Abstract
Background: The knowledge of sacral hiatus anatomy is crucial in clinical situations requiring caudal epidural block for various diagnostic and therapeutic procedures of the lumbosacral spine to avoid complications and failure rate. This study was undertaken to compare morphometric characteristics of sacral hiatus in human dry sacra and pelvic radiographs for placing the needle more accurately in the sacral hiatus landmarks to permit correct, painless, and uncomplicated caudal epidural accesses.
Materials and methods: The present study was done on 138 human adult dry sacra and 110 anteroposterior lumbosacral spine radiographs of the North Karnataka region of India. Sacral hiatus was evaluated in each sacrum based on its shape, level of its apex, and base according to sacral and coccygeal vertebrae, length, anteroposterior diameter at its apex, and transverse width at its base.
Results:The mean length of sacral hiatus in men and women was 27.81+1.17 mm and 24.73+2.21 mm, respectively. The mean anteroposterior diameter of the sacral hiatus at the apex was 6.24+2.73 mm in males and 6.63+2.81 mm in females. The transverse width of the sacral hiatus at the base was 17.56+1.81 mm in males and 17.92+2.59 mm in females. The location of the apex of sacral hiatus was the highest in number at the level of the fourth sacral vertebra (23.63%). The location of apex in radiographs of all lumbosacral spine S3 showed 49.09% maximum. The location of the base of the sacral hiatus was observed in the dry sacra at the level of the fifth sacral vertebra (64.54%). In the present study, different shapes of the sacral hiatus were recorded. The most common shape in males and females was inverted U shape (42.02%), followed by inverted V shape (26.08%) and dumbbell shape (12.31%). The least common shape was observed in the bifid sacra (5.07%). In 2.17% of cases, sacral hiatus was absent. Percentage of absence, agenesis, irregular, and bifid shapes were found rather in female than male sacra. An anteroposterior view of spine radiograph showed sacral hiatus agenesis in both females (7.81%) and males (4.34%). The anatomical knowledge of sacral hiatus and its variations are important in caudal epidural anesthesia, and it may improve the success rate of caudal epidural anesthesia.
Keywords:sacral hiatus, caudal epidural anaesthesia, anatomical variations.
INTRODUCTION
The sacrum is a triangular wedge-shaped bone that is composed of five fused sacral vertebrae. It articulates with the lumbar spine superiorly, the iliac bones laterally, and the coccyx inferiorly (1). The posterior midline crest represents the vertebral spines. Sacral and coccygeal cornua are important landmarks for the identification of the sacral hiatus (SH) and increase the success rate of the caudal epidural block (CEB) (2). These landmarks are easily palpable in infants and children, but can be very difficult in adults because of the increase in fat and tissue content in this area (3). Lack of fusion or the absence of the S4-S5 laminae gives rise to the SH. The SH contains: 1) a pair of the fifth sacral nerves, which groove the lateral parts of the fifth sacral vertebra; 2) a pair of coccygeal nerves; 3) filum terminale externa, which passes to the coccyx; and 4) fibrofatty tissue (3). Sacrococcygeal symphysis is stabilized by dense sacrococcygeal ligament as well as ligamentum flava (4, 5).
In the current study, we aimed to find out the anatomical variations of SH by morphometric and radiological measurements, as the reliability and success of caudal epidural anesthesia depend upon anatomical variations of sacral hiatus.
MATERIALS AND METHODS
The material for the present study consists of sacra from 138 adults (86 males and 52 females), which were collected from the Bone Bank Department of Anatomy, and 110 pelvis radiographs of normal adults (46 males and 64 females), which were collected from the Department of Radiology Faculty of Medicine, Shri B.M. Patil Medical College Hospital and Research Center, BLDE (deemed to be University) Vijayapur, India. All selected sacra were normal, fully mature and ossified, and devoid of any fractures or pathological changes. Methods of measurements, index calculation and statistical methods were used. The different parameters of each sacrum studied by us include: 1) SH shape, noted by appearance; 2) level of SH apex, noted with respect to the sacral vertebra; 3) SH length, measured from the midpoint of the base to the apex; 4) the measured SH anteroposterior depth of the apex; and 5) SH transverse width at the base, measured between the inner aspects of sacral cornua using a Digital Vernier Caliper.
1) Maximum length is the distance between the middle points on the anterosuperior margin of the promontory to the middle of the anteroinferior margin of the fifth sacral vertebrae.
2) Maximum sacral width is the most distant point on the sides of the ala of the sacrum.
3) Mid ventral curved length measured along the midline of the pelvic surface of the sacrum from the middle of the anterosuperior margin of the promontory to the middle of the anteroinferior margin of the fifth sacra by using a flexible measuring tape.
4) Transverse diameter of the body of the first sacral vertebra [S1]: measurements were taken from the right to the left end of the body of sacral promontory.
5) The anteroposterior diameter of the body of the first sacral vertebra [S1]: midpoint anterior border of sacral promontory to the midpoint of the posterior border of the first sacral vertebral body.
6) Sacral index = sacral width x 100/sacral ventral straight length
7) Corporo-basal index = transverse diameter of body of S1 x100/width of sacrum
RESULTS
Observations were made on 138 dry sacra and 110 anteroposterior sacral radiographs of both males and females, which showed significant morphological findings.
Assessment of sacral hiatus
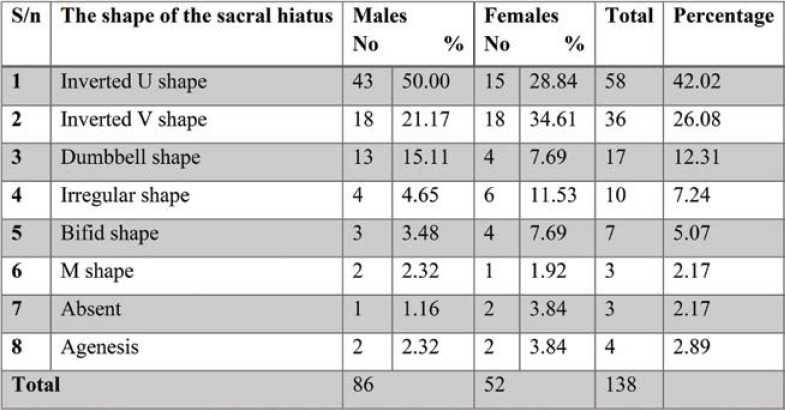
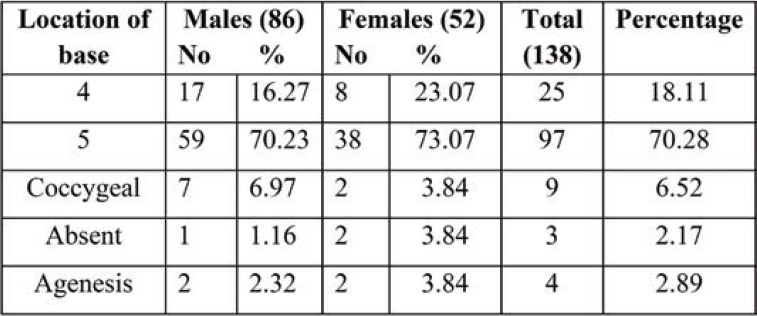
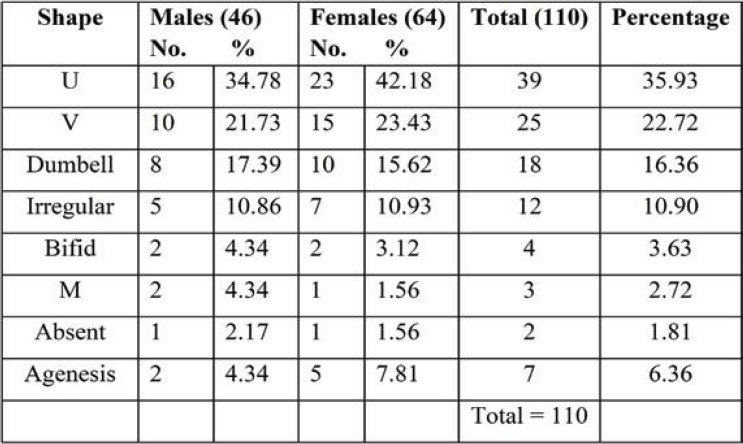
Out of the 138 dry sacra studied by us, the most common shape of SH recorded in the present study was inverted U shape (42.02%), followed by inverted V shape (26.08%), dumbbell (12.31%), irregular (7.24%) bifid (5.07%) and M shape (2.17%). The most common shapes of dry sacra were U, V and dumbbell shapes in men, and bifid and irregular shapes in women. Agenesis of sacral hiatus was detected in 2.89% of all sacra, out of which 3.84% in females. Thus, complete agenesis of the dorsal wall of sacrum is more common in females than males. In both male and female sacra, the least common shape was M (2.17%) and bifid shape (5.07%) (Table 2, Figure 4a). Absence of sacral hiatus was found in 3.84% of female sacra (Table 1).
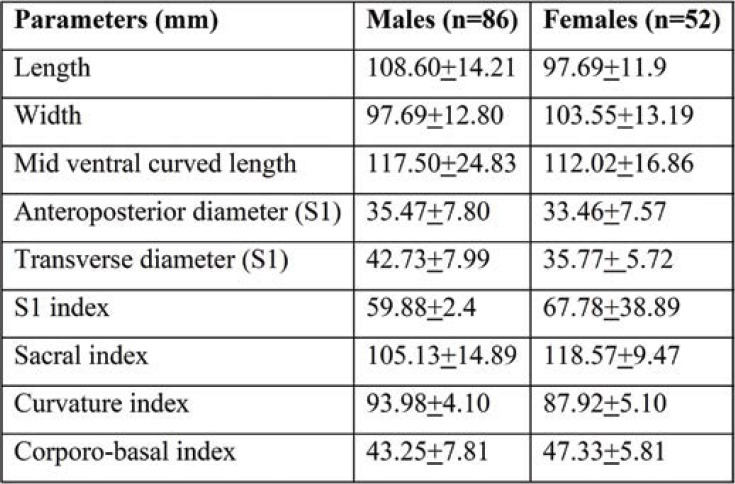
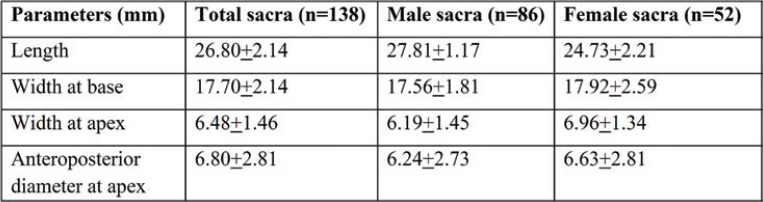
Table 1 shows the mean and standard deviation (SD) of maximum height, width, and anteroposterior diameter sacral index curvature index corporo-basal index of SH in all male and female sacra.
The mean length of dry sacra in males and females was 108.60+14.21 mm and 97.69+11.9 mm, respectively. The mean anteroposterior diameter of dry S1 sacra was 35.47+7.80 mm in males and 33.46+7.57 mm in females. The transverse width at the base of sacra was 97.69+12.80 mm in males and 103.55+13.19 mm in females. The maximum sacral width and sacral index were significantly increased in females comparatively with males, whereas the sacral length and midventral curved length and curvature index were significantly increased in male subjects.
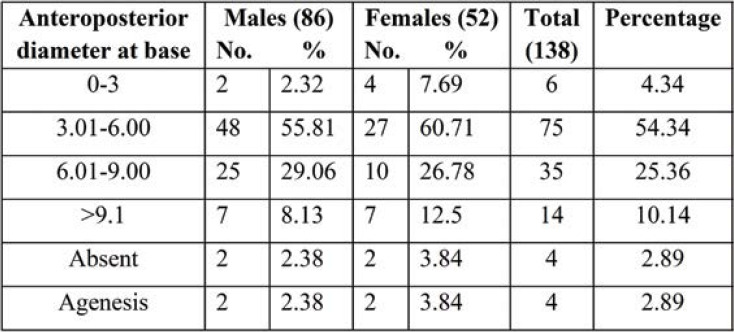
The mean length of SH was 26.80+2.14 mm. The mean transverse width at base was 17.70+2.14 mm and the mean anteroposterior diameter of SH 6.80+2.81 mm. The width at apex and base was 6.48+1.46 mm and 17.70+2.14 mm, respectively. The anteroposterior diameter was significantly increased in female dry sacra (6.63+2.81), and the length of SH was higher in male sacra (27.81+1.17 mm) (Table 3).
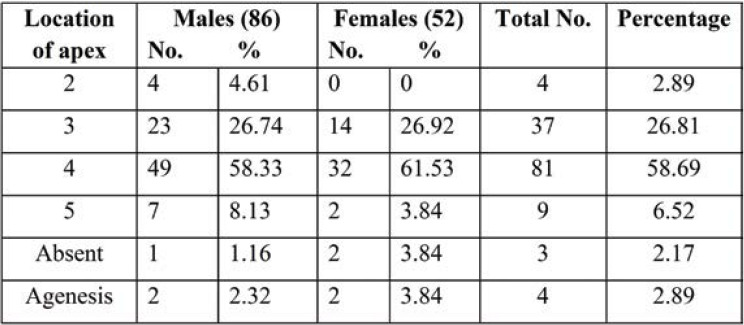
The levels of the apex and base of each SH of dry sacra were determined according to the sacral and coccygeal vertebrae (Tables 4-6; Figures 2-5). Among all sacra, the most common location of SH apex was found at the level of the fourth sacral vertebra (58.69%), followed by SH base (70.28% at the level of the fifth sacral vertebra in all sacra). The anteroposterior diameter of SH was 54.34%. All three parameters were significantly increased in female dry sacra.
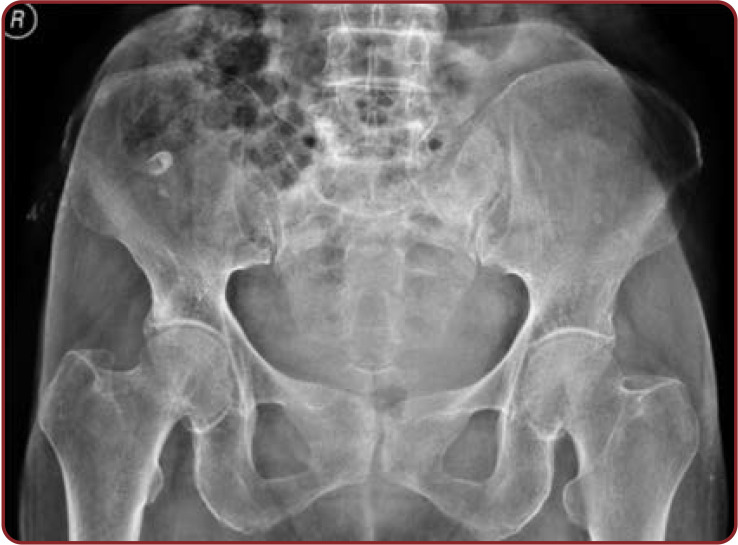
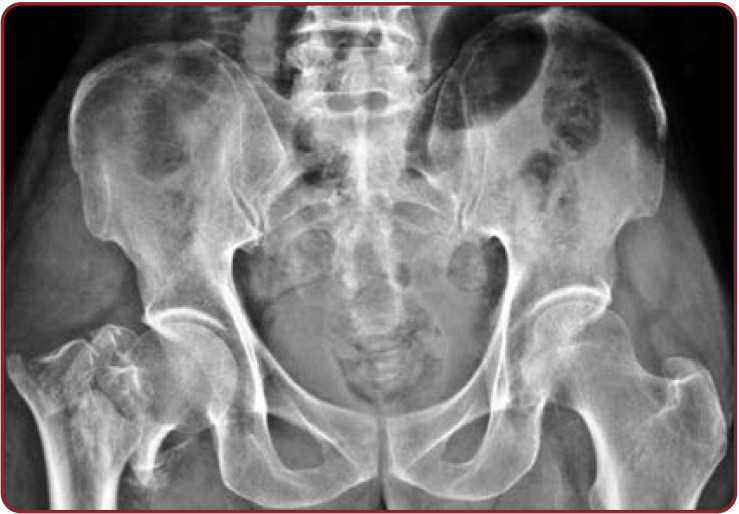
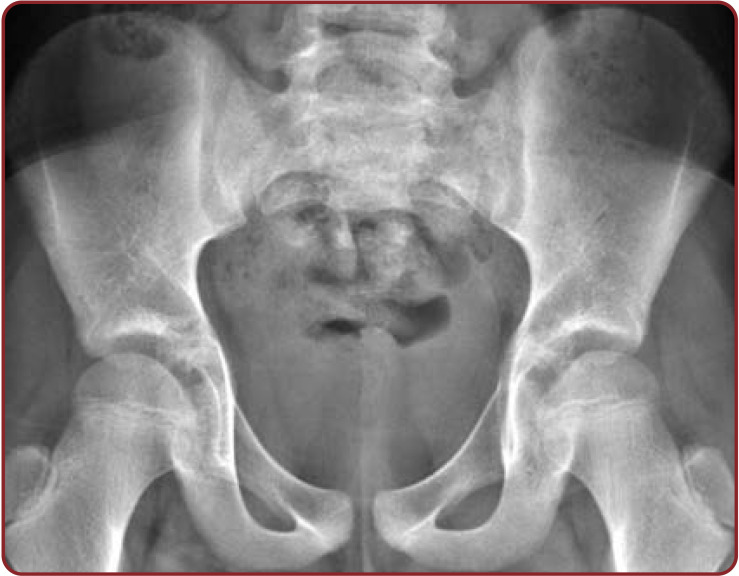
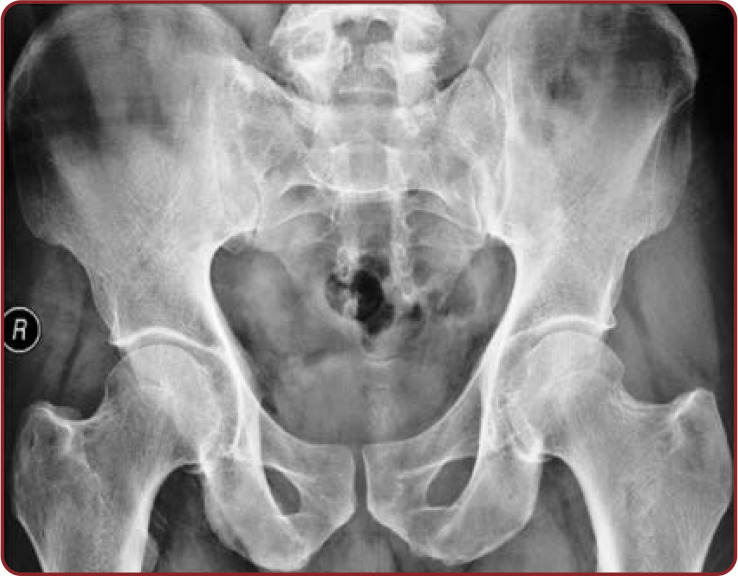
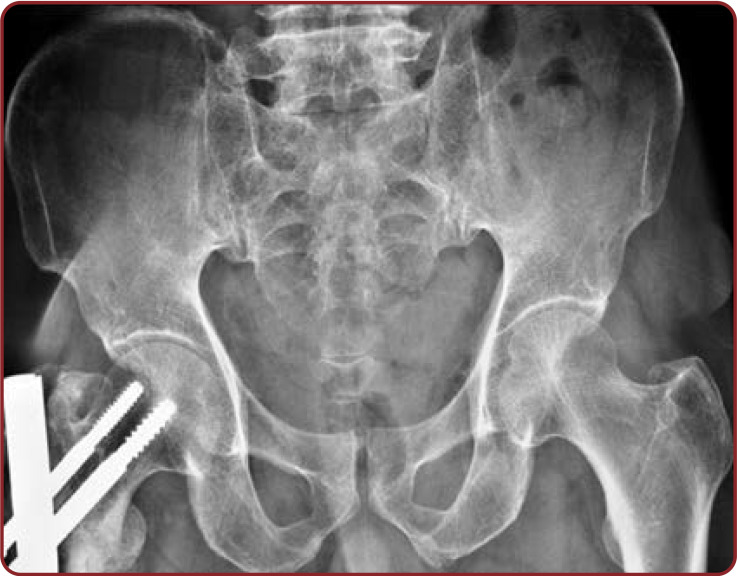
Radiographic observations of the pelvis showed U shaped SH in 35.93% of cases. V shape was the second commonest shape in 23.43% of female radiographs. Agenesis of SH in pelvic radiograph was significantly increased (7.81%) in Indian women. The remaining shapes showed by pelvic sacral radiographs were almost similar in both sexes (Table 7, Figures 6-10).
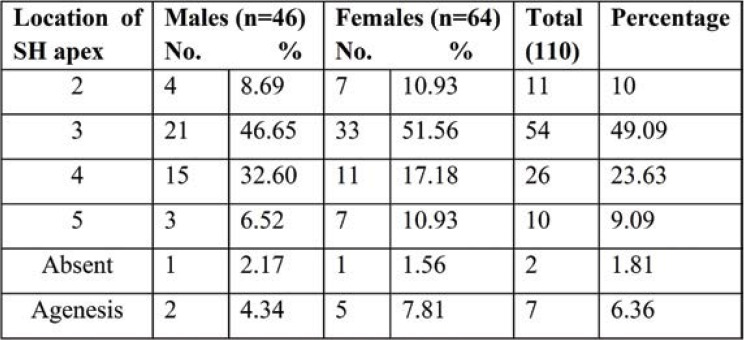
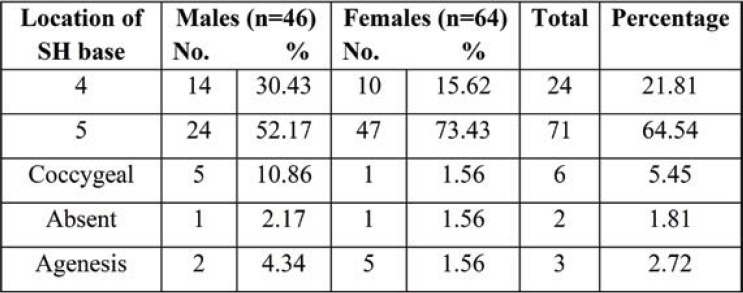
Tables 8 and 9 show the level of the apex and base of SH. The apex of SH was found to be most common at the level of the third sacral vertebra in 54 (49.09%) sacra and fourth sacral vertebra in 26 sacra bones (23.63%); the apex at the level of the second sacra was seen in 11 (10%) and at the level of the fifth sacral vertebra in 10 (9.09%). While the level of the base was common at the level of the fifth sacra vertebra in 71 (64.54%) sacra and that of the fourth sacra vertebra in 24 (21.81%), base at the level of coccyx also observed in six (5.45%) Indian adults’ pelvic radiographs of SH.
DISCUSSION
Anatomical variations in morphology and morphometry of sacral hiatus are more clinically and surgically important, since this route is frequently utilized for caudal epidural anesthesia (CEA) in perineal surgery and caudal analgesia for a painless delivery. The sacrum is a wedgeshaped triangular bone formed by the fusion of all sacral vertebra and completes the lower end of the skeleton of the vertebral column by protecting the meningeal part of the spinal cord. Sacral somites contribute to the development of the sacrum during the end of the first month of development. The secondary centers of ossification appear in adolescence. Any defect in the formation of primary centers may lead to incomplete formation of the sacral canal and incomplete ossification of the laminae (5). The incomplete fusion of posterior elements of the fifth or fourth sacral vertebra results in the formation of SH. Sacral hiatus is used for caudal epidural block in orthopedic therapeutic and diagnostic procedures (2). Epidural corticosteroid injections can be given into the SH to treat sciatica and chronic low back pain in clinical cases (6).
Shape of hiatus
Unilateral incomplete development of sacra, flattened SH, reduced sacral size, and presence of ossified sacrococcygeal ligament in SH is the causative factor for change in the shape of sacral hiatus. Different shapes of SH were studied, the most common being the inverted U shape: 58 (42.02%). This finding is similar to those reported by Aggarwal et al. (7) (40.35%), Seema et al. (8), and (42.47%) Desai et al. (9) (42.12%). U shape was significantly more frequent in male than female sacra (48.21%). The second commonest shape noted was the inverted V shape (26.08%). These findings were similar to those reported by Seema et al. (8) (27.51%) and Mustafa et al. (10) (24%). Inverted V shape was more significantly seen in female than male sacra (34.61%). We found dumbbell shaped sacra in both sexes: 15.11% in men and 17.39% in women. Sacral hiatus was absent in 2.17% of cases, which was close to the findings described by Seema et al. (8) (2.51%). In the study of Sengoku N et al. (11), absent SH was found in 4% of cases, which was a significantly higher percentage than ours. Sacral hiatus absence is one of the anatomical regions for caudal epidural anesthesia failure and needle damage (12). Radiograph of anteroposterior view of the pelvis showed the highest incidence (6.36%) of agenesis SH, and it was significant in female radiographs. Agenesis of SH be due to condyle regression syndrome, spina bifida, and Currarino syndrome.
Apex of the sacral hiatus
In the dry sacra, the apex of SH was located at the level of S4 in 81 (58.69%) of cases. Radiographs of anteroposterior view of the pelvic sacra showed apex of SH at S3 level vertebra in 54 (49.09%) of cases. Incidence of SH at S3 level was significantly higher in female 33 (51.56%) than male radiographs. The apex of SH is a notable landmark for carrying out the successful caudal epidural block. Knowledge about the apex of SH is important when it is located at the level of S2 or S3 vertebra because of more chances of puncture of the dural sac during CEA as the apex is very close to the lower limit of the dural sac (13). When it is extended up to lumbar spinous processes, it is important to develop the techniques to prevent neurological injury associated with neuraxial injections.
Base of sacral hiatus
In the present study, the base of SH was most commonly seen at the level of the fifth sacral vertebra in 97 (70.28%) of all sacra. The findings were more similar to those reported by Kumar et al. (14) (81.17%) and Seema et al. (8) (71.67%). On the other hand, it was least found at the level of coccyx: 9 (6.52%). Precise knowledge about the shape and extent of SH is important because it is the site where caudal anesthesia is given in surgical, obstetric and gynecological, and urological operations. Results of anteroposterior radiographic observations of pelvic sacra at the level of the fifth sacral vertebrae were observed in 71 (64.54%) of cases. Anteroposterior view of a radiograph of the base of SH at the level of the coccygeal vertebra was found in 6 (5.45%) of cases. The base of SH at the coccygeal vertebra level may lead to coccygeal ankylosis.
Length of the sacral hiatus
In the present study, the length of SH was 27.81±1.17 mm in males and 24.73±2.21 mm in females, similarly to the study of Kumar et al. 1992 (14), in which the mean length of SH was 20 mm. Similar results have been also noted in earlier studies of Trotter et al. 1944 (15), in which the length of SH varied between 0–60 mm, with a mean value of 22.5 mm. Nagar SK, Nadeem G, Sinha MB (16-18) reported the length of SH from 10-30 mm. Longer SH reduce the length of the sacral canal, increasing the possibility of needle puncture of the dural sac. Deposition of fat in other connective tissue reduces the length of SH in obese people, which makes it difficult to locate the SH and increases the likelihood of caudal epidural block (CEB) failure rate (19).
Width of sacral hiatus at the base
The width at the base of SH was 17.56±1.81 mm in males and 17.92±2.59 mm in females. This was almost similar to an earlier study conducted by Trotter and Letterman (15), in which the width at the base varied between 7–26 mm, with a mean of 17 mm, Lanier et al. (20), who reported a mean width at the base of 19.3±0.3 mm, and Pandey et al. (21), who reported 5-20 mm in male sacra and 8-18 mm in female sacra. Sekiguchi et al. (22) reported less than 10 mm of the width of SH at the base, while Patil Dhananjay et al. (23) found a width of SH ranging between 9–20 mm, with a mean of 13.71. Morphometry of SH at the base is important in caudal anesthesia, and it would not be safe to push the needle beyond 7 mm to avoid dural puncture.
Anteroposterior diameter
The anteroposterior diameter of the sacral canal at the apex of SH is important as it should be sufficiently large to introduce a needle. Various anteroposterior diameters of SH lead to subcutaneous deposition of an anesthetic drug. In the present study, the anteroposterior diameter at the apex of SH was 6.24±2.73 mm in males and 6.63±2.81 mm in females. Earlier studies reported the following values of the mean diameter of SH: 6.1±0.2 mm [Lanier et al. (20)], 5 mm in white people and 6 mm in black people [Trotter et al. (15)], and 5-20 mm (mean of 1.3 mm) in male sacra and 8-18 mm (mean of 1.25 mm) in female sacra [Vinod Kumar et al. (14)]. Sekiguchi et al. (22) found an anteroposterior diameter of the sacral canal less than 2 mm in 1% of specimens, which made it difficult to use a 22 gauze needle for caudal epidural block.
Caudal epidural anaesthesia
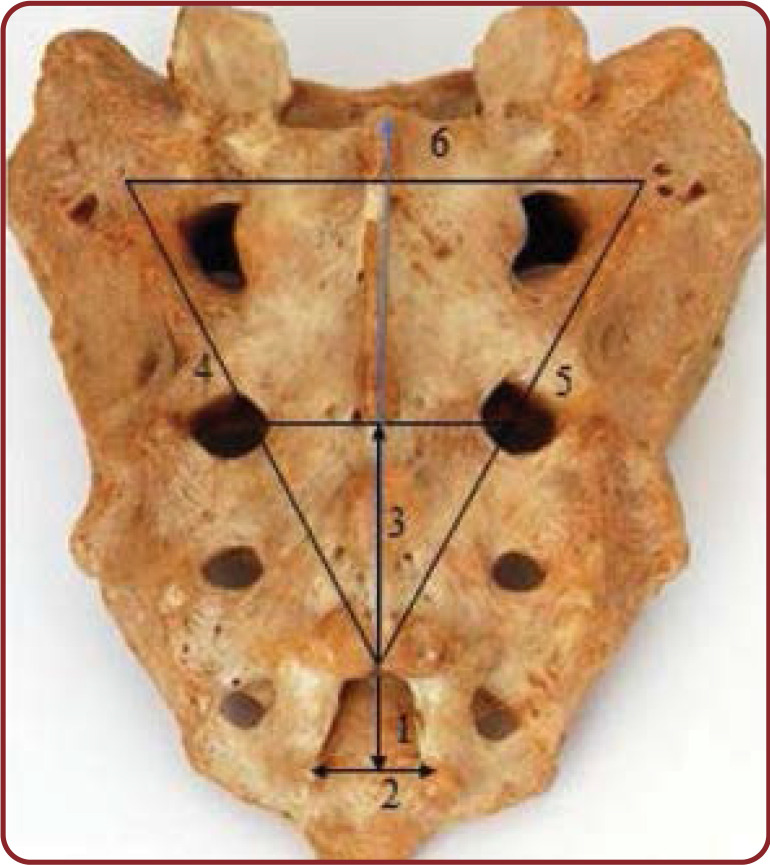
Sacrum provides a reliable and effective block for operations that involve low lumbar and sacral dermatomes. A caudal epidural block is a commonly used technique for surgical anesthesia in children and chronic pain management in adults, especially in control of intraoperative analgesia such as genitourinary surgery and labor pain. It is performed by inserting a needle through the SH to gain entrance into the sacral epidural space. For the caudal epidural catheter insertion, posterior superior iliac spines of the ilial part of the hip bone are palpated. The line between both the spine is called the Tuffiers line forms the base of the equilateral triangle of the sacrum (Figure 1). The success rate depends on accurate localization of SH, but these considerable anatomical difference in its size and shape makes the CEB difficult and increases the likelihood of failure.
CONCLUSION
The knowledge of anatomical variations of sacral hiatus is important while the administration of caudal epidural anesthesia. Variations in the shape and level of hiatus may lead to failure of caudal epidural block. Knowledge of these variations may improve the success of CEB. It is also important during the reporting of CT and MRI and for the differential diagnosis of low backache and other neurological symptoms, clinical cases related to the lumbosacral and sacrococcygeal region. This study has some limitations related to the fact that measurements were performed on the Indian adult dry sacra. Hence, a generalization of these results to other races is not straightforward and may limit their applicability.
Conflict of interests: none declared
Financial support: none declared.
Acknowledgements: We express our gratitude for the support and cooperation rendered by staff, Department of Anatomy and Department of Radiology, Shri B M Patil Medical College Research Center, BLDE (deemed to be University), Vijayapura, Karnataka, India.
FIGURE 1.
Measured parameters of the sacral hiatus. 1) height of sacral hiatus; 2) width of sacral hiatus at the level of sacral cornua; 3) distance from hiatus apex to the level of S2 foramina; 4) distance between the left superolateral sacral crest and sacral hiatus apex; 5) distance between the right superolateral sacral crest and sacral hiatus apex; and 6) distance between two superolateral sacral crests (base of the triangle)
TABLE 1.
Morphometric parameters of dry sacra
TABLE 2.
Different shapes of sacral hiatus in dry sacra
FIGURE 2.
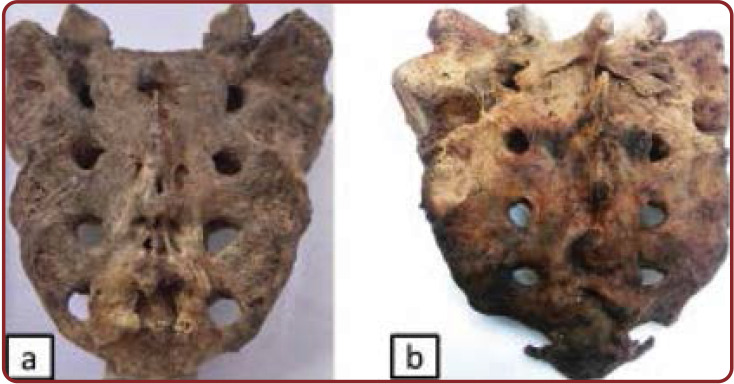
Posterior surface of sacra showing: (a) inverted U shaped sacral hiatus with its apex at the level of S4 and its base at S5 in in Indian female adults; and (b) inverted V shaped sacral hiatus with its apex at the level of S3 and its base at S5 in Indian male adults
FIGURE 3.
Posterior surface of sacrum showing: (a) dumbbell shaped sacral hiatus with its apex at the level of S4 and its base at S5 in Indian male adults; and (b) irregular shaped sacral hiatus at S3 and its base at S5 in in Indian female adults
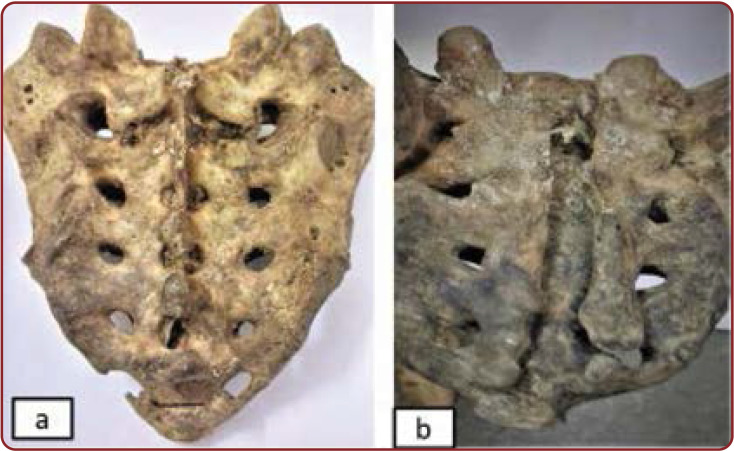
FIGURE 4.
Posterior surface of sacrum showing: (a) bifid shaped SH in Indian female adults; (b) M shaped sacral hiatus in in Indian male adults
FIGURE 5.
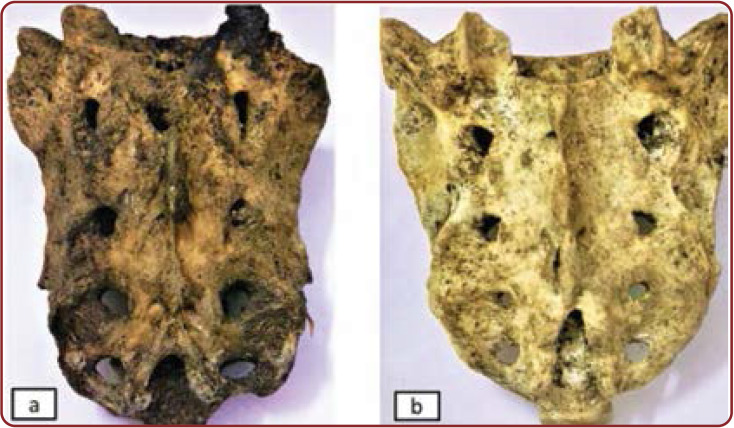
Posterior surface of sacrum showing: (a) absent sacral hiatus in Indian female adults; b) complete agenesis of the sacral canal in Indian male adults
TABLE 3.
Length width and anteroposterior diameter of sacral hiatus
TABLE 4.
Different locations of sacral hiatus apex in adult dry sacra
TABLE 5.
Different locations of sacral hiatus base in relation to sacral and coccygeal vertebrae in adult dry sacra
TABLE 6.
Anteroposterior diameter of sacral hiatus apex in adult dry sacra
TABLE 7.
Different shapes of sacral hiatus in anteroposterior lumbosacral spine radiographs of Indian adults
FIGURE 6.
Anteroposterior view of male lumbosacral spine radiograph showing inverted U shaped sacral hiatus and its apex at the level of S3 and its base at S5
FIGURE 7.
Anteroposterior view of male lumbosacral spine radiograph showing inverted V shaped sacral hiatus and its apex at the level of S4 and its base at the level of the coccyx
FIGURE 8.
Anteroposterior view of female lumbosacral spine radiograph showing dumbbell shaped sacral hiatus and its apex located at the level of S4 and its base at the level of the coccyx
FIGURE 9.
Anteroposterior view of female lumbosacral spine radiograph showing agenesis of sacral hiatus
FIGURE 10.
Anteroposterior view of male lumbosacral spine radiograph showing incomplete sacral hiatus and its apex at the level of S4 and its base at S5
TABLE 8.
Different locations of sacral hiatus apex in anteroposterior lumbosacral spine radiographs of Indian adults
TABLE 9.
Different locations of sacral hiatus base in sacral and coccygeal vertebrae in anteroposterior lumbosacral spine radiographs of Indian adults
Contributor Information
Ishwar B. BAGOJI, Department of Anatomy, SRI B.M. Patil Medical College, BLDE (deemed to be University), Vijayapur, Karnataka, India
Ambadasu BHARATHA, Faculty of Medical Sciences, University of The West Indies, Cave Hill Campus, Barbados, West Indies.
KG PRAKASH, Department of Anatomy, Azeezia Institute of Medical Sciences and Research,Meeyyannoor, Kollam, Kerala, India.
Gavishiddappa A. HADIMANI, Department of Anatomy, SRI B.M. Patil Medical College, BLDE (deemed to be University), Vijayapur, Karnataka, India
Vikas DESAI, Department of Dentistry, SHRI B.M. Patil Medical College, Hospital and Research Centre, Vijayapura, Karnataka, India.
R. S. BULGOUD, Department of Anatomy, SRI B.M. Patil Medical College, BLDE (deemed to be University), Vijayapur, Karnataka, India
References
- 1.Standring S. The Back. Gray’s Anatomy: The Anatomical Basis of Clinical Practices, Standring S, Ellis H, Healy JC, et al, 40th edition, Elsevier, Churchill Livingstone, New York, NY, USA. 2008. pp. 24–28.
- 2.Susie SD. Morphometric measurements of sacral hiatus in South Indian dry human sacra for safe caudal epidural block. Int J Anat Res. 2019;3:6911–6917. [Google Scholar]
- 3.Joseph SC, Song SK. Anatomy of the sacrum. Neurosurg Focus. 2003;2:1–4. doi: 10.3171/foc.2003.15.2.3. [DOI] [PubMed] [Google Scholar]
- 4.Saluja PG. The incidence of ossification of the sacrococcygeal joint. J Anat. 1988;156:11–15. [PMC free article] [PubMed] [Google Scholar]
- 5.Jason TkW, Stringer MD. The anatomy of the sacrococcygeal cornual region and its clinical relevance. Anat Sci Int. 2014;89:207–214. doi: 10.1007/s12565-013-0222-x. [DOI] [PubMed] [Google Scholar]
- 6.Abdi S, Datta S, Trescot AM, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;1:185–212. [PubMed] [Google Scholar]
- 7.Aggarwal A, Kaur H, Batra YK, et al. Anatomic consideration of caudal epidural space: A cadaver study. Clin Anat. 2009;22:730–737. doi: 10.1002/ca.20832. [DOI] [PubMed] [Google Scholar]
- 8.Seema, Singh M, and Mahajan A. An anatomical study of variations of sacral hiatus in sacra of North Indian origin and its clinical significance. Int J Morphol. 2013;1:110–114. [Google Scholar]
- 9.Desai Rajeev R, Jadhav Surekha D, Doshi Medha A, et al. Variations in anatomical features of the sacral hiatus in Indian dry sacra. Int J Med Res Health Sci. 2014;3:634–638. [Google Scholar]
- 10.Mustafa Mohamed S, Omayma M Mahmoud, Hoda H A El Raouf, et al. Morphometric study of sacral hiatus in adult human Egyptian sacra: Their significance in caudal epidural anesthesia. Saudi J Anaesth. 2012;6:350–357. doi: 10.4103/1658-354X.105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senoglu N, Senoglu M, Oksuz H, et al. Landmarks of the sacral hiatus for caudal epidural block: an anatomical study. Bri J Anaesth. 2005;95:692–695. doi: 10.1093/bja/aei236. [DOI] [PubMed] [Google Scholar]
- 12.Sheng-Chin Kao, Chia-Shiang Lin. Caudal Epidural Block: An Updated Review of Anatomy and Techniques. Biomed Res Int. 2017;2017:9217145. doi: 10.1155/2017/9217145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nastoulis E, Karakasi MV, Pavlidis P, et al. Anatomy and clinical significance of sacral variations: a systematic review. Folia Morphol. 2019;78:651–667. doi: 10.5603/FM.a2019.0040. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, Pandey Sn, Bajpai RN, et al. Morphometrical study of sacral hiatus. Journal of Anatomical Society of India. 1992;41:7–13. [Google Scholar]
- 15.Trotter M, Letterman GS, Gordon S. Variations of female sacrum. Their significance in continuous caudal anesthesia. Surg Gyneac Obstet. 1944;78:419–424. [Google Scholar]
- 16.Nagar SK. A study of sacral hiatus in dry human sacra. J Anat Soc India. 2004;2:18–21. [Google Scholar]
- 17.Nadeem G. Importance of knowing the level of sacral hiatus for caudal epidural anesthesia. Int J Morphol. 2014;1:9–13. [Google Scholar]
- 18.Sinha MB, Rathore M, Sinha HR. A study of variation of sacra hiatus in dry bone in the central Indian region. Int J of Healthcare and Biomedical Research. 2014;4:46–52. [Google Scholar]
- 19.Njihia BN, Awori KO, Gikenye G. Morphology of the Sacral Hiatus in an African Population – Implications for Caudal Epidural Injections. Ann African Surg. 2011;1:20–23. [Google Scholar]
- 20.Lanier PF, Trotter M. The volume of the sacral canal. Am J Phys Anthropol. 1946;2:227–234. doi: 10.1002/ajpa.1330040216. [DOI] [PubMed] [Google Scholar]
- 21.Pandey M. A Morphometric Study of Variations in Sacral Hiatus of Dry Human Bones. Int J Anat Res. 2016;3:2804–2808. [Google Scholar]
- 22.Sekiguchi M, Yabuki S, Satoh K, Kikuchi S. An Anatomical Study of the Sacral Hiatus: A Basis for Successful Caudal Epidural Block. Clinical Journal of Pain. 2004;1:51–54. doi: 10.1097/00002508-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Patil Dhananjay S. Anatomical study of sacral hiatus for caudal epidural block. National Journal of Medical Research. 2012;2:272. [Google Scholar]