Abstract
Objectives: Carotid artery dissection represents a common cause of stroke among people aged 30-45. We present two clinical cases and a review of the literature concerning the management of internal carotid artery dissections (ICADs).
Materials and methods: The two patients are a 54-year-old male and a 40-year-old female. The first patient presented to our Neurology Department for one-week-old intense occipital headache. His clinical examination revealed left-sided miosis and upper eyelid ptosis. He underwent cerebral-cervical computed tomography (CT) and computed tomography angiography (CTA) scans and the latter revealed hemodynamically significant narrowing of both ICAs (right C1-C5 and left C1-C2 segments). Transcranial Doppler ultrasonography and Doppler ultrasonography (DUS) of the cervical-cerebral arteries showed right ICA occlusion at its origin (dissection fold and intraluminal thrombosis). Cervical magnetic resonance imaging (MRI) and time-of-flight magnetic resonance angiography (MRA) revealed a semilunar-shaped T2-weighted hypersignal present in the walls of the C1-C5 segments of the right ICA and of the C1-C2 segments of the left ICA, with bilaterally reduced intraluminal flow (right more than left). These findings indicated the presence of bilateral ICA intramural hematomas caused by subacute bilateral ICAD. The second patient presented to our Neurology Department for recurrent episodes of headache and lateral cervical pain on both sides. She underwent transcranial DUS and DUS of the cervicalcerebral arteries. They revealed right ICAD fold in its upper cervical segments. The CTA scan of the supra-aortic trunks showed hemodynamically significant narrowing with subsequent diminished blood flow in the upper cervical segments of right ICA. The patient was diagnosed with right ICAD.
Results:Both patients were treated using antiplatelet therapy for primary prevention of ischaemic events. Follow-up at seven months and at six months, respectively, by means of CTA of the supra-aortic trunks or MRA of the cervical region, revealed the restoration of arterial patency with subsequent normal blood flow in both cases.
Conclusions: The long-term outcomes of ICADs should be kept in mind when assigning medical or endovascular management on a case-by-case basis. Antiplatelet or anticoagulant therapy is a safe and effective first-line strategy in such patients, especially in cases that do not warrant particular management.
Keywords:carotid artery dissection, antithrombotic therapy.
INTRODUCTION
Dissection of the cervical arteries (carotid and vertebral arteries) is a frequent cause of stroke before the age of 45 (1–3), carotid artery dissection (CAD) being considered the underlying stroke etiology in about 2.5% of all strokes and 5–25% of strokes under the age of 30–45 (4–6). Furthermore, CAD is the most common cause of stroke in males under the age of 45 (7). The literature reports reveal an incidence of about 2.6–2.9 per 100 000 cases of CAD for all age groups, with mean age being 42-44 years (1, 8-11). Carotid artery dissection has an associated mortality of up to 5%, full luminal permeability being reached in 90% of cases (12).
Management of internal carotid artery dissections (ICADs) consists of strategies that combine different approaches, mainly medical or endovascular (13). We present the cases of two patients and a short review of the literature concerning the management of ICADs.
CASE 1
A 54-year old male patient with history of grade 2 arterial hypertension (treated with olmesartan medoxomil, amlodipine, hidroclorotiazide and indapamide), umbilical and right inguinal hernias (surgically resolved seven years ago) presented to our Neurology Department for one-week-old intense occipital headache which was felt at first as left side hemicrania, but eventually extended to both sides. This pain was partially alleviated by metamizole and, for a few days, it was associated with inconstant blurred vision in both eyes (left more than right). Furthermore, the patient asserted that he had suffered repeated neck trauma, years on end, during his job as furniture courier.
On the same day of his admittance to our clinic, the patient had presented to an outpatient care setting, where he underwent cerebral-cervical computed tomography (CT) and CT angiography (CTA) scans. The latter revealed hemodynamically significant narrowing of both internal carotid arteries (ICAs) associated with bilateral intramural hematomas (right C1-C5 and left C1-C2 segments). The aforementioned imaging elements were suggestive for bilateral ICAD.
The general physical and neurological examinations revealed the presence of left-sided partial Horner’s syndrome (miosis and upper eyelid ptosis).
His blood tests depicted dyslipidemia with hypertriglyceridemia.
Transcranial Doppler ultrasonography and Doppler ultrasonography of the cervical-cerebral arteries were carried out (Figure 1) and showed right ICA occlusion at its origin from the common carotid artery (dissection fold and intraluminal thrombosis), negative flow in the right ophthalmic artery, low diastolic velocities in the left ICA and positive flow in the left ophthalmic artery.
Cervical magnetic resonance imaging (MRI) scan with and without contrast and time-of-flight magnetic resonance angiography (MRA) revealed a semilunar-shaped T2-weighted hypersignal present in the walls of the C1-C5 segments of the right ICA and of the C1-C2 segments of the left ICA, with bilaterally reduced intraluminal flow (right more than left) (Figure 2). These findings indicated the presence of bilateral ICA intramural hematomas caused by subacute bilateral ICAD.
The cerebral MRI scan showed no recent vascular lesions or areas with restricted diffusion. Furthermore, it highlighted few isointense T1-weighted and hyperintense T2-weighted millimetric oval lesions showing no contrast enhancement, located in the frontal-parietal white matter in both hemispheres, and dilated Virchow- Robin spaces.
Taking into consideration the patient history and all the aforementioned investigations, the man was diagnosed with bilateral ICAD. He started treatment for primary prevention of ischaemic events with antiplatelet therapy. Furthermore, he began treatment with Atorvastatin 40 mg/day and was discharged eight days later.
mg/day and was discharged eight days later. Follow-up at seven months, by means of CTA of the supra-aortic trunks, revealed complete recanalization of both ICAs (Figure 2).
CASE 2
A 40-year old female patient with history of autoimmune thyroiditis presented to our Neurology Department for recurrent episodes of headache. The patient mentioned that, for many years, she had frontal-temporal headache of mild to moderate intensity, felt usually during stressful situations and without accompanying nausea, emesis, phono- or photophobia. Furthermore, the episodes of headache had become more frequent in the last two years. Two weeks prior to her current presentation, the woman had an episode of diffuse headache that felt different from her normal episodes because it had very high intensity and was accompanied by nausea. Even though the cephalalgia remitted under treatment with Dexamethasone and Paracetamol in six days, she developed lateral cervical pain on both sides.
One day before her admittance to our clinic, the woman had presented to an outpatient care setting where she underwent cervical region and cerebral MRI scans. They revealed hemodynamically significant narrowing of the right ICA (C3-C5 segments) and the presence of an intramural hematoma, which were suggestive for spontaneous right ICA dissection in its C1-C2 segments.
The general physical and neurological examinations revealed no abnormalities and the blood tests showed mildly elevated fibrinogen.
Transcranial DUS and DUS of the cervical-cerebral arteries were performed. They revealed right ICA dissection fold in its upper cervical segments. Transcranial Doppler monitoring for microemboli detection has been also performed and it showed no abnormalities.
The CTA scan of the supra-aortic trunks showed hemodynamically significant narrowing with subsequent diminished blood flow in the upper cervical segments of right ICA (Figure 3).
Taking into consideration the patient history and all the aforementioned investigations, the woman was diagnosed with right ICA dissection. She started treatment for primary prevention of ischaemic events with antiplatelet therapy and was discharged eight days later.
Follow-up at two months, by means of cervical region MRA, revealed a reduced intramural hematoma in the right ICA with subsequent increased blood flow. The CTA performed at six months showed complete recanalization of the right ICA.
DISCUSSION
The majority of ICADs exhibit spontaneous mural healing (5). Nedeltchev et al. reported that the rate of complete recanalization was 16%, 50% and 60% at one month, three months and six months, respectively. Complete recanalization occurred in most cases within the first six months (14). Furthermore, Baracchini et al. (15) found that in spontaneous CAD (internal carotid and vertebral arteries) with occlusion or hemodynamically significant stenosis (>50%), recanalization rates were 76.7%%, with complete recanalization (55%) occurring in the first nine months (16).
Associations between the initial clinical and imaging features have been underlined: ICA occlusion reduced the odds of recanalization, whereas initial presentation characterized by local symptoms and signs has been only independently associated with complete recanalization (5, 6, 14, 17, 18).
The natural history and long-term outcome of ICAD with or without stenosis, occlusion or (pseudo)aneurysm has been targeted by many studies. In those conducted by Touze (19) and Guillon (20), none of the patients with aneurysmal form of ICAD had recurrent ischemic events, rupture, or local compressive signs under antiplatelet or anticoagulant treatment after a mean follow-up of more than three years. Also, 82% of patients had either complete resolution of the ICAD or documented stable residual luminal irregularity in the first year, the median time to ascertainment of imaging criteria being 0.29 years (17).
The long-term anatomical outcome seems to depend on the initial angiographic form of the ICAD: occlusive, stenotic or aneurysmal (21). Furthermore, aneurysmal ICAD can be associated with stenosis, occlusions can lead to residual stenoses and initially stenotic or occluded arteries can give rise to residual aneurysms in the post-acute phase (14). Internal carotid artery dissection represents a dynamic process with imaging changes varying in a matter of hours to days (6). Some of these changes can account for worsening and they can be seen in the acute phase as well (21). Even in that case, stenotic lesions have been found to resolve in up to 70-90% of patients and recanalization of occluded arteries occurred in up to 60% of patients (6, 11, 21). Recanalization usually occurs within the first weeks and seems to be exceptional beyond the first 3-6 months (12). Dissecting aneurysms are reported to resolve in up to 40% of patients, decrease in size in up to 33%, or remain unchanged in up to 65% of patients (6, 19, 20). Recanalization within six weeks seemed to have no influence on the neurological outcome (9). The overall risk of ICAD recurrence is low (0.3-1.4%) (10), the highest incidence of recurrence being reported within the first month (11).
The main factors that guide CAD management consider whether the dissection is located in the intracranial or extracranial segments of the ICA, which is the degree of intraluminal stenosis or occlusion, and whether it has determined subsequent ischaemic events or not and how acute they are (23).
Treatment goals of ICAD are to prevent ischaemic events and further damage of the ICA (24, 25). Management choices include a conservative approach (thrombolysis, anticoagulation or antiplatelet therapy), endovascular therapy or surgical treatment (6). Given the gap in the guidelines and literature caused by lack of randomized clinical trial data, a case-by-case basis approach is the norm (5).
Previous studies suggested that thromboembolism, rather than hemodynamic compromise, is the main mechanism of stroke in ICAD (26, 27). Thus, anticoagulation or antiplatelet therapy should be the first line of treatment in the conservative approach of patients with ICAD and no acute ischaemic complications. Up to the CADISS trial (28), there were no randomized trials that compared anticoagulants with antiplatelet drugs in treatment of acute extracranial carotid artery dissections (12). Furthermore, non-randomized studies did not reveal any significant differences between the two types of therapy (4, 29-31). The CADISS trial showed that there was no difference in stroke prevention, residual stenosis or occlusion in patients with cervical artery dissection who received either antiplatelet therapy or anticoagulant treatment. Furthermore, the risk of recurrent stroke up to one year was low (28).
Up to date, there is no consensus regarding the optimum duration of antithrombotic therapy (16, 18). Follow-up imaging by means of MRA, CTA and carotid ultrasound, showing arterial wall healing, might be used when considering the duration of treatment (6). Antithrombotic therapy is maintained for no longer than six months on the basis of the natural history of ICADs and the fact that symptoms tend to reoccur in case of anticoagulant treatment discontinuation within the first three to six months after the onset of dissection, but rarely later on (11, 17, 21). Long-term antiplatelet therapy can be used in cases of ICA residual stenosis, occlusion or aneurysm (6), despite the fact that such patients were not been proven to have a higher risk of recurrent ischaemic events (18).
Surgical or endovascular treatment should be reserved for patients who have contraindications to antiplatelet or anticoagulation therapy in CAD (where medical management for six months failed), if carotid aneurysms and/or tight carotid stenosis persist or develop de novo, in iatrogenic dissections developed during intravascular procedures, in patients with hemodynamic hypoperfusion (involvement of multiple vessels or poor collateral vessels), and expanding pseudoaneurysm formation (11, 22, 32, 33). Moreover, Cohen et al. (34) suggested that stenting would be an option in symptomatic patients with imaging evidence of significant viable penumbral region that would otherwise evolve into irreversible infarction.
Surgical treatment consists of proximal ligation or clipping of the ICA coupled with in situ or extracranial-to-intracranial bypass or thromboendarterectomy with patch angioplasty (6, 16, 34). The above-mentioned procedures are technically demanding, as surgical management of ICAD requires extensive exposure of the ICA at the base of the skull and sometimes the fracture of the styloid bone at its base (32). Furthermore, there is a high-associated morbidity rate (34): in the retrostyloid space, ICA, the jugular vein, the IXth, Xth, XIth and XIIth cranial nerves and the sympathetic chain lie in close vicinity, thus increasing the probability of neural and vascular periprocedural events. In the series of Muller et al. (32), perioperative minor recurrent stroke and cranial nerve injury summed up to 68% of patients. Given that surgical management involves many periprocedural risks, high morbidity rate and great difficulty, it is almost never taken into consideration nowadays.
Endovascular treatment by means of percutaneous balloon angioplasty, stent placement, embolization, with various materials and combinations of such approaches, has increasingly become the therapy of choice in cases of conservative management failure (24, 35-37). Drawbacks concerning the endovascular treatment of ICAD can be dichotomized in technique-related and postprocedural approach. Among technical difficulties, selective microcatheterization of the true arterial lumen can prove to be challenging. Furthermore, there is a risk of distal embolization (22). Stenting would require postprocedural antiplatelet therapy in order to avoid thromboembolic ischaemic events (6, 35), which would have been the case even without exposing the patient to endovascular procedural risks. Some studies reported endovascular management of ICADs failure due to intimal hyperplasia causing hemodynamically significant in-stent stenosis and thromboembolic complications summing up to about 5.2% (6, 38). The review made by Xianjun et al. found that the perioperative major cardiovascular event rate was 4% and 3.3% of patients had intimal hyperplasia, in-stent restenosis or occlusion following endovascular management. Furthermore 2.1% of patients had recurrent transient ischaemic attacks ipsilateral to the treated ICAD (22).
The study conducted by Kremer et al had indirectly challenged the rationale of surgical or endovascular therapy in patients with ICAD (39). The authors tackled the natural history of ICAD resulting in persistent or transient stenosis or occlusion. Patients with permanent ICA stenosis or occlusion had a 2.1%/year chance of stroke, while those with transient ICA stenosis or occlusion had a 0.9% annual rate of stroke.
Taking into account the aforementioned percentages involving the long-term prognosis of ICADs, with low rates of ipsilateral ICA territory and of any type stroke and the fact that stroke rate in ICAD is not related to the persistence of severe ICA stenosis or occlusion, possible indications of surgical or endovascular therapy seem to become more restricted.
CONCLUSONS
Long-term outcomes of ICADs should be kept in mind when assigning medical or endovascular management on a case-by-case basis. Exposing patients to potential unnecessary complications, which are more frequent in invasive or minimally invasive procedures, should not be the norm. The current literature highlights conservative management using either antiplatelet or anticoagulant therapy as a prudent and effective strategy in patients with ICAD who do not have otherwise specific criteria for different management.
Conflict of interests: none declared
Financial support: none declared.
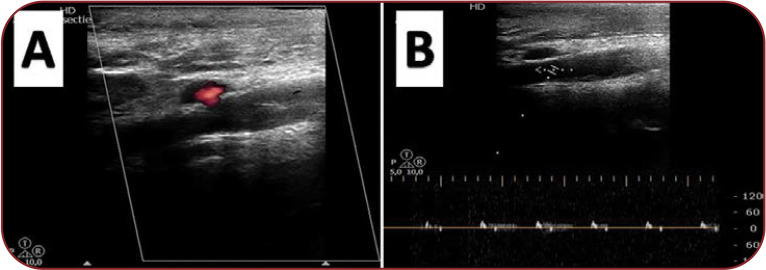
FIGURE 1.
Doppler ultrasonography of the cervical-cerebral arteries revealing dissection fold with intraluminal thrombosis occluding the right internal carotid artery (A) and velocimetric complexes suggestive for right internal carotid artery occlusion (B).
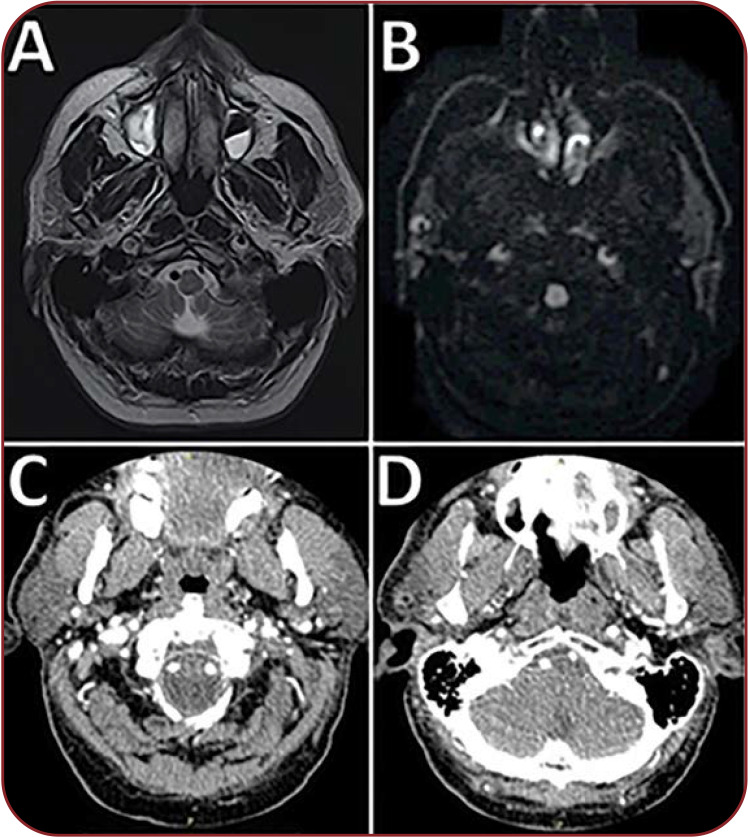
FIGURE 2.
(A) Cervical magnetic resonance imaging scan (cross section) T2-weighted hypersignal present in the walls of C1-C5 segments of the right internal carotid artery and of C1-C2 segments of the left internal carotid artery (suggestive for intramural hematomas), and dissection fold that occludes the lumen of the right carotid artery. (B) Cervical magnetic resonance imaging scan (cross section) – diffusion-weighted imaging – revealing the semilunar shape of the bilateral internal carotid artery intramural hematomas. (C and D) Computed tomography angiography of the supra-aortic trunks showing patent lumina of both internal carotid arteries.
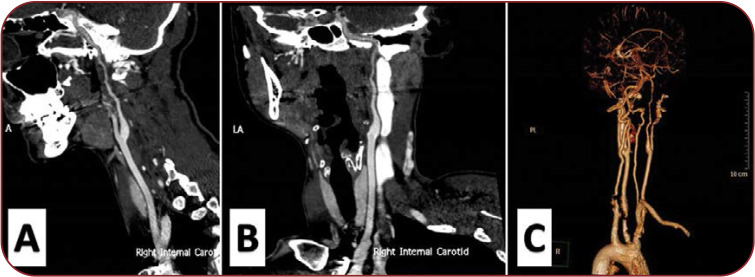
FIGURE 3.
(A, B and C) Computed tomography angiography of the supra-aortic trunks revealing hemodynamically significant narrowing with subsequent diminished blood flow in the upper cervical segments of right ICA.
Contributor Information
Ionut-Flavius BRATU, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Athena Cristina RIBIGAN, Department of Neurology, Emergency University Hospital, Bucharest, Romania; ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Daniela STEFAN, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Cristina Rebeca DAVIDOIU, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Raluca Stefania BADEA, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Florina Anca ANTOCHI, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
References
- 1.Baumgartner RW, Bogousslavsky J, Caso V, Paciaroni M. Handbook on Cerebral Artery Dissection. Basel: KARGER. 2005. pp. 175–178.
- 2.Bogousslavsky J, Regli F. Ischemic Stroke in Adults Younger Than 30 Years of Age: Cause and Prognosis. Arch Neurol. 1987;5:479–482. doi: 10.1001/archneur.1987.00520170009012. [DOI] [PubMed] [Google Scholar]
- 3.Leys D, Bandu L, Hénon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;1:26–33. doi: 10.1212/wnl.59.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Engelter ST, Lyrer PA, Kirsch EC, Steck AJ. Long-term follow-up after extracranial internal carotid artery dissection. Eur Neurol. 2000;4:199–204. doi: 10.1159/000008236. [DOI] [PubMed] [Google Scholar]
- 5.Lumsden S, Rosta G, Bismuth J, et al. Spontaneous Recanalization After Carotid Artery Dissection: The Case for an Ultrasound-Only Monitoring Strategy. Methodist Debakey Cardiovasc J. 2017;4:243–247. doi: 10.14797/mdcj-13-4-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RR, Adam R, Maldjian C, et al. Cervical carotid artery dissection: Current review of diagnosis and treatment. Cardiol Rev. 2012;20:145–152. doi: 10.1097/CRD.0b013e318247cd15. [DOI] [PubMed] [Google Scholar]
- 7.Lyrer P, Engelter S. Antithrombotic Drugs for Carotid Artery Dissection. Stroke. 2004;2:613–614. doi: 10.1161/01.STR.0000112970.63735.FC. [DOI] [PubMed] [Google Scholar]
- 8.Stapf C, Elkind MS V., Mohr JP. Carotid Artery Dissection. Annu Rev Med. 2000;1:329–347. doi: 10.1146/annurev.med.51.1.329. [DOI] [PubMed] [Google Scholar]
- 9.Caso V, Paciaroni M, Corea F, et al. Recanalization of Cervical Artery Dissection: Influencing Factors and Role in Neurological Outcome. Cerebrovasc Dis. 2004;2-3:93–97. doi: 10.1159/000075775. [DOI] [PubMed] [Google Scholar]
- 10.Dziewas R, Konrad C, Dräger B, et al. Cervical artery dissection - Clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;10:1179–1184. doi: 10.1007/s00415-003-0174-5. [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. NEJM. 2001;12:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury MM, Sabbagh CN, Jackson D, et al. Antithrombotic Treatment for Acute Extracranial Carotid Artery Dissections: A Meta-Analysis. European Journal of Vascular and Endovascular Surgery. 2015;2:148–156. doi: 10.1016/j.ejvs.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Schulman S. Cervical artery dissection: Pathology, epidemiology and management. Thrombosis Research. 2009;6:810–821. doi: 10.1016/j.thromres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Nedeltchev K, Bickel S, Arnold M, et al. R2-recanalization of spontaneous carotid artery dissection. Stroke. 2009;2:499. doi: 10.1161/STROKEAHA.108.519694. [DOI] [PubMed] [Google Scholar]
- 15.Baracchini C, Tonello S, Meneghetti G, Ballotta E. Neurosonographic monitoring of 105 spontaneous cervical artery dissections: A prospective study. Neurology. 2010;21:1864–1870. doi: 10.1212/WNL.0b013e3181feae5e. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind D. Spontaneous cerebral and cervical artery dissection: Treatment and prognosis. UpToDate. 2020.
- 17.Lee VH, Brown RD, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: A population-based study. Neurology. 2006;10:1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 18.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;7:668–678. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 19.Touzé E, Randoux B, Méary E, et al. Aneurysmal forms of cervical artery dissection: Associated factors and outcome. Stroke. 2001;2:418–423. doi: 10.1161/01.str.32.2.418. [DOI] [PubMed] [Google Scholar]
- 20.Guillon B, Brunereau L, Biousse V, et al. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology. 1999;1:117–122. doi: 10.1212/wnl.53.1.117. [DOI] [PubMed] [Google Scholar]
- 21.Touzé E, Gauvrit JY, Meder JF, Mas JL. Prognosis of cervical artery dissection. Frontiers of neurology and neuroscience. Front Neurol Neurosci. 2005;20:129–139. doi: 10.1159/000088157. [DOI] [PubMed] [Google Scholar]
- 22.Xianjun H, Zhiming Z. A Systematic Review of Endovascular Management of Internal Carotid Artery Dissections. Interv Neurol. 2013;3-4:164–170. doi: 10.1159/000353124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodfriend S, Tadi P, Kouri R. Carotid Artery Dissection - StatPearls - NCBI Bookshelf. Treasure Island (FL): StatPearls Publishing. 2020. [PubMed]
- 24.Fava M, Meneses L, Loyola S, et al. Carotid artery dissection: Endovascular treatment. Report of 12 patients. Catheter Cardiovasc Interv. 2008;5:694–700. doi: 10.1002/ccd.21483. [DOI] [PubMed] [Google Scholar]
- 25.Chern JJ, Chamoun RB, Mawad ME, et al. Endovascular stenting of traumatic extracranial carotid artery dissections in the pediatric population: A case report. Cases J. 2009;10:171. doi: 10.1186/1757-1626-2-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benninger DH, Georgiadis D, Kremer C, et al. Mechanism of Ischemic Infarct in Spontaneous Carotid Dissection. Stroke. 2004;2:482–485. doi: 10.1161/01.STR.0000109766.27393.52. [DOI] [PubMed] [Google Scholar]
- 27.Morel A, Naggara O, Touzé E, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. 2012;5:1354–1361. doi: 10.1161/STROKEAHA.111.643338. [DOI] [PubMed] [Google Scholar]
- 28.Markus HS, Levi C, King A, et al. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: The cervical artery dissection in stroke study (cadiss) randomized clinical trial final results. JAMA Neurol. 2019;6:657–664. doi: 10.1001/jamaneurol.2019.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arauz A, Hoyos L, Espinoza C, et al. Dissection of cervical arteries: Long-term follow-up study of 130 consecutive cases. Cerebrovasc Dis. 2006;2-3:150–154. doi: 10.1159/000093244. [DOI] [PubMed] [Google Scholar]
- 30.Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;10:1122–1127. doi: 10.1136/jnnp.2007.138800. [DOI] [PubMed] [Google Scholar]
- 31.Georgiadis D, Arnold M, Von Buedingen HC, et al. Aspirin vs anticoagulation in carotid artery dissection: A study of 298 patients. Neurology. 2009;21:1810–1815. doi: 10.1212/WNL.0b013e3181a2a50a. [DOI] [PubMed] [Google Scholar]
- 32.Müller BT, Luther B, Hort W, et al. Surgical treatment of 50 carotid dissections: Indications and results. J Vasc Surg. 2000;5:980–988. doi: 10.1067/mva.2000.104586. [DOI] [PubMed] [Google Scholar]
- 33.Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383–388. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen JE, Leker RR, Gotkine M, et al. Emergent stenting to treat patients with carotid artery dissection: clinically and radiologically directed therapeutic decision making. Stroke. 2003;12:e254. doi: 10.1161/01.STR.0000101915.11128.3D. [DOI] [PubMed] [Google Scholar]
- 35.Donas KP, Mayer D, Guber I, et al. Endovascular Repair of Extracranial Carotid Artery Dissection: Current Status and Level of Evidence. J Vasc Interv Radiol . 2008;12:1693–1698. doi: 10.1016/j.jvir.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Biggs KL, Chiou AC, Hagino RT, Klucznik RP. Endovascular repair of a spontaneous carotid artery dissection with carotid stent and coils. J Vasc Surg. 2004;1:170–173. doi: 10.1016/j.jvs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Kadkhodayan Y, Jeck DT, Moran CJ, et al. Angioplasty and Stenting in Carotid Dissection with or without Associated Pseudoaneurysm. Am J Neuroradiol. 2005;9:2328–2335. [PMC free article] [PubMed] [Google Scholar]
- 38.Ansari SA, Parmar H, Ibrahim M, et al. Cervical Dissections: Diagnosis, Management, and Endovascular Treatment. Neuroimaging Clin N Am. 2009;19:257–270. doi: 10.1016/j.nic.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Kremer C, Mosso M, Georgiadis D, et al. Carotid dissection with permanent and transient occlusion or severe stenosis: Long-term outcome. Neurology. 2003;2:271–275. doi: 10.1212/01.wnl.0000043580.70857.92. [DOI] [PubMed] [Google Scholar]