Abstract
BACKGROUND AND AIMS:
Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive hepatic fat accumulation. Increased hepatic saturated fats and decreased hepatic polyunsaturated fats may be particularly lipotoxic, contributing to metabolic dysfunction. We compared hepatic lipid subspecies in adults with and without NAFLD, and examined links with hallmark metabolic and clinical characteristics of NAFLD.
METHODS AND RESULTS:
Nineteen adults with NAFLD (total hepatic fat:18.8 ± 0.1%) were compared to sixteen adults without NAFLD (total hepatic fat: 2.1 ± 0.01%). 1H-MRS was used to assess hepatic lipid subspecies. Methyl, allylic, methylene, and diallylic proton peaks were measured. Saturation, unsaturation, and polyunsaturation indices were calculated. Whole-body phenotyping in a subset of participants included insulin sensitivity (40 mU/m2 hyperinsulinemic-euglycemic clamps), CT-measured abdominal adipose tissue depots, exercise capacity, and serum lipid profiles. Participants with NAFLD exhibited more saturated and less unsaturated hepatic fat, accompanied by increased insulin resistance, total and visceral adiposity, triglycerides, and reduced exercise capacity compared to controls (all P < 0.05). All proton lipid peaks were related to insulin resistance and hypertriglyceridemia (P < 0.05).
CONCLUSION:
Participants with NAFLD preferentially stored excess hepatic lipids as saturated fat, at the expense of unsaturated fat, compared to controls. This hepatic lipid profile is accompanied by an unhealthy metabolic phenotype.
Keywords: magnetic resonance spectroscopy, insulin resistance, saturated fats
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a chronic condition characterized by excessive fat accumulation in the liver. It is estimated that NAFLD affects up to 27% of the general population in the industrialized world[1]. Metabolic dysfunction is a concerning consequence, as NAFLD and type 2 diabetes often develop simultaneously[2]. Even more alarming, NAFLD can have fatal consequences by progressing to nonalcoholic steatohepatitis (NASH), which is a condition characterized by hepatic inflammation and apoptosis. For 20% of patients, NASH may progress to liver cirrhosis, eventually leading to liver-related fatality[3].
There is considerable variation in the impact of hepatic fat accumulation on health; some cases of NAFLD remain benign, while others progress to more advanced liver disease. One source of disease variation may be related to differences in hepatic lipid storage type[4, 5]. Hepatic lipids can differ by the degree of saturation, or the amount of double or single bonds present in various fatty acid functional groups. Increased hepatic saturated fats measured by gas-liquid chromatography have been previously linked to NAFLD and NASH[6, 7]. Conversely, reduced levels of dietary polyunsaturated fats may trigger hepatic fat accumulation processes[8]. Furthermore, dietary supplementation with polyunsaturated fat has been shown to improve biochemical, ultrasonographic, and hemodynamic features of hepatic steatosis[9]. These findings are suggestive of a lipotoxic profile that is characterized by increased saturated fats and reduced polyunsaturated fats.
Recent methodological advances have created new opportunities for addressing questions related to storage forms of hepatic lipids. Experiments using proton magnetic resonance spectroscopy (1H-MRS) are now being utilized for measurement of in vivo hepatic lipids[10]. Hepatic fatty acids can be quantified by measurement of proton peaks representative of various proton functional groups. The main contributors to the hepatic proton signal include methylene (−CH2) and methyl (−CH3) functional groups, albeit not exclusively, representative of saturated fatty acids. The smallest contributor to the proton signal comes from the diallylic peak, which is exclusively representative of polyunsaturated fatty acids. When expressed as ratios of total hepatic fat, these proton peaks can be used to describe preferential lipid storage forms, or subspecies, as indices of saturated, unsaturated, and polyunsaturated fats.
It has been suggested that high levels of saturated fats and low levels of polyunsaturated fats promote the progression of liver disease. The purpose of this study was to apply novel methods of 1H-MRS-based hepatic lipid assessments to adults with and without NAFLD. It was hypothesized that hepatic saturated fats would be elevated in participants with NAFLD, while polyunsaturated fats would be reduced. In addition, we explored relationships between hepatic lipids and hallmark characteristics of NAFLD including insulin resistance, abdominal adiposity, low exercise capacity, and dyslipidemia.
METHODS:
Study Population:
This was a cross-sectional study in which hepatic lipid subspecies were measured using 1H-MRS in 35 adults with suspected NAFLD (55 ± 13.4 yrs). Nineteen participants met the diagnostic criteria for NAFLD (total hepatic triglyceride content ≥ 5.56%)[11], and 16 participants were below the diagnostic threshold. Participants were required to be weight stable (< 2 kg of weight change) for at least 6 months prior to testing. All participants gave informed consent prior to study involvement. This study was approved by the Cleveland Clinic Institutional Review Board. A subset of study participants was included in a previously published study10.
Testing occurred over 2 consecutive days. Day 1 involved collection of anthropometric data (height, weight) and a DXA (body composition, GE Healthcare, Madison, Wisconsin) scan. Abdominal adipose tissue depots were quantified using computerized tomography (CT) scans. For the purpose of metabolic control, participants stayed overnight in the Clinical Research Unit and were given a standardized evening meal (55% carbohydrate, 30% fat, and 15% protein). On the following morning (Day 2), participants underwent hepatic 1H-MRS scans, and hyperinsulinemic-euglycemic clamp testing. After completion of these procedures, participants were given lunch and discharged.
Hepatic Lipid Subspecies:
1H-magnetic resonance spectroscopy (1H-MRS) scans were performed on the liver, as previously described[10, 12] in 35 participants. Proton spectra were used to measure hepatic lipid subspecies including total hepatic triglyceride content, as well as saturation, unsaturation, and polyunsaturation indices.
All scans were completed using a Siemens Verio 3T MRI. Participants were fasted during scans. It should be noted, however, that hepatic lipids are not sensitive to changes in short-term feeding[10]. Participants were scanned in a prone position. A spine array coil and a body surface array coil were used to acquire the MRS datasets. Following localization scans, an 8-cm3 voxel was positioned within the right posterior lobe of the liver. Manual shimming was performed at ~40 Hz to obtain spectra delineating water and lipid subspecies. Spectra with and without water suppression were acquired using a long repetition time (TR = 5000 ms) and a short echo time (TE = 30 ms). Thirty-two averages were acquired to obtain sufficient signal for assessment of lipid components. Scan time was approximately 30 minutes. Data were Fourier-transformed, filtered, baseline corrected, and phase modulated to obtain high quality spectra. Peak height was used to assess the relative proton density of each species.
Indices of hepatic lipid subspecies were calculated as [10, 12]:
Insulin Resistance:
The hyperinsulinemic-euglycemic clamp technique was used to assess peripheral insulin resistance in 30 participants. In brief, a continuous infusion of insulin (40 mU/m2/min−1) was administered via catheter placed in an antecubital vein. Glucose (20% dextrose) was subsequently infused for 120 minutes at variable rates in order to maintain plasma glucose concentrations at 90 mg/dL. The opposite hand was warmed to 60°C for the purpose of arterializing blood. Plasma glucose was analyzed using YSI 2300 STAT Plus analyzer (Yellow Springs, OH). Blood samples for plasma insulin were obtained in 10-min increments at baseline (−30, −20, −10 min), and in 15-min increments throughout the clamp procedure (from 0–180 min), and were analyzed using a radioimmunoassay (Millipore, Billerica, MA). Data are reported as glucose metabolized “M”, which is the glucose disposal rate after space correction[13].
Blood Analysis:
Fasting blood lipids were assessed using enzymatic analysis (Roche Modular Diagnostics) and included triglycerides, total cholesterol, HDL, LDL, and VLDL in 30 participants.
Fasting serum liver enzymes aspartate transaminase (AST) and alanine transaminase (ALT), were assessed using Cobra Integra Alanine Aminotransferase (ALTL) and Aspartate Aminotransferase (ASTL) tests (Roche Diagnostics, Indianapolis, IN), as previously described[14].
Adipose Tissue Distribution:
DXA scans were performed for assessment of whole-body adiposity distribution, in which fat mass and fat-free mass percentages were determined in 29 participants. Gynoid and android adipose distribution were also evaluated.
Abdominal adipose depots were assessed using CT scans (Somotom Sensation 16 scanner; Siemens Medical Solutions, Malvern Pennsylvania), in which 5-mm slices were obtained at the L4 region, as described previously[15, 16] in 26 participants. Regions of interest were identified from each scan, including abdominal perimeter, subcutaneous fascia, outer abdominal musculature membrane, and inner abdominal musculature. From these regions, the following fat depots were calculated: total abdominal adipose tissue (AT), total subcutaneous AT, superficial subcutaneous AT, deep subcutaneous AT, and visceral AT.
Exercise Capacity
Maximal oxygen consumption (VO2max) was measured using an incremental, graded treadmill exercise test in 25 participants. Criteria for determination of a maximal test were as follows: 1) oxygen consumption plateau (< 150 ml/min), 2) heart rate within 15 beats of age-predicted max, 3) respiratory exchange ratio > 1.15, and/or 4) volitional fatigue. Participants were required to achieve 3 of 4 criteria in order for the test to be considered maximal[17].
Statistical Analysis
Data are expressed as mean ± SD, unless otherwise noted. Data were analyzed using GraphPad Prism v7. Data were tested for normality using the Shapiro-Wilk test. Student’s unpaired t-test was used for between group differences for normally distributed data. Mann-Whitney U tests were used when data were not normally distributed. Pearson’s correlation was used to test relationships between hepatic lipid subspecies and outcome variables. Data were log transformed when non-normally distributed for correlation analyses. Significance was accepted at P < 0.05.
RESULTS:
Nineteen of the 35 participants recruited for the study met the diagnostic criteria for NAFLD. Three participants within the NAFLD group had a fasting plasma glucose ≥ 126 mg/dL. Participant characteristics are shown in Table 1.
Table 1.
Participant Physical and Biochemical Characteristics
| Control | NAFLD | P-value | |
|---|---|---|---|
| N | 16 | 19 | - |
| Age | 51 ± 15 | 59 ± 12 | 0.11 |
| Height (cm) | 167 ± 12 | 168 ± 8 | 0.89 |
| Sex (M/F) | 6/10 | 7/12 | - |
| Weight (kg) | 78 ± 19 | 97 ± 14 | 0.004 |
| BMI (kg/m2) | 27 ± 5 | 34 ± 4 | 0.0003 |
| Body Fat (%) | 39 ± 9% | 46 ± 6% | 0.01 |
| Fasting Glucose (mg/dL) | 95 ± 13 | 115 ± 20 | 0.003 |
| Fasting Insulin (μU/mL) | 17 ± 6 | 26 ± 22 | 0.22 |
| Aspartate Aminotransferase (U/I) | 22 ± 7 | 34 ± 22 | 0.07 |
| Alanine Aminotransferase (U/I) | 23 ± 16 | 37 ± 24 | 0.07 |
| Cholesterol (mg/dL) | 182 ± 6 | 185 ± 35 | 0.82 |
| LDL (mg/dL) | 108 ± 36 | 105 ± 32 | 0.83 |
| HDL (mg/dL) | 55 ± 19 | 47 ± 10 | 0.14 |
| VLDL (mg/dL) | 19 ± 6 | 32 ± 14 | 0.002 |
| VO2max (ml/kg/min) | 33 ± 8 | 21 ± 5 | 0.0004 |
Values are presented as mean ± SD.
Hepatic Lipid Subspecies:
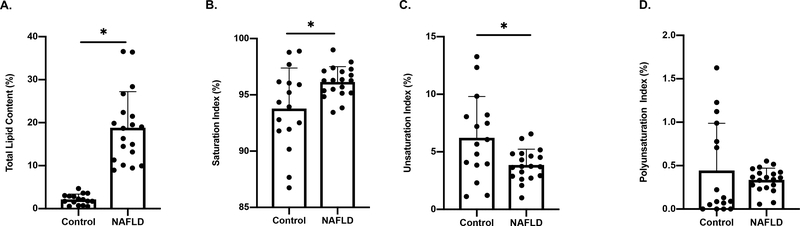
Total hepatic lipid content in NAFLD versus controls is displayed in Fig 1; Panel A, (P<0.005). Saturation index was higher in the NAFLD group compared to controls (Fig 1; Panel B; P=0.01). Conversely, the unsaturation index was lower in the NAFLD group compared to controls (Fig 1; Panel C, P=0.01). No group differences were observed in the polyunsaturation index (Fig 1; Panel D, P =0.362).
Fig 1:
Panel A: Participants categorized into either control (n=19) or NAFLD (n=16) groups based on total hepatic lipid content (NAFLD ≥ 5.56%). Panel B: Increased saturation index in NAFLD compared to a control group. Panel C: Decreased unsaturation index in NAFLD compared to controls. Panel D: No significant differences in polyunsaturation index. Data shown as mean ± SD. * indicates P ≤ 0.05.
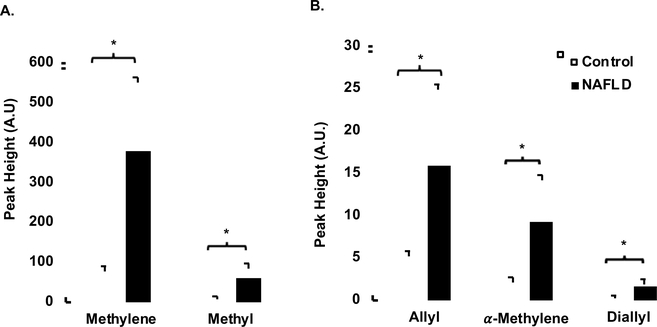
All proton peaks were significantly elevated in the NAFLD group compared to controls, as shown in Fig 2, including methylene (Panel A, P=1.0 × 10−7), methyl (Panel A, P=5.0 × 10−6), allyl (Panel B, P=1.1 × 10−5) α-methylene (Panel B, P=6.0 × 10−6), and diallylic (Panel B, P=5.0 × 10−6) peaks.
Fig 2:
Panel A: Average methylene and methyl peak heights in NAFLD group (n=16) compared to a control group (n=19). Panel B: Average allylic, α-methylene, and diallylic peak heights in NAFLD compared to controls. Data shown as mean ± SD. * indicates P ≤ 0.05.
Abdominal Fat Depots:
Abdominal perimeter was higher in the NAFLD group (Control: 104 ± 10 vs. NAFLD: 119 ± 8 cm; P=0.0005). In addition, participants with NAFLD had increased total abdominal adipose tissue (Control: 436 ± 107 vs. NAFLD: 571 ± 118 cm2; P=0.01), as well as visceral fat (Control: 68 ± 32 vs. NAFLD: 119 ± 53 cm2; P=0.01). No differences were observed in total subcutaneous fat (Control: 368 ± 85 vs. NAFLD: 452 ± 120 cm2; P=0.08), or when separated by deep (Control: 168 ± 48 vs. NAFLD: 195 ± 50 cm2; P=0.2) and superficial (Control: 200 ± 60 vs. NAFLD: 257 ± 87 cm2; P=0.09) depots. Among all participants, total hepatic triglyceride content was positively related to total adipose tissue (r=0.48, P=0.012), deep subcutaneous adipose tissue (r=0.43, P=0.29), visceral adipose tissue (r=0.54, P=0.007), and abdominal perimeter (r=0.54, P=0.004).
Peripheral Insulin Resistance:
Participants with NAFLD had increased peripheral insulin resistance compared to controls (Figure 3, Panel A, P=5.0 × 10−5). In addition, increased insulin resistance was significantly related to methylene, methyl, α-methylene, allylic, and diallylic proton peaks (Fig 3, Panels B–F, all P > 0.05).
Fig 3:
Panel A: Average glucose metabolized (M) during 150–180 minutes of a euglycemic-hyperinsulinemic clamp in NAFLD (n=17) represented by closed circles, and controls (n=13) represented by open circles. Panel B: Correlation between log transformed methylene peak height and M. Panel C: Correlation between log transformed methyl peak height and M. Panel D: Correlation between log transformed α-methylene peak height and M. Panel E: Correlation between log transformed allyic peak height and M. Panel F: Correlation between log transformed diallylic peak height and M.
Lipid Profile:
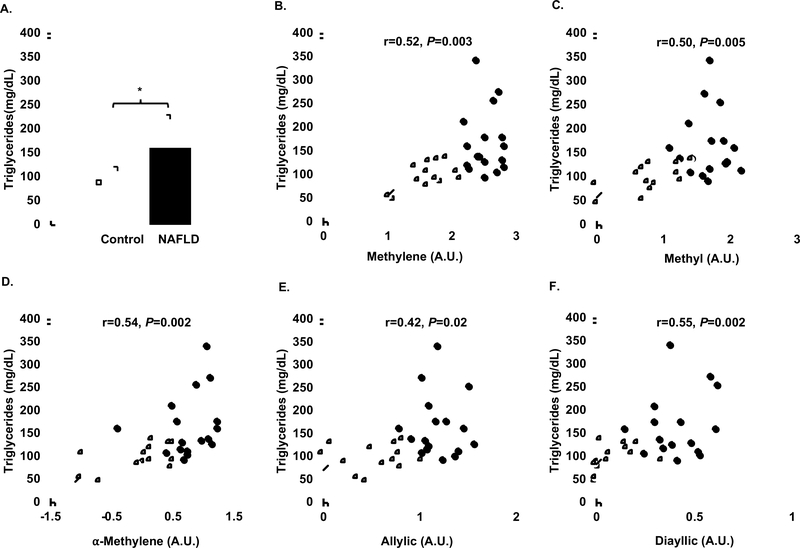
Triglycerides were elevated in the NAFLD group compared to controls (Fig 4, Panel A, P=0.002). In addition, triglycerides were positively related to methylene, methyl, α-methylene, allylic, and diallylic proton peaks (Fig 4, Panels B–F, all P > 0.05). Analogously, VLDL was positively related to methylene, methyl, α-methylene, allylic, and diallylic proton peaks (all P > 0.05).
Fig 4:
Panel A: Average serum triglycerides in NAFLD group (n=17) represented by closed circles compared to controls (n=13) represented by open circles. Panel B: Correlation between log transformed methylene peak height and triglycerides. Panel C: Correlation between log transformed methyl peak height and triglycerides. Panel D: Correlation between log transformed α-methylene peak height and triglycerides. Panel E: Correlation between log transformed allylic peak height and triglycerides. Panel F: Correlation between log transformed diallylic peak height and triglycerides.
Additional Correlations:
Absolute VO2 (L/min) was significantly correlated with polyunsaturation index (r=0.49, P=0.02).
DISCUSSION:
We found that participants with NAFLD had more hepatic lipids stored as saturated fats, and inversely, less lipids stored as unsaturated fats when assessed non-invasively by 1H-MRS, compared to control participants. These findings are in agreement with reports of direct assessment of human hepatic tissue. For example, lipidomic comparisons of hepatic biopsies from patients with liver disease versus controls consistently report increased saturated and monounsaturated fatty acid species, along with reduced polyunsaturated fatty acid species, when measured with gas liquid chromatography [7, 18], capillary gas chromatography [6], and mass spectrometry lipidomics [19]. While a low abundance of hepatic lipids creates some measure difficulties for in vivo non-invasive assessment, consistent findings between invasive and non-invasive methodologies supports the application of 1H-MRS scans for the study of study human liver disease. Furthermore, we found that this hepatic lipid profile was accompanied by hallmark characteristics of NAFLD including greater insulin resistance, low exercise capacity, increased total and visceral adipose tissue depots, and increased systemic triglycerides compared to controls. Our study extends previous work[10] by applying hepatic lipid subspecies assessments to a larger cohort of subjects with elevated body fat (46% vs. 36%) and hepatic fat (19% vs. 15%). In addition, we demonstrate that this potentially lipotoxic profile characterized by elevated saturated fats and low polyunsaturated fats, occurs in the presence of more dysfunctional whole-body metabolism.
Data showing more saturated lipids in NAFLD compared to controls is consistent with the view that NAFLD is a highly lipotoxic clinical condition. For example, saturated free fatty acids in circulation have been shown to play a causal role in hepatic lipotoxicity[20], and in vitro experiments demonstrate that saturated free fatty acids are toxic to hepatocytes, promoting apoptosis through ER stress[21]. These events clinically manifest as NASH, which is characterized by hepatocyte inflammation, cell death, and eventual liver failure[22]. The exact mechanisms regulating disease progression from NAFLD to NASH remain unknown, but given the role of saturated fats in promoting hepatic apoptosis, it is plausible that a higher degree of saturated fats increases patient susceptibility for NAFLD to NASH progression. Such findings would amplify the clinical relevancy of hepatic saturated lipid assessments, as its non-invasive nature allows for repeat measurements with low participant burden. Thus, the 1H-MRS technique for measuring hepatic lipid subspecies has the potential to fulfill an unmet need by providing a non-invasive biomarker to track hepatic disease progression from NAFLD to NASH.
NAFLD is characterized by insulin resistance in both the liver and skeletal muscle, often contributing to the simultaneous development of type 2 diabetes[2]. While the exact mechanisms of insulin resistance are still being uncovered, there is evidence supporting a link between fatty acid saturation of various bodily tissues and whole-body insulin action[23]. The large amount of saturated hepatic lipids in the NAFLD group, was accompanied by increased peripheral insulin resistance, measured with the gold-standard hyperinsulinemic-euglycemic clamp technique. In addition, we found significant relationships between insulin resistance and all proton peaks, with the strongest correlation observed with the methylene peak, which is primarily reflective of saturated fatty acids (r=−0.67, P=4.5×10−5). Although the 1H-MRS measurements used in this study do not distinguish specific fatty acid composition, these data corroborate the intriguing pattern that lipid saturation in bodily tissues is related to whole-body insulin action.
In addition to insulin resistance, another detrimental consequence of NAFLD is the ‘spillover’ of hepatic lipids into systemic circulation, i.e., dyslipidemia[24]. This is concerning because dyslipidemia further increases risk for developing cardiovascular disease in patients with NAFLD[25]. It has been hypothesized that high levels of saturated fat and low levels of hepatic polyunsaturated fat lead to reduced hepatic fatty acid oxidation and triacylglycerol export and increased triglyceride synthesis[26]. In support of this hypothesis, the increased levels of hepatic saturated fats observed in the NAFLD group occurred in the presence of significantly elevated levels of circulating triglycerides. In addition, we observed significant associations between circulating triglycerides and all proton peaks. Thus, these data support the notion that a hepatic lipid profile characterized by elevated saturated lipids is related to downstream metabolic consequences, including hypertriglyceridemia.
In conclusion, we found that participants with NAFLD preferentially store hepatic lipid in the form of saturated fat, at the expense of unsaturated fat, compared to adults who were age-matched, healthy, and leaner. This hepatic lipid profile is consistent with direct assessments of human liver tissue. These data extend our previous work by linking a lipotoxic profile characterized by high levels of saturated fats and low levels of polyunsaturated fats with whole-body metabolic dysfunction. Most notably, we observed a significant relationship between all proton peaks and insulin resistance, as well as hypertriglyceridemia. These findings support the hypothesis that saturated lipid may play a role in the pathogenesis of NAFLD and related co-morbidities. Whether the saturation index can be used as a non-invasive biomarker for tracking liver disease progression remains to be determined, but further research is warranted given the irreversible and potentially fatal health consequences of NAFLD.
HIGHLIGHTS.
Non-alcoholic fatty liver disease is characterized by excessive hepatic fat accumulation
Hepatic fats can be stored as saturated, unsaturated, or polyunsaturated lipids
Participants with NAFLD preferentially store excess fats as saturated lipids
This hepatic lipid profile is associated with whole-body metabolic dysfunction
Acknowledgments
FUNDING: This work was supported by the Clinical Translational Science Collaborative Awards UL1 RR024989-01 and UL1 RR024989-02 and NIH T32 HL007887-05.
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors of the enclosed manuscript [Melissa L. Erickson Ph.D., Jacob M. Haus Ph.D., Steven K. Malin Ph.D., Chris A. Flask Ph.D., Arthur J. McCullogh M.D., and John P. Kirwan Ph.D.] wish to confirm that there are no known conflicts of interest associated with the submitted manuscript entitled, “Non-Invasive Assessment of Hepatic Lipid Subspecies Matched with Non-Alcoholic Fatty Liver Disease Phenotype.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Weiss J, Rau M, Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Deutsches Arzteblatt international. 2014;111:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bril F, Cusi K. Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Call to Action. Diabetes care. 2017;40:419–30. [DOI] [PubMed] [Google Scholar]
- [3].McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. Journal of clinical gastroenterology. 2006;40 Suppl 1:S17–29. [DOI] [PubMed] [Google Scholar]
- [4].Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic Lipid Partitioning and Liver Damage in Nonalcoholic Fatty Liver. The Journal of biological chemistry. 2009;284:5637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert review of gastroenterology & hepatology. 2009;3:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2007;46:1081–90. [DOI] [PubMed] [Google Scholar]
- [7].Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clinical science (London, England : 1979). 2004;106:635–43. [DOI] [PubMed] [Google Scholar]
- [8].Clarke SD. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. American journal of physiology Gastrointestinal and liver physiology. 2001;281:G865–9. [DOI] [PubMed] [Google Scholar]
- [9].Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Alimentary pharmacology & therapeutics. 2006;23:1143–51. [DOI] [PubMed] [Google Scholar]
- [10].Johnson NA, Walton DW, Sachinwalla T, Thompson CH, Smith K, Ruell PA, et al. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology (Baltimore, Md). 2008;47:1513–23. [DOI] [PubMed] [Google Scholar]
- [11].Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. American journal of physiology Endocrinology and metabolism. 2005;288:E462–8. [DOI] [PubMed] [Google Scholar]
- [12].Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. The Journal of clinical endocrinology and metabolism. 2013;98:E1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology. 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- [14].Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, et al. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. Journal of applied physiology (Bethesda, Md : 1985). 2012;113:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. Journal of applied physiology (Bethesda, Md : 1985). 2006;100:1584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kelly KR, Navaneethan SD, Solomon TP, Haus JM, Cook M, Barkoukis H, et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Medicine and science in sports and exercise. 2014;46:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kullman EL, Kelly KR, Haus JM, Fealy CE, Scelsi AR, Pagadala MR, et al. Short-term aerobic exercise training improves gut peptide regulation in nonalcoholic fatty liver disease. Journal of applied physiology (Bethesda, Md : 1985). 2016;120:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elizondo A, Araya J, Rodrigo R, Poniachik J, Csendes A, Maluenda F, et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity (Silver Spring, Md). 2007;15:24–31. [DOI] [PubMed] [Google Scholar]
- [19].Gorden DL, Ivanova PT, Myers DS, McIntyre JO, VanSaun MN, Wright JK, et al. Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PloS one. 2011;6:e22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Progress in lipid research. 2013;52:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American journal of physiology Endocrinology and metabolism. 2006;291:E275–81. [DOI] [PubMed] [Google Scholar]
- [22].Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G852–8. [DOI] [PubMed] [Google Scholar]
- [23].Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of Fatty acids and insulin action. Annals of the New York Academy of Sciences. 2002;967:183–95. [DOI] [PubMed] [Google Scholar]
- [24].Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. European heart journal. 2011;32:1345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free radical biology & medicine. 2004;37:1499–507. [DOI] [PubMed] [Google Scholar]