Abstract
Herein, we present a general synthetic strategy for the preparation of 3-, 4-, 5- and 6-membered heterocyclic unnatural amino acid derivatives by exploiting facile Mannich-type reactions between readily available N-alkyl- and N-aryl-substituted diisopropyl iminomalonates and a wide range of soft anionic C-nucleophiles without using any catalyst or additive. Fully substituted aziridines were obtained in a single step when enolates of α-bromo esters were employed as nucleophiles. Enantiomerically enriched azetidines, γ-lactones and tetrahydroquinolines were obtained via a two-step catalytic asymmetric reduction and cyclization sequence from ketone enolate-derived adducts. Finally, highly substituted γ-lactams were prepared in one pot from adducts obtained using acetonitrile-derived carbanions. Overall, this work clearly demonstrates the utility of iminomalonates as highly versatile building blocks for the practical and scalable synthesis of structurally diverse heterocycles.
Graphical Abstract

INTRODUCTION
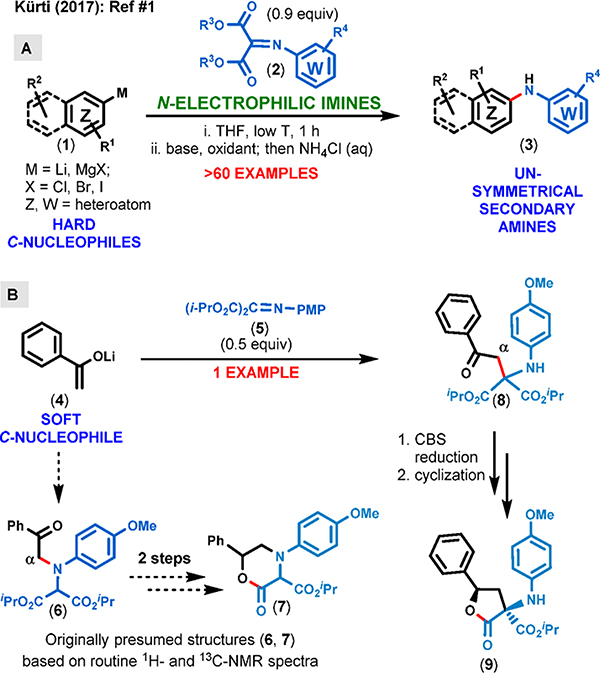
We recently reported the convenient preparation of novel N-electrophilic diisopropyl iminomalonates (2) derived from readily available primary amines and their application for the synthesis of a large number of secondary amines (3) using organometallic C-nucleophiles such as Grignard reagents and alkyl- and aryllithiums (1).1 These hard C-nucleophiles preferentially attacked the nitrogen atom of the imine moiety in the absence of catalysts or additives (Figure 1A). The experimental findings were also supported by DFT computational studies. In the same report we also disclosed the result of adding the lithium enolate of acetophenone 4 to the N-PMP-substituted diisopropyl iminomalonate 5 (Figure 1B). Based on routine NMR spectra (1H and 13C) of the major product, we assigned putative structure 6 in which the α position of acetophenone was aminated. Subsequent ketone reduction and cyclization afforded presumed compound 7; this 6-membered lactone structure was also in good agreement with routine NMR spectra. Since the focus of this past work was the unique N-selective addition of hard C-nucleophiles to iminomalonates, no in-depth spectroscopic studies were performed on compound 7 at that time.
Figure 1.
Exploring the reactivity of hard and soft nucleophiles with iminomalonates.
During the follow-up studies, when we focused our attention on exploring the reactivity of iminomalonates with softer C-nucleophiles such as enolates derived from ketones and esters along with carbanions obtained from acetonitrile derivatives, we performed 1D and 2D NMR studies on presumed compound 7. These studies strongly pointed to the presence of a 5-membered lactone scaffold (9) instead of the originally presumed 6-membered lactone scaffold (7). The only way a 5-membered lactone product (9) could have formed was to have a preferential attack occur at the iminomalonate carbon atom during the enolate addition step to afford compound 8.
This surprising switch in chemoselectivity was in stark contrast with the predominant N-attack of hard C-nucleophiles on a wide variety of N-substituted iminomalonates.1 Even though we originally anticipated predominant N-attack by most C-nucleophiles, based on the demonstrated reactivity pattern by Grignard reagents and alkyl/aryl lithium compounds, in-depth computational studies (vide infra) on the reactivity of iminomalonates toward soft C-nucleophiles confirmed our new experimental findings (Figure 1B).
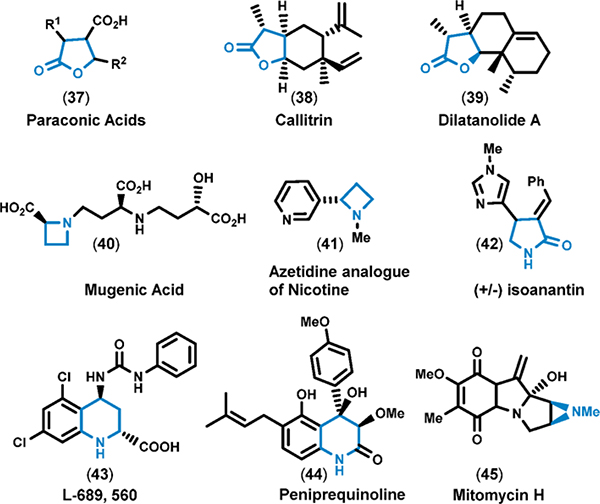
We identified three unique structural features of the 5-membered lactone product 9, namely: (a) two stereogenic centers with one being fully substituted, (b) an aminoaryl substituent in the α-position of the lactone carbonyl group, and (c) the generation of a highly substituted, unnatural cyclic amino acid derivative (Figure 1B). In fact, nonproteinogenic amino acids have become important tools for modern pharmaceutical research.2 While some occur naturally (e.g., ornithine, from urea cycle), the vast majority of these are chemically synthesized. Owing to their remarkable structural and functional diversity, they are often used as building blocks for drug discovery.2
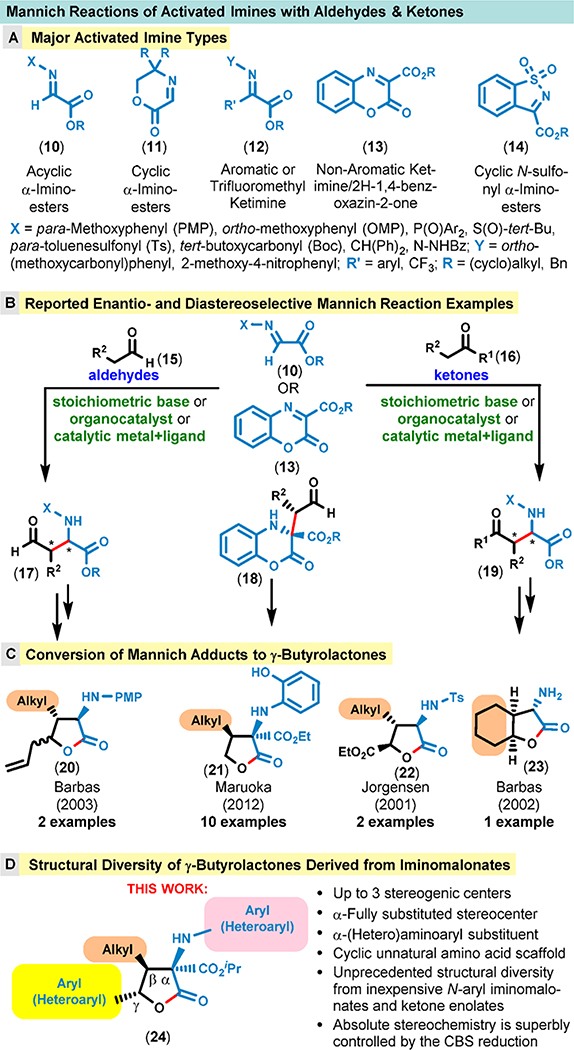
We also realized that there are only a few reported examples of these highly substituted 5-membered lactone scaffolds, and even fewer methods exist for their synthesis.3 A survey of reported Mannich reactions between activated imines (10–14) and aldehydes/ketones is shown in Figure 2. The Mannich reaction is one of the classic methods for the synthesis of β-amino carbonyl compounds.4–9 The versatility of this important carbon–carbon (C–C) bond-forming reaction lies in its potential to create both functional and structural diversity that generated a lot ofinterest in the synthetic community. However, the overwhelming majority of reported reactions produces compounds that have limited structural diversity with regard to both the imine and the carbonyl coupling partners.6 Even fewer reports attempted to make use of the initial Mannich adducts and convert these to more elaborated building blocks (i.e., γ-butyrolactones, Figure 2C).10 Of particular concern is the dearth of structural diversity with respect to the substituents on the nitrogen atom as well as in the γ-position of the lactone scaffold.
Figure 2.
Comparison of achievable structural diversity in known Mannich reactions and this study.
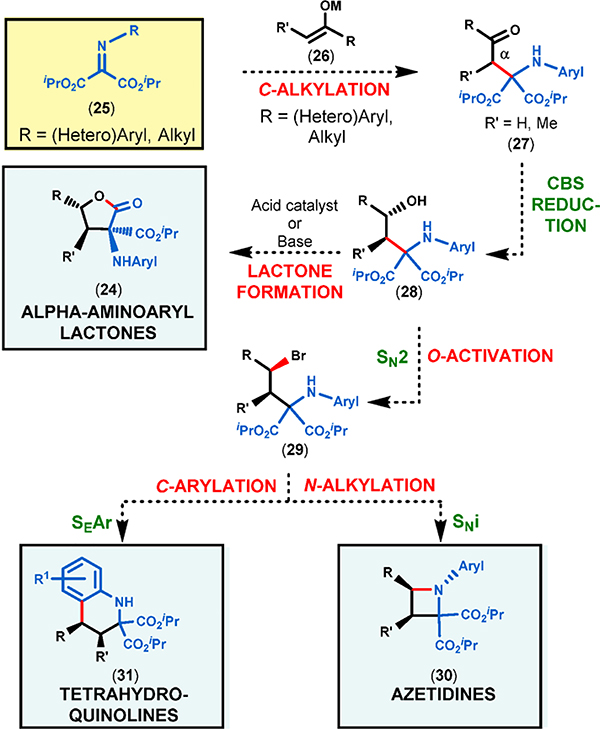
In contrast to known reports, we fully recognized the opportunity to convert our initial iminomalonate/ketone Mannich adducts into γ-butyrolactones (24) in which the nature of the substituents at each position (i.e., α, β, and γ), including the substituent on the nitrogen atom, can be widely varied (Figure 2D). This wide variation in the structure of substituents is possible because nearly all primary arylamines can be easily converted to the corresponding N-aryl iminomalonates1 (25) and virtually any enolizable ketone (26) can be coupled to these in the absence of catalysts or additives. We argued that the absolute stereochemistry could be readily controlled in the next step by the well-known and predictable CBS reduction of the carbonyl group (27 → 28, Figure 3).11
Figure 3.
Proposed synthesis of chiral nonracemic azetidines, tetrahydroquinolines, and lactones from iminomalonates via enantio- merically enriched secondary alcohols (28).
Moreover, the resulting enantiomerically enriched secondary alcohols (28) could open up a number of exciting possibilities for the synthesis of densely functionalized N- and O-heterocycles. For instance, activation of the benzylic alcohol moiety by inversion would allow for a subsequent intramolecular nucleophilic (SNi) substitution reaction by the N atom to furnish the corresponding azetidines (30) in which the benzylic stereocenter would retain its original configuration (i.e., double inversion). Alternatively, the aromatic ring attached to the nitrogen could also act as a nucleophile and perform the benzylic substitution with inversion in order to afford tetrahydroquinolines (31). A third option is to use the benzylic alcohol OH group as a nucleophile as we have already observed in our recent study (Figure 1B). Thus, an intramolecular nucleophilic acyl substitution reaction (SNAc; i.e., lactonization) is expected to take place with one of the isopropyl ester groups to yield α-aminoaryl γ-lactones (24) under either acid-catalyzed or base-mediated conditions (Figure 3).
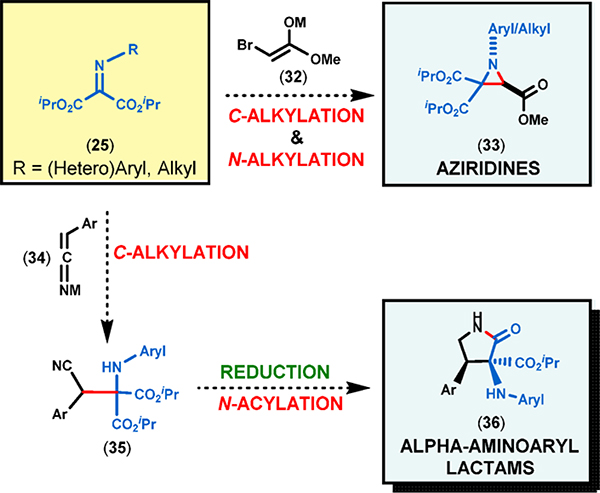
Additional synthetic possibilities for iminomalonates include a one-pot aza-Darzens-type reaction12 with haloester enolates to form aziridines 33 (Figure 4) and also a two-step sequence that involves the addition of nitrile enolates. Reduction of the nitrile to a primary amine followed by intramolecular N-acylation is expected to furnish α-aminoaryl γ-lactams 36 (Figure 4).
Figure 4.
Proposed synthesis of aziridines and lactams from iminomalonates (25).
These exciting synthetic possibilities prompted us to investigate the scope of enolates that would also exclusively attack the imine carbon of the iminomalonate substrates 25. Accordingly, we selected readily available esters, ketones, and nitriles and carried out the reaction ofiminomalonates with their corresponding enolates (32) or alkylnitrile-derived carbanions (34) under a variety of conditions. While simple ester enolates did not react with either N-aryl or N-alkyl iminomalonates, the Li enolate of the nonsterically hindered methyl bromoacetate quickly furnished the corresponding N-aryl- as well as N-alkylaziridines (33). The mechanism of this process is necessarily similar to an aza-Darzens reaction.12
A thorough literature search revealed that there were very few examples involving the addition of alkylnitrile-derived carbanions (e.g., 34) to activated imines such as α-iminoesters.13 From a synthetic perspective, alkylnitriles are versatile building blocks as they can be readily converted to primary amines, aldehydes, amides, and carboxylic acids.
Gratifyingly, N-aryl iminomalonates (25) displayed remarkable reactivity toward arylacetonitrile-derived carbanions (34), yielding β-nitriloamines (35). As anticipated, reduction of the nitrile moiety followed by in situ cyclization allowed us to transform these compounds into highly sought-after γ-lactams (36), which are popular targets among medicinal chemists.14 As in the case of γ-lactones, a fully substituted α-stereocenter could be generated bearing an aminoaryl and an alkoxycarbonyl group. The resulting γ-lactams all feature α-amino acid backbones and these compounds can be utilized as amino acid surrogates.
Based on these encouraging initial findings we decided to explore the full synthetic potential of adding soft C-nucleophiles to iminomalonates. Herein we provide the detailed report for the successful synthesis of structurally diverse 3-, 4-, 5-, and 6-membered heterocycles via sequential Mannich reaction and cyclization. Since these heterocyclic scaffolds are prevalent in a wide variety of natural products and drug molecules (Figure 5), a unified synthetic strategy that exploits readily available and inexpensive iminomalonates1 and enolates as starting materials would be a valuable addition to the toolbox of synthetic as well as medicinal chemists.
Figure 5.
Natural products and active pharmaceutical ingredients that contain 3-, 4-, 5-, and 6-membered heterocycles.
RESULTS AND DISCUSSION
The catalytic asymmetric synthesis of tetrasubstituted carbon stereocenters remains a significant challenge in organic synthesis.15 The stereoselective Mannich reaction between prochiral ketimines and enolates affords highly substituted chiral amines that are potentially valuable building blocks for further elaboration provided that the activating group, often present on the nitrogen, can be removed.10e Our investigation began with the systematic study of the Mannich-type reaction between nonprochiral (i.e., symmetrical) N-aryl iminomalonates and ketone-derived enolates (Figure 3) as this approach would allow the control of absolute stereochemistry in a variety of ways.
First, we combined 2 equiv of preformed acetophenone-derived lithium enolate with 1 equiv of p-methoxyphenyl iminomalonate (25a; Ar = PMP) at −78 °C in THF. The reaction proceeded well and exclusively furnished the C-addition product 27a in 66% yield (see the SI, pp S5 and S6). The nature of the lithium base used for the preformation of the ketone enolate in THF was important: LiHMDS afforded the highest isolated yield of 27a (66%), while n-BuLi as well as LDA resulted in a reduced yield of the Mannich adduct (54% and 22%, respectively). Next, the impact of the reaction temperature was evaluated in THF as the solvent. Increasing the temperature from −78 °C to first −40 °C and then to −20 °C led to decreasing yields of 27a (36% and 0%, respectively), and at −20 °C the reaction mixture became too complex.
Switching the solvent from THF to 2-Me-THF significantly lowered the isolated yield (66% → 27%). Other ethereal solvents such as diethyl ether, MTBE, dioxane, and DME were inferior compared to THF when LiHMDS was used as the base for the preparation of the preformed enolate (see the SI, pp S5 and S6).
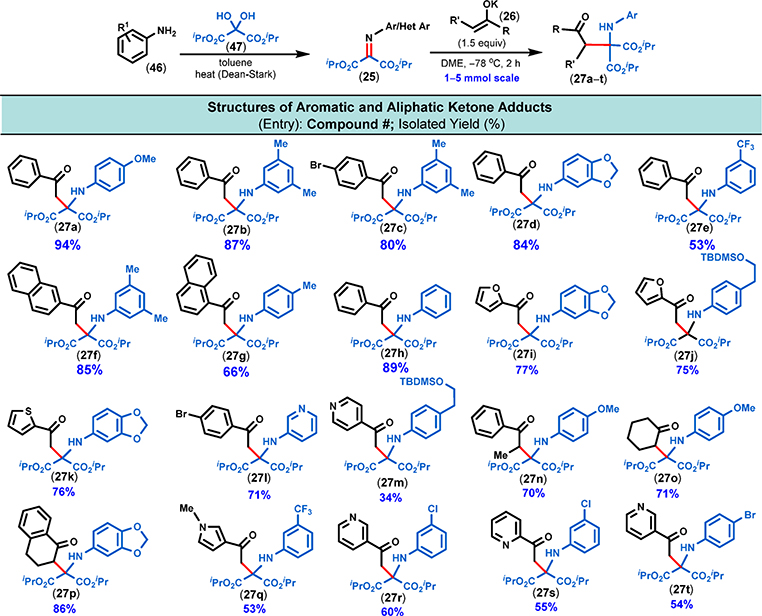
However, when KHMDS was employed as a base first in MTBE and then in DME at −78 °C, the yield of 27a dramatically improved to 61% and 70%, respectively. Subsequently, we examined the effect of the amount of enolate coupling partner on the isolated yield of 27a. Reducing the amount of the enolate from 2 to 1.1 equiv decreased the yield slightly (70%→ 63%); however, we found that employing 1.5 equiv of enolate coupling partner 26 was optimal, as it afforded 27a in 94% isolated yield. Changes in the concentration (0.1–0.3 M) did not have a significant effect on the isolated yield. Thus, the optimal Mannich coupling conditions called for the combination of reactants (i.e., 1:1.5 ratio of iminomalonate/potassium enolate) as a 0.2 M solution in DME at −78 °C (Figure 6).
Figure 6.
Scope of substrates using iminomalonates as electrophiles. All iminomalonates were prepared from the corresponding amines by simple condensation with ketomalonate hydrate. The enolate addition reactions were conducted on a 1–5 mmol scale with 0.2 M concentration of the iminomalonate at the indicated temperature and were considered complete upon the full consumption of the individual iminomalonates as determined by TLC analysis.
We were pleased to find that under the optimized coupling conditions a variety of N-aryl-substituted iminomalonates (25) smoothly reacted with different aromatic, heteroaromatic, and aliphatic ketone enolates (26), and the corresponding bench-stable Mannich adducts (27) were isolated in good to excellent yields. Ultimately, we explored the reactivity of 13 different ketone enolates with a diverse set of aromatic and heteroaromatic iminomalonates (10 structurally different examples, Figure 6).
Simple aromatic and aliphatic ketone-derived enolates afforded the corresponding Mannich adducts (27a–t, Figure 6) in excellent yields. However, in the case of nitrogen-containing heteroaromatic ketone enolates (e.g., pyridines and pyrroles; 27m, 27q–t), the yields were lower compared to oxygen- and sulfur-containing heteroaromatics (e.g., furans and thiophenes; 27i–k). Surprisingly, N-aliphatic iminomalonates were found to be unreactive with both aromatic and aliphatic ketone-derived enolates. This prompted us to use density functional theory (DFT) calculations to examine the reactivity and selectivity for enolate additions to these iminomalonates.
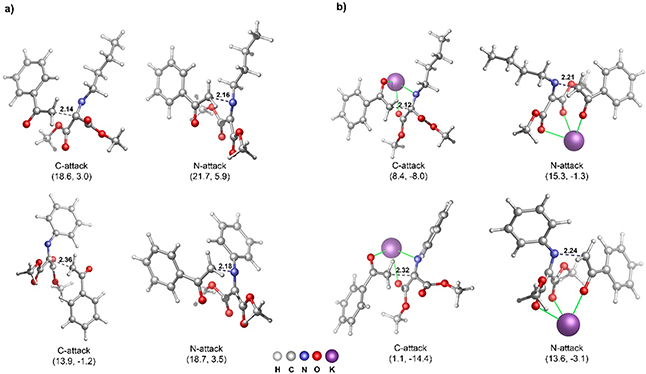
In Gaussian 09,26 we used the M06–2X27 functional with the def2-TZVP28 basis set for final SCF energies and 6–31G(d,p) and [LANL2DZ for Br] for geometries and thermochemical analysis. Tetrahydrofuran was used as a substitute for DME with the continuum SMD29 model. We used the N-Bu and N-Ph iminomalonates with methyl ester groups as model systems. For the enolates, we used the acetophenone-derived enolate (Figure 8a) as well as the potassium–oxygen stabilized version (Figure 8b).
Figure 8.
M06–2X/def2-TZVP//M06–2X/6–31G(d,p) transition-state structures and energies (Gibbs energy, enthalpy at 298 K) for (a) acetophenone-derived enolate and phenyl isopropyl ketone-derived enolate addition to N-Bu and N-Ph iminomalonates and (b) potassium-coordinated enolate addition to N-Bu and N-Ph iminomalonates. Energies are relative to separated reactants and in kcal/mol.
As expected from the outcome of the reactions presented in Figure 6, enolate addition to the N-Bu and N-Ph iminomalonate carbon is lower in Gibbs free energy (and enthalpy) than addition to the nitrogen. For the unstabilized enolate model, the C-attack transition states are 3–4 kcal/mol lower in energy. Interestingly, using the potassium-stabilized enolate model, the selectivity is much higher: the C-attack transition states are ~7–12 kcal/mol lower in energy than N-attack transition states, which is consistent with the experimental observation of C-attack products. The transition states also confirm that the N-Bu iminomalonate is significantly less reactive than the N-Ph derivative. For example, the unstabilized enolate C-attack transition state for N-Bu iminomalonate requires ΔG‡ = 18.6 kcal/mol, while for the N-Ph derivative ΔG‡ = 13.9 kcal/mol. The potassium-enolate model transition states for C-attack show a >7 kcal/mol lower barrier for the N-Ph iminomalonate.
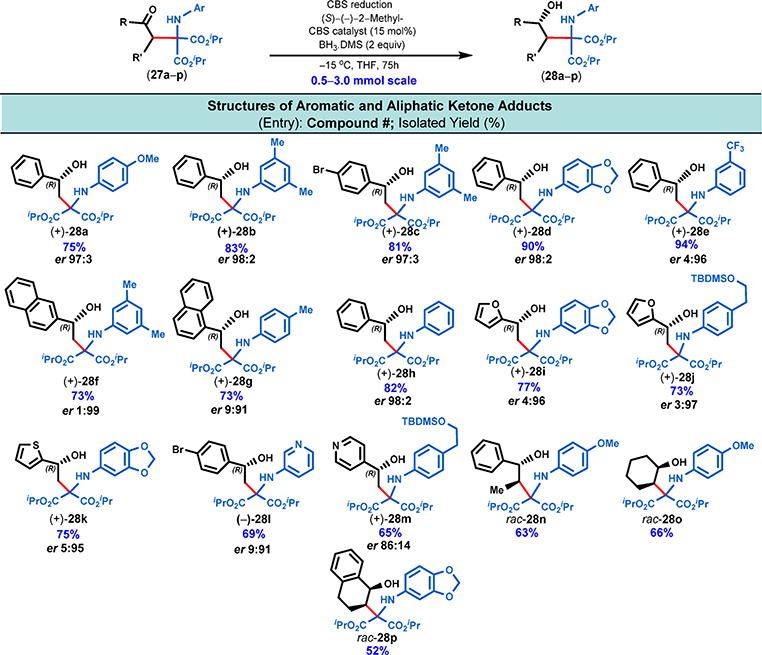
Next we proceeded to reduce the carbonyl group of the Mannich adducts utilizing the well-established CBS reduction (Figure 7).11 In order to identify the optimal conditions for the desired highly enantioselective carbonyl reduction (27 → 28), we carefully evaluated several combinations of chiral oxazaborolidine catalysts and borane reducing agents to obtain the corresponding secondary benzylic alcohols (see the SI, pp S35 and S36). A quick temperature study (between 25 and −20 °C) revealed that at −15 °C with 15 mol % catalyst loading the highest enanatioselectivity (94% ee) could be achieved. Any further increase in the catalyst loading as well as further lowering of the reaction temperature resulted in inferior isolated yields and significantly longer reaction times (e.g., at −20 °C the reaction took >96 h to go to completion). Ultimately, we found that the combination of 15 mol % of (S)-2-methyl oxazaborolidine catalyst, 2 equiv of BH3·DMS in THF at −15 °C were optimal for the efficient reduction of ketones (i.e., up to 94% isolated yield). With these optimized conditions in hand, we proceeded with the asymmetric carbonyl reduction of over one dozen Mannich adducts and obtained the corresponding amino alcohols (28a–m, Figure 7) in good-to-excellent isolated yields and with very high enantioselectivities (up to 98% ee as in the case of 28f).
Figure 7.
Scope of substrates for the reduction of ketone adducts. All chiral and racemic alcohols were prepared from the corresponding ketone adducts by simple reduction using BH3·DMS in the presence of CBS catalyst. The reductions were conducted on a 0.5–3 mmol scale with 0.2 M concentration of the ketone adduct at the indicated temperature and considered complete upon full consumption of the individual ketone adducts as determined by TLC analysis.
Both the isolated yields and enantioselectivities were slightly lowered in the case of pyridine-containing substrates (28l and 28m, Figure 7). The absolute configuration of these chiral 1,3-amino alcohols was unambiguously determined to be (R) by Mosher ester analysis16 (see Experimental section). The three Mannich adducts that were prepared by the addition of α-branched ketone enolates to N-aryl iminomalonates (27n–p, Figure 6) were subjected to simple borane reduction (BH3·DMS, no CBS catalyst was employed), and the corresponding racemic 1,3-amino alcohols (28n–p, Figure 7) were obtained as single diastereomers. With the over one dozen enantiomerically enriched 1,3-amino alcohols in hand (28a–m), we were in the position to evaluate two possible intramolecular cyclization pathways to either furnish azetidines or tetrahydroquinolines (28 → 29 → 30 and 31, Figure 3 and Figure 9).
Figure 9.
Cyclization of chiral alcohols to corresponding azetidines and tetrahydroquinolines. The chiral alcohols obtained by reduction of ketone adducts were subjected to the above-mentioned conditions with 0.05 M concentration of starting material for cyclization to corresponding azetidines and tetrahydroquinolines considered complete upon full consumption of the individual chiral alcohols by TLC analysis.
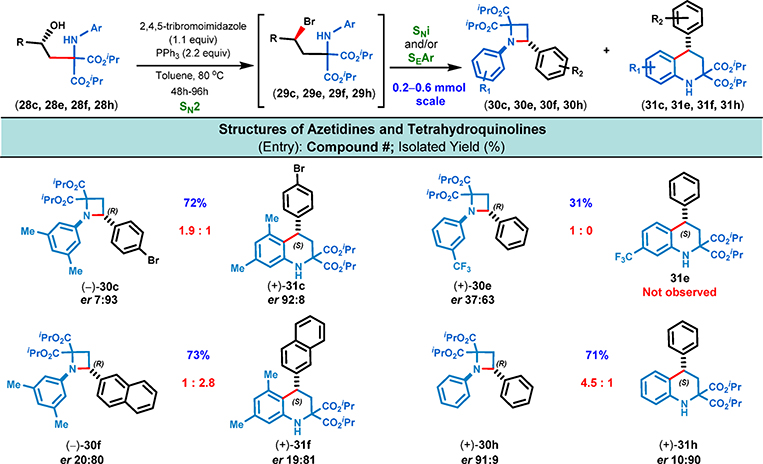
Azetidines are one of the privileged classes of heterocycles in medicinal chemistry.17 This four-membered and rigid azaheterocycle displays higher metabolic stability, ligand efficiency, and physicochemical profiles compared to its higher homologues. Owing to the challenges associated with the formation of this strained four-membered ring, relatively few methods are available for their synthesis.17 Likewise, the tetrahydroquinoline ring system is a common structural motif prevalent in numerous natural products and therapeutic agents.18 Although several methods have been developed for the enantioselective synthesis of 2-substituted tetrahydroquinolines, synthetic routes leading to 3- and 4-substituted derivatives have garnered significantly less attention despite their industrial importance.19 Recently Han et al. and Ghosh et al. independently reported the synthesis of malonate-derived azetidines and tetrahydroquinolines by the ring-opening of donor–acceptor cyclopropanes.20 Thus, we surmised that our versatile iminomalonates are the best precursors for the construction of these important structural moieties via sequential Mannich-type reaction, asymmetric carbonyl reduction, and cyclization. Initially, 1,3-amino alcohol 28h was treated with mesyl chloride (1.5 equiv) in the presence of triethylamine (1.5 equiv) at room temperature with the expectation of activating the benzylic alcohol moiety toward the subsequent intramolecular nucleophilic attack by conversion to the corresponding mesylate. While some of the desired cyclized products (30h and 31h) were formed, most of the starting material remained unreacted even after several hours of stirring (see Table S4, p S8). When the amounts of mesyl chloride and base were both increased (1.5 → 3.0 equiv) and the temperature was elevated (25 → 50 °C), complete consumption of the 1,3-amino alcohol substrate (28h) was observed and the corresponding O-mesylate was obtained as the major product. In this case, the anticipated cyclization was incomplete as only 25% of the O-mesylate intermediate was converted to the products 30h and 31h (obtained as a mixture of 2:1, respectively). In order to improve the efficiency of the intramolecular cyclization step, we decided to alter the nature of the leaving group by converting the benzylic alcohol moiety to the corresponding 2° alkyl bromide (29) via inversion of configuration. Two different bromination conditions were explored (PPh3 with either CBr4 or 2,4,5-tribromoimidazole; see Table S4, p S8), and the combination of triphenylphosphine with 2,4,5-tribromoimidazole was found to be optimal.
With the optimized cyclization conditions in hand, we examined the further reaction of four representative examples (Figure 9). We found that the aromatic substituent on the nitrogen had a significant impact on both the product distribution (i.e., azetidine or tetrahydroquinoline) and the stereoselectivity of the cyclization step. For example, the N-(3-trifluoromethylphenyl)-substituted amino alcohol (28e) did not furnish the anticipated tetrahydroquinoline (31e); only the azetidine product (30e) was observed. This suggests that the distribution of the electron density between the nitrogen substituent and the π system of the aryl ring dictates which one of these moieties will perform the nucleophilic displacement of bromine to afford either the azetidine or tetrahydroquinoline as the major product. If π-stacking interaction is possible during the cyclization (e.g., 28f → 31f), this secondary interaction can completely switch the product distribution compared to substrates in which such an interaction cannot occur (cf. 28h → 30h and 28c → 30c, Figure 9).
The γ-lactone moiety has attracted great interest in the synthetic organic chemistry community for two main reasons: (a) the molecules that contain this moiety exhibit a diverse range of biological activities and (b) its synthesis presents challenges. In fact, the γ-lactone substructure is present in more than 15000 natural products, and it is also a valuable building block for the preparation of structurally complex molecules as well as active pharmaceutical ingredients.21 While there are a number of efficient synthetic methods available for the preparation of structurally simple γ-lactones, access to highly substituted members of this family is limited. For example, to the best of our knowledge, there have been only a few reports for the synthesis of α-amino-substituted γ-lactones (see Figure 2C).10 Since our group found that N-aryliminomalonates reacted efficiently with ketone-derived enolates (Figure 6) and the resulting Mannich adducts afforded the corresponding 1,3-amino alcohols in high enantiomeric excess (Figure 7), the main question was how we could efficiently convert these amino alcohols (28a–p, Figures 10 and 11) to the corresponding γ-lactones (24)?
Figure 10.
Cyclization of chiral alcohols to corresponding lactones. The chiral alcohols, obtained by the reduction of ketone adducts, were subjected to the conditions A or B for cyclization with 0.2 M concentration of alcohol to the corresponding lactones and considered complete upon the full consumption of the individual alcohols by TLC analysis.
Figure 11.
Cyclization of chiral alcohols to the corresponding lactones. The enantiomerically enriched and racemic 2° alcohols, obtained by reductions of ketone adducts, were subjected to the conditions A or B for cyclization with 0.2 M concentration of alcohol to corresponding to lactones and considered complete upon the full consumption of the individual chiral and achiral alcohols by TLC analysis.
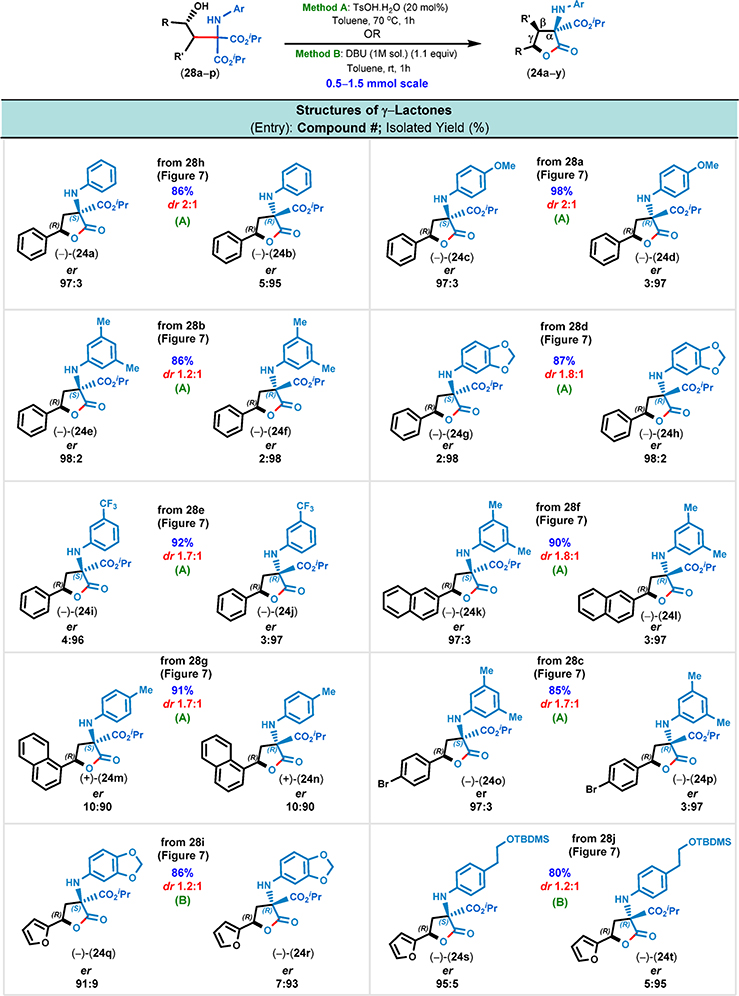
Importantly, when the solution of 1,3-amino alcohol 28a in toluene was treated with 20 mol % of TsOH·H2O at 50 °C, the lactonization (i.e., between the benzylic alcohol and isopropyl ester moieties) took place in 24 h and the easy-to-separate diastereomeric lactones (24c and 24d) were obtained in a 2:1 ratio. Increasing the reaction temperature (e.g., 70 and 110 °C) resulted in significantly faster lactonization–at 70 °C, the lactonization was fast (1 h) and had minimal impurities. In order to increase the diastereoselectivity during lactonization, we turned to using chiral Brønsted acid (i.e., phosphoric acid) catalysts.22 It was anticipated that the use of a chiral phosphoric acid catalyst would lead to an efficient cyclization process in a highly stereoselective manner owing to the ability of the chiral phosphate counterion to influence the stereochemistry-determining step. Ultimately, we screened a total of seven chiral Brønsted acid catalysts (e.g., chiral phosphoric acids and camphor sulfonic acids) and identified (R)-3,3′-bis[3,5-bis-(trifluoromethyl)phenyl]-1,1′-binaphthyl-2,2′-diylhydrogen phosphate to be the most efficient, as it afforded the cyclized γ-lactone products 24c and 24d in a 3.3:1 diastereomeric ratio at 100 °C in just 2 h (see the SI, Table S5, pp S9 and S10).
All of the other combinations of solvent, temperature, and Brønsted acid furnished the γ-lactones in about a ~2:1 diastereomeric ratio. These results clearly indicated that the presence of a chiral counterion did not significantly improve the diastereoselectivity of the lactonization step (e.g., 2:1 dr → 3.3:1 dr, which represents only a 1.65× increase). Such a modest increase in the diasteromeric ratio could not justify the use of an expensive chiral phosphoric acid catalyst. Thus, we shifted our focus toward using cheaper achiral Bransted acids–p-toluenesulfonic acid monohydrate (TsOH·H2O) was found to be the optimal catalyst in toluene at 70 °C as the reaction proceeded to completion in just 1 h.
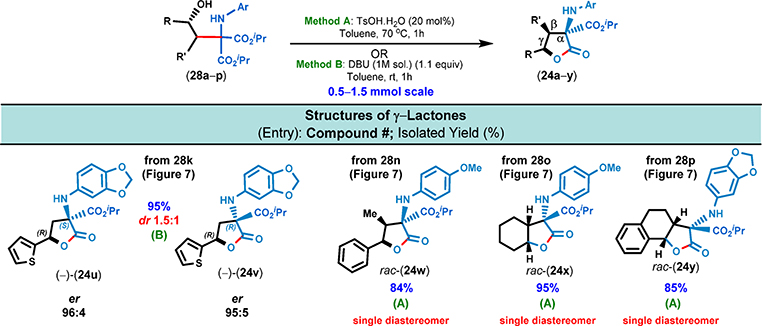
With these optimized acid-catalyzed lactonization conditions in hand, we proceeded to cyclize 14 amino alcohols (Figures 10 and 11). Without exception, each of the isolated γ-lactones was obtained in excellent enantiomeric excess (up to 98:2 er). Surprisingly, the acid-catalyzed cyclization conditions were unsuitable for heterocycle-containing substrates and invariably led to complex reaction mixtures. For these heteroaromatic substrates, base-mediated conditions (e.g., DBU) were found to be optimal, and the desired lactone products were obtained in high isolated yields while the diastereoselectivities varied from 1.2:1 → 1.5:1 (24q,r, 24s,t, and 24u,v, Figures 10 and 11). The modest diastereoselectivity in the lactonization step can be attributed to the lack of substituents at the β-position of the γ-lactone moiety. However, amino alcohols 28n, 28o, and 28p underwent lactonization with complete diastereoselectivity; in all three cases, the product γ-lactones were substituted at their β-position (Figure 11).
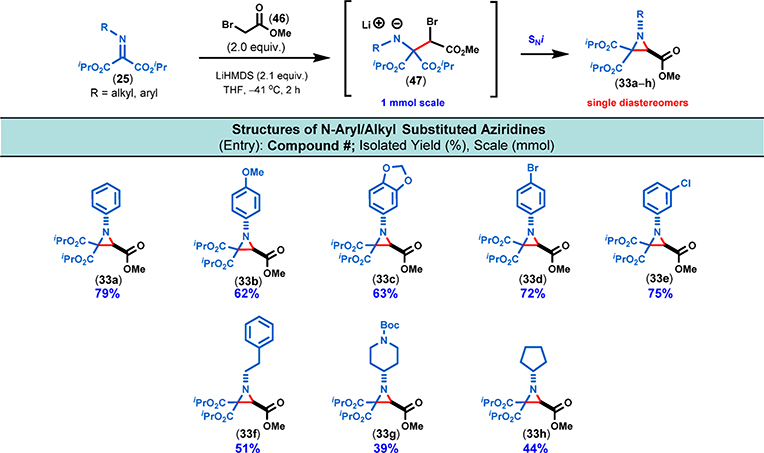
Diversely substituted aziridines are not only valuable synthetic intermediates but they also occur as substructures in natural products and druglike molecules.12a,23 Among these diverse families of aziridines, aziridine-2,2-diesters are special (i.e., donor–acceptor aziridines) as they serve as precursors of azomethine ylide intermediates that undergo a variety of cycloaddition reactions.24,25 The synthesis of fully substituted aziridines remains a significant synthetic challenge.23b Since synthetic access to structurally diverse donor–acceptor aziridines is fairly limited,25 we saw an opportunity to access these valuable building blocks using an aza-Darzens reaction between haloester enolates and N-alkyl as well as N-aryl diisopropyl iminomalonates (Figure 12). We briefly reported about this transformation in our previous publication to show the additional synthetic possibilities from iminomalonates.1 As we briefly mentioned (vide infra), simple ester enolates did not react with iminomalonates, but α-halogenated ester enolates 4 reacted quickly and efficiently affording the aziridine-2,2,3-triesters as single diastereomers. In fact, DFT calculations 4 showed that the trans-1-phenyl-3-methoxycarbonyl aziridine diastereomer (33a, Figure 12) is favored over the cis-diastereomer by ~4 kcal/mol (see the SI, pp S19–S35).
Figure 12.
Scope of substrates using aryl/alkyl iminomalonates as electrophiles. All of the aromatic as well as aliphatic iminomalonates have been prepared from the corresponding amines by simple condensation with ketomalonate hydrate. The aziridination reactions were conducted on a 1 mmol scale with 0.1 M concentration of iminomalonate at the indicated temperature and considered complete upon full consumption of the individual iminomalonates by TLC analysis.
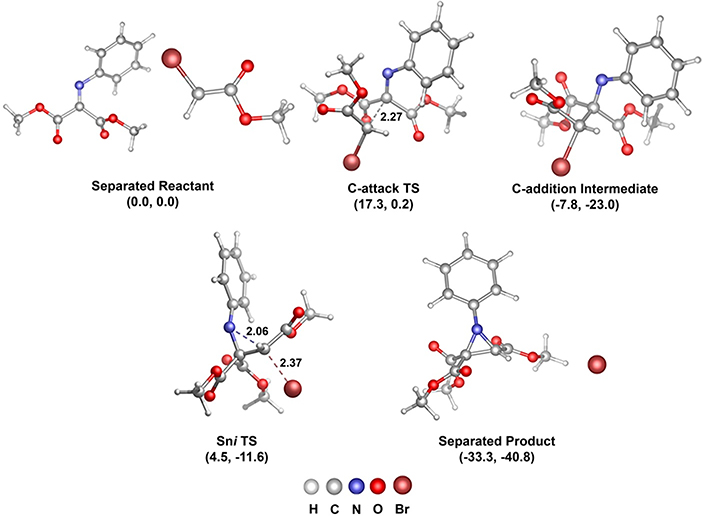
We also carried out DFT calculations on the reaction pathway for aziridine formation. Similar to what we showed in Figure 8, the enolate derived from ester 46 has a lower energy transition state for C-attack compared to N-attack. The calculated structures along the aziridine reaction pathway are shown in Figure 15. After the C-attack, with a barrier of 17 kcal/mol, the resulting intermediate can then induce intramolecular nucleophilic substitution and bromide ejection with a barrier of 12 kcal/mol. This lower barrier for intramolecular substitution is consistent with the lack of observation of the addition intermediate (47).
Figure 15.
//M06–2X/6–31G(d,p)[LANL2DZ for Br] calculated reaction pathway structures and energies (Gibbs free energy, enthalpy at 298 K) for 46-derived enolate addition to N-Ph iminomalonate.
For the optimization study (see the SI Table S6, p S10), we have chosen methyl bromoacetate and N-phenyl iminomalonate as coupling partners. Various ethereal solvents and strong bases were screened: when either n-BuLi or NaHMDS was used as base, a complex reaction mixture was obtained and no desired aziridine product (33a) was isolated. When KHMDS was used, only 3% of product was isolated at −78 °C. Ultimately, LiHMDS was identified as the most suitable base and THF as the best solvent at −41 °C (i.e., acetonitrile–dry ice bath); using these conditions, 33a was isolated in 79% yield. With these optimized conditions in hand, we proceeded to explore the scope and limitations of this transformation (Figure 12). Both aliphatic and aromatic iminomalonates worked well as reaction partners, and the aziridines were isolated in moderate to excellent yields.
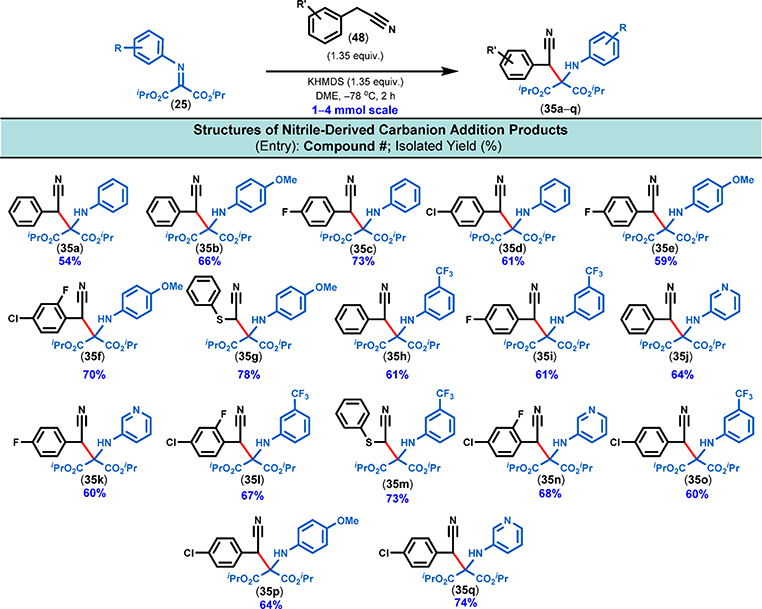
We then studied the reactivity of nitrile-derived carbanions with iminomalonates (Figure 13). For the optimization study, 4-fluorophenylacetonitrile (48i) was chosen as the coupling partner. Various solvents and bases were thoroughly screened at −78 °C. A very strong base, such as n-BuLi, only led to decomposition. Weaker bases, such as HMDS-derived bases, were then applied with varying results. LiHMDS and NaHMDS were totally ineffective: the 4-fluorophenylacetonitrile substrate was completely recovered in both THF and DME.
Figure 13.
Scope of substrates using iminomalonates as electrophiles. All iminomalonates were prepared from the corresponding amines by simple condensation with ketomalonate hydrate. The carbanion addition reactions were conducted on a 1–4 mmol scale with 0.13 M concentration of the iminomalonate at the indicated temperature and considered complete upon full consumption of the individual iminomalonate as determined by TLC analysis.
With the combination of KHMDS and either THF, Et2O, or 1,4-dioxane, the product could only be obtained in low yields (8–11%). However, when the reaction was carried out with KHMDS in DME as the solvent, the yield improved significantly (58%). We also determined that decreasing the amount of carbanion from 1.5 to 1.35 equiv made the reaction much cleaner, hence simplifying the purification process. It was observed that use of over 1.5 equiv of the carbanion generally resulted in more complex reaction mixtures. With the optimized reaction conditions in hand (1.35 equiv of KHMDS and 1.35 equiv of nitrile at −78 °C in DME), we embarked upon exploring the generality of this method with a variety of nitrile coupling partners (Figure 13). The isolated yields of adduct 35 ranged from good to excellent on a 1–4 mmol scale. There was no dramatic substituent effect on the nitrogen atom of the imine partner on the yields. Imines with both electron-withdrawing groups and electron-donating groups on the nitrogen atom were tolerated (Figure 13). The structure of the acetonitrile coupling partner was also widely varied. While arylacetonitriles worked well, aliphatic acetonitriles either performed poorly or did not react. The use of very strong bases (e.g., n-BuLi) with these aliphatic acetonitriles only led to their complete decomposition even at −78 °C. From these results, it is clear that both the stability and reactivity of the acetonitrile-derived anions are important; it can be opined that the nature of the anion from the acetonitrile is of paramount importance as the metal counterion can significantly alter the reactivity pattern. The successful acetonitriles were those that had anion-stabilizing substituents; thus, an aromatic ring or a heavier atom such as sulfur in the α-position was found to be critical.
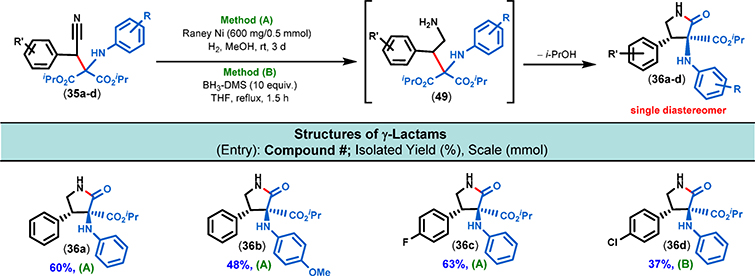
We then sought to find a method which would enable us to perform the reduction of the nitrile functionality (35 → 49, Figure 14) and the subsequent cyclization (49 → 36) to the corresponding γ-lactam in one step. After carefully exploring a number of methods, we found that the nickel-mediated hydrogenation of the nitrile addition products afforded the desired lactams in good isolated yields. It is important to note that some of the nitrile addition products, those with a chlorine substituent on their aromatic rings, failed to cyclize to the desired lactams. Presumably, the presence of nickel may have led to the decomposition of these substrates. To address these shortcomings, an alternative reduction method was performed on these substrates. Accordingly, treatment with excess borane was followed by an aqueous quench and subsequent reflux to facilitate a clean lactam formation (Figure 14). Both of these methods (A and B) delivered the γ-lactam products as single diastereomers. This is in contrast to the formation of γ-lactones (Figure 10 and 11) that were formed as a mixture of fully separable diastereomers. We believe that this difference in diastereoselectivity can be attributed to the presence or absence of substituents in the β-position (i.e., relative to the carbonyl group) of the 5-membered lactam ring.
Figure 14.
Scope of substrates for cyclization using nitrile adducts. All of the nitrile adducts were prepared by carbanion addition to the iminomalonate. These nitrile adducts were reduced and subsequently cyclized to the corresponding lactams using the above-mentioned conditions and considered complete upon the full consumption of the individual nitrile adducts by TLC analysis.
CONCLUSION
In summary, we have developed a practical and operationally simple protocol for the synthesis of five different classes of heterocycles (i.e., with ring sizes 3–6) using N-substituted iminomalonates as the common coupling partners. Each heterocycle features an unnatural amino acid backbone. The Mannich adducts, obtained via reaction with soft C-nucleophiles such as enolates of ketones, nitriles, and haloesters, were further functionalized (e.g., CBS reduction and cyclization) to afford structurally diverse heterocyclic moieties which are valuable building blocks for the preparation of biologically relevant molecules such as active pharmaceutical ingredients and structural analogs of natural products. The complete switch of chemoselectivity (i.e., predominant N-attack versus exclusive C-attack of the iminomalonate C=N bond) depending on the nature of the C-nucleophile (i.e., hard or soft) is unprecedented in the literature, and our experimental findings have been corroborated by detailed DFT calculations. Studies are currently underway in our laboratories to explore the reactivity of iminomalonates with additional soft C-nucleophiles and our findings will be reported in due course.
EXPERIMENTAL SECTION
General Information.
Reagents were purchased at the highest quality from commercially available sources and used without further purification. 1,2-Dimethoxyethane (DME), tetrahydrofuran (THF), and toluene for the reactions were obtained from pure process technology solvent system by passing the previously degassed solvents through an activated alumina column under argon. All reactions were carried out in flame-dried glassware under an atmosphere of argon with magnetic stirring. All reactions were monitored by either 1H NMR or thin-layer chromatography (TLC) carried out on 0.25 mm precoated E. Merck silica plates (60F-254) using shortwave UV light as a visualizing agent and KMnO4 or phosphomolybdic acid (PMA) and heat as developing agents. Flash column chromatography was performed using a Biotage Isolera One automated chromatograph with prepacked KP-Sil cartridges. 1D 1H, 13C and DEPT-135 13C NMR spectra were recorded on Bruker Avance III HD 600 and Bruker Avance III 500 spectrometers operating at 600 and 500 MHz for 1H and 151 and 126 MHz for 13C. The spectra were calibrated using either TMS or the solvent as an internal reference (residual CHCl3: 7.26 ppm 1H NMR; CDCl3: 77.00 ppm 13C NMR). 2D COSY, NOE, HSQC, and HMBC experiments were done on the 500 MHz spectrometer. 19F NMR spectra at 471 MHz were recorded on the 500 MHz spectrometer, and the chemical shifts were relative to CF35Cl3 defined as 0 ppm. For reporting NMR peak multiplicities, the following abbreviations were used: s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, hept = heptet, m = multiplet. High-resolution mass spectra (HRMS) were recorded on an Agilent UHPLC TOF mass spectrometer using electrospray ionization time-of-flight (ESI-TOF) or chemical ionization time-of-flight (CI-TOF) reflectron experiments. Melting points and ranges were recorded on Mettler Toledo MP50 melting point system.
General Procedure for the Synthesis of Mannich Adducts Using Ketone Enolates, Figure 6.
In a thick-walled flame-dried reaction vial, ketone (1.5 equiv) was dissolved in anhydrous DME (0.4 M) under argon and cooled to −78 °C using dry ice/acetone bath. To this cooled reaction mixture was added commercially available KHMDS (1 M solution in THF) (1.6 equiv) dropwise and the resulting mixture stirred for 45 min. Then iminomalonate (1.0 equiv) dissolved in anhydrous DME (0.4M) was added slowly dropwise over 5 min to the enolate solution and stirring continued for 2 h or until the complete consumption of starting material at −78 °C. Then the reaction was quenched using saturated NH4Cl solution (5 mL).
Workup and Purification.
After quenching, the reaction mixture was diluted with brine (20 mL), and the organic layer was separated. The aqueous layer was then extracted with EtOAc twice (2 × 30 mL), and the combined organic layers were washed with brine (30 mL) once, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by automated flash column chromatography.
Diisopropyl 2-((4-Methoxyphenyl)amino)-2-(2-oxo-2-phenylethyl)malonate (27a).
The general procedure was followed using acetophenone (0.72 mL, 1.5 mmol), KHMDS (1 M solution in THF) (6.6 mL, 1.6 mmol), and p-methoxyphenyl iminomalonate (1.27 g, 4.1 mmol) as starting materials to afford the ketone adduct as a brown waxy solid (1.65 g, 94%). The compound was purified by flash column chromatography (Rf = 0.41 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.85 (d, J = 8.4 Hz, 2H), 7.50 (t, J = 7.4 Hz, 1H), 7.38 (t, J = 7.8 Hz, 2H), 6.66 (d, J = 8.9 Hz, 2H), 6.60 (d, J = 9.0 Hz, 2H), 5.11 (h, J = 6.3 Hz, 3H), 4.02 (s, 2H), 3.67 (s, 3H), 1.20 (d, J = 6.3 Hz, 6H), 1.15 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.3, 168.8, 153.3, 138.0, 36.5, 133.1, 128.4, 127.8, 117.7, 114.4, 70.1, 67.0, 55.4, 40.9, 21.3. HRMS (eSI-TOF) m/z: [M + H]+ calcd for C24H30NO6 428.2068, found 428.2069. Note: The evidence for C-attack by the enolate was the generation of a quaternary aliphatic carbon, as shown by the presence of a signal at δ 67.0 in the standard 13C spectrum (p. S38) and the absence of this signal in the DEPT-135 13C spectrum (p. S39).
Diisopropyl 2-((3,5-Dimethylphenyl)amino)-2-(2-oxo-2-phenylethyl)malonate (27b).
The general procedure was followed using acetophenone (0.40 mL, 3.4 mmol), KHMDS (1 M solution in THF) (3.66 mL, 3.6 mmol), and 3,5-dimethylphenyl iminomalonate (0.7 g, 2.2 mmol) as starting materials to afford the ketone adduct as a pale yellow viscous oily liquid (0.85 g, 87%). The compound was purified by flash column chromatography (Rf = 0.57 (20% EtOAc/hexanes). 1H NMR (600MHz, CDCl3): δ 7.89 (d, J = 7.4Hz, 2H), 7.51 (t, J = 7.4 Hz, 1H), 7.40 (t, J = 7.8 Hz, 2H), 6.36 (s, 1H), 6.26 (s, 2H), 5.35 (s, 1H), 5.13 (hept, J = 6.2 Hz, 2H), 4.13 (s, 2H), 2.18 (s, 6h), 1.22 (d, J = 6.3 Hz, 6H), 1.15 (d, J =6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.2, 168.6, 144.1,138.4, 136.5, 133.1, 128.3, 127.8, 120.4, 112.5, 70.1, 66.1, 40.8, 21.27, 21.25, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H32NO5 426.2275, found 426.2275.
Diisopropyl 2-(2-(4-Bromophenyl)-2-oxoethyl)-2-((3,5-dimethylphenyl)amino)malonate (27c).
The general procedure was followed using 4-bromoacetophenone (0.68 g, 3.4 mmol), KHMDS (1 M solution in THF) (3.66 mL, 3.6 mmol), and 3,5-dimethylphenyl iminomalonate (0.7 g, 2.2 mmol) as starting materials to afford the ketone adduct as a pale yellow waxy solid (0.92 g, 80%). The compound was purified by flash column chromatography (Rf = 0.57 (20% EtOAc/hexanes). 1H NMR (600MHz, CDCl3): δ 7.73 (d, J = 8.5 Hz, 2H), 7.53 (d, J = 8.5 Hz, 2H), 6.36 (s, 1H), 6.22 (s, 2H), 5.29 (s, 1H), 5.11 (hept, J = 6.1 Hz, 2H), 4.05 (s, 2H), 2.17 (s, 6H), 1.21 (d, J = 6.3 Hz, 6H), 1.14 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.5, 168.6, 144.1, 138.6, 135.3, 131.7, 129.5, 128.4, 120.6, 112.6, 70.4, 66.2, 40.8, 21.38, 21.36, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H31BrNO5 504.1380, found 504.1378.
Diisopropyl 2-(Benzo[d][1,3]dioxol-5-ylamino)-2-(2-oxo-2-phenylethyl)malonate (27d).
The general procedure was followed using acetophenone (0.49 mL, 4.2 mmol), KHMDS (1 M solution in THF) (4.56 mL, 4.5 mmol), and 3,4-methylenedioxyphenyl iminomalonate (0.91 g, 2.8 mmol) as starting materials to afford the ketone adduct as a yellow waxy solid (1.05 g, 84%). The compound was purified by flash column chromatography (Rf = 0.49 (20% EtOAc/hexanes). 1H NMR (600MHz, CDCl3): δ 7.86 (d, J = 7.3 Hz, 2H), 7.51 (t, J = 7.4 Hz, 1H), 7.39 (t, J = 7.8 Hz, 2H), 6.53 (d, J = 8.3 Hz, 1H), 6.25 (d, J = 2.3 Hz, 1H), 6.06 (dd, J = 8.3, 2.3 Hz, 1H), 5.78 (s, 2h), 5.17 (s, 1H), 5.11 (hept, J = 6.2 Hz, 2H), 4.02 (s, 2H), 1.20 (d, J = 6.3 Hz, 6H), 1.16 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.2, 168.7, 148.0, 140.8, 139.3, 136.4, 133.2, 128.4, 127.8, 108.1, 107.9, 100.5, 99.1, 70.2, 66.8, 40.7, 21.3, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H28NO7 442.1860, found 442.1868.
Diisopropyl 2-(2-Oxo-2-phenylethyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (27e).
The general procedure was followed using acetophenone (0.58 mL, 4.9 mmol), KHMDS (1 M solution in THF) (5.32 mL, 5.3 mmol), and 3-trifluoromethylphenyl iminomalonate (1.1 g, 3.3 mmol) as starting materials to afford theketone adduct as a white solid (0.82 g, 53%) (mp 132–134 °C). The compound was purified by flash column chromatography (Rf = 0.53 (20% EtOAc/hexanes). 1H NMR (600MHz, CDCl3): δ 7.89 (d, J =8.4Hz, 2H), 7.52 (t, J = 7.4 Hz, 1H), 7.41 (t, J = 7.8 Hz, 2H), 7.19 (t, J = 7.9 Hz, 1h), 6.93 (d, J = 7.6 Hz, 1H), 6.83 (s, 1H), 6.77 (d, J = 8.2 Hz, 1H), 5.70 (s, 1H), 5.13 (hept, J = 6.3 Hz, 2H), 4.12 (s, 2H), 1.21 (d, J = 6.3 Hz, 6H), 1.13 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.0, 168.2, 144.6, 136.3, 133.4, 131.4 (q, 2JCF = 31.9 Hz), 129.6, 128.6, 128.0, 124.0 (q, 1Jcf = 272.4 Hz), 117.3, 114.9 (q, 3Jcf = 3.9 Hz), 110.5 (q, 3Jcf = 3.9 Hz), 70.8, 66.0, 40.6, 21.28, 21.22. 19F NMR (471 MHz, CDCl3): δ −62.0 (S). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H27F3NO5 466.1836, found 466.1827.
Diisopropyl 2-((3,5-Dimethylphenyl)amino)-2-(2-(naphthalen-2-yl)-2-oxoethyl)malonate (27f).
The general procedure was followed using 2-acetonaphthone (0.48 g, 2.8 mmol), KHMDS (1 M solution in THF) (3.01 mL, 3.0 mmol), and 3,5-dimethylphenyl iminomalonate (0.57 g, 1.8 mmol) as starting materials to afford the ketone adduct as a yellowish brown viscous gummy oily liquid (0.75 g, 85%). The compound was purified by flash column chromatography (Rf = 0.5 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.37 (s, 1H), 7.95 (dd, J = 8.6, 1.5 Hz, 1H), 7.85 (d, J =8.1 Hz, 1H), 7.81 (d, J = 8.7 Hz, 2H), 7.55 (ddd, J = 8.2, 6.8, 1.4 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 6.38 (s, 1H), 6.32 (s, 2H), 5.41 (s, 1H), 5.19 (hept, J = 6.3 Hz, 2h), 4.28 (s, 2H), 2.19 (s, 6H), 1.26 (d, J = 6.3 Hz, 6H), 1.20 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 196.3, 168.7, 144.1, 138.5, 135.4, 133.8, 132.1, 129.8, 129.3, 128.3, 128.1, 127.5, 126.5, 123.3, 120.5, 112.6, 70.2, 66.3, 40.8, 21.27, 21.21. HRMS (ESI-TOF) m/z:[M + H]+ calcd for C29H34NO5 476.2431, found 476.2433.
Diisopropyl 2-(2-(Naphthalen-1-yl)-2-oxoethyl)-2-(p-tolylamino)malonate (27g).
The general procedure was followed using 1-acetonaphthone (0.47 g, 3.0 mmol), KHMDS (1 M solution in THF) (3.29 mL, 3.3 mmol), and 4-methylphenyl iminomalonate (0.6 g, 2.0 mmol) as starting materials to afford the ketone adduct as a yellow viscous oily liquid (0.61 g, 66%). The compound was purified by flash column chromatography (Rf = 0.53 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.45 (d, J = 7.8 Hz, 1H), 7.91 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 9.0 Hz, 1H), 7.68 (d, J = 7.1 Hz, 1H), 7.49 (p, J = 6.1, 5.5 Hz, 2H), 7.38 (t, J = 7.7 Hz, 1H), 6.93 (d, J = 8.1 Hz, 2H), 6.61 (d, J = 8.4 Hz, 2H), 5.40 (s, 1H), 5.18 (hept, J = 6.2 Hz, 2H), 4.21 (s, 2H), 2.20 (s, 3H), 1.28 (d, J = 6.3 Hz, 6H), 1.19 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 200.4, 168.8, 141.9, 135.3, 133.6, 132.6, 129.8, 129.5, 128.2, 128.1, 127.7, 127.5, 126.3, 125.5, 124.1, 115.5, 70.2, 66.7, 44.2, 21.37, 21.30, 20.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H32NO5 462.2275, found 462.2277.
Diisopropyl 2-(2-Oxo-2-phenylethyl)-2-(phenylamino)malonate(27h).
The general procedure was followed using acetophenone (0.62 mL, 5.3 mmol), KHMDS (1 M solution in THF) (5.68 mL, 5.6 mmol), and phenyl iminomalonate (0.98 g, 3.5 mmol) as starting materials to afford the ketone adduct as a beige solid (mp 65–68 °C) (1.25 g, 89%). The compound was purified by flash column chromatography (Rf = 0.53 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.87 (d, J = 8.2 Hz, 2H), 7.50 (t, J = 6.8 Hz, 1H), 7.38 (t, J = 7.2 Hz, 2H), 7.10 (t, J = 7.9 Hz, 2h), 6.70 (t, J = 7.3 Hz, 1H), 6.63 (d, J = 8.1 Hz, 2H), 5.46 (s, 1H), 5.12 (hept, J = 6.2 Hz, 2H), 4.14 (s, 2h), 1.21 (d, J = 6.3 Hz, 6H), 1.12 (d, J = 6.3 Hz, 6H). 13C{1H}NMR (151 MHz, CDCl3): δ 196.2, 168.6, 144.3, 136.5, 133.2, 129.0, 128.4, 127.9, 118.5, 114.5, 70.3, 66.1, 40.8, 21.29, 21.24. HRMS (ESI-TOf) m/z: [M + H]+ calcd for C23H28NO5 398.1962, found 398.1958.
Diisopropyl 2-(Benzo[d][1,3]dioxol-5-ylamino)-2-(2-(furan-2-yl)-2-oxoethyl)malonate (27i).
The general procedure was followed using 2-acetylfuran (0.33 g, 3.0 mmol), KHMDS (1 M solution in THF) (3.23 mL, 3.2 mmol), and 3,4-methylenedioxyphenyl iminomalonate (0.65 g, 2.0 mmol) as starting materials to afford the ketone adduct as a dark brown viscous oily liquid (0.66 g, 77%). The compound was purified by flash column chromatography (Rf = 0.27 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.49 (s, 1H), 7.06 (d, J = 3.5 Hz, 1H), 6.53 (d, J = 8.3 Hz, 1H), 6.43 (dd, J = 3.5,1.7 Hz, 1h), 6.24 (d, J = 2.3 Hz, 1H), 6.05 (dd, J = 8.3, 2.3 Hz, 1H), 5.79 (s, 2h), 5.08 (hept, J = 6.3 Hz, 3H), 3.85 (s, 2H), 1.20 (d, J = 6.3 Hz, 6H), 1.13 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 185.0, 168.4, 152.2, 147.9, 146.5, 140.8, 139.3, 117.5, 112.2, 108.1, 107.8, 100.5, 99.0, 70.3, 66.7, 40.5, 21.29, 21.27. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26NO8 432.1653, found 432.1657.
Diisopropyl 2-((4-(2-((tert-Butyldimethylsilyl)oxy)ethyl)phenyl)-amino)-2-(2-(furan-2-yl)-2-oxoethyl)malonate (27j).
The general procedure was followed using 2-acetylfuran (0.27 g, 2.4 mmol), KHMDS (1 M solution in THF) (2.64 mL, 2.6 mmol) and ((tert-butyldimethylsilyl)oxy)ethyl)phenyl iminomalonate (0.72 g, 1.6 mmol) as starting materials to afford the ketone adduct as a brown viscous oily liquid (0.67 g, 75%). The compound was purified by flash column chromatography (Rf = 0.44 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.45 (s, 1H), 7.02 (d, J = 3.5 Hz, 1H), 6.91 (d, J = 8.3 Hz, 2H), 6.52 (d, J = 8.4 Hz, 2H), 6.38 (dd, J = 3.6, 1.7 Hz, 1H), 5.25 (s, 1H), 5.08 (h, J = 6.3 Hz, 2H), 3.91 (s, 2H), 3.66 (t, J = 7.1 Hz, 2H), 2.63 (t, J = 7.0 Hz, 2H), 1.20 (d, J = 6.3 Hz, 6H), 1.09 (d, J = 6.3 Hz, 6H), 0.82 (s, 9H), −0.08 (s, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 184.9, 168.4, 152.1, 146.4, 142.4, 170.2, 66.1, 64.5, 40.4, 38.5, 25.7, 21.2, 21.1, 18.1, −5.5. HRMS (ESI-TOf) m/z: [M + H]+ calcd for C29H44NO7Si 546.2882, found 546.2883.
Diisopropyl 2-(Benzo[d][1,3]dioxol-5-ylamino)-2-(2-oxo-2-(thio-phene-2-yl)ethyl)malonate (27k).
The general procedure was followed using 2-acetylthiophene (0.40 mL, 3.7 mmol), KHMDS (1 M solution in THF) (3.98 mL, 3.9 mmol), and 3,4-methylenedioxyphenyl iminomalonate (0.8 g, 2.4 mmol) as starting materials to afford the ketone adduct as a dark brownish yellow viscous gummy substance (0.84 g, 76%). The compound was purified by flash column chromatography (Rf = 0.38 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.60 (ddd, J = 14.6, 4.3, 0.8 Hz, 2H), 7.04 (dd, J = 4.8, 3.9 Hz, 1H), 6.55 (d, J =8.3 Hz, 1H), 6.25 (d, J = 2.3 Hz, 1H), 6.07 (dd, J = 8.3, 2.3 Hz, 1H), 5.80 (s, 2H), 5.15–5.07 (m, 3H), 3.94 (s, 2H), 1.22 (d, J = 6.3 Hz, 6h), 1.17 (s, 6h). 13C{1H} NMR (151 MHz, CDCl3): δ 189.1, 168.5, 148.0, 143.8, 140.9, 139.3, 134.1, 132.2, 128.0, 108.2, 107.8, 100.6, 99.1, 70.3, 66.9, 41.2, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26NO7S 448.1424, found 448.1425.
Diisopropyl 2-(2-(4-Bromophenyl)-2-oxoethyl)-2-(pyridin-3-ylamino)malonate (27l).
The general procedure was followed using 4-bromoacetophenone (0.85 g, 4.3 mmol), KHMDS (1 M solution in THF) (4.59 mL, 4.5 mmol), and 3-pyridyl iminomalonate (0.8 g, 2.8 mmol) as starting materials to afford the ketone adduct as a reddish brown viscous gummy substance (0.97 g, 71%). The compound was purified by flash column chromatography (Rf = 0.16 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.98 (s, 1H), 7.92–7.87 (m, 1H), 7.65 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2h), 6.96–6.91 (m, 1H), 6.84 (d, J = 7.0 Hz, 1H), 5.47 (s, 1H), 5.04 (hept, J = 6.2 Hz, 2H), 3.99 (s, 2H), 1.12 (d, J = 6.1 Hz, 6H), 1.04 (d, J = 6.1 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 194.7, 167.7, 140.2, 139.9, 137.1, 134.8, 131.7, 129.2, 128.5, 123.2, 120.2, 70.6, 65.6, 40.2, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26BrN2O5 477.1020, found 477.1021.
Diisopropyl 2-((4-(2-((tert-Butyldimethylsilyl)oxy)ethyl)phenyl)-amino)-2-(2-oxo-2-(pyridin-4-yl)ethyl)malonate (27m).
The general procedure was followed using 4-acetylpyridine (0.20 mL, 1.8 mmol), KHMDS (1 M solution in THF) (1.94 mL, 1.9 mmol), and ((tert-butyldimethylsilyl)oxy)ethyl)phenyl iminomalonate (0.53 g, 1.2 mmol) as starting materials to afford the ketone adduct as a brown viscous oily liquid (0.22 g, 34%). The compound was purified by flash column chromatography (Rf = 0.37 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.70 (d, J = 5.9 Hz, 2H), 7.58 (d, J = 5.9 Hz, 2H), 6.92 (d, J = 8.3 Hz, 2H), 6.52 (d, J = 8.3 Hz, 2H), 5.27 (s, 1H), 5.10 (hept, J = 6.1, 5.2 Hz, 2H), 4.05 (s, 2H), 3.66 (t, J = 7.0 Hz, 2H), 2.63 (t, J = 7.0 Hz, 2H), 1.20 (d, J = 6.3 Hz, 6H), 1.12 (d, J = 6.3 Hz, 6H), 0.82 (s, 9H), −0.09 (s, 6H). 13C{1H} NMR (151 MHz, CDCl3):δ 196.1, [168.4, 150.7, 142.3, 142.1, 129.8, 129.7, 120.7, 114.9, 70.5, 66.2, 64.6, 40.8, 38.5, 25.8, 21.35, 21.30, 21.2, 18.2, −5.5. HRMS (ESI-TOf) m/z:[M + H]+ calcd for C30H45N2O6Si 557.3041, found 557.3041.
Diisopropyl 2-((4-Methoxyphenyl)amino)-2-(1-oxo-1-phenylpro-pan-2-yl)malonate (27n).
The general procedure was followed using propiophenone (0.42 mL, 3.1 mmol), KHMDS (1 M solution in THF) (3.38 mL, 3.3 mmol), and p-methoxyphenyl iminomalonate (0.65 g, 2.1 mmol) as starting materials to afford the ketone adduct as a yellow viscous oily liquid (0.65 g, 70%). The compound was purified by flash column chromatography (Rf = 0.36 (15% EtOAc/hexanes). 1H NMR L (500 MHz, CDO3):δ 7.84–7.88 (m, 2H, H-2/H-6); 7.49–7.53 (m, 1H, H-4); 7.38–7.42 (m, 2H, H-3/H-5); 6.68 (apparent s, all 4-methoxyphenyl ring protons); 5.19 (s, broad, NH); 4.67 (q, J = 7.2 Hz, 1H, CH next to quaternary aliphatic carbon); 3.69 (s, 3H, methoxy); 1.41 (d, J = 7.2 Hz, 3H, methyl bonded to methine next to quaternary aliphatic carbon); isopropoxy group 1:5.23 (hept, J = 6.3 Hz, 1H, methine), 1.31 (d, J = 6.3 Hz, 3H, methyl), 1.23 (d, J = 6.3 Hz, 3H, methyl); isopropoxy group 2:4.99 (hept, J = 6.3 Hz, 1H, methine), 1.14 (d, J = 6.3 Hz, 3H, methyl), 1.01 (d, J = 6.3 Hz, 3H, methyl). 13C{1H} NMR (126 MHz, CDCl3): δ 201.7 (ketone carbonyl); isopropoxy- carbonyl group 1:169.3 (carbonyl), 70.2 (CH), 21.6 (methyl correlating with methyl 1H signal at δ1.31 in HSQC spectrum), 21.5 (methyl correlating with methyl 1H signal at δ1.23 in HSQC spectrum); isopropoxycarbonyl group 2:168.7 (carbonyl), 70.0 (CH), 21.31 (methyl), 21.29 (methyl); 153.6 (C-4′); 138.3 (C-1′); 136.5 (C-1); 133.0 (C-4); 128.5 (C-3/C-5); 128.3 (C-2/C-6); 118.7 (C-2′/C-6′); 114.3 (C-3′/C-5′); 71.1 (quaternary aliphatic carbon); 55.5 (methoxy); 43.9 (CH next to quaternary aliphatic carbon); 14.7 (methyl bonded to CH next to quaternary aliphatic carbon). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H32NO6 442.2224, found 442.2219.
Note: The evidence for C-attack by the enolate was the generation of a quaternary aliphatic carbon, as shown by the presence of a signal at δ 71.1 in the standard 13C spectrum (SI p S78) and the absence of this signal in the DEPT-135 13C spectrum (SI p S79). Detailed chemical shift assignments could be made through a combination of 1H (SI p. S77), 13C, DEPT-135 13C, and DEPT-90 13C (both optimized for 1Jch = 145 Hz), COSY (SI p S81), NOE with a 0.3 s mixing time SI p 80), HSQC optimized for 1Jch = 145 Hz (SI p S83), and HMBC optimized for 1JCH = 145 Hz and nJCH = 6.25 Hz (SI p S82) experiments.
Diisopropyl 2-((4-Methoxyphenyl)amino)-2-(2-oxocyclohexyl)-malonate (27o).
The general procedure was followed using cyclohexanone (0.25 mL, 2.4 mmol), KHMDS (1 M solution in THF) (2.56 mL, 2.5 mmol), and p-methoxyphenyl iminomalonate (0.49 g, 1.6 mmol) as starting materials to afford the ketone adduct as a brown viscous oily liquid (0.46 g, 71%). The compound was purified by flash column chromatography (Rf = 0.37 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDO3): δ 6.66–6.71 (m, AA′BB′ pattern, 4H, aromatic) ringprotons); isopropoxy group 1:5.05 (hept, J = 6.3 Hz, 1H, methine), 1.20 (d, J = 6.3 Hz, 3H, methyl), 1.19 (d, J = 6.3 Hz, 3H, methyl); isopropoxy group 2:4.97 (hept, J = 6.3 Hz, 1H, methine), 1.16 (d, J = 6.3 Hz, 3H, methyl), 0.99 (d, J = 6.3 Hz, 3H, methyl); 4.92 (s, broad, NH); 3.72 (s, 3H, methoxy); 3.51 (ddd, J = 12.6, 5.2, 1.0 Hz, 1H, methine next to ketone carbonyl); 2.55 and 1.86 [m, 2H, ring 3-CH2 (next to ring methine)]; 1.94 and 1.72 (m, 2H, ring 4-CH2); 2.06 and 1.63 (m, 2H, ring 5-CH2); 2.37 and 2.26 [ring 6-CH2 (next to ketone carbonyl)]. 13C{1H} NMR (126 MHz, CDCl3): δ 210.1 (ketone carbonyl); isopropoxycarbonyl group 1:169.2 (carbonyl), 70.0 (CH), 21.44 (methyl), 21.44 (methyl); isopropoxycarbonyl group 2:168.1 (carbonyl), 69.7 (CH), 21.44 (methyl), 21.19 (methyl); 153.6 (C-4); 139.3 (C-1); 119.1 (C-2/C-6); 114.1 (C-3/C-5); 69.9 (quaternary aliphatic carbon); 56.6 (ring methine carbon); 55.6 (methoxy); 42.5 (ring 6-CH2); 30.4 (ring 3-CH2); 27.5 (ring 5-CH2); 25.4 (ring 4-CH2). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H32NO6 406.2224, found 406.2227. The evidence for C-attack by the enolate was the generation of a quaternary aliphatic carbon, as shown by the presence of a signal at δ 69.9 in the standard 13C spectrum (SI p S85) and the absence of this signal in the DEPT-135 13C spectrum (SI p S86) and the DEPT-90 13C spectrum. Detailed chemical shift assignments could be made through a combination of 1H (SI p S84), 13C, DEPT-135 13C, DEPT-90 13C, COSY (SI p S87), HSQC (SI p S89), and HMBC (SI p S88) experiments (with key parameters the same as for 27n).
Diisopropyl 2-(Benzo[d][1,3]dioxol-5-ylamino)-2-(1-oxo-1,2,3,4-tetrahydronaphthalen-2-yl)malonate (27p).
The general procedure was followed using α-tetralone (0.30 mL, 2.2 mmol), KHMDS (1 M solution in THF) (2.43 mL, 2.4 mmol), and 3,4-methylenedioxyphenyl iminomalonate (0.49 g, 1.5 mmol) as starting materials to afford the ketone adduct as a brown gummy substance (0.61 g, 86%). The compound was purified by flash column chromatography (Rf = 0.37 (20% EtOAc/hexanes). 1H NMR (600MHz, CDCl3): δ 7.98–7.96 (m, 1H), 7.43 (td, J = 7.5, 1.3 Hz, 1H), 7.28–7.24 (m, 1H), 7.20 (d, J = 7.6 Hz, 1H), 6.56 (d, J = 8.4 Hz, 1H), 6.39 (d, J = 2.3 Hz, 1H), 6.21 (dd, J = 8.4, 2.3 Hz, 1H), 5.81 (s, 2H), 5.14 (dq, J = 12.5, 6.6 Hz, 2H), 4.98 (hept, J = 6.2 Hz, 1H), 3.71 (dd, J = 13.5, 4.0 Hz, 1H), 3.11 (td, J = 15.0, 13.1,4.0 Hz, 1H), 2.99 (dt, J = 16.6, 3.3 Hz, 1H), 2.70 (dq, J = 11.2, 3.9 Hz, 1H), 2.16 (qd, J = 13.0, 4.1 Hz, 1H), 1.24 (d, J = 6.3 Hz, 3H), 1.15 (dd, J = 16.3, 6.3 Hz, 6H), 0.99 (d, J = 6.3 Hz, 3h). 13C{1H} NMR (151 MHz, CDCl3): δ 196.6, 169.1, 167.7,147.6, 143.5, 140.9, 140.7, 133.4, 132.4, 128.3, 127.3, 126.5, 109.4, 107.8, 100.5, 100.0, 70.7, 70.3, 69.6, 54.3, 29.4, 26.5, 21.37, 21.32, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H30NO7 468.2017, found 468.2016.
Diisopropyl 2-(2-(1-Methyl-1H-pyrrol-3-yl)-2-oxoethyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (27q).
The general procedure was followed using 3-acetyl-1-methyl pyrrole (0.41 mL, 3.4 mmol), KHMDS (1 M solution in THF) (3.7 mL, 3.7 mmol), and 3-trifluoromethylphenyl iminomalonate (0.8 g, 2.3 mmol) as starting materials to afford the ketone adduct as a white solid (mp 160–162 °C) (0.57 g, 53%). The compound was purified by flash column chromatography (Rf = 0.31 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.18 (t, J = 7.9 Hz, 1H),7.07 (t, J = 1.7 Hz, 1H), 6.92 (d, J = 7.6 Hz, 1H), 6.80 (s, 1H), 6.78–6.73 (m, 1H), 6.53–6.44 (m, 2h), 5.66 (s, 1H), 5.12 (hept, J = 6.3 Hz, 2H), 3.82 (s, 2H), 3.58 (s, 3h), 1.23 (d, J = 6.3 Hz, 6H), 1.13 (d, J = 6.3 Hz, 6H). 13C{1H}NMR (151 MHz, CDCl3): δ 190.7, 168.4, 144.9, 131.3 (q, 2JCF = 31.8 Hz), 129.4, 127.0, 125.5, 124.1 (q, 1JCF = 272.3 Hz), 123.4, 117.6, 114.5 (q, 3Jcf = 3.9 Hz), 110.4 (q, 3Jcf = 3.9 Hz), 109.2, 70.5, 66.1, 41.0, 36.5, 21.33, 21.27. 19F NMR (471 MHz, CDCl3): δ −61.9 (s). HRMS (ESI-TOf) m/z: [M + H]+ calcd for C23H28F3N2O5 469.1945, found 469.1944.
Diisopropyl 2-((3-Chlorophenyl)amino)-2-(2-oxo-2-(pyridin-3-yl)ethyl)malonate (27r).
The general procedure was followed using 3-acetylpyridine (0.45 mL, 4.1 mmol), KHMDS (1 M solution in THF) (4.43 mL, 4.4 mmol), and 3-chlorophenyl iminomalonate (0.86 g, 2.7 mmol) as starting materials to afford the ketone adduct as a viscous gummy oily liquid (0.71 g, 60%). The compound was purified by flash column chromatography (Rf = 0.20 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 9.05 (s, 1H), 8.67 (s, 1H), 8.07 (d, J = 7.7 Hz, 1H), 7.32–7.27 (m, 1H), 6.95 (t, J = 8.0 Hz, 1H), 6.60 (d, J = 7.6 Hz, 1H), 6.54 (s, 1H), 6.44 (d, J = 7.8 Hz, 1H), 5.52 (s, 1H), 5.07 (hept, J = 6.3 Hz, 2H), 4.06 (s, 2H), 1.16 (d, J = 6.2 Hz, 6h), 1.08 (d, J = 6.2 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.0, 167.8, 153.6, 149.2, 145.1, 135.0, 134.6, 131.5, 130.0, 123.3, 118.4, 114.0, 112.2, 70.6, 65.6, 40.6, 21.15, 21.11. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26ClN2O5 433.1525, found 433.1525.
Diisopropyl 2-((3-Chlorophenyl)amino)-2-(2-oxo-2-(pyridin-2-yl)ethyl)malonate (27s).
The general procedure was followed using 2-acetylpyridine (0.43 mL, 3.8 mmol), KHMDS (1 M solution in THF) (4.10 mL, 4.1 mmol), and 3-chlorophenyl iminomalonate (0.80 g, 2.5 mmol) as starting materials to afford the ketone adduct as a yellow solid (mp 110–113 °C) (0.61 g, 55%). The compound was purified by flash column chromatography (Rf = 0.62 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.68–8.55 (m, 1H), 7.94 (d, J = 7.7 Hz, 1H), 7.77 (t, J = 7.1 Hz, 1H), 7.48–7.36 (m, 1H), 7.00 (t, J = 7.8 Hz, 1h), 6.65 (d, J = 7.8 Hz, 1H), 6.54 (d, J = 7.5 Hz, 1H), 5.57 (s, 1H), 5.13 (hept, J = 6.2 Hz, 2H), 4.43 (s, 2H), 1.23 (d, J = 6.1 Hz, 6h), 1.14 (d, J = 6.1 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 197.6, 168.1, 152.5, 148.7, 145.5, 136.6, 134.4, 129.8, 127.2, 121.4, 118.2, 114.3, 112.5, 70.3, 65.9, 40.0, 21.2, 21.1. HRMS (ESI-TOF)m/z: [M + H]+ calcd for C22H26ClN2O5 433.1525, found 433.1527.
Diisopropyl 2-((4-Bromophenyl)amino)-2-(2-oxo-2-(pyridin-3-yl)ethyl)malonate (27t).
The general procedure was followed using 3-acetylpyridine (0.37 mL, 3.3 mmol), KHMDS (1 M solution in THF) (3.59 mL, 3.5 mmol), and 4-bromophenyl iminomalonate (0.80 g, 2.2 mmol) as starting materials to afford the ketone adduct as a yellow solid (mp 127–130 °C) (0.58 g, 54%). The compound was purified by flash column chromatography (Rf = 0.21 (30% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 9.05 (s, 1H), 8.70 (d, J = 4.1 Hz, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.31 (dd, J = 7.8, 4.9 Hz, 1H), 7.15 (d, J = 8.6 Hz, 2H), 6.47 (d, J = 8.6 Hz, 2H), 5.46 (s, 1H), 5.08 (hept, J = 6.2 Hz, 2H), 4.06 (s, 2h), 1.17 (d, J = 6.3 Hz, 6H), 1.10 (d, J = 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 195.1, 168.0, 153.6, 149.3, 143.0, 135.0, 131.8, 131.6,123.4, 115.9, 110.5, 70.7, 65.8, 40.5, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26BrN2O5 477.1020, found 477.1017.
General Procedure for the Asymmetric Reduction of Ketone Adducts via CBS Reduction (Figure 7).
In a thick-walled flame-dried reaction vial, Mannich ketone adduct (1.0 equiv) was dissolved in anhydrous THF (0.2 M) under argon and cooled to –15 °C using a NESLAB CC 100 immersion cooler with isopropanol as bath solvent. To this cooled reaction mixture was added (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.15 equiv) dropwise and the mixture stirred for 5 min. Then BH3·DMS solution (2 M solution in THF) (2.0 equiv) was added slowly dropwise to the reaction mixture and continued stirring for 75 h at −15 °C or until complete consumption of the starting material ketone. Then the reaction was cooled to 0 °C and quenched using saturated NH4Cl solution or methanol (3 mL).
Workup and Purification.
After quenching, the reaction mixture was diluted with brine (10 mL), and the organic layer was separated. The aqueous layer was then extracted with EtOAc twice (2 × 20 mL), and the combined organic layers were washed with brine (20 mL) once, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by automated flash column chromatography.
Diisopropyl (R)-2-(2-Hydroxy-2-phenylethyl)-2-((4-methoxyphenyl)amino)malonate (28a).
The general procedure was followed using 27a (0.1 g, 0.2 mmol) (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.035 mL, 0.03 mmol), and BH3·DMS solution (2 M solution in THF) (0.23 mL, 0.4 mmol) as starting materials to afford the alcohol as a beige viscous oily liquid (0.075g, 75%). The compound was purified by flash column chromatography (Rf = 0.33 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.35–7.31 (m, 4H), 7.28–7.24 (m, 1H), 6.82 (d, J = 8.9 Hz, 2H), 6.76 (d, J = 9.0 Hz, 2H), 5.16 (s, 1H), 5.11 (hept, J = 6.3 Hz, 1H), 4.95 (hept, J = 6.3 Hz, 1H), 4.85 (d, J = 10.0 Hz, 1H), 3.98 (s, 1H), 3.74 (s, 3H), 2.74–2.62 (m, 2H), 1.29 (d, J = 6.3 Hz, 3H), 1.19 (d, J = 6.3 Hz, 3H), 1.13 (d, J = 6.3 Hz, 3H), 1.02 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (126 MHz, CDCl3): δ 169.1, 168.4, 154.1, 144.1, 137.5, 128.3, 127.3, 125.5, 118.8, 114.5, 70.9, 70.2, 69.9,69.0, 55.5, 41.7, 21.5, 21.36, 21.32, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H32NO6 430.2224, found 430.2228. HPLC analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 13.6 min, tminor = 15.2 min, ee 94%, er 97:3; [α]D20 = +42.3 (C = 1, CHCl3).
Determination of Absolute Configuration of Chiral Alcohol Using Mosher Ester Analysis.
Preparation of (S)- and (R)-MTPA Esters.16
To a stirred solution of the alcohol (28a, 10 mg, 0.023 mmol) and dry pyridine (5.8 μL, 0.07 mmol, 3.1 equiv) in dry DCM (0.5 mL, 0.046 M) at room temperature was added S-(+)-MTPA-Cl (8.2 μL, 0.04 mmol, 1.9 equiv). The reaction progress was monitored by thin-layer chromatography (TLC) on silica gel. After 5 h of stirring at room temperature, the reaction mixture was quenched by the addition of water (~1 mL) and ethyl acetate (~3 mL). The aqueous layer was extracted with two additional portions of ethyl acetate (~3 mL), and the combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography to give the R-MTPA ester. 1H NMR (600 MHz, CDCl3): 7.37 (ddd, J = 8.5,4.0, 2.1 Hz, 1H), 7.34–7.28 (m, 7H), 7.26 (s, 2H), 6.70 (d, J = 8.9 Hz, 2H), 6.53 (d, J = 8.9 Hz, 2H), 6.08 (t, J = 6.7 Hz, 1H), 4.87–4.80 (m, 2H), 4.64 (dp, J = 12.6, 7.2, 6.3 Hz, 1H), 3.73 (s, 3H), 3.37 (s, 3H), 3.06 (dd, J = 15.5, 7.0 Hz, 1H), 2.94 (dd, J = 15.5, 6.4 Hz, 1H), 1.19 (d, J = 6.3 Hz, 3H), 1.01 (d, J =5.7 Hz, 6H), 0.95 (d, J = 6.2 Hz, 3H). In an entirely analogous fashion, the S-MTPA ester was prepared using R-(–)-MTPA-Cl. 1H NMR (600 MHz, CDCl3): 7.37–7.29 (m, 2H), 7.26 (h, J = 4.6 Hz, 4H), 7.21 (d, J = 7.7 Hz, 2H), 7.18 (dd, J = 7.6, 1.7 Hz, 2H), 6.73 (d, J = 8.9 Hz, 2H), 6.61 (d, J = 8.9 Hz, 2H), 5.95 (t, J = 6.6 Hz, 1H), 4.99 (s, 1H), 4.95 (hept, J = 12.5, 6.3 Hz, 1H), 4.65 (hept, J = 6.3 Hz, 1H), 3.74 (s, 3H), 3.40 (s, 3H), 3.08 (dd, J = 15.6,7.2 Hz, 1H), 2.91 (dd, J = 15.6, 6.1 Hz, 1H), 1.11 (d, J = 6.3 Hz, 3H), 1.05 (d, J = 6.2 Hz, 3H), 0.98 (d, J = 6.3 Hz, 3H), 0.95 (d, J = 6.2 Hz, 3H).
Diisopropyl (R)-2-((3,5-Dimethylphenyl)amino)-2-(2-hydroxy-2-phenylethyl)malonate (28b).
The general procedure was followed using 27b (0.5 g, 1.18 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.18 mL, 0.18 mmol), and BH3·DMS solution (2 M solution in THF) (1.18 mL, 2.3 mmol) as starting materials to afford the alcohol as a brown viscous gummy substance (0.42g, 83%). The compound was purified by flash column chromatography (Rf = 0.47 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.36–7.30 (m, 4H), 7.29–7.23 (m, 1H), 6.49 (s, 1H), 6.42 (s, 2H), 5.35 (s, 1H), 5.09 (hept, J = 6.3 Hz, 1H), 5.02 (hept, J = 6.3 Hz, 1H), 4.84 (t, J = 6.3 Hz, 1H), 3.42 (s, 1H), 2.75 (d, J = 6.4 Hz, 2H), 2.23 (s, 6H), 1.26 (dd, J = 17.6, 6.3 Hz, 6h), 1.11 (d, J = 6.3 Hz, 3h), 1.05 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (126 MHz, CDCl3): δ 169.0, 168.8, 144.24, 144.22, 138.6, 128.2, 127.2, 125.5, 121.6, 113.8, 70.6, 70.1, 70.0, 68.0, 41.7, 21.5,21.3,21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H34NO5 428.2431, found 428.2431. HPLC analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 8.8 min, tminor = 9.4 min, ee 97%, er 98.4:1.6; [α]D20 = +32.7 (C = 1, CHCl3).
Diisopropyl (R)-2-(2-(4-Bromophenyl)-2-hydroxyethyl)-2-((3,5-1dimethylphenyl)amino)malonate (28c).
The general procedure was followed using 27c (0.27 g, 0.53 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.08 mL, 0.08 mmol), and BH3·DMS solution (2 M solution in THF) (0.53 mL, 1.07 mmol) as starting materials to afford the alcohol as a pale yellow foamy solid (0.22g, 81%). The compound was purified by flash column chromatography (Rf = 0.55 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.43 (d, J = 8.4 Hz, 2H), 7.16 (d, J =8.3 Hz, 2H), 6.49 (s, 1H), 6.38 (s, 2H), 5.32 (s, 1H), 5.08 (hept, J = 6.2 Hz, 1h), 5.00 (hept, J = 6.2 Hz, 1H), 4.78 (dd, J = 8.3, 3.9 Hz, 1H), 3.60 (s, 1H), 2.74–2.63 (m, 2H), 2.22 (s, 6H), 1.27 (d, J = 6.3 Hz, 3H), 1.23 (d, J = 6.3 Hz, 3H), 1.11 (d, J = 6.3 Hz, 3H), 1.04 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 168.9, 168.7, 144.0, 143.2, 138.7, 131.3, 127.2, 121.8, 120.9, 113.8, 70.2, 70.1, 70.0, 68.0, 41.6, 21.5, 21.3, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H33BrNO5 506.1537, found 506.1536. HPLC analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor =8.1 min, tminor = 8.7 min, ee 94%, er 97:3; [α]D20 = +26.8 (C = 1, CHCl3).
Diisopropyl (R)-2-(Benzo[d][1,3]dioxol-5-ylamino)-2-(2-hydroxy-2-phenylethyl)malonate (28d).
The general procedure was followed using 27d (0.32 g, 0.73 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.11 mL, 0.11 mmol), and BH3·DMS solution (2 M solution in THF) (0.73 mL, 1.46 mmol) as starting materials to afford the alcohol as a pale yellow waxy solid (0.28g, 90%). The compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.26–7.20 (m, 4H), 7.19–7.14 (m, 1H), 6.54 (d, J = 8.3 Hz, 1H), 6.36 (d, J = 2.2 Hz, 1H), 6.17 (dd, J = 8.3, 2.3 Hz, 1h),5.77 (s,2H), 5.12 (s, 1H), 5.00 (hept, J = 6.2 Hz, 1H), 4.91 (hept, J = 6.3 Hz, 1H), 4.74 (dd, J = 9.5, 2.8 Hz, 1H), 3.58 (s, 1H), 2.66–2.52 (m, 2H), 1.19 (d, J = 6.3 Hz, 3H), 1.14 (d, J = 6.3 Hz, 3h), 1.05 (d, J = 6.3 Hz, 3H), 0.99 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.0, 168.5, 148.0, 144.1, 141.6, 139.0, 128.3, 127.3, 125.5, 109.0, 108.2, 100.7, 99.9, 70.7, 70.2, 70.0, 68.7, 41.5, 21.5, 21.34, 21.30. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H30NO7 444.2017, found 444.2022. HPLC analysis: Chiralpak IB, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 14.0 min, tminor =15.3 min, ee 96%, er 98:2; [α]D20 = +36.2 (C = 1, CHCl3).
Diisopropyl (R)-2-(2-Hydroxy-2-phenylethyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (28e).
The general procedure was followed using 27e (0.40 g, 0.87 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.13mL, 0.13 mmol), and BH3·DMS solution (2 M solution in THF) (0.87 mL, 1.75 mmol) as starting materials to afford the alcohol as a colorless viscous gummy substance (0.385g, 94%). The compound was purified by flash column chromatography (Rf = 0.43 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.33 (t, J = 7.3 Hz, 2H), 7.31–7.25 (m, 4H), 7.07 (d, J = 7.5 Hz, 1H), 6.99 (s, 1H), 6.88 (d, J = 7.8 Hz, 1H), 5.77 (s, 1H), 5.13 (hept, J = 6.3 Hz, 1h), 5.06 (hept, J = 6.4 Hz, 1H), 4.84 (s, 1h), 2.85–2.72 (m, 3H), 1.33 (d, J = 6.2 Hz, 3H), 1.26 (d, J = 6.2 Hz, 3h), 1.17 (d, J = 6.2 Hz, 3H), 1.07 (d, J = 6.2Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.0, 168.6, 144.9, 144.0, 131.5 (q, 2JCF = 31.9 Hz), 129.7, 128.4, 127.5, 125.5, 124.0 (q, 1JCF = 272.3 Hz), 117.5, 115.3 (q, 3Jcf = 3.6 Hz), 111.5 (q, 3Jcf = 3.6 Hz), 70.6, 70.3, 70.2, 67.2, 41.4, 21.33, 21.30, 21.2, 21.1. 19F NMR (471 MHz, CDCl3): δ −61.9 (S). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H29F3NO5 468.1992, found 468.1993. HPLC analysis: Chiralpak IB, 2% IP/hexanes, continuous flow at 0.4 mL/min, 230 nm; tmajor = 21.1 min, tminor = 18.8 min, ee 92%, er 4:96; [α] D20 = +6.2 (C = 1, CHCl3).
Diisopropyl (R)-2-((3,5-Dimethylphenyl)amino)-2-(2-hydroxy-2-(naphthalen-2-yl)ethyl)malonate (28f).
The general procedure was followed using 27f (0.6 g, 1.26 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.19 mL, 0.19 mmol), and BH3·DMS solution (2 M solution in THF) (1.26 mL, 2.52 mmol) as starting materials to afford the alcohol as a pale yellow foamy solid (0.44g, 73%). The compound was purified by flash column chromatography (Rf = 0.51 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.87–7.75 (m, 4H), 7.51–7.44 (m, 2H), 7.41 (dd, J = 8.5, 1.8 Hz, 1H), 6.52 (s, 1H),6.45 (s,2H), 5.41 (s, 1H),5.11 (hept, J = 6.4 Hz, 1H), 5.07–4.99 (m, 2H), 3.55 (s, 1H), 2.91–2.79 (m,2H), 2.25 (s, 6H), 1.29 (dd, J = 14.3, 6.3 Hz, 6H), 1.13 (d, J = 6.2 Hz, 3h), 1.07 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.0, 168.9, 144.2, 141.6, 138.7, 133.2, 132.8, 128.0, 127.9, 127.5, 125.9, 125.6, 124.1, 123.9, 121.7, 113.8, 70.7, 70.2, 70.1, 68.1, 41.7, 21.5, 21.4, 21.3, 21.27, 21.21. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H36NO5 478.2588, found 478.2589. HPLC analysis: Chiralpak IB, 2% IP/hexanes, continuous flow at 0.4 mL/min, 230 nm; tmajor = 26.7 min, tminor = 25.4 min, ee 98%, er 1:99; [α]D20 = +18.8 (C = 1, CHCl3).
Diisopropyl (R)-2-(2-Hydroxy-2-(naphthalen-1-yl)ethyl)-2-(ptolylamino)malonate (28g).
The general procedure was followed using 27g (0.64g, 1.40 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.21 mL, 0.21 mmol), and BH3·DMS solution (2 M solution in THF) (1.40 mL, 2.80 mmol) as starting materials to afford the alcohol as a beige solid (mp 95–98 °C) (0.47g, 73%). The compound was purified by flash column chromatography (Rf = 0.48 (20% EtOAc/hexanes). 1HNMR (600 MHz, CDCl3): δ 7.84 (d, J = 8.2 Hz, 1H), 7.75 (t, J = 8.5 Hz, 2H), 7.66 (d, J = 8.5 Hz, 1H), 7.48 (t, J = 7.7 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.29–7.22 (m, 1h), 7.03 (d, J =8.1 Hz, 2h), 6.75 (d, J = 8.3 Hz, 2h), 5.68 (d, J = 10.7 Hz, 1H), 5.47 (s, 1H), 5.14–5.04 (m, 2H), 3.31 (s, 1H), 3.00 (d, J = 15.4 Hz, 1H), 2.79 (dd, J = 15.4, 10.8 Hz, 1H), 2.31 (s, 3H), 1.29 (dd, J = 15.6, 6.3 Hz, 6H), 1.12 (dd, J = 21.7, 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.3, 141.8, 140.0, 133.5, 129.8, 129.7, 128.8, 128.6, 127.125.5, 125.4, 125.2, 122.9, 122.8, 116.0, 70.2, 70.1, 67.9, 67.2, 40.6, 21.4, 21.36, 21.31, 20.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H34NO5 464.2431, found 464.2434. HPLC analysis: Chiralpak ID, 91% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor = 12.8 min, tminor = 11.7 min, ee 83%, er 8.6:91.4; [α]D20 = +28.5 (C =1, CHCl3).
Diisopropyl (R)-2-(2-Hydroxy-2-phenylethyl)-2-(phenylamino)- malonate (28h).
The general procedure was followed using 27h (0.45 g, 1.13 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.17 mL, 0.17 mmol), and BH3·DMS solution (2 M solution in THF) (1.13 mL, 2.27 mmol) as starting materials to afford the alcohol as a colorless viscous oily liquid (0.37g, 82%). The compound was purified by flash column chromatography (Rf = 0.42 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.34–7.23 (m, 5H), 7.18 (dd, J = 8.4, 7.5 Hz, 2H), 6.82 (t, J = 7.3 Hz, 1H), 6.77 (d, J = 7.7 Hz, 2h), 6.77 (d, J = 7.7 Hz, 1H), 5.05 (ddt, J = 20.7, 12.6, 6.3 Hz, 2H), 4.84 (dt, J = 6.5, 3.2 Hz, 1H), 3.16 (s, 1H), 2.79–2.71 (m, 2H), 1.25 (dd, J = 6.3, 1.9 Hz, 6h), 1.06 (dd, J = 11.3, 6.3 Hz, 6H). 13C{1H} NMR (126 MHz, CDCl3): δ 169.0, 168.9, 144.3, 144.1, 129.1, 128.3, 127.3, 125.5, 119.6, 115.6, 70.5, 70.2, 67.9, 41.7, 21.5, 21.3, 21.25, 21.22. HRMS (eSI-TOF) m/z: [M + H]+ calcd for C23H30NO5 400.2118, found 400.2120. HPLC analysis: Chiralpak IC, 14% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor = 14.8 min, tminor = 16.3 min, ee 97%, er 98.4:1.6; [α]D20 = +26.2 (c = 1, CHCl3).
Diisopropyl (R)-2-(benzo[d][1,3]dioxol-5-ylamino)-2-(2-(furan-2- yl)-2-hydroxyethyl)malonate (28i).
The general procedure was followed using 27i (0.53 g, 1.24 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.18 mL, 0.18 mmol), and BH3·DMS solution (2 M solution in THF) (1.24 mL, 2.48 mmol) as starting materials to afford the alcohol as a pale yellow wax (0.39g, 77%). The compound was purified by flash column chromatography (Rf= 0.26 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.33 (dd, J = 1.8, 0.9 Hz, 1H), 6.59 (d, J = 8.3 Hz, 1H), 6.38 (d, J = 2.3 Hz, 1H), 6.29 (dd, J = 3.1, 1.8 Hz, 1h), 6.24–6.16 (m, 2H), 5.83 (s, 2H), 5.14 (s, 1H), 5.00 (ddt, J = 15.7, 12.5, 6.3 Hz, 2H), 4.88 (dd, J = 10.0, 2.6 Hz, 1H), 3.35 (s, 1H), 2.88–2.74 (m, 2h), 1.21 (d, J = 6.4 Hz, 6H), 1.08 (dd, J = 16.5, 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 168.8, 168.7, 155.9, 148.0, 141.8, 141.4, 139.0, 110.0, 108.6, 108.2, 105.6, 100.7, 99.6, 70.2, 70.1, 68.1, 64.3, 37.9, 21.36, 21.30, 21.27, 21.25. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H28NO8 434.1809, found 434.1811. HPLC analysis: Chiralpak IB, 20% IP/hexanes, continuous flow at 0.8 mL/min, 250 nm; tmajor =14.1 min, tminor = 10.3 min, ee 92%, er 4:96; [α]D20 = +12.0 (c = 1, CHCl3).
Diisopropyl-(R)-2-((4-(2-((tert-butyldimethylsilyl)oxy)ethyl)phenyl)amino)-2-(2-(furan-2-yl)-2-hydroxyethyl)malonate (28j).
The general procedure was followed using 27j (0.55 g, 1.00 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1M solution in toluene) (0.15 mL, 0.15 mmol) and BH3·DMS solution (2M solution in THF) (1.00 mL, 2.01 mmol) as starting materials to afford the alcohol as a colorless viscous oily liquid (0.40g, 73%). Compound was purified by flash column chromatography (Rf = 0.37 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.33 (s, 1H), 6.98 (d, J = 8.1 Hz, 2H), 6.66 (d, J = 8.1 Hz, 2H), 6.32–6.28 (m, 1H), 6.22 (d, J = 2.9 Hz, 1H), 5.28 (s, 1H), 5.01 (pd, J = 6.1, 2.1 Hz, 2H), 4.90–4.85 (m, 1H), 3.70 (t, J = 7.1 Hz, 2H), 3.03 (s, 1H), 2.94–2.83 (m, 2H), 2.69 (t, J = 7.1 Hz, 2H), 1.22 (dd, J = 10.3, 6.3 Hz, 6H), 1.05 (dd, J = 28.9, 6.2 Hz, 6H), 0.86 (s, 9H), −0.03 (s, 6H). 13C{1H} NMR (126 MHz, CDCl3): δ 169.0, 168.9, 155.9, 142.4, 141.8, 130.2, 129.7, 115.7, 110.0, 105.7, 70.3, 70.1, 67.5, 64.7, 64.3, 38.6, 37.9, 25.8, 21.4, 21.3, 21.26, 21.25, 18.2, −5.4. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C29H46NO7Si 548.3038; found 548.3040. HPLC Analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5mL/min, 250 nm; tmajor = 13.7 min, tminor = 8.4 min, ee 94%, er 3:97; [α]D20 = +1.5 (C = 1, CHCl3).
Diisopropyl-(R)-2-(benzo[d][1,3]dioxol-5-ylamino)-2-(2-hydroxy-2-(thiophen-2-yl)ethyl)malonate (28k).
The general procedure was followed using 27k (0.51 g, 1.15 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1M solution in toluene) (0.17 mL, 0.17 mmol) and BH3·DMS solution (2M solution in THF) (1.15 mL, 2.31 mmol) as starting materials to afford the alcohol as a dark brown colored foamy gummy substance (0.39g, 75%). Compound was purified by flash column chromatography (Rf = 0.32 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.22 (dd, J = 5.0, 1.2 Hz, 1H), 6.95 (dd, J = 5.0, 3.5 Hz, 1H), 6.91 (d, J = 3.4 Hz, 1H), 6.62 (d, J = 8.3 Hz, 1H), 6.43 (d, J = 2.3 Hz, 1H), 6.24 (dd, J = 8.3, 2.3 Hz, 1H), 5.86 (s, 2H), 5.16 (s, 1H), 5.12–5.04 (m, 2H), 4.99 (hept, J = 6.2 Hz, 1H), 3.71 (s, 1H), 2.86–2.75 (m, 2H), 1.25 (d, J = 6.3 Hz, 3H), 1.22 (d, J = 6.3 Hz, 3H), 1.12 (d, J = 6.3 Hz, 3H), 1.08 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (126 MHz, CDCl3): δ 168.8, 168.4, 148.1, 148.0, 141.7, 138.8, 126.5, 124.3, 122.9, 109.1, 108.3, 100.8, 99.9, 70.3, 70.2, 68.6, 67.0, 41.7, 21.5, 21.37, 21.33. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C22H28NO7S 450.1581; found 450.1581. HPLC Analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.8mL/min, 250 nm; tmajor = 12.3 min, tminor = 10.6 min, ee 90%, er 5:95; [α]D20 = +20.3 (C = 1, CHCl3).
Diisopropyl (R)-2-(2-(4-bromophenyl)-2-hydroxyethyl)-2-(pyridin-3-ylamino)malonate (28l).
The general procedure was followed using 27l (0.53 g, 1.11 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.16 mL, 0.16 mmol), and BH3·DMS solution (2 M solution in THF) (1.11 mL, 2.22 mmol) as starting materials to afford the alcohol as a white foamy solid (0.37g, 69%). The compound was purified by flash column chromatography (Rf = 0.30 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.04 (s, 1H), 7.91 (d, J = 5.2 Hz, 1H), 7.38 (d, J =8.1 Hz, 2H), 7.19 (dd, J =8.1, 5.7 Hz, 1H), 7.06 (d, J = 8.1 Hz, 2H), 7.03 (d, J = 8.2 Hz, 1H), 6.00 (s, 1H), 5.11 (hept, J = 6.3 Hz, 1H), 5.02 (hept, J = 6.4 Hz, 1H),4.69 (d, J = 9.5 Hz, 1H), 2.70–2.48 (m, 4H), 1.29 (d, J = 6.2 Hz, 3H), 1.20 (t, J = 6.3 Hz, 6h), 1.06 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (126 MHz, CDCl3): δ 168.3, 167.6, 142.6, 142.1, 136.9, 134.5, 131.5, 127.1, 125.2, 121.8, 121.4, 71.3, 70.9, 69.4, 66.3, 40.4, 21.3, 21.29, 21.27. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H28BrN2O5 479.1176, found 479.1173. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 11.1 min, tminor = 9.8 min, ee 82%, er 9:91; [α]D20 = −14.7 (C = 1, CHCl3).
Diisopropyl (R)-2-((4-(2-((tert-Butyldimethylsilyl)oxy)ethyl)-1 phenyl)amino)-2-(2-hydroxy-2-(pyridin-4-yl)ethyl)malonate (28m).
The general procedure was followed using 27m (0.5 g, 0.89 mmol), (S)-(–)-2-methyl-CBS-oxazaborolidine solution (1 M solution in toluene) (0.13 mL, 0.13 mmol), and BH3·DMS solution (2 M solution in THF) (0.9 mL, 1.8 mmol) as starting materials to afford the alcohol as a beige foamy solid (0.32g, 65%). The compound was purified by flash column chromatography (Rf = 0.20 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.44 (d, J = 6.3 Hz, 2H), 7.33 (d, J = 6.3 Hz, 2H), 7.01 (d, J = 8.3 Hz, 2H), 6.64 (d, J =8.3 Hz, 2H), 5.30 (s, 1H), 5.08 (hept, J = 6.2 Hz, 1H), 4.97 (hept, J = 6.2 Hz, 1H), 4.88 (d, J =10.2 Hz, 1H), 4.03 (s, 1H), 3.71 (t, J = 7.0 Hz, 2H), 2.69 (t, J = 6.9 Hz, 2H), 2.57 (dd, J = 15.2, 10.6 Hz, 2H), 1.25 (d, J = 6.3 Hz, 3H), 1.20 (d, J = 6.3 Hz, 3H), 1.08 (d, J = 6.3 Hz, 3H), 1.01 (d, J = 6.3 Hz, 3H), 0.85 (s, 9H), −0.03 (s, 6H). 13C{1H} NMR (126 MHz, CDCl3): δ 168.5, 168.3, 157.1, 147.1, 141.7, 131.2, 129.9, 121.9, 115.9, 70.65, 70.60, 68.8, 67.8, 64.4, 40.8, 38.5, 25.8, 21.5, 21.28, 21.21, 21.1, 18.2, −5.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C30H47N2O6Si 559.3198, found 559.3197. HPLC analysis: Chiralpak IC, 15% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 16.2 min, tminor = 18.2 min, ee 72%, er 86:14; [α]D20 = +5.5 (C = 1, CHCl3).
General Procedure for the Synthesis of Racemic Alcohols from Ketone Adducts (Figure 7).
In a thick-walled flame-dried reaction vial, Mannich ketone adduct (1.0 equiv) was dissolved in anhydrous THF (0.2 M) under argon. Then BH3·DMS solution (2 M solution in THF) (2.0 equiv) was added slowly dropwise to the reaction mixture at room temperature under constant stirring. Then the reaction mixture was heated to 50 °C and stirring was continued until the complete consumption of the starting material ketone. Then the reaction was cooled to 0 °C and quenched using saturated NH4Cl solution or methanol (3 mL).
Workup and Purification.
After quenching, the reaction mixture was diluted with brine (10 mL) and the organic layer was separated. The aqueous layer was then extracted with EtOAc twice (2 × 20 mL), and the combined organic layers were washed with brine (20 mL) once, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by automated flash column chromatography.
Diisopropyl 2-((1R,2R)-1-Hydroxy-1-phenylpropan-2-yl)-2-((4–1 methoxyphenyl)amino)malonate (28n).
The general procedure was followed using 27n (0.34 g, 0.77 mmol) and BH3·DMS solution (2 M solution in THF) (0.78 mL, 1.55 mmol) as starting materials to afford the alcohol as a dark yellowish brown viscous oily liquid (0.21g, 61%). The reaction was finished in 3.5 h. The compound was purified by flash column chromatography (Rf = 0.39 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.33 (dt, J = 15.3, 7.6 Hz, 4H), 7.22 (t, J = 7.2 Hz, 1H), 6.81–6.74 (m, 4H), 5.51 (s, 1H), 5.10 (hept, J = 6.2 Hz, 2H), 4.96 (hept, J = 6.0 Hz, 1H), 3.74 (s, 3H), 3.37 (s, 1H), 2.86 (q, J = 7.0 Hz, 1H), 1.26 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 1.17 (d, J = 6.3 Hz, 3H), 0.94 (dd, J = 14.0, 6.7 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.9, 168.5, 153.9, 143.6, 138.4, 127.9, 126.6, 125.5, 118.7, 114.5, 72.3, 71.9, 70.5, 69.8, 55.6, 43.8, 21.5, 21.45, 21.40, 21.1, 7.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H34NO6 444.2381, found 444.2386. HPLC analysis: racemic mixture.
Diisopropyl 2-((1R, 2R)-2-Hydroxycyclohexyl)-2-((4–1 methoxyphenyl)amino)malonate (28o).
The general procedure was followed using 27o (0.31 g, 0.77 mmol) and BH3·DMS solution (2 M solution in THF) (0.77 mL, 1.54 mmol) as starting materials to afford the alcohol as a yellow viscous oily liquid (0.207g, 66%). The reaction was finished in 14 h. The compound was purified by flash column chromatography (Rf = 0.32 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 6.69 (d, J = 8.8 Hz, 2H), 6.63 (d, J = 8.8 Hz, 2H), 5.03 (hept, J = 5.9 Hz, 1H), 5.03 (hept, J = 5.9 Hz, 2H), 4.26 (s, 1H), 3.69 (s, 3H), 3.10 (s, 1H), 2.50 (d, J = 11.7 Hz, 1H), 1.82 (q, J = 13.0 Hz, 3H), 1.65 (dq, J = 26.3, 12.7, 11.4 Hz, 2H), 1.36 (dt, J = 45.4, 13.8 Hz, 3H), 1.22 (d, J = 6.2 Hz, 3H), 1.14 (d, J = 6.2 Hz, 3H), 1.08 (d, J = 6.2 Hz, 3H), 0.91 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.6, 168.8, 153.4, 138.7, 117.5, 114.3, 72.1, 70.1, 69.7, 66.6, 55.5, 45.7, 33.4, 26.4, 23.0, 21.4, 21.3, 21.2, 21.1, 19.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H34NO6 408.2381, found 408.2381. HPLC analysis: racemic mixture.
Diisopropyl 2-(Benzo[d][1,3]dioxol-5-ylamino)-2-((1S,2R)-1-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)malonate (28p).
The general procedure was followed using 27p (0.34 g, 0.72 mmol) and BH3·DMS solution (2 M solution in THF) (0.72 mL, 1.45 mmol) as starting materials to afford the alcohol as a beige foamy solid (0.178g, 52%). The reaction was finished in 12 h. The compound was purified by flash column chromatography (Rf = 0.27 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.28 (d, J = 5.9 Hz, 1H), 7.24 (t, J = 6.9 Hz, 1H), 7.21–7.16 (m, 2H), 6.60 (d, J = 8.4 Hz, 1H), 6.38 (d, J = 2.1 Hz, 1h), 6.19 (dd, J = 8.3, 2.1 Hz, 1H), 5.85 (d, J = 3.0 Hz, 2H), 5.25–5.14 (m, 2H), 5.09 (s, 1H), 5.00 (hept, J = 6.1, 5.6 Hz, 1H), 2.97 (qd, J = 16.8, 15.7, 7.7 Hz, 3h), 2.38 (s, 1h), 2.27 (d, J = 10.3 Hz, 1H), 2.15–2.04 (m, 1H), 1.33 (d, J = 6.2 Hz, 3H), 1.29 (d, J = 6.2 Hz, 3H), 1.21 (d, J = 6.2 Hz, 3H), 1.00 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 170.0, 168.3, 148.0, 140.9, 139.8, 137.8, 136.8, 130.3, 128.8, 128.0, 126.0, 108.3, 108.0, 100.6, 99.0, 70.76, 70.72, 69.5, 67.8, 44.6, 30.5, 21.6, 21.46, 21.40, 21.3, 20.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H32NO7 470.2173, found 470.2172. HPLC analysis: racemic mixture.
General Procedure for the Cyclization of Chiral Alcohols to the Corresponding Azetidines and Tetrahydroquinolines (Figure 9).
This procedure was adopted from the literature procedure.30,31 In a thick-walled, flame-dried reaction vial, chiral alcohol (1.0 equiv) was dissolved in anhydrous toluene (0.05 M) under argon. To this reaction mixture was added PPh3 (2.2 equiv) in one portion followed by the addition of 2,4,5-tribromoimidazole (1.1 equiv) and continued stirring for 48 h or in general until the complete consumption of starting material by heating at 80 °C. After the complete consumption of starting material was confirmed, the heating was stopped and the reaction mixture was brought to room temperature.
Workup and Purification.
After the reaction mixture was brought to room temperature, the solids that were separated out were filtered and the solids were washed with ethyl acetate twice (2 × 5 mL). The combined organic layers were concentrated under reduced pressure. The crude product was purified by automated flash column chromatography.
Note: For the substrates that we made, the reaction times ranged from 48 to 96 h.
Diisopropyl (R)-4-(4-Bromophenyl)-1-(3,5-dimethylphenyl)-azetidine-2,2-dicarboxylate (30c) and Diisopropyl (S)-4-(4-Bromophenyl)-5,7-dimethyl-3,4-dihydroquinoline-2,2(1H)-dicarboxylate (31c).
The general procedure was followed using 28c (0.20 g, 0.39 mmol), PPh3 (0.23 g, 0.87 mmol), and 2,4,5-tribromoimidazole (0.13 g, 0.43 mmol) as starting materials to afford azetidine (30c) as a white wax (0.093 g) (Rf = 0.40 (10% EtOAc/hexane)) and tetrahydroquinoline (31c) as a pale yellow viscous oily liquid (0.048 g) (Rf = 0.35 (10% EtOAc/hexane)) in a ratio of 1.9:1 after isolation (0.14 g in total, 72%). The reaction was finished in 48 h. The compound was purified by column chromatography to separate azetidine and tetrahydroqinoline. Azetidine (30c). 1H NMR (500 MHz, CDCl3): δ 7.49 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 8.3 Hz, 2H), 6.46 (s, 1H), 6.32 (s, 2H), 5.27 (hept, J = 6.2 Hz, 1H), 5.11 (t, J = 7.6 Hz, 1H), 5.03 (hept, J = 6.2 Hz, 1H), 3.14 (dd, J = 11.1, 8.4 Hz, 1H), 2.46 (dd, J = 11.2, 7.0 Hz, 1H), 2.17 (s, 6H), 1.36 (t, J = 6.1 Hz, 6H), 1.21 (d, J = 6.2 Hz, 3H), 1.07 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.6, 168.5, 147.0, 141.6, 138.0, 131.8, 127.5, 121.7, 121.4, 111.5, 72.0, 69.7, 69.2, 61.5, 36.3, 21.68, 21.66, 21.64, 21.48, 21.32. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H31BrNO4 488.1431, found 488.1460. HPLC analysis: Chiralpak IE, 3% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor = 13.5 min, tminor = 12.9 min, ee 86%, er 7:93; [α]D20 = −23.8 (C = 1, CHCl3).
Tetrahydroquinoline (31c).
1H NMR (500 MHz, CDCl3): δ 7.31 (d, J =8.4 Hz, 2H), 6.89 (d, J = 8.4 Hz, 2h), 6.49 (s, 1H), 6.42 (s, 1H), 5.02 (hept, J = 6.2 Hz, 1H), 4.76 (s, 1H), 4.43 (hept, J = 6.2 Hz, 1H), 4.21 (dd, J = 6.5, 4.2 Hz, 1H), 2.77–2.64 (m,2H), 2.24(s,3H), 1.79 (s, 3H), 1.21 (d, J = 6.3 Hz, 6H), 1.06 (d, J = 6.2 Hz, 3H), 0.83 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.5, 168.6, 143.3, 142.8, 137.4, 137.3, 131.1, 130.2, 122.2, 119.8, 116.9, 114.1, 69.8, 69.7, 63.2, 37.5, 35.1, 21.46, 21.42, 21.1, 21.0, 20.9, 19.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H31BrNO4 488.1431, found 488.1455. HPLC analysis: Chiralpak IF, 5% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 15.7 min, tminor = 16.5 min, ee 84%, er 92:8; [α]D20 = +65.6 (C = 1.04, CHCl3).
Diisopropyl (R)-4-Phenyl-1-(3-(trifluoromethyl)phenyl)azetidine-2,2-dicarboxylate (30e).
The general procedure was followed using 28e (0.27 g, 0.58 mmol), PPh3 (0.34 g, 1.29 mmol), and 2,4,5-tribromoimidazole (0.19 g, 0.64 mmol) as starting materials to afford only azetidine as a beige viscous oily liquid (0.084 g, 31%). In this reaction, the starting material was not totally consumed even after stirring at 80 °C for 62 h and at 100 °C for 34 h. The compound was purified by flash chromatography (Rf = 0.39 (10% EtOAc/hexanes). Azetidine (30e). 1H NMR (600 MHz, CDCl3): δ 7.47 (d, J = 7.3 Hz, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.32 (t, J = 7.3 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H), 7.14 (s, 1H), 7.03 (d, J = 7.6 Hz, 1H), 6.74 (d, J = 7.7 Hz, 1H), 5.29 (hept, J = 6.2 Hz, 1H), 5.19 (t, J = 7.7 Hz, 1H), 5.04 (hept, J = 6.2 Hz, 1H), 3.15 (dd, J = 11.3, 8.4 Hz, 1H), 2.67 (dd, J =11.3, 7.1 Hz, 1H), 1.38 (dd, J = 6.2, 2.1 Hz, 6H), 1.21 (d, J = 6.3 Hz, 3H), 1.07 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.2, 168.2, 147.6, 141.4, 130.8 (q, 2JCF = 31.8 Hz), 129.0, 128.9, 128.1, 125.9, 124.2 (q, 1JCF = 272.3 Hz), 116.5, 115.8 (q, 3JCF = 3.7 Hz), 110.6 (q, 3JCF = 3.7 Hz), 72.2, 70.3, 69.8, 62.7, 36.5, 21.61, 21.60, 21.5, 21.2. 19F NMR (471 MHz, CDCl3): δ −61.86 (t, J = 0.8 Hz). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H27F3NO4 450.1887, found 450.1889. HPLC analysis: Chiralpak IA, 2% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor =11.4 min, tminor = 10.7 min, ee 26%, er 37:63; [α]D20 = +4.0 (C = 1, CHCl3).
Diisopropyl (R)-1-(3,5-dimethylphenyl)-4-(naphthalen-2-yl)-azetidine-2,2-dicarboxylate (30f) and Diisopropyl (S)-5,7-dimethyl-4-(naphthalen-2-yl)-3,4-dihydroquinoline-2,2(1H)-dicarboxylate (31f).
The general procedure was followed using 28f (0.10 g, 0.20 mmol), PPh3 (0.12 g, 0.46 mmol), and 2,4,5-tribromoimidazole (0.070 g, 0.23 mmol) as starting materials to afford azetidine (30f) as a beige viscous oily liquid (0.018g) (Rf = 0.33 (10% EtOAc/hexanes) and tetrahydroquinoline (31f) as beige foam (0.051 g) (Rf = 0.28 (10% EtOAc/hexanes) in a ratio of 1:2.8 after isolation (0.070 g in total, 73%). Reaction took 48 h to finish. The compound was purified by column chromatography to separate azetidine and tetrahydroqinoline.
Azetidine (30f).
1H NMR (600 MHz, CDCl3): δ 7.93 (s, 1H), 7.85 (t, J = 8.7 Hz, 3H), 7.59 (d, J =8.3 Hz, 1H), 7.51–7.44 (m, 2H), 6.44 (s, 1H), 6.39 (s, 2H), 5.29 (dq, J =18.5, 6.9, 6.0 Hz, 2H), 5.06 (hept, J = 6.0 Hz, 1H), 3.20 (dd, J = 11.0, 8.5 Hz, 1H), 2.57 (dd, J = 11.2,7.1 Hz, 1H), 2.15 (s, 6H), 1.37 (dd, J = 15.0, 6.2 Hz, 6H), 1.23 (d, J = 6.2 Hz, 3H), 1.08 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.8, 168.8, 147.5, 140.1, 138.0, 133.4, 133.1, 128.6, 128.0, 127.7, 126.1, 125.8, 124.5, 123.8, 121.5, 111.6, 72.2, 69.7, 69.2, 62.4, 36.5, 21.76, 21.72, 21.5, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H34NO4 460.2482, found 460.2479. HPLC analysis: Chiralpak IE, 3% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor = 17.4 min, tminor = 16.0 min, ee 60%, er 20:80; [α]D20 = −14.7 (C = 1, CHCl3).
Tetrahydroquinoline (31f).
1H NMR (600 MHz, CDCl3): δ 7.79–7.66 (m, 3H), 7.43–7.37 (m, 2H), 7.35 (s, 1H), 7.28 (d, J = 7.5 Hz, 1H), 6.56 (s, 1H), 6.45 (s, 1H), 5.04 (hept, J = 5.8 Hz, 1H), 4.83 (s, 1H), 4.43 (t, J =5.3 Hz, 1H), 4.13 (hept, J = 6.3 Hz, 1H), 2.87–2.78 (m, 2H), 2.29 (s, 3H), 1.81 (s, 3H), 1.22 (d, J = 6.2 Hz, 6H), 0.94 (d, J = 6.1 Hz, 3H), 0.48 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.5, 168.8, 143.0, 141.7, 137.7, 137.1, 133.2, 132.1, 127.8, 127.7, 127.3, 126.89, 126.85, 125.7, 125.2, 122.2, 117.5, 114.1, 69.6, 69.5, 63.4, 38.3, 35.4, 21.45, 21.41, 21.0, 20.5, 19.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H34NO4 460.2482, found 460.2486. HPLC analysis: Chiralpak IA, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 15.8 min, tminor = 9.6 min, ee 62%, er 19:81; [α]D20 = +66.8 (C = 1, CHCl3).
Diisopropyl (R)-1,4-Diphenylazetidine-2,2-dicarboxylate (30h) and Diisopropyl (S)-4-Phenyl-3,4-dihydroquinoline-2,2(1H)-dicarboxylate (31h).
The general procedure was followed using 28h (0.10 g, 0.25 mmol), PPh3 (0.144 g, 0.055 mmol) and 2,4,5-tribromoimidazole (0.084 g, 0.27 mmol) as starting materials to afford azetidine (30h) as beige viscous oily liquid (0.055g) (Rf = 0.4 (10% EtOAc/hexanes) and tetrahydroquinoline (31h) as yellow viscous oily liquid (0.013 g) (Rf = 0.34 (10% EtOAc/hexanes) in the ratio of 4.2:1 after isolation (0.070 g in total, 71%). Reaction took 48 h to finish. The compound was purified by column chromatography to separate azetidine and tetrahydroqinoline.
Azetidine (30h).
1H NMR (500 MHz, CDCl3): C-phenyl group: δ 7.46–7.49 (m, 2H, H-2/H-6), 7.34–7.38 (m, 2H, H-3/H-5), 7.26–7.31 (m, 1H, H-4); N-phenyl group: 7.08–7.13 (m, 2H, H-3′/H-5′), 6.75–6.79 (m, 1H, H-4′), 6.69–6.73 (m, 2H, H-2′/H-6′); isopropoxy group 1:5.26 (hept, J = 6.3 Hz, 1H, methine), 1.35 (d, 3H, methyl), 1.34 (d, 3H, methyl); isopropoxy group 2:5.00 (hept, J = 6.3 Hz, 1H, methine), 1.20 (d, J = 6.3 Hz, 3H, methyl), 0.99 (d, J = 6.3 Hz, 3H, methyl); 5.15 (dd, 1H, benzylicproton); 3.13 (dd, J = 11.3, 8.3 Hz, 1H, CH2 proton cis to the benzylic proton); 2.56 (dd, J = 11.3,7.2 Hz, 1H, CH2 proton cis to the adjacent phenyl group). 13C{1H} NMR (126 MHz, CDCl3): δ 169.80 (carbonyl); 168.71 (other carbonyl); 147.43 (quaternary aromatic); 142.29 (other quaternary aromatic); C-phenyl group: 128.80 (C-3/C-5), 127.84 (C-4), 125.97 (C-2/C-6) N-phenyl group: 128.52 (C-3′/C-5′), 119.50 (C-4′), 113.75 (C-2′/C-6′); 72.24 (quaternary aliphatic carbon); isopropoxy group 1:69.37 (methine), 21.71 (methyl correlating with methyl 1H signal at δ 1.34 in HSQC spectrum), 21.66 (methyl correlating with methyl 1H signal at δ 1.35 in HSQC spectrum); isopropoxy group 2:69.86 (methine), 21.71 (methyl correlating with methyl 1H signal at δ 1.20 in HSQC spectrum), 21.34 (methyl correlating with methyl 1H signal at δ0.99 in HSQC spectrum); 62.43 (benzylic CH), 36.54 (CH2). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H28NO4 382.2013, found 382.2021. HPLC analysis: Chiralpak IA, 2% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 12.6 min, tminor = 14.7 min, ee 82%, er 91:9; [α]D20 = +14.8 (C = 1, CHCl3).
Tetrahydroquinoline (31h).
1H NMR (500 MHz, CDCl3): δ 7.33 (t, J = 7.4 Hz, 2H), 7.28–7.25 (m, 1H), 7.21 (d, J = 7.1 Hz, 2H), 7.03 (dt, J = 8.2,4.1 Hz, 1H), 6.72 (d, J = 7.9 Hz, 1H), 6.60 (q, J = 3.1, 1.9Hz, 2H), 5.6 (ddq, J = 31.0, 12.5, 6.3 Hz, 2H), 4.91 (s, 1H), 4.13 (dd, J = 11.9, 5.3 Hz, 1H), 2.84 (ddd, J = 13.4, 5.3, 1.5 Hz, 1H), 2.25 (dd, J = 13.3, 12.0 Hz, 1H), 1.38–1.12 (m, 12H). 13C{1H} NMR (151 MHz, CDCl3): δ 169.7, 168.9, 144.2, 142.2, 129.0, 128.8, 128.5, 127.3, 126.7, 123.9, 118.4, 114.8, 70.0, 69.6, 64.8, 40.7, 35.6, 21.54, 21.52, 21.47, 21.43. HRMS (ESI-TOF) m/z: calcd for [M + H]+ C23H28NO4 382.2013, found 382.2034. HPLC analysis: Chiralpak IF, 3% IP/hexanes, continuous flow at 0.4 mL/min, 250 nm; tmajor = 23.7 min, tminor = 22.7 min, ee 80%, er 10:90; [α]D20 = +37.0 (C = 1, CHCI3).
Determination of Stereochemistry of the Azetidines.
The stereochemistry of the two phenyl groups was established through a series of experiments. First, a COSY experiment (p. S174 enabled one set of three aromatic 1H signals to be assigned to one phenyl group and the other set of three aromatic signals to be assigned to the other phenyl group. The single-intensity multiplets at δ 7.26–7.31 and δ 6.75–6.79 clearly resulted from the para protons, each of which exhibits a 3-contour pattern in the HSQC spectrum (at δ 127.84 and δ 119.50, respectively) from two (equal) ortho J couplings (p. S175). The two other three-contour patterns in the HSQC spectrum, at δ 128.80 and δ 128.52, result from two ortho J couplings and clearly are from the two pairs of phenyl C-3/C-5 carbons. The two 2-contour patterns in the HSQC spectrum, at δ 125.97 and δ 113.75, result from just one ortho J coupling and are clearly from the two pairs of phenyl C-2/C-6 carbons. The HSQC correlations thus establish that the signals at δ 7.46–7.49 result from one set of H-2/H-6 protons, that the signals at δ 6.69–6.73 result from the other set of H-2/H-6 protons, that the signals at δ 7.34–7.38 result from one set of H-3/H-5 protons, and that the signals at δ 7.08–7.13 result from the other set of H-3/H-5 protons.
With these secure assignments for the phenyl protons closest to the azetidine ring, NOE experiments then enabled the proximity of these phenyl protons to each other and to the benzylic (δ 5.15) and CH2 (δ 3.13 and δ 2.56) protons to be probed. The absence of any NOE between protons on different phenyl rings with a mixing time of 0.10 or 0.20 s (SI pp S176 and S177) strongly indicated that the two phenyl rings were not near each other, i.e., they had a trans orientation. If the rings were cis to each other, H-2/H-6 on one ring would be near H-2′/H-6′ on the other ring; H-3/H-5 on one ring would be near H-3′/H-5′ on the other ring; and strong NOE cross peaks would result, even at very short mixing times. A weak NOE between H-2/H-6 and H-2′/H-6′ is detected with a mixing time of 0.40 or 0.70 s (SI pp S178 and S179). Increasing the mixing time from 0.10 to 0.20 to 0.40 to 0.70 s results in a steadily increasing NOE between the H-2/H-6 protons at δ 7.46–7.49 and the CH2 proton at δ 2.56. In contrast, the H-2′/H-6′ protons at δ 6.69–6.73 do not show an NOE with this CH2 proton at any mixing time. Clearly, the CH2 proton at δ 2.56 is cis to the adjacent C-phenyl group and trans to the more remote N-phenyl group.
Pictorial representation of NOE correlation in 30h:

General Procedure for the Cyclization of Chiral Alcohols to the Corresponding γ-Lactones (Figures 10 and 11).
Method A:
In a thick-walled flame-dried reaction vial, chiral alcohol (28) (1.0 equiv) was dissolved in anhydrous toluene (0.2 M) under argon. To this reaction mixture was added TsOH·H2O (0.20 equiv) in one portion and stirring was continued for 1 h by heating to 70 °C. After confirming the complete consumption of starting material, the reaction was quenched using water (2 mL).
Note: This reaction can be heated to 110 °C and the reaction will be finished in less than 1 h without a change in the diastereoselectivity.
Workup and Purification.
After quenching, the reaction mixture was diluted with brine (3 mL), and the organic layer was separated. The aqueous layer was then extracted with EtOAc twice (2 × 10 mL), and the combined organic layers were washed with brine (10 mL) once, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by automated flash column chromatography.
Method B:
In a thick-walled flame-dried reaction vial, chiral alcohol (1.0 equiv) was dissolved in anhydrous toluene (0.1 M) under argon. To this reaction mixture was added DBU (1 M solution in EtOAc) (1.1 equiv) dropwise and stirring was continued for 1 h at room temperature. After confirming the complete consumption of starting material, the reaction was quenched using water (2 mL).
Workup and Purification.
After quenching, the reaction mixture was diluted with brine (2 mL), and the organic layer was separated. The aqueous layer was then extracted with EtOAc twice (2 × 5 mL), and the combined organic layers were washed with brine (10 mL) once, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by column chromatography.
Isopropyl (3S,5R) −2-Oxo-5-phenyl-3- (phenylamino)-tetrahydrofuran-3-carboxylate (24a) and Isopropyl (3R,5R)-2-Oxo-5-phenyl-3-(phenylamino)tetrahydrofuran-3-carboxylate (24b).
The general procedure (Method A) was followed using 28h (0.29 g, 0.72 mmol) and TsOH·H2O (0.027 g, 0.14 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 2:1, by crude 1HNMR) (0.21 g, 86%) which were separated by using flash column chromatography. 24a. Physical state: white solid (mp 79–81 °C). Rf = 0.52 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.45–7.39 (m, 4H), 7.39–7.35 (m, 1H), 7.18 (t, J = 7.9 Hz, 2H), 6.82 (t, J = 7.3 Hz, 1H), 6.64 (d, J = 8.0 Hz, 2H), 5.77 (dd, J =10.3, 6.1 Hz, 1H), 5.10–5.02 (m, 2H), 3.57 (dd, J = 13.1, 6.1 Hz, 1H), 2.59 (dd, J =13.0, 10.5 Hz, 1H), 1.27 (d, J =6.3 Hz, 3H), 1.02 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.9, 167.6, 144.2, 137.8, 129.2, 128.9, 128.8, 125.7, 119.6, 114.8, 80.1, 71.1, 67.2, 42.8, 21.4, 21.0. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C20H21NO4Na 362.1363, found 362.1365. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 13.9 min, tminor = 14.9 min, ee 94%, er 97:3; [α]D20 = −8.2 (C = 1, CHCl3).
24b.
Physical state: white solid (mp 132–134 °C). Rf = 0.46 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.46–7.34 (m, 5H), 7.23 (t, J = 7.7 Hz, 2H), 6.88 (t, J = 7.3 Hz, 1H), 6.67 (d, J = 7.9 Hz, 2H), 5.71 (t, J =7.5 Hz, 1H), 5.01 (hept, J = 6.0 Hz, 1H), 4.81 (s, 1H), 3.14 (dd, J = 13.8, 8.1 Hz, 1H), 3.06(dd, J = 13.8, 7.0 Hz, 1H), 1.08 (dd, J = 19.6, 6.2 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.4, 168.2, 143.7, 138.8, 129.3, 128.6, 128.5, 125.4, 120.0, 115.6, 78.9, 71.0, 66.3, 39.4, 21.1, 21.0. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for 1 C20H21NO4Na 362.1363, found 362.1365. HPLC analysis: Chiralpak ID, 40% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 16.5 min, tminor = 15.5 min, ee 90%, er 5:95; [α]D20 = −3.7 (C = 1, CHCl3).
Isopropyl (3S,5R)-3-((4-Methoxyphenyl)amino)-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24c) and Isopropyl (3R,5R)-3-((4-Methoxypheny)amino)-2-oxo-5-phenyitetrahydrofuran-3-carboxylate (24d).
The general procedure (Method A) was followed using 28a (0.48 g, 1.11 mmol) and TsOH·H2O (0.042 g, 0.22 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 2:1, by crude 1HNMR) (0.40 g, 98%) which were separated by using flash column chromatography. 24c. Physical state: beige waxy solid; Rf = 0.41 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.33–7.42 (m, phenyl, 5H), 6.75 (m, AA′ part of AA′BB′ pattern, 2H, H-3′/H-5′), 6.63 (m, BB′ part of AA′BB′ pattern, 2H, H-2′/H-6′), 5.70 (dd, J = 10.4, 6.1 Hz, 1H, benzylic), 5.04 (hept, J = 6.3 Hz, 1H, isopropoxy methine), 4.77 (s, broad, NH), 3.72 (s, 3H, methoxy), 3.48 (dd, J = 13.0, 6.1 Hz, 1H, one H of CH2), 2.55 (dd, J = 13.0, 10.4 Hz, 1H, other H of CH2), 1.26 (d, J = 6.3 Hz, 3H, one isopropyl methyl), 1.04 (d, J = 6.2 Hz, 3H, other isopropyl methyl); 13C NMR{1H} (126 MHz, CDCl3): 172.3 (ring carbonyl), 167.9 (isopropoxy carbonyl), 153.9 (C-4′ of p-disubstituted aromatic ring), 138.0 (phenyl C-1), 137.8 (C-1′ of p-disubstituted aromatic ring), 129.0 (phenyl C-4, JCH = 161.3 Hz), 128.9 (phenyl C-3/C-5, JCH ≈ 161.3 Hz), 125.9 (phenyl C-2/C-6, JCH = 158.6 Hz), 117.4 (C-2′/C-6′ of p-disubstituted aromatic ring, JCH = 157.5 Hz), 114.7 (C-3′/C-5′ of p-disubstituted aromatic ring, JCH = 159.8 Hz), 80.1 (benzylic CH, JCH = 154.9 Hz), 71.2 (isopropyl CH, JCH = 149.5 Hz), 68.1 (quaternary aliphatic carbon), 55.6 (methoxy, JCH = 143.4 Hz), 42.8 (CH2, JCH = 139.3 Hz with 1H at δ 3.48, JCH = 134.8 Hz with 1H at δ 2.55), 21.5 [isopropyl methyl (correlating with methyl 1H signal at δ 1.26 in HSQC spectrum), JCH = 127.1 Hz with 1H at δ 1.26], 21.3 [other isopropyl methyl (correlating with methyl 1H signal at δ 1.04 in HSQC spectrum), JCH = 127.3 Hz with 1H at δ 1.04]. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H24NO5 370.1649, found 370.1646; HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.7 mL/min, 250 nm; tmajor = 12.6 min, tminor = 13.8 min, ee 93%, er 96.5:3.5; [α]D20 = −6.5 (C =1, CHCI3).
The evidence for C-attack by the initial enolate followed by formation of a 5-membered-ring lactone (rather than N-attack by the initial enolate followed by formation of a 6-membered-ring lactone) was the generation of a quaternary aliphatic carbon, as shown by the presence of a signal at δ 68.1 in the standard 13C spectrum (p. S190) and the absence of this signal in the DEPT-135 13C spectrum (SI p S191) and in the DEPT-90 13C spectrum (both optimized for 1JCH = 145 Hz). Additional confirmation that the signal at δ 68.1 resulted from a quaternary carbon was provided by the absence of this signal in DEPT-135 13C spectra optimized for 1JCH =125 Hz and 1JCH =170 Hz and by the absence of a large 1JCH coupling for the signal at δ 68.1 in a 1H-coupled 13C spectrum. (Just two small couplings, each ≈4.4 Hz, were observed, apparently from equal 2JCH couplings with the adjacent CH2 protons.) Detailed chemical shift assignments could be made through a combination of 1H (SI p S189), 13C (with and without 1H decoupling), DEPT-135 13C, DEPT-90 13C, NOE (SI pp S192–S195), HSQC (SI p S197), and HMBC (SI p S196) experiments (with key parameters the same as for 27n).
The stereochemistry of phenyl and H on the benzylic carbon relative to the substituents on the quaternary aliphatic carbon and relative to the two inequivalent CH2 protons was established through NOE experiments with various mixing times (0.2, 0.3, 0.5, 0.7 s). An NOE experiment with a relatively short mixing time will minimize spin diffusion effects32 and thus enable a more secure identification of the interacting protons provided that cross peaks with sufficient signal-to-noise are generated. However, even with the longest mixing time, there is no NOE between any of the phenyl protons and an isopropyl methyl group, establishing that these protons are not near each other; similarly, there is no NOE between any of the phenyl protons and the more deshielded CH2 proton (δ 3.48). In contrast, with the shortest mixing time, NOE is detected between phenyl protons and the more shielded CH2 proton (δ 2.55), establishing their cis relationship, and between phenyl protons and the benzylic proton (δ 5.70). NOE is also detected between the methoxy protons (δ 3.72) and the aromatic protons at δ 6.75, while NOE is detected between the aromatic protons at δ 6.63 and NH and each of the CH2 protons. These observations enabled secure assignments of the aromatic protons at δ 6.75 (H-3′/H-5′) and at δ 6.63 (H-2′/H-6′).
Pictorial representation of NOE correlation in 24c:

24d.
Physical state: beige waxy solid. Rf = 0.36 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.33–7.42 (m, phenyl, 5H), 6.81 (m, AA′ part of AA′BB′ pattern, 2H, H-3′/H-5′), 6.74 (m, BB′ part of AA′BB′ pattern, 2H, H-2′/H-6′), 5.56 (dd, J ≈ 7.7, 7.6 Hz, 1H, benzylic), 5.02 (hept, J = 6.3 Hz, 1H, isopropoxy methine), 4.50 (s, broad, NH), 3.76 (s, 3H, methoxy), 3.04 (dd, J ≈ 13.8, 7.7 Hz, 1H, one H of CH2), 2.99 (dd, J ≈ 13.8, 7.6 Hz, 1H, other H of CH2), 1.13 (d, J = 6.3 Hz, 3H, one isopropyl methyl), 1.12 (d, J = 6.3 Hz, 3H, other isopropyl methyl). 13C{1H} NMR (126 MHz, CDCl3): 172.9 (ring carbonyl), 168.6 (isopropoxy carbonyl), 154.7 (C-4′ of p-disubstituted aromatic ring), 138.8 (phenyl C-1), 136.9 (C-1′ of p-disubstituted aromatic ring), 128.7 (phenyl C-3/C-5), 128.6 (phenyl C-4), 125.6 (phenyl C-2/C-6), 119.6 (C-2′/C-6′ of p-disubstituted aromatic ring), 114.7 (C-3′/C-5′ of p-disubstituted aromatic ring), 79.1 (benzylic CH), 71.0 (isopropyl CH), 67.5 (quaternary aliphatic carbon), 55.6 (methoxy), 39.6 (CH2), 21.34 [isopropyl methyl (correlating with methyl 1H signal at δ 1.13 in HSQC spectrum)], 21.26 [other isopropyl methyl (correlating with methyl 1H signal at δ 1.12 in HSQC spectrum)]. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H24NO5 370.1649, found 370.1648. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.7 mL/min, 250 nm; tmajor =11.0 min, tminor = 13.9 min, ee 93%, er 3.4:96.6; [α]D20 = −3.7 (C = 1, CHCl3).
The evidence for C-attack by the initial enolate followed by formation of a 5-membered-ring lactone (rather than N-attack by the initial enolate followed by formation of a 6-membered-ring lactone) was the generation of a quaternary aliphatic carbon, as shown by the presence of a signal at δ 67.5 in the standard 13C spectrum (SI p S199) and the absence of this signal in the DEPT-135 13C spectrum (SI p S200) and in the DEPT-90 13C spectrum (both optimized for 1JCH = 145 Hz). Detailed chemical shift assignments could be made through a combination of 1H (SI p S198), 13C, DEPT-135 13C, DEPT-90 13C, NOE (SI p S201–S204), HSQC (SI p S206), and HMBC (SI p S205) experiments (with key parameters the same as for 27n).
The stereochemistry of phenyl and H on the benzylic carbon relative to the substituents on the quaternary aliphatic carbon and relative to the two inequivalent CH2 protons was established through NOE experiments with various mixing times (0.2, 0.3, 0.5, 0.7 s). Compared to 24c, the critical difference for 24d is that with a mixing time of just 0.3 s, NOE is clearly detected between phenyl protons and an isopropyl methyl group, establishing that these protons can be near each other. With a mixing time of 0.3 s, NOE is also detected between phenyl protons and the more shielded CH2 proton (δ 2.99), establishing their cis relationship, and between phenyl protons and the benzylic proton (δ 5.56). NOE is also detected between the methoxy protons (δ 3.76) and the aromatic protons at δ 6.81, while NOE is detected between the aromatic protons at δ 6.74 and NH and each of the more deshielded CH2 protons. These observations enabled secure assignments of the aromatic protons at δ 6.81 (H-3′/H-5′) and at δ 6.74 (H-2′/H-6′).
Pictorial representation of NOE correlation in 24d:

Isopropyl (3S,5R)-3-((3,5-Dimethylphenyl)amino)-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24e) and Isopropyl (3R,5R)-3-((3,5-Dimethylphenyl)amino)-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24f).
The general procedure (method A) was followed using 28b (0.35 g, 0.82 mmol) and TsOH·H2O (0.031 g, 0.16 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.2:1, by crude 1HNMR) (0.26 g, 86%) which were separated by using flash column chromatography. 24e. Physical state: beige solid (mp 106–108 °C). Rf = 0.57 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.46–7.4] (m, 4H), 7.38 (ddd, J =8.5, 5T, 2.4 Hz, 1H), 6.48 (s, 1H), 6.28 (s, 2H), 5.78 (dd, J = 10.3, 6.3 Hz, 1H), 5.11 (hept, J = 6.2 Hz, 1H), 4.96(s, 1H), 3.49 (dd, J = 13.2, 6.3 Hz, 1H), 2.64 (dd, J = 13.2, 10.3 Hz, 1H), 2.23 (s, 6H), 1.31 (d, J = 6.3 Hz, 3H), 1.11 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.0, 167.7, 143.8, 138.7, 138.0, 128.8, 128.7, 125.6, 121.4, 112.8, 79.8, 71.7, 67.1, 42.2, 21.4, 21.2, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26NO4 368.1856, found 368.1859. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 12.9 min, tminor = 15.5 min, ee 96%, er 97.8:2.2; [α]D20 = −6.7 (C = 1, CHCl3). 24f. Physical state: pale yellow waxy solid; Rf = 0.52 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.47–7.41 (m, 4H), 7.39 (ddd, J = 8.5, 5.3, 2.1 Hz, 1H), 6.56 (s, 1H), 6.32 (s, 2H), 5.72 (t, J =7.6 Hz, 1H), 5.05 (hept, J = 6.3 Hz, 1H), 4.74 (s, 1H), 3.17 (dd, J = 13.8, 8.1 Hz, 1H), 3.08 (dd, J = 13.8, 7.1 Hz, 1H), 2.28 (s, 6H), 1.13 (dd, J = 16.4, 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.5, 168.3, 143.6, 138.9, 138.8, 128.6, 128.4, 125.4, 122.0, 113.6, 78.9, 70.9, 66.3, 39.5, 21.4, 21.1, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26NO4 368.1856, found 368.1859; HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 14.5 min, tminor = 12.8 min, ee 96%, er 2:98; [α]D20 = −3.3 (C = 1, CHCl3).
Isopropyl (3S,5R)-3-(Benzo[d][1,3]dioxol-5-ylamino)-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24g) and Isopropyl (3R,5R)-3-(Benzo[d][1,3]dioxol-5-ylamino)-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24h).
The general procedure (method A) was followed using 28d (0.27 g, 0.61 mmol) and TsOH·H2O (0.023 g, 0.12 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.8:1, by crude 1HNMR) (0.206 g, 87%) which were separated by using flash column chromatography. 24g. Physical state: beige solid (mp 91–94 °C); Rf = 0.39 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.38 (td, J = 12.3, 10.2, 5.2 Hz, 5H), 6.62 (d, J = 8.3 Hz, 1H), 6.29 (d, J = 2.2 Hz, 1H), 6.07 (dd, J = 8.3, 2.2 Hz, 1H), 5.85 (s, 2H), 5.70 (dd, J = 10.3, 6.1 Hz, 1H), 5.06 (hept, J = 6.2 Hz, 1H), 4.79 (s, 1H), 3.49 (dd, J = 13.0, 6.1 Hz, 1H), 2.53 (dd, J = 13.0, 10.4 Hz, 1H), 1.27 (d, J =6.3 Hz, 3H), 1.09 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.0, 167.6, 148.3, 141.5, 139.3, 137.8, 128.9, 128.8, 125.7, 108.3, 107.5, 100.8, 98.8, 80.0, 71.1, 67.9, 42.8, 21.4, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H22NO6 384.1442, found 384.1440. HPLC analysis: Chiralpak ID, 80% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 22.5 min, tminor = 21.2 min, ee 96%, er 2:98; [α]D20 = −9.2 (C = 1, CHCl3). 24h: Physical state: beige solid (mp 78–80 °C); Rf = 0.31 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.38 (dq, J = 14.5, 7.5 Hz, 5H), 6.67 (d, J =8.3 Hz, 1H), 6.38 (d, J = 2.1 Hz, 1H), 6.17 (dd, J = 8.3, 2.1 Hz, 1H), 5.90 (s, 2H), 5.61 (t, J = 7.6 Hz, 1H), 5.02 (hept, J = 6.2 Hz, 1H), 4.53 (s, 1H), 3.12–2.93 (m, 2H), 1.12 (d, J = 6.1 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.5, 168.3, 148.3, 142.3, 138.7, 138.4, 128.6, 128.5, 125.4, 109.7, 108.3, 100.9, 100.5, 78.9, 71.0, 67.2, 39.5, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H22NO6 384.1442, found 384.1441. HPLC analysis: Chiralpak ID, 80% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 18.6 min, tminor = 20.02 min, ee 96%, er 98:2; [α]D20 = −4.0 (C = 1, CHCl3).
Isopropyl (3S,5R)-2-Oxo-5-phenyl-3-((3-(trifluoromethyl)phenyl)-amino)tetrahydrofuran-3-carboxylate (24i) and Isopropyl (3R,5R)-2-Oxo-5-phenyl-3-((3-(trifluoromethyl)phenyl)amino)-tetrahydrofuran-3-carboxylate (24j).
The general procedure (method A) was followed using 28e (0.35 g, 0.76 mmol) and TsOH·H2O (0.029 g, 0.15 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.7:1, by crude 1HNMR) (0.285 g, 92%) which were separated by using flash column chromatography. 24i. Physical state: white solid (mp 79–81 °C). Rf = 0.50 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.45–7.35 (m, 5H), 7.31–7.23 (m, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.83 (s, 1H), 6.80 (d, J = 8.2 Hz, 1H), 5.80 (dd, J = 10.3, 6.2 Hz, 1H), 5.25 (s, 1H), 5.09 (hept, J = 6.2 Hz, 1H), 3.55 (dd, J = 13.1, 6.2 Hz, 1H), 2.56 (dd, J = 13.0, 10.4Hz, 1H), 1.28 (d, J =6.3 Hz, 3H), 1.06 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.5, 167.2, 144.6, 137.6, 131.6 (q, 2JCF = 32.0 Hz), 129.8, 129.0, 128.9, 125.7, 124.0 (q, 1JCF = 272.4 Hz), 117.9, 116.0 (q, 3JCF = 3.8 Hz), 110.8 (q, 3JCF = 3.8 Hz), 80.2, 71.6, 67.0, 42.6, 21.4, 21.0. 19F NMR (471 MHz, CDCl3): δ −61.9 (S). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H21F3NO4 408.1417, found 408.1420. HPLC analysis: Chiralpak ID, 10% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 15.8 min, tminor = 14.8 min, ee 93%, er 3.6:96.4; [α]D20 = −7.2 (C = 1, CHCl 3). 24j. Physical state: colorless viscous oily liquid. Rf =0.40 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.40 (dq, J = 13.3, 6.9 Hz, 5H), 7.31 (d, J = 7.9 Hz, 1H), 7.10 (d, J = 7.6 Hz, 1H), 6.87 (s, 1H), 6.82 (d, J =8.1 Hz, 1H), 5.74 (t, J = 7.5 Hz, 1H), 5.07–4.96 (m, 2H), 3.17–3.05 (m, 2H), 1.07 (dd, J = 14.4, 6.3 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.9, 167.8, 144.2, 138.5, 131.6 (q, 2 JCF = 32.0 Hz), 129.8, 128.8, 128.7, 125.4, 124.0 (q, 1JCF = 272.4 Hz), 118.2, 116.4 (q, 3JCH = 3.9 Hz), 111.7 (q, 3JCH = 3.9 Hz), 78.9, 71.4, 66.0, 39.7, 21.1, 21.0. 19F NMR (471 MHz, CDCl 3): δ −61.9 (S). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H21F3NO4 408.1417, found 408.1420. HPLC analysis: Chiralpak ID, 15% IP/hexanes, continuous flow at 0.6 mL/min, 250 nm; tmajor = 16.1 min, tminor = 12.6 min, ee 93%, er 3.4:96.6; [α]D20 = −4.3 (C = 1, CHCl 3).
Isopropyl (3S,5R)-3-((3,5-Dimethylphenyl)amino)-5-(naphthalen-2-yl)-2-oxotetrahydrofuran-3-carboxylate (24k) and Isopropyl (3R,5R)-3-((3,5-Dimethylphenyl)amino)-5-(naphthalen-2-yl)-2-oxo-tetrahydrofuran-3-carboxylate (24l).
The general procedure (method A) was followed using 28f (0.32 g, 0.66 mmol) and TsOH·H2O (0.025 g, 0.13 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.8:1, by crude 1HNMR) (0.25 g, 90%) which were separated by using flash column chromatography. 24k. Physical state: white foamy solid. Rf = 0.42 (15% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.93–7.89 (m, 2H), 7.87 (d, J = 6.3 Hz, 2H), 7.55–7.51 (m, 2H), 7.50 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 6.30 (s, 2H), 5.94 (dd, J = 10.1, 6.4 Hz, 1H), 5.13 (hept, J = 6.1 Hz, 1H), 4.99 (s, 1H), 3.55 (dd, J = 13.2, 6.3 Hz, 1H), 2.73 (dd, J = 13.1, 10.5 Hz, 1H), 2.22 (s, 6H), 1.34 (d, J = 6.2 Hz, 3H), 1.13 (d, J =6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.1, 167.8, 143.9, 138.8, 135.3, 133.3, 133.0, 128.9, 128.0, 127.7, 126.63, 126.60, 125.0, 122.9, 121.5, 112.9, 80.0, 71.2, 67.2, 42.2, 21.5, 21.3, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H28NO4 418.2013, found 418.2007. HPLC analysis: Chiralpak IB, 10% IP/hexanes, continuous flow at 0.4 mL/min, 230 nm; tmajor = 19.3 min, tminor = 22.9 min, ee 95%, er 97.3:2.6; [α]D20 = −14.2 (C = 1, CHCl3). 24l. Physical state: brown viscous gummy liquid. Rf = 0.37 (15% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.92 (d, J = 8.5 Hz, 1H), 7.87 (t, J =10.0 Hz, 3H), 7.57–7.46 (m, 3H), 6.56 (s, 1H), 6.33 (s, 2H), 5.86 (t, J =7.5 Hz, 1H), 5.02 (hept, J = 6.4 Hz, 1H), 4.77 (s, 1H), 3.22 (dd, J = 13.7, 8.2 Hz, 1h), 3.15 (dd, J = 13.8, 7.1 Hz, 1H), 2.28 (s, 6H), 1.07 (d, J = 6.2 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.6, 168.3, 143.6, 138.9, 136.1, 133.1, 132.9, 128.7, 127.9, 127.6, 126.5, 126.4, 124.6, 122.8, 122.0, 113.7, 79.1, 70.9, 66.3, 39.4, 21.4, 21.1, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H28NO4 418.2013, found 418.2017. HPLC analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 21.3 min, tminor = 17.7 min, ee 95%, er 2.6:97.3; [α]D20 = −8.2 (C = 1, CHCl3).
Isopropyl (3S,5R)-5-(Naphthalen-1-yl)-2-oxo-3-(p-tolylamino)-tetrahydrofuran-3-carboxylate (24m) and Isopropyl (3R,5R)-5-(Naphthalen-1-yl)-2-oxo-3-(p-tolylamino)tetrahydrofuran-3-carboxylate (24n).
The general procedure (method A) was followed using 28g (0.42 g, 0.90 mmol) and TsOH·H2O (0.034 g, 0.18 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.7:1, by crude 1HNMR) (0.333 g, 91%) which were separated by using flash column chromatography. 24m. Physical State: Yellow foamy gummy substance. Rf = 0.55 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.03 (d, J = 8.4Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.87 (d, J =8.2 Hz, 1H), 7.71 (d, J = 7.2 Hz, 1H), 7.62 (t, J = 7.4 Hz, 1H), 7.54 (dt, J = 20.6, 7.6 Hz, 2H), 7.00 (d, J = 8.1 Hz, 2H), 6.61 (d, J = 8.3 Hz, 2h), 6.56 (dd, J = 10.3, 6.0 Hz, 1H), 5.16 (hept, J = 6.2 Hz, 1H), 5.02 (s, 1H), 3.81 (dd, J = 13.1, 6.0 Hz, 1H), 2.68 (dd, J = 13.0, 10.6 Hz, 1H), 2.26 (s, 3H), 1.37 (d, J = 6.3 Hz, 3h), 1.13 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.9, 167.8, 141.6, 133.5, 129.64, 129.61, 129.0, 128.96, 128.91, 126.6, 125.9, 125.3, 122.2, 121.8, 115.2, 77.4, 71.1, 67.5, 42.2, 21.4, 21.1, 20.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H26NO4 404.1856, found 404.1854. HPLC analysis: Chiralpak IB, 9% IP/hexanes, continuous flow at 0.4 mL/min, 230 nm; tmajor = 21.8 min, tminor = 20.4 min, ee 80%, er 9.7:90.3; [α]D20 = +4.3 (C = 1, CHCl3). 24n. Physical State: Pink foamy gummy substance. Rf = 0.48 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.96–7.92 (m, 1H), 7.88 (d, J =8.1 Hz, 1H), 7.77–7.72 (m, 1H), 7.69 (d, J = 7.0 Hz, 1H), 7.58–7.50 (m, 3H), 7.08 (d, J = 7.9 Hz, 2H), 6.67 (d, J = 8.1 Hz, 2H), 6.39 (t, J = 7.3 Hz, 1H), 4.98 (hept, J = 6.3 Hz, 1H), 4.86 (s, 1H), 3.36 (dd, J = 13.7, 8.6 Hz, 1H), 3.09 (dd, J = 13.7, 6.3 Hz, 1H), 2.30 (s, 3H), 1.11 (d, J = 6.2 Hz, 3h), 1.03 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.0, 168.3, 141.3, 134.6, 133.8, 130.0, 129.7, 129.33, 129.30, 128.9, 126.7, 126.0, 125.3, 122.5, 122.2, 116.3, 76.8, 71.1, 66.3, 39.3, 21.35, 21.33, 20.58, 20.57. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H26NO4 404.1856, found 404.1857. HPLC analysis: Chiralpak ID, 85% IP/hexanes, continuous flow at 0.6 mL/min, 250 nm; tmajor = 13.4 min, tminor = 12.6 min, ee 80%, er 10:90; [α]D20 = +0.8 (C = 1, CHCl3).
Isopropyl (3S,5R)-5-(4-Bromophenyl)-3-((3,5-dimethylphenyl)-amino)-2-oxotetrahydrofuran-3-carboxylate (24o) and Isopropyl(3R,5R)-5-(4-Bromophenyl)-3-((3,5-dimethylphenyl)amino)-2-oxo-tetrahydrofuran-3-carboxylate (24p).
The general procedure (method A) was followed using 28c (0.37 g, 0.72 mmol) and TsOH·H2O (0.027 g, 0.14 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.7:1, by crude 1HNMR) (0.277 g, 85%) which were separated by using flash column chromatography. 24o. Physical state: white solid (mp 120–122 °C); Rf = 0.59 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.54 (d, J =8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 6.47 (s, 1H), 6.24 (s, 2H), 5.70 (dd, J = 10.2, 6.3 Hz, 1H), 5.08 (hept, J = 6.2 Hz, 1H), 4.92 (s, 1H), 3.48 (dd, J = 13.2, 6.3 Hz, 1H), 2.55 (dd, J = 13.2, 10.3 Hz, 1H), 2.21 (s, 6H), 1.29 (d, J = 6.3 Hz, 3H), 1.08 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.8, 167.6, 143.8, 138.8, 137.1, 131.9, 127.3, 122.8, 121.6, 112.9, 79.0, 71.2, 67.1, 42.1, 21.4, 21.3, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H25BrNO4 446.0961, found 446.0961. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 14.4 min, tminor = 17.3 min, ee 94%, er 97:3; [α]D20 = −12.2 (C = 1, CHCl3). 24p. Physical state: beige solid (mp 145–148 °C); Rf = 0.54 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.54 (d, J = 8.4 Hz, 2H), 7.28 (d, J =8.4 Hz, 2H), 6.53 (s, 1H), 6.27 (s, 2H), 5.63 (t, J = 7.5 Hz, 1H), 5.02 (hept, J = 6.2 Hz, 1H), 4.69 (s, 1H), 3.13 (dd, J = 13.8, 8.2 Hz, 1H), 3.00 (dd, J = 13.8, 7.0 Hz, 1H), 2.24 (s, 6H), 1.12 (d, J = 6.3 Hz, 3H), 1.07 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.3, 168.2, 143.5, 138.9, 138.0, 131.8, 127.1, 122.4, 122.1, 113.6, 78.2, 71.1, 66.2, 39.4, 21.4, 21.2, 21.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H25BrNO4 446.0961, found 446.0965. HPLC analysis: Chiralpak ID, 90% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 13.8 min, tminor = 12.4 min, ee 94%, er 3:97; [α]D20 = −5.2 (C = 1, CHCl3).
Isopropyl (3S,5R)-3-(Benzo[d][1,3]dioxol-5-ylamino)-5-(furan-2-yl)-2-oxotetrahydrofuran-3-carboxylate (24q) and Isopropyl (3R,5R)-3-(Benzo[d][1,3]dioxol-5-ylamino)-5-(furan-2-yl)-2-oxotetrahydrofuran-3-carboxylate (24r).
The general procedure (method B) was followed using 28i (0.073 g, 0.16 mmol) and DBU (1 M solution in ethyl acetate) (0.185 mL, 0.18 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.2:1, by crude 1HNMR) (0.054 g, 86%) which were separated by using flash column chromatography. 24q. Physical state: brown viscous oily liquid; Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.48 (d, J = 1.9 Hz, 1H), 6.65 (d, J = 8.3 Hz, 1H), 6.53 (d, J = 3.3 Hz, 1H), 6.41 (dd, J = 3.4, 1.8 Hz, 1H), 6.31 (d, J = 2.5 Hz, 1H), 6.08 (dd, J = 8.2, 2.4 Hz, 1H), 5.87 (s, 2H), 5.70 (dd, J = 10.5, 6.3 Hz, 1H), 5.06 (hept, J = 6.4 Hz, 1H), 4.81 (s, 1H), 3.27 (dd, J = 13.1, 6.3 Hz, 1H), 2.96 (dd, J = 13.1, 10.5 Hz, 1H), 1.27 (d, J =6.3 Hz, 3H), 1.10 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.3, 167.7, 149.0, 148.4, 144.1, 141.4, 139.1, 111.1, 110.7, 108.4, 107.2, 100.8, 98.5, 73.0, 71.3, 67.4, 37.8, 21.5, 21.3. HRMS (ESI-TOF) m/z: [M + h]+ calcd for C19H20NO7 374.1234. found 374.1234. HPLC analysis: Chiralpak IC, 20% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 24.7 min, tminor = 26.8 min, ee 82%, er 91:9; [α]D20= −7.3 (C = 1, CHCl3). 24r. Physical state: dark brown viscous oily liquid; Rf = 0.29 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.47 (d, J = 1.9 Hz, 1H), 6.67 (d, J = 8.3 Hz, 1H), 6.52 (d, J = 3.3 Hz, 1H), 6.43–6.35 (m, 2H), 6.18 (dd, J = 8.3, 2.3 Hz, 1H), 5.90 (s, 2H), 5.57 (t, J = 7.7 Hz, 1H), 5.07 (hept, J = 6.3 Hz, 1H), 4.48 (s, 1H), 3.35 (dd, J = 13.9, 7.7 Hz, 1H), 2.88 (dd, J = 13.9, 7.8 Hz, 1H), 1.25 (d, J = 6.3 Hz, 3H), 1.16 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.0, 168.2, 149.7, 148.3, 143.7, 142.5, 138.3, 110.7, 110.2, 110.1, 108.4, 101.0, 100.8, 72.6, 71.1, 67.2, 35.4, 21.5, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20NO7 374.1234, found 374.1249. HPLC analysis: Chiralpak IC, 35% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 24.5 min, tminor = 21.7 min, ee 86%, er 7:93; [α]D20 = −5.2 (C =1, CHCl3).
Isopropyl (3S,5R)-3-((4-(2-((tert-Butyldimethylsilyl)oxy)ethyl)-phenyl)amino)-5-(furan-2-yl)-2-oxotetrahydrofuran-3-carboxylate (24s) and Isopropyl (3R,5R)-3-((4-(2-((tert-Butyldimethylsilyl)oxy)-ethyl)phenyl)amino)-5-(furan-2-yl)-2-oxotetrahydrofuran-3-carboxylate (24t).
The general procedure (method B) was followed using 28j (0.211 g, 0.38 mmol) and DBU (1 M solution in ethyl acetate) (0.423 mL, 0.42 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.2:1, by crude 1HNMR) (0.15 g, 80%) which were separated by using flash column chromatography. 24s. Physical state: pale yellow viscous oily liquid. Rf =0.58 (20% EtOAc/hexanes); 1H NMR (600 MHz, CDCl3): δ 7.48 (s, 1H), 7.04 (d, J = 8.1 Hz, 2H), 6.58 (d, J = 8.2 Hz, 2H), 6.54 (d, J = 2.9 Hz, 1H), 6.41 (s, 1H), 5.73 (dd, J = 10.4, 6.4 Hz, 1H), 5.05 (hept, J = 7.3, 6.6 Hz, 1H), 4.96 (s, 1H), 3.74 (t, J = 7.1 Hz, 2H), 3.28 (dd, J = 13.2, 6.3 Hz, 1H), 3.03–2.95 (m, 1H), 2.72 (t, J = 7.0 Hz, 2H), 1.27 (d, J = 6.2 Hz, 3H), 1.07 (d, J = 6.2 Hz, 3H), 0.88 (s, 9H), −0.00 (s, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.3, 167.8, 149.1, 144.1, 142.1, 130.2, 129.9, 114.8, 111.0, 110.7, 72.9, 71.3, 66.9, 64.7, 38.6, 37.6, 25.9, 21.4, 21.2, 18.3, −5.3, −5.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H38NO6Si 488.2463, found 488.2464. HPLC analysis: Chiralpak IB, 2% IP/hexanes, continuous flow at 0.4 mL/min, 230 nm; tmajor = 19.4 min, tminor = 20.9 min, ee 90%, er 95:5; [α]D20 = −5 (C = 1, CHCl3). 24t. Physical state: pale yellow viscous oily liquid; Rf = 0.52 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.47 (s, 1H), 7.05 (d, J = 8.1 Hz, 2H), 6.61 (d, J = 8.2 Hz, 2H), 6.53 (d, J = 2.7 Hz, 1H), 6.41 (s, 1H), 5.63 (t, J = 7.6 Hz, 1H), 5.05 (hept, J =6.3 Hz, 1H), 4.65 (s, 1H), 3.74 (t, J = 7.0 Hz, 2H), 3.39 (dd, J = 13.8, 7.4 Hz, 1H), 2.93 (dd, J = 13.8, 8.0 Hz, 1H), 2.73 (t, J =6.9 Hz, 2H), 1.23 (d, J = 6.2 Hz, 3H), 1.11 (d, J = 6.2 Hz, 3H), 0.87 (s, 9H), −0.01 (s, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 172.0, 168.2, 149.8, 143.6, 141.8, 131.3, 129.9, 116.3, 110.6, 110.0, 72.6, 71.0, 66.4, 64.6, 38.6, 35.3, 25.9. 21.4, 21.2, 18.3, −5.41. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H38NO6Si 488.2463, found 488.2465. HPLC analysis: Chiralpak ID, 15% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 18.5 min, tminor = 17.2 min, ee 90%, er 5:95; [α]D20 = −4.3 (C = 1, CHCl 3).
Isopropyl (3S,5R)-3-(benzo[d][1,3]dioxol-5-ylamino)-2-oxo-5-(thiophene-2-yl)tetrahydrofuran-3-carboxylate (24u) and Isopropyl (3R,5R)-3-(Benzo[d][1,3]dioxol-5-ylamino)-2-oxo-5-(thiophene-2-yl)tetrahydrofuran-3-carboxylate (24v).
The general procedure (method B) was followed using 28k (0.288 g, 0.64 mmol) and DBU (1 M solution in ethyl acetate) (0.704 mL, 0.70 mmol) as starting materials to afford the diastereomeric mixture of γ-lactones (diastereomeric ratio = 1.5:1, by crude 1HNMR) (0.237 g, 95%) which were separated by using flash column chromatography. 24u. Physical state: yellow viscous oily liquid; Rf = 0.33 (20% EtOAc/hexanes); 1H NMR (600 MHz, CDCl3): δ 7.38 (d, J = 4.5 Hz, 1H), 7.17 (d, J = 3.2 Hz, 1H), 7.04–7.00 (m, 1H), 6.64 (d, J = 8.3 Hz, 1H), 6.30 (d, J = 2.2 Hz, 1H), 6.07 (dd, J = 8.3, 2.2 Hz, 1H), 5.93 (dd, J = 10.3, 6.1 Hz, 1H), 5.86 (s, 2H), 5.05 (hept, J = 6.2 Hz, 1H), 4.81 (s, 1H), 3.45 (dd, J = 13.1, 6.1 Hz, 1H), 2.74 (dd, J = 13.1, 10.5 Hz, 1H), 1.26 (d, J = 6.3 Hz, 3H), 1.09 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.2, 167.5, 148.3, 141.4, 139.9, 139.0, 127.2, 127.1, 127.0, 108.3, 107.3, 100.8, 98.7, 75.8, 71.2, 67.8, 42.1, 21.4, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20NO6S 390.1006, found 390.1011. HPLC analysis: Chiralpak IC, 25% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 21.1 min, tminor = 24.9 min, ee92%, er 96:4; [α]D20 = −8.7 (C = 1, CHCL3). 24v. Physical state: beige waxy solid; Rf = 0.25 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.37 (d, J = 4.9 Hz, 1H), 7.14 (d, J = 3.2 Hz, 1H), 7.03–7.00 (m, 1H), 6.67 (d, J =8.3 Hz, 1H), 6.38 (d, J = 2.0 Hz, 1H), 6.17 (dd, J = 8.2, 2.0 Hz, 1H), 5.89 (s, 2H), 5.80 (t, J = 7.6 Hz, 1H), 5.06 (hept, J = 6.2 Hz, 1H), 4.51 (s, 1H), 3.16 (dd, J = 13.9, 7.6 Hz, 1H), 3.05 (dd, J =13.9, 7.6 Hz, 1H), 1.21 (d, J = 6.3 Hz, 3H), 1.15 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 171.8, 168.2, 148.3, 142.4, 140.8, 138.2, 126.9, 126.6, 126.4, 110.0, 108.3, 100.9, 100.7, 75.3, 71.1, 67.4, 39.3, 21.3, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20NO6S 390.1006, found 390.1008. HPLC analysis: Chiralpak IB, 40% IP/hexanes, continuous flow at 0.5 mL/min, 250 nm; tmajor = 16.2 min, tminor = 19.1 min, ee 90%, er 95:5; [α]D20 = −4.7 (C = 1, CHCl3).
Isopropyl (3S,4S,5R)-3-((4-Methoxyphenyl)amino)-4-methyl-2-oxo-5-phenyltetrahydrofuran-3-carboxylate (24w).
The general procedure (method A) was followed using 28n (0.18 g, 0.40 mmol) and TsOH·H2O(0.015 g, 0.08 mmol) as starting materials to afford the fully substituted γ-lactone as a single diastereomer 24w (0.13 g, 84%) which was purified by using flash column chromatography. Physical state: beige viscous gummy substance. Rf = 0.51 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.31–7.43 (m, phenyl, 5H), 6.75 (m, AA′ part of AA′BB′ pattern, 2H, H-3′/H-5′), 6.57 (m, BB′ part of AA′BB′ pattern, 2H, H-2′/H-6′), 6.00 (d, J = 5.2 Hz, 1H, benzylic H), 4.93 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.71 (s, broad, NH), 3.78 (qd, J = 7.5, 5.2 Hz, 1H, ring H-C−CH3), 3.73 (s, 3H, methoxy), 1.19 (d, J = 6.3 Hz, 3H, isopropyl CH3), 0.82 (d, J = 6.2 Hz, 3H, other isopropyl CH3), 0.61 (d, J = 7.5 Hz, ring H−C−CH3). 13C{1H} NMR (151 MHz, CDCl3): δ 172.1, 167.1, 153.3, 138.6, 135.5, 128.5, 128.0, 125.2, 115.1, 114.7, 82.5, 71.5, 70.7, 55.5, 43.8, 21.4, 21.0, 9.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H26NO5 384.1805, found 384.1808.
The stereochemistry of the methyl group on the 5-membered ring was established with two NOE experiments with very short mixing times [0.1 s (SI p S266) and 0.2 s (SI p S267)]. This methyl group is clearly cis to the adjacent phenyl substituent. With a mixing time of just 0.1 s:
The benzylic proton (δ 6.00) has a significantly stronger NOE with the adjacent methine proton (δ 3.78) than with the adjacent methyl group (δ0.61).
NH (δ 4.71) exhibits NOE with the adjacent methyl group (δ0.61) but not with the adjacent methine proton (δ 3.78).
The protons on the monosubstituted phenyl ring (downfield of δ 7.3) exhibit a much stronger NOE with the adjacent methyl group (δ0.61) than with the adjacent methine proton (δ 3.78).
In addition, the very shielded nature of the methyl protons of ring H–C–CH3 is consistent with these observations. With the monosubstituted phenyl ring and the ring methyl group cis to each other, the ring methyl group is nearly above the monosubstituted phenyl ring, which would be expected to result in a shielding effect.
NOE is also detected between the methoxy protons (δ 3.73) and the aromatic protons at δ 6.75 and between the NH proton (δ 4.71) and the aromatic protons at δ 6.57. These observations enabled secure assignments of the aromatic protons at δ 6.75 (H-3′/H-5′) and at δ 6.57 (H-2′/H-6′).
The same observations were made with a mixing time of 0.2 s.
Pictorial representation of NOE correlation in 24w:

Isopropyl (3R,3aR,7aR)-3-((4-Methoxyphenyl)amino)-2-oxoocta-hydrobenzofuran-3-carboxylate (24x).
The general procedure (method A) was followed using 28o (0.149 g, 0.36 mmol) and TsOH·H2O (0.014 g, 0.07 mmol) as starting materials to afford the fully substituted γ-lactone as a single diastereomer 24x (0.12 g, 95%) which was purified by using flash column chromatography. Physical state: pale brown viscous oily liquid. Rf = 0.50 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 6.74 (m, AA′ part of AA′BB′ pattern, 2H, H-3′/H-5′), 6.57 (m, BB′ part of AA′BB′ pattern, 2H, H-2′/H-6′), 4.88 (m, 1H, bridgehead methine next to oxygen≡H-1), 4.87 (hept, J = 6.3 Hz, 1H, isopropyl methine), 4.57 (s, broad, NH), 3.73 (s, 3H, methoxy), 3.29–3.23 (m, 1H, bridgehead methine next to quaternary carbon≡H-2), 2.31–2.25 (m, 1H), 1.76–1.56 (overlapping m, 4H), 1.41–1.18 (overlapping m, 3H), 1.15 (d, J = 6.3 Hz, 3H, isopropyl methyl), 0.80 (d, J = 6.2 Hz, 3H, other isopropyl methyl). 13C{1H} NMR (126 MHz, CDCl3): δ 173.0 (carbonyl), 166.9 (carbonyl), 153.2 (C-4′), 139.0 (C-1′), 115.2 (C-2′/C-6′), 114.8 (C-3′/C-5′), 77.8 (C-1), 72.2 (quaternary aliphatic carbon), 70.4 (isopropyl CH), 55.7 (methoxy), 43.0 (C-2), 27.3 (C-6), 23.0 (C-4), 22.0 (C-3), 21.4 [isopropyl methyl (correlating with methyl 1H signal at δ 1.15 in HSQC spectrum)], 21.1 [other isopropyl methyl (correlating with methyl 1H signal at δ0.80 in HSQC spectrum)], 19.4 (C-5). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H26NO5 348.1805, found 348.1804. As with 24y, the key issue for 24x was determining the stereochemistry of the fusion of the 6-and 5-membered rings, i.e., the relative orientation of H-1 and H-2. The severe overlap of the heptet from the isopropyl methine proton and the multiplet from the bridgehead methine proton next to oxygen (H-1) complicates the analysis. However, detailed chemical shift assignments could be made through a combination of 1H (SI p S268), 13C (SI p S269), COSY (SI p S271), NOE (SI p S273–S276), and HSQC (SI p S272) experiments. Indeed, the pair of diastereotopic protons associated with each methylene carbon could be assigned as follows: C-3 CH2 (next to bridgehead methine next to quaternary carbon): δ 1.65 and δ 1.27 with δ 22.0, C-4 CH2: δ 1.73 and δ 1.27 with δ 23.0, C-5 CH2: δ 1.59 and δ 1.34 with δ 19.4, C-6 CH2 (next to bridgehead methine next to oxygen): δ 2.28 and δ 1.66 with δ 27.3. NOE experiments with various mixing times (0.15, 0.30, 0.50 s) were used to establish the relative orientation of H-1 and H-2. With the shortest mixing time, prominent cross peaks were detected between the multiplet centered at δ 4.88 (H-1) and the multiplets centered at δ 3.26 (H-2) and δ 2.28 (one of the H-6 protons). These multiplets can be differentiated from the cross peaks between the heptet centered at δ 4.87 (isopropyl CH) and the doublets centered at δ 1.15 and δ0.80 (SI p S274). As with 24y, the prominent cross peak between H-1 and H-2 with a very short mixing time indicated cis ring fusion. NOE is also detected between the aromatic protons at δ 6.57 and each of H-2 and NH and between the methoxy protons (δ 3.73) and the aromatic protons at δ 6.74. These observations enabled secure assignments of the aromatic protons at δ 6.57 (H-2′/H-6′) and at δ 6.74 (H-3′/H-5′). NOE is not detected between H-2′/H-6′ and the more remote H-1, even with a mixing time of 0.50 s. In general, lengthening the mixing time increased the intensity of various cross peaks and simply reinforced the analysis.
The NOE experiments also exhibited a cross peak at δ 4.57/δ 1.27 indicating spatial proximity between the amine proton at δ 4.57 and methylene proton at δ 1.27. This is consistent with interaction between the amine proton and one of the H-3 protons; the cross peak becomes noticeably stronger as the mixing time is lengthened from 0.15 to 0.30 s to 0.50 s and is consistent with a cis relationship of the 3-CH2 group and the −NH-C6H4-OCH3 group. The absence of any cross peak between the amine proton and ring junction H-2 at the three mixing times is consistent with a trans relationship of ring junction H-2 and the −NH-C6H4-OCH3 group.
Pictorial representation of NOE correlation in 24x:

Isopropyl (3R,3aR,9bS)-3-(Benzo[d][1,3]dioxol-5-ylamino)-2-oxo-2,3,3a,4,5,9b-hexahydronaphtho[1,2-b]furan-3-carboxylate (24y).
The general procedure (method A) was followed using 28p (0.134 g, 0.28 mmol) and TsOH·H2O (0.011 g, 0.05 mmol) as starting materials to afford the fully substituted γ-lactone as a single diastereomer 24y (0.099 g, 85%) which was purified by using flash column chromatography. Physical state: white foam. Rf = 0.51 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.46 (m, 1H, H-5), 7.26–7.33 (m, 2H, H-7 and H-6), 7.18 (m, 1H, H-8), 6.65 (d, J =8.3 Hz, 1H, H-5′), 6.28 (d, J = 2.4 Hz, 1H, H-2′), 6.05 (dd, J = 8.3, 2.4 Hz, 1H, H-6′), 5.88 (d, J = 1.4 Hz, 1H, one H of-OCH2O−), 5.87 (d, J = 1.4 Hz, 1H, other H of-OCH2O−), 5.78 (d, J = 5.0 Hz, 1H, H-4), 4.98 (hept, J = 6.3 Hz, 1H, isopropyl methine), 4.70 (s, broad, NH), 3.60 (ddd, J = 13.5, 5.0, 4.7 Hz, 1H, H-3), 2.72–2.86 (m, 2H, H-1ax, H-1eq), 1.86 (dddd, J ≈ 13.6, 4.7, 3.6, 3.6 Hz, 1H, H-2ax or H-2eq), 1.51 (dddd, J = 13.6, 13.6, 13.5, 4.7 Hz, 1H, H-2eq or H-2ax), 1.21 (d, J = 6.3 Hz, 3H, isopropyl methyl), 0.90 (d, J = 6.2 Hz, 3H, other isopropyl methyl). 13C{1H} NMR (151 MHz, CDCl3): δ 171.3, 167.2, 148.4, 140.9, 140.4, 137.9, 131.1, 130.0, 129.2, 128.6, 126.6, 108.5, 105.1, 100.7, 96.9, 78.0, 71.3, 70.7, 43.6, 27.8, 21.4, 21.1, 19.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H24NO6 410.1598, found 410.1599. The key issue was determining the stereochemistry of the fusion of the 6- and 5-membered rings, i.e., the relative orientation of H-4 and H-3. H-4 gave a distinctive, deshielded doublet at δ 5.78 (SI p S277). Detailed 1H chemical shift assignments could be made through COSY (SI p S280) and NOE (p. S281–S285) experiments. In particular, COSY confirmed that only the multiplet at δ 3.60 correlated with H-4 and thus resulted from H-3. COSY indicated that H-3 also correlated with the protons at δ 1.86 and δ 1.51, thus confirming their assignments as H-2.
NOE experiments with various mixing times (0.15, 0.30, 0.50 s) were used to establish the relative orientation of H-4 and H-3. With the shortest mixing time, a cross peak was not detected between the signals at δ 5.78 (H-4) and the H-2 proton at δ 1.51; a weak cross peak was detected between H-4 and the other H-2 proton (at δ 1.86), which established their closer spatial proximity. A significantly stronger cross peak was detected between the signals at δ 5.78 (H-4) and δ 3.60 (H-3), which strongly suggested a cis relationship and thus cis ring fusion. With this very short mixing time, the cross peak between H-4 and H-3 was stronger than (1) the cross peak between H-4 and the signal at δ 7.46 (thus identified as H-5), (2) the cross peak between the other benzylic protons (H-1) and the signal at δ 7.18 (thus identified as H-8), (3) the cross peaks between H-3 and the signals at δ 6.28 and δ 6.05 (consistent with their assignments as H-2′ and H-6′ on the other aromatic ring on the basis of the magnitude of their J values), and (4) the cross peaks between NH and H-2′ and H-6′. Thus, the relatively strong cross peak between H-4 and H-3 with a very short mixing time indicated cis ring fusion. Lengthening the mixing time increased the intensity of various cross peaks and simply reinforced the analysis.
With mixing times of 0.30 and 0.50 s, very weak cross peaks were also observed between H-4 (δ 5.78) and each of the isopropyl methyl groups (δ 1.21 and δ 0.90) and between the 2-CH2 protons (δ 1.86 and δ 1.51) and the amine NH (δ 4.70). These cross peaks were also observed with mixing times of 0.70 and 1.00 s. The cross peaks clearly indicate a cis relationship between H-4 and the isopropoxycarbonyl group and a cis relationship between 2-CH2 and the -NH-C6H4–OCH3 group.
Pictorial representation of NOE correlation in 24y:

General procedure for the synthesis of aziridine-2,2,3-triesters, Figure 12.
To a solution of methyl bromoacetate (2.0 equiv) in anhydrous THF (0.2 M) in a flame-dried reaction vial under argon was added LiHMDS (1 M solution in THF) (2.1 equiv) dropwise over 3 minutes via syringe at −41 °C. After stirring for 45 min, a solution of diisopropyl iminomalonate 25 (1.0 equiv) in anhydrous THF (0.2M) was added dropwise via syringe. After stirring for another 2 h at −41 °C and confirming the completion of the reaction by thin layer chromatography, the reaction was quenched with saturated aqueous NH4Cl and warmed to room temperature. The reaction mixture was then diluted with brine (10 mL) and extracted with ethyl acetate thrice (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by automated flash column chromatography to afford the N-aryl aziridine.
2,2-diisopropyl-3-methyl-(R)-1-phenylaziridine-2,2,3-tricarboxylate (33a).
The general procedure was followed using diisopropyl phenyl iminomalonate (0.3 g, 1.08 mmol), bromomethyl ester (0.206 mL, 2.16 mmol) and LiHMDS (1M solution in THF) (2.27 mL, 2.27 mmol) were used as starting materials to afford aziridine-2,2,3-triester as pale yellow colored viscous oily liquid (0.3 g, 79%). Compound was purified by flash chromatography (Rf = 0.45 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.22–7.26 (m, 2H, H-3/H-5), 7.02–7.06 (m, 1H, H-4), 6.95–6.98 (m, 2H, H-2/H-6), 5.22 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.86 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.81 (s, 4H, methoxy and aziridine ring methine), 1.34 (d, J = 6.3 Hz, 3H, one isopropyl CH3), 1.31 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.13 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 0.96 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 166.9 (carbonyl), 163.7 (carbonyl), 162.6 (carbonyl), 146.1 (sp2 C–N), 129.0 (C-2/C-6 or C-3/C-5), 124.1 (C-4), 119.5 (C-3/C-5 or C-2/C-6), 71.0 (isopropyl CH), 70.0 (other isopropyl CH), 53.1 (quaternary aliphatic), 52.8 (methoxy), 45.6 (aziridine ring CH), 21.6 (overlapping signals for two isopropyl CH3), 21.5 (third isopropyl CH3), 21.1 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H24NO6 350.1598; Found 350.1598.
The singlets for the methoxy protons and the aziridine ring proton completely overlap and give a sharp signal with a half-height linewidth of 0.63 Hz. Additional support for this assignment comes from 13C satellites exhibiting 1JCH values that are reasonable for these functional groups; the identification of these satellites is supported by the expected very small, negative, one-bond 13C vs. 12C isotope effect on the 1H chemical shift [1ΔH(13/12C)] that is also observed for each type of proton. One pair of satellites exhibits 1JCH = 148.0 Hz (consistent with methoxy) with 1ΔH(13/12C) = −0.0025 ppm. A weaker pair of satellites exhibits 1JCH = 176.6 Hz (consistent with aziridine ring H) with 1ΔH(13/12C) = −0.0020 ppm.
Comparing the standard and DEPT-135 13C spectra provided a secure assignment of the quaternary aromatic carbon (δ 146.1) and of the quaternary aliphatic carbon (δ 53.1) in the aziridine ring (through the absence of these signals in the DEPT-135 13C spectrum). Comparing the DEPT-135 and DEPT-90 13C spectra provided a secure assignment of the methoxy carbon (δ 52.8) and aziridine ring methine carbon (δ 45.6), as the methoxy carbon signal appeared in only the DEPT-135 13C spectrum while the aziridine ring methine carbon signal appeared in both spectra).
In principle, two isomers are possible: the phenyl group can be cis or trans to the aziridine ring methine proton. If both isomers are present and inversion about the nitrogen is very fast, just one set of signals (representing the average environment) will be present, as is observed with N-(p-methoxyphenyl)-2,2-dimethylaziridine above the coalescence temperature Tc of −26 °C.33 In general, conjugation of the nitrogen lone pair with an aromatic ring system is known to decrease the barrier to inversion about nitrogen.33–35 The electron-donating nature of the p-methoxy substituent (present in 33b) raises the barrier to inversion relative to an unsubstituted phenyl group (as is present in 33a); Tc = −49 °C for N-phenyl-2,2-dimethylaziridine.33 The single pair of 1H and 13C spectra of 33a does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33a formed when the enolate reacted with the diisopropyl iminomalonate. However, one can reasonably conclude that slow inversion about the nitrogen is not occurring with a mixture of cis and trans isomers of 33a, as only one set of 13C signals and only one set of 1H signals are observed. (This aspect will be further considered in the discussion of 33h.)
2,2-diisopropyl-3-methyl-(R)-1-(4-methoxyphenyl)aziridine-2,2,3-tricarboxylate (33b).
The general procedure was followed using diisopropyl p-methoxyphenyl iminomalonate (1 g, 3.25 mmol), bromomethyl ester (0.618 mL, 6.50 mmol) and LiHMDS (1 M solution in THF) (6.8 mL, 6.8 mmol) were used as starting materials to afford aziridine-2,2,3-triester as brown colored viscous oily liquid (0.758 g, 62%). The compound was purified by flash chromatography (Rf = 0.19 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 6.90 (m, AA′ part of AA′BB′ pattern, 2H), 6.77 (m, BB′ part of AA′BB′ pattern, 2H), 5.21 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.87 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.80 (s, 3H, methoxy), 3.78 (s, 1H, aziridine ring CH), 3.75 (s, 3H, methoxy), 1.34 (d, J = 6.3 Hz, 3H, one isopropyl CH3), 1.30 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.14 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 1.02 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 167.0 (carbonyl), 163.8 (carbonyl), 162.6 (carbonyl), 156.4 (sp2 C–O), 139.2 (sp2 C–N), 120.5 (C-2/C-6 or C-/C-5), 114.2 (C-3/C-5 or C-2/C-6), 70.9 (isopropyl CH), 69.9 (other isopropyl CH), 55.4 (methoxy), 53.4 (quaternary aliphatic), 52.8 (methoxy), 45.8 (aziridine ring CH), 21.64 (isopropyl CH3), 21.62 (second isopropyl CH3), 21.5 (third isopropyl CH3), 21.2 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H26NO7 380.1704; Found 380.1706.
Of the five N-aryl aziridines 33a–e, only 33b has two methoxy groups. The methoxy protons in 33a, 33c, 33d, and 33e give signals from δ 3.795–3.811 (dilute solutions in CDCl3). Thus, it is reasonable to assign the singlet in 33b at δ 3.80 to the ester and the singlet at δ 3.75 to the ether.
As with 33a, the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the quaternary aliphatic carbon (δ 53.4) in the aziridine ring, the aziridine ring methine carbon (δ 45.8), and the methoxy carbons (δ 55.4 and δ 52.8). The methoxy carbon signals can reasonably be assigned to the ether (δ 55.4) and ester (δ 52.8) functional groups in light of the signal for the ester in 33a, 33c, 33d, and 33e appearing at δ 52.8 or δ 52.9.
The single pair of 1H and 13C spectra of 33b does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33b formed when the enolate reacted with the diisopropyl iminomalonate. However, as with 33a, one can reasonably conclude that slow inversion about the nitrogen is not occurring with a mixture of cis and trans isomers of 33b, as only one set of 13C signals and only one set of 1H signals are observed.
2,2-diisopropyl-3-methyl-(R)-1-(benzo[d][1,3]dioxol-5-yl)aziridine-2,2,3-tricarboxylate (33c).
The general procedure was followed using diisopropyl p-methoxyphenyl iminomalonate (0.45 g, 1.40 mmol), bromomethyl ester (0.266 mL, 2.80 mmol) and LiHMDS (1 M solution in THF) (2.9 mL, 2.94 mmol) were used as starting materials to afford aziridine-2,2,3-triester as brown colored viscous oily liquid (0.346 g, 63%). Compound was purified by flash chromatography (Rf = 0.23 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 6.66 (dd, 3J = 8.3 Hz, 5J ~ 0.3 Hz, 1H, H-5), 6.52 (dd, 4J = 2.3 Hz, 5J ~ 0.3 Hz, 1H, H-2), 6.41 (dd, 3J = 8.3 Hz, 4J = 2.3 Hz, 1H, H-6), 5.91 (AB quartet whose protons have virtually identical chemical shifts, J ~ 1.4 Hz, -OCH2O–), 5.20 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.93 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.79 (s, 3H, methoxy), 3.75 (s, 1H, aziridine ring CH), 1.33 (d, J = 6.3 Hz, 3H, one isopropyl CH3), 1.30 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.17 (d, J = 6.2 Hz, 3H, third isopropyl CH3), 1.08 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 166.8 (methoxycarbonyl), 163.7 (isopropoxycarbonyl), 162.5 (other isopropoxycarbonyl), 148.0 (C-3 or C-4), 144.3 (C-4 or C-3), 140.8 (C-1), 111.8 (C-6), 108.1 (C-5), 101.5 (C-2), 101.3 (−OCH2O–), 71.0 (isopropyl CH, correlating with methine 1H signal at δ 4.93 in HSQC spectrum), 70.0 (other isopropyl CH, correlating with methine 1H signal at δ 5.20 in HSQC spectrum), 53.4 (quaternary aliphatic), 52.8 (methoxy), 46.0 (aziridine ring CH), 21.65 (isopropyl CH3, correlating with methyl 1H signal at δ 1.17 in HSQC spectrum), 21.61 (second isopropyl CH3, correlating with methyl 1H signal at δ 1.30 in HSQC spectrum), 21.5 (third isopropyl CH3, correlating with methyl 1H signal at δ 1.33 in HSQC spectrum), 21.3 (fourth isopropyl CH3, correlating with methyl 1H signal at δ 1.08 in HSQC spectrum). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H24NO8 394.1496; Found 394.1489.
As with 33a, the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the quaternary aliphatic carbon (δ 53.4) in the aziridine ring, the methoxy carbon (δ 52.8), and the aziridine ring methine carbon (δ 46.0). These experiments also provided a secure assignment of the -OCH2O- carbon (δ 101.3), which has a chemical shift very similar to that of one of the aromatic CH carbons (δ 101.5).
In light of the unambiguous assignments for H-2, H-5, and H-6 in the 1D 1H spectrum, the 1H–13C HSQC spectrum immediately provided the C-2, C-5, and C-6 assignments. It also enabled pairwise 1H/13C assignments for each of the two isopropyl CH groups and for each of the four isopropyl CH3 groups (SI p S299–S304). The 1H–13C HMBC spectrum (SI p S295) unambiguously differentiated the two quaternary aromatic carbons bonded to oxygen from the quaternary aromatic carbon bonded to nitrogen. Only the two more deshielded quaternary aromatic carbons (δ 148.0 and δ 144.3) correlate with the -OCH2O- protons at δ 5.91, while only the most shielded quaternary aromatic carbon (δ 140.8) correlates with the aziridine ring methine proton at δ 3.75 (a 3Jch correlation in each case; SI p S296). In addition, the HMBC experiment indicated that
the methoxy 1H signal at δ 3.79 correlated with the carbonyl signal at δ 166.8 (but not with either of the other carbonyl signals), which clearly established that the signal at δ 166.8 resulted from the methoxycarbonyl group (SI p S296).
the more deshielded isopropyl methine 1H signal at δ 5.20 correlated with the carbonyl signal at δ 163.7 (but not with either of the other carbonyl signals) and that the more shielded isopropyl methine 1H signal at δ 4.93 correlated with the carbonyl signal at δ 162.5 (but not with either of the other carbonyl signals) (SI p S296). Each of these seven-contour cross peaks displays a very noticeable tilt resulting from the six 3Jhh couplings experienced by the methine proton.36 Acquiring the spectrum under conditions of high digital resolution in the 13C dimension (0.074 ppm) enables the individual contours to be readily observed. The separation between contours in the 13C dimension = 3Jhh; the separation between contours in the 1H dimension = 3Jch. Tilted correlations are also observed for the correlations involving the isopropyl methyl carbons SI p S297). Similarly, the relatively large 3Jh-5,h-6 coupling enables tilted correlations to be observed for correlations involving H-5 and H-6.
the two more deshielded isopropyl methyl doublets at δ 1.33 and δ 1.30 correlated with the more shielded isopropyl methine carbon at δ 70.0 (page S381) and that the two more shielded isopropyl methyl doublets at δ 1.17 and δ 1.08 correlated with the more deshielded isopropyl methine carbon at δ 71.0 (page S313) Tilted correlations are again observed, exhibiting 3Jhh in the 13C dimension and 2JCH in the 1H dimension.
The single pair of 1H and 13C spectra of 33c does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33c formed when the enolate reacted with the diisopropyl iminomalonate. However, as with 33a and 33b, one can reasonably conclude that slow inversion about the nitrogen is not occurring with a mixture of cis and trans isomers of 33c, as only one set of 13C signals and only one set of 1H signals are observed.
2,2-diisopropyl-3-methyl-(R)-1-(4-bromophenyl)aziridine-2,2,3-tricarboxyiate (33d).
The general procedure was followed using diisopropyl p-bromophenyl iminomalonate (0.36 g, 1.01 mmol), bromomethyl ester (0.192 mL, 2.02 mmol) and LiHMDS (1 M solution in THF) (2.12 mL, 2.12 mmol) were used as starting materials to afford aziridine-2,2,3-triester as yellow viscous oily liquid (0.311 g, 72%). The compound was purified by flash chromatography (Rf = 0.32 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.36 (m, AA′ part of AA′BB′ pattern, 2H), 6.85 (m, BB′ part of AA′BB′ pattern, 2H), 5.21 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.90 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.81 (s, 3H, methoxy), 3.75 (s, 1H, aziridine ring CH), 1.34 (d, J = 6.3 Hz, 3H, one isopropyl CH3), 1.30 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.16 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 1.04 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDO3): δ 166.6 (carbonyl), 163.4 (carbonyl), 162.3 (carbonyl), 145.3 (sp2 C–N), 132.0 (C-2/C-6 or C-3/C-5), 121.2 (C-3/C-5 or C-2/C-6), 116.9 (sp2 C–Br), 71.3 (isopropyl CH), 70.2 (other isopropyl CH), 53.0 (quaternary aliphatic), 52.9 (methoxy), 45.7 (aziridine ring CH), 21.7 (isopropyl CH3), 21.6 (second isopropyl CH3), 21.5 (third isopropyl CH3), 21.2 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H23BrNO6 428.0703, found 428.0705.
As with 33a and 33c the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the quaternary aliphatic carbon (δ 53.0) in the aziridine ring, the methoxy carbon (δ 52.9), and the aziridine ring methine carbon (δ 45.7).
The single pair of 1H and 13C spectra of 33d does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33d formed when the enolate reacted with the diisopropyl iminomalonate. However, as with 33a–c, one can reasonably conclude that slow inversion about the nitrogen is not occurring with a mixture of cis and trans isomers of 33d, as only one set of 13C signals and only one set of LH signals are observed.
2.2-diisopropyl-3-methyl-(R)-1-(3-chlorophenyl)aziridine-2,2,3-tricarboxylate (33e).
The general procedure was followed using diisopropyl m-chlorophenyl iminomalonate (0.32 g, 1.02 mmol), bromomethyl ester (0.195 mL, 2.05 mmol) and LiHMDS (1 M. solution in THF) (2.1 mL, 2.15 mmol) were used as starting materials to afford aziridine-2,2,3-triester as pale yellow viscous oily liquid (0.297 g, 75%). The compound was purified by flash chromatography (Rf = 0.36 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.18 (ddd, 3Jh-5,h-4 = 8.0 Hz, 3Jh-5,h-6 = 8.0 Hz, 5JH-5,H-2 ~ 0.3 Hz, 1H, H-5), 7.03 (ddd, 3Jh-4,h-5 = 8.0 Hz, 4JH-4,H-2 = 2.0 Hz, 4JH-4,H-6 ~ 0.95 Hz, 1H, H-4), 6.97 (ddd, 4JH-2,H-4 = 2.0 Hz, 4JH-2,H-6 = 2.0 Hz, 5JH-2,H-5 ~ 0.3 Hz, 1H, H-2), 6.85 (ddd, 3JH‑6,H‑5 = 8.1 Hz, 4JH‑6,H‑2 = 2.2 Hz, 4JH‑6,H‑4 ~ 0.93 Hz, 1H, H-6), 5.21 (hept, J = 6.3 Hz, 1H, isopropyl CH), 4.92 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.81 (s, 3H, methoxy), 3.78 (s, 1H, aziridine ring CH), 1.34 (d, J = 6.3 Hz, 3H, one isopropyl CH3), 1.31 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.17 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 1.05 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3).; 13C{1H} NMR (126 MHz, CDCl3): δ 166.5 (carbonyl), 163.4 (carbonyl), 162.3 (carbonyl), 147.5 (sp2 C–N), 134.6 (sp2 C–Cl), 1 130.1 (C-5), 124.3 (C-4), 119.8 (C-2), 117.8 (C-6), 71.4 (isopropyl CH), 70.2 (other isopropyl CH), 53.0 (quaternary aliphatic), 52.9, (methoxy), 45.7 (aziridine ring CH), 21.64 (isopropyl CH3), 21.59 (second isopropyl CH3), 21.49 (third isopropyl CH3), 21.18 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H23ClNO6 384.1208, found 384.1211.
While H-2 and H-5 in the aromatic ring have distinctive coupling patterns, H-4 and H-6 have very similar coupling patterns and cannot be confidently differentiated just on the basis of chemical shift. However, literature 13C chemical shift data on 3-chloroaniline,37 3-chloro-N,N-1 dimethylaniline,38 and a more elaborate 3-chloro-N,N-dialkylaniline39 clearly show that C-6 is considerably more shielded than C-4 (with C-6 ranging from δ 113.9–110.4 vs C-4 ranging from δ 119.5–115.9), that C-2 is essentially in between (ranging from δ 115.8–112.0), and that C-5 is much more deshielded (ranging from δ 130.1–129.9). 1H chemical shift data were also reported for 3-chloroaniline37 and the elaborate derivative,39 and in both cases, H-6 is more shielded than H-4, and H-5 is significantly more deshielded than either.
An HSQC experiment on 33e gave the following correlations for the four aromatic CH groups: δc130.1/δh7.18, δc124.3/δh7.03, δc119.8/δh6.97, and δc117.8/δh6.85 (page S326). The δc130.1/δh7.18 correlation clearly results from C-5/H-5. The δc119.8/δh6.97 correlation clearly results from C-2/H-2. In light of the literature chemical shift data,37–39 the δc124.3/δh7.03 correlation can reasonably be assigned to C-4/H-4, and the δc117.8/δh6.85 correlation (the most shielded aromatic 13C and 1H nuclei) can reasonably be assigned to C-6/H-6. Highly expanded plots of the correlations for the two isopropyl CH groups, the methoxy and aziridine CH groups, and the four isopropyl CH3 groups are shown on pages S312–S314.
As with 33a, 33c and 33d, the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the quaternary aliphatic carbon (δ 53.0) in the aziridine ring, the methoxy, carbon (δ 52.9), and the aziridine ring methine carbon (δ 45.7). In the standard 13C spectrum, two signals of different intensity appear for C–Cl (page S309), reflecting the 1Δ13C(37/35Cl) isotope effect (4 ppb shielding upon replacement of 35Cl with 37Cl).40,41
The single pair of 1H and 13C spectra of 33e does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33e formed when the enolate reacted with the diisopropyl iminomalonate. However, as with 33a–d, one can reasonably conclude that slow inversion about the nitrogen is not occurring with a mixture of cis and trans isomers of 33e, as only one set of 13C signals and only one set of 1H signals are observed.
2,2-diisopropyl-3-methyl-(R)-1-phenethylaziridine-2,2,3-tricarboxylate (33f).
The general procedure was followed using diisopropyl Phenethyl iminomalonate (0.31 g, 1.01 mmol), bromomethyl ester (0.193 mL, 2.03 mmol) and LiHMDS (1 M solution in THF) (2.13 mL, 2.13 mmol) were used as starting materials to afford aziridine-2,2,3-triester as pale yellow colored viscous oily liquid (0.194 g, 51%). Compound was purified by flash chromatography (Rf = 0.29 (20% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.29–7.16 (m, phenyl, 5H), 5.12 (hept, J = 6.3 Hz, 1H, isopropyl CH), 5.11 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.70 (s, 3H, methoxy), 3.07 (s, 1H, aziridine ring CH), 3.06–2.99 (m, 3H, CH2), 2.87–2.77 (m, 1H, CH2), 1.29 (d, J = 6.3 Hz, 6H, overlapping doublets for two isopropyl CH3), 1.27 (d, J = 6.2 Hz, 3H, third isopropyl CH3), 1.22 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 167.5 (carbonyl), 164.1 (carbonyl), 163.6 (carbonyl), 138.9 (C-1), 128.8 (C-2/C-6 or C-3/C-5), 128.4 (C-3/C-5 or C-2/C-6), 126.4 (C-4), 71.0 (isopropyl CH), 69.5 (other isopropyl CH), 53.1 (N–CH2), 52.4 (methoxy), 51.8 (quaternary aliphatic), 47.8 (aziridine ring CH), 35.7 (phenyl C-CH2), 21.73 (isopropyl CH3), 21.55 (second isopropyl CH3), 21.54 (third isopropyl CH3), 21.49 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C20H28NO6 378.1911; Found 378.1909.
As with 33a and 33c-e, the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the quaternary aliphatic carbon (δ 51.8) in the aziridine ring, the methoxy carbon (δ 52.4), and the aziridine ring methine carbon (δ 47.8). The presence of inverted signals at δ 53.1 and δ 35.7 in the DEPT-135 13C spectrum and their absence in the DEPT-90 13C spectrum enabled their identification as CH2 signals. Their large chemical shift difference allowed the N–CH2 and phenyl C–CH2 assignments to easily be made.
Inversion about the nitrogen is known to be much slower, in general, in N-alkylaziridines than in N-arylaziridines.
In contrast to the coalescence temperature Tc = −49 °C for N-phenyl-2,2-dimethylaziridine (15 vol % solution in CF2Cl2),33 Tc = +60 °C for N-isopropyl-2,2-dimethylaziridine (1M in CDCl3),42 and Tc = +76 °C for N-methyl-2,2-dimethylaziridine (1M in CDCl3).42 Solvent has a noticeable effect, as the Tc values for N-isopropyl-2,2-dimethylaziridine and N-methyl-2,2-dimethylaziridine are significantly lower (+38 °C and +48 °C, respectively) in 1 M C6H12. The effect of substituents on the aziridine ring on the rate of inversion seems to vary widely with the nature of the N-substituent. Relatively little change in Tc occurs with N-phenyl (Tc = −40 °C for N-phenylaziridine in CS2),35 but a large increase in Tc occurs with N-methyl (Tc = +108 °C for N-methylaziridine, 1M in C6H12). We are not aware of any studies with several much bulkier C-substituents such as are in aziridines 33a-h and therefore do not know their effect on Tc.
Tc is the same within experimental error (+95 °C) for N-cyclohexylaziridine and N-(2-phenylethyl)aziridine.34 Since the latter compound has the same N-substituent as in 33f, it seems reasonable to conclude that inversion about the nitrogen in 33f is slow at ambient temperature and that a set of signals for each isomer would be observed if both isomers of 33f were present. However, the 1H and 13C spectra of 33f clearly exhibit signals for only one isomer. To confirm that only one isomer is present and undergoing slow inversion about the nitrogen, spectra would have to be obtained at much lower temperatures to determine if only one set of 1H signals and only one set of 13C signals continue to be observed.
The N-alkyl substituent in 33f has a noticeable effect on the 1H chemical shifts of the aziridine ring methine proton and the C-substituents on the aziridine ring and on the 13C chemical shifts of the aziridine ring carbons and their substituents, as shown in Table S7 (see SI page No. S25). Not surprisingly, the effect is most noticeable for the nuclei closest to the aziridine N: the two carbons in the aziridine ring and especially for the aziridine ring proton.
Even with the aziridine N-substituent apparently cis to one isopropoxycarbonyl group and trans to the other in 33f, the two isopropyl methine protons have nearly the same chemical shift. In contrast, the environments for the two isopropyl methine protons in N-arylaziridines 33a-e are noticeably different, as the isopropyl methine 1H chemical shifts differ by 0.27–0.36 ppm in 33a-e. Still, it does not seem possible to rigorously conclude from the single pair of 1H and 13C spectra for each of 33a-e whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer formed when the enolate reacted with the diisopropyl iminomalonate.
The N-alkyl substituent also has a striking effect on some of the 13C linewidths. Four of the signals are much shorter because they are much broader: the most shielded carbonyl, the N–CH2, the aziridine ring methine carbon, and the aziridine ring quaternary carbon (Table S8) (see SI Page No. S26). Indeed, significant signal averaging is required to clearly detect these signals above the baseline noise in the standard, DEPT-135, and DEPT-90 13C experiments.
In 33f, the signal for the aziridine ring quaternary carbon is significantly sharper (ν1/2 = 1.09 Hz) than the signals for the other two N–C carbons (ν1/2= 4.6 and 6.5 Hz) but is still broader than every other signal except for the most shielded carbonyl (ν1/2 = 2.7 Hz). In contrast, in 33a-e:
the signal for the aziridine ring quaternary carbon is very sharp (ν1/2 = 0.34−0.38 Hz, three times narrower than the corresponding signal in 33f),
the signal for the quaternary aromatic carbon bonded to aziridine N is sharp (ν1/2 = 0.55−0.62 Hz, seven times narrower than the corresponding signal in 33f),
the signal for the aziridine ring methine carbon is almost as sharp (ν1/2 = 0.75–0.81 Hz, eight times narrower than the corresponding signal in 33f), and
the signal for the most shielded carbonyl carbon is very sharp (ν1/2 = 0.34–0.37 Hz, seven times narrower than the corresponding signal in 33f).
Relaxation of the quadrupolar 14N appears to be significantly faster with the N-alkyl substituent in 33f than with any of the N-aryl substituents in 33a-33e.
2,2-diisopropyl-3-methyl-(R)-1-(1-(tert-butoxycarbonyl)-piperidin-4-yl)aziridine-2,2,3-tricarboxylate (33g).
The general procedure was followed using diisopropyl-1-(tert-butoxycarbonyl)-piperidin-4-yl iminomalonate (0.4 g, 1.04 mmol), bromomethyl ester (0.198 mL, 2.08 mmol) and LiHMDS (1M solution in THF) (2.18 mL, 2.18 mmol) were used as starting materials to afford aziridine-2,2,3-triester as pale yellow colored viscous oily liquid (0.183 g, 39%). Compound was purified by flash chromatography (Rf = 0.4 (30% EtOAc/hexanes). 1H (500 MHz, CDCl3): δ 5.12 (hept, J = 6.3 Hz, 1H, isopropyl CH), 5.10 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.93 (br s, 2H, a CH2 proton on each N–CH2), 3.72 (s, 3H, methoxy), 3.22 (s, 1H, aziridine ring CH), 3.00 (m, 2H, a CH2 proton on each N–CH2), 2.61 (m, 1H, N–CH in 6-membered ring), 1.75–1.50 (m, 4H, –CH2-CH–CH2-), 1.45 (s, 9H, t-butoxy), 1.29 (d, J = 6.3 Hz, 3H, isopropyl CH3), 1.28 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.26 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 1.22 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (151 MHz, CDCl3): δ 167.3 (carbonyl), 163.8 (carbonyl), 163.6 (carbonyl), 154.6 (N-Boc carbonyl), 79.2 (t-butoxy quaternary), 70.7 (isopropyl CH), 69.2 (other isopropyl CH), 55.9 (N–CH in 6-membered ring), 52.3 (methoxy), 51.8 (aziridine ring quaternary), 46.4 (aziridine ring CH), 41.6, 41.3, 40.7, and 40.5 (N–CH2), 30.8 (CH-CH2), 28.2 (t-butoxy methyl), 21.37 (isopropyl CH3), 21.34 (second isopropyl CH3), 21.32 (third isopropyl CH3), 21.30 (fourth isopropyl CH3). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C22H37N2O8 457.2544; Found 457.2548.
As with 33a and 33c-f, the combination of standard, DEPT-135, and DEPT-90 13C experiments provided a secure assignment of the t-butoxy quaternary carbon (δ 79.2) and the aziridine ring quaternary carbon (δ 51.8), the methoxy carbon (δ 52.3), and the 6-membered ring methine carbon (δ 55.9) and the aziridine ring methine carbon (δ 46.4). The presence of inverted signals at δ 41.6, 41.3, 40.7, 40.5, and 30.8 in the DEPT-135 13C spectrum and their absence in the DEPT-90 13C spectrum enabled their identification as CH2 signals. The large chemical shift differences for signals with the same multiplicity allowed assignments for the pair of quaternary aliphatic carbons, the pair of ring methine carbons, and the pair of methylene carbons to easily be made.
The severe broadening of the CH2 signals is obvious. We believe that the four broad signals near 41 ppm result from a combination of two factors: (1) inversion at nitrogen in the 6-membered ring occurring on a time scale comparable to the reciprocal of the frequency difference (in Hz) between the chemical shift of a given N–CH2 in the conformer with t-Boc equatorial and the chemical shift of that N–CH2 in the conformer with t-Boc axial (2) the presence of two diastereotopic N–CH2 carbons because of the chiral center in the aziridine ring. For the two diastereotopic CH-CH2 carbons, just one broad signal is observed at δ 30.8. The much higher sensitivity of the 600 MHz spectrometer’s cryoprobe optimized for 13C detection greatly facilitated detecting these extremely broad CH2 signals.
The 13C signals that are so broad in 33f narrow by approximately a factor of two in 33g (Table S8) (see SI page No. S26). In light of the very slow inversion about nitrogen for N-cyclohexylaziridine,34 it seems reasonable to conclude that inversion about the aziridine nitrogen in 33g is slow at ambient temperature and that a set of signals for each isomer would be observed if both isomers of 33g were present. However, as with 33f, the 1H and 13C spectra of 33g clearly exhibit signals for only one isomer.
2,2-diisopropyl-3-methyl-(R)-1-cyclopentylaziridine-2,2,3-tricarboxylate (33h).
The general procedure was followed using diisopropyl cyclopentyl iminomalonate (0.27 g, 1.002 mmol), bromomethyl ester (0.190 mL, 2.005 mmol) and LiHMDS (1 M solution in THF) (2.11 mL, 2.10 mmol) were used as starting materials to afford aziridine-2,2,3-triester as colorless viscous oily liquid (0.15 g, 44%). The Compound was purified by flash chromatography (Rf = 0.15 (10% EtOAc/hexanes). 1H (500 MHz, CDCl3): δ 5.12 (hept, J = 6.3 Hz, 1H, isopropyl CH), 5.10 (hept, J = 6.3 Hz, 1H, other isopropyl CH), 3.72 (s, 3H, methoxy), 3.21 (s, 1H, aziridine ring CH), 2.94 (m, 1H, N–CH in 5-membered ring), 1.94–1.63 (m, 5H, ring CH2 protons), 1.63–1.45 (m, 3H, ring CH2 protons), 1.28 (d, J = 6.3 Hz, 3H, isopropyl CH3), 1.270 (d, J = 6.3 Hz, 3H, second isopropyl CH3), 1.268 (d, J = 6.3 Hz, 3H, third isopropyl CH3), 1.21 (d, J = 6.3 Hz, 3H, fourth isopropyl CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 167.9 (methoxycarbonyl, ν1/2 = 0.51 Hz), 164.3 (isopropoxycarbonyl, ν1/2 = 0.47 Hz), 163.8 (other isopropoxycarbonyl, ν1/2 = 0.93 Hz), 70.6 (isopropyl CH, ν1/2 = 0.89 Hz, correlating with methine 1H signal at δ 5.12 in HSQC spectrum), 69.3 (other isopropyl CH, ν1/2 = 0.90 Hz, correlating with methine 1H signal at δ 5.10 in HSQC spectrum), 62.5 (N–CH in 5-membered ring, ν1/2 = 2.2 Hz), 52.8 (aziridine ring quaternary, ν1/2 = 0.48 Hz), 52.4 (methoxy, ν1/2 = 0.87 Hz), 47.8 (aziridine ring CH, ν1/2 = 2.5 Hz), 32.7 (–CH2-CH-CH2-, ν1/2 = 0.63 Hz), 32.3 (–CH2-CH-CH2-, ν1/2 = 0.63 Hz), 24.6 (–CH2-CH2-CH-, ν1/2 = 0.51 Hz), 24.5 (–CH-CH2-CH2-, ν1/2 = 0.50 Hz), 21.59 (isopropyl CH3, ν1/2 = 0.63 Hz, correlating with methyl 1H signal at δ 1.270 in HSQC spectrum), 21.553 (second isopropyl CH3, correlating with methyl 1H signal at δ 1.21 in HSQC spectrum), 21.548 (third isopropyl CH3, correlating with methyl 1H signal at δ 1.28 in HSQC spectrum), 21.49 (fourth isopropyl CH3, ν1/2 = 0.65 Hz, correlating with methyl 1H signal at δ 1.268 in HSQC spectrum). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H28NO6 342.1911; Found 342.1936.
As with the other aziridines, the combination of standard, DEPT-135, and DEPT-90 13C experiments differentiated among the CH3, CH2, CH, and quaternary carbons. A 1H–13C HSQC experiment (SI p S325) provided secure assignments and critical additional information:
The correlation at δH3.72/δC52.4 (SI p S326) clearly results from methoxy because the 1H singlet at δ 3.72 results from three protons and because the 13C signal at δ 52.4 appears in a DEPT-135 13C spectrum but is strongly attenuated in a DEPT-90 13C spectrum.
The correlation at δH3.21/δC47.8 (SI p S326) clearly results from the aziridine ring CH because the 1H singlet at δ 3.21 results from one proton and because the 13C signal at δ 47.8 is strong in DEPT-135 and DEPT-90 13C spectra.
The correlation at δH2.94/δC62.5 (SI p S326) clearly results from the cyclopentyl ring CH because the 1H multiplet at δ 2.94 results from one proton and because the 13C signal at δ 62.5 is strong in DEPT-135 and DEPT-90 13C spectra.
At each of the four different CH2 13C frequencies (δ 32.7, 32.3, 24.6, and 24.5), a different pattern of contours appears (SI p S327). Each CH2 group is clearly different, consistent with the presence of two pairs of diastereotopic CH2 groups because of the chiral center in the aziridine ring. The pattern is consistent with the relative intensities for the CH2 signals in the 1H spectrum: signals for five CH2 protons downfield of δ 1.65 and signals for three CH2 protons upfield of δ 1.65.
There are striking differences in the 13C spectra of 33g and 33h (SI p S319 and S322). First, the 13C signals that are relatively broad in 33f and narrow by about a factor of 2 in 33g continue to narrow in 33h (Table S9) (See SI p S11). The CH2 signals greatly sharpen in 33h and have half-height line widths comparable to many of the other signals. The two methylene carbons adjacent to the methine are diastereotopic because of the chiral center in the aziridine ring and give distinct signals at δ 32.7 and δ 32.3. The remaining two methylene carbons are diastereotopic for the same reason; because they are further from the chiral center, they exhibit a smaller chemical shift difference (δ 24.6 and δ 24.5).
An alternative explanation for these pairs of CH2 signals seems much less plausible—the presence of two diastereomers: one with the cyclopentyl group cis to the aziridine ring proton, the other with the cyclopentyl group trans to the aziridine ring proton, and very slow interconversion of the diastereomers (through inversion about nitrogen) because of the rigidity of the three-membered ring with an N-alkyl substituent. This explanation seems much less plausible because pairs of signals would then be expected for all carbons, which is clearly not observed. (A previous report43 has clearly shown different 13C chemical shifts for a wide range of cis- and trans-N-alkyl-2,3- disubstituted aziridines).
Because of the aziridine ring quaternary carbon′s proximity to the N-substituent and the 3-methoxycarbonyl substituent and because of the extreme sensitivity of 13C chemical shifts to even remote changes in stereochemistry,44 the chemical shift of the aziridine ring quaternary carbon should be particularly sensitive to the presence of cis and trans isomers. Because the signal for this carbon in 33h is very sharp (v1/2 = 0.48 Hz with 0.20 Hz of line broadening applied), observing a pair of signals from the aziridine ring quaternary carbon in the two diastereomers—if they were present—should be particularly easy. Such a pair is not seen. Clearly, only one isomer of33h is present. Note that in N-aryl aziridines 33a-e, the signal for the aziridine ring quaternary carbon is even sharper (with v1/2 ranging from 0.34 to 0.38 Hz), which would make observing a pair of signals even easier; however, each of 33a-e exhibits just one aziridine ring quaternary carbon signal. Again, as noted earlier, the single pair of 1H and 13C spectra for each of 33a-e does not indicate whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer of 33a-e formed when the enolate reacted with the diisopropyl iminomalonate.
The 1H spectra support the presence of only one isomer for each of N-alkyl aziridines 33f-h, as just one methoxy singlet and just one aziridine ring methine 1H singlet are observed (as is also the case for N-aryl aziridines 33a-e. In 33a, these singlets happen to coincide within our ability to measure.) Remarkably, the 1H half-height line widths of the methoxycarbonyl and aziridine ring methine proton singlets are remarkably similar in 33a-h (Table S9) (see SI p S11); the modest broadening in 33g might result from the relatively slow inversion about nitrogen in the 6-membered ring. Still, it does not seem possible to rigorously conclude from the single pair of 1H and 13C spectra for each of 33a-e whether (1) cis and trans isomers are present with very fast inversion about the nitrogen or (2) just one isomer formed when the enolate reacted with the diisopropyl iminomalonate.
We note that the much smaller chemical shift range in 1H NMR and the presence of multiplets for the other signals make 1H NMR a less sensitive probe for the presence of cis and trans isomers. Still, one could reasonably expect the chemical shift of the aziridine ring methine proton to be sensitive to whether it is cis or trans to the adjacent substituent on nitrogen. (For 33a, the probability of the cis and trans isomers both being present and both methoxy 1H singlets and both aziridine ring methine 1H singlets all occurring at the same frequency in the slow inversion limit seems extraordinarily low.) Indeed, 1H spectra of N-substituted aziridines have been reported with different 1H chemical shifts for the cis and trans isomers.43
Thus, with only one diastereomer of 33h formed when the enolate reacted with the diisopropyl iminomalonate, determining the stereochemistry was the next goal. NOE experiments have been previously used to determine the relative orientation of substituents on N-substituted aziridines.45–47 NOE experiments with mixing times of 0.6 and 1.0 s (SI p S328 and S329) clearly showed NOE between the aziridine ring proton at δ 3.21 and the cyclopentyl methine proton at δ 2.94. No cross peak was evident between the methoxy protons at δ 3.72 and the cyclopentyl methine proton at δ 2.94. These observations are consistent with the cyclopentyl ring cis to the aziridine ring proton and trans to the methoxycarbonyl group, which is clearly the sterically less hindered diastereomer.
A 1H–13C HMBC experiment (SI p S324) indicated that
the methoxy 1H signal at δ 3.72 correlated with the carbonyl signal at δ 167.9 (but not with either of the other carbonyl signals), which clearly established that the signal at δ 167.9 resulted from the methoxycarbonyl group;
the more deshielded isopropyl methine 1H signal at δ 5.12 correlated with the carbonyl signal at δ 163.8 (but not with either of the other carbonyl signals) and that the more shielded isopropyl methine 1H signal at δ 5.10 correlated with the carbonyl signal at δ 164.3 (but not with either of the other carbonyl signals);
the two more shielded isopropyl methyl doublets at δ 1.21 and δ 1.268 correlated with the more shielded isopropyl methine carbon at δ 69.3; and
the two more deshielded isopropyl methyl doublets at δ 1.270 and δ 1.28 correlated with the more deshielded isopropyl methine carbon at δ 70.6.
It was not clear which isopropoxycarbonyl group is cis to the aziridine ring proton and which is trans.
Finally, we note the sensitivity of the 1H and 13C chemical shifts of various functional groups to the presence of an N-aryl or an N-alkyl substituent on the aziridine (Table S7) (see SI p. S11). In general, small, but clearly noticeable, differences are observed for the two types of substituents. However, the chemical shift difference is large for the isopropyl methine protons as well as for the aziridine ring methine proton.
General Procedure for the Addition of Nitrile Carbanions onto Iminomalonates, Figure 13.
Ina thick-walled, flame-dried, 25 mL round-bottom flask, the corresponding acetonitrile (1.35 equiv) was dissolved in DME (0.23M) and cooled to −78 °C. KHMDS (1.0M in THF) (1.35 equiv) was added dropwise to this solution. Usually the solution turned yellow and slowly to reddish yellow. After 1hof stirring, a solution of the iminomalonate (1 equiv) in of DME (0.13M) was added slowly in a dropwise manner. The reaction mixture was stirred for 2 h. After confirming the complete consumption of starting material by TLC, saturated aqueous solution of ammonium chloride was added (5 mL) to quench the reaction and the reaction mixture was allowed to warm to room temperature. The organic layer was separated. The aqueous layer was extracted twice with diethyl ether (2 × 20 mL). The combined organic layers were dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The crude reaction mixture was purified by automated flash chromatography.
Note: For gram-scale reaction (for the synthesis of 35a) for acetonitrile-derived carbanion generation, the reaction mixture should be stirred for 2 h, after which the iminomalonate was added and the reaction mixture was stirred for another 3 h. The rest of the workup procedure is same as mentioned in the procedure described above.
Diisopropyl2-(Cyano(phenyl)methyl)-2-(phenylamino)malonate (35a).
The general procedure was followed using 2-phenylacetonitrile (0.079 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol) and phenyl iminomalonate (0.138 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.107 g, 54%). The compound was purified by flash column chromatography (Rf = 0.24 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.26 (td, J = 6.1, 5.0, 3.1 Hz, 1H), 7.21 (t, J = 7.4 Hz, 2H), 7.19–7.16 (m, 2H), 7.12–7.08 (m, 2H), 6.74 (t, J = 7.4 Hz, 1H), 6.57 (d, J = 7.8 Hz, 2H), 5.10 (hept, J = 6.2 Hz, 1H), 5.01 (p, J = 6.3 Hz, 1H), 4.93 (s, 1H), 4.87 (s, 1h), 1.39 (d, J =6.3 Hz, 3H), 1.18 (d, J = 6.3 Hz, 3H), 1.15 (d, J = 6.2 Hz, 3H), 0.95 (d, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 166.7, 166.0, 143.3, 130.7, 129.5, 129.3, 128.9, 128.4, 119.4, 118.8, 114.8, 72.3, 71.2, 70.4, 40.0, 21.6, 21.3, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H27N2O4 395.1965, found 395.1990.
Note: For gram-scale reaction, the general procedure was followed using 2-phenylacetonitrile (0.808 g, 6.91 mmol), KHMDS (1 M solution in THF) (6.91 mL, 6.91 mmol), and phenyl iminomalonate) (1.42 g, 5.12 mmol) as starting materials. The rest of the procedure is same as mentioned in the procedure described above.
Diisopropyl 2-(Cyano(phenyl)methyl)-2-((4-methoxyphenyl)-amino)malonate (35b).
The general procedure was followed using 2-phenylacetonitrile (0.079 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and p-methoxyphenyl iminomalonate (0.154 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a bright yellow viscous oily liquid (0.140 g, 66%). The compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.33–7.22 (m, 5H), 6.72 (d, J = 8.8 Hz, 2H), 6.59 (d, J = 8.8 Hz, 2H), 5.10 (hept, J = 6.2 Hz, 1H), 5.01 (hept, J = 6.7 Hz, 1H), 4.84 (s, 1h), 4.74 (s, 1H), 3.72 (s, 3h), 1.40 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 1.16 (d, J = 6.2 Hz, 3H), 1.0 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 166.9, 166.2, 153.5, 137.2, 130.8, 129.7, 129.0, 128.4, 119.1, 117.0, 114.8, 72.1, 71.23, 71.20, 55.6, 40.5, 21.6, 21.4, 21.3, 21.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H29N2O3 425.2100, found 425.2065.
Diisopropyl 2-(Cyano(4-fluorophenyl)methyl)-2-(phenylamino)-malonate (35c).
The general procedure was followed using 2-(4-fluorophenyl)acetonitrile (0.091 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol) and phenyl iminomalonate (0.138 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.150 g, 73%). The compound was purified by flash column chromatography (Rf = 0.24 (20% EtOAc/hexanes). 1HNMR (600 MHz, CDCl3): δ 7.22 (dd, J = 8.6, 5.2 Hz, 2H), 7.15 (t, J = 7.9 Hz, 2H), 6.96 (t, J =8.6 Hz, 2H), 6.80 (t, J = 7.3 Hz, 1H), 6.59 (d, J = 7.9 Hz, 2H), 5.15 (hept, J = 6.3 Hz, 1H), 5.05 (hept, J = 6.3 Hz, 1H), 4.99 (s, 1H), 4.92 (s, 1H), 1.44 (d, J = 6.3 Hz, 3H), 1.23 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 0.99 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.7, 166.0, 163.0 (d, 1JCF = 248.9 Hz), 143.2, 131.4 l (d, 3Jcf = 8.3 Hz), 129.4, 126.5 (d, 4Jcf = 3.4 Hz), 119.7, 118.7, 115. Five (d, 2Jcf = 21.8 Hz), 114.9, 72.5, 71.4, 70.5, 39.4, 21.6, 21.4, 21.3, 21.2. 19F NMR (471 MHz, CDCl3): δ –111.3 (tt, J = 8.4, 5.1 Hz). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H26FN2O4 413.1832, found 413.1866.
Diisopropyl 2-((4-Chlorophenyl)(cyano)methyl)-2-(phenylamino)malonate (35d).
The general procedure was followed using 2-(4-chlorophenyl)acetonitrile (0.102 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and phenyl iminomalonate (0.138 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.131 g, 61%). The compound was purified by flash column chromatography (Rf = 0.24 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.27 (d, J = 8.5 Hz, 2H), 7.18 (dd, J = 17.6, 8.2 Hz, 4H), 6.83 (t, J = 7.3 Hz, 1h), 6.62 (d, J = 7.9 Hz, 2H), 5.17 (hept, J = 6.3 Hz, 1H), 5.08 (hept, J = 6.2 Hz, 1H), 5.01 (s, 1H), 4.93 (s, 1H), 1.46 (d, J = 6.3 Hz, 3H), 1.25 (d, J = 6.3 Hz, 3H), 1.23 (d, J = 6.2 Hz, 3H), 1.01 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.5, 165.8, 143.1, 135.1, 130.9, 129.4, 129.2, 128.6, 119.6, 118.4, 114.8, 72.5, 71.4, 70.3, 39.4, 21.6, 21.3, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H26ClN2O4 429.1576, found 429.1582.
Diisopropyl 2-(Cyano(4-fluorophenyl)methyl)-2-((4-methoxyphenyl)amino)malonate (35e).
The general procedure was followed using 2-(4-fluorophenyl)acetonitrile (0.091 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and p-methoxyphenyl iminomalonate (0.154 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.130 g, 59%). The compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.26 (dd, J =8.2 Hz, 2H), 6.96 (t, J =8.5 Hz, 2H), 6.72 (d, J =8.8 Hz, 2H), 6.57 (d, J =8.8 Hz, 2H), 5.10 (hept, J = 6.2 Hz, 1H), 5.01 (hept, J = 6.1 Hz, 1H), 4.85 (s, 1H), 4.77 (s, 1H), 3.72 (s, 3h), 1.39 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 1.16 (d, J = 6.2 Hz, 3H), 0.99 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.8, 166.1, 163.0 (d, 1JCF = 248.9 Hz), 153.6, 137.0,131.5 (d, 3JCF = 8.3 Hz), 126.6 (d, 4Jcf = 3.3 Hz), 118.9, 117.0, 115.5 (d, 2Jcf = 21.9 Hz), 114.7, 72.2, 71.3, 71.1, 55.5, 39.9, 21.6, 21.4, 21.3, 21.2. 19F NMR (471 MHz, CDCl3): δ –111.3 (tt, J = 8.4, 5.2 Hz). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H28FN2O5 443.1973, found 443.1972.
Diisopropyl 2-((4-Chloro-2-fluorophenyl)(cyano)methyl)-2-((4- methoxyphenyl)amino)malonate (35f).
The general procedure was followed using 2-(4-chloro-2-fluorophenyl)acetonitrile (0.114 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and p-methoxyphenyl iminomalonate (0.154 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a bright yellow viscous oily liquid (0.167 g, 70%). The compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.34 (t, J =8.1 Hz, 1H), 7.08 (ddd, J = 23.2, 9.1, 1.9 Hz, 2H), 6.74–6.72 (m, 2H), 6.69–6.67 (m, 2H), 5.16–5.09 (m, 2H), 4.98 (hept, J =6.1 Hz, 1H), 4.74 (s, 1H), 3.73 (s, 3H), 1.38 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 1.12 (d, J = 6.2 Hz, 3H), 1.00 (d, J = 6.2 Hz, 3H). 13c{1H} (151 MHz, CDCl3): δ 166.5,166.2,160.2 (d, 1JCF = 253.2 Hz), 153.9, 137.1,136.2 (d, 3Jcf = 10.5 Hz), 132.2 (d,4 or 3Jcf = 3.4 Hz), 124.9 (d, 3 or 4Jcf = 3.5 Hz), 118.0, 117.6, 117.5 (d, 2Jcf = 13.9 Hz), 116.5 (d, 2Jcf = 25.8 Hz), 114.6, 72.3, 71.8, 71.2, 55.6, 34.8, 21.5, 21.2, 21.1. 19F NMR (471 MHz, CDCl3): δ –110.4 (t, J = 8.9 Hz). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H27ClFN2O5 477.1594, found 477.1586.
Diisopropyl 2-(Cyano(phenylthio)methyl)-2-((4-methoxyphenyl)- amino)malonate (35g).
The general procedure was followed using 2-(phenylthio)acetonitrile (0.100 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and p-methoxyphenyl iminomalonate (0.154 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellowish brown viscous oily liquid (0.178 g, 78%). The compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.44–7.38 (m, 2H), 7.34 (t, J = 7.2 Hz, 1H), 7.29 (t, J = 7.3 Hz, 2h), 6.81 (d, J = 8.9 Hz, 2h), 6.76 (d, J = 8.9 Hz, 2H), 5.16 (hept, J = 6.2 Hz, 1H), 5.09 (hept, J = 6.2 Hz, 1H), 5.01 (s, 1H),4.59(s, 1H), 3.74 (s, 3h), 1.28 (d, J = 6.3 Hz, 3H), 1.25 (d, J = 6.3 Hz, 3H), 1.20 (d, J =6.3 Hz, 3H), 1.16 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): 166.6, 165.4, 154.6, 136.0, 134.4, 130.7, 129.5, 129.3, 119.6, 117.6, 114.6, 72.1, 71.7, 70.4, 55.5, 42.4, 21.3, 21.2, 21.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H29N2O5S 457.1785, found 457.1794.
Diisopropyl 2-(Cyano(phenyl)methyl)-2-((3-(trifluoromethyl)- phenyl)amino)malonate (35h).
The general procedure was followed using 2-phenylacetonitrile (0.079 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and m-trifluoromethyl- phenyl iminomalonate (0.172 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.141 g, 61%). The compound was purified by flash column chromatography (Rf = 0.36 (10% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.287.14 (m, 6H), 6.95 (d, J = 7.7 Hz, 1H), 6.69 (dd, J = 8.2, 2.1 Hz, 1H), 6.57 (s, 1H), 5.22 (s, 1H), 5.12 (hept, J = 6.5 Hz, 1H), 4.95 (hept, J = 6.3 Hz, 1H), 4.84 (s, 1H), 1.37 (d, J = 6.3 Hz, 3H), 1.16 (d, J = 6.3 Hz, 3H), 1.14 (d, J = 6.3 Hz, 3H), 0.91 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.0, 165.9, 144.0, 131.6 (q, 2JCF = 32.1 Hz), 130.4, 129.8, 129.7, 129.2, 128.6, 123.9 (q, 1JCF = 272.4 Hz), 118.7, 117.8, 115.9 (q, 3Jcf = 3.8 Hz), 111.3 (q, 3Jcf = 3.8 Hz), 72.7, 71.8, 70.7, 40.8, 21.6, 21.4, 21.3, 21.1. 19F NMR (471 MHz, CDCl3): δ −61.9 (S). HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H26 F3N2O4463.1823, found 463.1815.
Diisopropyl 2-(Cyano(4-fluorophenyl)methyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (35i).
The general procedure was followed using 2-(4-fluorophenyl)acetonitrile (0.091 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol), and m-trifluoromethylphenyl iminomalonate (0.172 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.146 g, 61%). The compound was purified by flash column chromatography (Rf = 0.36 (10% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.32 (dd, J = 8.4, 5.2 Hz, 2H), 7.22 (t, J = 7.9 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 6.97 (t, J = 8.5 Hz, 2H), 6.73 (d, J = 8.0 Hz, 1H), 6.55 (s, 1H), 5.33 (s, 1H), 5.19 (hept, J = 6.2 Hz, 1H), 5.00 (hept, J = 6.1 Hz, 1H), 4.91 (s, 1H), 1.43 (d, J =6.3 Hz, 3H), 1.22 (d, J =5.7 Hz, 3H), 1.21 (d, J = 5.7 Hz, 3H), 0.94 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 165.9, 165.8, 163.2 (d, 1JCF = 249.5 Hz), 144.0, 131.8 (d, 3Jcf = 8.4 Hz), 131.7 (q, 2Jcf = 32.1 Hz), 129.7, 126.2 (d, 4Jcf =3.3 Hz), 123.9 (q, 1JCF = 272.4Hz), 118.5, 118.0, 116.1 (q, 3JCF = 3.9 Hz), 115.8 (d, 2Jcf = 21.9 Hz), 111.2 (q, 3Jcf = 3.7 Hz), 72.8, 71.9, 70.7, 40.4, 21.5, 21.3, 21.2, 21.0. 19F NMR(471 MHz, CDCl3): δ –62.04 (S), –110.9 (tt, J = 8.4, 5.2 Hz). HRMS (ESI-TOF) m/z: [M + H]+calcd for C24H25F4N2O4 481.1787, found 481.1785.
Diisopropyl 2-(cyano(phenyl)methyl)-2-(pyridin-3-ylamino)-malonate (35j).
The general procedure was followed using 2-phenylacetonitrile (0.079 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol) and 3-pyridyl iminomalonate (0.139 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.126 g, 64%). Compound was purified by flash column chromatography (Rf = 0.32 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.02 (s, 1H), 7.95 (s, 1H), 7.30–7.23 (m, 5H), 7.00 (m, 1H), 6.81 (d, J = 7.4 Hz, 1H), 5.14 (s, 1H), 5.13 (hept, J = 6.2 Hz, 1H), 5.00 (hept, J = 6.2 Hz, 1H), 4.85 (s, 1H), 1.39 (d, J = 6.3 Hz, 3H), 1.20 (d, J = 6.3 Hz, 3H), 1.15 (d, J = 6.2 Hz, 3H), 0.96 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 165.9, 165.8, 140.9, 138.3, 130.3, 129.7, 129.2, 128.7, 120.4, 118.7, 72.6, 71.8, 70.6, 40.8, 21.5, 21.4, 21.3, 21.2. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C22H26N3O4 [M+H]+ 396.2122; found 396.2101.
Diisopropyl 2-(cyano(4-fluorophenyl)methyl)-2-(pyridin-3-ylamino)malonate (35k).
The general procedure was followed using 2-(4-fluorophenyl)acetonitrile (0.091 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol) and 3-pyridyl iminomalonate (0.139 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow viscous oily liquid (0.124 g, 60%). Compound was purified by flash column chromatography (Rf = 0.32 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.05 (d, J = 4.3 Hz, 1H), 8.01–7.94 (m, 1H), 7.30 (dd, J = 7.6, 5.6 Hz, 2H), 7.00 (dt, J = 16.8, 5.9 Hz, 3H), 6.82–6.74 (m, 1H), 5.18 (s, 1H), 5.12 (hept, J = 6.2 Hz, 1H), 5.01 (hept, J = 6.1 Hz, 1H), 4.86 (s, 1H), 1.40 (d, J = 6.3 Hz, 3H), 1.22 (d, J = 6.2 Hz, 3H), 1.18 (d, J = 6.2 Hz, 3H), 0.98 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 165.8, 165.7, 163.2 (d, 1JCF = 249.7 Hz), 141.1, 139.9, 138.3, 131.6 (d, 3JCF = 8.4 Hz), 126.2 (d, 4JCF = 3.3 Hz), 123.4, 120.4, 118.5, 115.8 (d, 2JCF = 21.8 Hz), 72.8, 72.0, 70.7, 40.3, 21.5, 21.4, 21.3, 21.2. 19F NMR (471 MHz, CDCl3): δ −110.6 (tt, J = 8.4, 5.1 Hz). HRMS (ESI-TOF) m/z: [M+H]+ calcd for C22H25FN3O4 [M+H]+ 414.2034; found 414.2027.
Diisopropyl 2-((4-chloro-2-fluorophenyl)(cyano)methyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (35l).
The general procedure was followed using 2-(4-chloro-2-fluorophenyl)acetonitrile (0.114 g, 0.675 mmol), KHMDS (1 M solution in THF) (0.68 mL, 0.675 mmol) and m-trifluoromethylphenyl iminomalonate (0.172 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow oily liquid (0.172 g, 67%). The compound was purified by flash column chromatography (Rf = 0.35 (10% EtOAc/hexanes). 1H NMR (500 MHz, CDCl3): δ 7.33 (dd, 3JHH = 8.4 Hz, 4JHF = 7.8 Hz, 1H, H-6’); 7.27 (dd, 3JHH = 8.0 Hz, 3JHH = 7.9 Hz, 1H, H-5); 7.11 (ddd, 3JHH = 8.4 Hz, 4JHH = 2.1 Hz, 5JHH ≈ 0.7 Hz, 1H, H-5′); 7.065 (m, 1H, H-4); 7.062 (dd, 3JHF = 9.7 Hz, 4JHH = 2.1 Hz, 1H, H-3′); 6.88 (dd, 3JHH = 8.2 Hz, 4JHH = 2.4 Hz, 1H, H-6); 6.82 (s with unresolved long-range couplings, 1H, H-2); 5.22 (s, broad, NH); 5.16 [d, 5JHH (with H-5′) ≈ 0.7 Hz, 1H, H–C–CN]; isopropoxy group 1:5.19 (hept, J = 6.3 Hz, 1H, methine), 1.41 (d, J = 6.3 Hz, 3H, methyl), 1.16 (d, J = 6.3 Hz, 3H, methyl); isopropoxy group 2:5.03 (hept, J = 6.3 Hz, 1H, methine), 1.24 (d, J = 6.3 Hz, 3H, methyl), 1.01 (d, J = 6.3 Hz, 3H, methyl). 13C{1H} NMR (126 MHz, CDCl3): δ 160.2 (d, 1JCF = 253.4 Hz, C-2′); 143.9 (s, C-1); 136.6 (d, 3JCF = 10.5 Hz, C-4′); 132.0 (d, 3JCF = 3.4 Hz, C-6′); 131.6 (q, 2JCF = 32.2 Hz, C-3); 129.8 (s, C-5); 125.1 (d, 4JCF = 3.7 Hz, C-5′); 123.9 (q, 1JCF = 272.4 Hz, CF3); 118.1 (q, 5JCF = 1 Hz, C-6); 117.6 (s, nitrile); 117.1 (d, 2JCF = 14.0 Hz, C-1′); 116.6 (d, 2JCF = 25.6 Hz, C-3′); 116.4 (q, 3JCF = 3.9 Hz, C-4); 111.7 (q, 3JCF = 3.9 Hz, C-2); 70.4 (broadened—unresolved long-range JCF coupling, quaternary aliphatic carbon); 34.5 (d, 3JCF = 1.7 Hz, N≡C–CH); isopropoxy group 1:165.7 (s, carbonyl), 72.9 [s, CH (correlating with methine 1H signal at δ 5.19 in HSQC spectrum)], 21.5 [s, CH3 (correlating with methyl 1H signal at δ 1.41 in HSQC spectrum)], 21.23 [s, CH3 (correlating with methyl 1H signal at δ 1.16 in HSQC spectrum)]; isopropoxy group 2:165.9 (s, carbonyl), 72.4 [s, CH (correlating with methine 1H signal at δ 5.03 in HSQC spectrum)], 21.22 [d, JCF = 1 Hz, CH3 (correlating with methyl 1H signal at δ 1.24 in HSQC spectrum)], 21.1 [s, CH3 (correlating with methyl 1H signal at δ 1.01 in HSQC spectrum)]. 19F NMR (471 MHz, CDCl3): δ −62.03 (broad singlet with shoulders from unresolved long-range JHF couplings that are not recognizable in the 1H spectrum, CF3; satellite 1JCF = 272.4 Hz), δ−110.5 [broad dd, J ≈ 8 Hz, J ≈ 8 Hz from 3JHF with H-3′ and 4JHF with H-6′ (see above), ring CF; satellite 1JCF = 253 Hz)]. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C24H24ClF4N2O4 515.1447; found 515.1441.
Detailed chemical shift assignments could be made through a combination of 1H (p. S384), 13C (SI p S370), DEPT-135 13C (SI p S371), COSY (SI p S373), HSQC (SI p S375–S379), HMBC (SI p S374), and 19F (SI p S372) spectra. The 1H–13C HSQC spectra also enabled 1H–19F couplings to be readily recognized and the corresponding JHF values to be measured because of the distinctive effect that coupling to a passive spin (19F) has on the appearance of a 1H–13C HSQC spectrum, i.e., splitting the contour into two contours separated by the value of JCF in the 13C dimension and by the value of JHF in the 1H dimension (SI pp S393 and S394).48 For example, SI p S393 shows a highly expanded plot of part of the aromatic region in the HSQC spectrum and the corresponding regions in the 1D 1H and 13C spectra. The correlations for C-4/H-4 and C-3′/H-3′ are shown. The two contours at δC116.41 separated in the 1H dimension by about 7.2 Hz and centered at δH7.065 result from C-4 and H-4 on the CF3-substituted ring. The separation between the contours results from 3J(H-4,H-5). The 1D 13C spectrum (left side) indicates that C-4 is a quartet with 3JCF = 3.9 Hz. This splitting is too small to detect in the HSQC spectrum, where the 13C FID digital resolution is 5.2 Hz (1.3 Hz after zero-filling). The 4JHF splitting is much too small to detect in the 1H dimension, where the 1H digital resolution is 2.1 Hz. In contrast, the 1D 13C spectrum indicates that C-3′ is a doublet at δ 116.62 with 2JCF = 25.6 Hz. The corresponding contours in the HSQC spectrum are offset by ±12.8 Hz in the 13C dimension; the separation of the contours in the 13C dimension is 2JCF. The contours in the 1H dimension are centered at δ 7.062 and separated by 9.7 Hz (3JHF). The 4JHH coupling between H-3′ and H-5′ (2.1 Hz) is too small to detect in the 1H dimension of the HSQC spectrum. The severely overlapping 1H signals for H-4 and H-3′ in the 1D 1H spectrum (top) make this region difficult to interpret by sight, but the HSQC results enable the four relatively prominent signals to be recognized as a doublet (3JHF) of doublets (4JHH) for H-3′. The same doublet of doublets centered at δ 7.062 is observed in a 400 MHz 1H spectrum, providing further support for the interpretation. SI p S379 shows a highly expanded plot of the C-6′/H-6′ correlation in the HSQC spectrum and the corresponding regions in the 1D 1H and 13C spectra. The 1H spectrum (top) is a doublet of doublets with similar J values that are attributed to 3JHH = 8.4 Hz and 4JHF = 7.8 Hz. Three equidistant contours (separation ≈ 8.3 Hz) are correspondingly observed in the 1H dimension in the HSQC spectrum. In the 13C dimension, the most shielded and the most deshielded contours differ by 3.4 Hz, the value of 3JCF observed for the doublet in the 1D 13C spectrum (left side). There is no detectable 5JHF between H-5′ and the ring fluorine. Thus, the broad doublet of doublets (with the central peaks clearly overlapping) observed for the ring fluorine in the 19F spectrum can be attributed to 3JHF with H-3’ and 4JHF with H-6′. The 1D 13C spectrum exhibited a completely unexpected splitting of one of the isopropyl methyl signals (SI p S370) that was also evident on 400 and 600 MHz spectrometers. In contrast, the 1D 13C spectrum of the analogous compound 35o without the ring fluorine (i.e., a 4-chlorophenyl group) exhibits the expected four singlets for the four different isopropyl methyl carbons. Thus, in the 2-fluoro-4-chlorophenyl compound (35l), there is an unexpected coupling between one methyl carbon and the ring fluorine. A 13C–19F J coupling through eight bonds would not be expected. A through-space 13C–19F coupling of a single methyl carbon with the ring fluorine is also hard to explain. Additional work is required to determine the origin of this unexpected 13C–19F interaction in 35l.
Diisopropyl-2-(cyano(phenylthio)methyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (35m).
The general procedure was followed using 2-(phenylthio)acetonitrile (0.100 g, 0.675 mmol), KHMDS (1M solution in THF) (0.68 mL, 0.675 mmol) and m-trifluoromethylphenyl iminomalonate (0.172 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow colored viscous oily liquid (0.180 g, 73%). Compound was purified by flash column chromatography (Rf = 0.36 (10% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.44 (d, J = 7.2 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 7.32–7.26 (m, 3H), 7.09 (d, J = 7.7 Hz, 1H), 6.98 (s, 1H), 6.94 (d, J = 8.1 Hz, 1H), 5.50 (s, 1H), 5.19 (hept, J = 6.2 Hz, 1H), 5.15 (hept, J = 6.2 Hz, 1H), 4.68 (s, 1H), 1.32 (d, J = 6.3 Hz, 3H), 1.29 (d, J = 6.3 Hz, 3H), 1.19 (d, J = 6.2 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.0, 165.0, 143.2, 134.4, 132.4, 132.1, 131.7 (q, 2JCF = 32.1 Hz), 130.4, 129.8, 129.7, 129.5, 129.4, 128.9, 123.9 (q, 1JCF = 272.4 Hz), 118.4, 117.3, 116.6 (q, 3JCF = 3.8 Hz), 116.5, 112.7 (q, 3JCF = 3.8 Hz), 72.7, 72.3, 69.7, 41.9, 21.34, 21.32, 21.2, 21.1. 19F NMR (471 MHz, CDCl3): δ −62.0 (S). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C24H26F3N2O4S 495.1599; found 495.1595.
Diisopropyl-2-((4-chloro-2-fluorophenyl)(cyano)methyl)-2-(pyridin-3-ylamino)malonate (35n).
The general procedure was followed using 2-(4-chloro-2-fluorophenyl)acetonitrile (0.114 g, 0.675 mmol), KHMDS (1M solution in THF) (0.68 mL, 0.675 mmol) and p-pyridyl iminomalonate (0.139 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellow colored viscous oily liquid (0.152 g, 68%). Compound was purified by flash column chromatography (Rf = 0.32 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.06–8.03 (m, 2H), 7.32 (t, J = 8.1 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 7.08–7.03 (m, 3H), 5.16 (hept, J = 6.2 Hz, 1H), 5.12 (s, 1H), 5.07 (s, 1H), 5.01 (hept, J = 6.3 Hz, 1H), 1.38 (d, J = 6.3 Hz, 3H), 1.22 (d, J = 6.3 Hz, 3H), 1.12 (d, J = 6.3 Hz, 3H), 1.00 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 165.7, 165.5, 160.0 (d, 1JCF = 253.2 Hz), 141.3, 139.7, 138.5, 136.5 (d, 3JCF = 10.5 Hz), 131.8 (d, 4 or 3 JCF = 3.3 Hz), 125.1 (d, 3 or 4JCF = 3.5 Hz), 123.3, 120.8, 117.6, 117.0 (d, 2JCF = 13.9 Hz), 116.7 (d, 2JCF = 25.6 Hz), 72.8, 72.3, 70.2, 34.3, 21.4, 21.2, 21.1. 19F NMR (471 MHz, CDCl3): δ −110.6 (t, J = 8.3 Hz). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C22H24ClFN3O4 448.1434; found 448.1438.
Diisopropyl-2-((4-chlorophenyl)(cyano)methyl)-2-((3-(trifluoromethyl)phenyl)amino)malonate (35o).
The general procedure was followed using 2-(4-chlorophenyl)acetonitrile (0.102 g, 0.675 mmol), KHMDS (1M solution in THF) (0.68 mL, 0.675 mmol) and m-trifluoromethylphenyl iminomalonate (0.172 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a yellowish grey colored viscous oily liquid (0.149 g, 60%). Compound was purified by flash column chromatography (Rf = 0.36 (10% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.31–7.24 (m, 5H), 7.05 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 8.0 Hz, 1H), 6.62 (s, 1H), 5.35 (s, 1H), 5.21 (hept, J = 6.1 Hz, 1H), 5.04 (hept, J = 6.2 Hz, 1H), 4.93 (s, 1H), 1.45 (d, J = 6.3 Hz, 3H), 1.25 (d, J = 6.3 Hz, 3H), 1.23 (d, J = 6.4 Hz, 3H), 0.98 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 165.8, 165.7, 143.9, 135.5, 131.5 (q, 2JCF = 32.1 Hz), 131.1, 129.7, 128.9, 128.8, 123.8 (q, 1JCF = 272.5 Hz), 118.2, 117.9, 116.2 (q, 3JCF = 3.7 Hz), 111.2 (q, 3JCF = 3.7 Hz), 72.9, 71.9, 70.6, 40.4, 21.5, 21.3, 21.2, 21.0. 19F NMR (471 MHz, CDCl3): δ −62.0 (S). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C24H25ClF3N2O4 497.1480; found 497.1477.
Diisopropyl-2-((4-chlorophenyl)(cyano)methyl)-2-((4-methoxyphenyl)amino)malonate (35p).
The general procedure was followed using 2-(4-chlorophenyl)acetonitrile (0.102 g, 0.675 mmol), KHMDS (1M solution in THF) (0.68 mL, 0.675 mmol) and p-methoxyphenyl iminomalonate (0.154 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a dark brown colored viscous oily liquid (0.146 g, 64%). Compound was purified by flash column chromatography (Rf = 0.35 (20% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.27 (d, J = 7.9 Hz, 2H), 7.22 (d, J = 8.3 Hz, 2H), 6.74 (d, J = 8.7 Hz, 2H), 6.60 (d, J = 8.7 Hz, 2H), 5.13 (hept, J = 6.2 Hz, 1H), 5.03 (hept, J = 6.2 Hz, 1H), 4.85 (s, 1H), 4.77 (s, 1H), 3.75 (s, 3H), 1.41 (d, J = 6.2 Hz, 3H), 1.23 (d, J = 6.2 Hz, 3H), 1.19 (d, J = 6.1 Hz, 3H), 1.02 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 166.7, 166.0, 153.7, 136.9, 135.2, 131.0, 129.3, 128.7, 118.7, 117.0, 114.8, 72.3, 71.4, 71.1, 55.6, 40.0, 21.6, 21.4, 21.3, 21.2. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C24H28ClN2O5 459.1681; found 459.1674.
Diisopropyl-2-((4-chlorophenyl)(cyano)methyl)-2-(pyridin-3-ylamino)malonate (35q).
The general procedure was followed using 2-(4-chlorophenyl)acetonitrile (0.102 g, 0.675 mmol), KHMDS (1M solution in THF) (0.68 mL, 0.675 mmol) and 3-pyridyl iminomalonate (0.139 g, 0.5 mmol) as starting materials to afford the nitrile adduct as a brown colored viscous oily liquid (0.159 g, 74%). Compound was purified by flash column chromatography (Rf = 0.31 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 8.06 (d, J = 4.1 Hz, 1H), 8.00 (d, J = 2.8 Hz, 1H), 7.31–7.24 (m, 2H), 7.24–7.20 (m, 2H), 7.03 (dd, J = 8.3, 4.7 Hz, 1H), 6.82 (ddd, J = 8.4, 2.9, 1.1 Hz, 1H), 5.18 (s, 1H), 5.15 (hept, J = 6.2 Hz, 1H), 5.01 (hept, J = 6.2 Hz, 1H), 4.84 (s, 1H), 1.39 (d, J = 6.3 Hz, 3H), 1.21 (d, J = 6.3 Hz, 3H), 1.17 (d, J = 6.2 Hz, 1H), 0.98 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 165.6, 165.4, 140.9, 139.7, 138.1, 135.3, 130.9, 128.8, 128.7, 123.3, 120.4, 118.1, 72.6, 71.8, 70.4, 40.2, 21.4, 21.2, 21.1, 21.0. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C22H25ClN3O4 430.1671; found 430.1662.
General procedure for the cyclization of nitrile addition products to corresponding γ-lactams, Figure 14.
Method A:
In a thick-walled flame dried 25 mL round bottom flask, the acetonitrile addition product (1 equiv) was dissolved in of anhydrous methanol (0.07 M) at room temperature. Raney Nickel (600 mg), washed with water and ethanol, was added to this. The mixture was then stirred for 3 days under a hydrogen atmosphere (in form of a balloon). After confirming the complete consumption of the starting material by TLC, the reaction was stopped and the mixture was passed through a small pad of celite and the celite pad was rinsed with ethyl acetate (10 mL). All the solvent was removed under reduced pressure followed by automated flash chromatography.
Method B:
In a thick wall flame dried 25 mL round bottom flask; the acetonitrile addition product (1.0 equiv) was dissolved in of dry THF (0.83 M) at room temperature. BH3.Me2S (10 equiv) was added dropwise in this solution followed by reflux for 1 hour. After confirming the complete consumption of the starting material by TLC, the reaction mixture was brought to room temperature and 1 mL of water was added slowly dropwise to the reaction mixture. Evolution of hydrogen was noticed. The mixture was again refluxed for 6 hours. The reaction mixture was then allowed to cool to room temperature and ethyl acetate (10 mL) was added. The organic layer was separated and the aqueous layer was extracted twice with ethyl acetate (2 X 10 mL). The combined organic layer was then dried over anhydrous sodium sulphate. All the solvent was removed under reduced pressure followed by automated flash chromatography.
Isopropyl-2-oxo-4-phenyl-3-(phenylamino)pyrrolidine-3-carboxylate (36a).
The general procedure, Method A was followed using diisopropyl 2-(cyano(phenyl)methyl)-2-(phenylamino)malonate, 35a (0.197 g, 0.5 mmol) to afford the nitrile adduct as a white colored solid (m.p. 198–203 °C) (0.101 g, 60%). Compound was purified by flash column chromatography (Rf = 0.33 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.22–7.14 (m, 5H), 7.03 (t, J = 7.9 Hz, 2H), 6.66 (t, J = 7.3 Hz, 1H), 6.50 (d, J = 7.8 Hz, 2H), 6.47 (s, 1H), 4.94 (hept, J = 6.2 Hz, 1H), 4.68 (s, 1H), 4.52 (d, J = 5.9 Hz, 1H), 4.10 (dd, J = 9.7, 6.6 Hz, 1H), 3.50 (d, J = 9.7 Hz, 1H), 1.16 (d, J = 6.3 Hz, 3H), 0.82 (d, J = 6.3 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 172.0, 169.4, 145.3, 139.1, 128.6, 128.2, 128.0, 127.4, 118.5, 114.3, 70.2, 69.8, 49.1, 47.6, 21.5, 21.0. HRMS (ESI-TOF): calc’d for C20H23N2O3 [M+H]+ 339.1703; found 339.1703.
Isopropyl-3-((4-methoxyphenyl)amino)-2-oxo-4-phenylpyrrolidine-3-carboxylate (36b).
The general procedure, Method A was followed using diisopropyl 2-(cyano(phenyl)methyl)-2-((4-methoxyphenyl)amino)malonate, 35b (0.212 g, 0.5 mmol) to afford the nitrile adduct as a white colored solid (m.p. 185–187 °C) (0.088 g, 48%). Compound was purified by flash column chromatography (Rf = 0.35 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.23–7.15 (m, 5H), 6.76 (s, 1H), 6.63 (d, J = 8.7 Hz, 2H), 6.48 (d, J = 8.7 Hz, 2H), 4.95 (hept, J = 6.1 Hz, 1H), 4.47 (d, J = 5.5 Hz, 1H), 4.43 (s, 1H), 4.04 (dd, J = 9.6, 6.8 Hz, 1H), 3.68 (s, 3H), 3.50 (d, J = 9.7 Hz, 1H), 1.17 (d, J = 6.2 Hz, 3H), 0.88 (d, J = 6.2 Hz, 3H). 13C{1H} (151 MHz, CDCl3): δ 172.3, 169.6, 152.8, 139.1, 138.9, 128.2, 128.0, 127.3, 115.9, 114.2, 70.1, 70.0, 55.6, 49.2, 47.4, 21.5, 21.1. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C21H25N2O4 369.1809; found 369.1809.
Isopropyl-4-(4-fluorophenyl)-2-oxo-3-(phenylamino)pyrrolidine-3-carboxylate (36c).
The general procedure, Method A was followed using diisopropyl 2-(cyano(4-fluorophenyl)methyl)-2-(phenylamino)malonate, (35c) (0.206 g, 0.5 mmol) to afford the nitrile adduct as a white colored solid (m.p. 191–193 °C) (0.112 g, 63%). Compound was purified by flash column chromatography (Rf = 0.35 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.16 (AA′ part of AA′BB′X spin system, 2H, H-2′/H-6′), 7.04 (m, 2H, H-3/H-5), 6.85 (BB′ part of AA′BB′X spin system, 2H, H-3′/H-5′), 6.67 (m, 1H, H-4), 6.47 (m, 2H, H-2/H-6), 6.46 (s, br, amide NH), 4.93 (hept, J = 6.3 Hz, 1H, isopropyl methine), 4.71 (s, br, amine NH), 4.53 (d, J = 6.5 Hz, 1H, benzylic H), 4.12 (dd, J = 9.8, 6.6 Hz, 1H, one of the CH2 protons), 3.45 (ddd, J = 9.8, ≈1.7, ≈1.3 Hz, 1H, the other CH2 proton), 1.16 (d, J = 6.3 Hz, 3H, isopropyl methyl), 0.81 (d, J = 6.3 Hz, 3H, other isopropyl methyl). 13C{1H} (126 MHz, CDCl3): δ 171.8 (carbonyl), 169.3 (carbonyl), 161.9 (d, 1JCF = 245.8 Hz, C-4′), 145.1 (C-1), 135.1 (d, 4JCF = 3.4 Hz, C-1′), 129.5 (d, 3JCF = 8.0 Hz, C-2′/C-6′), 128.7 (C-3/C-5), 118.6 (C-4), 115.1 (d, 2JCF = 21.3 Hz, C-3′/C-5′), 114.2 (C-2/C-6), 70.4 (isopropyl methine), 69.7 (d, 6JCF ≈ 0.8 Hz, quaternary aliphatic carbon), 48.2 (s, br, unresolved 5JCF, benzylic CH), 47.9 (CH2), 21.5 [isopropyl methyl (correlating with the methyl 1H signal at δ 1.16 in HSQC spectrum)], 21.0 [other isopropyl methyl (correlating with the methyl 1H signal at δ 0.81 in HSQC spectrum)]. 19F NMR (471 MHz, CDCl3): δ −114.1 (X part of AA′BB′X spin system: tt, 3JHF = 8.6 Hz, 4JHF = 5.3 Hz). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C20H22 FN2O3 357.1609; found 357.1628.
The COSY spectrum (SI, p S408) immediately identifies the two pairs of coupled protons (H-2′/H-6′ and H-3′/H-5′) in the p-fluorophenyl ring and the three sets of coupled protons (H-2/H-6, H-3/H-5, and H-4) in the –NH-phenyl ring. In the cluster of signals centered at δ 6.85 (SI, p S505), the three tallest signals appear to result from splitting the two most prominent peaks of the BB′ part of the AA′BB′ pattern into two doublets (with the central components overlapping) exhibiting the 3JHF = 8.6 Hz separation seen in the 19F spectrum (SI, p S407). The HSQC spectrum (SI, pp. S409 and S410) shows that the 1H signal at δ 6.85 correlates with the 13C doublet centered at δ 115.1. This HSQC correlation for H-3′/C-3′ and H-5′/C-5′ exhibits the expected pair of tilted contours that reflect 3JHF in the 1H dimension and 2JCF in the 13C dimension (SI, p S410). The relatively shielded aromatic carbon adjacent to CF is consistent with the corresponding carbon in 4-fluorotoluene, where C-3/C-5 gives a signal at δ 114.0.49 In the cluster of signals centered at δ 7.16 (SI, p S411), the four tallest signals appear to result from splitting the two most prominent peaks of the AA′ part of the AA′BB′ pattern into two doublets exhibiting the 4JHF = 5.3 Hz separation seen in the 19F spectrum. The HSQC spectrum shows that the 1H signal at δ 7.16 correlates with the 13C doublet centered at δ 129.5. This HSQC correlation for H-2′/C-2′ and H-6′/C-6′ also exhibits the expected pair of tilted contours that reflect the smaller 4JHF in the 1H dimension and the smaller 3JCF in the 13C dimension (SI, p S411). The relative intensities of the 1H signals for the three aromatic multiplets of the –NH-phenyl group, the corresponding 13C chemical shifts, and the HSQC spectrum provided unambiguous assignments for these three types of CH groups. The single-intensity 1H multiplet at δ 6.67 can result only from H-4, while the very shielded aromatic 13C signal at δ 114.2 clearly results from C-2/C-6 in light of 13C chemical shift data for aniline and N-substituted anilines.53 Thus, the HSQC spectrum indicates that C-4 gives the signal at δ 118.6, while H-2/H-6 give the double-intensity multiplet at δ 6.47. By elimination, H-3/H-5 give the double-intensity multiplet at δ 7.04, and C-3/C-5 give the signal at δ 128.7. As expected, ordinary (i.e., untilted) contours are observed for the three HSQC correlations in the phenyl group of –NH-phenyl: at δH 6.47 / δC 114.2 for H-2/C-2 and H-6/C-6, at δH 7.04 / δC 128.7 for H-3/C-3 and H-5/C-5, and at δH 6.67 / δC 118.6 for H-4/C-4 (SI, pp. S409 and S412). The equal J values (9.8 Hz) exhibited by the multiplets at δ 4.12 and δ 3.45 indicated that these signals resulted from the CH2 protons; by elimination, the multiplet at δ 4.53 resulted from the benzylic CH proton. The additional J coupling for the CH2 proton at δ 3.45 (compared to that at δ 4.12) results from coupling with the amide proton, as shown by the COSY spectrum. The NOE observed with mixing times of 0.10, 0.20, and 0.40 s (SI, pp. S413–S415) between the –NH-phenyl H-2/H-6 protons at δ 6.47 and the amine proton at δ 4.71 is reasonable. The NOE observed between the –NH-phenyl H-2/H-6 protons at δ 6.47 and the benzylic proton at δ 4.53 suggests that –NH-phenyl and the benzylic proton have a cis relationship because it would be hard for the phenyl ring to approach the benzylic proton with the other stereochemistry. Similarly, the weak cross peak between the amine proton at δ 4.71 and the p-fluorophenyl H-2′/H-6′ protons at δ 7.16 suggests that the amine proton and p-fluorophenyl group have a trans relationship. With mixing times of 0.10, 0.20, and 0.40 s, the CH2 proton at δ 3.45 exhibits an NOE with the p-fluorophenyl H-2′/H-6′ protons at δ 7.16 but not with the phenyl H-2/H-6 protons at δ 6.47. Thus, the CH2 proton at δ 3.45 appears to be cis to the p-fluorophenyl group and trans to the –NH-phenyl group.
Finally, we note the clearly unequal peak heights for the two components of the 1JCF doublet centered at δ 161.9 in the standard 13C spectrum (SI, p. S405, 5.77s FID processed with just 0.2 Hz of line broadening). In contrast, the peak heights are essentially the same (at this level of S/N) for the long-range JCF doublets. The unequal peak heights for the two components of the 1JCF doublet apparently result from 13C–19F cross-correlated relaxation, as previously shown for the 1JCF splitting in 1,3-difluorobenzene.51
Pictorial representation of NOE correlation in 36c:

Isopropyl-4-(4-chlorophenyl)-2-oxo-3-(phenylamino)pyrrolidine-3-carboxylate (36d).
The general procedure, Method B was followed using diisopropyl 2-((4-chlorophenyl)(cyano)methyl)-2-(phenylamino)malonate, (35d) (0.214 g, 0.5 mmol) and BH3.Me2S (0.380 g, 5 mmol) to afford the nitrile adduct as a white colored solid (m.p. 184–186 °C) (0.069 g, 37%). Compound was purified by flash column chromatography (Rf = 0.35 (40% EtOAc/hexanes). 1H NMR (600 MHz, CDCl3): δ 7.13 (s, 4H, H-2′/H-6′, H-3′/H-5′), 7.05 (m, 2H, H-3/H-5), 6.68 (m, 1H, H-4), 6.47 (m, 2H, H-2/H-6), 6.43 (s, br, amide NH), 4.92 (hept, J = 6.3 Hz, 1H, isopropyl methine), 4.72 (s, br, amine NH), 4.53 (dd, J = 6.5, ≈0.8 Hz, 1H, benzylic H), 4.12 (dd, J = 9.8, 6.6 Hz, 1H, one of the CH2 protons), 3.43 (ddd, J = 9.8, ≈1.7, ≈1.1 Hz, 1H, the other CH2 proton), 1.16 (d, J = 6.3 Hz, 3H, isopropyl methyl), 0.79 (d, J = 6.3 Hz, 3H, other isopropyl methyl). 13C{1H} (126 MHz, CDCl3): δ 171.7 (carbonyl), 169.2 (carbonyl), 145.1 (C-1), 137.9 (C-1′), 133.1 (C-4′), 129.3 (C-2′/C-6′ or C-3′/C-5′), 128.8 (C-3/C-5), 128.4 (C-3′/C-5′ or C-2′/C-6′), 118.7 (C-4), 114.2 (C-2/C-6), 70.4 (isopropyl methine), 69.7 (quaternary aliphatic carbon), 48.3 (benzylic CH), 47.7 (CH2), 21.5 [isopropyl methyl (correlating with methyl 1H signal at δ 1.16 in HSQC spectrum)], 20.9 [other isopropyl methyl (correlating with methyl 1H signal at δ 0.79 in HSQC spectrum)]. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C20H22ClN2O3 373.1313; found 373.1309.
The 1H spectrum indicated that the p-chlorophenyl ring provided a very unusual example of H-2′/H-6′ and H-3′/H-5′ having the same chemical shift (δ 7.13) and thus giving just a singlet that exhibits no correlations in the COSY spectrum (SI, p. S419). Consequently, an HSQC spectrum (SI, p. S420) indicated that two carbons (δ 129.3 and δ 128.4) exhibited correlations at this 1H frequency. The equal J values (9.8 Hz) exhibited by the multiplets at δ 4.12 and δ 3.43 indicated that these signals resulted from the CH2 protons; by elimination, the multiplet at δ 4.53 resulted from the benzylic CH proton. The additional J coupling for the CH2 proton at δ 3.43 (compared to that at δ 4.12) results from coupling with the amide proton, as shown by a COSY spectrum. The relative intensities of the 1H signals for the three aromatic multiplets of the –NH-phenyl group, the corresponding 13C chemical shifts, and the HSQC spectrum provided unambiguous assignments for these three types of CH groups. The single-intensity 1H multiplet at δ 6.68 can result only from H-4, while the very shielded aromatic 13C signal at δ 114.2 clearly results from C-2/C-6 in light of 13C chemical shift data for aniline and N-substituted anilines (method B). Thus, the HSQC spectrum indicates that C-4 gives the signal at δ 118.7, while H-2/H-6 give the double-intensity multiplet at δ 6.47. By elimination, H-3/H-5 give the double-intensity multiplet at δ 7.05, and C-3/C-5 give the signal at δ 128.8. Thus, the NOE observed with mixing times of 0.10, 0.20, and 0.40 s (SI, pp. S421–S423) between the –NH-phenyl H-2/H-6 protons at δ 6.47 and the amine proton at δ 4.72 is reasonable. The NOE observed between the –NH-phenyl H-2/H-6 protons at δ 6.47 and the benzylic proton at δ 4.53 suggests that –NH-phenyl and the benzylic proton have a cis relationship because it would be hard for the phenyl ring to approach the benzylic proton with the other stereochemistry. Similarly, the weak cross peak between the amine proton at δ 4.72 and the p-chlorophenyl protons at δ 7.13 suggests that the amine proton and p-chlorophenyl group have a trans relationship. With mixing times of 0.10, 0.20, and 0.40 s, the CH2 proton at δ 3.43 exhibits an NOE with the p-chlorophenyl protons at δ 7.13 but not with the –NH-phenyl H-2/H-6 protons at δ 6.47. Thus, the CH2 proton at δ 3.43 appears to be cis to the p-chlorophenyl group and trans to the –NH-phenyl group.52
Supplementary Material
ACKNOWLEDGMENT
L.K. gratefully acknowledges the generous financial support of Rice University, the National Institutes of Health (R01 GM-114609-04), the National Science Foundation (CAREER:SusChEM CHE-1546097), the Robert A. Welch Foundation (grant C-1764), Amgen (2014 Young Investigators’ Award for LK) and Biotage (2015 Young Principal Investigator Award) that are greatly appreciated. D.H.E. thanks BYU and the Fulton Supercomputing Lab. X-Ray crystallographic data was collected at the Center for Nano-structured Materials at the University of Texas at Arlington.
Footnotes
The authors declare no competing financial interest.
SUPPORTING INFORMATION
The Supporting information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.joc.9b00681.
X-ray data for compound 27H (CIF)
Complete optimization tables, X-ray crystallography data, computational study details, 1H, 13C, DEPT-135 13C, DEPT-90 13C, COSY, NOE, HSQC, HMBC NMR spectra and HPLC chromatograms (PDF)
REFERENCES
- (1).(a) Kattamuri PV; Yin J; Siriwongsup S; Kwon D-H; Ess DH; Li Q; Li G; Yousufuddin M; Richardson PF; Sutton SC; Kürti L, Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation. J. Am. Chem. Soc 2017, 139, 11184–11196; [DOI] [PubMed] [Google Scholar]; (b) Kattamuri PV; Yin J; Siriwongsup S; Kwon D-H; Ess DH; Li Q; Li G; Yousufuddin M; Richardson PF; Sutton SC; Kürti L, Correction to “Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation”. J. Am. Chem. Soc 2019, 141, 3315–3315. [DOI] [PubMed] [Google Scholar]
- (2).(a) Stevenazzi A; Marchini M; Sandrone G; Vergani B; Lattanzio M, Amino acidic scaffolds bearing unnatural side chains: An old idea generates new and versatile tools for the life sciences. Bioorg. Med. Chem. Lett 2014, 24, 5349–5356; [DOI] [PubMed] [Google Scholar]; (b) Blaskovich MAT, Unusual Amino Acids in Medicinal Chemistry. J. Med. Chem 2016, 59, 10807–10836; [DOI] [PubMed] [Google Scholar]; (c) Saghyan Ashot S. and Langer Peter Eds. Asymmetric Synthesis of Nonproteinogenic Amino Acids. (Wiley-VCH, Weinheim, Germany, 2016); [Google Scholar]; (d) Ni S; Garrido-Castro AF; Merchant RR; de Gruyter JN; Schmitt DC; Mousseau JJ; Gallego GM; Yang S; Collins MR; Qiao JX; Yeung K-S; Langley DR; Poss MA; Scola PM; Qin T; Baran PS, A General Amino Acid Synthesis Enabled by Innate Radical Cross-Coupling. Angew. Chem. Int. Ed 2018, 57, 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Mao B; Fañanás-Mastral M; Feringa BL, Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev 2017, 117, 10502–10566; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Murauski KJR; Jaworski AA; Scheidt KA, A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones. Chem. Soc. Rev 2018, 47, 1773–1782. [DOI] [PubMed] [Google Scholar]
- (4).Burns NZ; Jacobsen EN Mannich Reaction In Science of Synthesis: Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups; Molander G, Ed.; Georg Thieme Verlag: New York, 2010; Chapter 2.16. [Google Scholar]
- (5).Saranya S; Harry NA; Krishnan KK; Anilkumar G, Recent Developments and Perspectives in the Asymmetric Mannich Reaction. Asian J. Org. Chem 2018, 7, 613–633 and references cited therein. [Google Scholar]
- (6).(a) Eftekhari-Sis B; Zirak M, α-Imino Esters in Organic Synthesis: Recent Advances. Chem. Rev 2017, 117, 8326–8419; [DOI] [PubMed] [Google Scholar]; (b) Yu J-S; Zhou J, A highly efficient Mukaiyama–Mannich reaction of N-Boc isatin ketimines and other active cyclic ketimines using difluoroenol silyl ethers catalyzed by Ph3PAuOTf. Organic & Biomolecular Chemistry 2015, 13, 10968–10972. [DOI] [PubMed] [Google Scholar]
- (7).Karimi B; Enders D; Jafari E, Recent Advances in Metal-Catalyzed Asymmetric Mannich Reactions. Synthesis 2013, 45, 2769–2812. [Google Scholar]
- (8).Cai X-H; Xie B, Recent Advances on Organocatalysed Asymmetric Mannich Reactions. Arkivoc 2013, 264–293. [Google Scholar]
- (9).Córdova A, The Direct Catalytic Asymmetric Mannich Reaction. Acc. Chem. Res 2004, 37, 102–112. [DOI] [PubMed] [Google Scholar]
- (10).(a) Juhl K; Gathergood N; Jørgensen KA, Catalytic Asymmetric Direct Mannich Reactions of Carbonyl Compounds with α-Imino Esters. Angew. Chem. Int. Ed 2001, 40, 2995–2997; [DOI] [PubMed] [Google Scholar]; (b) Córdova A; Notz W; Zhong G; Betancort JM; Barbas CF, A Highly Enantioselective Amino Acid-Catalyzed Route to Functionalized α-Amino Acids. J. Am. Chem. Soc 2002, 124, 1842–1843; [DOI] [PubMed] [Google Scholar]; (c) Córdova A; Barbas CF, Direct organocatalytic asymmetric Mannich-type reactions in aqueous media: one-pot Mannich-allylation reactions. Tetrahedron Lett 2003, 44, 1923–1926; [Google Scholar]; (d) Berkeš D; Kolarovič A; Manduch R; Baran P; Považanec F, Crystallization-induced asymmetric transformations (CIAT): stereoconvergent acid-catalyzed lactonization of substituted 2-amino-4-aryl-4-hydroxybutanoic acids. Tetrahedron: Asymmetry 2005, 16, 1927–1934; [Google Scholar]; (e) Taichi K; Sunhwa S; Yasushi K; Keiji M, Highly Diastereo- and Enantioselective Mannich Reactions of Synthetically Flexible Ketimines with Secondary Amine Organocatalysts. Angew. Chem. Int. Ed 2012, 51, 1191–1194. [DOI] [PubMed] [Google Scholar]
- (11).(a) Corey EJ; Bakshi RK; Shibata S, Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc 1987, 109, 5551–5553; [Google Scholar]; (b) Corey EJ; Helal CJ, Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem. Int. Ed 1998, 37, 1986–2012; [DOI] [PubMed] [Google Scholar]; (c) Helal CJ; Meyer MP in Lewis Base Catalysis in Organic Synthesis, 1st ed. (Eds.: Vedejs E; Denmark SE), Wiley-VCH, Weinheim, 2016, Chap 11, 387–456. [Google Scholar]
- (12).(a) Sweeney J, Aziridine Synthesis via Nucleophilic Attack of Carbene Equivalents on Imines: the Aza-Darzens Reaction. Eur. J. Org. Chem 2009, 2009, 4911–4919, and references cited therein; [Google Scholar]; (b) de los Santos JM; Ochoa de Retana AM; Martínez de Marigorta E; Vicario J; Palacios F, Catalytic Asymmetric Darzens and Aza-Darzens Reactions for the Synthesis of Chiral Epoxides and Aziridines. ChemCatChem 2018, 10, 5092–5114; [Google Scholar]; (c) Kattamuri PV; Xiong Y; Pan Y; Li G, N,N-Diisopropyl-N-phosphonyl imines lead to efficient asymmetric synthesis of aziridine-2-carboxylic esters. Org. Biomol. Chem 2013, 11, 3400–3408, and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Kawato Y; Kumagai N; Shibasaki M, Direct catalytic asymmetric addition of acetonitrile to N-thiophosphinoylimines. Chem. Commun 2013, 49, 11227–11229; [DOI] [PubMed] [Google Scholar]; (b) Arseniyadis S; Kyler KS; Watt DS, Addition and Substitution Reactions of Nitrile-Stabilized Carbanions. Org. React 1984, 31, 1–71; [Google Scholar]; (c) Fleming FF; Shook BC Tetrahedron 2002, 58, 1; [Google Scholar]; (d) Verkade JG; Kisanga PB, Recent Applications of Proazaphosphatranes in Organic Synthesis. Aldrichimica Acta 2004, 37, 3–14. [Google Scholar]
- (14).(a) Caruano J; Muccioli GG; Robiette R, Biologically active γ-lactams: synthesis and natural sources. Org. Biomol. Chem 2016, 14, 10134–10156; [DOI] [PubMed] [Google Scholar]; (b) Baldwin JE; Lynch GP; Pitlik J, γ-Lactam Analogues of β-Lactam Antibiotics. J. Antibiotics 1991, 44, 1–24; [DOI] [PubMed] [Google Scholar]; (c) Crossley MJ; Crumbie RL; Fung YM; Potter JJ; Pegler MA, γ-Lactam analogues of monocyclic β-lactam antibiotics. Tetrahedron Lett 1987, 28, 2883–2886. [Google Scholar]
- (15).(a) Bloch R, Additions of Organometallic Reagents to CN Bonds: Reactivity and Selectivity. Chem. Rev 1998, 98, 1407–1438; [DOI] [PubMed] [Google Scholar]; (b) Riant O; Hannedouche J, Asymmetric catalysis for the construction of quaternary carbon centres: nucleophilic addition on ketones and ketimines. Org. Biomol. Chem 2007, 5, 873–888; [DOI] [PubMed] [Google Scholar]; (c) Shibasaki M; Kanai M, Asymmetric Synthesis of Tertiary Alcohols and α-Tertiary Amines via Cu-Catalyzed C−C Bond Formation to Ketones and Ketimines. Chem. Rev 2008, 108, 2853–2873; [DOI] [PubMed] [Google Scholar]; (d) Cogan DA; Ellman JA, Asymmetric Synthesis of α,α-Dibranched Amines by the Trimethylaluminum-Mediated 1,2-Addition of Organolithiums to tert-Butanesulfinyl Ketimines. J. Am. Chem. Soc 1999, 121, 268–269; [Google Scholar]; (e) Clayden J; Donnard M; Lefranc J; Tetlow DJ, Quaternary centres bearing nitrogen ([small alpha]-tertiary amines) as products of molecular rearrangements. Chem. Commun 2011, 47, 4624–4639; [DOI] [PubMed] [Google Scholar]; (f) Zhou F; Liao F-M; Yu J-S; Zhou J, Catalytic Asymmetric Electrophilic Amination Reactions To Form Nitrogen-Bearing Tetrasubstituted Carbon Stereocenters. Synthesis 2014, 46, 2983–3003; [Google Scholar]; (g) Zhou F; Liu Y-L; Zhou J, Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Synth. Catal 2010, 352, 1381–1407; [Google Scholar]; (h) Matsunaga S; Yoshino T, Construction of contiguous tetrasubstituted chiral carbon stereocenters via direct catalytic asymmetric aldol and mannich-type reactions. The Chemical Record 2011, 11, 260–268. [DOI] [PubMed] [Google Scholar]
- (16).(a) Dale JA; Mosher HS, Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and.alpha.-methoxy-.alpha.-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc 1973, 95, 512–519; [Google Scholar]; (b) Seco JM; Quiñoá E; Riguera R, The Assignment of Absolute Configuration by NMR. Chem. Rev 2004, 104, 17–118; [DOI] [PubMed] [Google Scholar]; (c) Hoye TR; Jeffrey CS; Shao F, Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nature Protocols 2007, 2, 2451–2458. [DOI] [PubMed] [Google Scholar]
- (17).(a) Yoda H; Takahashi M; Sengoku T in Heterocycles in Natural Product Synthesis, 1st ed. (Eds.: Majumdar KC; Chattopadhyay SK), Wiley-VCH, Weinheim, 2011, 41–62; [Google Scholar]; (b) Brandi A; Cicchi S; Cordero FM, Novel Syntheses of Azetidines and Azetidinones. Chem. Rev 2008, 108, 3988–4035; [DOI] [PubMed] [Google Scholar]; (c) Antermite D; Degennaro L; Luisi R, Recent advances in the chemistry of metallated azetidines. Org. Biomol. Chem 2017, 15, 34–50; [DOI] [PubMed] [Google Scholar]; (d) Bott TM; West FG, Preparation and Synthetic Applications of Azetidines. Heterocycles 2012, 84, 223–264; [Google Scholar]; (e) Cardillo G; Gentilucci L; Tolomelli A in Asymmetric Synthesis of Nitrogen Heterocycles, Royer J Ed. (Wiley-VCH, Weinheim, Germany, 2009), pp. 3–50. [Google Scholar]
- (18).(a) Katritzky AR; Rachwal S; Rachwal B, Recent progress in the synthesis of 1,2,3,4,-tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070; [Google Scholar]; (b) Nammalwar B; Bunce RA, Recent Syntheses of 1,2,3,4-Tetrahydroquinolines, 2,3-Dihydro-4(1H)-quinolinones and 4(1H)-Quinolinones using Domino Reactions Molecules 2014, 19, 204–232; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sridharan V; Suryavanshi PA; Menéndez JC, Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev 2011, 111, 7157–7259. [DOI] [PubMed] [Google Scholar]
- (19).Rueping M; Theissmann T; Stoeckel M; Antonchick AP, Direct enantioselective access to 4-substituted tetrahydroquinolines by catalytic asymmetric transfer hydrogenation of quinolines. Org. Biomol. Chem 2011, 9, 6844–6850. [DOI] [PubMed] [Google Scholar]
- (20).(a) Han J-Q; Zhang H-H; Xu P-F; Luo Y-C, Lewis Acid and (Hypo)iodite Relay Catalysis Allows a Strategy for the Synthesis of Polysubstituted Azetidines and Tetrahydroquinolines. Org. Lett 2016, 18, 5212–5215; [DOI] [PubMed] [Google Scholar]; (b) Ghosh A; Mandal S; Chattaraj PK; Banerjee P, Ring Expansion of Donor–Acceptor Cyclopropane via Substituent Controlled Selective N-Transfer of Oxaziridine: Synthetic and Mechanistic Insights. Org. Lett 2016, 18, 4940–4943. [DOI] [PubMed] [Google Scholar]
- (21).(a) Janecki T Natural Lactones and Lactams, Synthesis, Occurrence and Biological Activity, Wiley-VCH, Weinheim, 2013; [Google Scholar]; (b) Murphy SK; Dong VM, Enantioselective Ketone Hydroacylation Using Noyori’s Transfer Hydrogenation Catalyst. J. Am. Chem. Soc 2013, 135, 5553–5556 and references cited therein. [DOI] [PubMed] [Google Scholar]
- (22).(a) Wilent J; Petersen KS, Enantioselective Desymmetrization of Diesters. J. Org. Chem 2014, 79, 2303–2307; [DOI] [PubMed] [Google Scholar]; (b) Qabaja G; Wilent JE; Benavides AR; Bullard GE; Petersen KS, Facile Synthesis of Versatile Enantioenriched α-Substituted Hydroxy Esters through a Brønsted Acid Catalyzed Kinetic Resolution. Org. Lett 2013, 15, 1266–1269. [DOI] [PubMed] [Google Scholar]
- (23).(a) Singh GS, Synthetic Aziridines in Medicinal Chemistry: A Mini-Review. Mini Reviews in Medicinal Chemistry 2016, 16, 892–904; [DOI] [PubMed] [Google Scholar]; (b) Degennaro L; Trinchera P; Luisi R, Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev 2014, 114, 7881–7929; [DOI] [PubMed] [Google Scholar]; (c) Bergmeier SC; Lapinsky DJ Progress in Heterocyclic Chemistry (Eds.:Gribble GW, Joule JA), Elsevier, Oxford, 2009; [Google Scholar]; (d) McMills MC; Bergmeier SC Comprehensive Heterocyclic Chemistry III (Ed.: Padwa A), Pergamon, Oxford, 2008; [Google Scholar]; (e) Ismail FMD; Levitsky DO; Dembitsky VM, Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem 2009, 44, 3373–3387; [DOI] [PubMed] [Google Scholar]; (f) Pellissier H, Recent developments in asymmetric aziridination. Tetrahedron 2010, 66, 1509–1555. [Google Scholar]
- (24).(a) Huisgen R; Scheer W; Mäder H, Azomethine Ylides from Aziridinedicarboxylic Esters: Kinetics of the cis-trans Isomerization and of the Ring Opening. Angew. Chem. Int. Ed 1969, 8, 602–604; [Google Scholar]; (b) Vaultier; Robert Carrié M, Comportement d’une aziridime substituee par deux groupements esters gemines en presence de perchlorate de lithium; etude physicochimique du complexe obtenu. Tetrahedron Lett 1978, 19, 1195–1198; [Google Scholar]; (c) Pohlhaus PD; Bowman RK; Johnson JS, Lewis Acid-Promoted Carbon−Carbon Bond Cleavage of Aziridines: Divergent Cycloaddition Pathways of the Derived Ylides. J. Am. Chem. Soc 2004, 126, 2294–2295; [DOI] [PubMed] [Google Scholar]; (d) Li L; Zhang J, Lewis Acid-catalyzed [3 + 2]Cyclo-addition of Alkynes with N-Tosyl-aziridines via Carbon–Carbon Bond Cleavage: Synthesis of Highly Substituted 3-Pyrrolines. Org. Lett 2011, 13, 5940–5943; [DOI] [PubMed] [Google Scholar]; (e) Paasche A; Arnone M; Fink RF; Schirmeister T; Engels B, Origin of the Reactivity Differences of Substituted Aziridines: CN vs CC Bond Breakages. J. Org. Chem 2009, 74, 5244–5249; [DOI] [PubMed] [Google Scholar]; (f) Liao Y; Liu X; Zhang Y; Xu Y; Xia Y; Lin L; Feng X, Asymmetric [3 + 2] cycloaddition of donor–acceptor aziridines with aldehydes via carbon–carbon bond cleavage. Chem. Sci 2016, 7, 3775–3779; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Wu X; Zhang J, Y(OTf)3-Catalyzed Diastereoselective [3+2] Cycloaddition of N-Tosylaziridines and Imines; Efficient Synthesis of Multisubstituted Imidazolidines. Synthesis 2012, 44, 2147–2154; [Google Scholar]; (h) Jiang Z; Wang J; Lu P; Wang Y, Diasteroselective synthesis of oxazolidines and imidazolidines via the Lewis acid catalyzed C–C cleavage of aziridines. Tetrahedron 2011, 67, 9609–9617; [Google Scholar]; (i) Li L; Wu X; Zhang J, Lewis acid-catalyzed formal [3+2] cycloadditions of N-tosyl aziridines with electron-rich alkenesvia selective carbon–carbon bond cleavage. Chem. Commun 2011, 47, 5049–5051; [DOI] [PubMed] [Google Scholar]; (j) Wu X; Li L; Zhang J, Nickel(ii)-catalyzed diastereoselective [3+2] cycloaddition of N-tosyl-aziridines and aldehydesvia selective carbon–carbon bond cleavage. Chem. Commun 2011, 47, 7824–7826. [DOI] [PubMed] [Google Scholar]
- (25).Wu X; Li L; Zhang J, Direct Aza-Darzens Aziridination of N-Tosylimines with 2-Bromomalonates for the Synthesis of Highly Functionalized Donor-Acceptor Aziridines. Adv. Synth. Catal 2012, 354, 3485–3489 and references cited therein. [Google Scholar]
- (26).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AF; Bloino J; Zheng G; Sonnenberg JL; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Montgomery JA; Peralta JE; Ogliaro F; Bearpark M; Heyd JJ; Brothers E; Kudin KN; Staroverov VN; Kobayashi R; Normand J; Raghavachari K; Rendell A; Burant JC; Iyengar SS; Tomasi J; Cossi M; Rega N; Millam JM; Klene M; Knox JE; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Martin RL; Morokuma K; Zakrzewski VG; Voth GA; Salvador P; Dannenberg JJ; Dapprich S; Daniels AD; Farkas; Foresman JB; Ortiz JV; Cioslowski J; Fox DJ, Gaussian 09, Revision B.01. Wallingford CT, 2009. [Google Scholar]
- (27).(a) Zhao Y; Truhlar DG, A New Local Density Functional for Main-Group Thermochemistry, Transition Metal Bonding, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Phys 2006, 125, 194101–1–194101–18. [DOI] [PubMed] [Google Scholar]; b) Zhao Y; Truhlar D, The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalents Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 other functionals. Theor. Chem. Acc 2008, 120, 215–241. [Google Scholar]; c) Zhao Y; Truhlar DG, Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res 2008, 41, 157–167. [DOI] [PubMed] [Google Scholar]
- (28).Def2-TZVP basis sets were downloaded from https://bse.pnl.gov/bse/portal (access date: 06/01/2018).; b) Weigend F; Ahlrichs R Phys. Chem. Chem. Phys 2005, 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- (29).Marenich AV; Cramer CJ; Truhlar DG, Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [DOI] [PubMed] [Google Scholar]
- (30).Balaban IE; Pyman FL, CIX.—Bromo-derivatives of glyoxaline. Journal of the Chemical Society, Transactions 1922, 121, 947–958. [Google Scholar]
- (31).Zhao Z; Liu H. w., Synthesis of a Deoxysugar Dinucleotide Containing an exo-Difluoromethylene Moiety As a Mechanistic Probe for Studying Enzymes Involved in Unusual Sugar Biosynthesis. J. Org. Chem 2001, 66, 6810–6815. [DOI] [PubMed] [Google Scholar]
- (32).Jacobsen NE NMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural Biology; John Wiley & Sons: Hoboken, New Jersey, 2007; p. 436. [Google Scholar]
- (33).Andose JD; Lehn J-M; Mislow K; Wagner J, Effect of substituents on the rate of pyramidal inversion of 1-aryl-2,2-dimethylaziridines. J. Am. Chem. Soc 1970, 92, 4050–4056. [Google Scholar]
- (34).Bottini AT; Roberts JD, Nuclear Magnetic Resonance Spectra. Nitrogen Inversion Rates of N-Substituted Aziridines (Ethylenimines)1. J. Am. Chem. Soc 1958, 80, 5203–5208. [Google Scholar]
- (35).Anet FAL; Osyany JM, Nuclear Magnetic Resonance Spectra and Nitrogen Inversion in 1-Acylaziridines. J. Am. Chem. Soc 1967, 89, 352–356. [Google Scholar]
- (36).García-Lopez V; Alemany LB; Chiang P-T; Sun J; Chu P-L; Martí AA; Tour JM, Synthesis of light-driven motorized nanocars for linear trajectories and their detailed NMR structural determination. Tetrahedron 2017, 73, 4864–4873. [Google Scholar]
- (37).Jayalakshmi KL; Gowda BT, Synthetic, Infrared and NMR (1H and 13C) Spectral Studies of N-(Substituted Phenyl)-Methanesulphonamides Z. Naturforsch 2004, 59a, 491–500. [Google Scholar]
- (38).Ströhl D; Thomas S; Kleinpeter E; Radeglia R; Brunn J, Substituent effects in 13C-NMR chemical shifts of polysubstituted benzene and naphthalene compounds: An incremental calculation Monatshefte für Chemie 1992, 123, 769–777. [Google Scholar]
- (39).Xiao Z; Yuan M; Zhang S; Wu J; Qi S; Li Q, Complete assignments of 1H and 13C NMR data for ten phenylpiperazine derivatives. Magn. Reson. Chem 2005, 43, 869–872. [DOI] [PubMed] [Google Scholar]
- (40).Morales-Rios MS; García-Martínez C; Joseph-Nathan P; Zepeda LG, 35Cl/37Cl one-bond isotope effects on 13C chemical shifts in a series of para-substituted chlorobenzenes. Magn. Reson. Chem 1995, 33, 149–151. [Google Scholar]
- (41).Matsubara K, Unambiguous signal assignments of 1H and 13C NMR spectra for chlorinated benzenes using 37Cl/35Cl isotope effects. Magn. Reson. Chem 2001, 39, 633–636. [Google Scholar]
- (42).Drakenberg T; Lehn JM, Nuclear magnetic resonance studies of rate processes and conformations. Part XX. Nitrogen inversion in the gas phase. J. Chem. Soc., Perkin Trans 2 1972, 532–535. [Google Scholar]
- (43).Anet FAL; Osyany JM, Nuclear Magnetic Resonance Spectra and Nitrogen Inversion in 1-Acylaziridines. J. Am. Chem. Soc 1967, 89, 352–356. [Google Scholar]
- (44).Alemany LB, Exceptional resolution and new signals detected in the 13C NMR spectra of alkanes. Magn. Reson. Chem 1989, 27, 1065–1073. [Google Scholar]
- (45).Åhman J; Jarevång T; Somfai P, Synthesis and Aza-[2,3]-Wittig Rearrangements of Vinylaziridines: Scope and Limitations. J. Org. Chem 1996, 61, 8148–8159. [DOI] [PubMed] [Google Scholar]
- (46).De Vitis L; Florio S; Granito C; Ronzini L; Troisi L; Capriati V; Luisi R; Pilati T, Stereoselective synthesis of heterosubstituted aziridines and their functionalization. Tetrahedron 2004, 60, 1175–1182. [Google Scholar]
- (47).Capriati V; Florio S; Luisi R; Musio B, Directed Ortho Lithiation of N-Alkylphenylaziridines. Org. Lett 2005, 7, 3749–3752. [DOI] [PubMed] [Google Scholar]
- (48).Schwalbe H; Samstag W; Engels JW; Bermel W; Griesinger C, Determination of 3J(C,P) and 3J(H,P) coupling constants in nucleotide oligomers with FIDS-HSQC. J. Biomol. NMR 1993, 3, 479–486. [Google Scholar]
- (49).Kalinowski H-O; Berger S; Braun S Carbon-13 NMR Spectroscopy; John Wiley & Sons: Chichester, 1988, p 326. [Google Scholar]
- (50).Stothers JB Carbon-13 NMR Spectroscopy; Academic Press: New York, 1972, p 197. [Google Scholar]
- (51).Alemany LB; Malloy TB Jr.; Nunes MM; Zaibaq NG, Importance of cross-correlated relaxation in the spectra of simple organofluorine compounds: Spectral complexity of A3B3X spin systems compared to ABX spin systems. J. Mol. Struct 2012, 1023, 176–188. [Google Scholar]
- (52).Corio PL Structure of High-Resolution NMR Spectra; Academic Press: New York, 1966, p 382. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.