Abstract
Study Objectives
To determine whether actigraphy-assessed indices of sleep are associated with cognitive performance in women, and explore whether these associations vary by race/ethnicity.
Methods
Participants were 1,126 postmenopausal community-dwelling females (mean age 65 years) from the observational Study of Women’s Health Across the Nation (SWAN); 25% were black, 46% white, 13% Chinese, 11% Japanese, and 5% Hispanic. Actigraphy-assessed sleep measures included total sleep time, wake after sleep onset (WASO), and fragmentation. Cognitive measures included immediate and delayed verbal memory, working memory, and information processing speed. All measures were assessed in conjunction with SWAN annual visit 15.
Results
Across the sample, after covariate adjustment, greater WASO and fragmentation were concurrently associated with slower information processing speed. Black participants had significantly worse sleep relative to other race/ethnic groups. Significant race/sleep interactions were observed; in black, but not white, participants, greater fragmentation was concurrently associated with worse verbal memory and slower information processing speed, and greater WASO was concurrently associated with slower information processing speed. Sleep-cognitive performance associations were not different in Chinese and Japanese participants relative to white participants.
Conclusions
Greater wakefulness and fragmentation during sleep are concurrently associated with slower information processing. Sleep continuity impacted concurrent cognitive performance in black, but not white, women. This effect may not have been detected in white women because their sleep was largely within the normal range. Future longitudinal studies in diverse samples are critical to further understand whether race/ethnicity moderates the influence of sleep on cognitive performance.
Keywords: actigraphy, cognitive function, sleep fragmentation, race, ethnicity, sleep duration, women, older adults
Statement of Significance.
Actigraphy-assessed wakefulness and fragmentation are concurrently associated with slower information processing speed in postmenopausal women. Race/ethnicity analyses revealed significant racial disparities; consistent with previous studies, black women had worse sleep relative to other races. Exploratory analyses of whether the sleep-cognitive performance relationship differed by race/ethnicity showed that overall, black women were more vulnerable to worse sleep relative to white women. Sleep-cognitive performance associations in Chinese and Japanese women were not different from white women, although results in these racial/ethnic groups may be limited by sample size and the greater magnitude of sleep impairment in black versus white women. Findings highlight the need for future work to disentangle determinants of racial disparities in the sleep-cognitive performance pathway, and interventions to reduce such disparities.
Introduction
As the population profile in the United States ages, the number of adults suffering from cognitive impairment is expected to rise precipitously, with estimates suggesting a doubling of the prevalence of dementia every 5–7 years [1]. Cognitive impairment and dementia are major contributors to morbidity and mortality in older adults [2, 3]. By virtue of their longer lifespan, women are disproportionately affected by dementia; indeed, more than two-thirds of Americans with dementia are women [4]. The menopausal transition is identified as a possible risk event that may interact with an underlying genetic vulnerability to significantly heighten the risk of cognitive impairment in women [5]. Indeed, findings from the Study of Women’s Health Across the Nation (SWAN), a large-scale, community-based cohort study of the menopause transition, have demonstrated that cognitive performance declines with age in midlife postmenopausal women [6]. Thus, it is critically important to identify potentially modifiable factors that influence cognitive functioning in women as they age.
Coincident with the rise in rates of cognitive impairment and dementia, sleep complaints increase with age. A recent study of SWAN participants showed a high prevalence of sleep complaints in postmenopausal women, with 45% of women reporting at least one problem with sleep [7]. Waking during the night was the most frequently endorsed sleep complaint, affecting 40% of women in this subgroup, followed by early morning awakenings (16%) and trouble falling asleep (15%) [7].
Sleep plays a substantial role in cognitive functioning across the lifespan. Importantly, a number of sleep parameters are modifiable and may, thus, represent a possible opportunity for intervention. Several cohort studies of older adults have examined relationships between behaviorally assessed indices of sleep (i.e. wrist actigraphy) and cognitive performance using both cross-sectional and prospective methodologies in exclusively female or mixed male/female samples. The Study of Osteoporotic Fractures (SOF), a female-only cohort study, found cross-sectional associations among poorer sleep efficiency, longer sleep onset latency, and more time spent awake during the night and poorer performance on a measure of gross cognitive function (Mini-Mental Status Exam) and executive functioning (Trail Making Test Part B) [8]. In a follow-up study with this cohort, after removing participants who had dementia, participants who spent the longest time awake after sleep onset (WASO) had lower delayed and total verbal recall on the California Verbal Learning Test and lower scores on the Trail Making Test Part B relative to those who spent the least amount of time awake [9]. A prospective examination of data across 5 years of SOF showed that the risk for developing Mild Cognitive Impairment or dementia in a sample of older women was significantly higher for women with the lowest sleep efficiency and those with a longer sleep onset latency, both of which are indices of sleep fragmentation (SF) [10]. Findings from the National Social Life, Health, and Aging Project (NSHAP) cohort, which enrolled both males and females, identified greater WASO, greater SF, and lower sleep efficiency as both cross-sectional and prospective (5 years later) correlates of impairment on a measure of global cognitive changes (Montreal Cognitive Assessment) [11]. SF was also linked to impairments in global cognitive functioning, working memory, semantic memory, visuospatial abilities, and perceptual speed, but not episodic memory, in a smaller cohort study of 700 men and women [12].
Although the results from these large-scale studies using actigraphy-assessed sleep measures suggest an association between sleep and cognitive performance in older adults, as noted in a recent review, more studies are needed to fully elucidate the relationship between objective sleep and cognitive functioning in older adults [13]. Importantly, the demographics of the extant literature limit the generalizability of these studies to normative aging across a diverse female population, including the older age of the participants (i.e. mean age > 80 years, with one exception [11]) and racial/ethnical homogeneity in the samples (i.e. > 80% white participants). The inclusion of a racially diverse population in studies of sleep and cognitive performance in women is particularly important, as relative to white women, black women are significantly more likely to experience sleep disturbances [14] and black adults have a twofold risk for developing dementia relative to white adults [15, 16].
Thus, in the present study, our goal was to build upon the existing literature by examining associations between behaviorally assessed sleep and cognitive performance in a comparatively younger and more racially/ethnically diverse sample of community-dwelling women, and to explore whether these associations differ by race/ethnicity. Studies exploring whether the associations between postmenopausal women’s sleep and cognitive performance differs by race/ethnicity are uncommon. Specifically, we measured cognitive performance within the domains of verbal memory, information processing speed, and working memory, and actigraphy was used to quantify three key indices of sleep: total sleep time (TST), WASO, and SF. We hypothesized that shorter sleep duration, longer time spent awake during the night, and greater SF would be associated with worse performance across all cognitive domains measured in the study. Further, we explored whether associations between sleep and cognitive performance differed by race/ethnicity.
Methods
Study design
Data for the present study were collected as part of the SWAN, a community-based, multisite longitudinal cohort study of women [17]. Participants provided written informed consent, and approval was obtained from Institutional Review Boards at each of the seven SWAN sites (Boston, MA; Chicago, IL; Detroit, MI; Davis and Los Angeles, CA; Newark, NJ; and Pittsburgh, PA). Each site enrolled white women and women of one other race/ethnicity (black women at four sites and Chinese, Hispanic, and Japanese women at one site each). Data for this cross-sectional analysis were collected at the SWAN follow-up visit 15, from 2015 to 2016. We chose this time point to (1) minimize the influence of menopause transition effects (all women were post-menopausal [18]) and (2) avoid practice effects on the cognitive tests. Practice effects on cognitive tests diminish with each retest, such that by the third testing occasion, practice effects should be minimal [6, 19]; most participants had taken the cognitive tests at least twice prior to SWAN follow-up visit 15.
Participants
Of the 3,302 women enrolled in SWAN at baseline, 2,091 (63.3%) participated in follow-up visit 15, and wrist actigraphy was collected on a subset of 1,217 (36.9%). At visit 15, all women were postmenopausal (defined as more than 12 months since their last menstrual bleed). Because of financial constraints as well as timing of the weeklong actigraphy study (see below) embedded in the context of the core protocol, not all women were asked to participate in the actigraphy portion of the protocol. Rather, each site was committed to recruit between 141 and 225 women, with minorities overrepresented. The overall percent of target enrolled averaged 83.7%, ranging from 72.3% to 91.4% by site. Participants who were wheelchair bound, blind, or who had plans to travel across time zones during the protocol period were excluded from the actigraphy protocol. To be included in the analysis for the present study, participants had to have at least 4 days of actigraphy data (N = 1,183). We excluded 47 women who were missing one of the cognitive tests and an additional 10 who were missing covariate information (education level and body mass index [BMI]), for a final analytical sample of 1,126. The Michigan, New Jersey, and Pittsburgh sites did not administer one of the cognitive tests (Rey Auditory Verbal Learning Test [RAVLT]); analyses involving this test were, therefore, completed with a subset of women (N = 687). As Hispanic women were enrolled only at the New Jersey site, the RAVLT results exclude all Hispanic women.
Sleep measures
Wrist actigraphy was utilized to behaviorally characterize sleep–wake patterns. Participants wore an Actiwatch-2 (AW-2, Philips Respironics, Bend, OR) on their nondominant wrist for seven consecutive days. Participants completed a daily diary in the morning and evening on each day of actigraphy. Participants were provided with instructions from study staff, which included a slide set presentation, on how to complete diaries; to press the actigraph event marker to indicate when they went to bed with the intention of going to sleep and when they rose from bed to start their day; and to wear the Actiwatch-2 device continuously. The evening diary included items regarding naps taken that day; the morning diary included items regarding when they got into bed, time they tried to go to sleep, length of time to fall asleep, number of awakenings during the night, length of time they were awake during the night, time when they woke for the day, time when they rose from bed to start their day, as well as hot flashes or night sweats during the night, use of medication for sleep that night, and overall sleep quality.
The AW-2 accelerometer was set at 0.05 g for 3–11 Hz. The analog signal was digitized by the digital integration method. The wake threshold was set at 40 counts/min and data were sampled in 1-min epochs. Data were processed, evaluated for quality, and scored at the University of Pittsburgh study site with the sleep diary “in-hand” in conjunction with the standard sleep detection algorithm in Actiware 5.0.9 (Philips Respironics), using procedures consistent with Society of Behavioral Sleep Medicine guidelines [20]. Start and stop times were initially detected by the Actiware algorithm, and hand-edited when needed to adjust misidentified sleep–wake times. Rest intervals that occurred within 30 min of a participants’ primary rest interval were incorporated into the primary rest interval. Actigraphy data were averaged across the measurement period. Measures derived from actigraphy used in the present study include TST, WASO (total minutes within the sleep period scored as wake), and the movement and fragmentation index. The movement and fragmentation index is the proportion of total sleep epochs which were scored as movement. Epochs were scored as movement if the number of activity counts recorded was greater than, or equal to, the epoch length in 15-s intervals [21]. Higher scores on this index indicate greater fragmentation of sleep and is an indicator of restlessness during sleep. Hereafter the movement and fragmentation index is referred to as SF.
Cognitive performance measures
Participants were able to choose between English and an alternative language in which the cognitive test battery was administered at the following sites: Los Angeles (Japanese); Davis (Chinese); and New Jersey (Spanish). Verbal episodic memory was tested using the RAVLT [22], completed by a subset of participants. Participants are read a list of 15 words and asked to recall as many of the words from the list as possible immediately (immediate recall) and again after a delay of approximately 20-min (delayed recall). The primary outcome is the number of words recalled immediately and after the delay; score range, 0–15. Information processing speed was assessed using the Symbol Digit Modalities Test (SDMT), a task in which participants are provided a master key from which they are to match as many numbers to symbols as possible within 90 s [23]. The score reflects the total number of accurate matches; range, 0–110. Working memory was measured by the digit span backwards (DSB) task, in which participants are read strings of single-digit numbers and asked to repeat the string backwards, with two trials at each string length, increasing from 2 to 7. The test is discontinued after errors in both trials at any string length [24]. The primary outcome for the DSB is the number of correct trials; score range, 0–12. Higher scores on all of the cognitive measures indicate better cognitive performance.
Demographic variables and covariates
Demographic variables included self-reported race/ethnicity (Black, Chinese, Hispanic, Japanese, or White), age, and education level. Covariates were selected based on a priori knowledge of demographic variables known to influence cognitive performance in the general population and previous SWAN investigations [6, 18] (i.e. age, education, race/ethnicity, and study site), as well as those who had significant unadjusted associations with the cognitive measures. We tested the following variables for possible inclusion as covariates: BMI (calculated as weight in kilograms divided by height in meters squared); current reported use of sleep apnea treatment; STOP-BANG [25] score of ≥ 3; presence of clinically significant depression (positive if score ≥ 16 on the Center for Epidemiological Studies Depression Scale [26]); current use of anxiolytics, antihistamines, or trazodone; proportion of nights used sleep medication per sleep diary; number of times each cognitive test had previously been taken; presence of any vasomotor symptoms per diary (i.e. hot flashes or night sweats); and number of health comorbidities. The number of health comorbidities was based on the number of self-reports of any of the following conditions: anemia, diabetes, hypertension, high cholesterol, migraines, osteoarthritis, hyper- or hypo-active thyroid, heart attack, angina, osteoporosis, cancer, pulmonary embolism, deep vein thrombosis, stroke, or congestive heart failure. The comorbidity variable was then trichotomized as 0, 1, or ≥2 conditions reported. The following variables were retained as covariates as they demonstrated an association with cognitive measures: BMI, depression, and number of health comorbidities. Thus, final adjusted models included the following covariates: age, education, race/ethnicity, study site, BMI, depression, and number of health comorbidities. All covariates were measured at visit 15, except for education, which was collected at baseline (1996–1997).
Statistical analyses
Statistical significance was defined at α < 0.05; all tests were two-tailed. Analyses were performed using RStudio version 1.1.456 [27]. Means and standard deviations (SDs) were calculated for continuous covariates and frequencies for categorical covariates in the full analytical sample and the subsample that took the RAVLT, which was not administered at the Michigan, New Jersey, and Pittsburgh sites. We examined the cognitive measures for skewness and found them all to be approximately normally distributed. Means and SDs were also calculated for each of the sleep predictors and cognitive measures overall and by race/ethnicity. We did not present results separately for Hispanic women due to their small number (n = 48, 4.3%). We used ANOVA and ANCOVA with Tukey post hoc adjustment (honestly significant difference; HSD) to determine if the sleep and cognitive measures, respectively, differed significantly by race/ethnicity. TST and WASO were converted to hours by dividing minutes of sleep or WASO by 60 to aid the interpretation of estimates. SF was log-transformed in all analyses due to its right skew. TST was treated as a continuous variable because there were few women with long sleep; only 5.33% had TST >8 h.
To assess the associations between sleep and cognitive performance, separate linear models regressed each cognitive outcome on each sleep measure, first unadjusted and then adjusted for race/ethnicity, site, education level, depressive symptoms, BMI, and number of comorbidities. A sensitivity analysis was completed for all models of the SDMT and DSB within the subsample that also had taken the RAVLT. As the results did not differ significantly from the analyses conducted in the larger sample, we present results from the larger sample where available.
Next, to explore whether the sleep/cognitive associations differed by race/ethnicity, we fit the adjusted models with interactions between each of the sleep measures and race/ethnicity, with white participants as the referent group. Due to the small number of Hispanic women in the full sample, we excluded Hispanic women from the race/ethnicity-specific analyses. For models with significant interactions, we calculated the race-specific associations between sleep and cognition from the adjusted interaction models and plotted the estimated associations to further explore the nature of the interaction.
Results
Sample characteristics
Reported race/ethnicity in the sample is as follows: 25% Black, 46% White, 13% Chinese, 11% Japanese, and 5% Hispanic. On average, participants were 65.4 years old (SD = 2.6 years) and most had completed at least some college when examined across the entire sample (see Table 1 for demographic characteristics for the entire sample and by race/ethnicity group). Very few (5%) used hormone therapy at the time of the study. Approximately half of the sample had two or more medical comorbidities.
Table 1.
Characteristics of participants at follow-up visit 15 (2015–2016) overall and by race/ethnicity in the SWAN
| Characteristic | Overall, N = 1,126 |
Race/ethnicity | P-value | ||||
|---|---|---|---|---|---|---|---|
| White, N = 523 |
Black, N = 286 |
Chinese, N = 143 |
Japanese, N = 126 |
Hispanic, N = 48 |
|||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 65.4 (2.6) | 65.4 (2.6) | 65.2 (2.6) | 65.7 (2.5) | 65.6 (2.7) | 66.1 (2.8) | 0.160a |
| BMI (kg/m2) | 28.7 (6.9) | 28.8 (6.4)2,3,4,5 | 32.8 (7.0)1,3,4 | 23.8 (3.7)1,2,5 | 23.5 (4.2)1,2,5 | 32.3 (6.6)1,3,4 | <0.001a |
| Days actigraphy | 7.9 (0.9) | 8.0 (0.9)3,4 | 8.0 (0.7)3,4 | 8.3 (0.8)1,2,4,5 | 7.2 (0.5)1,2,3,5 | 7.7 (1.2)3,4 | <0.001a |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Site | <0.001b | ||||||
| Michigan | 167 (14.8) | 73 (14.0) | 94 (32.9) | – | – | – | |
| Boston | 146 (13.0) | 95 (18.2) | 51 (17.8) | – | – | – | |
| Chicago | 147 (13.1) | 73 (14.0) | 74 (25.9) | – | – | – | |
| Davis | 204 (18.1) | 61 (11.7) | – | 143 (100.0) | – | – | |
| Los Angeles | 195 (17.3) | 69 (13.2) | – | – | 126 (100.0) | – | |
| New Jersey | 75 (6.7) | 27 (5.2) | – | – | – | 48 (100.0) | |
| Pittsburgh | 192 (17.1) | 125 (23.9) | 67 (23.4) | – | – | – | |
| Education level | <0.001b | ||||||
| Some high school | 51 (4.5) | 6 (1.1) | 9 (3.1) | 14 (9.8) | 0 (0.0) | 22 (45.8) | |
| High school | 169 (15.0) | 64 (12.2) | 57 (19.9) | 22 (15.4) | 19 (15.1) | 7 (14.6) | |
| Some college | 349 (31.0) | 143 (27.3) | 118 (41.3) | 29 (20.3) | 45 (35.7) | 14 (29.2) | |
| College | 256 (22.7) | 131 (25.0) | 44 (15.4) | 44 (30.8) | 34 (27.0) | 3 (6.2) | |
| Post-college | 301 (26.7) | 179 (34.2) | 58 (20.3) | 34 (23.8) | 28 (22.2) | 2 (4.2) | |
| Menopausal status | 0.002b | ||||||
| Natural post | 1,033 (91.8) | 487 (93.1) | 246 (86.3) | 135 (94.4) | 122 (96.8) | 43 (89.6) | |
| Surgical post | 92 (8.2) | 36 (6.9) | 39 (13.7) | 8 (5.6) | 4 (3.2) | 5 (10.4) | |
| Hormone user | 0.001b | ||||||
| No | 1,064 (94.5) | 480 (91.8) | 277 (96.9) | 142 (99.3) | 117 (92.9) | 48 (100.0) | |
| Yes | 62 (5.5) | 43 (8.2) | 9 (3.1) | 1 (0.7) | 9 (7.1) | 0 (0.0) | |
| Depressive symptoms (CES-D ≥ 16) | 0.234c | ||||||
| No | 1,004 (89.2) | 472 (90.2) | 254 (88.8) | 131 (91.6) | 106 (84.1) | 41 (85.4) | |
| Yes | 122 (10.8) | 51 (9.8) | 32 (11.2) | 12 (8.4) | 20 (15.9) | 7 (14.6) | |
| Comorbidities | <0.001c | ||||||
| 0 | 239 (21.2) | 124 (23.7) | 28 (9.8) | 48 (33.6) | 35 (27.8) | 4 (8.3) | |
| 1 | 280 (24.9) | 131 (25.0) | 66 (23.1) | 41 (28.7) | 34 (27.0) | 8 (16.7) | |
| ≥2 | 607 (53.9) | 268 (51.2) | 192 (67.1) | 54 (37.8) | 57 (45.2) | 36 (75.0) | |
| Testing language | <0.001b | ||||||
| English | 982 (87.2) | 523 (100.0) | 286 (100.0) | 92 (64.3) | 79 (62.7) | 2 (4.2) | |
| Non-English | 144 (12.8) | – | – | 51 (35.7) | 47 (37.3) | 46 (95.8) | |
CES-D = Center for Epidemiological Studies Depression Scale.
aANOVA.
bFisher’s exact test.
cChi-square test. Tukey’s HSD
1Different from white women.
2Different from black women.
3Different from Chinese women.
4Different from Japanese women.
5Different from Hispanic women.
Sleep and cognitive performance
Participants completed an average of nearly eight consecutive days of actigraphy (range = 4–16). After completion of visit 15, 91.3% of participants had taken the RAVLT test ≥ 3 times (mean = 2.9 ± 0.3), and 98.9% of participants had taken the SDMT and DSB ≥ 3 times (means = 7.2 ± 1.4 and 7.1 ± 1.5, respectively). Sleep and cognitive performance results are summarized across the sample and by race/ethnic group in Tables 2 and 3, respectively. All sleep variables differed by race/ethnic group per ANOVA; Tukey’s HSD tested differences by race/ethnic group (all ps < 0.05). White participants had the longest TST; between 0.5 and 0.7 h longer than the other groups. Black participants had the longest WASO (0.2 h longer than the other groups) and the greatest SF (between 5.9 and 6.5 points greater than the other groups). All cognitive variables differed by race/ethnic group after adjustment for age and education per ANCOVA; Tukey’s HSD tested differences by race/ethnic group (all ps < 0.05). Japanese participants had the highest scores on the RAVLT delayed recall (between 0.7 and 2 points higher than the other groups), RAVLT immediate recall (between 1.1 and 2.6 points higher than the other groups), and SDMT (between 3.5 and 11.5 points higher than the other groups). White participants had the highest score on DSB (between 0.2 and 1.5 points higher than the other groups).
Table 2.
Means and standard deviations of sleep measures overall and by race/ethnicity at follow-up visit 15 in the SWAN (N = 1,126)
| Race/ethnicity | ||||||
|---|---|---|---|---|---|---|
| Overall | White (A) | Black (B) | Chinese (C) | Japanese (D) | Tukey’s HSD* | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| TST (h) | 6.5 (1.0) | 6.8 (0.9) | 6.3 (1.0) | 6.2 (1.0) | 6.1 (1.0) | A > B, C, D |
| WASO (h) | 0.9 (0.4) | 0.8 (0.4) | 1.0 (0.4) | 0.8 (0.4) | 0.8 (0.3) | A, C, D < B |
| SF† | 32.2 (14.8) | 30.7 (15.6) | 36.6 (16.1) | 30.3 (10.8) | 30.1 (9.9) | A, C, D < B |
TST, total sleep time; WASO, wake after sleep onset; SF, sleep fragmentation (higher scores indicate more fragmentation).
*All measures differed by race/ethnicity (ANOVA p < 0.001). Differences between race/ethnic groups were determined by Tukey’s HSD tests p < 0.05. Race/ethnic groups are denoted by the letter in parentheses next to each group.
†SF was right-skewed; overall median = 29.7, overall interquartile range = 22.8–38.5.
Table 3.
Adjusted estimated means and standard error of cognitive measures overall and by race/ethnicity at follow-up visit 15 in the SWAN (N = 1,126)
| Race/ethnicity | ||||||
|---|---|---|---|---|---|---|
| Overall | White (A) | Black (B) | Chinese (C) | Japanese (D) | Tukey’s HSD* | |
| Mean (SD) | Mean (SD) | Mean (SE) | Mean (SD) | Mean (SD) | ||
| RAVLT-I† | 7.7 (0.08) | 8.0 (0.12) | 6.7 (0.18) | 7.2 (0.17) | 8.7 (0.18) | A, D > B, C; D > A |
| RAVLT-D† | 5.6 (0.09) | 5.8 (0.13) | 4.3 (0.20) | 5.2 (0.19) | 6.9 (0.20) | A, C, D > B; A, D > C; D > A |
| SDMT | 54.7 (0.32) | 56.5 (0.43) | 49.4 (0.58) | 57.5 (0.82) | 61.0 (0.87) | D > A, B, C; A, C > B |
| DSB | 6.7 (0.06) | 7.2 (0.09)2,5 | 5.7 (0.12)1,3,4 | 7.0 (0.17)2,5 | 6.7 (0.18)2,5 | A, C, D > B |
Estimated means adjusted for age and education. SE, standard error; RAVLT-I, Rey Auditory Verbal Learning Test, immediate recall; RAVLT-D, Rey Auditory Verbal Learning Test, delayed recall; SDMT, Symbol Digit Modalities Test; DSB, Digit Span Backwards; HSD, honestly significant difference.
*All measures differed by race/ethnicity (ANCOVA p < 0.05). Differences between race/ethnic groups were determined by Tukey’s HSD tests p < 0.05. Race/ethnic groups are denoted by the letter in parentheses next to each group.
† N = 687.
Results for the main sleep-cognitive performance models are presented in Table 4. Please see Supplementary Materials for tables with the following results: models with each covariate tested individually; models adjusted for base demographics (age, race/ethnicity, site, education—“base demographic model”); and the base demographic model adjusted for depression, BMI, and comorbidities individually. Prior to adjustment, longer TST was associated with better performance on DSB (β = 0.13, p = 0.044). Greater WASO was associated with lower immediate and delayed recall RAVLT scores (β = −0.62, p = 0.005; β = −0.59, p = 0.015, respectively). Greater SF was associated with lower immediate and delayed recall RAVLT scores (β = −0.68, p = 0.002; β = −0.61, p = 0.016, respectively), worse performance on SDMT (β = −4.85, p ≤ 0.001), and lower DSB scores (β = −0.59, p ≤ 0.001). However, after adjustment, only WASO (β = −1.85, p = 0.016) and SF (β = −1.99, p = 0.008) remained significantly associated with poorer performance on SDMT; there was a trend for the SF to be associated with lower immediate recall scores on the RAVLT (β = −0.39, p = 0.063).
Table 4.
Unadjusted and adjusted linear estimates regressing cognitive measures on sleep measures at follow-up visit 15 (2015–2016) in the SWAN
| RAVLT (immediate)* | RAVLT (delayed)* | SDMT† | DSB† | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| TST | ||||||||
| Unadjusted | 0.02 | 0.796 | 0.01 | 0.896 | 0.54 | 0.122 | 0.13 | 0.044 |
| (−0.15, 0.19) | (−0.17, 0.20) | (−0.14, 1.23) | (0.00, 0.27) | |||||
| Adjusted‡ | −0.001 | 0.993 | −0.002 | 0.984 | 0.18 | 0.547 | −0.01 | 0.903 |
| (−0.16, 0.16) | (−0.18, 0.17) | (−0.41, 0.78) | (−0.13, 0.12) | |||||
| WASO | ||||||||
| Unadjusted | −0.62 | 0.005 | −0.59 | 0.015 | −5.20 | <0.001 | −0.59 | <0.001 |
| (−1.06, −0.19) | (−1.07, −0.12) | (−6.91, −3.49) | (−0.92, −0.26) | |||||
| Adjusted‡ | −0.28 | 0.175 | −0.11 | 0.621 | −1.85 | 0.016 | −0.09 | 0.577 |
| (−0.68, 0.12) | (−0.55, 0.33) | (−3.35, −0.35) | (−0.41, 0.23) | |||||
| Log SF | ||||||||
| Unadjusted | −0.69 | 0.002 | −0.61 | 0.016 | −4.85 | <0.001 | −0.59 | <0.001 |
| (−1.14, −0.25) | (−1.10, −0.12) | (−6.52, −3.18) | (−0.92, −0.27) | |||||
| Adjusted‡ | −0.39 | 0.063 | −0.19 | 0.399 | −1.99 | 0.008 | −0.15 | 0.332 |
| (−0.80, 0.02) | (−0.64, 0.26) | (−3.45, −0.52) | (−0.47, 0.16) | |||||
TST, total sleep time; WASO, wake after sleep onset; SF, sleep fragmentation; RAVLT, Rey Auditory Verbal Learning Test; SDMT, Symbol Digit Modalities Test; DSB, Digit Span Backwards.
*N = 687.
† N = 1,126.
‡Adjusted for age, race/ethnicity, site, education, depression, BMI, and number of comorbidities.
Table 5 summarizes the results for the adjusted race/ethnicity interaction models; parameter estimates for significant models are plotted in Figure 1. When compared to white participants, in black participants, longer TST was associated with better performance on DSB (β = 0.38, p = 0.020), longer WASO was linked to worse SDMT performance (β = −3.92, p = 0.021), and SF was associated with worse performance on SDMT (β = −4.90, p = 0.004) and delayed recall RAVLT (β = −1.49, p = 0.015), with a trend for immediate recall RAVLT (β = −1.03, p = 0.063). Sleep-cognitive performance associations in Chinese and Japanese participants were not significantly different from white participants.
Table 5.
Adjusted linear regression estimates between sleep and cognitive measures excluding Hispanic women with interactions between race/ethnicity and sleep at follow-up visit 15 (2015–2016) in the SWAN
| RAVLT (immediate) (N = 687) | RAVLT (delayed) (N = 687) | SDMT (N = 1,078) | DSB (N = 1,078) | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Total sleep model | ||||||||
| Total sleep | 0.04 | 0.767 | −0.12 | 0.406 | 0.33 | 0.488 | −0.18 | 0.073 |
| (−0.22, 0.29) | (−0.39, 0.16) | (−0.60, 1.25) | (−0.38, 0.02) | |||||
| Total sleep * Race/ethnicity | ||||||||
| Total sleep * black | −0.21 | 0.393 | 0.01 | 0.973 | −0.48 | 0.526 | 0.38 | 0.020 |
| (−0.69, 0.27) | (−0.52, 0.54) | (−1.95, 1.00) | (0.06, 0.70) | |||||
| Total sleep * Chinese | −0.01 | 0.963 | 0.27 | 0.235 | 0.76 | 0.414 | 0.28 | 0.162 |
| (−0.42, 0.40) | (−0.18, 0.72) | (−1.06, 2.58) | (−0.11, 0.67) | |||||
| Total sleep * Japanese | −0.03 | 0.904 | 0.23 | 0.338 | 0.18 | 0.854 | 0.29 | 0.169 |
| (−0.46, 0.40) | (−0.24, 0.70) | (−1.73, 2.09) | (−0.12, 0.70) | |||||
| Total sleep * white | reference | reference | reference | reference | ||||
| WASO Model | ||||||||
| WASO | −0.16 | 0.609 | 0.06 | 0.865 | −0.02 | 0.983 | −0.16 | 0.502 |
| (−0.76, 0.45) | (−0.61, 0.72) | (−2.24, 2.19) | (−0.65, 0.32) | |||||
| WASO * Race/ethnicity | ||||||||
| WASO * black | −0.66 | 0.186 | −0.75 | 0.171 | −3.92 | 0.021 | 0.11 | 0.771 |
| (−1.64, 0.32) | (−1.82, 0.32) | (−7.27, −0.58) | (−0.62, 0.83) | |||||
| WASO * Chinese | 0.05 | 0.936 | 0.14 | 0.819 | −4.32 | 0.088 | −0.03 | 0.956 |
| (−1.06, 1.15) | (−1.07, 1.35) | (−9.29, 0.64) | (−1.11, 1.05) | |||||
| WASO * Japanese | 0.47 | 0.508 | 0.04 | 0.955 | 1.76 | 0.590 | 1.04 | 0.141 |
| (−0.92, 1.85) | (−1.47, 1.56) | (−4.64, 8.16) | (−0.35, 2.43) | |||||
| WASO * White | reference | reference | reference | reference | ||||
| Log SF Model | ||||||||
| Log SF | −0.19 | 0.540 | 0.16 | 0.632 | −0.38 | 0.716 | −0.05 | 0.832 |
| (−0.79, 0.41) | (−0.50, 0.82) | (−2.40, 1.65) | (−0.49, 0.39) | |||||
| Log SF * Race/ethnicity | ||||||||
| Log SF * black | −1.03 | 0.063 | −1.49 | 0.015 | −4.90 | 0.004 | −0.40 | 0.289 |
| (−2.13, 0.06) | (−2.68, −0.29) | (−8.27, −1.53) | (−1.13, 0.34) | |||||
| Log SF * Chinese | −0.20 | 0.719 | −0.07 | 0.907 | −3.13 | 0.208 | −0.17 | 0.756 |
| (−1.30, 0.90) | (−1.27, 1.13) | (−8.00, 1.74) | (−1.23, 0.89) | |||||
| Log SF * Japanese | 0.31 | 0.618 | −0.31 | 0.649 | 0.45 | 0.871 | 0.36 | 0.553 |
| (−0.91, 1.53) | (−1.64, 1.02) | (−5.01, 5.91) | (−0.83, 1.55) | |||||
| Log SF * White | Reference | reference | reference | reference | ||||
TST, total sleep time; WASO, wake after sleep onset; SF, sleep fragmentation; RAVLT, Rey Auditory Verbal Learning Test; SDMT, Symbol Digit Modalities Test; DSB, Digit Span Backwards. All models adjusted for age, site, education, depression, BMI, and number of comorbidities.
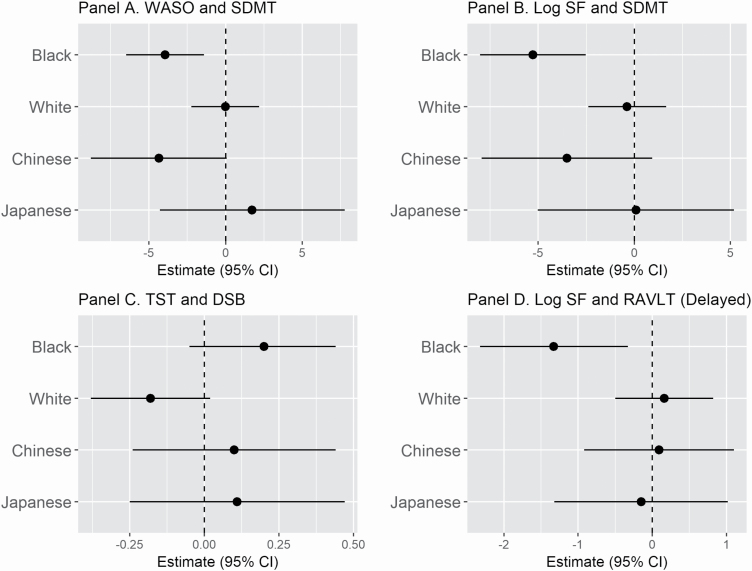
Figure 1.
Adjusted race-specific linear associations between sleep and cognitive measures from models with significant interactions between sleep and race/ethnicity. All models adjusted for age, site, education, depressive symptoms, BMI, and number of comorbidities. WASO, wake after sleep onset; SF, sleep fragmentation; TST, total sleep time; RAVLT, Rey Auditory Verbal Learning Test; SDMT, Symbol Digit Modalities Test; DSB, Digit Span Backwards. (A) Negative estimate = greater WASO, lower SDMT. (B) Negative estimate = greater SF, lower SDMT. (C) Positive estimate = longer TST, greater DSB. (D) Negative estimate = greater SF, lower RAVLT.
For models with significant race/ethnicity interactions, we also examined race-specific associations for black and white participants to further assess the nature of the interactions. Although the TST-DSB model estimates were not significant for both black (β = 0.20, p = 0.122) and white participants (β = −0.18, p = 0.073), the interaction was significant due to the opposing direction of the estimates. The WASO–SDMT estimate was significant for black participants (β = −3.95, p = 0.002), with longer WASO associated with poorer SDMT performance, and nonsignificant for white participants (β = −0.02, p = 0.983). The SF-SDMT estimate was significant for black participants (β = −5.28, p ≤ 0.001), such that more SF was associated with poorer SDMT performance, and nonsignificant for white participants (β = −0.38, p = 0.716). The SF-delayed recall RAVLT estimate was significant for black participants (β = −1.33, p = 0.009), with more SF linked to worse delayed recall on the RAVLT and non-significant in white participants (β = 0.16, p = 0.632). The SF-immediate recall RAVLT estimate was significant for black participants (β = −1.22, p = 0.009), such that more SF was associated with worse immediate recall on the RAVLT, and non-significant for white participants (β = −0.19, p = 0.540).
Discussion
In this study of actigraphy-assessed sleep and cognitive performance in a large cohort of racially and ethnically diverse community-dwelling older women, we found that more disruption in the sleep continuity measures of SF and time spent awake during the night was concurrently associated with poorer cognitive performance, specifically in the domain of information processing speed, with a signal for the possible impact of SF on immediate verbal memory. However, our other hypotheses were not supported within the group as a whole after we adjusted for possible covariates; specifically, TST was not associated with cognitive performance, and none of the actigraphy-assessed indices of sleep duration and fragmentation evaluated in the present report were associated with working memory or delayed verbal memory.
We also explored whether associations between actigraphy-assessed sleep and cognitive performance differed by race/ethnicity. Due to relatively small samples in some of the race/ethnic subgroups studied (i.e. Japanese and Chinese), our analyses were necessarily exploratory due to power limitations. Sleep-cognitive performance associations in Japanese and Chinese women were not different from those in white women. However, relative to white women, worse sleep in black women was associated with poorer cognitive performance in a manner largely consistent with the hypotheses we had tested in the entire sample: greater SF was linked to worse performance in verbal memory and information processing speed, and more wakefulness during the night was associated with slower information processing speed. In most cases, these interaction effects were due to significant associations in black women and nonsignificant associations in white women, which may be attributed to notably worse sleep in black women relative to white women.
Previous SWAN investigations have found that: poor sleep (shorter duration, lower sleep efficiency, and greater WASO) was linked to higher levels of inflammatory markers in black, but not white, women [28]; and that black women have lighter sleep per polysomnography as evidenced by decreased EEG power in the δ band and increased EEG power in the β band during NREM sleep [29], which could represent pathways by which sleep influences cognitive functioning in black women. However, the exact mechanisms for why sleep would be associated with cognitive functioning in black, but not white, women remain unknown. Black women in our sample had worse sleep relative to white participants, consistent with other investigations of racial disparities in sleep in the SWAN cohort [14, 29]. It is possible that we were able to detect the effects of sleep continuity on cognitive performance in black women only because they had worse sleep relative to other groups, perhaps falling below a threshold important for cognitive performance. Sleep and cognitive performance are both strongly influenced by the complex socioeconomic and cultural factors which underlie racial disparities, such as poor sleeping environment, limited access to health care, and less adequate, poorer quality of treatment for diseases known to affect cognitive functioning (e.g. hypertension and diabetes), which could underlie our findings [15].
Our results, which require confirmation by longitudinal studies, have significant implications for public health. Ensuring that black women have equal access to high-quality health care is fundamentally important; moreover, specialized programs to support sleep health, including strategies to improve sleep continuity and increase sleep duration, as well as early identification and evidence-based treatment for sleep disturbances, targeted to, and tailored for, the black population, are crucial to reducing these disparities. Such interventions could be informed by previous SWAN work, which showed that health problems, vasomotor symptoms, waist circumference, number of stressful events, and financial hardship mediated differences in sleep continuity in black women versus white women [14]. Ultimately, future work to more fully disentangle these relationships is vitally important to our understanding of the determinants of these racial disparities in the sleep-cognitive performance pathway and to inform public health strategies.
Overall, our results suggest that sleep continuity (SF and WASO) is concurrently associated with the cognitive domain of information processing speed in community-dwelling older women; performance in the other domains we assessed was not reliably associated with actigraphy-assessed indices of sleep in SWAN participants. As a longitudinal SWAN study of cognitive aging found that processing speed was the most sensitive to age-related changes [6], it may be that only those tests capable of detecting more subtle changes would detect sleep-related impairment in this cognitively intact, younger cohort. Consistent with our findings, SF [11, 12] and related sleep measures (i.e. low sleep efficiency and more time spent awake during the night [8–10]) have been consistently associated with lower cognitive function in older cohorts of women. In contrast, only one previous study has found associations with TST as measured by actigraphy [9]. It is possible that inconsistent findings between studies examining TST and cognitive performance are due to discrepancies in how TST is measured; for example, no relationship was shown when sleep duration was measured via polysomnography [30], whereas relationships were found between sleep duration and cognitive performance in studies using daily self-report [31] and questionnaire methodologies [32]. Our results further bolster the theory that sleep continuity is more closely linked to impairments in cognitive performance compared to sleep duration [8]. Future studies in the sleep-cognitive functioning domain should include measures of sleep continuity to ensure that this putative mechanism is captured along with more established measures of risk for cognitive impairment including education [33], race [15, 16], and history of major depression [34].
Potential mechanisms linking sleep continuity and worse cognitive performance include impaired synaptic plasticity caused by disruption of neuronal pathways (e.g. GABA and cAMP), and greater amyloid-β accumulation and the promotion of inflammation due to poor sleep [35]. Support for sleep continuity as a mechanism for this pathway in humans is found in recent studies: greater actigraphically measured WASO was associated with amyloid deposition in a sample of cognitively intact middle-aged adults [36], and the intensity of actigraphy-assessed sleep continuity in cognitively intact adults mediated the relationship between fronto-hippocampal hypometabolism (brain regions most affected by aging and Alzheimer’s disease) and poorer executive functioning [37]. Our study utilized a cross-sectional design and therefore precludes conclusions regarding the temporal relationship between sleep and cognitive performance. Importantly, as the relationship between sleep and cognitive performance is bidirectional [36, 38, 39]—sleep may influence cognitive performance, and cognitive decline may also lead to sleep disturbances (e.g. many diseases that cause cognitive decline also negatively affect sleep), it is possible that our findings are capturing sleep problems caused by diseases of cognitive decline and not vice versa.
Whereas strengths of the study include a large sample of women who are postmenopausal, yet younger and more racially/ethnically diverse than the other samples studied with actigraphy-assessed sleep measures and objective quantification of cognitive function, limitations of the present study in addition to the cross-sectional design must be acknowledged. First, our exclusively female sample limits the generalizability of our findings for males. Second, our sample included too few women who self-identified as Hispanic to allow us to examine ethnic differences in this subgroup; further, the site which enrolled Hispanic women did not administer the verbal memory measure, and Hispanic women were therefore excluded from analyses in this cognitive domain. Similarly, we had relatively small numbers of women who self-identified as Japanese or Chinese, so the absence of findings in these subgroups may be due to limited power to detect differences.
In summary, in this study of racially/ethnically diverse, postmenopausal, community-dwelling women in the United States, actigraphy-assessed sleep continuity was concurrently associated with information processing speed, a dimension of cognitive performance highly sensitive to age-related changes. Racial disparities were observed, wherein poor sleep was significantly, concurrently associated with worse cognitive performance in black women relative to white women, with poorer sleep linked to worse verbal memory, working memory, and information processing speed. Future studies of these associations should enroll diverse samples of older adults large enough to examine race/ethnic effects more broadly (e.g. using large samples of black, Japanese, Chinese, and Hispanic women), as results from samples with a majority of white participants may obscure important relationships relevant to other racial/ethnic groups. Other potential avenues for future work include longitudinal studies to disentangle factors that influence racial disparities in the sleep-cognitive performance pathway, to examine the directionality and temporal associations, and to assess whether interventions, including increasing access to sleep health care for underserved populations, reduce these disparities.
Supplementary Material
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Supplementary material
Supplementary material is available at SLEEP online.
Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). Other sources of funding for this publication include K23HL122461 (Swanson), K24HL123565 (Thurston), and R01AG053838 (Joffe).
Clinical centers
University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office
National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory
University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center
University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee
Susan Johnson, Current Chair; Chris Gallagher, Former Chair. We thank the study staff at each site and all the women who participated in SWAN.
Disclosure statements
Financial disclosure: Dr Joffe is a recipient of grants from Merck, Pfizer, Que-Oncology, and NeRRe/KaNDy; she is a consultant for NeRRe/KaNDy, Merck, Sojournix, Eisai, Jazz Pharmaceutical. Dr Joffe’s spouse is a consultant for Arsenal Biosciences and holds equity in Arsenal Biosciences and Tango and Iomx. Dr Thurston is a consultant for Astellas, Pfizer, Procter & Gamble, and Virtue Health.
Non-financial disclosure: none.
References
- 1. Prince M, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2. [DOI] [PubMed] [Google Scholar]
- 2. Bassuk SS, et al. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151(7):676–688. [DOI] [PubMed] [Google Scholar]
- 3. Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 4. Prince, Martin et al. World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends; 2015.
- 5. Riedel BC, et al. Age, APOE and sex: triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karlamangla AS, et al. Evidence for cognitive aging in midlife women: study of women’s health across the nation. PLoS One. 2017;12(1):e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kravitz HM, et al. Sleep trajectories before and after the final menstrual period in the Study of Women’s Health Across the Nation (SWAN). Curr Sleep Med Rep. 2017;3(3):235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackwell T, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–410. [DOI] [PubMed] [Google Scholar]
- 9. Spira AP, et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8). doi: 10.1093/sleep/zsx073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diem SJ, et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McSorley VE, et al. Associations of sleep characteristics with cognitive function and decline among older adults. Am J Epidemiol. 2019;188(6):1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim AS, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5):633–640b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brewster GS, et al. Sleep and cognition in community-dwelling older adults: a review of literature. Healthcare. 2015;3(4):1243–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matthews KA, et al. Racial/ethnic disparities in women’s sleep duration, continuity, and quality, and their statistical mediators: Study of Women’s Health Across the Nation. Sleep. 2019;42(5). doi: 10.1093/sleep/zsz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, et al. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y). 2018;4:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajan KB, et al. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sowers M, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Lobo R, Marcus R, Kelsey J, eds. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000:175–188. [Google Scholar]
- 18. Greendale GA, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171(11):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartels C, et al. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ancoli-Israel S, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13Suppl 1:S4–S38. [DOI] [PubMed] [Google Scholar]
- 21. Mezick EJ, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt M Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 23. Smith A Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Service; 1982. [Google Scholar]
- 24. Wechsler D Wechsler Adult Intelligence Scale-III. San Antonio, CA: Psychological Corporation, Harcourt, Brace & Company; 1997. [Google Scholar]
- 25. Chung F, et al. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638. [DOI] [PubMed] [Google Scholar]
- 26. Radloff LS The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27. RStudio. Integrated Development for R [computer program]. Boston, MA: RStudio, Inc.; 2015. [Google Scholar]
- 28. Matthews KA, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women’s Health across the Nation sleep study. Sleep. 2010;33(12):1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall MH, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson DA, et al. Greater cognitive deficits with sleep-disordered breathing among individuals with genetic susceptibility to Alzheimer disease. The Multi-Ethnic Study of Atherosclerosis. Ann Am Thorac Soc. 2017;14(11):1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gamaldo AA, et al. Exploring the within-person coupling of sleep and cognition in older African Americans. Psychol Aging. 2010;25(4):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Virta JJ, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36(10):1533–41, 1541A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu W, et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53(5):3113–3123. [DOI] [PubMed] [Google Scholar]
- 34. Diniz BS, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yaffe K, et al. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 36. Ju YE, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. André C, et al. Brain and cognitive correlates of sleep fragmentation in elderly subjects with and without cognitive deficits. Alzheimers Dement (Amst). 2019;11:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brzecka A, et al. Sleep disorders associated with Alzheimer’s disease: a perspective. Front Neurosci. 2018;12:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin YQ, et al. RBD: a red flag for cognitive impairment in Parkinson’s disease? Sleep Med. 2018;44:38–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.