Abstract
Background:
Grayscale ultrasound (US) is the most common imaging modality for the assessment of thyroid nodules.
Objective:
This research aimed to assess the value of using the elasticity index (EI), obtained using shear wave elastography (SWE), to discriminate between malignant and benign thyroid nodules.
Materials and methods:
A total of 86 patients (94 distinct thyroid nodules) were operated on at Vietnam National Cancer Hospital from June 2018 to June 2019. Comparisons of the grayscale ultrasound (US) findings and the EI values between the benign and malignant groups were performed using the Chi-square test and Student’s t-test, respectively. The discrimination abilities of EI were determined through receiver operating characteristic (ROC) curve analysis, with the computation of optimal cut-off points.
Results:
The EI values of the benign and malignant groups were 37.6 ± 26.1 kPa and 105.4 ± 48.8 kPa, respectively. The area under the ROC curve (AUROC) value for discrimination between groups based on EI values was 0.889 when using an optimal cut-off point of 74.5 kPa, which resulted in a sensitivity of 74.3% and a specificity of 90%. Logistic multivariate regression analysis found that EI and microcalcification were significant factors for the discrimination between groups, with an odds ratio (OR): 1.487 [95% confidence interval (95% CI): 1.124–1.968, p = 0.005] and OR: 12.119 (95% CI: 2.031–72.323, p = 0.006), respectively. Combining grayscale US imaging with SWE can increase the specificity of the diagnosis but does not increase the accuracy.
Conclusion:
SWE can be helpful for predicting the malignancy of thyroid nodules, although the accuracy of this method is only moderate.
Keywords: shear wave elastography, thyroid nodule, malignancy, elasticity index
1. INTRODUCTION
Grayscale ultrasound (US) is the most common imaging modality for the assessment of thyroid nodules (1). Although grayscale US is a very sensitive method for the detection of thyroid nodules, the accuracy (Acc) of grayscale US for the differentiation between malignant and benign thyroid nodules remains limited. Reported ultrasound features that have been associated with thyroid nodule malignancy include: microcalcification, marked hypoechogenicity, irregular margins, and a taller-than-wide shape (2). However, these characteristic features are not specific and can also be found in benign thyroid nodules. Separately, each of these features has low accuracy for the prediction of thyroid nodule malignancy. When these features are combined, specificity increases, but sensitivity decreases (3).
Ultrasound elastography is a new tool capable of complementing grayscale US assessments by evaluating tissue stiffness. The results are presented as color images for qualitative assessments, strain ratios for semi-quantitative assessments, and elasticity indices (EIs) for quantitative assessments (4). Among the various available elastography technologies, shear wave elastography (SWE) is a newly developed form of elastography that assesses the elasticity of tissues by measuring the velocity of a shear wave generated through the acoustic radiation force impulse (ARFI). This technique is thought to depend less on the skill of the individual operator than other techniques and is both quantitative and reproducible (3). Several studies have reported that SWE can be useful for differentiating between malignant and benign thyroid nodules, indicating that EIs represent significant parameters for the differentiation of malignant from benign lesions, which are independent of grayscale US parameters. Four meta-analyses, performed by Lin et al. (5), Zhan et al. (6), Dong et al. (7) and Liu et al. (8-11), which included more than 6,000 nodules, revealed that SWE had high Acc, with an area under the receiver operating characteristic curve (AUROC) value ranging from 0.91–0.94, a sensitivity (Se) from 80%–86%, and a specificity (Sp) of 84%–90%.
However, in recent years, some studies have reported contrasting results. Bardet et al. found that the SWE parameters were similar between benign and malignant nodules, including the EI mean (20.2 vs. 19.6 kPa), the EI max (34.3 vs. 32.5 kPa), and the ratio between EI of nodule and that of normal-appearing parenchyma (1.57 vs. 1.38), respectively, and the EI values failed to discriminate between benign and malignant thyroid nodules (12). Swan et al. found that the EI values displayed large overlaps between malignant and benign nodules and that difference between the two groups was not significant. The cut-off point identified for EI for the prediction of malignancy was not found to be clinically meaningful (13). These discrepancies suggested that the stiffness of tissue is a complex issue that requires further study.
2. AIM
The aim of present research was to evaluate the role of SWE in differentiating malignant from benign thyroid nodules.
3. METHODS
Patients
The present study was approved by the Ethical Review Committee of Hanoi Medical University. All patients signed written informed consent. The study was conducted from June 2018 to June 2019 at Vietnam National Cancer Hospital, Hanoi, Vietnam, and the data were prospectively assessed. A total of 86 patients, featuring 94 thyroid nodules, were enrolled in the study. All patients underwent thyroidectomy, and the histopathological results of the resected specimens were obtained. All patients were examined by grayscale US and SWE, which were both administered by the same investigator. The inclusion criteria included thyroid nodules ≥ 10 mm in the largest dimension. The exclusion criteria included nodules with predominant cystic characteristics or the inability to obtain SWE registration.
Ultrasound examination
Thyroid US examinations were performed by a radiologist with 10 years of experience in ultrasound using a 4–15 MHz linear array transducer (LOGIQ S8 XDclear 2.0, GE Healthcare, the USA). The data for grayscale US and SWE findings were prospectively recorded.
The grayscale US finding included: echogenicity, margin, calcification, and shape. The vascularity of the lesion on Color-Flow Doppler US was also recorded. Echogenicity findings were categorized as hyperechoic, isoechoic, hypoechoic, or marked hypoechoic, based on the comparison between the nodule and the thyroid gland or the surrounding strap muscle (marked hypoechogenicity was defined as decreased echogenicity compared with the surrounding strap muscle) (2). The margins of the lesions were categorized as smooth or irregular. Irregular or lobulated margins were defined as the presence of many small lobules on the surface of a nodule. Calcification was classified as the presence or absence of microcalcification, which was defined as tiny, punctate hyperechoic foci, either with or without acoustic shadows. The shape was divided into either wider-than-tall or taller-than-wide. Thyroid nodules that presented one or more characteristic findings (marked hypoechogenicity, lobulated, microcalcification, and taller-than-wide) were regarded as malignant according to the grayscale US evaluation (2).
SWE was performed on the thyroid nodules immediately following grayscale US by the same radiologist. In patients with multiple nodules, SWE was performed on each nodule, in turn. The SWE images were obtained in the longitudinal plane to reduce the motion artifacts generated by the trachea and carotid artery. The size of the acquisition box was adjusted to cover the entire thyroid nodule. A color-coded image was displayed, which showed soft tissue in blue and stiff tissue in red. The EI was automatically calculated and expressed in kilo-pascals (kPa). For each thyroid nodule, five measurements were performed, and the average of all five measurements was calculated. The US probe was used without the application of pressure by the radiologist.
Statistical analysis
SPSS version 26 (IBM corp., New York, USA) was applied. The EI were expressed as mean values ± standard deviations. Chi-square tests and Student’s t-test were used to compare the grayscale US findings and the EI values between two groups with benign and malignant thyroid nodules, respectively. The diagnostic ability of EI to differentiate malignant from benign thyroid nodules was evaluated using AUROC analysis. The optimal cut-off points were identified based on the Youden index, which were then used to calculate the Se, Sp, and Acc values. Logistic multivariate regression was performed to estimate the ability to correctly assess the malignancy of the thyroid nodules using both the grayscale US and EI variables. McNemar’s test was used to compare the diagnostic performances of grayscale US alone compared with the diagnostic performance of grayscale US combined with EI. A p-value of < 0.05 was considered significant.
4. RESULTS
In the present study, 86 patients were examined, including 9 men and 77 women, aged between 22–73 years, with a mean ± standard deviation age of 46.94 ± 12.69 years. Men ranged in age from 32-73 years, with a mean age of 46.44 ± 16.26 years, whereas women ranged in age from 22–72 years, with a mean age of 47.00 ± 12.34 years. No age difference was observed between men and women (p = 0.902).
A total of 94 thyroid nodules were identified, comprised of 20 benign and 74 malignant nodules. Benign thyroid nodules included 14 follicular adenomas and 6 adenomatous hyperplasias. All of the malignant thyroid nodules were papillary carcinomas.
The US characteristics of the nodules are presented in Table 1. Malignant nodules were significantly more likely to feature taller-than-wide shape (45.9% vs. 15%), irregular margins (68.9% vs. 30%), marked hypoechogenicity (31.1% vs. 5%), and microcalcification (66.2% vs. 10%) than benign nodules (p < 0.05). No differences in vascularity were identified between the two groups. Table 1 also shows the EI values for both malignant and benign thyroid nodules. The mean EI value for malignant thyroid nodules (105.4 ± 48.8 kPa) was significantly higher than that for benign thyroid nodules (37.6 ± 26.1 kPa).
Table 1. Grayscale ultrasound features and elasticity index values for benign and malignant thyroid nodules.
| Examined parameters | Benign (n=20) | Malignant (n=74) | P |
|---|---|---|---|
| Taller-than-wide shape | 0.012a | ||
| Yes | 3 (15) | 34 (45.9) | |
| No | 17 (85) | 40 (54.1) | |
| Irregular margin | 0.002a | ||
| Yes | 6 (30) | 51 (68.9) | |
| No | 14 (70) | 23 (31.1) | |
| Marked hypoechogenicity | 0.018a | ||
| Yes | 1(5) | 23 (31.1) | |
| No | 19 (95) | 51 (68.9) | |
| Microcalcification | < 0.001a | ||
| Yes | 2 (10) | 49 (66.2) | |
| No | 18 (90) | 25 (33.8) | |
| Intranodular vascularity | 0.713a | ||
| Yes | 12 (60) | 41 (55.4) | |
| No | 8 (40) | 33 (44.6) | |
| Elasticity index (kPa) | 37.6 ± 26.1 | 105.4 ± 48.8 | 0.002b |
Data for grayscale ultrasound findings are reported as the number (percentage). Data for the elasticity index are reported as the mean ± standard deviation.
a p-values were calculated by Chi-square test
b p-values were calculated by Student’s t-test.
P-values shown in bold indicate significance.
To evaluate the ability of the EI to differentiate malignant from benign thyroid nodules, receiver operating characteristic (ROC) curve analysis was performed. Figure 1 shows that the optimal cut-off value for EI was determined to be 74.5 kPa. Using this threshold value, SWE correctly diagnosed 55/74 malignant thyroid nodules and 18/20 benign thyroid nodules. The Se, Sp, and Acc for SWE alone were 74.3%, 90%, and 77.7%, respectively.
Figure 1. ROC curve, indicating the sensitivity, specificity, and optimal cut-off value of the elasticity index-based differentiation between benign and malignant thyroid nodule groups.
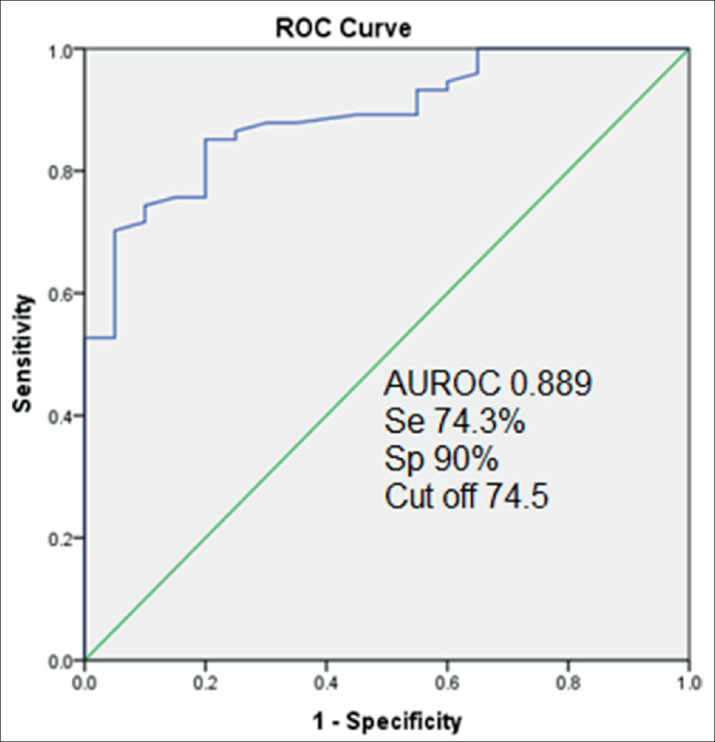
Upon logistic multivariate regression analysis for all imaging parameters that showed significant differences between the two groups, only microcalcification [odds ratio (OR): 12.119; 95% confidence index (95% CI): 2.031–72.323; p = 0.006] and EI (OR per 10-kPa increase: 1.487; 95% CI: 1.124–1.968; p = 0.005) were found to be significant factors for the discrimination between groups (Table 2). The identification of microcalcification or an EI increase of 10 kPa increased the probability of correctly differentiating malignant thyroid nodules by 12-fold and 1.5-fold, respectively.
Table 2. Univariate and multivariate logistic regression models evaluating the use of various grayscale ultrasound imaging parameters and the elasticity index to discriminate malignant from benign thyroid nodules. 95% CI: 95% confidence interval. P-values shown in bold indicate significance.
| Tested parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Taller-than-wide shape (yes-no) | 4.817 (1.300–17.847) | 0.019 | 5.342 (0.952–29.966) | 0.057 |
| Irregular margin (yes-no) | 5.174 (1.765–15.169) | 0.003 | 1.015 (0.199–5.189) | 0.986 |
| Marked hypoechogenicity (yes-no) | 8.569 (1.081–67.923) | 0.042 | 2.379 (0.202–28.043) | 0.491 |
| Microcalcification (yes-no) | 17.640 (3.788–82.139) | < 0.001 | 12.119 (2.031–72.323) | 0.006 |
| Elasticity index per 10 kPa | 1.565 (1.265–1.936) | < 0.001 | 1.487 (1.124–1.968) | 0.005 |
Using the grayscale US-based morphologic criteria (taller-than-wide shape, irregular margin, marked hypoechogenicity, and microcalcification) to differentiate malignant from benign thyroid nodules resulted in the correct diagnosis of 71/74 malignant thyroid nodules and 10/20 benign thyroid nodules. The Se, Sp, and Acc of using grayscale US criteria alone were 95.9%, 50%, and 86.2%, respectively (Table 3).
Table 3. The abilities of grayscale ultrasound, elasticity index, and the combination of grayscale ultrasound with elasticity index to differentiate malignant from benign thyroid nodules.
| Se | Sp | Acc | |
|---|---|---|---|
| Gray scale US | 95.9 (71/74) | 50 (10/20) | 86.2 (81/94) |
| Elasticity index | 74.3 (55/74) | 90 (18/20) | 77.7 (73/94) |
| Combined use of GSU with EI | 74.3 (55/74) | 90 (18/20) | 77.7 (73/94) |
When the grayscale US criteria were combined with SWE, we defined malignant thyroid nodules as those that featured one or more characteristic morphologic features and had an EI value ≥ 74.5 kPa. Using these criteria, the combination of SWE and grayscale US correctly diagnosed 55/74 malignant thyroid nodules and 18/20 benign thyroid nodules, with Se, Sp, and Acc values of 74.3%, 90%, and 77.7%, respectively. The diagnostic performance of grayscale US alone was compared with the diagnostic performance of combining grayscale US with SWE using McNemar’s test. The Se of grayscale US alone was significantly higher than that for the combination of grayscale US with SWE (p < 0.0001). In contrast, the Sp of the combination of grayscale US with SWE was significantly higher than that of grayscale US alone (p = 0.007). No significant difference was identified between the Acc values of grayscale US alone and the combination of grayscale US with SWE (p = 0.169).
Se, selectivity; Sp, specificity; Acc, accuracy; GSU, grayscale ultrasound; EI, elasticity index. For selectivity, data are presented as the percentage (correctly identified/total) of correctly identified malignant tumors identified. For specificity, data are presented as the percentage (correctly identified/total) of correctly identified benign tumors. For Acc, the data are presented as the percentage (correctly identified/total) of correctly identified tumors.
5. DISCUSSION
SWE is a novel technique that is believed to show promise for the differentiation between malignant and benign thyroid nodules. Previous studies have demonstrated the EI values were significantly different between benign and malignant groups. Sebag et al. performed SWE in 146 thyroid nodules and found that the mean EI of malignant nodules (150 ± 95 kPa) was significantly higher than that of benign nodules (36 ± 30 kPa). The optimal cut-off SWE value of 65 kPa achieved an AUROC of 0.94, with the Se of 85.2% and the Sp of 93.9% (3). According to Baig et al. (14), the SWE indices (including E max and E mean) of malignant nodules (85.2 ± 8.1 kPa and 26.6 ± 2.5 kPa, respectively) were significantly higher than those of benign nodules (50.3 ± 3.1 kPa and 20.2 ± 1 kPa, respectively). The optimal cut-off values identified the distinction between benign and malignant nodules for EI max and EI mean were 67.3 kPa and 23.1 kPa, respectively. Applying these cut-off values produced AUROC values of 0.785 and 0.71, Se values of 70.4% and 74.1%, and Sp values of 70.2% and 66.7% for EI max and EI mean, respectively.
However, large disparities were observed in the EI values associated with malignant lesions, and large differences in the identified cut-off values were reported by different studies (15–18 kPa). The optimal cut-off values reported for EI mean ranged from 23.1 kPa in the study by Baig et al. [14] to 85.2 kPa in the study by Park et al. (19). Applying these different cut-off points resulted in different AUROC, Se, and Sp values. These wide variations interfere with the widespread application of thyroid SWE in clinical practice.
The differences in the optimal EI values reported by various studies are likely due to heterogeneity among methodological studies. First, the malignant nodule rate among the total nodules in each study, as well as the papillary thyroid carcinoma (PTC) rate within the malignant group, could affect the results. PTC appears to be stiffer than other malignant thyroid nodules (12). According to Bhatia et al., the discrimination between malignant and benign nodules can be performed using a threshold of 42.1 kPa, which produced an AUROC value of 0.61, with 52.9% Se and 77.8% Sp. If non-PTC malignancy were excluded from the analysis, the Acc increased, with an AUROC value of 0.74, an Se of 76.9%, and an Sp of 71.1% (20). In contrast, the highest cut-off value of 85.2 kPa was reported by Park et al., who studied 379 malignant nodules among 476 total thyroid nodules (19). The high EI value identified in this study might have been influenced by the large proportion of malignant nodules. In the present study, up to 74 of the 94 total nodules were malignant, and the cut-off value was determined to be 74.5 kPa.
Second, the uniaxial compression (pre-compression) level could affect the EI values that were measured by SWE. Studying 67 surgically resected thyroid samples, Lyshchik et al. found that the EI values of the normal parenchyma, benign thyroid tumors, and PTC were correlated with an increase in the pre-compression value. At a pre-compression value of 5%, the EI values of these samples were 9.0 ± 4.0 kPa, 15.0 ± 5.3 kPa, and 44.5 ± 27.8 kPa, respectively, which increased to 23.9 ± 7.4 kPa, 53.0 ± 30.5 kPa, and 373.4 ± 219.0 kPa with a pre-compression value of 20% (21). Studying the influence of pre-compression on the EI values of thyroid nodules, Lam et al. found that the EI values of normal thyroid glands, benign hyperplastic nodules, and PTCs were 10.3 ± 3.3 kPa, 17.7 ± 7.6 kPa, and 22.2 ± 11.9 kPa at pre-compression (0% strain), respectively, which increased to 21.1 ± 4.2 kPa, 42.3 ± 16.0 kPa, and 97.6 ± 46.8 kPa at high pre-compression (22%–30% strain) (22).
Third, the EI measurements could be affected by nodule size. Studying 81 focal thyroid lesions, Bhatia et al. found that lesion size was positively correlated with EI values among benign nodules but not for PTC nodules (20). In contrast, Liu et al. found that nodule size showed a positive correlation with EI values in malignant nodules but not in benign nodules (8). Kim et al. (23) reported no correlations between nodule size and EIs, in either benign or malignant thyroid nodules. Therefore, this issue remains controversial.
Finally, the cut-off values differed depending on the shear wave speed imaging system used. After performing measurements in 140 focal thyroid nodules using the Toshiba SWE (T-SWE; 60 Toshiba Medical System, Tochigi, Japan) and the SuperSonic SWE (S-SWE; Aix en Provence, France), He et al. reported cut-off values of 26.6 kPa and 42.9 kPa, respectively (24).
In clinical practice, SWE is not used alone but in combination with grayscale US. Grayscale US is always the first modality of choice for differentiating between malignant and benign thyroid nodules. Although no single suspicious finding has been found to be reliable for differentiating between malignant and benign tumors, the classification of a malignant nodule based on the presence of any suspicious finding was found to be highly sensitive, reaching a sensitivity of 93.8% (2). In contrast, SWE has been determined to be a highly specific diagnostic modality. Therefore, combining SWE with grayscale US could increase the Sp compared with grayscale US alone. The results reported by Baig et al., who examined 111 thyroid nodules (including 27 malignant and 84 benign nodules), showed that grayscale US alone had a high Se (96.3%), a low Sp (46.4%), and an overall Acc of 58.5% for the discrimination between benign and malignant nodules. When grayscale US was combined with SWE parameters (cut-off EI max value of 67.3 kPa and a cut-off EI mean value of 23.1 kPa), the overall Acc increased to 80.2% and 78.4%, with Se values of 70.4% and 74.1%, and Sp values of 83.3% and 79.8%, respectively (14). Veyrieres et al. performed SWE analysis in 297 thyroid nodules (including 35 malignant nodules) and found that the optimal cut-off value was 66 kPa. Applying this cut-off point, the AUROC, Se, and Sp were 0.852, 80%, and 90.5%, respectively. However, by combining the SWE with grayscale US, the Se and positive predictive value (PPV) were excellent, at 97% and 99.5%, respectively, whereas the Sp and negative predictive value (NPV) were low, at 55.30% and 22.5%, respectively (15). According to Dobruch-Sobczak et al., an EI max value of 67 kPa was a significant parameter for differentiating between benign and malignant lesions, with an Se of 42% and an Sp of 88.24%. However, no significant difference was found between the diagnostic performance of using grayscale US alone and using the combination of grayscale US and SWE (AUROC for grayscale US alone was 0.854, whereas the AUROC for combined grayscale US and SWE parameters was 0.872, p = 0.22) (18). Thus, although the combination of SWE with grayscale US appears promising, the diagnostic effectiveness of this combination has not been consistent across studies. The results of our study showed that compared with grayscale US alone, combining an EI cut-off value 74.5 kPa resulted in a significant increase in Sp, from 50% to 90%, accompanied by a simultaneous decrease in Se, from 95.9% to 74.3%. However, the Acc was not significantly different between these two diagnostic models. These results were likely affected by the high proportion of malignant thyroid nodules in the present study. The reduction in Se would result in the failure to identify a large proportion of malignant nodules, whereas the increase in Sp would result in the correct identification of only a few benign nodules.
In addition to studies supporting the use of SWE for differentiating between malignant and benign thyroid nodules, some recent studies have presented results contradicting the usefulness of SWE for this purpose. In SWE, shear waves are produced in tissues stimulated by ARFI. Although SWE is generally considered to be less operator-dependent and more reproducible than other techniques, the data about it remains limited. By examining inter-rater and intra-rater agreement, along with a day-to-day agreement, Swan et al. recently presented suboptimal reproducibility data. The inter-rater and intra-rater agreements were low, and the EI varied from day-to-day. These findings indicated that SWE might be unreliable for the differentiation of malignant from benign nodules in individual patients (25). To assess the role of SWE in the differentiation of malignant from benign thyroid nodules, Nattabi et al. performed a meta-analysis of 14 studies, including 2,851 thyroid nodules (1,092 malignant and 1,759 benign nodules) from 2,139 patients. They found that the diagnostic performance of quantitative SWE for the malignancy risk stratification of thyroid nodules was suboptimal, with mediocre Se and Sp (26). In the most recent study, Swan et al. described a pessimistic view of SWE for the purposes of diagnosing malignant thyroid nodules. Among 413 thyroid nodules, the EI values showed a large degree of overlap between malignant and benign nodules (ranges for EI mean: malignant 3–100 kPa, benign 4–182 kPa), and differences between malignant and benign nodules were not found for any EI parameter. No association between EI values and histological diagnosis was identified by ROC analysis (AUROC 0.51–0.56). The author concluded that defining an EI cut-off point for the prediction of malignancy was not clinically meaningful (13).
Our study has some limitations. First, only a small number of patients were examined. Second, because all patients underwent thyroid surgery, more malignant thyroid nodules were identified than benign thyroid nodules. These numbers are not representative of the prevalence of benign and malignant thyroid nodules in the general population. The high portion of malignant nodules could result in a high EI cut-off value (19).
6. CONCLUSIONS
In conclusion, the present study showed the EI value obtained from SWE was an independent predictor of thyroid malignancy. Combining grayscale US with SWE can improve the specificity of the differentiation between malignant and benign thyroid nodules. However, this combination did not increase the Acc of diagnosis compared with grayscale US alone, and the threshold EI values reported in the literature is vast. Therefore, SWE should only be considered as a supplemental tool to complement grayscale US when assessing thyroid nodules.
Acknowledgments:
The authors are grateful to the study participants and the medical staff for their assistance.
Author contributions:
NMD and PAT contributed equally to this article therefore considered as co-first authors. NMD and PAT prepared, drafted, and revised the manuscript critically, for important intellectual content. NMD and PAT contributed substantially to the acquisition, analysis, and interpretation of data. Each author gave final approval to the version of the manuscript submitted for publication and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest:
The authors declare that they have no conflict of interest.
Funding:
The authors received no external financial support for the research.
REFERENCES
- 1.Sipos JA. Advances in ultrasound for the diagnosis and management of thyroid cancer. Thyroid. 2009;19(12):1363–1372. doi: 10.1089/thy.2009.1608. [DOI] [PubMed] [Google Scholar]
- 2.Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178(3):687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 3.Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95(12):5281–528. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 4.Moraes PHM, Sigrist R, Takahashi MS, et al. Ultrasound elastography in the evaluation of thyroid nodules: evolution of a promising diagnostic tool for predicting the risk of malignancy. Radiol Bras. 2019;52(4):247–253. doi: 10.1590/0100-3984.2018.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin P, Chen M, Liu B, et al. Diagnostic performance of shear wave elastography in the identification of malignant thyroid nodules: a meta-analysis. Eur Radiol. 2014;24(11):2729–2738. doi: 10.1007/s00330-014-3320-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhan J, Jin JM, Diao XH, et al. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules-A meta-analysis. Eur J Radiol. 2015;84(11):2181–2186. doi: 10.1016/j.ejrad.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Dong FJ, Li M, Jiao Y, et al. Acoustic radiation force impulse imaging for detecting thyroid nodules: a systematic review and pooled meta-analysis. Med Ultrason. 2015;17(2):192–199. doi: 10.11152/mu.2013.2066.172.hyr. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Liang J, Zheng Y, et al. Two-dimensional shear wave elastography as promising diagnostic tool for predicting malignant thyroid nodules: a prospective single-centre experience. Eur Radiol. 2015;25(3):624–634. doi: 10.1007/s00330-014-3455-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Liang J, Zhou L, et al. Shear wave elastography in the diagnosis of thyroid nodules with coexistent chronic autoimmune Hashimoto’s thyroiditis. Otolaryngol Head Neck Surg. 2015;153(5):779–785. doi: 10.1177/0194599815600149. [DOI] [PubMed] [Google Scholar]
- 10.Liu BJ, Li DD, Xu HX, et al. Quantitative shear wave velocity measurement on acoustic radiation force impulse elastography for differential diagnosis between benign and malignant thyroid nodules: A meta-analysis. Ultrasound Med Biol. 2015;41(12):3035–3043. doi: 10.1016/j.ultrasmedbio.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu BJ, Xu HX, Zhang YF, et al. Acoustic radiation force impulse elastography for differentiation of benign and malignant thyroid nodules with concurrent Hashimoto’s thyroiditis. Med Oncol. 2015;32(3):50. doi: 10.1007/s12032-015-0502-5. [DOI] [PubMed] [Google Scholar]
- 12.Bardet S, Ciappuccini R, Pellot-Barakat C, Monpeyssen H, Michels JJ, Tissier F, et al. Shear wave elastography in thyroid nodules with indeterminate cytology: Results of a rosppective bicentric study. Thyroid. 2017;27(11):1441–1449. doi: 10.1089/thy.2017.0293. [DOI] [PubMed] [Google Scholar]
- 13.Swan KZ, Bonnema SJ, Jespersen ML, et al. Reappraisal of shear wave elastography as a diagnostic tool for identifying thyroid carcinoma. Endocr Connect. 2019;8(8):1195–1205. doi: 10.1530/EC-19-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baig F, Liu S, Lam HC, et al. Shear wave elastography combining with conventional grey scale ultrasound improves the diagnostic accuracy in differentiating benign and malignant thyroid nodules. Appl Sci. 2017;7(11):1103. [Google Scholar]
- 15.Veyrieres JB, Albarel F, Lombard JV, et al. A threshold value in shear wave elastography to rule out malignant thyroid nodules: A reality? Eur J Radiol. 2012;81:3965–3972. doi: 10.1016/j.ejrad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Szczepanek-Parulska E, Woliński K, Stangierski A, et al. Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy. PloS one. 2013;8(11):e81532. doi: 10.1371/journal.pone.0081532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan SB, Yu J, Li X, et al. Diagnostic value of two-dimensional shear wave elastography in papillary thyroid microcarcinoma. Onco Targets Ther. 2016;9:1311–1317. doi: 10.2147/OTT.S98583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobruch-Sobczak K, Zalewska EB, Guminska A, et al. Diagnostic performance of shear wave elastography parameters alone and in combination with conventional B-mode ultrasound parameters for the characterization of thyroid nodules: A prospective, dual-center study. Ultrasound Med Biol. 2016;42(12):2803–2811. doi: 10.1016/j.ultrasmedbio.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Park AY, Son EJ, Han K, et al. Shear wave elastography of thyroid nodules for the prediction of malignancy in a large scale study. Eur J Radiol. 2015;84(3):407–412. doi: 10.1016/j.ejrad.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia KS, Tong CS, Cho CC, et al. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol. 2012;22(11):2397–2406. doi: 10.1007/s00330-012-2495-1. [DOI] [PubMed] [Google Scholar]
- 21.Lyshchik A, Higashi T, Asato R, et al. Elastic moduli of thyroid tissues under compression. Ultrasonic imaging. 2005;27:101–110. doi: 10.1177/016173460502700204. [DOI] [PubMed] [Google Scholar]
- 22.Lam AC, Pang SW, Ahuja AT, et al. The influence of precompression on elasticity of thyroid nodules estimated by ultrasound shear wave elastography. Eur Radiol. 2016;26(8):2845–2852. doi: 10.1007/s00330-015-4108-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Kim JA, Son EJ, et al. Quantitative assessment of shear-wave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. Eur Radiol. 2013;23(9):2532–2537. doi: 10.1007/s00330-013-2847-5. [DOI] [PubMed] [Google Scholar]
- 24.He YP, Xu HX, Wang D, et al. First experience of comparisons between two different shear wave speed imaging systems in differentiating malignant from benign thyroid nodules. Clin Hemorheol Microcirc. 2017;65(4):349–361. doi: 10.3233/CH-16197. [DOI] [PubMed] [Google Scholar]
- 25.Swan KZ, Nielsen VE, Bibby BM, et al. Is the reproducibility of shear wave elastography of thyroid nodules high enough for clinical use? A methodological study. Clin Endocrinol. 2017;86(4):606–613. doi: 10.1111/cen.13295. [DOI] [PubMed] [Google Scholar]
- 26.Nattabi HA, Sharif NM, Yahya N, et al. Is diagnostic performance of quantitative 2D-shear wave elastography optimal for clinical classification of benign and malignant thyroid nodules?: A systematic review and meta-analysis. Acad Radiol. 2017 doi: 10.1016/j.acra.2017.09.002. S1076-6332(17)30369-0. [DOI] [PubMed] [Google Scholar]


