most normal tissues [82]. After a successful phase I trial evaluating the CA9/12-selective small molecule inhibitor SLC-0111 for safety and tolerability in patients with advanced solid tumors (NCT02215850), a follow-up trial is evaluating SLC-0111 in combination with gemcitabine for PDAC in patients with CA9-positive tumors (NCT03450018). HIF1 transcriptional activity is increased by redox signaling via Apurinic/Apyrimidinic Endonuclease-1-Reduction/oxidation Effector Factor 1 (APE1/Ref-1), that converts HIF oxidized transcription factor to a reduced state, leading to upregulation of tumor-promoting genes [83]. APX3330, a direct small molecule inhibitor of APE1/Ref-1 is highly selective for APE1/Ref-1 redox signaling activity in tumor cells [83]. An ongoing clinical trial (NCT03375086) will establish its tolerability and appropriate dosing in patients with solid tumors, including PDAC. CA9 expression has also been correlated with insulin-like growth factor-1 (IGF1) expression in pancreatic specimens. Under hypoxia, CAFs were shown to stimulate the invasion activity of PDAC cells through paracrine IGF1/IGF1R signaling, which was shown to be inhibited using IGF1R inhibitors [84].
Natural products such as β-elemene (1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane), a compound isolated from an extract of the traditional Chinese medicinal herb Curcuma wenyujin has been shown to ameliorates malignant peritoneum effusion in pancreatic cancer patients by inhibiting the HIF1A/VEGFA pathway [85]. Emodin (1,3,8-trihydroxy-6-methylanthracene-9,10-dione) and rhein (4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic acid), components of Rheum palmatum or Chinese rhubarb were shown to inhibit HIF1α expression in human pancreatic cancer cells by decreasing phosphorylation of protein kinases B (p-AKT) and ERK1/2 (p-ERK1/2) in vitro and have been shown to attenuate cancer cachexia in vivo that is caused by high hepatic gluconeogenesis and skeletal-muscle proteolysis [49].
3.1. Hypoxia and drug delivery platforms
Conventional therapies have shown some success in the treatment of PDAC but overall survival times of PDAC patients remain unconscionably low. This is attributed to physicochemical properties of the therapeutic agents resulting in poor bioavailability, toxicity and overall, reduced efficacy. Alternate platforms that can increase the efficacy and reduce toxicity of chemotherapeutic agents is needed. Nanotechnology based drug delivery platforms is a viable strategy as it provides the versatility to manipulate the loading capacity, plasma profiles, and surface functionalization for targeting. The platform can be used to deliver small molecules and well as macromolecules and can also be used to co-deliver multiple therapeutic agents. Currently, there are two nanotechnology-based therapeutics approved by the FDA, albumin-bound paclitaxel nanoparticles, Abraxane® or nab-paclitaxel, and liposomal formulation of irinotecan, Onivyde™, that have been approved for PDAC treatment in combinations with other chemotherapeutic agents [86]. Abraxane is a human serum albumin-based encapsulation of paclitaxel, which allows for the enhanced delivery of paclitaxel to the cancer cells leading to increased bioavailability and alleviated toxicity to normal tissue compared to cremophor-based paclitaxel formulation [87]. Another nanomedicine formulation approved for use as a second-line therapy for patients with metastatic PDAC who progressed after gemcitabine-based chemotherapy is liposomal irinotecan in combination with 5-FU and folinic acid [86]. However, these improve the overall survival of patients only modestly. Having established the crucial role of hypoxia above, novel approaches employing targeting hypoxia along with chemotherapeutic agents will be discussed in this section.
Iron chelation results in stabilization and overexpression of HIF1α through the inhibition of prolyl hydroxylases, iron-dependent enzymes responsible for HIF1α degradation. Abnormal iron metabolism in pancreatic cancer is considered as a therapeutic target as significantly higher iron content was found in clinical pancreatic cancer tissues compared to adjacent tissues. Also, the expression of iron metabolism-related proteins by immunohistochemistry in a tissue microarray consisting of 96 human pancreatic cancer specimens showed that the pancreatic cancer cells possess an abnormally high level of intracellular iron, which performs an important role in proliferation and that iron chelation may be an effective therapeutic strategy to treat these tumors. Thus, a combination of a HIF1α inhibitor to block the HIF1α overexpression that accompanies iron chelation was studied. Transferrin receptor targeting liposomes loaded with iron chelator deferoxamine and lificiguat, a HIF1α inhibitor, showed significant tumor growth reduction in both subcutaneous and orthotopic pancreatic cancer xenografts [88].
Oxygen being essential in the process of cancer cell destruction makes it obvious for the hypoxic tumor to be resistance to radiotherapy, chemotherapy, and photodynamic therapy [74]. Tumor oxygenation, resulting in relieving or even reversing the hypoxic microenvironment via delivering oxygen to hypoxic regions of solid tumors to further attenuate resistance to various therapies has received considerable attention [89]. One such platform is an oxygen-self-produced nanoplatform with a modified fluorocarbon (FC)- chain-mediated oxygen delivery protocol established in the mesoporous organosilica nanoplatform that can provide sufficient storage capacity and binding sites for sonosensitizers (IR780) and oxygen [89]. Based on the same principle of tumor oxygenation, CaO2 NPs coated with a pH-sensitive polymer to enable the controlled generation of molecular oxygen as a function of pH were prepared which were activated only as a function of low pH in hypoxic regions of solid tumors. The polymer coated NPs significantly increased the tumor pO2 levels in pancreatic xenograft tumors in a period of 10–30 min following administration [90]. Similarly, to increase the sensitivity of hypoxic tumor cells to radiotherapy, a tungsten oxide nanoparticle (NP) modified with ligands targeting the CCL-28, a chemokine overexpressed in the hypoxic microenvironment [91], were formulated. The NPs were covalently bound using a matrix metalloproteinase-2 (MMP-2) cleavable peptide (ProLeu-Gly-Val-Arg-Gly) to increase size and thus the overall plasma circulation. The particles were shown to be taken up due to the enhanced permeability and retention (EPR) effect and the up-regulated expression of the MMP-2 enzyme in the tumor microenvironment triggered the destruction of the larger NPs to release smaller NPs with the CCL-28 ligands, which facilitated deeper tumor penetration due to their smaller sizes [91]. Ultrasound mediation of stimuli responsive microdroplet loaded with LLL12, a small molecule STAT3 inhibitor and oxygen gas showed increased drug and oxygen deposition in pancreatic cancer cells and enhanced therapeutic outcome [92]. To minimize the risk involved with intravenous injection of microbubbles or droplets, an orally delivered suspension of surfactant-stabilized oxygen nanobubbles was developed and tested in a xenograft tumor model for human pancreatic cancer [93]. Single dose of these oxygen nanobubbles showed a reduction of 75% and 25% in the transcriptional and translational expression of HIF1α, respectively, along with a significant reduction in the expression of vascular endothelial growth factor (VEGF) and corresponding increase in the expression of arrest-defective protein 1 homolog A (ARD1A) [93]. Sonodynamic microbubbles loaded with antimetabolite, 5-fluorouracil and oxygen also showed significant tumor reduction of pancreatic tumors in mice compared to antimetabolite alone [94].
Lipid nanoparticles comprising a synthesized hypoxia-responsive, PEGylated lipid and an iRGD peptide encapsulating the anticancer drug gemcitabine in the aqueous core were formulated. The NP released 65% of the encapsulated contents under hypoxic conditions in 2 h and showed decreased cell viability with increased penetration to the hypoxic cores in cultured pancreatic cancer cell spheroids [95]. Similarly, a hypoxia-responsive, diblock copolymer poly(lactic acid)-azobenzene-poly(ethylene glycol), which self-assembled to form polymersomes loaded with gemcitabine and erlotinib were formulated [96]. In three-dimensional spheroid cultures of pancreatic cancer cells, polymersomes released the anticancer drugs in 50 min under hypoxic condition compared to none in normoxia [96].
4. Conclusion
Despite the lack of success in treatment of PDAC due to the genetic mutations which lead to its progression and aggressiveness, the contribution of tumor microenvironment to its poor outcome is substantial. Hypoxia is a key determinant amongst the tumor microenvironment driving pancreatic tumor malignancy. Extensive research has identified hypoxia to drive a number of pathways in PDAC that lead to invasiveness, induction of EMT, metastasis, promote ECM deposition, activation of macrophages, and stellate cells that contribute to desmoplasia. Moreover, it is also a barrier to chemo- and radiotherapy. However, all these findings point to hypoxia as an important therapeutic target in PDAC, a disease in urgent need of novel therapeutic approaches.
Funding
V.M.S is supported by grant support from the Brenden-Colson Center for Pancreatic Care at OHSU. R.C.S acknowledges grant support from the NIH (1U01 CA224012, U2C CA233280, U54 CA209988, R01 CA196228, and R01 CA186241) and the Brenden-Colson Center for Pancreatic Care at OHSU. A.W.G is supported by grant support from NIH (1R15 CA227754, 1R37 CA234006).
Footnotes
Declaration of competing interest
None.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012, Int. J. Canc 136 (5) (2015) E359–E386. [DOI] [PubMed] [Google Scholar]
- [2].English IA, Sears RC, Deconstructing pancreatic adenocarcinoma by targeting the conductor, MYC, Canc. Discov 10 (4) (2020) 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, Fishman EK, Hruban RH, Yu J, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J, Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection, Ann. Surg 270 (2) (2019) 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooperman AM, Iskandar ME, Wayne MG, Steele JG, Prevention and early detection of pancreatic cancer, Surg. Clin 98 (1) (2018) 1–12. [DOI] [PubMed] [Google Scholar]
- [5].Buscail L, Bournet B, Cordelier P, Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer, Nat. Rev. Gastroenterol. Hepatol 17 (3) (2020) 153–168. [DOI] [PubMed] [Google Scholar]
- [6].Pu N, Chen Q, Gao S, Liu G, Zhu Y, Yin L, Hu H, Wei L, Wu Y, Maeda S, Lou W, Yu J, Wu W, Genetic landscape of prognostic value in pancreatic ductal adenocarcinoma microenvironment, Ann. Transl. Med 7 (22) (2019) 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES, Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets, Nat. Commun 6 (2015) 6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grutzmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA, Australian I Pancreatic Cancer Genome, A.J. Gill, J.R. Eshleman, C. Pilarsky, A. Scarpa, E. A. Musgrove, J.V. Pearson, A.V. Biankin, S.M. Grimmond, Whole genomes redefine the mutational landscape of pancreatic cancer, Nature 518 (7540) (2015) 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dal Molin M, Zhang M, de Wilde RF, Ottenhof NA, Rezaee N, Wolfgang CL, Blackford A, Vogelstein B, Kinzler KW, Papadopoulos N, Hruban RH, Maitra A, Wood LD, Very long-term survival following resection for pancreatic cancer is not explained by commonly mutated genes: results of whole-exome sequencing analysis, Clin. Canc. Res 21 (8) (2015) 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ, Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma, Nat. Genet 47 (10) (2015) 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shah VM, Nguyen DX, Al Fatease A, Patel P, Cote B, Woo Y, Gheewala R, Pham Y, Huynh MG, Gannett C, Rao DA, Alani AWG, Liposomal formulation of hypoxia activated prodrug for the treatment of ovarian cancer, J. Contr. Release 291 (2018) 169–183. [DOI] [PubMed] [Google Scholar]
- [12].McKeown SR, Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response, Br. J. Radiol 87 (1035) (2014) 20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M, Pancreatic tumors show high levels of hypoxia, Int. J. Radiat. Oncol. Biol. Phys 48 (4) (2000) 919–922. [DOI] [PubMed] [Google Scholar]
- [14].Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC, Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation, Mol. Cell Biol 23 (24) (2003) 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ziello JE, Jovin IS, Huang Y, Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia, Yale J. Biol. Med 80 (2) (2007) 51–60. [PMC free article] [PubMed] [Google Scholar]
- [16].Jun JC, Rathore A, Younas H, Gilkes D, Polotsky VY, Hypoxia-inducible factors and cancer, Curr Sleep Med Rep 3 (1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song Z, Ren H, Gao S, Zhao X, Zhang H, Hao J, The clinical significance and regulation mechanism of hypoxia-inducible factor-1 and miR-191 expression in pancreatic cancer, Tumour Biol 35 (11) (2014) 11319–11328. [DOI] [PubMed] [Google Scholar]
- [18].Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD, Liang TB, Hypoxia-inducible factor 1alpha expression and its clinical significance in pancreatic cancer: a meta-analysis, Pancreatology 14 (5) (2014) 391–397. [DOI] [PubMed] [Google Scholar]
- [19].Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, Cai XJ, Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1alpha as a prognostic role in gastric cancer, World J. Gastroenterol 20 (4) (2014) 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M, Metzger R, Hoelscher AH, Danenberg KD, Prenzel KL, Danenberg PV, High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF, Neoplasia 10 (7) (2008) 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Ren H, Zhao T, Ma W, Dong J, Zhang S, Xin W, Yang S, Jia L, Hao J, Single nucleotide polymorphism in the microRNA-199a binding site of HIF1A gene is associated with pancreatic ductal adenocarcinoma risk and worse clinical outcomes, Oncotarget 7 (12) (2016) 13717–13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Criscimanna A, Duan LJ, Rhodes JA, Fendrich V, Wickline E, Hartman DJ, Monga SP, Lotze MT, Gittes GK, Fong GH, Esni F, PanIN-specific regulation of Wnt signaling by HIF2alpha during early pancreatic tumorigenesis, Canc. Res 73 (15) (2013) 4781–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou X, Guo X, Chen M, Xie C, Jiang J, HIF-3 alpha promotes metastatic phenotypes in pancreatic cancer by transcriptional regulation of the RhoC-ROCK1 signaling pathway, Mol. Canc. Res 16 (1) (2018) 124–134. [DOI] [PubMed] [Google Scholar]
- [24].Nguyen DX, Bos PD, Massague J, Metastasis: from dissemination to organ-specific colonization, Nat. Rev. Canc 9 (4) (2009) 274–284. [DOI] [PubMed] [Google Scholar]
- [25].Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S, The epithelial to mesenchymal transition in pancreatic cancer: a systematic review, Pancreatology 15 (3) (2015) 217–225. [DOI] [PubMed] [Google Scholar]
- [26].Maier HJ, Wirth T, Beug H, Epithelial-mesenchymal transition in pancreatic carcinoma, Cancers 2 (4) (2010) 2058–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thiery JP, Acloque H, Huang RY, Nieto MA, Epithelial-mesenchymal transitions in development and disease, Cell 139 (5) (2009) 871–890. [DOI] [PubMed] [Google Scholar]
- [28].Yang Y, Zheng H, Zhan Y, Fan S, An emerging tumor invasion mechanism about the collective cell migration, Am J Transl Res 11 (9) (2019) 5301–5312. [PMC free article] [PubMed] [Google Scholar]
- [29].Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, Bar-Sagi D, Stanger BZ, EMT subtype influences epithelial plasticity and mode of cell migration, Dev. Cell 45 (6) (2018) 681–695 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan W, Sun Q, Xu J, Wu Z, Wu E, Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner, Mol. Canc 12 (2013) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shimojo Y, Akimoto M, Hisanaga T, Tanaka T, Tajima Y, Honma Y, Takenaga K, Attenuation of reactive oxygen species by antioxidants suppresses hypoxia-induced epithelial-mesenchymal transition and metastasis of pancreatic cancer cells, Clin. Exp. Metastasis 30 (2) (2013) 143–154. [DOI] [PubMed] [Google Scholar]
- [32].Salnikov AV, Liu L, Platen M, Gladkich J, Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J, Schemmer P, Buchler MW, Herr I, Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential, PloS One 7 (9) (2012) e46391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chang Q, Jurisica I, Do T, Hedley DW, Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer, Canc. Res 71 (8) (2011) 3110–3120. [DOI] [PubMed] [Google Scholar]
- [34].Buchler P, Reber HA, Lavey RS, Tomlinson J, Buchler MW, Friess H, Hines OJ, Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model, J. Surg. Res 120 (2) (2004) 295–303. [DOI] [PubMed] [Google Scholar]
- [35].Rankin EB, Nam JM, Giaccia AJ, Hypoxia: signaling the metastatic cascade, Trends Cancer 2 (6) (2016) 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang X, Trinh T, Aljoufi A, Broxmeyer HE, Hypoxia signaling pathway in stem cell regulation: good and evil, Curr Stem Cell Rep 4 (2) (2018) 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Katagiri T, Kobayashi M, Yoshimura M, Morinibu A, Itasaka S, Hiraoka M, Harada H, HIF-1 maintains a functional relationship between pancreatic cancer cells and stromal fibroblasts by upregulating expression and secretion of Sonic hedgehog, Oncotarget 9 (12) (2018) 10525–10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schwartz DL, Bankson JA, Lemos R Jr., Lai SY, Thittai AK, He Y, Hostetter G, Demeure MJ, Von Hoff DD, Powis G, Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer, Mol. Canc. Therapeut 9 (7) (2010) 2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aguilera KY, Brekken RA, Hypoxia studies with pimonidazole in vivo, Bio Protoc 4 (19) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J, The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma, Clin. Gastroenterol. Hepatol 6 (10) (2008) 1155–1161. [DOI] [PubMed] [Google Scholar]
- [41].Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H, Kleeff J, Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma, Neoplasia 11 (5) (2009) 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T, Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer, Am. J. Physiol. Gastrointest. Liver Physiol 295 (4) (2008) G709–G717. [DOI] [PubMed] [Google Scholar]
- [43].Spivak-Kroizman TR, Hostetter G, Posner R, Aziz M, Hu C, Demeure MJ, Von Hoff D, Hingorani SR, Palculict TB, Izzo J, Kiriakova GM, Abdelmelek M, Bartholomeusz G, James BP, Powis G, Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer, Canc. Res 73 (11) (2013) 3235–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, Iovanna JL, Tomasini R, Vasseur S, Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma, Proc. Natl. Acad. Sci. U. S. A 110 (10) (2013) 3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T, Yu F, Johnson KR, Powers R, Hollingsworth MA, Singh PK, MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer, Proc. Natl. Acad. Sci. U. S. A 109 (34) (2012) 13787–13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM, Wang YH, Yang MW, Fu XL, Zhang XX, Li Q, Pang XF, Huo YM, Li J, Zhang JF, Lee HY, Lee SJ, Qin WX, Gu JR, Sun YW, Zhang ZG, Increased serotonin signaling contributes to the warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice, Gastroenterology 153 (1) (2017) 277–291, e19. [DOI] [PubMed] [Google Scholar]
- [47].Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL, Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia, J. Biol. Chem 283 (16) (2008) 10892–10903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [48].Kim JW, Tchernyshyov I, Semenza GL, Dang CV, HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia, Cell Metabol. 3 (3) (2006) 177–185. [DOI] [PubMed] [Google Scholar]
- [49].Hu L, Cui R, Liu H, Wang F, Emodin and rhein decrease levels of hypoxia-inducible factor-1alpha in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells, Oncotarget 8 (50) (2017) 88008–88020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, Whiting SH, Yeh MM, Yee C, Riddell SR, Pillarisetty VG, Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment, PloS One 9 (5) (2014) e96565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Padoan A, Plebani M, Basso D, Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity, Int. J. Mol. Sci 20 (3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Kloppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J, StellaTUM: current consensus and discussion on pancreatic stellate cell research, Gut 61 (2) (2012) 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Harashima N, Takenaga K, Akimoto M, Harada M, HIF-2alpha dictates the susceptibility of pancreatic cancer cells to TRAIL by regulating survivin expression, Oncotarget 8 (26) (2017) 42887–42900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG, Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma, Clin. Transl. Med 8 (1) (2019) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC, Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia, Canc. Discov 6 (3) (2016) 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Barsoum IB, Smallwood CA, Siemens DR, Graham CH, A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells, Canc. Res 74 (3) (2014) 665–674. [DOI] [PubMed] [Google Scholar]
- [57].Wobben R, Husecken Y, Lodewick C, Gibbert K, Fandrey J, Winning S, Role of hypoxia inducible factor-1alpha for interferon synthesis in mouse dendritic cells, Biol. Chem 394 (4) (2013) 495–505. [DOI] [PubMed] [Google Scholar]
- [58].Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S, Naldini A, Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1alpha and PI3K/Akt pathway, J. Cell. Physiol 229 (12) (2014) 2067–2076. [DOI] [PubMed] [Google Scholar]
- [59].Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK, Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa, Proc. Natl. Acad. Sci. U. S. A 109 (41) (2012) E2784–E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC, Reactive oxygen species regulate activation-induced T cell apoptosis, Immunity 10 (6) (1999) 735–744. [DOI] [PubMed] [Google Scholar]
- [61].Balachandran VP, Beatty GL, Dougan SK, Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities, Gastroenterology 156 (7) (2019) 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tsujikawa T, Kumar S, Borkar RN, Azimi V, Thibault G, Chang YH, Balter A, Kawashima R, Choe G, Sauer D, El Rassi E, Clayburgh DR, Kulesz-Martin MF, Lutz ER, Zheng L, Jaffee EM, Leyshock P, Margolin AA, Mori M, Gray JW, Flint PW, Coussens LM, Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis, Cell Rep. 19 (1) (2017) 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr., Brockstedt DG, Jaffee EM, Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer, J. Clin. Oncol 33 (12) (2015) 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L, Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation, Cancer Immunol Res 2 (7) (2014) 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang SY, Song BQ, Dai SL, Yang KX, Jin Z, Shi KW, Effects of hypoxia-inducible factor-1alpha silencing on drug resistance of human pancreatic cancer cell line Patu8988/5-Fu, Hepato-Gastroenterology 61 (136) (2014) 2395–2401. [PubMed] [Google Scholar]
- [66].Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M, Higashino F, Hamuro J, Okada F, Kobayashi M, Nakagawa K, Koide H, Kobayashi M, Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin, Canc. Res 67 (7) (2007) 3345–3355. [DOI] [PubMed] [Google Scholar]
- [67].Abe T, Toyota M, Suzuki H, Murai M, Akino K, Ueno M, Nojima M, Yawata A, Miyakawa H, Suga T, Ito H, Endo T, Tokino T, Hinoda Y, Imai K, Upregulation of BNIP3 by 5-aza-2’-deoxycytidine sensitizes pancreatic cancer cells to hypoxia-mediated cell death, J. Gastroenterol 40 (5) (2005) 504–510. [DOI] [PubMed] [Google Scholar]
- [68].Limani P, Linecker M, Kron P, Samaras P, Pestalozzi B, Stupp R, Jetter A, Dutkowski P, Mullhaupt B, Schlegel A, Nicolau C, Lehn JM, Petrowsky H, Humar B, Graf R, Clavien PA, Development of OXY111A, a novel hypoxia-modifier as a potential antitumor agent in patients with hepato-pancreato-biliary neoplasms - protocol of a first Ib/IIa clinical trial, BMC Canc. 16 (1) (2016) 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, Doroshow JH, Kummar S, Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors, Canc. Chemother. Pharmacol 73 (2) (2014) 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, Li J, Wang X, Gao S, Qian D, Huang C, Hao J, Inhibition of HIF-1alpha by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma, Oncotarget 6 (4) (2015) 2250–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL, Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth, Proc. Natl. Acad. Sci. U. S. A 105 (50) (2008) 19579–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H, Triptolide and its derivatives as cancer therapies, Trends Pharmacol. Sci 40 (5) (2019) 327–341. [DOI] [PubMed] [Google Scholar]
- [73].Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, Thiruganasambandam SC, Moats CR, Lin M, Luo W, Tran PT, Herman JM, Semenza GL, Ewald AJ, Vogelstein B, Geschwind JF, Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer, Clin. Canc. Res 20 (24) (2014) 6406–6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wilson WR, Hay MP, Targeting hypoxia in cancer therapy, Nat. Rev. Canc 11 (6) (2011) 393–410. [DOI] [PubMed] [Google Scholar]
- [75].Babiker HM, Riaz IB, Shah SR, Von Hoff DD, Borad MJ, Hypoxia-activated prodrugs in the treatment of advanced pancreatic adenocarcinoma, Anti Canc. Drugs 28 (2) (2017) 127–132. [DOI] [PubMed] [Google Scholar]
- [76].Spiegelberg L, Houben R, Niemans R, de Ruysscher D, Yaromina A, Theys J, Guise CP, Smaill JB, Patterson AV, Lambin P, Dubois LJ, Hypoxia-activated prodrugs and (lack of) clinical progress: the need for hypoxia-based biomarker patient selection in phase III clinical trials, Clin Transl Radiat Oncol 15 (2019) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lohse I, Rasowski J, Cao P, Pintilie M, Do T, Tsao MS, Hill RP, Hedley DW, Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302, Oncotarget 7 (23) (2016) 33571–33580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Baran N, Konopleva M, Molecular pathways: hypoxia-activated prodrugs in cancer therapy, Clin. Canc. Res 23 (10) (2017) 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shah VM, Nguyen DX, Alfatease A, Bracha S, Alani AW, Characterization of pegylated and non-pegylated liposomal formulation for the delivery of hypoxia activated vinblastine-N-oxide for the treatment of solid tumors, J. Contr. Release 253 (2017) 37–45. [DOI] [PubMed] [Google Scholar]
- [80].Mangraviti A, Raghavan T, Volpin F, Skuli N, Gullotti D, Zhou J, Asnaghi L, Sankey E, Liu A, Wang Y, Lee DH, Gorelick N, Serra R, Peters M, Schriefer D, Delaspre F, Rodriguez FJ, Eberhart CG, Brem H, Olivi A, Tyler B, HIF-1alpha-targeting acriflavine provides long term survival and radiological tumor response in brain cancer therapy, Sci. Rep 7 (1) (2017) 14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nagaraju GP, Zakka KM, Landry JC, Shaib WL, Lesinski GB, El-Rayes BF, Inhibition of HSP90 overcomes resistance to chemotherapy and radiotherapy in pancreatic cancer, Int. J. Canc 145 (6) (2019) 1529–1537. [DOI] [PubMed] [Google Scholar]
- [82].McDonald PC, Chafe SC, Brown WS, Saberi S, Swayampakula M, Venkateswaran G, Nemirovsky O, Gillespie JA, Karasinska JM, Kalloger SE, Supuran CT, Schaeffer DF, Bashashati A, Shah SP, Topham JT, Yapp DT, Li J, Renouf DJ, Stanger BZ, Dedhar S, Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia, Gastroenterology 157 (3) (2019) 823–837. [DOI] [PubMed] [Google Scholar]
- [83].Logsdon DP, Shah F, Carta F, Supuran CT, Kamocka M, Jacobsen MH, Sandusky GE, Kelley MR, Fishel ML, Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1 dramatically affects pancreatic cancer cell survival, Sci. Rep 8 (1) (2018) 13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hirakawa T, Yashiro M, Doi Y, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Kimura K, Amano R, Hirakawa K, Pancreatic fibroblasts stimulate the motility of pancreatic cancer cells through IGF1/IGF1R signaling under hypoxia, PloS One 11 (8) (2016) e0159912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhu J, Li B, Ji Y, Zhu L, Zhu Y, Zhao H, Betaelemene inhibits the generation of peritoneum effusion in pancreatic cancer via suppression of the HIF1AVEGFA pathway based on network pharmacology, Oncol. Rep 42 (6) (2019) 2561–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhu L, Staley C, Kooby D, El-Rays B, Mao H, Yang L, Current status of biomarker and targeted nanoparticle development: the precision oncology approach for pancreatic cancer therapy, Canc. Lett 388 (2017) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim G, nab-Paclitaxel for the treatment of pancreatic cancer, Canc. Manag. Res 9 (2017) 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lang J, Zhao X, Wang X, Zhao Y, Li Y, Zhao R, Cheng K, Li Y, Han X, Zheng X, Qin H, Geranpayehvaghei M, Shi J, Anderson GJ, Hao J, Ren H, Nie G, Targeted Co-delivery of the iron chelator deferoxamine and a HIF1alpha inhibitor impairs pancreatic tumor growth, ACS Nano 13 (2) (2019) 2176–2189. [DOI] [PubMed] [Google Scholar]
- [89].Chen J, Luo H, Liu Y, Zhang W, Li H, Luo T, Zhang K, Zhao Y, Liu J, Oxygen-self-produced nanoplatform for relieving hypoxia and breaking resistance to sonodynamic treatment of pancreatic cancer, ACS Nano 11 (12) (2017) 12849–12862. [DOI] [PubMed] [Google Scholar]
- [90].Sheng Y, Nesbitt H, Callan B, Taylor MA, Love M, McHale AP, Callan JF, Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours, J. Contr. Release 264 (2017) 333–340. [DOI] [PubMed] [Google Scholar]
- [91].Huo D, Liu S, Zhang C, He J, Zhou Z, Zhang H, Hu Y, Hypoxia-targeting, tumor microenvironment responsive nanocluster bomb for radical-enhanced radiotherapy, ACS Nano 11 (10) (2017) 10159–10174. [DOI] [PubMed] [Google Scholar]
- [92].Xu J, Yuan S, Tian J, Martin KA, Song J, Li C, Wang Z, Lin J, Si T, Xu RX, Ultrasound mediated delivery of oxygen and LLL12 loaded stimuli responsive microdroplets for the treatment of hypoxic cancer cells, Sci. Rep 7 (2017) 44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Owen J, McEwan C, Nesbitt H, Bovornchutichai P, Averre R, Borden M, McHale AP, Callan JF, Stride E, Reducing tumour hypoxia via oral administration of oxygen nanobubbles, PloS One 11 (12) (2016) e0168088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].McEwan C, Kamila S, Owen J, Nesbitt H, Callan B, Borden M, Nomikou N, Hamoudi RA, Taylor MA, Stride E, McHale AP, Callan JF, Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle, Biomaterials 80 (2016) 20–32. [DOI] [PubMed] [Google Scholar]
- [95].Kulkarni P, Haldar MK, Katti P, Dawes C, You S, Choi Y, Mallik S, Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic cancer cell spheroids, Bioconjugate Chem. 27 (8) (2016) 1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kulkarni P, Haldar MK, You S, Choi Y, Mallik S, Hypoxia-responsive polymersomes for drug delivery to hypoxic pancreatic cancer cells, Biomacromolecules 17 (8) (2016) 2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]


