Abstract
Purpose of review:
The purpose of this review is to describe the mechanobiological mechanisms of tendon repair as well as outline current and emerging tools in mechanobiology that might be useful for improving tendon healing and regeneration. Over 30 million musculoskeletal injuries are reported in the US per year and nearly 50% involve soft tissue injuries to tendons and ligaments. Yet current therapeutic strategies for treating tendon injuries are not always successful in regenerating and returning function of the healing tendon.
Recent findings:
The use of rehabilitative strategies to control the motion and transmission of mechanical loads to repairing tendons following surgical reattachment is beneficial for some, but not all, tendon repairs. Scaffolds that are designed to recapitulate properties of developing tissues show potential to guide the mechanical and biological healing of tendon following rupture. The incorporation of biomaterials to control alignment and reintegration, as well as promote scar-less healing, are also promising. Improving our understanding of damage thresholds for resident cells and how these cells respond to bioelectrical cues may offer promising steps forward in the field of tendon regeneration.
Summary:
The field of orthopaedics continues to advance and improve with the development of regenerative approaches for musculoskeletal injuries, especially for tendon, and deeper exploration in this area will lead to improved clinical outcomes.
Keywords: Tendon, stem cells, muscle, scaffolds, immobilization, rehabilitation
Introduction
Tendon Pathology
Tendons are highly susceptible to injury and are difficult to treat. Of the 33 million musculoskeletal injuries reported in the US per year, approximately 50% involve soft tissue injuries, such as tendon injury (1). A variety of conditions are associated with tendon injuries, from acute tendon tear to tendinopathies to full-width tendon lacerations. Tendonitis, characterized by inflammation or irritation of tendon, is initiated by mechanical overuse and can result in pain, tenderness, tendon damage, and collagen breakdown. Because of its hierarchical structure, tendon inflammation can occur in the tissue surrounding the tendon sheath (paratenon) or inside the tendon (intratenon)(2). Chronic deterioration of tendon, known as tendinosis, results from chronic overuse and is primarily responsible for decreased strength and flexibility, as well as pain(3). Tendinosis results from accumulated damage initiated by structural and mechanical overuse and is most often seen in athletic, male runners between 35-45 years of age(4). In patients with chronic Achilles pain, ~70% have tendinosis(5). The recommended time for return-to-sport is 3-6 months and dependent on rehabilitative strategies, magnitude of tendon injury and pain, and level of sport performance(4). Achilles tendon rupture and rotator cuff tears are the most common and detrimental tendon injuries (6,7). The primary focus of this review is to improve tendon healing following rupture, which is the most common tendon injury that often require surgical repair.
Increased age and participation in sports are the most common causes of tendon injuries (8). Sports with abrupt changes in speed and footwork are at risk for Achilles tendon rupture. Although surgical approaches have improved in recent years and can lead to improvements in pain management (9), there remains a low return-to-sport rate following tendon repair. Achilles injuries have the lowest return-to-play as well as the worst post-operative performance compared to other tendon repairs following injury (10). For example, studies of National Basketball Association players experiencing tendon injuries showed, on average, that return-to-sport rates were upwards of 11 months (11). Tendon injuries also influence the level of competition that athletes return to following injury. For example, athletes that experience rotator cuff tears do not typically return to the same level as they were prior to their injury (12). It remains unknown what the exact type or magnitude of loading leads to tendon ruptures, and the physiological history of the tendon also predisposes tendon to damage and injury. In this review, we examine the role that mechanical loading plays in susceptibility for tendon injuries, such as ruptures, and highlight current approaches for improving tendon healing. In addition, we discuss emerging approaches for understanding tissue damage and remodeling from adjacent fields that could be translated to the tendon field in the future.
Mechanical Loading and Overuse Leads to Tendon Injury
Tendons are critical for transmitting contractile forces from muscle to bone in order to move joints as well as provide postural stability. The structure of tendon is a hierarchical cord-like network of aligned collagen fibers that provides a flexible and inextensible connection for load transmission, joint mobility, and joint stability. During growth, tendons are highly adaptable to changes in the magnitude and direction of applied mechanical loads(13,14). This adaptive response to applied mechanical load results in tissue-scale adaptations such as increased cross-sectional area, inclusion of compressive-resistant inclusions (fibrocartilage), and tendon length. This can also lead to changes in cellular morphology, density, and behavior, as well as extracellular matrix composition. The developing tendon is highly cellular and actively remodels to meet the applied mechanical demands. During skeletal growth, the tendon lengthens in parallel with bone length and rapidly remodels to accommodate forces from skeletal muscle. However, once the skeleton stops growing, so too does the tendon, and its ability to continuously renew dramatically slows down. This was elegantly illustrated in work by Heinemeier and colleagues with C14 bomb-pulse experiments of human tendons(15). In this study, forensic tendon samples from cadavers were analyzed from humans of varying ages that were alive between 1955-1963, when levels of C14 were elevated because of nuclear bomb tests. Tendons from human cadavers that were alive during atomic bomb testing were analyzed for radioactive C14, and the levels of C14 in tendon was correlated with environmental C14 levels. The tendons from humans that were <17 years of age during this time had low levels of C14, suggesting that the resident tendon cells were able to turnover the C14-labeled tissue at young ages, whereas tendons from older human cadavers had elevated C14 levels, indicating that tendon cores did not renew after skeletal growth commenced(15). Although mature tendons are also capable of adapting and remodeling, this adaptive response is damped because the dense connective tissue of tendon carries much of the mechanical load, shielding the embedded stromal cells, such as tendon fibroblasts, tendon-derived stem cells (TDSCs), and resident inflammatory cells to such loads. The stress-shielding behavior of matrix-dense tendon may influence the longevity of its resident cell populations. In fact, bomb-pulse studies have recently shown that adult tendon does not undergo renewal, which may influence the inherent regenerative capacity of tendon following injury(15).
Changes in the mechanical environment, such as overuse and overloading, are common causes of tendinopathies in mature and aging tendons. Following overuse, the mechanical properties of the tendons deteriorate, as collagen microtears, inflammation, and damage accumulates and are not sufficiently repaired (16,17). Adult tendon is unable to regenerate its native structure following injury or damage accumulation, and following injury, the mechanical properties of healed tendon are nearly an order of magnitude lower than the native, healthy tendon (18). Loading not only alters the structure and function of tendon, it also changes the cellular phenotypes and biological response. Increased mechanical loading leads to increased strain and tissue/microscale damage to the structure and organization of tendon. Tendon overloading leads to reduced microscale collagen alignment as well as nuclear disorganization (19). Overuse also results in an increased density of mast cells, which induce a focal inflammatory response and release of prostaglandins and inflammatory cytokines (20). Overuse leads to increased expression of mechanically induced growth factors as well as proliferation of TDSCs(21). Understanding how mechanical load plays a role in tendon injury and repair can allow scientists and clinicians to develop rehabilitative approaches in order to heal injured tendons.
There is a need to address tendon healing, including research to understand healing as well as nonsurgical methods to heal tendinopathies. Cells and tissues sense and respond to mechanical and chemical cues from their surrounding extracellular matrix environment, and innovative tools in mechanobiology have emerged as potential therapeutics for treating tendon injuries. This review will highlight current clinical interventions as well as explore potential therapies that could be applied to tendons, including innovative tools that build and mechanically load tissues, guide cellular behavior, and ultimately aim to regenerate the injured tendon.
Current strategies to improve tendon repair
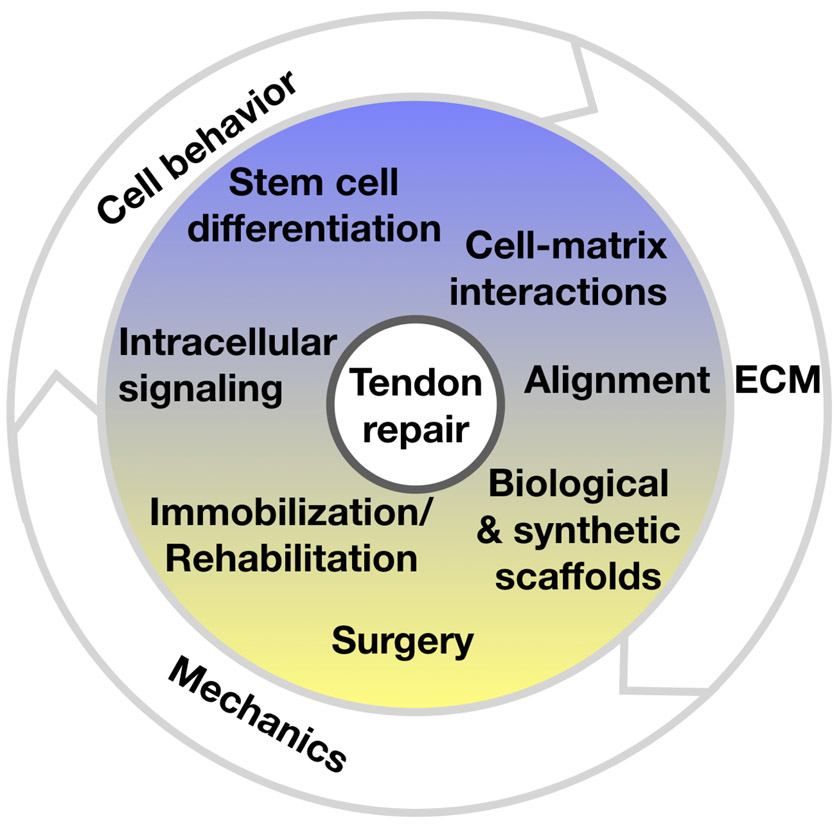
Tendon heals by forming scar tissue, and many of the current approaches for restoring tendon function following injury aim to intervene in the healing process of tendon (1). Nonsurgical treatment of tendon injury has varied success rates and depend on the patient and their expectations of the level of functional return that they hope to achieve. Current strategies to improve tendon repair focus on controlling the mechanical environment, extracellular matrix, and cellular behavior of stromal and stem cells (Figure 1).
Figure legend:
Tendon repair is mediated by the mechanical environment, native and extrinsic cell behavior, and the structural and material properties of the extracellular matrix (ECM).
Controlled mechanical loading through immobilization
Immobilization is a common strategy used in most treatment protocols for tendinopathy. However, its efficacy in tendon healing is debated. A framework has been prescribed by the American Shoulder and Elbow Therapists for a rehabilitation strategy of a short-term (2-week) strict immobilization period following arthroscopic rotator cuff repair (22). Following immobilization, controlled loading is often beneficial for promoting healing and reintegration of tendon back to its bony footprint (23). Yet for chronic rotator cuff tears, there remains no clear advantage or disadvantage for implementing passive mobility as a therapy regime following rotator cuff tendon repair (24). Early mobility (at ~8wks post repair in humans) following immobilization with a cast or boot is typically considered beneficial following Achilles tendon rupture regardless of the tendon underwent surgically repaired(25) as it can reduce complication rates and improve return-to-function(26). The prescribed time of immobilization varies across treatment protocols and the type of tendon injury. In addition, immobilization may only be beneficial until a certain point, after which its prolonged effects can be detrimental. In animal models of tendon rupture, prolonged immobilization can limit return of function and lead to impaired biomechanical properties similar to that of native tendon following rupture(27,28). Thus, as a strategy to improve tendon repair, long-term immobilization may not be preferred. The healing process following tendon injury can be delayed with immobilization, as nerve regeneration, blood circulation, and tissue regeneration surrounding the injury site are inhibited (27). In basic science studies that use external structures or wire framing to immobilize the rat Achilles tendon, the healing tendon repairs with inferior tensile strength, reduced failure load, and decreased stiffness(29,30). Immobilization using casting had similar outcomes, as well as increased collagen degradation and decreased collagen mass in the injured tendon/ligament (31). Additionally, rigid immobilization may lead to atrophy of the surrounding muscle, posing a risk for potential re-rupture (31).
The prescribed implementation and duration of immobilization depends on injury and tendon type. A short bout of immobilization following injury can limit gap formation and improve reintegration between injured tendon stumps (32). The effects of tendon immobilization on healing also has varying, age-depending effects. For example, following flexor tendon rupture, immobilization and early remobilization of neonatal tendons did not have differential impacts on range of motion, re-rupture, or risk of adhesion, which differs from adult tendons that benefit from early mobilization (33). Thus, depending on the tendon type, immobilization may be detrimental to the healing process, leading to detrimental changes in the structural integrity of the surrounding muscle and bone.
Surgical repair and reattachment of tendons post-injury
Surgical treatment of tendon injuries is dependent on the age and health of the patient and the type of tendon tear and involves both surgical approach and post-operative rehabilitation (34). For young adults, surgical repair remains the main treatment plan given a limited success with non-surgical intervention without rehabilitation (35). Surgical approach is largely more successful than a non-surgical approach alone. Nonsurgical treatment of acute Achilles tendon ruptures, followed by functional rehabilitation, maintained an equivalent rate of re-rupture with surgical treatment (35). Surgical repair can also reduce the rate of return to work (34). However, these approaches are not without its limitations and complications. Surgical interventions increase the risk of complications and the risk of infection, nerve injury, and deep vein thrombosis are significantly increased after operation (36). Surgical repair procedures, in addition to increased risk of complication, may also not be the logical choice depending on the type of injury, especially not for large and massive tears. For example, success rates, classified by ultrasound or magnetic resonance imaging, of outcomes following rotator cuff repair differ depending on the tear size. Small to medium (1-3 cm) tear repairs typically have high success rates, while the success rates of large tear (3-5 cm) and massive tear (2 or more tendons) repairs are substantially lower (37). Depending on the size of the tear, variability in repair approach and outcomes can vary. A need exists for post-operative or non-surgical early intervention (38) and protecting the repaired tendon following repair.
Potential tools to guide mechanobiology during tendon repair
The transfer of loads from tissues to cellular and intracellular compartments can dramatically influence how cells respond and adapt to physical cues. Cells convert physical cues into biochemical signals, changes in gene expression, and altered interactions with neighboring cells. In addition, physical deformation of intracellular organelles, such as nuclear compression, can have a significant influence on gene transcription by changing the physical positioning or stretching of genes as well as altering the transport of transcription factors across the nuclear envelope (39-41). Tissue remodeling in response to applied mechanical loads also relies on plasma membrane integrity and transport. Fibroblasts, which are phenotypically similar to resident tendon cells, transport collagen fibrils across the plasma membrane using non-myosin II-powered transport (42) and cell surface-directed steps of collagen fibrillogenesis (43,44).
Stem cell populations that guide extracellular matrix (ECM) composition and scar remodeling
Tendon stem/progenitor cells (TSPCs) are responsible for maintaining tendon cell populations throughout life and replenishing the tissue-resident cell pool following injury. TSPCs have universal stem cell characteristics with capacity to form clonal populations, undergo multipotent differentiation, and self-renew(45). This stem/progenitor pool is organized by its surrounding extracellular matrix (ECM), and the fate of these cells depends on cues from the ECM niche, such as biglycan and fibromodulin(45). Recently, unique markers of stem/progenitor cells in tendon that contribute to regeneration have been identified using sophisticated approaches, including single-cell RNAsequencing and lineage tracing approaches(46). Stem cell-mediated regeneration is dependent on cells that express both tubulin polymerization-promoting protein family member 3 (Tppp3+) and platelet-derived growth factor receptor alpha (Pdgfra+), and cells that are Tppp3− contribute to injury-associated fibrosis(46). Previously, cell-surface markers of TSPCs included: Scal+ (marker of stem cells), Cd90+ and Cd44+(markers of fibroblasts), Cd18− (marker of leukocyte), Cd34− (marker of vascular/hematopoietic cells), Cd106− (marker of endothelial cells), and Cd133− (marker of perivascular cells) (47,48). Historically, TDSCs enhance tendon healing following injury in small animal models, but the role that these cells play in scar formation and remodeling following injury still remains unknown. Conversely, SI00 calcium-binding protein a4 (S100a4+) has emerged as a potential marker for a large proportion of tendon resident cells that contribute to scar formation during healing(49,50). The discrete populations of cells that are differentially marked by Scx and S100a4 suggests that there likely exists a discrete population of cells that may contribute to either regenerative or scar-mediated healing of tendon following injury(50).
Recapitulating the mechanobiology of development for stem cell differentiation
Mechanical load is necessary for proper development of the musculoskeletal system (51-53). Tendon development is biphasic: muscle must first anchor to tendon and then the attached muscle can apply mechanical force for tendon elongation (54). Myogenic signals from the developing skeletal muscle also promote tendon development (55) and coordinate the formation of preliminary matrix of fibronectin, laminin, and decorin from tendon progenitor cells (56,57). Both Scleraxis (Scx) and Mohawk (Mkx) promote collagen production, the major component of the tendon ECM, and these transcription factors also promote differentiation of stem-like cells to the tendon progenitor cell fate (58-64). Tendon ECM is rich in structural proteins that become crosslinked into a lattice to surround fibrils (65) and fibril-associated collagens with interrupted triple helices (FACIT) stabilize the ECM and its interaction with cellular integrins (66). Proteoglycan bridges composed of decorin, fibromodulin, and biglycan are integral for transmitting and resisting tensile stress and collagen maintenance in tendon (59,65,67,68).
Dynamic changes in ECM stiffness and alignment during tendon development influences the behavior and mode of cell proliferation, recruitment, and migration, the latter of which may be mediated by actomyosin contractility (69). Tendon elongation depends on the recruitment and migration of mesenchymal progenitor cells via Scx(70). Differentiation of mesenchymal progenitors is mediated by transforming growth factor (TGF)-β and fibroblast growth factor signaling(71-73). Tendon elongation occurs in parallel with skeletal growth once skeletal muscle is anchored to tendon (13,74). Applied mechanical loading from contractile muscle leads to reorganization of collagen fibrils into parallel and hierarchical fiber structures (65,67). Under strain, collagen fibril size and volume fraction increase in developing tendons in accordance with Wolff’s Law to generate tendon of higher tissue strength and stiffness (75-77). Increased size of collagen fibers during modeling requires remodeling of nascent tissues through matrix metalloprotease (MMP) that unwind and break collagen fibrils (78-80). Mature collagen fibrils harbor functional integrin-binding sites called latency-associated peptides (LAP) structures (81) that also release TGFβ (82). TGFβ activates Smad2/3 signaling pathways to promote the additional release of TGFβ from the ECM, and this release decreases with increasing magnitudes of mechanical load (83-85). Larger, highly organized collagen fibrils provide tendon with a capacity to handle increased tensile loads (86). Collagen remodeling relies on active matrix metalloproteinase (MMP) activity (87,88), and a balance between collagen matrix production and breakdown is important during the healing process. The dynamic change in matrix stiffness during development can guide stem-like cells toward a fibroblastic cell fate (89). Collagen synthesis in biological and synthetic ECM, such as scaffolds, can be fine-tuned with localized growth factor signaling, such as platelet derived growth factor (PDGF) receptor or PDGF-BB(90,91) and insulin-like growth factor (IGF-1) (90,92).
Biomimetic scaffolds for guided tendon repair
The use of scaffolds to bridge tendon defects, guide remodeling, and accelerate healing has been a promising approach for preclinical studies but has demonstrated mixed results for improving patient outcomes when translated to the clinic. In fact, there are few long-term studies that have supported the use of various scaffolds for tendon repair. Nonetheless, scaffolds offer a mechanical advantage over more simple reattachment repair approaches, especially for rotator cuff repair (93-95). In addition, over 50,000 patches derived from extracellular matrix derivatives (e.g., dermis, amniotic membrane) are used in the clinic each year to repair soft tissue injuries (37). However, although some biologically (ECM)-derived scaffolds enhance cell attachment and new tissue formation, these benefits come at the cost of mechanical integrity and durability. A balance is needed in the design of biomimetic scaffolds to not only provide mechanical strength and durability but also to promote stem cell differentiation, stromal migration, and limit scarring and immunogenic responses.
The most common sources of naturally-derived ECM used for tendon-mimetic scaffolds include small intestine submucosa, amniotic membrane(96), collagen(97), gelatin (denatured collagen)(98), glycosaminoglycans(97), silk(99), hyaluronic acid, and fibrin (described in more detail in a recent review by Freedman and Mooney(100)). Additionally, synthetic ECM has also been used for developing scaffolds for tendon repair, including poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(ethylene glycol) (PEG)(100), poly(ε-caprolactone) (PCL)(101), and combinations of these (e.g., poly(1-lactide-co-ε-caprolactone) (PLCL)(102)), as well as polyacrylamide(100). The selection of ECM-derived and synthetic materials for use in tendon repair depends on the wide range of mechanical properties both in tension and compression of these materials. Additionally, each material possesses different microscale interactions of cells with ECM, and these properties can be exploited depending on the cell type, density of ECM and cells, and processing used for manufacturing the scaffolds. Rigorous in vitro and preclinical testing of the ECM scaffolds are essential for each iteration of scaffold design.
Cell adhesion, migration, and proliferation are critical for regenerating and revitalizing tissue replacements, and these processes are mediated by cell-cell and cell-matrix interactions. These cellular behaviors are also dependent on the mechanical and biological properties of two- and three-dimensional substrates upon which the cells reside. Cells sense mechanical loads from their surrounding ECM via integrins (83,84). Two- and three-dimensional microenvironments, both from synthetic and biologically derived sources, have been used to identify and exploit the cell-cell and cell-matrix interactions. For example, integrin binding sites (e.g., RGD) are commonly used in synthetic materials, such as hydrogels, to promote cell adhesion and invasion in otherwise biologically-inert substrates (103). Cell morphology and differentiation depends on these binding sites, as well as matrix compliance (103,104). Factors that should be considered when using stem cells in conjunction with biomimetic scaffolds include stem cell priming (i.e., potential benefits and costs of pre-implantation differentiation towards a tenogenic cell fate) and temporal exposure to binding sites that promote proliferation, differentiation, and ECM production.
The development of custom-built bioreactors has helped to define how various parameters of dynamic stretch (e.g., amplitude, frequency, and duration) influence cellular behaviors and phenotypes of tendon fibroblasts and stem cells in vitro (105-108). Various approaches have been used to build tendon mimics, including directed self-assembly of dermal fibroblasts (106) or primary tendon fibroblasts (109) or contraction of fibrin gels(110).
Bioelectricity
Aside from clinical diagnosis of tendinosis, tendon damage is not well described. However, the ability of tendon-resident cells to sense and respond to mechanical forces, including the cell-scale response to tendon overuse, is a potential target for abrogating the accumulation of damage and improving healing outcomes. A major process of mechanobiology across all tissues, including tendon, relies on membrane permeability to ions and early intracellular communication (111). Typically, neurons and myocytes are considered “activatable” cells as they respond to suprathreshold changes in the transport of ions across the membrane via action potentials. However, other cells types, including those found in musculoskeletal tissues, also respond to changes in membrane potential by changes in gene transcription, receptor/protein phosphorylation, and changes in mitochondrial function. Ion transport across the plasma membrane is controlled in part via plasma membrane integrity, and has been identified in vivo as an important process for bone cell (e.g., osteocyte) mechanosensation (112). Plasma membrane disruptions can regulate expression of early response proteins, such as c-fos, in various cell types, supporting the “damage sensor” hypothesis that can explain how cells initiate adaptative responses to injurious but not lethal mechanical stress(112,113). This hypothesis poses that transient plasma membrane stretch that induces small breaks or tears in the plasma membrane can induce a mechanosensitive cascade of cell signaling if the number or magnitude of tears are large enough. While some plasma disruptions may be beneficial for driving adaptation in response to mechanical load, a threshold likely exists that, when exceeded, results in cell death if the plasma membrane disruption is not repaired. This damage threshold reduces in disease and in aging, which is postulated to increase risk of unrecoverable injury in diseased or aged tissues, such as muscle and bone. Potential therapeutics for maintaining or repairing plasma membrane integrity include antioxidant treatment (e.g., Vitamin E(114) or Vitamin C(112)), fetuin A treatment(115), or surfactant poloxamers (such as P-188)( 116,117), and these treatments show promise in maintaining cell viability for muscle, bone, or cartilage following tissue- or cell-scale damage. Although the damage sensing mechanisms in osteocytes and skeletal myocytes have been translated and validated in vivo, it has yet to be demonstrated as a model of mechanobiology in tendon.
In addition to membrane integrity, voltage-gated transport of ions across the membrane (e.g., transient receptor potential cation channel subfamily V member 4, TRPV4; voltage-operated calcium channel, VOCC) may also be controlled via mechanical stress and/or extracellular stiffness (118,119). In non-musculoskeletal cells (e.g., cardiomyocytes), fibroblast growth factor homologs modulate the trafficking of calcium and sodium ion channels to the plasma membrane, which suggests a coordinated role maintaining homeostasis in activatable (and perhaps previously considered “non-activatable”) cells (120).
Conclusions
The progress towards improving tendon healing with translatable therapeutics is promising, and control of cellular and extracellular processes may provide useful and effective treatments for improving repair outcomes. Engineered materials from natural or synthetic sources may be useful for guiding cellular remodeling and improving biomechanical reintegration of ruptured tendon. The incorporation of biological cues that mimic development may be useful for guiding differentiation of stem cells towards a tenogenic fate and accelerate tendon healing. The control of biological processes (e.g., bioelectricity) could potentially be leveraged to encourage tendon regeneration driven by tendon-resident stromal cells. The field of orthopaedics continues to advance and improve with the development of regenerative approaches for musculoskeletal injuries, especially for tendon, and deeper exploration in this area will lead to improved clinical outcomes.
Acknowledgments:
Research was supported by the University of Delaware Research Foundation; the Delaware Space Grant Consortium (NNX15AI19H), the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R03HD094594 and K12HD073945 and the National Institute for General Medical Sciences under Award Number P30GM103333.
Footnotes
Conflict of Interest: Dr. Killian, Ms. Sullivan, Ms. Soulas and Mr. Leek have nothing to disclose.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.James R, Kesturu G, Balian G, Chhabra AB. Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. The Journal of Hand Surgery. 2008. January;33(1):102–12. [DOI] [PubMed] [Google Scholar]
- 2.Leadbetter WB, Buckwalter JA, Gordon SL, Foundation for Sports Medicine Education and Research, American Orthopaedic Society for Sports Medicine, National Institute of Arthritis and Musculoskeletal and Skin Diseases (U.S.), editors. Sports-induced inflammation: clinical and basic science concepts. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1990. 799 p. [Google Scholar]
- 3.Khan KM, Cook JL, Taunton JE, Bonar F. Overuse Tendinosis, Not Tendinitis: Part 1: A New Paradigm for a Difficult Clinical Problem. The Physician and Sportsmedicine. 2000. May;28(5):38–48. [DOI] [PubMed] [Google Scholar]
- 4.Alfredson H, Lorentzon R. Chronic Achilles Tendinosis: Recommendations for Treatment and Prevention. Sports Medicine. 2000;29(2):135–46. [DOI] [PubMed] [Google Scholar]
- 5.Aström M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995. July;(316):151–64. [PubMed] [Google Scholar]
- 6.Wiseman SP, Nelson SJ, Tyagi V, Kovacevic D, Blaine TA. Current trends in orthobiologics and shoulder surgery: Current Orthopaedic Practice. 2017;28(2):135–41. [Google Scholar]
- 7.Albers S, Zwerver J, van den Akker-Scheek I. 7 Incidence And Prevalence Of Lower Extremity Tendinopathy In The General Population: Abstract 7 Table 1. Br J Sports Med. 2014. Sep;48(Suppl 2):A5.1–A5. [Google Scholar]
- 8.Wertz J, Galli M, Borchers JR. Achilles Tendon Rupture: Risk Assessment for Aerial and Ground Athletes. Sports Health. 2013. September;5(5):407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consigliere P, Polyzois I, Sarkhel T, Gupta R, Levy O, Narvani AA. Preliminary Results of a Consecutive Series of Large & Massive Rotator Cuff Tears Treated with Arthroscopic Rotator Cuff Repairs Augmented with Extracellular Matrix. Arch Bone Jt Surg. 2017. January;5(1):14–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Minhas SV, Kester BS, Larkin KE, Hsu WK. The Effect of an Orthopaedic Surgical Procedure in the National Basketball Association. Am J Sports Med. 2016. April;44(4):1056–61. [DOI] [PubMed] [Google Scholar]
- 11.Saxena A, Ewen B, Maffulli N. Rehabilitation of the Operated Achilles Tendon: Parameters for Predicting Return to Activity. The Journal of Foot and Ankle Surgery. 2011. January;50(1):37–40. [DOI] [PubMed] [Google Scholar]
- 12.Klouche S, Lefevre N, Herman S, Gerometta A, Bohu Y. Return to Sport After Rotator Cuff Tear Repair: A Systematic Review and Meta-analysis. Am J Sports Med. 2016. July;44(7):1877–87. [DOI] [PubMed] [Google Scholar]
- 13.Huang AH, Riordan TJ, Pryce B, Weibel JL, Watson SS, Long F, et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development. 2015. July 15;142(14):2431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtwark GA, Wilson AM. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. Journal of Experimental Biology. 2006. November 1;209(21):4379–88. [DOI] [PubMed] [Google Scholar]
- 15.Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J. 2013. May;27(5):2074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Neer Award 1999: Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. Journal of Shoulder and Elbow Surgery. 2000. March;9(2):79–84. [PubMed] [Google Scholar]
- 17.Herod TW, Veres SP. Development of overuse tendinopathy: A new descriptive model for the initiation of tendon damage during cyclic loading. J Orthop Res. 2018;36(1):467–76. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Maffulli N. The Future: Rehabilitation, Gene Therapy, Optimization of Healing. Foot and Ankle Clinics. 2005. June;10(2):383–97. [DOI] [PubMed] [Google Scholar]
- 19.Freedman BR, Rodriguez AB, Leiphart RJ, Newton JB, Ban E, Sarver JJ, et al. Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission. Sci Rep. 2018. December;8(1):10854.This work highlights the multiscale response of tendon following dynamic loading and during healing using sophisticated imaging techniques and mechanical characterization. Findings from this work provides insight into how endogenous and/or therapeutic cells experience the tendon microenvironment.
- 20.Pingel J, Wienecke J, Kongsgaard M, Behzad H, Abraham T, Langberg H, et al. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports. 2013. December;23(6):e353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komatsu I, Wang JH-C, Iwasaki K, Shimizu T, Okano T. The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model. Acta Biomater. 2016. 15;42:136–46. [DOI] [PubMed] [Google Scholar]
- 22.Thigpen CA, Shaffer MA, Gaunt BW, Leggin BG, Williams GR, Wilcox RB. The American Society of Shoulder and Elbow Therapists’ consensus statement on rehabilitation following arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2016. April;25(4):521–35. [DOI] [PubMed] [Google Scholar]
- 23.Hsu JE, Horneff JG, Gee AO. Immobilization After Rotator Cuff Repair: What Evidence Do We Have Now? Orthop Clin North Am. 2016. January;47(1):169–77. [DOI] [PubMed] [Google Scholar]
- 24.Keener JD, Galatz LM, Stobbs-Cucchi G, Patton R, Yamaguchi K. Rehabilitation following arthroscopic rotator cuff repair: a prospective randomized trial of immobilization compared with early motion. J Bone Joint Surg Am. 2014. January 1;96(1):11–9. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson-Helander K, Silbernagel KG, Thomeé R, Faxén E, Olsson N, Eriksson BI, et al. Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010. November;38(11):2186–93. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Wang C, Ma X, Wang X, Zhang C, Chen L. Rehabilitation regimen after surgical treatment of acute Achilles tendon ruptures: a systematic review with meta-analysis. Am J Sports Med. 2015. April;43(4):1008–16. [DOI] [PubMed] [Google Scholar]
- 27.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009. October;18(5):669–75. [DOI] [PubMed] [Google Scholar]
- 28.Murrell GA, Lilly EG, Goldner RD, Seaber AV, Best TM. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res. 1994. July;12(4):582–91. [DOI] [PubMed] [Google Scholar]
- 29.Hillin CD, Fryhofer GW, Freedman BR, Choi DS, Weiss SN, Huegel J, et al. Effects of immobilization angle on tendon healing after achilles rupture in a rat model. J Orthop Res. 2019;37(3):562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murrell GA, Lilly EG, Goldner RD, Seaber AV, Best TM. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res. 1994. July;12(4):582–91. [DOI] [PubMed] [Google Scholar]
- 31.Amiel D, Akeson WH, Harwood FL, Frank CB. Stress deprivation effect on metabolic turnover of the medial collateral ligament collagen. A comparison between nine- and 12-week immobilization. Clin Orthop Relat Res. 1983. February;(172):265–70. [PubMed] [Google Scholar]
- 32.Killian ML, Cavinatto L, Shah SA, Sato EJ, Ward SR, Havlioglu N, et al. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res. 2014. March;32(3):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkisson I, Dahlin L, Rosberg H. Early mobilization compared with immobilization after repair of a flexor tendon injury in children: A retrospective long time follow-up. Hand Microsurg. 2017;1. [Google Scholar]
- 34.Wu F, Nerlich M, Docheva D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017. July;2(7):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Joint Surg Am. 2012. December 5;94(23):2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochen Y, Beks RB, van Heijl M, Hietbrink F, Leenen LPH, van der Velde D, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019. January 7;364:k5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratcliffe A, Butler DL, Dyment NA, Cagle PJ, Proctor CS, Ratcliffe SS, et al. Scaffolds for Tendon and Ligament Repair and Regeneration. Ann Biomed Eng. 2015. March;43(3):819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsson N, Nilsson-Helander K, Karlsson J, Eriksson BI, Thomée R, Faxén E, et al. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2011. August;19(8):1385–93. [DOI] [PubMed] [Google Scholar]
- 39.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nature Mater. 2016. December;15(12):1287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brickner JH, Walter P. Gene Recruitment of the Activated INO1 Locus to the Nuclear Membrane. Tom Misteli, editor. PLoS Biol. 2004. September 28;2(11):e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008. March;452(7184):243–7. [DOI] [PubMed] [Google Scholar]
- 42.Kalson NS, Starborg T, Lu Y, Mironov A, Humphries SM, Holmes DF, et al. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc Natl Acad Sci USA. 2013. December 3;110(49):E4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith SM, Zhang G, Birk DE. Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis. Matrix Biol. 2014. January;33:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, et al. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem. 2006. December 15;281(50):38592–8. [DOI] [PubMed] [Google Scholar]
- 45.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007. October;13(10):1219–27. [DOI] [PubMed] [Google Scholar]
- 46.Harvey T, Flamenco S, Fan C-M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol. 2019. December;21(12):1490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mienaltowski MJ, Adams SM, Birk DE. Regional Differences in Stem Cell/Progenitor Cell Populations from the Mouse Achilles Tendon. Tissue Engineering Part A. 2013. January;19(1–2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate: Tendon-derived stem/progenitor cell aging. Aging Cell. 2010. October;9(5):911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackerman JE, Best KT, O’Keefe RJ, Loiselle AE. Deletion of EP4 in S100a4-lineage cells reduces scar tissue formation during early but not later stages of tendon healing. Sci Rep. 2017. December;7(1):8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Best KT, Loiselle AE. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. The FASEB Journal. 2019. July;33(7):8578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. Journal of Rehabilitation Research and Development. 2000. April;37(2):127–133. [PubMed] [Google Scholar]
- 52.Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Pitsillides AA. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. Journal of Musculoskeletal & Neuronal Interactions. 2002. September;2(5):448–456. [PubMed] [Google Scholar]
- 53.Sharir A, Stern T, Rot C, Shahar R, Zelzer E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development (Cambridge, England). 2011. August;138(15):3247–3259. [DOI] [PubMed] [Google Scholar]
- 54.Zelzer E, Blitz E, Killian ML, Thomopoulos S. Tendon-to-Bone Attachment: From Development to Maturity. Birth defects research Part C, Embryo today : reviews. 2014. March;102(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edom-Vovard F, Duprez D. Signals regulating tendon formation during chick embryonic development. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 2004. March;229(3):449–457. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development (Cambridge, England). 2015. December;142(24):4191–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kannus P Structure of the tendon connective tissue. Scandinavian Journal of Medicine & Science in Sports. 2000. December;10(6):312–320. [DOI] [PubMed] [Google Scholar]
- 58.Kannus P, Jozsa L, Järvinen TAH, Jarvinen TLN, Kvist M, Natri A, et al. Location and Distribution of Non-collagenous Matrix Proteins in Musculoskeletal Tissues of Rat. The Histochemical Journal. 1998. November;30(11):799–810. [DOI] [PubMed] [Google Scholar]
- 59.Birk DE, Zycband EI, Woodruff S, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 1997. March;208(3):291–298. [DOI] [PubMed] [Google Scholar]
- 60.Huang AH, Lu HH, Schweitzer R. Molecular regulation of tendon cell fate during development. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2015. June;33(6):800–812. [DOI] [PubMed] [Google Scholar]
- 61.Berthet E, Chen C, Butcher K, Schneider RA, Alliston T, Amirtharajah M. Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2013. September;31(9):1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010. June;107(23):10538–10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016. January;30(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Yin Z, Chen J, Shen W, Liu H, Tang Q, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Scientific Reports. 2012. December;2:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Provenzano PP, Vanderby R. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biology: Journal of the International Society for Matrix Biology. 2006. March;25(2):71–84. [DOI] [PubMed] [Google Scholar]
- 66.Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Research Part C, Embryo Today: Reviews. 2008. September;84(3):228–244. [DOI] [PubMed] [Google Scholar]
- 67.Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proceedings of the National Academy of Sciences of the United States of America. 2013. April;110(16):6370–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphries SM, Lu Y, Canty EG, Kadler KE. Active Negative Control of Collagen Fibrillogenesis in Vivo Intracellular Cleavage of the Type I Procollagen Propeptides in Tendon Fibroblasts Without Intracellular Fibrils. Journal of Biological Chemistry. 2008. May;283(18):12129–12135. [DOI] [PubMed] [Google Scholar]
- 69.Wang WY, Davidson CD, Lin D, Baker BM. Actomyosin contractility-dependent matrix stretch and recoil induces rapid cell migration. Nat Commun. 2019. December;10(1):1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang AH, Watson SS, Wang L, Baker BM, Akiyama H, Brigande JV, et al. Requirement for scleraxis in the recruitment of mesenchymal progenitors during embryonic tendon elongation. Development. 2019. October 4;146(20).This paper used sophisticated lineage tracing approaches to identify the requirement of Scleraxis in the recruitment of mesenchymal progenitor cells during rapid elongation of tendon.
- 71.Sakabe T, Sakai K, Maeda T, Sunaga A, Furuta N, Schweitzer R, et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J Biol Chem. 2018. 20;293(16):5766–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theodossiou SK, Tokle J, Schiele NR. TGFβ2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem Biophys Res Commun. 2019. 15;508(3):889–93.This work characterized cell-cell junction proteins during tenogenic differentiation of mesenchymal stem cells. The authors identified temporal changes in N-cadherin, cadherin-11, and connexin-43 during tenogenesis induced by TGFβ2.
- 73.Brown JP, Galassi TV, Stoppato M, Schiele NR, Kuo CK. Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res Ther. 2015. May 9;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henderson JH, Carter DR. Mechanical induction in limb morphogenesis: the role of growth-generated strains and pressures. Bone. 2002. December;31(6):645–653. [DOI] [PubMed] [Google Scholar]
- 75.Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE. Slow Stretching That Mimics Embryonic Growth Rate Stimulates Structural and Mechanical Development of Tendon-Like Tissue In Vitro. Developmental Dynamics. 2011. November;240(11):2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore MJ, De Beaux A. A quantitative ultrastructural study of rat tendon from birth to maturity. Journal of Anatomy. 1987. August;153:163–169. [PMC free article] [PubMed] [Google Scholar]
- 77.Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proceedings of the Royal Society of London Series B, Biological Sciences. 1978. December;203(1152)305–321. [DOI] [PubMed] [Google Scholar]
- 78.Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis and Rheumatism. 2006. March;54(3):832–842. [DOI] [PubMed] [Google Scholar]
- 79.Gotoh M, Mitsui Y, Shibata H, Yamada T, Shirachi I, Nakama K, et al. Increased matrix metalloprotease-3 gene expression in ruptured rotator cuff tendons is associated with postoperative tendon retear. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2013. August;21(8):1807–1812. [DOI] [PubMed] [Google Scholar]
- 80.Holmes DF, Tait A, Hodson NW, Sherratt MJ, Kadler KE. Growth of Collagen Fibril Seeds from Embryonic Tendon: Fractured Fibril Ends Nucleate New Tip Growth. Journal of Molecular Biology. 2010. May;399(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. Journal of Biochemistry. 2012. October;152(4):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exercise and sport sciences reviews. 2015. April;43(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nature Genetics. 1997. November;17(3):318–323. [DOI] [PubMed] [Google Scholar]
- 84.Wang H-V, Chang L-W, Brixius K, Wickström SA, Montanez E, Thievessen I, et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. The Journal of Cell Biology. 2008. March;180(5):1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conti FJ, Felder A, Monkley S, Schwander M, Wood MR, Lieber R, et al. Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle. Development (Cambridge, England). 2008. June;135(11):2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McBride DJ, Trelstad RL, Silver FH. Structural and mechanical assessment of developing chick tendon. International Journal of Biological Macromolecules. 1988. August;10(4):194–200. [Google Scholar]
- 87.Maeda E, Shelton JC, Bader DL, Lee DA. Differential regulation of gene expression in isolated tendon fascicles exposed to cyclic tensile strain in vitro. Journal of Applied Physiology (Bethesda, Md: 1985). 2009. February;106(2):506–512. [DOI] [PubMed] [Google Scholar]
- 88.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002. March;35(3):303–9. [DOI] [PubMed] [Google Scholar]
- 89.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of Cell Science. 2011. April;124(Pt 8):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthr Cartil. 1999. January;7(1):141–53. [DOI] [PubMed] [Google Scholar]
- 91.Meier Bürgisser G, Evrova O, Calcagni M, Scalera C, Giovanoli P, Buschmann J. Impact of PDGF-BB on cellular distribution and extracellular matrix in the healing rabbit Achilles tendon three weeks post-operation. FEBS Open Bio. 2019. September 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turlo AJ, Mueller-Breckenridge AJ, Zamboulis DE, Tew SR, Canty-Laird EG, Clegg PD. Insulin-like growth factor binding protein (IGFBP6) is a cross-species tendon marker. Eur Cell Mater. 2019. September 24;38:123–36. [DOI] [PubMed] [Google Scholar]
- 93.Baker AR, McCarron JA, Tan CD, Iannotti JP, Derwin KA. Does augmentation with a reinforced fascia patch improve rotator cuff repair outcomes? CORR. 2012. September;470(9):2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. JBJS Am. 2006. December;88(12):2665–72. [DOI] [PubMed] [Google Scholar]
- 95.Derwin KA, Baker AR, Codsi MJ, Iannotti JP. Assessment of the canine model of rotator cuff injury and repair. J Shoulder Elbow Surg. 2007;16(5 Suppl):S140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hortensius RA, Ebens JH, Dewey MJ, Harley BAC. Incorporation of the Amniotic Membrane as an Immunomodulatory Design Element in Collagen Scaffolds for Tendon Repair. ACS Biomater Sci Eng. 2018. December 10;4(12):4367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grier WK, Sun Han Chang RA, Ramsey MD, Harley BAC. The influence of cyclic tensile strain on multi-compartment collagen-GAG scaffolds for tendon-bone junction repair. Connective Tissue Research. 2019. November 2;60(6):530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Echave MC, Domingues RMA, Gómez-Florit M, Pedraz JL, Reis RL, Orive G, et al. Biphasic Hydrogels Integrating Mineralized and Anisotropic Features for Interfacial Tissue Engineering. ACS Appl Mater Interfaces. 2019. December 26;11(51):47771–84. [DOI] [PubMed] [Google Scholar]
- 99.Font Tellado S, Chiera S, Bonani W, Poh PSP, Migliaresi C, Motta A, et al. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018;72:150–66. [DOI] [PubMed] [Google Scholar]
- 100.Freedman BR, Mooney DJ. Biomaterials to Mimic and Heal Connective Tissues. Adv Mater. 2019. May;31(19):1806695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gniesmer S, Brehm R, Hoffmann A, de Cassan D, Menzel H, Hoheisel AL, et al. Vascularization and biocompatibility of poly(ε-caprolactone) fiber mats for rotator cuff tear repair. Zhao F, editor. PLoS ONE. 2020. January 13;15(1):e0227563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu W, Feng Z, Ou-Yang W, Pan X, Wang X, Huang P, et al. 3D printing of implantable elastic PLCL copolymer scaffolds. Soft Matter. 2020;10.1039.C9SM02396H. [DOI] [PubMed] [Google Scholar]
- 103.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010. June;9(6):518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006. August 25;126(4):677–89. [DOI] [PubMed] [Google Scholar]
- 105.Youngstrom DW, LaDow JE, Barrett JG. Tenogenesis of bone marrow-, adipose-, and tendon-derived stem cells in a dynamic bioreactor. Connect Tissue Res. 2016;57(6):454–65. [DOI] [PubMed] [Google Scholar]
- 106.Schiele NR, Koppes RA, Chrisey DB, Corr DT. Engineering cellular fibers for musculoskeletal soft tissues using directed self-assembly. Tissue Eng Part A. 2013. May;19(9–10):1223–32. [DOI] [PubMed] [Google Scholar]
- 107.Mubyana K, Corr DT. Cyclic Uniaxial Tensile Strain Enhances the Mechanical Properties of Engineered, Scaffold-Free Tendon Fibers. Tissue Eng Part A. 2018. August 3; [DOI] [PubMed] [Google Scholar]
- 108.Doroski DM, Levenston ME, Temenoff JS. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng Part A. 2010. November;16(11):3457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006. November;12(11):3149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012. February;18(3–4):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. JMNI. 2005. March;5(1):70–84. [PubMed] [Google Scholar]
- 112.Yu K, Sellman DP, Bahraini A, Hagan ML, Elsherbini A, Vanpelt KT, et al. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J Orthop Res. 2018;36(2):653–62.This work highlights a new mechanism of the mechanical responsiveness of osteocytes (the resident cells of bone). Using both in vitro and in vivo experiments, the authors show that osteocytes are capable of detecting damage via plasma membrane disruptions, and propose that control of membrane damage using pharmacological methods could be used to modify skeletal adaptation.
- 113.Grembowicz KP, Sprague D, McNeil PL. Temporary disruption of the plasma membrane is required for c-fos expression in response to mechanical stress. Mol Biol Cell. 1999. April;10(4):1247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. [DOI] [PMC free article] [PubMed]
- 115.Mellgren RL, Huang X. Fetuin A Stabilizes m-Calpain and Facilitates Plasma Membrane Repair. J Biol Chem. 2007. December 7;282(49):35868–77. [DOI] [PubMed] [Google Scholar]
- 116.Isaac DI, Golenberg N, Haut RC. Acute repair of chondrocytes in the rabbit tibiofemoral joint following blunt impact using P188 surfactant and a preliminary investigation of its long-term efficacy. J Orthop Res. 2009;n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 117.Merchant FA, Holmes WH, Capelli-Schellpfeffer M, Lee RC, Toner M. Poloxamer 188 Enhances Functional Recovery of Lethally Heat-Shocked Fibroblasts. Journal of Surgical Research. 1998. February;74(2):131–40. [DOI] [PubMed] [Google Scholar]
- 118.Gilchrist CL, Leddy HA, Kaye L, Case ND, Rothenberg KE, Little D, et al. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc Natl Acad Sci USA. 2019. May;116(6):1992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Magra M, Hughes S, El Haj AJ, Maffulli N. VOCCs and TREK-1 ion channel expression in human tenocytes. American Journal of Physiology-Cell Physiology. 2007. March;292(3):C1053–60. [DOI] [PubMed] [Google Scholar]
- 120.Hennessey JA, Wei EQ, Pitt GS. Fibroblast Growth Factor Homologous Factors Modulate Cardiac Calcium Channels. Circ Res. 2013. August 2;113(4):381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]