Abstract
Visceral pain is of great concern to the medical community because it remains particularly resistant to current clinical treatments. A serendipitous and initially unexplainable clinical finding that a punctate midline dorsal column lesion is effective in eliminating visceral pain, however, has initiated a resurgence of interest in the study of the basic mechanisms of visceral nociception. Clinical and anatomic findings have determined that visceral pain either of thoracic or pelvic origin can be relieved by carefully placed lesions directed at the lateral edge or the medial edge of the gracile fasciculus, respectively. Studies are demonstrating that visceral pain is quite unique from cutaneous pain.
Recent clinical evidence has shown that pelvic cancer pain can be successfully treated by a limited or punctate surgical midline myelotomy [1,2,3••,4]. Thoracic visceral pain was relieved by a punctate lesion at the lateral edge of the gracile fasciculus (1 mm lateral) at T1 [5]. The patients can be tapered off morphine and maintained in most instances on non-narcotic analgesics. Postmortem examination in this laboratory of the spinal cord tissue from 3 of their 12 patients with pelvic visceral pain revealed that the neurosurgical lesions performed by Hirshberg et al. [1] and Nauta et al. [3••] interrupted only a limited portion of the axons of the dorsal column adjacent to the midline (Fig. 1). In addition to histologic verification of the limited myelotomy, the Hirshberg et al. [1] clinical report of pelvic pain relief provided preliminary anatomic demonstration in rats of a new visceral nociceptive pathway in the midline of the dorsal column (Fig. 2).
Figure 1.
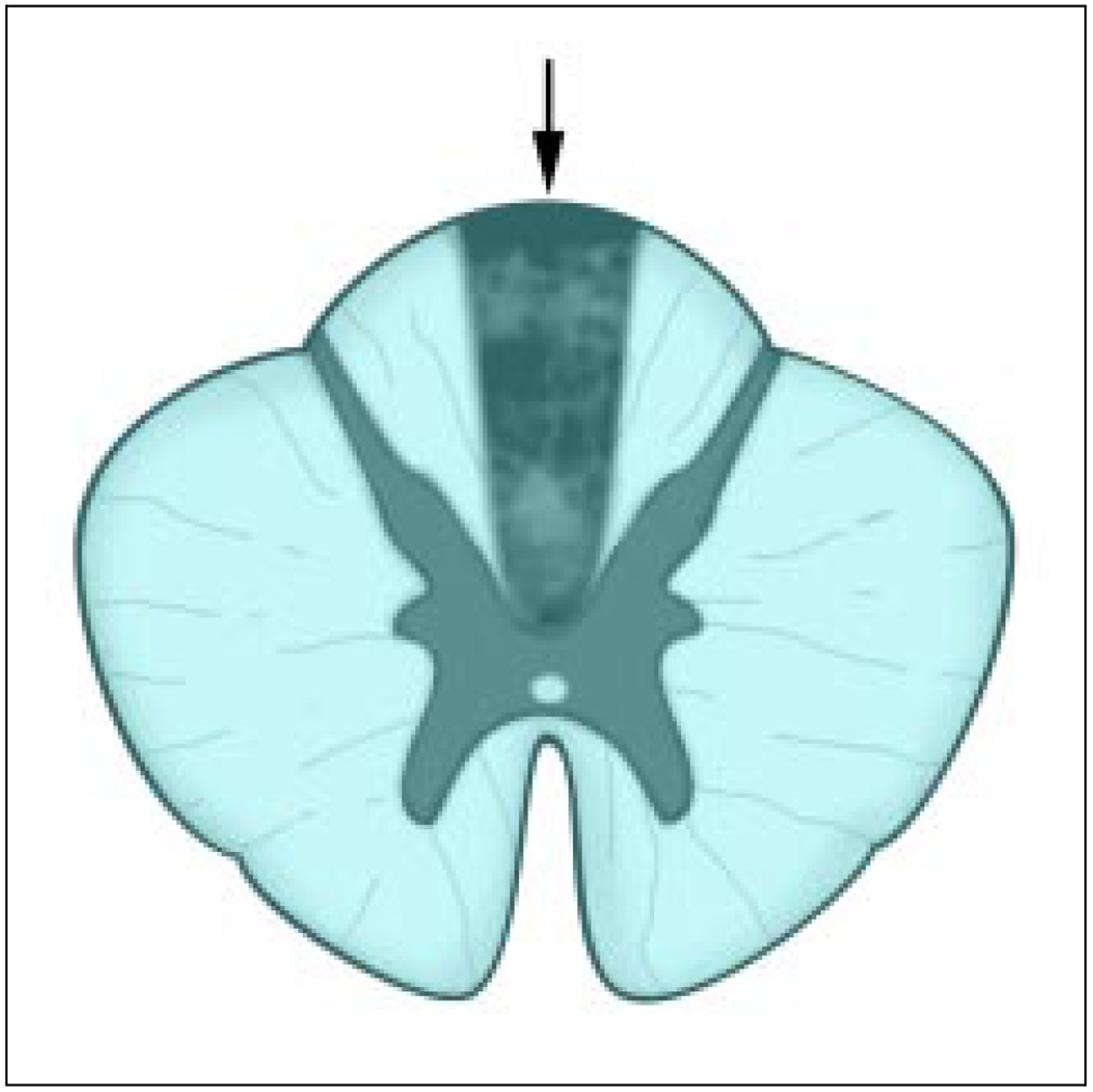
The surgical lesion of the dorsal column midline at T7-T8 made by Dr. Hank Nauta at the University of Texas Medical Branch that produced relief of rectal pain caused by a pre-sacral small cell carcinoma. After surgery the patient reported pain relief, and no narcotics were required for 11 months. When the cancerous mass extended into the sacral canal the patient was treated by resection of the sacrum and coccyx. The arrow indicates the blackened region indicative of the lesioned zone of demyelination, which was 1 mm in width.
Figure 2.
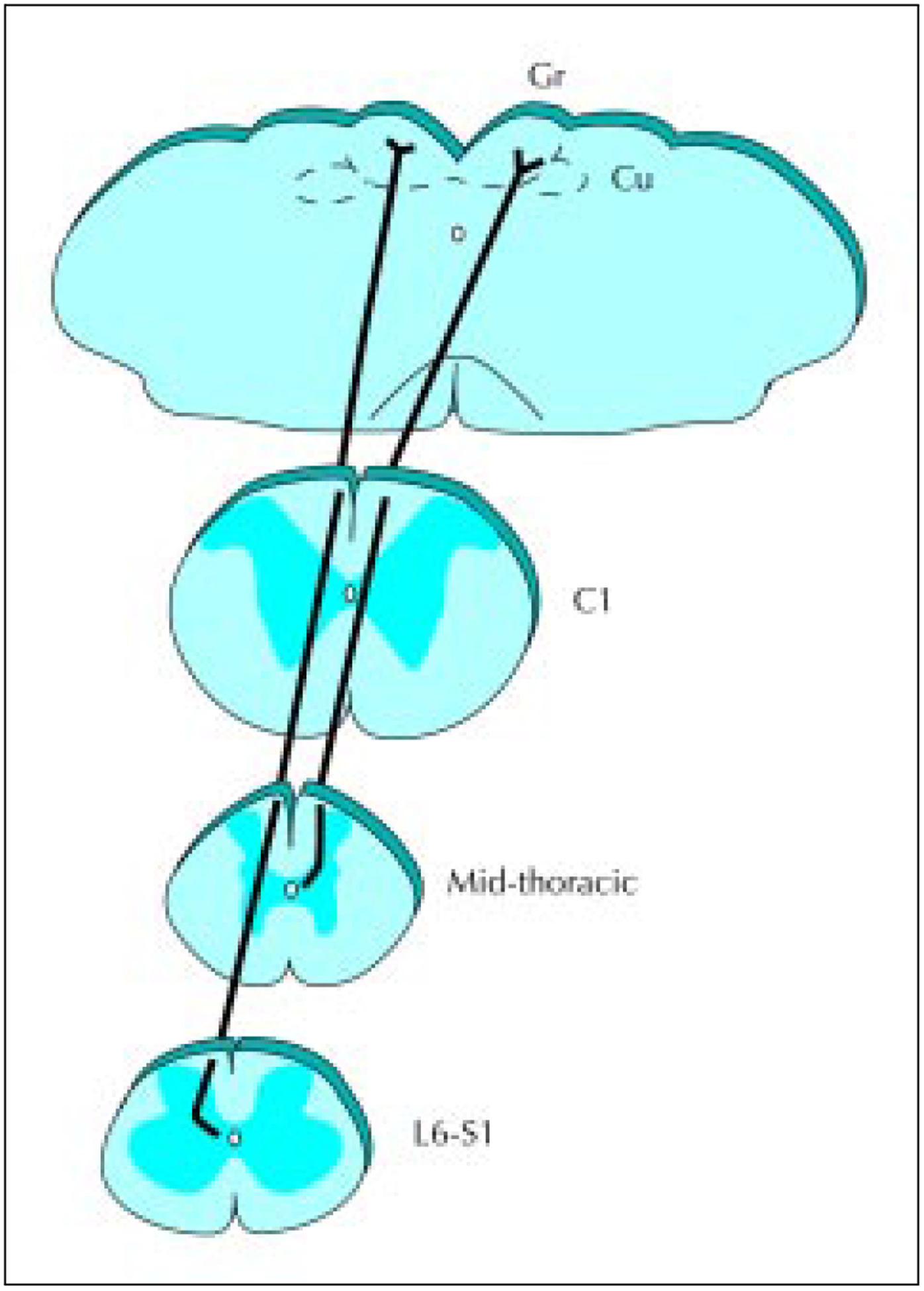
The visceral nociceptive pathway arises from postsynaptic dorsal column (PSDC) projection neurons located around the central canal. This diagram depicts the route taken by axons ascending as separate pathways in the dorsal column. The pathway was visualized after small injections of anterograde transport of Phaseolus vulgaris leucoagglutinin were made in the gray matter near the central canal. The visceral pathway arising from the lumbosacral cord travels in the midline. The ascending fibers from thoracic levels travel between the gracile and cuneate fasciculi. Cu—cuneate; Gr—gracile.
Using retrograde and anterograde neuronal tract tracers, the anatomic data demonstrated existence of a column of cells around the central canal with axonal projections to the gracile nucleus [1,6]. These cells are classified as postsynaptic dorsal column (PSDC) cells and are particularly evident in the lumbosacral spinal cord. PSDC cells have been described in this region through the length of the spinal cord, but previously focus has been solely on the lamina III PSDC cells that are not involved in the transmission of information about pain [7–9]. Further studies, described next, have revealed an ascending visceral nociceptive pathway. This visceral pain pathway is viscerotopically organized into two distinct ascending bundles traveling on either side of the fasciculus gracilis with the visceral afferent information received in the thoracic or the lumbosacral level of the spinal cord, respectively [10•].
In a series of collaborative electrophysiologic studies by Willis et al. [11], strong evidence has been accumulated demonstrating the physiologic responsiveness of neurons of the visceral pain pathway to noxious visceral input. It was shown in these studies that neurons in the midline of the sacral cord respond to noxious colorectal distension (Fig. 3). The visceral information is then transmitted through a long ascending axonal pathway to responsive neurons in the dorsal column nuclei. From the dorsal column nucleus, the information in turn is relayed to the thalamus on the opposite side. These studies in rats were later confirmed in monkeys [12•].
Figure 3.
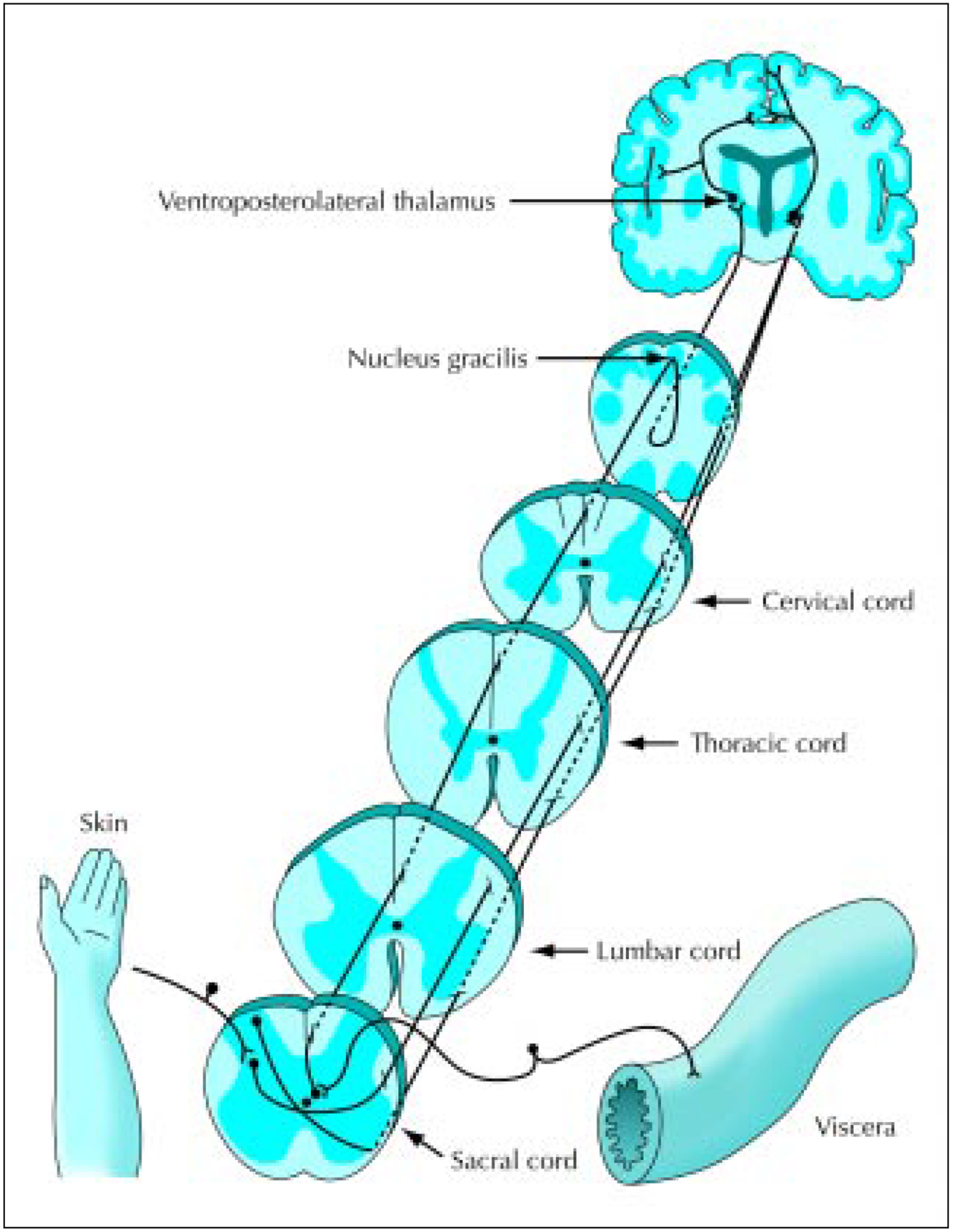
Two separate pathways to higher brain centers are taken by cutaneous and visceral pain information. It is well-known that cutaneous inputs are relayed in the spinal cord onto spinothalamic tract cells that send their axons up the ventrolateral white matter on the opposite side. Recent clinical and basic studies have shown that visceral pain is relayed onto postsynaptic dorsal column cells in the spinal grey midline. Many of these postsynaptic dorsal column neurons send their axons through the dorsal column to relay their information at the dorsal column nuclei. The information travels with the lemniscal system to the thalamus and then to the cortex on the opposite side.
It was demonstrated in the animal studies that responses of neurons in the thalamus to noxious colorectal distention, gastric distension, and pancreatic activation with bradykinin are drastically reduced (80%) by a lesion (C1, T10) in the dorsal column [13–16]. The control lesions in the ventrolateral white matter interrupting the spinothalamic tract decreased thalamic tract cell responses by only 20%. Blockade of transmission of noxious visceral signals to higher centers by anesthesia of spinal neurons with intrathecal morphine has confirmed that a synaptic relay exists in the thoracic [14–16] and sacral spinal cord [13,17,18]. Thus, noxious visceral information is relayed to PSDC neurons in the medial spinal cord for transmission to the dorsal column nucleus. These cells are distinctly different from the lamina III PSDC neurons that are not believed to be involved in the transmission of nociception but are likely to be involved in mechanoreception [8]. Thus, the visceral nociceptive PSDC pathway is unique among all other known sensory pathways.
Cutaneous Sensory Pathways
It has been known for many years that large, heavily myelinated fibers ascending in the dorsal column are involved in the transmission of discriminative information related to light touch, vibration, and position in space (Fig. 3). Most of the cutaneous sensory information is carried by these large myelinated afferent nerve fibers into the spinal cord dorsal column pathway where they ascend uncrossed, directly to the dorsal column nucleus. From there this cutaneous sensory information is transmitted as the medial lemniscal pathway to the sensory relay nucleus in the contralateral thalamus (ventral posterolateral nucleus).
Cutaneous temperature and nociceptive information, conversely, are relayed across at least one synapse in the spinal cord onto spinothalamic tract cells located primarily in laminae I, IV, and V [19]. Sensory processing of nociceptive and temperature information received from cutaneous tissue initially involves small unmyelinated (C fibers) and small finely myelinated (Aδ fibers) afferent nerve fibers. Spinothalamic tract cells in the dorsal horn receiving this information send their axons across the midline to the lateral or ventrolateral white matter (Fig. 3). They send their axonal fibers directly to the thalamus and from there to regions of the somatosensory cortex [19]. It has been assumed previously that visceral nociceptive information is relayed in a similar manner to higher brain centers, although substantial evidence was lacking as to the specifics.
Transmission of Visceral Sensation
It was the crossing of the spinothalamic tract axons in the midline that was initially the target of some of the surgical procedures attempting to relieve cancer pain. Numerous studies have reported extensive midline neurosurgical lesions designed for this purpose [20]. It was determined by Hirshberg et al. [1], however, that pelvic cancer pain could be successfully alleviated with a tiny midline incision in the dorsal column even when performed at the thoracic spinal level. His lesions in the thoracic cord were well away from crossing axons that carried nociceptive information about pelvic pain. Because the lesion was small, the patients’ lower body cutaneous sensation, vibratory, and position sense remained intact. Because it had been assumed that visceral nociceptive information was relayed to the thalamus through the ventrolateral spinothalamic tract, this classic notion could not explain the relief from pelvic cancer pain experienced by these patients.
Successful pain reduction for patients with visceral pain has been reported not only by neurosurgeons in Galveston, Texas, and Houston, Texas, but also in Germany and Korea [4,5]. Limited or punctate midline myelotomies in these patients allow narcotic levels to be tapered or discontinued. The surgical procedure allowed resumption of activities, such as going fishing on the day prior to death in one instance. Clinical description of the limited myelotomy enabling patients’ freedom of activity, elimination of thalamic neuronal responses in animals after dorsal column lesions, and the anatomic tract tracing studies together suggested that pelvic visceral pain is relayed by a new nociceptive pathway in the dorsal column midline located between, but unique and separate from, the classically described axons carrying mechanoreceptive information. Thus, the clinical and animal studies provide a firm basis to support the hypothesis recently articulated that visceral pain is a separate entity from cutaneous pain [21,22]. Some of the recent information available in the literature relevant to differentiation of the new visceral pain pathway is discussed next.
Defining a New Visceral Pain Pathway
Visceral afferent fibers and their receptor pharmacology
Anatomic considerations
The peripheral endings of visceral primary afferent fibers are localized in the wall or in the parenchyma of internal organs, in the vessels supplying the viscera, and in the serosal membranes that cover the internal organs. Their cell bodies are located in spinal dorsal root ganglia and cranial nerve ganglia. It has been known for many years that the perception of visceral pain is diffuse, poorly localized, and often “referred” to another body site. Most of the visceral afferent fibers are considered “silent” afferent fibers because they are minimally responsive under normal conditions. Very little nociceptive information is transmitted from the visceral afferent fibers other than about the state of distension, even in the face of frank damage. It is likely, however, that studies will continue to be forthcoming that indicate that responses of visceral afferents are dramatically altered in an inflammatory state.
Neuromediators of visceral nociception
The neuromediators involved in normal versus hypersensitive states have been extensively reviewed recently [23••]. Findings reported more recently since that review for only a few specific mediators are mentioned in a brief summary next.
Although cutaneous afferent fibers are of both the nonpeptidergic and peptidergic type, visceral afferent fibers appear to be primarily of the peptidergic variety [24]. This difference in the distribution of peptide expression in visceral afferents suggests a relatively more important role for peptides in the transmission of information from the viscera that may have implications for the development of therapy for visceral pain. It is very likely that peptidergic afferent fibers are involved in both visceral and cutaneous nociception, and some functional relationship between these two modalities may exist. For example, Wesselmann and Lai [25] have found that dye extravasation in the cutaneous receptive field that would have been induced by vascular release of calcitonin gene-related peptide (CGRP) and substance P, occurs in an inflammatory uterine pain model. A “by-stander” effect was also evident in studies in which retrograde infection of somatic neurons led to activation of sensory and autonomic circuits innervating the bladder, resulting in neurogenic inflammation of the bladder [26•].
Peptide receptors
Peptide content in visceral afferents also includes some of the same peptides found in cutaneous nerves such as the tachykinin, substance P. Although some of the peptide content may be similar, different receptor subtypes may be functionally relevant at different sites. Transgenic mice that lack the receptor for substance P do not develop hyperalgesia after some types of visceral inflammation and noxious chemical stimuli, whereas they do develop hyperalgesia after inflammation of somatic tissues [27,28].
Data is also available to suggest that specific peptide receptor subtypes will differentially affect control of gastrointestinal motility and modulation of visceral pain. After the development of inflammation, NK1 receptors mediate gastric emptying inhibition and NK2 receptor inhibition reduces the nociceptive responses [29]. Peripheral NK3 receptors are involved in the mediation of both visceral nociception and gastrointestinal disorders, particularly in the colon [30]. The NK1 and NK2 but not NK3 receptors are involved in the distension pain response in the rat jejunum, however [31]. Although clinical trials for substance P antagonists have not proved to be effective for some types of pain, these studies suggest they may be effective in the treatment of some forms of visceral pain, including gastrointestinal pain.
The neurokinin-mediated gastrointestinal responses also involve the release of CGRP. CGRP has been shown to be important in the sensitization of capsaicin-sensitive visceral afferent nerves of gastrointestinal origin [32]. This study has shown that a significantly increased number of abdominal contractions occur in response to colorectal distension as a result of intracolonic irritation with mild acetic acid or intravenous CGRP. Complete reversal of the sensitizing effect of acetic acid was observed after use of the intravenous peptide antagonist, CGRP [8,9,10•,11,12•,13–22,23•,24,25,26•,27–37].
Monoaminergic receptors
In addition to peptides, a number of monoaminergic agents are present in inflammatory states and are responsible for the sensitization of nerve endings in the viscera, including 5-hydroxytryptamine and histamine. Serotonin is involved in the sensitization of primary afferent fibers. Various serotonin receptor subunit antagonists have also been found to be effective in the reduction of visceral nociception. Antinociceptive effects of serotonin receptor antagonists have been compared recently in gut distension models [33]. Whereas activation of 5-HT1A receptors in the central nervous system has been shown recently to increase gastric tone as well as decrease gastric sensitivity to distension [34], the majority of previous studies has focused on peripheral pharmacology. Antinociceptive responses to the serotonin 5-HT3 receptor antagonist have been shown to be effective when administered either centrally or peripherally [35]. Antagonism of peripheral 5-HT4 receptors reduces both visceral and cutaneous pain in mice, and induces visceral analgesia after simultaneous inactivation of 5-HT3 receptors [36].
The role of another amine, specifically histamine H1 receptors, in both somatic and visceral pain perception has been demonstrated using a mouse knockout model [37]. A report of enhanced receptor responses in the inflamed state is clearly demonstrated in a study in which interleukin-1β sensitizes abdominal visceral afferents of cats to histamine [38].
Opiate receptors
Activation of peripheral kappa-opioid receptors with agonists has been found to be highly effective in reducing nociception in a dose-dependent manner in an animal model of combined colonic irritation and distension [39]. Noxious urinary bladder distension was also effectively controlled with a kappa but not with mu or delta opioid receptor agonists [40]. These basic studies imply that kappa-opioid receptors might potentially be effective for use peripherally in a variety of visceral pain conditions.
Spinal neurons activated by visceral afferent nerve fibers
Anatomic studies
Localization of neurons that express the Fos protein in various animal models of visceral pain has proved to be a useful marker for the identification of neurons responsive to visceral activation. Although relatively little Fos protein is found in the spinal cord under normal conditions, bladder or colon distension and other visceral pain models produce an increase in the numbers of Fos-labeled neurons. Fos-labeled cells defining sites of renal pain processing after ureteral obstruction have been reported in laminae I, lateral IV–V, and medial VII and X [41].
Another recent study found that after cyclophosphamide-induced bladder inflammation, bladder distension now produced a greater number of spinal dorsal horn neurons expressing the Fos protein [42••]. They also noted that the distribution pattern of Fos-labeled neurons in the lumbosacral spinal cord is altered after distension when the bladder is inflamed. In addition to increased numbers of Fos-labeled cells in the dorsal horn, after bladder inflammation a large population of Fos-labeled cells (45%) is located around the central canal in the dorsal commissural nucleus (lamina X). The activation of the Fos protein in this cell population along with our electrophysiologic recordings of increased activity after visceral stimulation in PSDC neurons around the central canal suggest that this population is the second-order spinal cord neurons relaying visceral input to higher brain centers.
Pharmacologic studies
Pharmacologic evidence has been found for the spinal activation of visceroresponsive neurons mediated by glutamate receptors. In fact, N-methyl-D-aspartate (NMDA) receptor activation contributes significantly more to noxious than to non-noxious colorectal distension-induced Fos expression [43]. Spinal neuronal activity induced by colorectal distention was reduced 35% and 65%, respectively, by selective NMDA and non-NMDA glutamate receptor antagonists [44], demonstrating a role for both of these receptors in the modulation of visceral nociceptive processing. When combined with intrathecal lidocaine, 6-cyano-7-nitroquinox-aline-2,3-dione (CNQX) produced significant visceral antinociception to colorectal distension in response to doses to which there were no responses when given alone [45].
Response states of spinal neurons
Comparison of the response state of spinal neurons before and after activation of visceral afferent fibers has also revealed a number of interesting findings. In a model of experimental urinary calculosis that produced muscle hyperalgesia, spinal neurons receiving muscle input displayed changes indicative of central sensitization, including increased background activity and a significantly increased number of responsive cells [46]. Cells in the spinal cord have been identified that were either inhibited by the distension of an inflamed colon or activated in either an abrupt manner or with a sustained increase in baseline activity [47]. Colonic inflammation with acetic acid provoked significant increases in the responses of the cells with sustained firing, but not after pinching the somatic receptive field [48].
Prior inflammatory insults also greatly increase activation of spinal visceroresponsive neurons long term. Dorsal horn neurons of neonatal rats exposed to complete Freund’s adjuvant have long-standing functional increases in firing rates in response to brush and noxious pinch [49••]. Increases in afferent nerve innervation of the dorsal horn were also evident. In a model of uterine/vaginal inflammation, it was found that the activation of spinal neurons after previous exposure to vaginal inflammation (9 days prior) produced a marked increase in Fos expression compared with that of rats with no previous insult [50••]. These studies provide strong evidence that a prior state of sensitization, particularly early life experience, has a significant affect on responsiveness to noxious input.
Central pathways relaying visceral information to higher brain centers
Studies reported in a series of manuscripts by the Galveston/Houston pain group have demonstrated that a population of visceroresponsive PSDC neurons is scattered around the central canal of the spinal cord that send their axons through the dorsal column to the dorsal column nucleus [1,3••, 13–15,17,18]. These PSDC neurons respond to innocuous and noxious cutaneous as well as to mechanical and chemical noxious visceral stimuli [17]. Electrophysiologically characterized responses of medial PSDC and thalamic neurons to electrical and chemical activation of thoracic and pelvic visceral afferent fibers have been recorded.
Although our studies describing the new visceral pain pathway provide clear evidence that information about visceral pain is transmitted by axons of PSDC cells, literature is available in support of the notion that visceromotor information is also transmitted through the lateral and ventrolateral white matter of the spinal cord [7,20,51, 52,53•]. Visceroresponsive neurons in the spinal cord described previously in the literature are primarily localized in the dorsal horn, are responsive primarily to cutaneous input, and include spinothalamic tract, spinohypothalamic, spinoreticular, spinoparabrachial, and spinoamygdalar neurons [19,54]. The pathways described next by us recently, however, are those portions of the input sent to higher centers arising from neurons situated around the spinal cord central canal in a region of the spinal cord known to be associated with visceral function.
Midline dorsal column visceral pain pathway
The axons of PSDC neurons transmitting pelvic visceral nociception travel uncrossed in the dorsal column [1,10•]. The primary termination of the visceral input of the PSDC cells is the dorsal column nucleus. These third-order neurons relay the information to the contralateral thalamus as the medial lemniscal pathway. Other major brain sites receiving direct spinal input from neurons revealed by these and other recent tract tracing studies included the medullary reticular formation, the raphe, the periaqueductal gray, the parabrachial nucleus, the hypothalamus, the central nucleus of the amygdala, and medial regions of the thalamus [10•,55–57]. These brain regions are known to be concerned with the control of visceral function and the affective perception of pain. They have been shown to express the Fos protein after activation of visceral afferent nerve fibers in a variety of animal models [58]. Fos protein expression has also been shown to appear in the ventral reticular formation and the central nucleus of the amygdala after cyclophosphamide-induced inflammation of the bladder [59] and colorectal distension [60].
Although the initial clinical reports indicated that pelvic visceral pain is relieved by midline dorsal column lesions, the question remained as to whether this surgical method would be useful for the control of pain arising from thoracic levels. Because nociceptive information from the pelvic viscera is relayed by a medial PSDC pathway, it might have been reasonable to assume that midline dorsal column lesions could also alter the transmission of neuronal information transmitted to higher centers from thoracic spinal cord levels. Both a clinical report and studies in animals, however, are providing important clarification distinguishing the specific route taken by ascending neuronal input from thoracic viscera.
Intermediate dorsal column visceral pain pathway
Detailed neuronal tract tracing studies began with the comparison of the parallel ascending projections from the medial PSDC neurons located in both the thoracic and sacral spinal levels [10•]. A small injection of Phaseolus vulgaris leucoagglutinin (PHA-L) was made into the region around the central canal at the T7 level of the spinal cord. The marker is taken into the spinal neurons and transported along the length of their ascending axonal fibers. This method provided direct visualization of the route taken by the axonal pathway and the axonal termination sites in higher brain centers. The map of the distribution of ascending axons and terminations of the PSDC pathway revealed the “viscerotopic” relationship that exists between the pelvic (S1) and thoracic (T7) visceral nociceptive pathways in the dorsal column (Fig. 2). While the visceral nociceptive pathway arising from lumbosacral regions of the spinal cord sends axons in the dorsal column midline adjacent to the dorsal median septum, axons of the thoracic PSDC visceral pathway travel at the lateral edge of the fasciculus gracilis. This position is adjacent to the dorsal intermediate septum separating the gracile and cuneate fasciculi.
Ventrolateral pathway to the brain stem
Neuronal tract tracing and lesion studies have shown that cells around the central canal of the spinal cord also send axons via another route, in the ventrolateral white matter [10•,53•]. The visceroreceptive pathway traveling in the ventrolateral white matter sends a large number of axonal terminations to the medullary reticular formation on the ipsilateral side. More importantly, the Ness study [53•] extended our previous anatomic studies by demonstrating that although lesions of the dorsal column attenuated thalamic responses to noxious colorectal distention, the pseudoaffective visceromotor/cardiovascular responses and evoked activity recorded from medullary neurons were only eliminated by bilateral and ipsilateral lesions of the lateral spinal white matter, respectively.
Functional significance of the thoracic visceral pain pathway
Recently, models of pancreatic pain in rats have been developed in our laboratory with the aim of devising a means of abrogating pathophysiologic pancreatic input with therapeutic applicability. With acute pancreatitis models, the entire pancreas had clinically relevant inflammatory, histopathologic, and behavioral abnormalities [15,16]. Since our anatomic data determined that the intermediate dorsal column is a route for the relay of pancreatic nociceptive input to sensory processing centers in the brain with a trajectory situated more lateral to the anatomic position held by the recently described midline dorsal column pelvic relay, studies were begun to determine if more laterally placed dorsal column lesions could significantly reduce behavioral and thalamic neuronal responses to noxious inflammatory pancreatic stimulation.
The results of the pancreatic inflammation studies have determined that reductions in homecage behavioral activity were significantly abrogated by dorsal column pathway lesions if the lesion included the lateral edge of the fasciculus gracilis [16]. Thus, disruption of the pancreatic nociceptive transmission with lesions situated in the intermediate dorsal column visceral pathway can restore behavioral activity reduced by noxious stimulation of the pancreas.
Responses of thalamic neurons
Responses of thalamic neurons to stimulation of the pancreas with bradykinin have also been compared before and after dorsal column lesions to determine the extent of alteration from baseline induced by activation of pancreatic afferent fibers (Figs. 4 and 5) [15]. Lesions situated medially (as control lesions) in the dorsal column were not as effective as lesions that included the intermediate portion of the dorsal column at the border between the gracile and cuneate fascicles. These studies determined that carefully placed dorsal column lesions significantly reduced responses of neurons in the dorsal column nucleus and thalamus to pancreatic stimulation. Thus, because lesions of the intermediate dorsal column can completely or significantly abrogate pain-related responses to noxious stimulation of the pancreas as well as gastric distension [14], it is assumed that this is a primary route for the transmission of visceral input from the thoracic levels.
Figure 4.
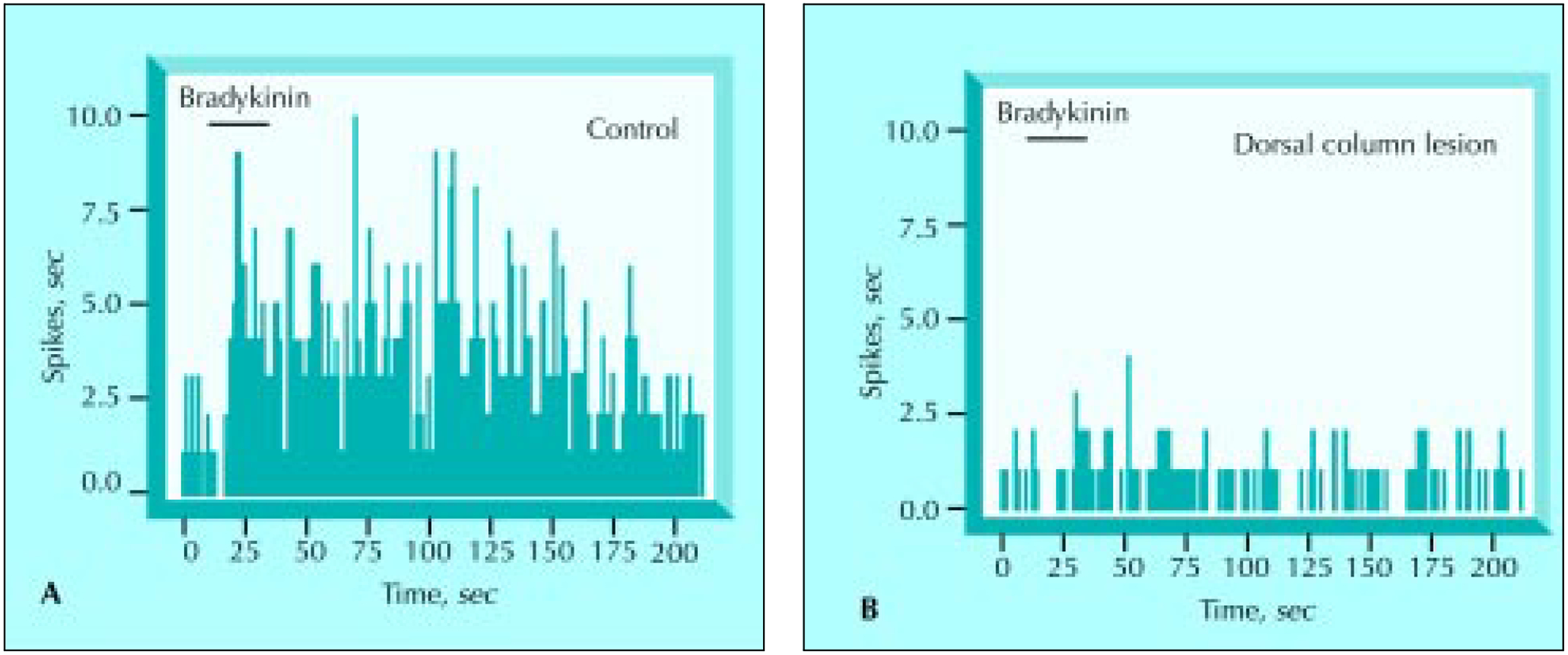
Rate histograms showing the responses of a thalamic cell to bradykinin (10−5 m, 20 sec) application both A, before and B, after dorsal column lesions. (From Houghton et al. [15].)
Figure 5.
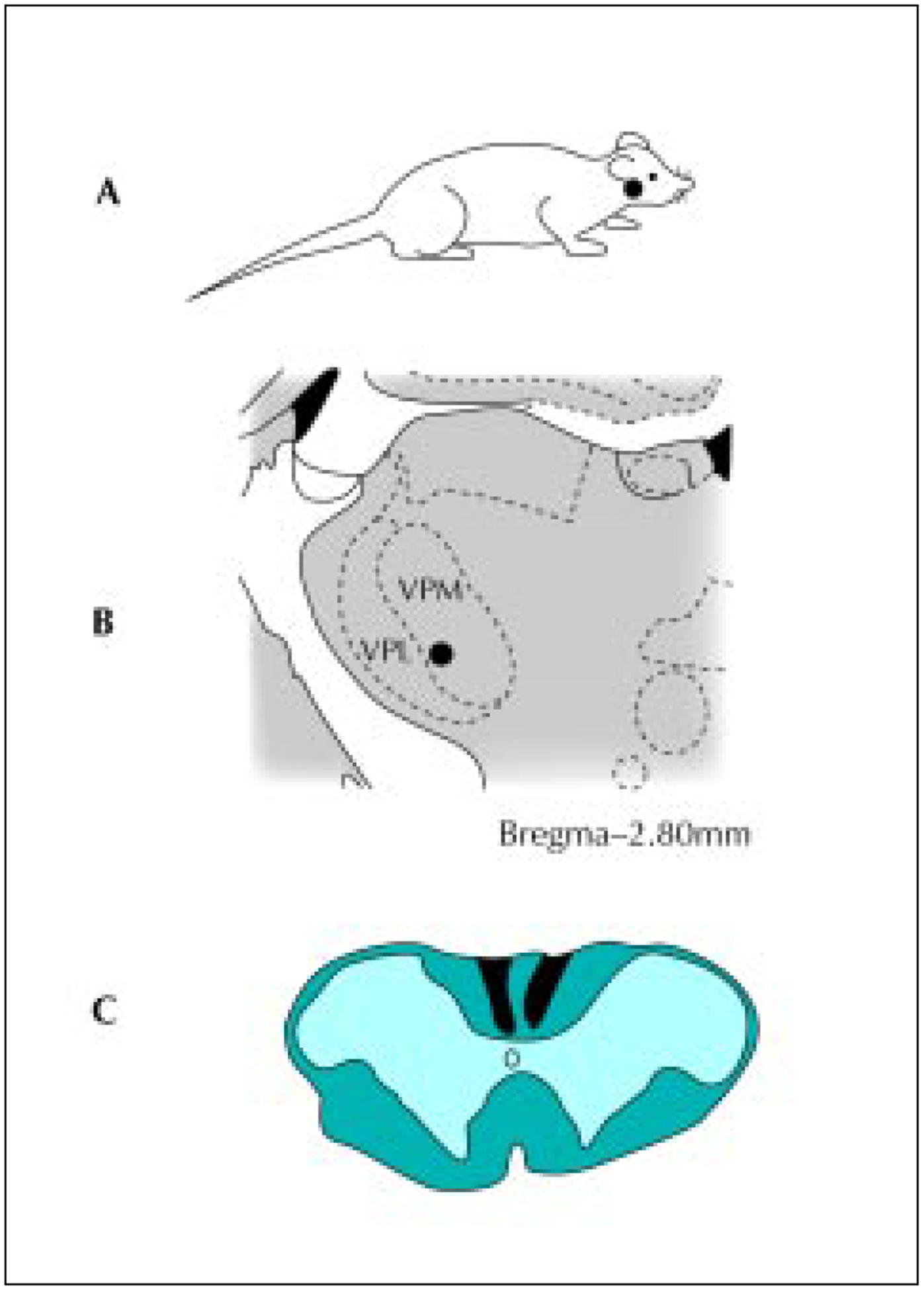
A, The dark area delineates the cutaneous receptive field on the face of a thalamic cell responsive to bradykinin activation of the pancreas. B, The location of the thalamic cell in the ventroposteromedial (VPM) nucleus. C, Camera lucida drawing of the lesion site (black area) reconstructed from multiple spinal cord sections from the same animal. VPL—ventroposterolateral.(From Houghton et al. [15].)
Clinical Significance
The anatomic and physiologic studies have confirmed the significance of the route taken by the neuronal pathway mediating the transmission of thoracic nociceptive information. These studies have determined that lesions must be placed along the lateral edge of the gracile fasciculus rather than the dorsal midline in order to disrupt the transmission of noxious visceral nociceptive information from the thoracic regions. The clinical significance of a separate visceral pain pathway located relatively accessibly in the dorsal column is enormous as an alternate means of pain control for patients with few alternative therapies.
Kim and Kwon [5] recently described their attempts to eliminate severe stomach cancer pain in patients with difficult failed pain control using high thoracic (T1 or T2) dorsal column myelotomies. It should be noted that at T1 there is no cuneate fasciculus, which is only found at cervical levels of the spinal cord. For elimination of the stomach pain, these neurosurgeons made their myelotomy with a neurosurgical microdissector inserted approximately 1 mm lateral to the midline down to a depth of 5 mm. Specific mention is given that efforts were made to avoid injury to the fasciculus gracilis. In fact, only one patient is reported to have experienced dorsal column signs and paresthesia below T4 after the myelotomy, in a case in which it was noted that the surgical lesion was large. In two other patients, transient paresthesias were successfully treated with corticosteroids. Nonetheless, it was possible to successfully relieve thoracic pain in five of their eight patients and spare other dorsal column functions of the gracile fasciculus. Based on our studies in animals, it is likely that these off-midline myelotomies eliminated the ascending axons of the thoracic visceral PSDC pathway traveling at the lateral edge of the gracile fasciculus at the T1-T2 spinal cord level (Fig. 3.) [10•]. Thus, a wealth of recent clinical and animal studies is now available to provide a firm basis to support the idea that visceral pain is a separate anatomic and mechanistic entity from cutaneous pain. These exciting studies should provide further stimulus for study into insights that may improve therapies for currently intractable pains caused by visceral cancer, and obstructive and inflammatory conditions.
Conclusions
Several clinical studies have reported that both pelvic and thoracic visceral pain is relieved by specifically placed punctate dorsal column lesions.
Information about visceral pain is primarily transmitted to higher brain centers from spinal cord PSDC neurons located around the central canal (lamina X, dorsal commissural nucleus).
Thoracic visceral nociceptive input travels to the brain stem at the lateral edge of the fasciculus gracilis.
Pelvic visceral nociceptive input travels to the brain stem in the dorsal column midline.
Activation of spinal neurons by visceral nociceptive input is increased in states of inflammation as indicated by increased numbers of Fos-labeled cells, including many more lamina X cells. Neonatal nociceptive insults can result in increases in primary afferent innervation and visceral nociceptive reactivity.
Comparisons and contrasts of cutaneous and visceral nociception are noted that may be relevant to specific treatments for visceral pain. Pharmacology of visceral activation includes peptide and aminergic receptor neuromediators. In contrast to cutaneous nociceptive input, kappa opiate receptor activation is effective in inducing visceral pain.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hirshberg RM, Al-Chaer ED, Lawand NB, et al. : Is there a pathway in the posterior funiculus that signals visceral pain? Pain 1996, 67:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauta HJ, Hewitt KN, Westlund KN, Willis WD: Surgical interruption of a midline dorsal column visceral pain pathway. J Neurosurg 1997, 86:538–542. [DOI] [PubMed] [Google Scholar]
- 3.••.Nauta HJ, Soukup VM, Fabian RH, et al. : Punctate midline myelotomy for the relief of visceral cancer pain. J Neurosurg 2000, 92:125–130. [DOI] [PubMed] [Google Scholar]; This study offers clinical support for the concept that neurosurgical interruption of a midline posterior column pathway with a punctate midline myelotomy provides significant pain relief of pelvic visceral pain without causing adverse neurologic sequelae. Clinical case reports for six patients, including histologic verification of the lesion site from one patient, are presented.
- 4.Becker R, Sure U, Bertalanffy H: Punctate midline myelotomy. A new approach in the management of visceral pain. Arch Neurochir (Wien) 1999, 141:881–883. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Kwon SJ: High thoracic midline dorsal column myelotomy for severe visceral pain due to advanced stomach cancer. Neurosurgery 2000, 46:85–90. [PubMed] [Google Scholar]
- 6.Westlund KN, Hirshberg RM, Lawand NB, et al. : Anatomical evidence for a visceral pain pathway in the dorsal column. Abstr Int Assoc Stud Pain 1996, 8:447. [Google Scholar]
- 7.Angaut-Petit D: The dorsal column system. II. Functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp Brain Res 1975, 22:471–493. [DOI] [PubMed] [Google Scholar]
- 8.Giesler GJ Jr, Cliffer KD: Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res 1985, 326:347–356. [DOI] [PubMed] [Google Scholar]
- 9.Cliffer KD, Giesler GJ Jr: Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J Neurosci 1989, 9:3146–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Wang C-C, Willis WD, Westlund KN: Ascending projections from the area around the spinal cord central canal: a phaseolus vulgaris leucoagglutinin study in rats. J Comp Neurol 1999, 415:341–367. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the differential projections of the thoracic and lumbosacral PSDC neurons that are found in the visceral processing region around the central canal of the spinal cord. The cells arethe origin of a new visceral pain pathway that travels in the dorsal column midline from lumbosacral levels of the spinal cord and are sandwiched intermediately in the dorsal column between the gracile and cuneate fasciculi when arising from thoracic levels following a viscerotopic organization.
- 11.Willis WD, Al-Chaer ED, Quast MJ, Westlund KN: A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci U S A 1999, 96:7675–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.•.Al-Chaer ED, Feng Y, Willis W: A role for the dorsal column in nociceptive visceral input into the thalamus of primates. J Neurophysiol 1998, 79:3143–3150. [DOI] [PubMed] [Google Scholar]; These studies extend previous demonstrations of a new visceral pain pathway in the dorsal column to the primate. They confirm that a small electrolytic or chemical lesion with kianic acid in the dorsal column nucleus dramatically reduces responses of thalamic neurons to colorectal distension.
- 13.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD: Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 1996a, 76:2661–2674. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Cui M, Al-Chaer ED, Willis WD: Epigastric antinociception by cervical dorsal column lesion in rats. J Anesthesiol 1998, 89:411–420. [DOI] [PubMed] [Google Scholar]
- 15.Houghton AK, Wang C-C, Westlund KN: Do nociceptive signals from the pancreas travel in the dorsal column? Pain 2000, in press. [DOI] [PubMed] [Google Scholar]
- 16.Houghton AK, Kadura S, Westlund KN: Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport 1997, 8:3795–3800. [DOI] [PubMed] [Google Scholar]
- 17.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD: Pelvic visceral input to the nucleus gracilis is largely mediated by postsynaptic dorsal column pathway. J Neurophysiol 1996b, 76:2675–2690. [DOI] [PubMed] [Google Scholar]
- 18.Al-Chaer ED, Westlund KN, Willis WD: Nucleus gracilis: an integrator for visceral and somatic information. J Neurophysiol 1997, 78:521–527. [DOI] [PubMed] [Google Scholar]
- 19.Willis WD, Coggeshall RE: Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. [Google Scholar]
- 20.Gybels JM, Sweet WH: Neurosurgical Treatment of Persistent Pain. Basel, Switzerland: Karger; 1989. [PubMed] [Google Scholar]
- 21.McMahon SB: Are there fundamental differences in the peripheral mechanisms of visceral and somatic pain? Behav Brain Sci 1997, 20:381–381. [DOI] [PubMed] [Google Scholar]
- 22.Willis WD,Westlund KN: Anatomy and physiology of pain In Textbook of Stereotactic and Functional Neurosurgery. Edited by Guildenberg P, Tasker R. Ontario, Canada: McGraw Hill; 1997:1289–1310. [Google Scholar]
- 23.••.Bueno L, Fioramonti J, Delvaux M, et al. : Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology 1997, 112:1717–1743. [DOI] [PubMed] [Google Scholar]; This comprehensive review of mediators of visceral pain and the relevant animal models is useful for clinical comparisons and includes over 300 references. The review highlights the paramount importance of sensitization of afferent nerve endings by serotonin, bradykinin, tachykinins, CGRP, and neurotrophins. It reviewsthe current understanding of these mediators and their potential usefulness for clinical efficacy.
- 24.Perry MJ, Lawson SN: Differences in expression of oligosaccharides, neuropeptides, carbonic anhydrase and neurofilament in rat primary afferent neurons retrogradely labeled via skin, muscle or visceral. Neuroscience 1998, 85:293–310. [DOI] [PubMed] [Google Scholar]
- 25.Wesselmann U, Lai J: Mechanisms of referred visceral pain: uterine inflammation in the adult virgin rat results in neurogenic plasma extravasation in the skin. Pain 1997, 73:309–317. [DOI] [PubMed] [Google Scholar]
- 26.•.Jasmin L, Janni G, Manz HJ, Rabkin SD: Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J Neurosci 1998, 18:10016–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report presents a model of neurogenic cyctitis induced by viral infection of specific circuits of the central nervous system. In this model, retrograde viral infection by the pseudorabies virus of somatic neurons neighboring those that innervate the bladder leads to activation of sensory and autonomic visceral circuits innervating the bladder and resulting in a neurogenic inflammation of the bladder. Infection of the bladder does not occur if the bladderis denervated or its central nervous system circuits are disrupted.
- 27.Cervero F, Laird MA: Pain: visceral pain. Lancet 1999, 353:2145–2148. [DOI] [PubMed] [Google Scholar]
- 28.Laird JM, Olivar T, Roza C, et al. : Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 2000, 98:345–352. [DOI] [PubMed] [Google Scholar]
- 29.Julia V, Bueno L: Tachykininergic mediation of viscerosensitive responses to acute inflammation in rats: role of CGRP. Am J Physiol 1997, 272:G141–146. [DOI] [PubMed] [Google Scholar]
- 30.Julia V, Su X, Bueno L, Gebhart GF: Role of neurokinin 3 receptors on responses to colorectal distention in the rat: electrophysiological and behavioral studies. Gastroenterology 1999, 116:1124–1131. [DOI] [PubMed] [Google Scholar]
- 31.McLean PG, Garcia-Villar R, Fioramonti J, Bueno L: Effectsof tackykinin receptor antagonists on the jejunal distension pain response. Eur J Pharmacol 1998, 345:247–252. [DOI] [PubMed] [Google Scholar]
- 32.Plourde V, St-Pierre S, Quirion R: Calcitonin gene-related peptide in viscerosensitive responses to colorectal distension in rats. Am J Physiol 1997, 273:G191–196. [DOI] [PubMed] [Google Scholar]
- 33.Pappas TN, Mangel AW, Lawson C: Review article: evaluation of drugs in experimental gut distension models. Alimentary Pharmacol Ther 1999, 13:54–56. [PubMed] [Google Scholar]
- 34.Rouzarde ML, Fioramonti J, Bueno L: Decrease in gastric sensitivity to distension by 5-HT1A receptor agonists in rats. Dig Dis Sci 1998, 43:2048–2054. [DOI] [PubMed] [Google Scholar]
- 35.Miura M, Lawson DC, Clary EM, et al. : Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci 1999, 44:20–24. [DOI] [PubMed] [Google Scholar]
- 36.Espejo EF, Gil E: Antagonism of peripheral 5-HT4 receptors reduces visceral and cutaneous pain in mice, and induces visceral analgesia after simultaneous inactivation of 5-HT3 receptors. Brain Res 1998, 788:20–24. [DOI] [PubMed] [Google Scholar]
- 37.Mobarakeh JI, Sakurada S, Katsuyama S, et al. : Role of histamine H(1) receptor in pain perception: a study of the receptor gene knockout mice. Eur J Pharmacol 2000, 391:81–89. [DOI] [PubMed] [Google Scholar]
- 38.Fu LW, Longhurst JC: Interleukin-1beta sensitizes abdominal visceral afferents of cats to ischemia and histamine. J Physiol 1999, 521:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langlois A, Diop L, Friese N, et al. : Fedotozine blocks hypersensitive visceral pain in conscious rats: action at peripheral kappa-opioid receptors. Eur J Pharmacol 1997, 324:211–217. [DOI] [PubMed] [Google Scholar]
- 40.Su X, Sengupta JN, Gabhart GF: Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 1997, 77:1566–1580. [DOI] [PubMed] [Google Scholar]
- 41.Avelino A, Cruz F, Coimbra A: Sites of renal pain processing in the rat spinal cord. A c-fos study using a percutaneous method to perform uretal obstruction. J Auton Nerv Syst 1997, 67:60–66. [DOI] [PubMed] [Google Scholar]
- 42.••.Vizzard MA: Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 2000, 119:R1027–1039. [DOI] [PubMed] [Google Scholar]; This paper describes a greater increase in numbers and an altered topographic distribution for Fos expression in neurons when cystitis accompanies intravesicular distension with saline. After induction of cystitis by intravesicular cyclophosphamide, a large percentage (45%) of Fos-labeled neurons were distributed in the medial spinal cord (dorsal commissure).
- 43.Zhai QZ, Traub RJ: The NMDA receptor antagonist MK-801 attenutates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain 1999, 83:321–329. [DOI] [PubMed] [Google Scholar]
- 44.Song XJ, Zhao ZQ: Involvement of NMDA and non-NMDA receptors in transmission of spinal visceral nociception in cat. Chung Kuo Yao Li Hsueh Pao 1999, 20:308–312. [PubMed] [Google Scholar]
- 45.Imamachi N, Saito Y, Hara K, et al. : The non-NMDA glutamate receptor antagonist CNQX augments lidocaine antinociception through a spinal action in rats. Anesth Analg 1999, 89:416–421. [DOI] [PubMed] [Google Scholar]
- 46.Giamberardino MA, Valente R, Affaitati G, Vecchiet L: Central neuronal changes in recurrent visceral pain. Int J Clin Pharmacol Res 1997, 17:63–66. [PubMed] [Google Scholar]
- 47.Ness TJ, Gebhart GF: Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett 2000, 281:131–134. [DOI] [PubMed] [Google Scholar]
- 48.Olivar T, Cervero F, Laird JM: Responses of rat spinal neurons to natural and electrical stimulation of colonic afferents: effects of inflammation. Brain Res 2000, 866:168–177. [DOI] [PubMed] [Google Scholar]
- 49.••.Ruda MA, Ling Q-D, Hohmann AG, et al. : Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 2000, 289:628–630. [DOI] [PubMed] [Google Scholar]; This study investigated the impact of neonatal tissue injury and pain on the development of nociceptive circuitry and dorsal horn neuronal responsivity. Increases in afferent nerve innervation of the dorsal horn were evident. Dorsal horn neurons of neonatal rats exposedto complete Freund’s adjuvant had long-standing functional increases in firing rates in response to brush and noxious pinch.
- 50.••.Wesselmann U, Czakanski PP, Sanders C: Altered CNS Processing of nociceptive messages from the vagina in rats that have recovered from uterine inflammation In Proceedings of the 9th World Congress on Pain. Progress in Pain Research and Management. Edited by Devor M et al. Seattle, Washington: IASP Press; 2000, 17:1–8. [Google Scholar]; This manuscript reviews the clinical aspects of chronic pelvic pain, a debilitating pain problem for women. Previous studies with a recently developed animal model for inflammatory uterine pain are reviewed. The enhanced activation of visceroresponsive neurons to prior exposure of inflammation is reported.
- 51.Foreman RD: Organization of the spinothalamic tract as a relay for cardiopulmonary sympathetic afferent fiber activity. Prog Sensory Physiol 1989, 9:1–51. [Google Scholar]
- 52.Berkeley KJ, Hubscher CH: Are there separate central nervous system pathways for touch and pain? Nat Med 1995, 1:766–773. [DOI] [PubMed] [Google Scholar]
- 53.•.Ness TJ: Evidence for ascending visceral nociceptive information in the dorsal midline and lateral spinal cord. Pain 2000, 87:83–88. [DOI] [PubMed] [Google Scholar]; This study determined that colorectal distention–evoked activity in the thalamus is attenuated by lesions in the dorsal midline, whereas pseudoaffective visceromotor/cardiovascular responses were eliminated by bilateral lesions of the lateral spinal white matter. Responses of medullary neurons in the ventral reticular formation to colorectal distention were affected by ipsilaterally situated lesions of the lateral white matter.
- 54.Willis WD, Westlund KN: Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 1997, 40:968–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernard JF, Huang GF, Besson JM: The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol 1994, 71:1646–1660. [DOI] [PubMed] [Google Scholar]
- 56.Katter JT, Dado RJ, Kostarczyk E, Giesler GJ Jr: Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. 2: responses to cutaneous and visceral stimuli. J. Neurophysiol 1996, 75:2606–2628. [DOI] [PubMed] [Google Scholar]
- 57.Jasmin L, Burkey AR, Card JP, Basbaum AI: Transneuronal labeling of a nociceptive pathway, the spino(trigemino-) parabrachio-amygdaloid, in the rat. J Neurosci 1997, 17:3751–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodella L, Rezzani R, Gioia M, et al. : Expression of Fos immunoreactivity in the rat supraspinal regions following noxious visceral stimulation. Brain Res Bull 1998, 47:357–366. [DOI] [PubMed] [Google Scholar]
- 59.Bon K, Lanteri-Minet M, Michiels JF, Menetrey D: Cyclophos-phamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP). Exp Brain Res 1998, 122:165–174. [DOI] [PubMed] [Google Scholar]
- 60.Traub RJ, Silva E, Gebhart GF, Solodkin A: Noxious colorectal distention induced c-Fos protein in limbic brain structures in the rat. Neurosci Lett 1996, 215:165–168. [DOI] [PubMed] [Google Scholar]


