Abstract
Study Objectives
More than half of young adults at risk for alcohol-related harm report symptoms of insomnia. Insomnia symptoms, in turn, have been associated with alcohol-related problems. Yet one of the first-line treatments for insomnia (Cognitive Behavioral Therapy for Insomnia or CBT-I) has not been tested among individuals who are actively drinking. This study tested (1) the feasibility and short-term efficacy of CBT-I among binge-drinking young adults with insomnia and (2) improvement in insomnia as a predictor of improvement in alcohol use outcomes.
Methods
Young adults (ages 18–30 years, 75% female, 73% college students) who met criteria for Insomnia Disorder and reported 1+ binge drinking episode (4/5+ drinks for women/men) in the past month were randomly assigned to 5 weekly sessions of CBT-I (n = 28) or single-session sleep hygiene (SH, n = 28). All participants wore wrist actigraphy and completed daily sleep surveys for 7+ days at baseline, posttreatment, and 1-month follow-up.
Results
Of those randomized, 43 (77%) completed posttreatment (19 CBT-I, 24 SH) and 48 (86%) completed 1-month follow-up (23 CBT-I, 25 SH). CBT-I participants reported greater posttreatment decreases in insomnia severity than those in SH (56% vs. 32% reduction in symptoms). CBT-I did not have a direct effect on alcohol use outcomes; however, mediation models indicated that CBT-I influenced change in alcohol-related consequences indirectly through its influence on posttreatment insomnia severity.
Conclusions
CBT-I is a viable intervention among individuals who are actively drinking. Research examining improvement in insomnia as a mechanism for improvement in alcohol-related consequences is warranted.
Trial Registration
U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT03627832, registration #NCT03627832
Keywords: alcohol, insomnia, sleep, treatment, mechanism
Statement of Significance.
In this randomized pilot trial of binge-drinking young adults with insomnia, Cognitive Behavioral Therapy for Insomnia (CBT-I) was associated with greater reductions in insomnia symptoms than single-session sleep hygiene. Change in insomnia symptoms at posttreatment also mediated CBT-I effects on alcohol-related consequences at 1 month. Findings suggest that CBT-I is a viable treatment for young adults with insomnia who are actively drinking and may have downstream effects on alcohol-related problems.
Introduction
Insomnia disorder affects 6%–15% of the general population [1], with rates as high as 58% among those with alcohol use disorder (AUD) [2]. It is associated with a range of medical and psychiatric comorbidities, including depression and suicidal ideation [3], and creates a burden on the healthcare system [4]. Although cognitive behavioral therapies (CBT) are widely accepted as an important component of insomnia treatment [5], research to date has not examined the efficacy of CBT for insomnia (CBT-I) among individuals who are actively drinking. Indeed, abstinence (or a goal of abstinence) is often considered a prerequisite for insomnia treatment among individuals with AUD [2]. This creates a challenge for insomnia treatment among young adults, approximately 30% of whom report binge drinking (5+ drinks in one occasion) [6] and alcohol-related problems [7], but few of whom perceive a need for abstinence or alcohol treatment [8]. Unfortunately, insomnia treatment may be critical for binge-drinking young adults because sleep disturbance is a predictor of future alcohol use and related problems [9–14]. If sleep disturbance is indeed a contributor to alcohol-related problems, then treatment of insomnia among individuals who have not yet developed severe AUD could theoretically alter the trajectory of the disorder [15].
A number of biological and behavioral factors may contribute to the association between sleep and alcohol-related outcomes. Among individuals with AUD and insomnia, rates of alcohol use as a sleep aid are relatively high (55%) [16]. However, young adults tend to report more social than coping motives for alcohol use [17, 18], in which case this mechanism may not explain the association between sleep and alcohol outcomes in young adults. One alternative explanation involves the effect of sleep on neurocognitive functioning. Specifically, impairments in sleep may lead to impairments in higher order cognitive functioning (working memory performance [19]) and decreased ability to regulate emotions [20, 21]. These impairments, in turn, may decrease one’s ability to avoid substance use or problems related to use. Indeed, sleep disturbance has been linked prospectively to alcohol use and related problems among adolescents and young adults [9–14]. However, only one study has examined the extent to which the reverse association may be true; that is, the extent to which treatment of sleep problems may mitigate alcohol-related harm among young adults. That study did not find significant differences between the sleep intervention and control condition [22]; however, it also tested a novel (rather than empirically supported) treatment and did not target individuals with insomnia. Thus, research to date has not examined the efficacy of a first-line insomnia treatment among young adults who are actively drinking.
As a first step in determining the potential for insomnia treatment to mitigate alcohol-related harm among young adults, this study tested the feasibility and short-term efficacy of CBT-I among binge-drinking young adults with insomnia. First, because CBT-I has not been tested within this population, we examined the feasibility and acceptability of a five-session, face-to-face CBT-I protocol. Second, because CBT-I is efficacious among individuals in recovery from AUD [23–25] and young adults who do not engage in binge drinking [26], we hypothesized that CBT-I would be more effective than single-session sleep hygiene (SH) education in reducing insomnia symptoms among binge-drinking young adults with insomnia. Finally, based on research linking sleep disturbance to subsequent alcohol use and related problems [9–14, 22], we hypothesized that improvement in insomnia would lead to improvements in drinking and related problems (i.e. improvement in insomnia would mediate CBT-I effects on alcohol use outcomes).
Methods
Participants and procedure
Power analysis
Based on previous studies, we expected moderate to large effects on insomnia symptoms [27] and small to moderate effects on alcohol use [23, 24]. A priori power analyses (G*Power 3.1.9) indicated that 44 participants would provide sufficient power to detect moderate (f = 0.25) within-between interactions using repeated-measures ANOVA (α = 0.05, groups = 2, repetitions = 3, correlation = 0.50). We utilized multilevel modeling to increase power to detect small effects. Research examining CBT-I among young adults who do not engage in binge drinking [26] reported 15% posttreatment attrition. Anticipating higher attrition in a binge-drinking sample, we aimed to recruit 56 participants to obtain a final sample of 44.
Recruitment
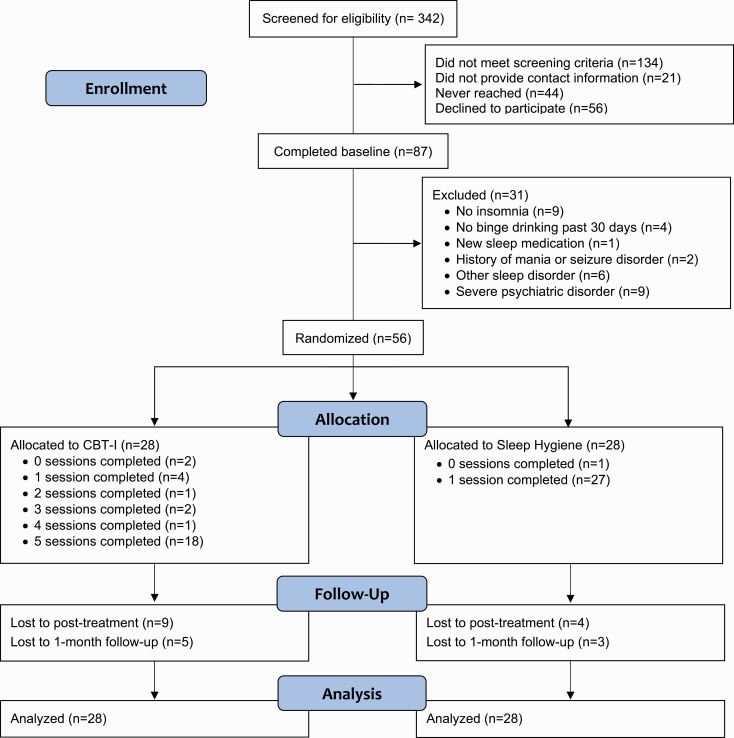
Figure 1 depicts participant recruitment and retention. Young adults (age 18–30 years) in a Midwestern college town were recruited through community advertising (flyers, university email, and Facebook). Advertisements stated, “We are looking for men and women between the ages of 18 and 30 who drink alcohol to participate in an insomnia treatment study.” Recruitment ran August 2018 through June 2019. Interested individuals (n = 342) completed an online screening survey. Research staff contacted those who met screening criteria (n = 208) to provide them with additional information and schedule the baseline assessment. Of those, 56 (27%) declined to participate, primarily due to time constraints. Eighty-seven participants completed the in-person baseline assessment.
Figure 1.
CONSORT flow diagram.
Participant enrollment/eligibility
At baseline, participants provided written informed consent, completed a clinician-administered sleep assessment and the MINI International Neuropsychiatric Interview for DSM-5 (MINI) [28] with a trained assessor, and completed baseline measures. They also wore an actiwatch and completed online sleep diaries for at least 7 days. Individuals were eligible to participate if they (1) were between 18 and 30 years; (2) reported consumption of 4/5+ drinks (for women/men) on a single occasion at least once in the past 30 days1; and (3) met research and DSM-5 criteria for Insomnia Disorder. Criteria for insomnia included >30 minutes falling asleep, staying asleep, or waking up too early on 3+ nights per week for 3+ months [29] and daytime impairment, operationalized as scores ≥10 on the Insomnia Severity Index [30]. Participants were not excluded for use of sleep medication; however, to avoid confounds between CBT-I and medication effects, participants had to be stabilized on sleep medications for 6+ weeks at baseline. Participants were excluded if they reported contraindications for CBT-I (mania or seizure disorder), reported other symptoms requiring immediate clinical attention (e.g. severe sleep apnea/PTSD or suicidal intent), or were receiving treatment for insomnia or alcohol use.
Of the 87 baseline participants, 56 met all inclusion criteria. Individuals who were excluded from the study did not differ from those included in terms of age, sex, race/ethnicity, college status, sleep parameters, drinking quantity, or alcohol-related consequences. Participant demographics are presented in Table 1.
Table 1.
Group demographics at baseline (n = 56)
| Full sample (n = 56) | CBT-I (n = 28) | Sleep hygiene (n = 28) | |
|---|---|---|---|
| Demographic characteristics | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) |
| Age | 22.4 (2.7) | 22.3 (2.7) | 22.5 (2.8) |
| Female (vs. male) | 42 (75%) | 22 (79%) | 20 (71%) |
| Race* | – | – | – |
| European American | 51 (91%) | 26 (93%) | 25 (89%) |
| African American | 6 (11%) | 2 (7%) | 4 (14%) |
| Asian American | 2 (4%) | 1 (4%) | 1 (4%) |
| Nat. Am. or Nat. Al. | 3 (5%) | 2 (7%) | 1 (4%) |
| Nat. Haw. or Pac. Isl. | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 1 (2%) | 0 (0%) | 1 (4%) |
| Hispanic/Latinx | 2 (4%) | 2 (7%) | 0 (0%) |
| Highest level of education | – | – | – |
| Grade 12 or GED | 3 (5%) | 2 (7%) | 1 (4%) |
| Some college/tech. school | 36 (64%) | 19 (68%) | 17 (61%) |
| College graduate | 17 (30%) | 7 (25%) | 10 (36%) |
| Current college enrollment | 41 (73%) | 21 (75%) | 20 (71%) |
| Use of any sleep medication | 12 (21%) | 3 (11%) | 9 (32%) |
| Trazodone | 1 (2%) | 0 (0%) | 1 (4%) |
| Doxylamine | 2 (4%) | 0 (0%) | 2 (7%) |
| Melatonin | 5 (9%) | 1 (4%) | 4 (14%) |
| OTC (e.g. diphenhydramine) | 8 (14%) | 2 (7%) | 6 (21%) |
| Motives for substance use | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) |
| Social motives† | 3.44 (0.91) | 3.41 (0.93) | 3.48 (0.92) |
| Anxiety coping motives† | 2.26 (0.91) | 2.33 (1.01) | 2.19 (0.81) |
| Depression coping motives† | 1.62 (0.79) | 1.70 (0.92) | 1.54 (0.65) |
| Enhancement motives† | 2.81 (0.98) | 2.94 (1.07) | 2.69 (0.88) |
| Conformity motives† | 1.44 (0.73) | 1.55 (0.87) | 1.33 (0.55) |
| Alcohol to help with sleep | 7 (13%) | 2 (7%) | 5 (18%) |
| Cannabis to help with sleep | 9 (16%) | 6 (21%) | 3 (11%) |
| MINI diagnoses | n (%) | n (%) | n (%) |
| AUD | 47 (84%) | 25 (89%) | 22 (79%) |
| Moderate AUD | 14 (25%) | 9 (32%) | 5 (18%) |
| Severe AUD | 5 (9%) | 4 (14%) | 1 (4%) |
| Substance use disorder (SUD) | 5 (9%) | 1 (4%) | 4 (14%) |
| Moderate SUD | 4 (7%) | 1 (4%) | 3 (11%) |
| Severe SUD | 0 (0%) | 0 (0%) | 0 (0%) |
| Comorbid diagnosis‡ | 24 (43%) | 14 (50%) | 10 (36%) |
MINI, MINI International Neuropsychiatric Interview DSM-5; OTC, over-the-counter.
*Not mutually exclusive.
†From the Modified Drinking Motives Questionnaire Revised.
‡Comorbid diagnoses included current major depressive disorder, panic disorder, agoraphobia, social anxiety disorder, obsessive-compulsive disorder, PTSD, bulimia, and generalized anxiety disorder.
Randomization, assessment, and compensation
Participants were randomly assigned in parallel design to CBT-I (n = 28) or SH (n = 28; see Figure 1). M.B.M. generated random allocation (1:1 ratio) using a random number generator. After the 5-week treatment period (“posttreatment”) and 1 month after posttreatment, participants completed follow-up assessments, which included 7+ days of actigraphy and daily sleep diaries. Participants were compensated for each assessment ($20 for baseline, $25 for post, and $25 for follow-up), with additional compensation based on percent compliance with daily diaries (an additional $5 at baseline and an additional $15 at post and follow-up).
Blinding
The principal investigator and study therapists were blinded to assessment outcomes, and outcome assessors were blinded to participant condition. All participants were informed that they received treatment for insomnia (either “brief” or “more intense”) in order to blind them to condition assignment.
Interventions
Cognitive behavioral therapy for insomnia
A five-session, individual CBT-I manual was derived from existing, efficacious protocols [31, 32]. Session 1 introduced treatment rationale and SH recommendations (see later section). Session 2 targeted sleep restriction (limiting time in bed) and stimulus control (using bed/bedroom only for sleep). Session 3 presented relaxation techniques (diaphragmatic breathing, progressive muscle relaxation, and visual imagery). Session 4 targeted cognitive therapy (thoughts logs and behavioral experiments). Session 5 reviewed treatment rationale/progress and discussed prevention of future insomnia episodes. Sleep diaries and treatment adherence were reviewed in all sessions.
Sleep hygiene
A single-session SH control condition was chosen to model “usual care.” Similar to the sleep information that participants may receive during routine primary care visits, SH participants received a one-page handout of SH practices listed on the National Sleep Foundation (2018) website. Recommendations included limiting naps, avoiding caffeine and nicotine before bedtime, moderating alcohol use before bedtime, exercising, avoiding foods that disrupt sleep before bedtime, exposing oneself to natural sunlight, establishing a bedtime routine, and creating a pleasant sleep environment (Table 2). Therapists reviewed the handout with participants and answered questions.
Table 2.
Percentage of treatment days that CBT-I and SH participants complied with SH and partial stimulus control recommendations (n = 49)
| Recommendation | CBT-I (n = 25) | Sleep hygiene (n = 24) | t(df) |
|---|---|---|---|
| Limit naps to 30 min | 92.3 (9.0) | 90.0 (9.0) | −1.16 (47) |
| Limit caffeine to 3 (8 oz) doses | 95.7 (13.7) | 93.5 (9.8) | −0.63 (47) |
| Avoid caffeine after 12 p | 66.1 (29.4) | 64.2 (24.8) | −0.24 (47) |
| Avoid nicotine within 2 h of bedtime | 96.9 (11.8) | 98.7 (3.1) | 0.74 (47) |
| Avoid alcohol within 2 h of bedtime | 92.6 (12.4) | 87.3 (20.5) | −1.09 (47) |
| Avoid exercise† within 2 h of bedtime | 99.4 (1.7) | 98.3 (3.3) | −1.54 (47) |
| Avoid heavy meals within 2 h of bedtime | 92.4 (7.8) | 85.4 (13.7) | −2.18 (36‡)* |
| Avoid bright light before bedtime | 63.0 (35.0) | 63.3 (32.2) | 0.03 (47‡) |
| Engage in relaxing bedtime routine | 53.8 (33.7) | 53.8 (33.7) | 0.72 (47) |
| Get out of bed during awakenings | 46.3 (30.4) | 37.0 (30.3) | −1.08 (47) |
†Defined as at least moderate-intensity aerobic activity.
‡Equal variances not assumed.
*p < 0.05.
Alcohol intervention
Neither intervention was modified to target alcohol use. However, participants in both conditions reviewed the general SH recommendation to limit alcohol consumption before bedtime. Moreover, because daily diaries included an item assessing number of standard alcoholic drinks consumed, participants in both conditions self-monitored alcohol consumption throughout the trial.
Treatment integrity
Both treatments were delivered in person by predoctoral clinical/counseling psychology students. Participant interactions were supervised by a licensed clinical psychologist (M.B.M.), who was supervised by a clinical psychologist board certified in behavioral sleep medicine (C.S.M.). Treatment integrity was assured in three steps:[33] (1) interventionists received initial training via mock therapy sessions, and sessions were audiotaped and reviewed in ongoing training and supervision; (2) participants received a workbook of treatment materials; and (3) completion of assignments and barriers to compliance were reviewed each week.
Measures
Treatment satisfaction
Participants rated their satisfaction with treatment during the posttreatment assessment. The Client Satisfaction Questionnaire [34] is an eight-item measure that has been validated in substance use treatment settings [35]. Items are scored from 1 to 4, with higher scores indicating greater satisfaction. For example, response options for the item, “How would you rate the quality of treatment you received?” ranged from 1 (poor) to 4 (excellent).
Insomnia severity
Insomnia severity was measured at baseline, posttreatment, and 1-month follow-up using the seven-item Insomnia Severity Index [36]. The first three items assess the severity of insomnia symptoms (problems falling asleep, staying asleep, and waking too early). Remaining items assess dissatisfaction with sleep, distress, noticeability, and daily interference. Responses range 0–4, with higher scores indicating more severe insomnia.
Sleep diary
Participants completed 7+ consecutive days of sleep diaries at baseline, posttreatment, and 1-month follow-up. To control for assessment reactivity, participants in both conditions were also instructed (but not incentivized) to complete daily diaries for the 5-week treatment phase of the study. The study sleep diary included all elements of the consensus sleep diary [37]. Participants estimated what time they got into bed, what time they tried to go to sleep (bedtime), how long it took them to fall asleep (sleep onset latency [SOL]), total duration of nighttime awakenings (wake after sleep onset [WASO]), time of their final awakening (waketime), and what time they got out of bed for the day. Sleep efficiency (range 0%–100%) was calculated by dividing the amount of time actually spent sleeping by the amount of time spent in bed. Participants rated their subjective sleep quality on a scale from 0 (very poor) to 4 (very good).
In order to help CBT-I interventionists identify behaviors that could negatively affect sleep, sleep diaries also included several items assessing compliance with recommendations. Participants reported the timing and duration of any daytime naps; the number of caffeine doses consumed (e.g. 1 oz espresso, 8 oz coffee, and 12 oz soda) and what time they finished their last caffeinated drink; the number of full cigarettes consumed and what time they finished their last cigarette; and the number of standard alcoholic drinks consumed (e.g. 12 oz beer), the approximate time they started drinking, and the approximate time they finished their last alcoholic drink. They also indicated (yes/no) if they engaged in 30+ min of moderate-intensity aerobic activity (and, if so, what time they finished exercising); if they consumed a heavy meal within 2 h of bedtime; if they avoided bright light in the 30 min before bedtime; and if they did something routine or relaxing in the 30 min before bedtime. Due to an oversight by the principal investigator, participants in both groups who reported waking up during the night were also asked to indicate if they had gotten up out of bed and returned to bed only when sleepy (i.e. if they had complied with partial stimulus control instructions). These data are reported in Table 2 for descriptive purposes.
Actigraphy
The Actiwatch Spectrum Plus (Philips Respironics) was used to measure sleep objectively. Actigraphy has been validated against polysomnography as an accurate measure of WASO, total sleep time, and sleep efficiency among individuals with insomnia [38, 39]. Data were analyzed in 30-s epochs using medium sensitivity settings. Because actigraphy is less reliable in estimating the major sleep period among individuals with insomnia, sleep diaries were used in conjunction with actigraphy to estimate the times that participants got into and out of bed [40]. Diaries were used in lieu of event markers to reduce participant burden. Diary data were available for the majority (96%) of cases; however, if diary data were not available, algorithm-generated start and end times for the sleep period were visually inspected for consistency with light and activity data. In all but one case, the algorithm-generated start/end time was used.
Drinking quantity
Past-month drinking quantity was measured using the Daily Drinking Questionnaire (DDQ) [41]. Participants indicated how many standard drinks (e.g. 12 oz beer) they consumed on each day of a typical week in the past month. Responses were summed to estimate the number of drinks consumed per week.
Alcohol-related consequences
Alcohol-related consequences were measured using the 24-item Brief Young Adult Alcohol Consequences Questionnaire [42]. Participants indicated (yes/no) if they had experienced events such as having a hangover or missing work/class in the past month. Responses were summed to create a total score.
Substance use motives
Participants’ motives for alcohol use were measured at baseline using the 28-item Modified Drinking Motives Questionnaire Revised (MDMQ-R) [17]. The MDMQ-R includes five subscales assessing social (e.g. “as a way to celebrate”), anxiety coping (e.g. “to relax”), depression coping (e.g. “to numb my pain”), enhancement (e.g. “because I like the feeling”), and conformity (e.g. “to fit in with a group I like”) motives for alcohol use. Participants indicated how often they consume alcohol for each of the specified reasons on a scale from 1 (almost never/never) to 5 (almost always/always). Subscale scores were obtained by summing relevant items. Internal consistency for the social, depression-coping, enhancement, and conformity subscales were in the good to excellent range (α = 0.81–0.94); and reliability for the anxiety-coping subscale was in the acceptable range (α = 0.76). Two additional items assessing (yes/no) use of (1) alcohol and (2) cannabis to help with sleep were included on the daily sleep diaries. Substance use motives are reported in Table 1 for descriptive purposes.
Data screening and analysis
Missing data
A total of 13 participants were missing data at either the posttreatment (n = 13) or follow-up (n = 8) assessments. Linear multiple regression with 20 imputations was used to estimate missing outcome values for primary outcomes [43, 44]. Predictors for imputation models included study variables (treatment group and baseline levels of the outcome), baseline variables correlated with missingness (insomnia severity, race, diary SOL, negative affect, and dysfunctional sleep beliefs), and baseline variables associated with drinking/sleep in other studies (sex, education, college enrollment, age, drinking quantity, cigarette use, PTSD symptoms, and anxiety symptoms).
Data analysis
All analyses were conducted in SPSS version 25. Multilevel models were used because they account for nesting within data and require fewer assumptions than general linear models [45]. Unconditional models were conducted to determine the intraclass correlation coefficient (ICC) for each outcome. For primary outcomes, ICCs indicated that 39% of variance in insomnia severity, 45% of variance in actigraphy sleep efficiency, 69% in diary sleep quality, 78% in drinking quantity, and 65% in alcohol-related consequences occurred between individuals (Level 2). Remaining variance occurred within individuals over time (Level 1).
Separate models were conducted for each outcome (Table 3). For each model, time points (Level 1) were nested within persons (Level 2). Treatment condition (CBT-I = 1; SH = 0) was included as a fixed effect at Level 2. Demographic variables (e.g. sex) were not included as covariates because participants were randomized. For the model predicting alcohol-related consequences, baseline drinking quantity was included as a Level 2 covariate and was grand mean centered to capture between-person variability [46]. Intercepts were specified as random to allow for individual differences in mean levels of each outcome at baseline. All other effects were fixed. Pairwise comparisons were examined, using Bonferroni adjustment to control for inflation in Type I error (α = 0.05/5 = 0.01). Effect sizes were calculated using Cohen’s d (0.20 small, 0.50 medium, and 0.80 large). To be consistent with insomnia research reporting guidelines [29], descriptive data for subjective and objective measures of commonly reported sleep variables are included in Table 4.
Table 3.
Descriptive and inferential statistics for primary sleep and alcohol use outcomes (n = 56)
| Baseline | Post | 1 Month | Group | Time | G × T | Cohen’s d (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | F | F | BL to Post | BL to 1 mo | |
| Insomnia severity | – | – | – | – | – | – | 5.19* | 149.40* | 9.73* | 1.20 | 0.60 |
| CBT-I | 16.43 | 4.06 | 7.15WB | 4.02 | 6.82W | 4.01 | (0.64, 1.76) | (0.08, 1.13) | |||
| Sleep hygiene | 15.93 | 2.92 | 10.74W | 3.37 | 9.25W | 4.62 | |||||
| Actigraphy SE | – | – | – | – | – | – | 0.01 | 0.28 | 2.42† | 0.50 | −0.01 |
| CBT-I | 76.73 | 6.33 | 78.27 | 6.79 | 75.92 | 9.59 | (−0.04, 1.03) | (0.00, 0.09) | |||
| Sleep hygiene | 77.99 | 6.54 | 76.07 | 6.98 | 77.29 | 7.21 | |||||
| Diary sleep quality | – | – | – | – | – | – | 0.10 | 41.72* | 4.05* ‡ | −0.73 | −0.04 |
| CBT-I | 1.98 | 0.58 | 2.22W | 0.65 | 2.47W | 0.63 | (0.23, 1.27) | (0.00, 0.26) | |||
| Sleep hygiene | 1.83 | 0.54 | 2.35W | 0.60 | 2.34W | 0.53 | |||||
| Drinks per week | – | – | – | – | – | – | 0.09 | 14.34* | 0.14 | −0.08 | −0.13 |
| CBT-I | 12.14 | 6.02 | 10.84 | 8.36 | 8.99W | 7.17 | (−0.45, 0.60) | (−0.39, 0.66) | |||
| Sleep hygiene | 13.11 | 8.40 | 11.38 | 9.20 | 9.28W | 8.91 | |||||
| Consequences§ | – | – | – | – | – | – | 2.05 | 20.90* | 2.71 | 0.67 | 0.21 |
| CBT-I | 8.29 | 6.50 | 4.25W | 5.86 | 4.90W | 5.25 | (0.16, 1.20) | (0.00, 0.72) | |||
| Sleep hygiene | 5.93 | 3.61 | 4.52 | 4.30 | 3.33W | 3.03 |
Cohen’s d interpreted as 0.20 small, 0.50 medium, and 0.80 large (negative effect size indicates change in favor of the control group). 1 mo, 1 month follow-up; BL, baseline; CI, confidence interval; G × T, group by time interaction; Post, posttreatment; SE, sleep efficiency.
*p < 0.05.
†Discrepant from nonimputed data (F = 3.71, p = 0.03).
‡Discrepant from nonimputed data (F = 1.60, p = 0.21).
§Model controlled for between-person variability in drinks per week at baseline.
WSignificant (p ≤ 0.01) within-group change from baseline.
BSignificant (p ≤ 0.01) between-group difference at that time point.
Table 4.
Descriptive statistics for commonly reported sleep variables (n = 56)
| Baseline | Posttreatment | 1 month | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Diary SOL | – | – | – | – | – | – |
| CBT-I | 39.50 | 27.26 | 15.81 | 8.50 | 15.90 | 7.45 |
| Sleep hygiene | 41.34 | 22.41 | 22.55 | 15.69 | 24.05 | 17.64 |
| Diary WASO | – | – | – | – | – | – |
| CBT-I | 19.96 | 17.83 | 13.71 | 14.58 | 11.23 | 2.56 |
| Sleep hygiene | 21.36 | 18.41 | 13.03 | 16.05 | 11.22 | 2.25 |
| Diary TST | – | – | – | – | – | – |
| CBT-I | 8.47 | 1.28 | 7.61 | 0.92 | 8.12 | 0.96 |
| Sleep hygiene | 8.53 | 1.80 | 8.42 | 1.57 | 8.40 | 1.15 |
| Diary SE | – | – | – | – | – | – |
| CBT-I | 88.82 | 7.09 | 93.79 | 3.64 | 94.66 | 2.71 |
| Sleep hygiene | 88.60 | 5.34 | 93.15 | 4.62 | 93.82 | 2.97 |
| Actigraphy SOL | – | – | – | – | – | – |
| CBT-I | 48.83 | 30.85 | 28.99 | 19.61 | 36.45 | 19.32 |
| Sleep hygiene | 47.68 | 29.38 | 50.48 | 33.03 | 40.79 | 19.13 |
| Actigraphy WASO | – | – | – | – | – | – |
| CBT-I | 47.41 | 16.61 | 41.66 | 12.30 | 43.16 | 18.16 |
| Sleep hygiene | 45.14 | 15.90 | 38.21 | 15.38 | 44.42 | 21.85 |
| Actigraphy TST | – | – | – | – | – | – |
| CBT-I | 7.02 | 0.83 | 6.60 | 0.81 | 6.70 | 0.89 |
| Sleep hygiene | 7.06 | 0.83 | 6.63 | 1.17 | 7.01 | 0.78 |
Reports based on all available data (not imputed). SE, sleep efficiency; SOL, sleep onset latency (min); TST, total sleep time (h); WASO, wake after sleep onset (min).
Mediation was tested using bootstrapped confidence intervals for indirect effects in the PROCESS 2.04 macro [47, 48]. Posttreatment insomnia symptoms were modeled as a mediator of the association between treatment group and 1-month alcohol-related consequences, controlling for baseline insomnia severity, alcohol-related consequences, and drinks per week. Because they limit assumptions and Type I error while increasing power to detect effects in smaller samples [49, 50], asymmetric 95% bootstrap confidence intervals with 5,000 sampling estimates were used to test indirect effects.
Results
Treatment feasibility and acceptability
Follow-up rates are depicted in Figure 1. Treatment compliance with SH and partial stimulus control instructions is reported for the 88% of participants (25 CBT-I and 24 SH) who completed treatment diaries (Table 2). At posttreatment (n = 43), CBT-I participants (M = 28.58, SD = 2.97) reported greater satisfaction with treatment than SH participants (M = 21.71, SD = 5.05), t(41) = 5.78, p = 0.02. A larger proportion of CBT-I than SH participants also agreed with the statements, “The techniques I learned are logical treatments for insomnia” (100% vs. 79%), “I am confident that the techniques I learned will improve my insomnia” (100% vs 71%), and “I would recommend this treatment to other people with insomnia” (100% vs 54%). Thus, CBT-I was associated with greater treatment credibility and participant satisfaction than SH. A smaller proportion of CBT-I than SH participants still met study criteria for insomnia (average diary SOL or WASO >30 min and ISI ≥10) at both posttreatment (7% vs 29%; χ2 = 4.38, p = 0.04) and 1-month follow-up (4% vs 21%; χ2 = 4.08, p = 0.04).
Primary outcomes
Sleep outcomes
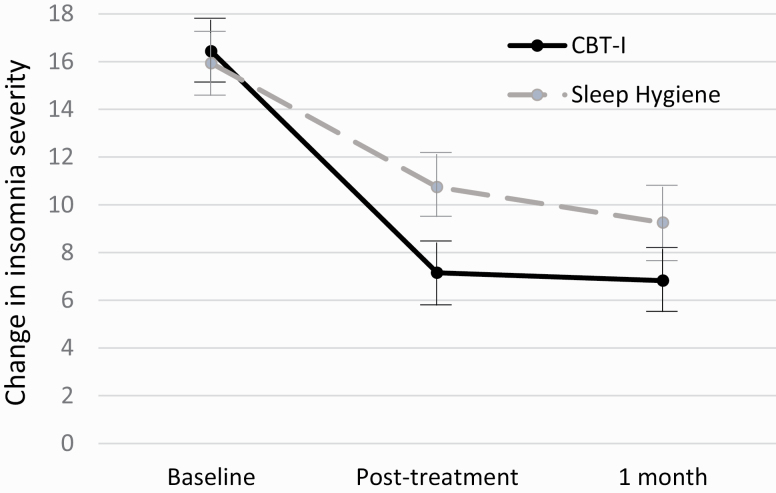
Descriptive and inferential statistics for all primary sleep outcomes are depicted in Table 3. In the prediction of insomnia severity, there was a significant group by time interaction (Table 3 and Figure 2). From baseline to posttreatment, participants in the CBT-I group reported greater decreases in insomnia severity (−9.61, SE = 0.84, p < 0.001) than those in the SH group (−6.68, SE = 0.84, p < 0.001; d = 1.20); but neither group changed significantly from posttreatment to 1-month follow-up. There were no significant main or interaction effects for actigraphy-assessed sleep efficiency. For sleep quality, there was a significant group by time interaction (Table 3). Both groups reported improved sleep quality from baseline to posttreatment (CBT-I +0.49, SE = 0.09, p < 0.001; SH +0.51, SE = 0.09, p < 0.001), and the CBT-I group reported continued improvements from posttreatment to 1-month follow-up (CBT-I +0.25, SE = 0.09, p = 0.01; SH −0.01, SE = 0.09, p = 0.89).
Figure 2.
Group change in insomnia severity over time (n = 56), with error bars reflecting 95% CIs.
Alcohol use outcomes
Descriptive and inferential statistics for alcohol use outcomes are also depicted in Table 3. In the prediction of drinking quantity, there was a significant effect of time: both CBT-I (−3.15, SE = 0.92, p = 0.001) and SH groups (−3.82, SE = 0.92, p < 0.001) reported decreases in drinking quantity from baseline to 1-month follow-up.2
In the prediction of alcohol-related consequences, the group by time interaction failed to reach statistical significance (p = 0.07); however, there was a significant effect of time (Table 3). Participants in the CBT-I condition reported a significant decrease in consequences from baseline to posttreatment (−4.03, SE = 0.81, p < 0.001) that was maintained at 1 month, while participants in the SH condition reported decreases from baseline to 1 month (−2.60, SE = 0.68, p < 0.001).
Secondary analyses: indirect (mediated) treatment effects
Change in insomnia symptoms were examined as a mediator of the association between treatment and change in drinking quantity. CBT-I was associated with greater posttreatment decreases in insomnia severity (a = −3.85, SE = 0.85; 95% CI = [−5.56, −2.14]). However, posttreatment change in insomnia symptoms was not significantly associated with 1-month change in drinking quantity (b = 0.26, SE = 0.22; 95% CI = [−0.17, 0.70]). CBT-I did not have a significant total (0.69, SE = 1.34, p = 0.61; 95% CI = [−2.00, 3.38]), direct (c = 1.70, SE = 1.58; 95% CI = [−1.46, 4.87]), or indirect (ab = −1.01, 1.02; 95% CI = [−3.27, 0.75]) effect on change in 1-month drinking quantity.
This model was then replicated examining change in insomnia symptoms as a mediator of the association between treatment and change in alcohol-related consequences, controlling for baseline levels of drinking. CBT-I was associated with greater posttreatment decreases in insomnia severity (a = −4.30, SE = 0.86; 95% CI = [−6.03, −2.57]), and posttreatment change in insomnia symptoms was significantly associated with 1-month change in alcohol-related consequences (b = 0.35, SE = 0.13; 95% CI = [0.08, 0.61]). CBT-I did not have a significant total (0.36, SE = 0.86, p = 0.68; 95% CI = [−1.32, 2.08]) or direct effect (c = 1.85, SE = 0.99; 95% CI = [−0.14, 3.84]) on change in 1-month alcohol-related consequences. However, CBT-I had an indirect (mediated) effect on alcohol-related consequences through its influence on insomnia symptoms (ab = −1.49, SE = 0.58; 95% CI = [−2.79, −0.53]).
Sensitivity analyses
We conducted sensitivity analyses to examine the impact of noncompliance and data imputation on primary outcomes. First, because time spent in treatment may impact treatment outcomes, we reanalyzed all models controlling for number of treatment sessions completed. Completing more treatment sessions was associated with greater improvements in insomnia severity (B = −1.23, SE = 0.24; 95% CI = [−1.71, −0.75]), actigraphy-assessed sleep efficiency (B = 2.08, SE = 0.49; 95% CI = [1.09, 3.07]), and alcohol-related consequences (B = −0.88, SE = 0.35; 95% CI = [−1.59, −0.17]). It was not significantly associated with change in sleep quality (B = 0.07, SE = 0.05; 95% CI = [−0.04, 0.17]) or drinks per week (B = −0.38, SE = 0.72; 95% CI = [−1.83, 1.06]). Inclusion of this covariate did not change the pattern of results for any time by group interaction (as depicted in Table 3). To determine if results differed when using an alternative method for handling missing data, we also replicated analyses without imputing data. Again, treatment effects were largely consistent (see discrepancies noted in Table 3).
Discussion
CBT-I outperformed SH in reducing insomnia symptoms, which in turn were associated with reductions in alcohol-related problems among young adults at risk for alcohol-related harm. This suggests not only that CBT-I is effective in reducing insomnia symptoms, but also that improvements in insomnia may have downstream effects on alcohol-related problems. However, one in four participants (27%) who screened eligible for the study declined to participate, and only 64% of CBT-I participants completed the entire treatment protocol. Thus, a five-session, in-person treatment may not be feasible for all young adults who engage in binge drinking. In this study, participants frequently cited being “too busy” as their reason for disinterest or treatment drop-out. Although posttreatment effects tend to be smaller, CBT-I has demonstrated efficacy when delivered by phone [51] or internet [52]. Thus, these modalities and shorter protocols may be considered in future treatment or clinical settings.
On average, at the end of treatment, CBT-I participants reported a 56% reduction in insomnia severity, whereas those receiving SH reported 32% reduction. This represented a large treatment effect (Cohen’s d = 1.20), so we were surprised that CBT-I did not have stronger effects on other measures of sleep impairment. While the change was not statistically significant (and, therefore, should be interpreted with caution), CBT-I seemed to have a moderate effect on actigraphy-assessed sleep efficiency at posttreatment relative to SH. Interestingly, participants in both groups reported increases in sleep quality at posttreatment, with SH participants reporting nonsignificantly larger increases in sleep quality than CBT-I participants. We speculate that we did not find stronger CBT-I effects due in part to this unexpected level of sleep improvement among SH participants.
SH education is not recommended as a stand-alone treatment for insomnia [53] and tends to have significantly smaller effects than CBT-I [54]. However, in this study, participants in both groups reviewed SH recommendations every day while completing sleep diaries. Moreover, due to an oversight, SH (as well as CBT-I) participants were prompted on daily diaries to indicate if they had gotten out of bed during nighttime awakenings. While participants in both conditions reported relatively low compliance with this recommendation (~30%), it seems likely that diaries prompted SH participants to engage in partial stimulus control, as the diaries were the only part of the study that provided this instruction to SH participants. Moreover, research in both the sleep [55] and alcohol [56] fields has found that daily monitoring of behavior may impact these behaviors, at least in the short-term. Thus, daily self-monitoring may have facilitated improvements in insomnia in both treatment groups.
While we did not find a significant treatment effect on alcohol-related consequences, improvements in insomnia were associated with reductions in alcohol-related consequences. The potential for insomnia treatment to influence alcohol-related consequences has significant implications for the prevention and treatment of problematic alcohol use among young adults. Individuals tend to report a preference for insomnia treatment relative to other mental health treatment (e.g. alcohol use, depression, and PTSD) [57, 58]. Moreover, the majority of heavy drinkers do not seek help for alcohol use, even if they report problems [8]. Thus, identifying other forms of treatment that either influence alcohol outcomes or open the door to alcohol-related treatment is critical. The results of this study indicate that insomnia treatment may facilitate improvements in alcohol-related outcomes and, therefore, may be an ideal first step toward treatment among binge-drinking young adults with insomnia.
Limitations
This study tested the efficacy of CBT-I in a high-risk sample that is likely to be generalizable, given the limited number of exclusion criteria. It also examined change in insomnia as a potential mechanism for improvement in alcohol-related problems. This is novel methodologically and has high potential impact; however, there were limitations to the study design. First, we compared CBT-I to the least stringent control condition that was ethical to use (single-session SH). While this design is appropriate for the mechanistic trials that this study aims to inform [59], we did not compare CBT-I to a treatment matched for time and content; therefore, observed results may be attributable in part to nonspecific therapy effects (e.g. more therapy hours in CBT-I). Also, because CBT-I was more credible than SH and associated with greater participant satisfaction, it is possible that placebo effects accounted in part for treatment outcomes. Future studies may determine if there is something unique to CBT-I (vs other treatments or placebo) that elicits change in sleep and alcohol-related outcomes within this population. Additional research is also needed to examine the duration of treatment effects, as outcomes reported here were limited to 1 month.
Certain aspects of our sample also limit generalizability. For example, the sample was relatively small and comprised primarily of women and European Americans. Given the diversity of young adults in the United States, research examining the generalizability of these results among more diverse groups is needed. Finally, although a large number of participants met criteria for AUD, the majority of cases (28/47; 60%) were mild, and only 5 reported experiencing 2+ withdrawal symptoms in the past year. Moreover, participants reported primarily social (as opposed to coping) motives for alcohol use, and only 7 (13%) reported using alcohol to help with sleep. Individuals with more severe AUD and those going through alcohol withdrawal may have different motives for alcohol use, and alcohol may have different physiological effects on sleep in this population [60]. As such, research examining the extent to which findings may generalize to individuals in treatment for moderate to severe AUD is needed. Although use of alcohol as a sleep aid was uncommon in this sample, future research may also examine the extent to which use of alcohol as a sleep aid impacts treatment adherence and outcomes, as individuals with insomnia who use large doses of alcohol before bedtime may persist in using alcohol to help with sleep, even when the acute benefits of such alcohol use diminish with repeated use [61].
Conclusion
This study documents that CBT-I is more effective than “usual care” (single-session SH recommendations) in reducing symptoms of insomnia among young adults who are actively drinking, even in the absence of alcohol intervention. The potential for insomnia treatment to facilitate improvements in other forms of mental health (e.g. alcohol-related consequences) is especially important in this population, who demonstrate high rates of alcohol-related problems and low perceived need for treatment. Additional research is needed to determine if early intervention on sleep problems may delay onset of alcohol use or prevent progression to problematic use.
Acknowledgments
The National Institutes of Health and the Department of Defense had no role in study design; data collection, analysis, or interpretation; manuscript preparation; or the decision to submit the paper for publication. This trial was registered on clinicaltrials.gov prior to data collection (NCT03627832).
Footnotes
The original alcohol use inclusion criterion was score ≥4/5 on the Alcohol Use Disorder Identification Test. This was revised because three of the first seven participants recruited using this criterion did not report past-month binge drinking (4/5+ drinks for women/men) at baseline.
DDQ and daily diary estimates of drinking quantity were strongly correlated (r = 0.75, p < 0.001). Models using the daily diary estimate in place of the DDQ estimate of drinking quantity produced similar results, with a significant main effect for time [F(2,62) = 3.31, p = 0.04], no significant effect for group [F(1,56) = 0.49, p = 0.49], and no significant group by time interaction [F(2,62) = 1.25, p = 0.29].
Funding
This work was supported by funding from the University of Missouri System Research Board Office (PI Miller). Investigator contributions to this project were also supported by the National Institute on Alcohol Abuse and Alcoholism (K23AA026895, PI Miller; R21AA025175, PI Miller; T32AA013526, PI Sher) and the Department of Defense (AR190047, PI McCrae).
Conflict of interest statement. None declared.
Author contributions
M.B.M. designed the study and wrote the protocol and funding proposal, in collaboration with C.S.M. and P.K.S. M.B.M., L.K.F., C.J.P., and N.A.H. implemented the research plan and wrote the first draft of the manuscript. M.B.M. and C.B.D. conducted the statistical analyses. All authors contributed to and have approved the final manuscript.
References
- 1.Ohayon MM. Epidemiological overview of sleep disorders in the general population. Sleep Medicine Research. 2011;2:1–9. [Google Scholar]
- 2.Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49(4):417–427. [DOI] [PubMed] [Google Scholar]
- 3.Bryan CJ, et al. Depression mediates the relation of insomnia severity with suicide risk in three clinical samples of U.S. Military personnel. Depress Anxiety. 2015;32(9):647–655. [DOI] [PubMed] [Google Scholar]
- 4.Sarsour K, et al. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schutte-Rodin S, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Patrick ME, et al. Age-specific prevalence of binge and high-intensity drinking among U.S. young adults: changes from 2005 to 2015. Alcohol Clin Exp Res. 2017;41(7):1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hingson R, et al. Alcohol-induced blackouts as predictors of other drinking related harms among emerging young adults. Alcohol Clin Exp Res. 2016;40(4):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells JE, et al. Reasons why young adults do or do not seek help for alcohol problems. Aust N Z J Psychiatry. 2007;41(12):1005–1012. [DOI] [PubMed] [Google Scholar]
- 9.Wong MM, et al. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 2009;10(7):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong MM, et al. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcohol Clin Exp Res. 2015;39(2):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieters S, et al. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J Youth Adolesc. 2015;44(2):379–388. [DOI] [PubMed] [Google Scholar]
- 12.Hasler BP, et al. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. J Stud Alcohol Drugs. 2016;77(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MB, et al. Prospective association between sleep and the initiation of substance use in adolescents. Journal of Adolescent Health. 2017;60:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MB, et al. Adequate sleep moderates the prospective association between alcohol use and consequences. Addict Behav. 2016;63:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob GF, et al. Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology. 2020;45(1):141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brower KJ, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158(3):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant VV, et al. Psychometric evaluation of the five-factor modified drinking motives questionnaire–revised in undergraduates. Addict Behav. 2007;32(11):2611–2632. [DOI] [PubMed] [Google Scholar]
- 18.Howell AN, et al. Anxiety sensitivity, distress tolerance, and discomfort intolerance in relation to coping and conformity motives for alcohol use and alcohol use problems among young adult drinkers. Addict Behav. 2010;35(12): 1144–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas AG, et al. Sleep problems across development: a pathway to adolescent risk taking through working memory. J Youth Adolesc. 2015;44(2):447–464. [DOI] [PubMed] [Google Scholar]
- 20.Mauss IB, et al. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cogn Emot. 2013;27(3):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum KT, et al. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55(2):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fucito LM, et al. Using sleep interventions to engage and treat heavy-drinking college students: a randomized pilot study. Alcohol Clin Exp Res. 2017;41:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnedt JT, et al. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currie SR, et al. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99(9):1121–1132. [DOI] [PubMed] [Google Scholar]
- 25.Chakravorty S, et al. Cognitive behavioral therapy for insomnia in alcohol-dependent veterans: a randomized, controlled pilot study. Alcohol Clin Exp Res. 2019;43(6):1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor DJ, et al. A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behav Ther. 2014;45(3):376–389. [DOI] [PubMed] [Google Scholar]
- 27.Edinger JD, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheehan DV, et al. Reliability and validity of the MINI International Neuropsychiatric Interview (MINI) according to the SCID-P. European Psychiatry. 1997;12:232–241. [Google Scholar]
- 29.Edinger JD, et al. ; American Academy of Sleep Medicine Work Group. Derivation of research diagnostic criteria for insomnia: report of an American academy of sleep medicine work group. Sleep. 2004;27(8):1567–1596. [DOI] [PubMed] [Google Scholar]
- 30.Morin CM, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manber R, et al. Cognitive Behavioral Therapy for Insomnia in Veterans: Therapist Manual. Washington, DC: U.S. Department of Veterans Affairs; 2014. [Google Scholar]
- 32.McCrae CS, et al. Cognitive behavioral treatments for insomnia (CBT-I) and pain (CBT-P) in adults with comorbid chronic insomnia and fibromyalgia: the SPIN randomized controlled trial. Sleep. 2019;42(3). doi: 10.1093/sleep/zsy234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichstein KL, et al. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Therapy. 1994;16:1–29. [Google Scholar]
- 34.Larsen DL, et al. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2(3):197–207. [DOI] [PubMed] [Google Scholar]
- 35.Kelly PJ, et al. The client satisfaction questionnaire-8: psychometric properties in a cross-sectional survey of people attending residential substance abuse treatment. Drug Alcohol Rev. 2018;37(1):79–86. [DOI] [PubMed] [Google Scholar]
- 36.Bastien CH, et al. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 37.Carney CE, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushida CA, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. [DOI] [PubMed] [Google Scholar]
- 39.Lichstein KL, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 40.Ancoli-Israel S, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. [DOI] [PubMed] [Google Scholar]
- 41.Collins RL, et al. Social determinants of alcohol consumption: the effects of social interaction and model status on the self-administration of alcohol. J Consult Clin Psychol. 1985;53(2):189–200. [DOI] [PubMed] [Google Scholar]
- 42.Kahler CW, et al. Validation of the 30-day version of the brief young adult alcohol consequences questionnaire for use in longitudinal studies. J Stud Alcohol Drugs. 2008;69(4):611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham JW, et al. How many imputations are really needed? some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 44.Hallgren KA, et al. Missing data in alcohol clinical trial: a comparison of methods. Alcoholism: Clinical and Experimental Research. 2013;37:2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heck RH, et al. Multilevel and Longitudinal Modeling with IBM SPSS Second Edition. New York, NY: Routledge; 2014. [Google Scholar]
- 46.Enders CK, et al. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138. [DOI] [PubMed] [Google Scholar]
- 47.Mackinnon DP, et al. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes AF Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 49.Fritz MS, et al. Explanation of two anomalous results in statistical mediation analysis. Multivariate Behav Res. 2012;47(1):61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes AF, et al. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci. 2013;24(10):1918–1927. [DOI] [PubMed] [Google Scholar]
- 51.Arnedt JT, et al. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36(3):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blom K, et al. Internet-vs. group-delivered cognitive behavior therapy for insomnia: a randomized controlled non-inferiority trial. Behav Res Ther. 2015;70:47–55. [DOI] [PubMed] [Google Scholar]
- 53.Morgenthaler T, et al. ; American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American academy of sleep medicine report. Sleep. 2006;29(11): 1415–1419. [PubMed] [Google Scholar]
- 54.Chung KF, et al. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35(4):365–375. [DOI] [PubMed] [Google Scholar]
- 55.Todd J, et al. The role of self-monitoring and response inhibition in improving sleep behaviours. Int J Behav Med. 2014;21(3):470–477. [DOI] [PubMed] [Google Scholar]
- 56.Buu A, et al. Examining measurement reactivity in daily diary data on substance use: results from a randomized experiment. Addict Behav. 2020;102:106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutner CA, et al. Going direct to the consumer: examining treatment preferences for veterans with insomnia, PTSD, and depression. Psychiatry Res. 2018;263:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anton RF, et al. ; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. [DOI] [PubMed] [Google Scholar]
- 59.Freedland KE, et al. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med. 2011;73(4):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–125. [PMC free article] [PubMed] [Google Scholar]
- 61.Roehrs T, et al. Insomnia as a path to alcoholism: tolerance development and dose escalation. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy091 [DOI] [PMC free article] [PubMed] [Google Scholar]