Abstract
Background:
In biomarker-based studies, collecting repeated biospecimens per participant can decrease measurement error, particularly for biomarkers displaying high within-subject variability. Guidelines to combine such repeated biospecimens do not exist.
Aims:
To compare the efficiency of several designs relying on repeated biospecimens to estimate exposure over 7 days.
Methods:
We quantified triclosan and bisphenol A (BPA) in all urine voids (N=427) collected over seven days from eight individuals. We estimated the volume-weighted concentrations for all urine samples collected during a week and compared these gold standards with the concentrations obtained for equal-volume pools (standardized or not for urine dilution), unequal-volume pools (based on sample volume or creatinine concentration), and for the mean of the creatinine-standardized concentrations measured in each spot sample.
Results:
For both chemicals, correlations with gold standards were similar for equal- and unequal-volume pooling designs. Only for BPA, correlation coefficients were markedly lower after standardization for specific gravity or creatinine of concentrations estimated in equal-volume pools. Averaging BPA creatinine-standardized concentrations measured in each spot sample led also to lower correlations with gold standards compared to those obtained for unstandardized pooling designs.
Conclusion:
For BPA and triclosan, considering individual urine sample volume or creatinine concentrations when pooling is unnecessary because equal-volume pool adequately estimates concentrations in gold standards. Standardization for specific gravity or creatinine of the concentrations assessed in equal-volume pool as well as averaging creatinine-standardized concentrations measured in each individual spot sample are not suitable for BPA. These results provide a practical framework on how to combine repeated biospecimens in epidemiological studies.
Keywords: Biomarkers, bisphenol A, measurement error, pooling designs, repeated biospecimens
BACKGROUND
Most of the epidemiological studies on the health effects of short biological elimination half-lives chemicals (e.g., bisphenol A (BPA), phthalates, triclosan, pyrethroids) have relied on a relatively small number of urine samples per participant to assess exposure. Because these biomarkers present high intra-individual variability in urine concentrations (reviewed by Casas et al. 2018), snapshot assessments imperfectly reflect the average exposure over a day or longer time periods, leading to classical measurement error and biased effect estimates (Brunekreef et al. 1987; Perrier et al. 2016). Several recent cohorts aiming to reduce measurement error and associated bias in effect estimates (LaKind et al. 2019; Perrier et al. 2016), collected multiple biospecimens per participant during target exposure windows (Lyon-Caen et al. 2019; Shin et al. 2019; Warembourg et al. 2019).
For chemicals with high intra-individual variability (e.g., intraclass correlation coefficient (ICC) ≤ 0.2), up to 30 samples per subject may be required to adequately estimate a long-term exposure, such as during pregnancy (Perrier et al. 2016; Vernet et al. 2019). To limit assay costs, one can, for each participant, pool an equal volume of each spot sample collected over the period of interest and analyze the pool instead of individual samples (Schisterman and Vexler 2008; Vernet et al. 2019; Weinberg and Umbach 1999). Assuming no pooling error, biomarker concentrations obtained from equal-volume pooling are equivalent to the average of concentrations in each spot sample. However, such assumption is not valid when applying a standardization to account for urine dilution; which often involves dividing the biomarker concentration by the creatinine concentration. Averaging the creatinine-standardized concentrations measured in each spot sample will lead to different concentrations than those obtained by dividing the biomarker by the creatinine concentrations assessed in the pool of these samples (Rosen Vollmar et al. 2020). In addition, the equal-volume pooling design does not account for variations in urine dilution across spot samples and may over-represent spot collections with the smallest volumes. How this over-representation may affect biomarker concentrations compared to other pooling designs relying on unequal volumes of biospecimens (Weinberg et al. 2019) has not been studied yet.
OBJECTIVES
Our aim was to compare the efficiency of several designs relying either on pooling spot urine biospecimens or on averaging biomarker concentrations measured in multiple spot samples to estimate concentrations in a volume-weighted concentration for all urine samples collected during a week (or 24 hour) deemed to be the gold standard. We considered two chemicals with contrasted intra-individual variability and correlation patterns with creatinine and specific gravity: triclosan and BPA.
METHODS
Study population
Details regarding the study population have been described before (Li et al. 2010; Preau et al. 2010; Ye et al. 2011). Briefly, in October-November 2005, eight adults (four males, four females) between 26 and 58 years of age, healthy, nonsmokers, and living in the metropolitan Atlanta area in Georgia (USA) were recruited to participate in a study. The study, designed to examine the temporal variability in urinary concentrations of polycyclic aromatic hydrocarbon biomarkers, was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board. All participants signed an informed consent.
Urine collection
The study research team provided non-vinyl, non-polycarbonate plastic urine collection cups. Participants were asked to collect all urine voids produced over a week and to record for each void the volume and collection time. Study participants collected a total of 427 urine specimens and missed 23 samples (Ye et al. 2011). Among the 427 samples collected, 10 had no volume recorded and were excluded from this study. Participants decanted approximately 50 mL of urine to a prelabeled, sterile, polypropylene/polyethylene urine collection cup and stored it in an ice cooler containing frozen ice packs until collection by the study staff (daily or after the weekend). Urine was then aliquoted into polypropylene cryovials and frozen at −70°C until analysis. Using the time elapsed between two urine voids (t) and the volume of the second void (Vi), we computed an urinary flow rate (UFRi) (Middleton et al. 2016):
Quantification of BPA, triclosan, specific gravity (SG) and creatinine
Urine samples were analyzed at the CDC using a method based on online solid phase extraction (SPE) coupled to high performance liquid chromatography-atmospheric pressure chemical ionization-isotope dilution tandem mass spectrometry (HPLC-APCI-MS/MS)(Ye et al. 2005). Briefly, 100 μL of urine spiked with the appropriate reagents and standards was incubated to hydrolyze the biomarkers urinary conjugates. The procedure for extracting the deconjugated biomarkers from the urine involved concurrent online SPE-HPLC operation with peak focusing followed by APCI-MS/MS. In addition to study samples, each analytical run included high- and low-concentration quality control materials (QCs) and reagent blanks to assure accuracy and reliability of the data. The concentrations of the QCs were evaluated using standard statistical probability rules. The limits of detection (LODs) were 2.3 μg/L (triclosan) and 0.4 μg/L (BPA). For analysis, concentrations below the LOD were replaced by a value equal to LOD/√2 (Hornung and Reed 1990).Urinary specific gravity and creatinine were measured at the CDC using a handheld digital refractometer and a Roche Hitachi 912 Chemistry Analyzer (Hitachi, Pleasanton, CA), respectively.
Temporal variability
We used linear mixed models with a random intercept for participant to compute Intraclass Correlation Coefficients (ICCs) between concentrations measured in the spot urine samples.
Construction of the exposure proxies
For each participant and both BPA and triclosan, using the biomarker urinary concentrations and volume of each collected void, we constructed the exposure proxies described below.
- Pool of the whole volume of all individual spot samples collected over a week. The concentration assessed in this pool was equivalent to the concentration that would have been obtained in cumulative urine voids during a week. This concentration, named the volume-weighted concentration for all urine samples collected during a week, was considered as the gold standard:
where ConcAi was the concentration of biomarker A in the urine sample i, and volumei was the sample volume. - Equal-volume pool (EVP). We simulated the biomarker concentration that would have been obtained after pooling the exact same volume of each individual spot sample:
where N was the number of spot samples collected by the participant over a week. -
Unequal-volume pool (EVP)
-
3.1)Volume-based pool (VBP). In this pool, the volume of each spot sample was equal to the ratio of its volume to the entire volume of urine collected over a week:
When all the samples collected over the period of interest are included in the pool, volume-based pool is equivalent to the gold standard. -
3.2)Creatinine-based pool (CBP, (Weinberg et al. 2019)). In this case, the volume of each spot included in the pool (Volume_pooledi) depends on its creatinine concentration. Samples with higher creatinine concentration contribute less volume, and those with lower creatinine concentration contribute more volume:
Where Volumeref and Conccreat_ref are the volume and creatinine concentration, respectively, of a selected reference spot sample whose creatinine concentration, for each participant, was near the creatinine median concentration of the spot samples collected over a week (Weinberg et al. 2019). Conccreat_i was the creatinine concentration in the considered spot sample. We then computed the biomarker concentration in the creatinine-based pool as follows:
-
3.1)
- Equal-volume pool standardized for SG or creatinine. Because SG and creatinine standardization are commonly used to account for urine dilution varying across samples, we derived two additional exposure proxies from the concentrations estimated in the equal-volume pools. Creatinine standardization was done by dividing the EVP biomarker concentration by the EVP creatinine concentration while for SG standardization we used the following formula (Philippat et al. 2013):
where SGmeanEVP was the SG arithmetic mean of the equal-volume pools in the study population and SGEVP equaled the SG in the considered equal-volume pool. -
For each biomarker we also computed an average of the creatinine-standardized concentrations measured in each spot sample. This approach did not involve pooling but has been used in previous epidemiological studies that relied on biomarker concentrations obtained from multiple urine samples per person (Braun et al. 2011).
Collecting all urine voids produced over a week can be a considerable burden for participants and might limit participation rate or lead to selection bias in epidemiological studies. For this reason, we also considered a scenario in which we constructed the above exposure proxies using a limited number (2, 5 or 10) of voids randomly selected among the N voids collected over a week for each participant.
Comparison of biomarker concentrations in the exposure proxies and gold standard
We relied on Spearman correlation coefficients to compare the biomarker concentrations estimated for the exposure proxies (equal-volume pool standardized or not for creatinine and SG, volume-based pool, creatinine-based pool and mean of the creatinine-standardized concentrations measured in each spot) with the volume-weighted concentrations for all urine samples collected during a week, considered as the gold standard. For the scenarios relying on a limited number of randomly selected urine samples, Spearman correlation coefficients and their confidence intervals were estimated using 1000 bootstraps.
Sensitivity analysis
In sensitivity analysis, we explored a shorter time window, specifically 24-h urine collection. For each 24-h period and for each participant, we constructed the exposure proxies described above and computed correlation coefficients between each exposure proxy and the urine concentrations estimated in the 24-h urine collection. As for the main analysis, we considered a situation where only a few (2 or 3) of the collected spot samples were included in the pool.
Analysis were carried out using STATA/SE, version 15.1 (StataCorp, College Station, TX, USA) and R version 4.0.2. The code is available in the public repository of the Team of Environmental Epidemiology applied to Reproduction and Respiratory Health (https://gricad-gitlab.univ-grenoble-alpes.fr/iab-env-epi).
RESULTS
Out of the 427 urine samples collected, we excluded 10 (2%) from the analyses because of missing volume data. The number of samples with volume information available ranged between 27 and 68, depending on the participant. The average volume per void and for a 24-h urine collection were 417 mL (Standard Deviation (SD): 267) and 2017 mL (SD: 832), respectively. We detected BPA and triclosan in 91% and 72% of the spot samples, respectively. Median concentrations in the spot samples were 1.7 (25th, 75th percentiles: 0.8, 3.6) and 9.4 (< LOD, 32.8) μg/L for BPA and triclosan, respectively. Biomarker urinary concentrations assessed in spots are displayed for each participant in Figure 1. ICC were relatively low for BPA (ICC = 0.14, 95%CI: 0.05; 0.33), creatinine (ICC = 0.21, 95%CI: 0.08; 0.44) and SG (ICC = 0.21, 95%CI: 0.08; 0.44) and relatively high for triclosan (ICC = 0.77, 95%CI: 0.56; 0.90).
Figure 1:
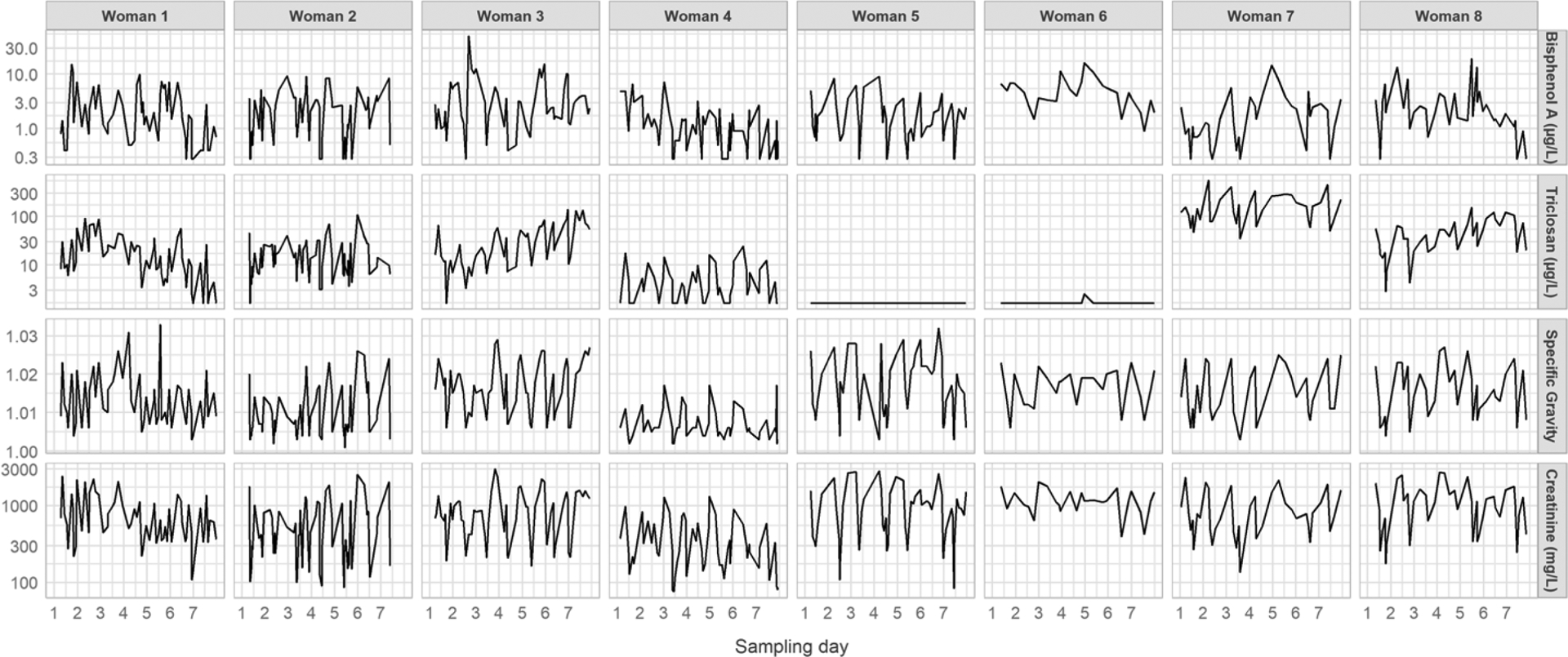
Biomarker concentrations for all participants in all spots collected over the week of urine collection
Within sample correlations across markers assessed in the study
UFR was negatively correlated with SG (rho = −0.80), creatinine (rho = −0.87), BPA (rho = −0.62), and triclosan (rho = −0.41). Void volume was also negatively associated with these biomarkers, however the absolute values of the correlation coefficients were lower (rho = −0.41, −0.37, −0.28, −0.24 for SG, creatinine, BPA, and triclosan, respectively) than those observed for the UFR. Time elapsed since the last void, SG and creatinine were all positively correlated with both BPA and triclosan concentrations; the absolute value of the correlation coefficient was higher for triclosan than for BPA (Table 1).
Table 1:
Within sample Spearman correlation coefficients for the different markers assessed in the current study
| Void volume | Urinary flow rate | Time since last void | Specific gravity | Creatinine | BPA | |
|---|---|---|---|---|---|---|
| Void volume | 1.00 | |||||
| Urinary flow rate | 0.46 | 1.00 | ||||
| Time since last void | 0.31 | −0.66 | 1.00 | |||
| Specific gravity | −0.41 | −0.80 | 0.48 | 1.00 | ||
| Creatinine | −0.37 | −0.87 | 0.59 | 0.90 | 1.00 | |
| BPA | −0.28 | −0.62 | 0.40 | 0.57 | 0.64 | 1.00 |
| Triclosan | −0.24 | −0.41 | 0.25 | 0.33 | 0.37 | 0.24 |
N = 417 urine spot samples, except for the correlations with urinary flow rate that were restricted to the 400 samples with available data for time since last void. Abbreviation: BPA: bisphenol A.
Equal-volume pool
For all participants, but one for triclosan and two for BPA, equal-volume pool estimated concentrations were higher than the volume-weighted concentrations for all urine samples collected during a week by 1% to 42%, depending on the participant (Figure 2). For both triclosan and BPA, when all the collected spot samples were included in the equal-volume pools, correlations with the gold standard were high (rho = 0.98 for triclosan and 0.93 for BPA). Limiting the number of samples used in the equal-volume pools had little impact for triclosan: correlation coefficients between equal-volume pools and the volume-weighted concentrations for all urine samples collected during a week were > 0.90, regardless of the number of samples (2, 5 or 10) included in the pool (Table 2). Limiting the number of urine samples included in equal-volume pools had a stronger impact for BPA: correlation with gold standard was 0.47 (95% confidence interval (CI): −0.12; 0.88), 0.63 (95%CI: 0.10; 0.95) and 0.75 (95%CI: 0.38; 0.95) when 2, 5 and 10 samples were included, respectively.
Figure 2:
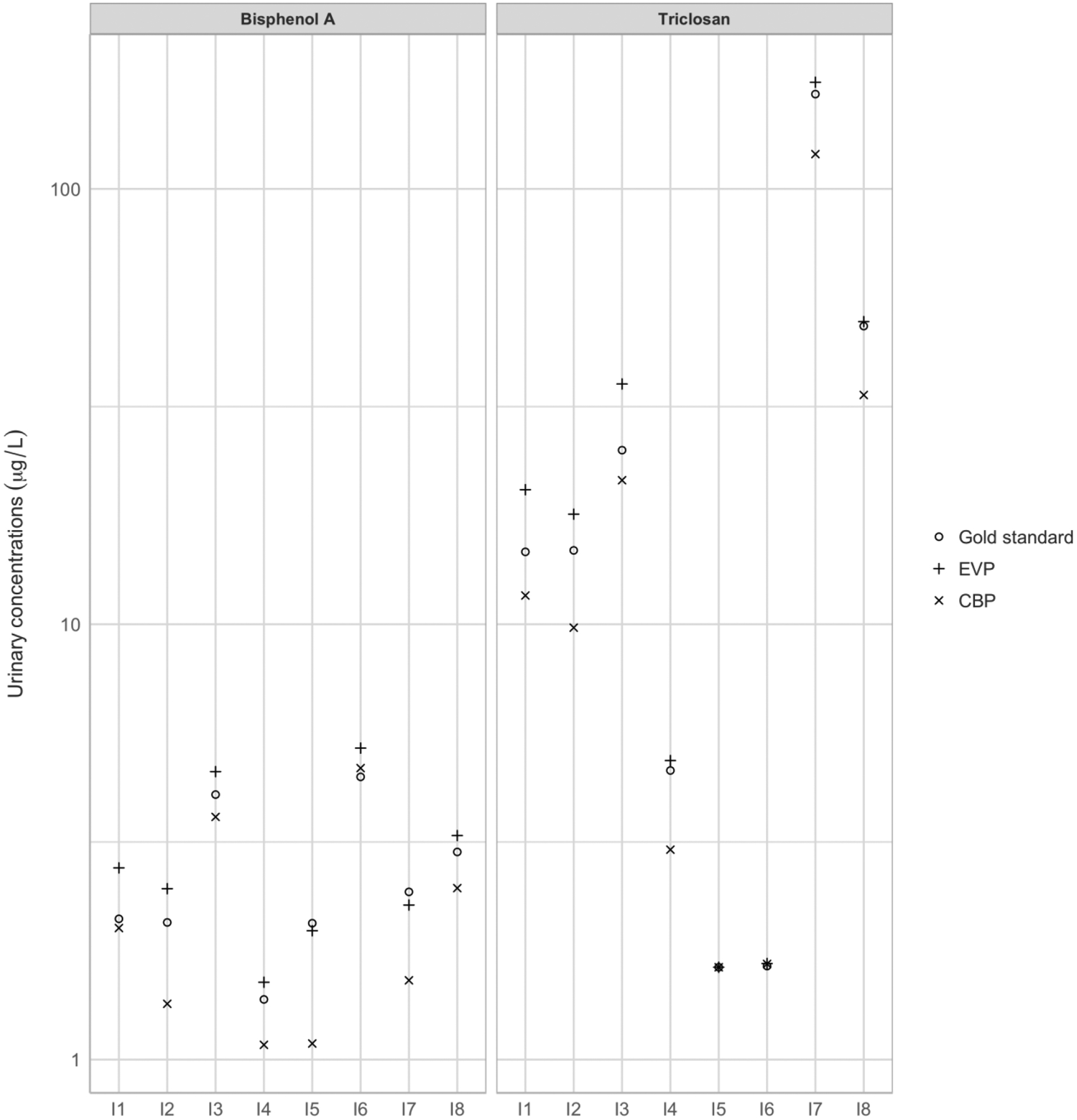
Estimated BPA and triclosan concentrations for each individual in gold standard (volume-weighted concentrations for all urine samples collected during a week), equal-volume (EVP) and creatinine-based (CBP) pools.
Table 2:
Spearman correlation coefficients between the volume-weighted concentrations for all urine samples collected during a week (gold standard) and the concentrations estimated from different protocols using pooling of spot samples or averaging creatinine-standardized concentrations measured in each spot sample
| All voids used to construct exposure proxies | 2 randomly selected voids used to construct exposure proxies | 5 randomly selected voids used to construct exposure proxies | 10 randomly selected voids used to construct exposure proxies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triclosan | BPA | Triclosan | BPA | Triclosan | BPA | Triclosan | BPA | |||||||
| rho | rho | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | |
| Equal-volume pool | 0.98 | 0.93 | 0.93 | [0.78; 0.99] | 0.47 | [−0.12; 0.88] | 0.96 | [0.85; 1.00] | 0.63 | [0.10; 0.95] | 0.97 | [0.92; 1.00] | 0.75 | [0.38; 0.95] |
| Volume-based pool | 1.00 | 1.00 | 0.93 | [0.79; 1.00] | 0.45 | [−0.17; 0.88] | 0.96 | [0.88; 1.00] | 0.58 | [0.07; 0.93] | 0.98 | [0.92; 1.00] | 0.70 | [0.29; 0.95] |
| Creatinine-based pool | 0.98 | 0.98 | 0.92 | [0.76; 1.00] | 0.50 | [−0.12; 0.90] | 0.96 | [0.86; 1.00] | 0.67 | [0.19; 0.95] | 0.97 | [0.92; 1.00] | 0.79 | [0.48; 0.98] |
| SG standardized equal-volume pool | 0.98 | 0.52 | 0.92 | [0.76; 1.00] | 0.21 | [−0.40; 0.74] | 0.95 | [0.83; 1.00] | 0.29 | [−0.29; 0.81] | 0.96 | [0.88; 1.00] | 0.37 | [−0.17; 0.81] |
| Creatinine-standardized equal-volume pool Average of the spot creatinine-standardized | 0.98 | 0.45 | 0.90 | [0.76; 1.00] | 0.11 | [−0.50; 0.71] | 0.94 | [0.81; 1.00] | 0.18 | [−0.36; 0.74] | 0.95 | [0.88; 1.00] | 0.22 | [−0.31; 0.71] |
| concentrations | 0.98 | 0.12 | 0.91 | [0.76; 1.00] | 0.09 | [−0.50; 0.69] | 0.94 | [0.83; 0.98] | 0.13 | [−0.43; 0.71] | 0.96 | [0.88; 0.98] | 0.14 | [−0.33; 0.69] |
estimated using 1000 bootstraps
Abbreviation: BPA: bisphenol A, CI: confidence interval, rho: Spearman correlation coefficient, SG: specific gravity
Unequal-volume pool
Volume-based pool:
Regardless of the number of samples considered in the volume-based pools, correlation with the gold standard was high for triclosan (rho ≥ 0.93) and moderate to high (ranged between 0.45 (95%CI: −0;17; 0.88) when two samples were used to 0.70 (95%CI: 0.29; 0.95) when 10 samples were used) for BPA. Our results suggested that considering void volumes when pooling had little impact on biomarker concentration estimates. Spearman correlations with the gold standard for volume-based pools were indeed similar (triclosan, rho ≥ 0.93) or slightly lower compared to those of equal-volume pools. For BPA, when 10 samples were included in the pool, correlations with the gold standard were 0.70 (95%CI: 0.29; 0.95) for volume-based pools and 0.75 (95%CI: 0.38; 0.95) for equal-volume pools, respectively.
Creatinine-based pool:
Biomarker concentrations in the creatinine-based pools were overall lower than the volume-weighted concentrations for all urine samples collected during a week (except for two participants for triclosan and one for BPA (Figure 2)). Compared to the equal-volume pool design, creatinine-based pools only slightly increased correlation coefficients with gold standards for BPA, while both approaches gave similar results for triclosan (Table 2). For example, for BPA, when 10 samples were included in the pool, correlation coefficients with volume-weighted concentrations for all urine samples collected during a week were 0.79 (95%CI: 0.48; 0.98) for creatinine-based pools and 0.75 (95%CI: 0.38; 0.95) for equal-volume pools.
Standardization of equal-volume pools for urine dilution using creatinine or SG:
Creatinine and SG standardization had little impact for triclosan. Regardless of the number of spot samples considered, creatinine- and SG-standardized equal-volume pools led to similar (all samples used) or slightly lower (limited number of samples included in the pool) Spearman correlations with the gold standardthan those observed for the unstandardized equal-volume pools (all rho ≥ 0.90, Table 2). The impact of creatinine and SG standardization was more pronounced for BPA (Table 2). Regardless of the number of samples considered, correlation coefficients with gold standard were markedly weaker with than without standardization (Table 2). For example, when using 10 samples, Spearman correlation coefficients with the volume-weighted concentrations for all urine samples collected during a week were 0.22 (95%CI: −0.31; 0.71) and 0.37 (95%CI: −0.17; 0.81) for the creatinine- and SG-standardized equal-volume pools, respectively, compared to 0.75 (95%CI: 0.98; 0.95) for the non-standardized equal-volume pools. Overall, for both biomarkers, correlation coefficients with gold standard were slightly higher for the SG-standardized than for the creatinine-standardized equal-volume pools.
Assessing concentrations in each spot and using the average instead of pooling
Compared to all the pooling designs evaluated, assessing biomarker and creatinine concentrations in each spot sample and using the average of the creatinine-standardized concentrations led to weaker correlations with the gold standard for BPA (all rho ≤ 0.14 (Table 2)). For triclosan, correlation coefficients obtained with this design were high (≥ 0.91, regardless of the number of samples used) and similar to those obtained with the evaluated pooling designs.
Sensitivity analysis
Overall, relying on the 24-h urine collection instead of weekly urine collection as the gold standard, led to similar conclusions. While concentration distributions overlapped, when all the collected spot samples were included in the pools, triclosan and BPA medians tended to be higher in equal-volume pools and lower in creatinine-based pools compared to the 24-h urine collection (Figure 3). Pooling unequal spot volumes (based on their original creatinine concentration or volume) gave equivalent correlation coefficients with the 24-urine collection to equal-volume pools (Table 3). For BPA, standardization of equal-volume pools for SG or creatinine or using the average of the creatinine-standardized concentrations in each spot markedly decreased correlation coefficients with the 24-h urine collection compared to the other tested designs.
Figure 3:
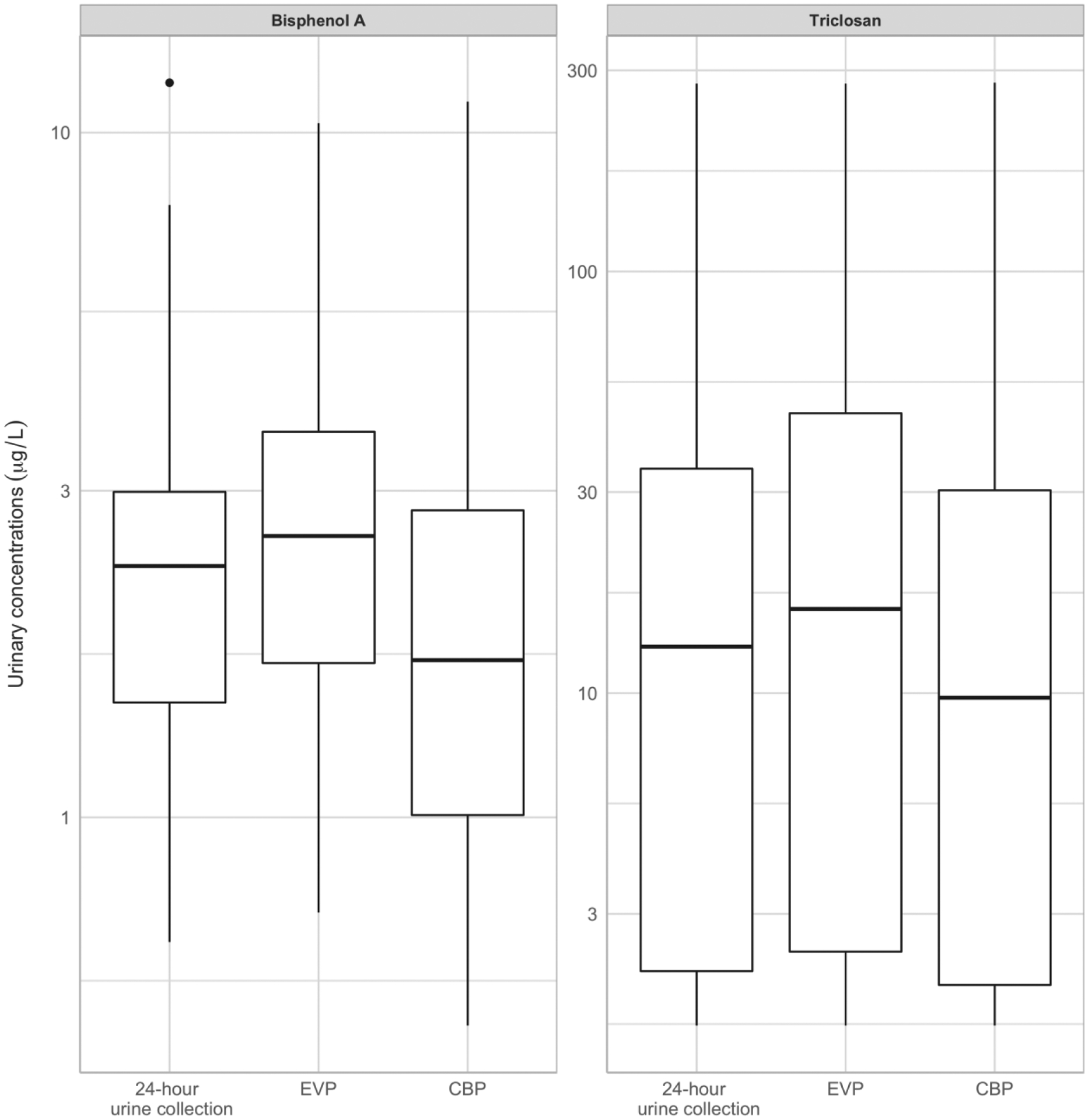
Distribution of BPA and triclosan concentrations estimated in 24-h urine collection (gold standard), equal-volume (EVP) and creatinine-based (CBP) pools among the eight study participants
Boxes lines represent 75th (upper line), 50th (middle line) and 25th (lower line) percentiles.
Table 3:
Spearman correlation coefficients between BPA and triclosan concentrations estimated from 24-h urine collections (gold standard) and from different protocols using pooling of spot samples or averaging of the creatinine-standardized concentrations measured in each spot sample
| All voids used to construct exposure proxies | Only 2 randomly selected voids used to construct exposure proxies | Only 3 randomly selected voids used to construct exposure proxies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Triclosan | BPA | Triclosan | BPA | Triclosan | BPA | |||||
| rho | rho | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | rhoa | 95% CIa | |
| Equal-volume pool | 0.99 | 0.95 | 0.95 | [0.94 ; 0.97] | 0.72 | [0.59 ; 0.82] | 0.97 | [0.95 ; 0.98] | 0.80 | [0.70 ; 0.88] |
| Volume-based pool | 1.00 | 1.00 | 0.95 | [0.93 ; 0.97] | 0.72 | [0.58 ; 0.83] | 0.97 | [0.95 ; 0.98] | 0.80 | [0.71 ; 0.88] |
| Creatinine-based pool | 0.98 | 0.91 | 0.95 | [0.92 ; 0.97] | 0.71 | [0.57 ; 0.82] | 0.96 | [0.94 ; 0.98] | 0.79 | [0.69 ; 0.86] |
| SG standardized equal volume pool | 0.96 | 0.76 | 0.94 | [0.93 ; 0.96] | 0.60 | [0.44 ; 0.73] | 0.95 | [0.93 ; 0.96] | 0.66 | [0.52 ; 0.77] |
| Creatinine-standardized equal volume pool | 0.94 | 0.66 | 0.93 | [0.91 ; 0.95] | 0.51 | [0.35 ; 0.67] | 0.94 | [0.92 ; 0.95] | 0.57 | [0.43 ; 0.69] |
| Average of the spot creatinine-standardized concentrations | 0.95 | 0.61 | 0.93 | [0.91 ; 0.95] | 0.53 | [0.36 ; 0.66] | 0.94 | [0.92 ; 0.95] | 0.57 | [0.43 ; 0.69] |
estimated using 1000 bootstraps
Abbreviation: BPA: bisphenol A, rho: Spearman correlation coefficients, SG: specific gravity, CI: Confidence Interval
DISCUSSION
In this study, we used correlation coefficients to compare urinary concentrations estimated from several designs (based on pooling or averaging of the creatinine-standardized concentration assessed in spot samples) with the volume-weighted concentrations for all urine samples collected during a week or a day. For both BPA and triclosan, we observed similar correlations with these gold standards for equal-volume, volume-based and creatinine-based pools, suggesting that accounting for sample volumes or creatinine concentrations when pooling might not be necessary. In addition, for BPA, a chemical with high within-subject variability (ICC = 0.14) and high correlations with creatinine and SG (rho ≥ 0.57) equal-volume pools standardized for SG and creatinine as well as averaging of the creatinine-standardized concentrations assessed in each spot should be avoided because for these designs correlations with the gold standard were considerably lower than those obtained for unstandardized pooling designs. Such standardization had little impact for triclosan, a chemical characterized by a moderate intra-individual variability ((ICC = 0.77) and moderate correlation with SG and creatinine (rho ≤ 0.37).
Pooling of the equal or unequal spot sample volumes
For both BPA and triclosan, regardless of the number of samples included in the pool, correlations with the gold standard were comparable for equal-volume, volume-based and creatinine-based pools, suggesting that these three pooling designs are equivalent for estimating the volume-weighted concentrations for all urine samples collected during a week or a day. Compared to equal-volume pools, unequal-volume pools are more prone to technical errors because the volume of each spot included in the pool varies according to its original volume or creatinine concentrations. Another limitation of the creatinine-based pooling design is its cost as the quantification of creatinine concentration in each spot is needed before pooling. For these practical reasons, equal-volume pools might be preferred over creatinine- and volume-based pools in the framework of epidemiological studies relying on biomarker assessments. Noteworthy, while equal-volume, volume-based and creatinine-based pools overall preserved the ranking of the individuals compared to the volume-weighted concentrations for all urine samples collected during a week or a day (i.e. correlation coefficients > 0.90 when all the spot samples were included in the pool), the absolute biomarker concentrations varied. When all the spot samples collected were included in the pool, equal-volume pools tended to overestimate the urinary concentrations while creatinine-based pools underestimated them compared to the gold standards. This important fact should be considered when comparing urinary concentrations across studies which have relied on different pooling designs.
Number of samples included in the pool
As expected, regardless of the design used, when a limited number of urine samples was included in the pools, correlation coefficients with the gold standard were higher for triclosan than for BPA, a compound showing rather high intra-individual variability. For triclosan, a pool of as few as two samples adequately represented the volume-weighted concentration for all urine samples collected during a day or a week (correlation coefficients > 0.92) in our study population. For BPA, such high correlation was never achieved suggesting that more than 10 urine samples were needed to correctly estimate exposure over seven days, and more than three urine samples to estimate exposure over a day. This finding is in line with a previous simulation study suggesting that for a chemical with high intra-individual variability such as BPA, about 35 individual urine samples would be required to reduce bias in effect estimates to < 10% when studying associations with a continuous outcome (Perrier et al. 2016). Casas et al. estimated that four pools of 20 spot samples each would be needed to properly estimate (defined as an ICC ≥ 0.80) women exposure to BPA during a nine-month period (Casas et al. 2018).
Standardization of equal-volume pools for creatinine or SG
While standardization of the equal-volume pools for creatinine or SG had little impact for triclosan in our study, for BPA correlations with the gold standard drastically dropped. These results suggested that standardization in equal-volume pools was inappropriate for BPA. Of note, this result was in agreement with a study comparing standardized equal-volume pools and creatinine-based pools in the framework of a case control study design (Weinberg et al. 2019). Using simulated data, Weinberg et al. reported a lower confidence interval coverage (i.e., proportion of simulated datasets where the confidence interval of the predicted effect estimate included the true effect) for the standardized equal-volume pools than for creatinine-based pools.
Averaging of the standardized biomarker concentrations quantified in each spot sample
Quantifying biomarker and creatinine concentrations in several spot samples per individual allows to assess intra-individual variability (Vernet et al. 2018; Ye et al. 2011) and can be used in models such as regression calibration and SIMEX to correct an exposure-health outcome association for measurement error (Carroll et al. 1995). Despite the fact that such models limit bias in the effect estimates, their use is still rare in biomarkers-based studies (Jackson-Browne et al. 2018) and an average of the biomarker concentrations measured in each spot has been sometimes used as a proxy of exposure (Braun et al. 2011; Philippat et al. 2018; Shin et al. 2018). When no standardization for creatinine is performed and assuming no error during preparation of the pools or chemicals assessments, such approach is equivalent to the equal-volume pool and considerably limits measurement error compared to the situation when a spot sample is used (Perrier et al. 2016; Vernet et al. 2019). However, in our study, using the average of the creatinine-standardized instead of the crude concentration led to poor correlation coefficients with the BPA concentration estimated in the gold standard. This suggested that this approach should be used with caution.
Strengths and limitations
Strengths of the study include the assessment of urine volume void and measurement of exposure biomarkers, creatinine and SG in all spot urine samples collected over a week. The average volume of the 24-h urine collection in our study population (2017 mL (SD: 832)) was similar to that reported in a subsample of the 2013 National Health and Nutrition Examination Survey participants (Terry et al. 2016) suggesting that the participants correctly recorded their individual void volumes. Such detailed data are quite rare and challenging to collect. The downside of this extensive data collection is the sample size (N = 8) which limits result generalization to other populations. In addition, our conclusions only apply to biomarkers with similar intra-individual variability and correlation with creatinine and SG as BPA and triclosan. We empirically estimated the urinary concentrations that would have been observed in theoretical pools from the urinary concentrations quantified in the collected spot samples. Because our approach excluded processing errors potentially introduced by the pooling process (Lyles et al. 2015), correlation coefficients estimated in our analyses might have been overestimated. However, such errors are more likely to occur in pools relying on different urine volumes compared to those relying on equal volumes. Therefore, even if such errors would have taken place, they should not have affected our main findings suggesting that equal-volume pools were as efficient as creatinine-based and volume-based pools to estimate the volume-weighted concentration for all urine samples collected during a week or a day, and that no standardization for SG and creatinine should be made on equal-volume pools.
CONCLUSION
Our results suggest that the equal-volume pooling design performs well in estimating the volume-weighted concentration for all urine samples collected during a week or a day for two biomarkers, BPA and triclosan, with stark differences in terms of intra-individual variability and correlation with creatinine and SG. Furthermore, standardization for SG or creatinine is not recommended for equal-volume pools, at least for BPA and perhaps other chemicals with similarly relatively high intra-individual variability and high correlation with SG and creatinine. Last, averaging of the creatinine-standardized biomarker concentrations measured in each spot sample of an individual is not suitable for BPA. These findings will help epidemiologists to optimize their use of repeated urine samples in biomarker-based studies.
Acknowledgements:
We thank Andreas Sjodin for designing the original study to evaluate temporal variability of polycyclic aromatic hydrocarbon biomarkers, measuring specific gravity, and providing the urine samples, the late Xiaoyun Ye for the bisphenol A and triclosan biomarkers data, and Pam Olive for the creatinine measures. We thank Paulina Jedynak for her useful comments on the manuscript and Matthieu Rolland for the design of figures.
Sources of funding: Funding was provided by the U.S. government to CDC. Claire Philippat is supported by ANR (19-CE36-0003-01) and The French National Research Program for Environmental and Occupational Health of Anses (2019/1/039).
Abbreviations:
- BPA
bisphenol A
- CBP
creatinine-based pool
- EVP
equal-volume pool
- LOD
limit of detection
- VBP
volume-based pool
- SG
specific gravity
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
REFERENCES
- Aylward LL, Hays SM, Zidek A. 2017. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry. J Expo Sci Environ Epidemiol 27:582–590; doi: 10.1038/jes.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye XY, Dietrich KN, et al. 2011. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics 128:873–882; doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Noy D, Clausing P. 1987. Variability of exposure measurements in environmental epidemiology. American journal of epidemiology 125: 892–8. [DOI] [PubMed] [Google Scholar]
- Carroll RJ, Ruppert D, Stefanski LA. 1995. Measurement Error in Nonlinear Models, A Modern Perspective, Second Edition Chapman & Hall.:London. [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, et al. 2018. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int 121:561–573; doi: 10.1016/j.envint.2018.09.046. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 5:46–51; doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, et al. 2018. Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environmental health perspectives 126:057001; doi: 10.1289/ehp2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos R, Cocker J, et al. 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day samPling Period. Part 2: Personal care product ingredients. Toxicology letters 231:261–9; doi: 10.1016/j.toxlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Idri F, Naiman DQ, Verner M-A. 2019. Biomonitoring and Nonpersistent Chemicals-Understanding and Addressing Variability and Exposure Misclassification. Curr Environ Health Rep 6:16–21; doi: 10.1007/s40572-019-0227-2. [DOI] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebæk NE, et al. 2013. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environ Res 126:164–170; doi: 10.1016/j.envres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, et al. 2010. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol 20:526–535; doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles RH, Van Domelen D, Mitchell EM, Schisterman EF. 2015. A Discriminant Function Approach to Adjust for Processing and Measurement Error When a Biomarker is Assayed in Pooled Samples. Int J Environ Res Public Health 12:14723–14740; doi: 10.3390/ijerph121114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon-Caen S, Siroux V, Lepeule J, Lorimier P, Hainaut P, Mossuz P, et al. 2019. Deciphering the Impact of Early-Life Exposures to Highly Variable Environmental Factors on Foetal and Child Health: Design of SEPAGES Couple-Child Cohort. Int J Environ Res Public Health 16; doi: 10.3390/ijerph16203888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, Variability, and Predictors of Urinary Concentrations of Phenols and Parabens among Pregnant Women in Puerto Rico. Environmental science & technology 47:3439–47; doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton DRS, Watts MJ, Lark RM, Milne CJ, Polya DA. 2016. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ Health 15:68; doi: 10.1186/s12940-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology (Cambridge, Mass) 27:378–88; doi: 10.1097/ede.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Barkoski J, Tancredi DJ, Elms B, Barr DB, Ozonoff S, et al. 2018. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. International journal of hygiene and environmental health; doi: 10.1016/j.ijheh.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. 2013. Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environmental health perspectives 121:1225–1231; doi: 10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack AZ, Perkins NJ, Mumford SL, Ye A, Schisterman EF. 2013. Correlated biomarker measurement error: an important threat to inference in environmental epidemiology. American journal of epidemiology 177:84–92; doi: 10.1093/aje/kws209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau J r JL, Wong LY, Silva MJ, Needham LL, Calafat AM. 2010. Variability over One Week in the Urinary Concentrations of Metabolites of Diethyl Phthalate and Di(2-Ethylhexyl) Phthalate among 8 Adults: an Observational Study. Environmental health perspectives 118: 1748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen Vollmar AK, Johnson CH, Weinberg CR, Deziel NC, Baird DD, Wilcox AJ, et al. 2020. Accounting for urinary dilution in peri-implantation samples: implications for creatinine adjustment and specimen pooling. J Expo Sci Environ Epidemiol; doi: 10.1038/s41370-020-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A. 2008. To pool or not to pool, from whether to when: applications of pooling to biospecimens subject to a limit of detection. Paediatr Perinat Epidemiol 22:486–96; doi: 10.1111/j.1365-3016.2008.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, et al. 2019. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 122:222–230; doi: 10.1016/j.envint.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Schmidt RJ, Tancredi D, Barkoski J, Ozonoff S, Bennett DH, et al. 2018. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health 17:85; doi: 10.1186/s12940-018-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AL, Cogswell ME, Wang C-Y, Chen T-C, Loria CM, Wright JD, et al. 2016. Feasibility of collecting 24-h urine to monitor sodium intake in the National Health and Nutrition Examination Survey. Am J Clin Nutr 104:480–488; doi: 10.3945/ajcn.115.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, et al. 2019. An Empirical Validation of the Within-subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-based Studies. Epidemiology 30:756–767; doi: 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Calafat AM, Ye X, Lyon-Caen S, Siroux V, et al. 2018. Within-Day, Between-Day, and Between-Week Variability of Urinary Concentrations of Phenol Biomarkers in Pregnant Women. Environ Health Perspect 126:037005; doi: 10.1289/EHP1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg C, Basagaña X, Seminati C, de Bont J, Granum B, Lyon-Caen S, et al. 2019. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int J Hyg Environ Health 222:446–454; doi: 10.1016/j.ijheh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Shi M, O’Brien KM, Umbach DM. 2019. Adjustment for urinary creatinine or serum lipids for analytes assayed in pooled specimens. Epidemiology; doi: 10.1097/EDE.0000000000001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Umbach DM. 1999. Using pooled exposure assessment to improve efficiency in case-control studies. Biometrics 55: 718–26. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem 77:5407–5413; doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. 2011. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environmental health perspectives 119:983–8; doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]


