Abstract
Background: The aim of this study was to evaluate the frequency distribution of antibiotic therapy according to lumbar puncture outcome in hospitalized children.
Methods:This study was conducted on 94 children undergoing lumbar puncture. All data were extracted from medical records. Administration of primary treatments and initial diagnosis including febrile convulsion, meningitis, and encephalitis in these patients were based on a physician’s opinion.
Results:The majority of subjects were diagnosed with febrile convulsion. Antibiotic treatment before lumbar puncture was taken by 58 children. After lumbar puncture, 35 children discontinued antibiotic therapy, two patients were switched to another antibiotic treatment and 21 subjects continued antibiotic medication. In addition, 36 children did not take antibiotics. Positive PCR was found in four cases from the encephalitis group.
Conclusion: After lumbar puncture, antibiotic treatment was continued in 23 cases, whereas administration of antibiotics could be justified only in four cases based on positive PCR. Given that antibiotic treatment of our subjects was initiated prior to lumbar puncture but it was changed in two cases and continued in 21 cases after the medical procedure, conducting lumbar puncture seemed to be not very useful in these patients, assuming that laboratory signs or symptoms could justify an antibiotic treatment.
Keywords:antibiotic therapy, cerebrospinal fluid, lumbar puncture, children.
INTRODUCTION
Lumbar puncture (LP) is a common and routine procedure in anaesthesia and medical emergency (1). It is an invasive method used to collect a sample of cerebrospinal fluid (CSF).
Lumbar puncture (LP) is a common and routine procedure in anaesthesia and medical emergency (1). It is an invasive method used to collect a sample of cerebrospinal fluid (CSF). Cerebrospinal fluid reduces the effective weight of the brain from 1,500 g to around 50 g, which causes the brain to float (2). It also provides a medium for transferring nutrients and waste products to and from the brain tissue (2). Assessment of CSF plays a main role in the normal function of brain (2), and changes in its constituents, pressure and flow can negatively influence the normal function of the brain. Equally, an abnormal cerebral function can influence the CSF. Thus, collecting CSF samples and analyzing them can provide valuable information to help neurological diagnosis (2). Analysis of CSF was used for the diagnosis of infectious diseases such as bacterial meningitis, encephalitis and subarachnoid hemorrhage as well as for therapeutic purposes such as treatment of pseudotumor cerebri. Also, CSF analysis can be useful in diagnosing various other conditions such as carcinomatous meningitis and demyelinating diseases. The effects of antibiotic pretreatment on CSF chemical and cellular profiles were reported in some studies (3). A short period of antibiotic prior to LP may not alter CSF white blood cell count, protein, or glucose (3); however, patients with bacterial meningitis need early antibiotic treatment (4, 5). The choice of antibiotic was dependent on the isolated organisms. In most cases, empirical treatment is considered as the initial treatment. However, the selected antibiotic should have the activity against bacteria in the CSF (6, 7).
According to findings, patients with Gram negative bacillary meningitis and pneumococcal meningitis who are treated with bacteriostatic antibiotics may have poor clinical outcome (8). Studies based on animal models showed that a bactericidal effect of antibiotics was necessary for survival and CSF sterilization (6). Other studies have also shown that addition of antibiotics decreased the release of bacterial toxins (7). Recent studies reported that some factors affected the activity of antibiotics, including their penetration into the CSF, concentration, and intrinsic activity in fluid. The concentration of antibiotics in CSF which is required for optimal bactericidal activity is unknown (7).
Delays in performing LP after administration of antibiotics may alter the results of CSF cultures because antibiotics may potentially sterilize the CSF, decreasing the likelihood of making a microbiologic diagnosis (9). Given that few studies have been conducted regarding the role of antibiotic therapy in children before and after LP and no such studies have been undertaken in our region, the aim of the present research was to evaluate the frequency distribution of antibiotic therapy based on CSF outcome in lumbar puncture in hospitalized children.
MATERIALS AND METHODS
This descriptive-analytical study was conducted on all children admitted in Shahid Sadoughi hospital in 2017 who were subjected to LP. Patients with incomplete medical records or those with unknown diagnosis and paraclinical information were excluded from the study. After obtaining consent from each child’s parents, the current study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences. Data regarding age, gender, laboratory findings including white blood cell count (WBC), erythrocyte sedimentation rate (ESR), absolute neutrophil count (ANC), cell count, positive PCR, culture and smear, clinical signs including fever, seizure, lethargy, headache, vomiting, neck stiffness, and tenderness was extracted from the patients’ medical records. Initial diagnosis including febrile convulsion (FC), meningitis, and encephalitis, as well as administration of primary treatments (ceftriaxone, acyclovir, ceftriaxone + acyclovir, ceftriaxone + vancomycin, ceftriaxone + acyclovir + vancomycin) were based on a study physician’s opinion.
Statistical analysis
Data were entered to SPSS, version 19. Frequency comparison of antibiotic intervention regarding age, gender, laboratory symptoms and clinical signs was done according to Chi square test. P < 0.05 was assumed as statistically significant.
RESULTS
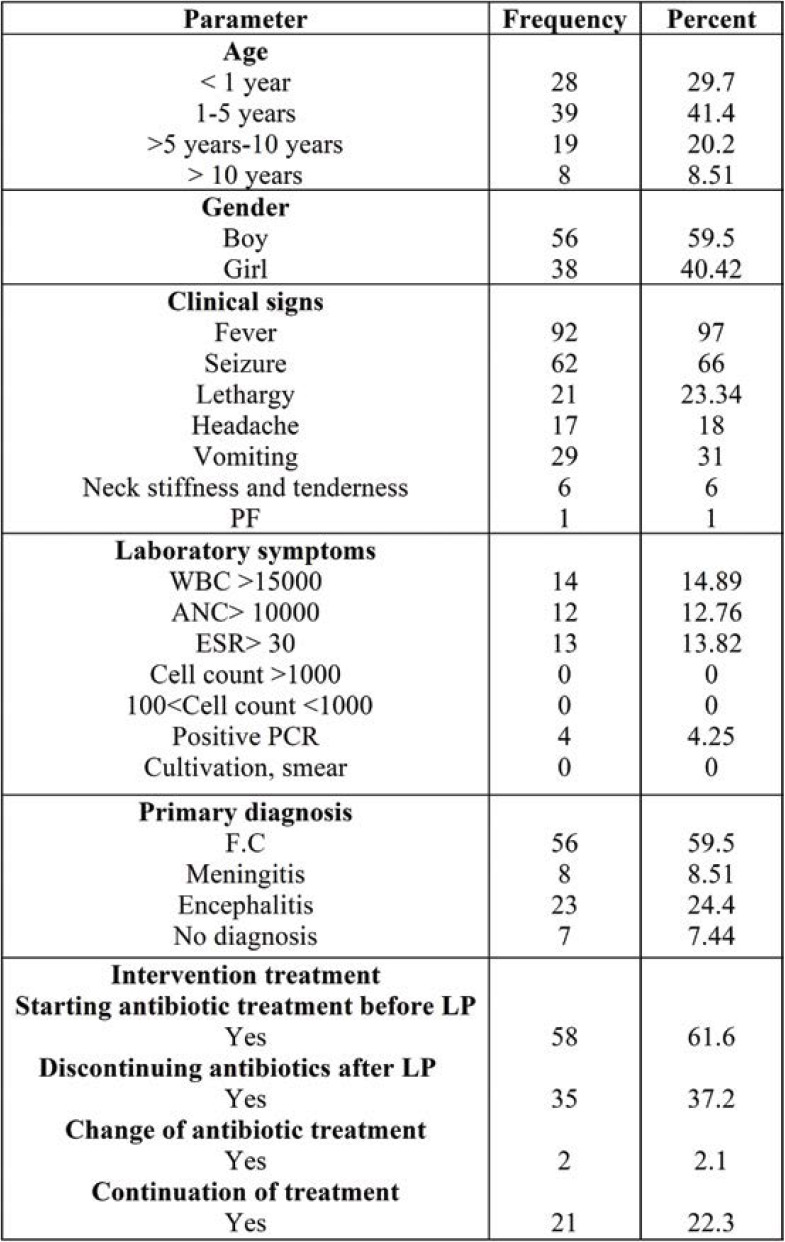
The current study was conducted on 94 patients who underwent lumbar puncture. Table 1 shows the frequency distribution of patients according to individual parameters including age, gender, clinical signs, laboratory findings, primary diagnosis and interventional treatment.
As shown in Table 1, 41.4% of patients were boys aged 1-5. The most frequently reported clinical sign in children was fever (97%). The majority of children (59.5%) were diagnosed by FC. Of all children, 61.6% were taking an antibiotic treatment before performing LP.
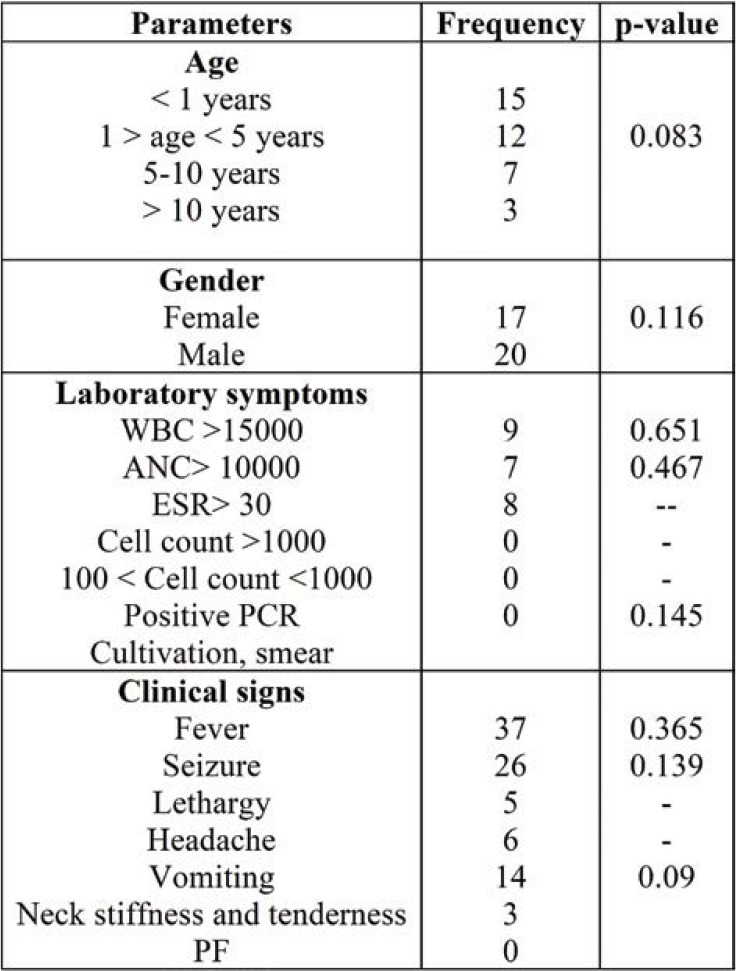
Frequency comparison of antibiotic intervention regarding age, gender, laboratory symptoms and clinical signs is shown in Table 2.
As shown in Table 2, no significant difference was seen between frequency distribution of antibiotic intervention regarding age, gender, clinical signs, and laboratory symptoms (p >0.05).
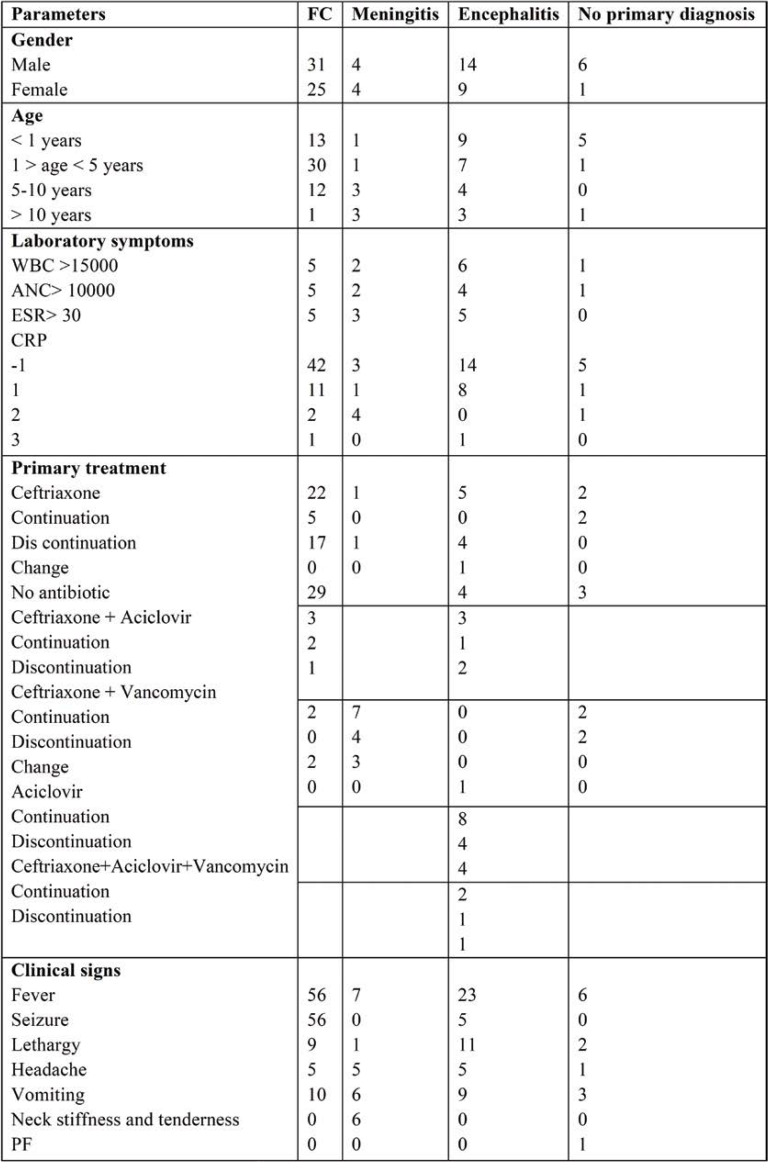
The frequency of patients with primary diagnosis (meningitis, encephalitis, FC and no primary diagnosis) in terms of gender, age, laboratory symptoms, primary treatment and clinical signs is shown in Table 3.
In the FC group, changes in treatment intervention were observed in 20 cases (all of them included discontinuation of antibiotics after observing LP findings). In addition, 29 cases did not receive antibiotic before and after LP, and continuation of treatment was observed in seven cases.
In the meningitis group, changes in treatment intervention were observed in four cases, all of which included discontinuation of antibiotics. Moreover, continuation of treatment was observed in four cases.
In the encephalitis group, changes in therapeutic intervention were observed in 13 cases (in 11 cases, antibiotics were discontinued and in two cases, the antibiotic was changed). In addition, six cases continued treatment.
In the group without initial diagnosis, change in therapeutic intervention was not observed in any cases. In addition, four cases continued treatment.
Totally, 21 cases continued treatment, two cases changed antibiotic treatment and 35 cases discontinued treatment. In addition, 36 cases did not take any antibiotic.
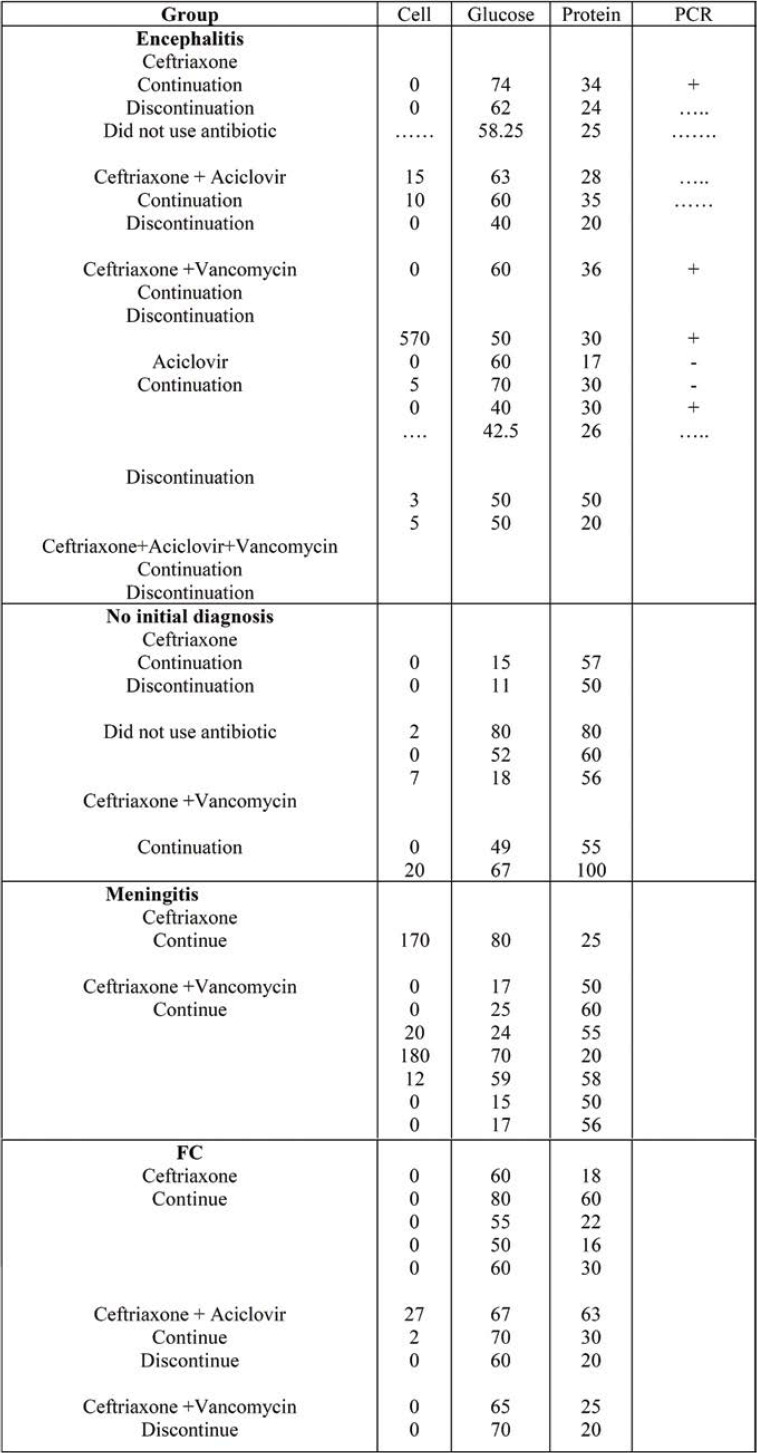
Table 4 shows the frequency of patients in all four groups (encephalitis, no initial diagnosis, meningitis and FC) regarding laboratory findings, including cell, glucose, protein and PCR.
DISCUSSION
In our study, antibiotic treatment was taken by 61% of patients before LP. Bauchner et al. evaluated the effect of antibiotic pretreatment in children with meningitis and administered antibiotic 72 hours before performing LP in 35% of patients (3). McMaster et al. assessed pneumococcal meningitis implications and reported that 55% of patients were subjected to LP before taking antibiotics and in 45% of them, antibiotic was administered before LP (10). Talen et al. reported that, if bacterial meningitis was suspected, intravenous antibiotics were necessary before obtaining CSF (11); moreover, they reported that short time antibiotic therapy before LP did not alter CSF protein, white blood cell count or glucose.
In our study, 8% of children were diagnosed with meningitis. Ehsanipour et al. (12) evaluated meningitis in hospitalized children and reported the 3.6% of them had meningitis. Tavasoli et al. found that in children from Tehran, the prevalence of meningitis was 4.5% (13). In the two above-cited studies, the prevalence of meningitis was lower than that found by us. Both studies were conducted in Tehran, whereas ours was performed in a different geographic region. Moreover, ceftriaxone + vancomycin and ceftriaxone were considered as primary treatments in the meningitis group. Change in treatment intervention has been also observed in four cases, which discontinued their antibiotics. Viladrich et al. evaluated the effect of vancomycin and ceftriaxone therapy on pneumococcal meningitis and found that four patients in the vancomycin group experienced a therapeutic failure, which eventually led to change of vancomycin (14), while therapeutic failure was not observed in the ceftriaxone group; however, the mortality rate in the two groups was equal. In addition, the number of patients who were cured in the ceftriaxone and vancomycin groups was 14 and 6, respectively. Suntur et al. evaluated mono- and combination therapy for cephalosporin-resistant meningitis and reported that vancomycin + ceftriaxone could be synergistic in a rabbit model (15). Ribes et al. assessed the effect of ceftriaxone and vancomycin, either alone or in combination therapy, on an experimental model of meningitis and reported that ceftriaxone plus vancomycin appeared to be effective in treatment of pneumococcal meningitis (16). Cabellos C. et al. selected a rabbit model of meningitis and evaluated the efficacy of ceftriaxone or vancomycin in the presence and absence of dexamethasone on CFS. They found no statistically significant differences between the two groups which were treated with dexamethasone and without dexamethasone (17). However, in the vancomycin treated groups we found statistically significant lower CFS vancomycin levels at two hours in the dexamethasone-treated rabbits and differences in bacterial killing. Elyasi et al. have also reported that administration of high dose vancomycin in meningitis increased the efficacy and penetration into the CSF and accelerated the answer to treatment in meningitis (18). Taheri et al. reported that continuous infusion of vancomycin led to a higher concentration of vancomycin in the CSF, although it had no effect on penetration of vancomycin (19).
In our study, change in therapeutic intervention in the encephalitis group was observed in 13 cases (antibiotic were discontinued in 11 cases and changed in two cases). Moreover, ceftriaxone and acyclovir were considered as primary treatments in children with encephalitis. In addition, one case under treatment with ceftriaxone + vancomycin changed to acyclovir. Kneen et al. conducted a study on children with encephalitis who received acyclovir (20); however, in one third of patients, acyclovir was administered without a rational basis and decision to begin treatment was done without proper investigation (20). Seedat and Winnett reported that intravenous ceftriaxone and acyclovir could be administered in patients with encephalitis (21). Vomiero et al. evaluated a combination of ceftriaxone and acyclovir in children with encephalitis and reported that the degree of kidney impairment was correlated with the dose of acyclovir, while no correlation was seen with the dose of ceftriaxone (22). Therefore, a guideline for the management of patients with suspected viral encephalitis should be very useful.
Moreover, febrile convulsion (FC) was diagnosed in about 60% of patients undergoing LP. Kamali et al. reported that FC was the most common neurological disease in 2 to 5% of children (23). Laino et al. reported that the exact cause of FC was still unknown, although some studies indicated a possible association between environmental and genetic factors (24). Fever as a normal response to infection associated with the release of high levels of cytokines during fever may alter normal brain activity triggering seizures (24). Moreover, in the FC group, the change in treatment intervention was observed in 20 cases (for all of them, discontinuation of antibiotics was decided after observing LP findings). In addition, ceftriaxone was administered in 39% of patients with FC. Mahmood et al. evaluated the management of febrile seizures in children and reported that ceftriaxone was used in 32% of patients with febrile seizures (25). O’Leary et al. (26) conducted a study on patients with febrile seizures who received 500 mg ceftriaxone every 12 hours; after switching them to an oral cephalosporin, patients’ symptoms began to improve with oral intake on the third day of admission. The difference between O’Leary’s study and ours was that we did not change ceftriaxone to another antibiotic, while they replaced ceftriaxone with oral cephalosporin. Several factor such as duration of antibiotic taking, dosage, and age of children may cause this difference.
CONCLUSION
After performing LP, antibiotic treatment was continued in 23 cases, whereas administration of antibiotics can be justified only in four cases, according to positive PCR. Given that antibiotic treatment of these individuals has been initiated prior to LP and treatment process was changed in two cases and continued in other cases, it seemed that conducting LP was not very necessary in these patients, assuming that laboratory signs or symptoms can justify antibiotic treatment.
Conflict of interests: none declared
Financial support: none declared.
Acknowledgments: The authors would like to thank the staff of Shahid Sadoughi Hospital.
TABLE 1.
Frequency distribution of patients according to individual parameters
TABLE 2.
Frequency comparison of antibiotic intervention regarding age, gender, laboratory symptoms and clinical signs
TABLE 3.
The frequency of patients with primary diagnosis in terms of parameters
TABLE 4.
Frequency of patients in four groups regarding laboratory findings
Contributor Information
Farzad FERDOSIAN, Department of Pediatrics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Alireza Eghbali KHEYRABADI, Department of Pediatrics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Zahra NAFEI, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
References
- 1.Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgrad Med J. 2006;82:713–716. doi: 10.1136/pgmj.2006.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright BLC, Lai JTF, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol. 2012;8:1530–1545. doi: 10.1007/s00415-012-6413-x. [DOI] [PubMed] [Google Scholar]
- 3.Nigrovic LE, Macias C. Effect of Antibiotic Pretreatment on Cerebrospinal Fluid Profiles of Children With Bacterial Meningitis. Pediatrics. 2008;122:726–730. doi: 10.1542/peds.2007-3275. [DOI] [PubMed] [Google Scholar]
- 4.Heyderman RS, O’Sullivan H. Early management of suspected bacterial meningitis and meningococcal septicaemia in adults. J Infect. 2003;46:75–80. doi: 10.1053/jinf.2002.1110. [DOI] [PubMed] [Google Scholar]
- 5.Benedict M, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;6:433–438. doi: 10.1136/emj.2009.075598. [DOI] [PubMed] [Google Scholar]
- 6.Scheld WM. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Invest. 1983;75:1–10. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashir H, Booy R. Diagnosis and treatment of bacterial meningitis. Arch Dis Child. 2003;8:88–98. doi: 10.1136/adc.88.7.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherubin CE, Sierra MF. Listeria and gram-negative bacillary meningitis in New York City, 1972–1979: frequent causes of meningitis in adults. Am J Med. 1981;71:199–209. doi: 10.1016/0002-9343(81)90106-6. [DOI] [PubMed] [Google Scholar]
- 9.Balouch A. Do Delays in Performing Lumbar PunctureAfter Administration of Antibiotics Alter the Results of CSF Cultures? Curr Infect Dis Rep. 2011;4:305–307. doi: 10.1007/s11908-011-0188-6. [DOI] [PubMed] [Google Scholar]
- 10.McMaster P, Mcintyre P, Glimour, R, Gilbert L. The emergence of pneumococcal meningitis - Implications for empiric therapy. Arch Dis Child. 2002;3:207–210. doi: 10.1136/adc.87.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talan D. Role of Empiric Parenteral Antibiotics Prior to Lumbar Puncture in Suspected Bacterial Meningitis: State of the Art. Review of Infectious Disease. 1988;2:1–12. doi: 10.1093/clinids/10.2.365. [DOI] [PubMed] [Google Scholar]
- 12.Ehsanipour F, Aslani Z. The prevalence of meningitis in children with febrile seizure hospitalized at Hazrat Rasoul Hospital (1997-2002). Razi J Med Sci. 2005;44:907–911. [Google Scholar]
- 13.Tavasoli A, Edraki A. Frequency of meningitis in children presenting with febrile seizures at Ali-Asghar Children’s Hospital. Iran J Child Neuro. 2014;1:51–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Viladrich PF1, Liñares J, Pallarés R, et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis.”. Antimicrob Agents Chemother. 1991;12:1761–1768. doi: 10.1128/aac.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suntur B, Yurtseven T, Sipahi OR, et al. Rifampicin+ceftriaxone versus vancomycin+ceftriaxone in the treatment of penicillin- and cephalosporin-resistant pneumococcal meningitis in an experimental rabbit model. Int J Antimicrob Agents. 2005;3:258–260. doi: 10.1016/j.ijantimicag.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Ribes S, Domenech A, Cabellos C, et al. Evaluation of ceftriaxone, vancomycin and rifampicin alone and combined in an experimental model of meningitis caused by highly cephalosporin-resistant. Streptococcus pneumoniae J Antimicrob Chemother. 2005;5:979–982. doi: 10.1093/jac/dki323. [DOI] [PubMed] [Google Scholar]
- 17.Cabellos C, Martinez-Lacasa J, Martos A, et al. Influence of dexamethasone on efficacy of ceftriaxone and vancomycin therapy in experimental pneumococcal meningitis. Animicrob Agents Chemother. 1995;9:2158–2160. doi: 10.1128/aac.39.9.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elyasi S, Dashti-Khavidaki S, EmadiKoochak H. Conventional- versus high-dose vancomycin regimen in patients with acute bacterial meningitis: a randomized clinical trial. Expert Opin Pharmacother. 2015;16:297–307. doi: 10.1517/14656566.2015.999042. [DOI] [PubMed] [Google Scholar]
- 19.Taheri M, Shokouhi S, Ebrahimzadeh K, et al. Administration of Vancomycin at High Doses in Patients with Post Neurosurgical Meningitis: A Comprehensive Comparison between Continuous Infusion and Intermittent Infusion. Iran J Pharm Res. 2018;17(Suppl 2):195–205. [PMC free article] [PubMed] [Google Scholar]
- 20.Kneen R, Mithyantha R, Riordan A, Solomon T. The management of infants and children treated with aciclovir for suspected viral encephalitis. Arch Dis Child. 2010;95:100–106. doi: 10.1136/adc.2008.144998. [DOI] [PubMed] [Google Scholar]
- 21.Seedat A, Winnett G. Acyclovir-induced acute renal failure and the importance of an expanding waist line. BMJ Case Rep. 2012;12:2012–2019. doi: 10.1136/bcr-2012-006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vomiero G Carpenter B, Filler G. Combination of ceftriaxone and acyclovir - an underestimated nephrotoxic potential? Pediatr Nephrol. 2002;8:233–235. doi: 10.1007/s00467-002-0867-5. [DOI] [PubMed] [Google Scholar]
- 23.Aghdam MK, Sadeghzadeh M, Fakhimi S, Eftekhari K. Evaluation of Aseptic Meningitis Following Measles-Mumps-Rubella Vaccine in Children Admitted due to Febrile Convulsion. Int J Pediatics. 2018;56:8147–8152. [Google Scholar]
- 24.Laino D, Esposito S. Management of Pediatric Febrile Seizures. Int J Environ Res Public Health. 2018;10:1–10. doi: 10.3390/ijerph15102232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood KT, Fareed T, Tabbasum R. Management of Febrile Seizures in Children. J Biomed Sci and Res. 2011;1:353–363. [Google Scholar]
- 26.O’Leary MF, Chappell JD, Stratton CW, et al. Complex Febrile Seizures Followed by Complete Recovery in an Infant with High-Titer 2009 Pandemic Influenza A (H1N1) Virus Infection. J Clin Microbiol. 2010;10:3803–3805. doi: 10.1128/JCM.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]