Supplemental Digital Content is Available in the Text.
Key Words: HIV, COVID-19, comorbidities, health disparities, medical monitoring project
Background:
Health inequities among people with HIV may be compounded by disparities in the prevalence of comorbidities associated with an increased risk of severe illness from COVID-19.
Setting:
Complex sample survey designed to produce nationally representative estimates of behavioral and clinical characteristics of adults with diagnosed HIV in the United States.
Methods:
We estimated the prevalence of having ≥1 diagnosed comorbidity associated with severe illness from COVID-19 and prevalence differences (PDs) by race/ethnicity, income level, and type of health insurance. We considered PDs ≥5 percentage points to be meaningful from a public health perspective.
Results:
An estimated 37.9% [95% confidence interval (CI): 36.6 to 39.2] of adults receiving HIV care had ≥1 diagnosed comorbidity associated with severe illness from COVID-19. Compared with non-Hispanic Whites, non-Hispanic Blacks or African Americans were more likely [adjusted PD, 7.8 percentage points (95% CI: 5.7 to 10.0)] and non-Hispanic Asians were less likely [adjusted PD, −13.7 percentage points (95% CI: −22.3 to −5.0)] to have ≥1 diagnosed comorbidity after adjusting for age differences. There were no meaningful differences between non-Hispanic Whites and adults in other racial/ethnic groups. Those with low income were more likely to have ≥1 diagnosed comorbidity [PD, 7.3 percentage points (95% CI: 5.1 to 9.4)].
Conclusions:
Among adults receiving HIV care, non-Hispanic Blacks and those with low income were more likely to have ≥1 diagnosed comorbidity associated with severe COVID-19. Building health equity among people with HIV during the COVID-19 pandemic may require reducing the impact of comorbidities in heavily affected communities.
BACKGROUND
Contrary to the early prediction that COVID-19 would be “the great equalizer,”1 the disease burden in the United States falls disproportionately on non-Hispanic Blacks or African Americans (Blacks), Hispanics or Latinos of any race (Hispanics), people with low income, and those with certain chronic illnesses.
Relative to their proportion of the US population, Blacks and Hispanics have higher COVID-19 attack rates and hospitalization rates, and Blacks have higher death rates than non-Hispanic Whites (Whites).2–9 Blacks constitute 13% of the US population but accounted for 16% of COVID-19 cases and 20% of COVID-19 deaths, as of November 15, 2020.9 Hispanics, at 18% of the population, accounted for 26% of cases and 16% of deaths nationwide. Among those with diagnosed COVID-19, Blacks and people with low income are more likely to have severe illness and die.10–13 Among people with HIV, those who are Black or Hispanic, or have low income, are also disproportionately affected by COVID-19.14,15
In addition to these sociodemographic risk factors, several chronic health conditions increase the risk of COVID-19 requiring hospitalization, eg, obesity, chronic kidney disease (CKD), type 2 diabetes, chronic obstructive pulmonary disease (COPD), immunocompromised state from solid organ transplantation, heart disease, and sickle cell disease.13,16 Hospitalizations are 6 times higher and deaths 12 times higher for people with underlying illnesses.7
Although the Centers for Disease Control and Prevention (CDC) lists an immunocompromised state due to HIV as a condition that might increase the risk for severe illness from COVID-19,16 the available literature in North America and Europe suggests that HIV, in itself, does not increase the risk of SARS-CoV-2 infection or severe illness.17–22 However, several studies have found that most people with diagnosed HIV and COVID-19 have other comorbidities,14,15,17,20,23 suggesting these conditions, rather than HIV, may be the driver of severe illness in this population.
With HIV, as with COVID-19, Blacks, Hispanics, and people with low income bear a disproportionate burden of disease in the United States.24 Compared with Whites, the prevalence of HIV in 2018 was 7.2 times higher among Blacks and 3.0 times higher among Hispanics.25 Among those with recent HIV infection, 42% were Black and 28% Hispanic. Blacks and Hispanics have poorer outcomes than Whites at all stages of the HIV care continuum, including linkage to HIV care, retention in care, and viral suppression.26 HIV is also a disease of poverty. Among adults with diagnosed HIV, 43% had a household income below the poverty threshold and one in 5 went without food during the past year because of lack of money.27
The burden of poor health outcomes from HIV in heavily affected communities may be compounded by similar patterns in the distribution of COVID-19 and by disparities in the prevalence of comorbidities associated with an increased risk for severe illness from COVID-19 among people with HIV.28 To examine the prevalence of these comorbidities among people with HIV, we analyzed HIV surveillance data on adults receiving HIV care to determine the percentage with ≥1 diagnosed comorbidity and assessed differences by race/ethnicity, income level, and health insurance type.
METHODS
Design and Data Collection
The Medical Monitoring Project (MMP) is an annual cross-sectional survey designed to produce nationally representative estimates of the behavioral and clinical characteristics of adults with diagnosed HIV in the United States. This analysis presents estimates of adults receiving HIV care drawn from this sample. MMP data collection constitutes routine public health surveillance and was thus determined by the CDC to be nonresearch. This activity was conducted consistent with the applicable federal law and CDC policy.29 When required, participating states or territories obtained local institutional review board approval to collect data. All participants provided informed consent. MMP uses 2-stage sampling in which, during the first stage, 16 states and one territory, including 6 separately funded metropolitan areas within selected states, were sampled from all states, the District of Columbia, and Puerto Rico. During the second stage, simple random samples of people with diagnosed HIV aged ≥18 years were drawn annually for each participating area from the National HIV Surveillance System, a census of people with diagnosed HIV in the United States. People sampled during the 2016–2018 data collection cycles were recruited for a phone or face-to-face interview and medical record abstraction at their usual place of HIV medical care. Data were collected beginning in June of each cycle until the following May. Medical record data included selected laboratory test results and all diagnoses recorded in the problem list or assessment section of medical encounters during a 2-year, retrospective observation period ending on the interview date. For the 2016–2018 data collection cycles, this period spanned June 2014–May 2019.
All sampled areas and separately funded jurisdictions within states participated in the MMP, including California (including Los Angeles County and San Francisco), Delaware, Florida, Georgia, Illinois (including Chicago), Indiana, MI, Mississippi, New Jersey, New York (including New York City), North Carolina, Oregon, Pennsylvania (including Philadelphia), Puerto Rico, Texas (including Houston), Virginia, and Washington. Annual response rates for adults with diagnosed HIV were 44%–45%. During the 2016–2018 data collection cycles, 12,310 sampled people were interviewed (Fig. 1). Of these, 12,189 reported receiving HIV medical care or had medical record documentation of at least one element of HIV care (eg, an HIV laboratory result or antiretroviral prescription) during the past 24 months. We excluded 627 participants without a medical record abstraction containing at least one diagnosed condition. The analytic data set included 11,562 participants who received HIV medical care in the past 24 months and had diagnosis data abstracted (referred to as “adults receiving HIV care”).
FIGURE 1.
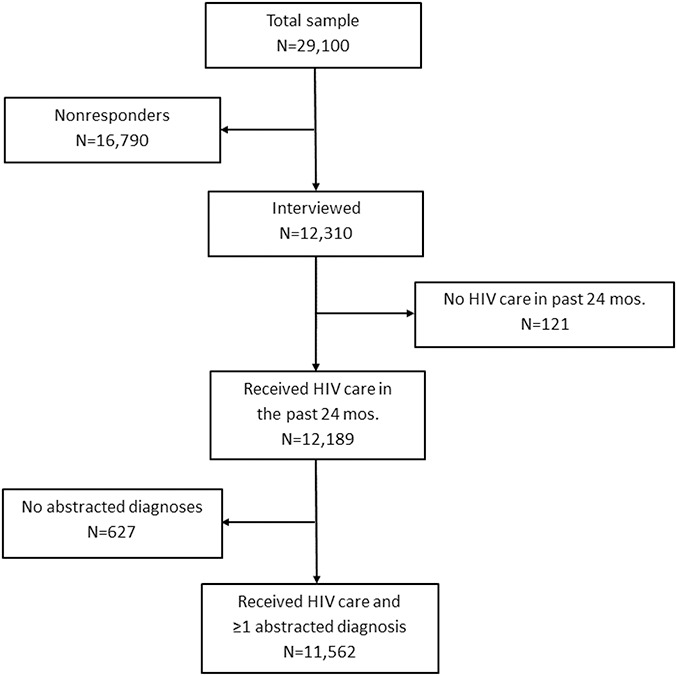
Sample disposition for this analysis, the Medical Monitoring Project, 2016–2018 data collection cycles.
Measures
Sociodemographic variables included age, race/ethnicity (non-Hispanic American Indian/Alaska Native, Asian, Black, Native Hawaiian/other Pacific Islander, White, or multiracial, and Hispanic of any race), household income above or below the federal poverty threshold during the past 12 months, and health insurance or coverage type during the past 12 months [any private insurance, public insurance alone, Ryan White HIV/AIDS Program (RWHAP) assistance alone, unspecified, and no insurance/coverage]. We ascertained whether facilities identified by participants as their usual place of HIV care received any funding from the RWHAP.30 Clinical variables included diagnosed comorbidities identified by the CDC on July 15, 2020, as associated with an increased risk of severe illness from COVID-19, defined as requiring hospitalization (obesity, CKD, diabetes, COPD, immunocompromised from solid organ transplantation, heart disease, and sickle cell disease)16 as well as diagnosed AIDS-defining illnesses.31 We ascertained obesity from recorded diagnoses of obesity, without consideration of body mass index. Because medical records often do not differentiate type 1 and type 2 diabetes,32 we measured diagnosed diabetes, irrespective of type. Diagnosed heart disease included atherosclerotic heart disease, congestive heart failure, cardiomyopathy, pulmonary hypertension or cor pulmonale, and arrhythmia. Other clinical variables included CD4+ T-lymphocyte cell count (CD4 count) and HIV RNA level.
Outcomes
The primary outcome was having ≥1 diagnosed comorbidity associated with an increased risk for severe illness from COVID-19 recorded in the medical record during the 24 months before being interviewed. Because the risk increases with age33 and possibly with immunocompromised state secondary to HIV, secondary outcomes included having ≥1 diagnosed comorbidity along with combinations of older age (≥50 or ≥65 years) and poorly controlled HIV, defined as lowest CD4 count <200 cells/µL, any HIV RNA ≥200 copies/mL, or diagnosis of an AIDS-defining illness during the past 24 months.
Statistical Analysis
Data were weighted based on known probabilities of selection at the state or territory and person levels, adjusted for nonresponse, and poststratified to known population totals by age, race/ethnicity, and sex from the National HIV Surveillance System. This design allows inference to all adults with diagnosed HIV in the United States.27
We estimated the prevalence, with 95% confidence intervals (CIs), of having ≥1 diagnosed comorbidity associated with an increased risk for severe illness from COVID-19 by race/ethnicity, income, type of health insurance, and RWHAP facility funding. We used logistic regression with predicted marginals to estimate prevalence differences (PDs), with corresponding 95% CIs and t test P values, for having ≥1 comorbidity by race/ethnicity, income, health insurance type, and RWHAP facility funding.
Because the median age of Whites was higher than for other race/ethnicities and older adults are more likely to have diagnosed comorbidities, we adjusted the association between race/ethnicity and having ≥1 diagnosed comorbidity for age. To assess whether the association between race/ethnicity and having ≥1 diagnosed comorbidity differed by income level, we added poverty as an interaction term to the race/ethnicity model. For income, health insurance type, and RWHAP-funding status, we reported unadjusted measures of association. We considered a PD ≥5 percentage points to be meaningful from a public health perspective.
RESULTS
The sociodemographic characteristics of people receiving HIV care during June 2014–May 2019 are displayed in Table 1. Clinical characteristics are displayed in Table 2. An estimated 12.3% (95% CI: 11.4 to 13.1) had diagnosed obesity, 11.7% (95% CI: 10.8 to 12.6) had CKD, 12.6% (95% CI: 11.9 to 13.4) had diabetes, 10.1% (95% CI: 9.4 to 10.8) had COPD, 0.1% (95% CI: 0.1 to 0.2) were immunocompromised from organ transplantation, 9.3% (95% CI: 8.7 to 10.0) had heart disease, and 37.9% (95% CI: 36.6 to 39.2) had ≥1 diagnosed comorbidity associated with an increased risk for severe illness from COVID-19 (Table 2). An estimated 51.6% (95% CI: 50.4 to 52.8) were aged ≥50 years, 8.3% (95% CI: 7.5 to 9.0) were aged ≥65 years, and 35.8% (95% CI: 34.4 to 37.2) had poorly controlled HIV. Prevalence estimates of combinations of these characteristics are presented in Table 2. PDs for having ≥1 diagnosed comorbidity are displayed in Table 3 and Figure 2. Compared with White adults, Black adults were more likely [adjusted PD (APD), 7.8 percentage points (95% CI: 5.7 to 10.0)] and Asian adults were less likely [APD, −13.7 percentage points (95% CI, −22.3 to −5.0)] to have ≥1 diagnosed comorbidity, after adjusting for age differences (for the median age of categories within each variable see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B588). There were no meaningful differences in the prevalence of having ≥1 diagnosed comorbidity between White adults and adults of any other race/ethnicity. APDs for race/ethnicity were not substantially different among those with incomes above vs. at or below the poverty threshold (see Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B588). The likelihood of having ≥1 diagnosed comorbidity was higher among those with incomes at or below vs. above the poverty threshold [PD, 7.3 percentage points (95% CI: 5.1 to 9.4)]. Compared with people with any private insurance, those with public insurance alone were more likely [PD, 10.2 percentage points (95% CI: 8.3 to 12.2)] and those with RWHAP assistance alone were less likely [PD, −8.4 percentage points (95% CI, −11.5 to −5.2)] to have ≥1 diagnosed comorbidity. The likelihood of having ≥1 diagnosed comorbidity did not differ among adults receiving HIV care at RWHAP-funded vs. non–RWHAP-funded facilities.
TABLE 1.
Sociodemographic Characteristics of Adults Receiving HIV Care in the United States, 2014–2019, the Medical Monitoring Project
| Characteristic | Sample Size | Weighted Col % (95% CI) |
| Total | 11,562 | |
| Race/ethnicity | ||
| American Indian/Alaska Native, non-Hispanic | 55 | 0.6 (0.4 to 0.8) |
| Asian, non-Hispanic | 114 | 1.0 (0.7 to 1.2) |
| Black or African American, non-Hispanic | 4877 | 40.8 (35.3 to 46.2) |
| Hispanic or Latino of any race | 2540 | 22.7 (18.0 to 27.4) |
| Native Hawaiian/Other Pacific Islander, non-Hispanic | 25 | 0.2 (0.1 to 0.2) |
| White, non-Hispanic | 3318 | 29.2 (25.7 to 32.7) |
| Multiracial, non-Hispanic | 630 | 5.6 (4.7 to 6.4) |
| Age | ||
| 18–29 yrs | 945 | 8.4 (7.7 to 9.2) |
| 30–39 yrs | 1840 | 16.8 (16.0 to 17.6) |
| 40–49 yrs | 2648 | 23.2 (22.2 to 24.1) |
| ≥50 yrs | 6129 | 51.6 (50.4 to 52.8) |
| Sex | ||
| Male | 8412 | 74.6 (73.1 to 76.1) |
| Female | 2946 | 23.7 (22.3 to 25.2) |
| Transgender | 195 | 1.6 (1.4 to 1.9) |
| Highest education level | ||
| Less than high school | 2021 | 17.2 (16.0 to 18.4) |
| High school | 3040 | 26.6 (25.4 to 27.8) |
| Greater than high school | 6464 | 56.2 (54.4 to 58.1) |
| Income*† | ||
| Above the poverty threshold | 5997 | 57.2 (54.8 to 59.6) |
| At or below the poverty threshold | 4690 | 42.8 (40.4 to 45.2) |
| Health insurance*‡ | ||
| Any private insurance | 3987 | 35.1 (33.1 to 37.1) |
| Public insurance alone | 6428 | 54.3 (52.0 to 56.6) |
| RWHAP assistance alone | 919 | 9.0 (7.4 to 10.6) |
| Unspecified insurance | 49 | 0.4 (0.3 to 0.6) |
| No insurance or coverage | 102 | 1.1 (0.8 to 1.4) |
| RWHAP-funded facility§ | ||
| Yes | 7997 | 69.1 (63.7 to 74.6) |
| No | 3462 | 30.9 (25.4 to 36.3) |
During the past 12 months.
Poverty guidelines as defined by the Department of Health and Human Services: https://aspe.hhs.gov/frequently-askedquestions-related-poverty-guidelines-and-poverty
Participants could select more than 1 response for health insurance or coverage for medications (including antiretroviral medications).
Whether the usual place of care where participants received HIV care during the past 24 months received any funding from the RWHAP (parts A, B, C, D, or F).
TABLE 2.
Clinical Characteristics of Adults Receiving HIV Care in the United States, 2014–2019, the Medical Monitoring Project
| Risk Factor for Severe COVID-19 | Sample Size | Weighted Col % (95% CI) |
| Total | 11,562 | |
| Comorbidities in the past 24 months* | ||
| Obesity | 1476 | 12.3 (11.4 to 13.1) |
| Chronic kidney disease | 1390 | 11.7 (10.8 to 12.6) |
| Diabetes | 1503 | 12.6 (11.9 to 13.4) |
| Chronic obstructive pulmonary disease | 1168 | 10.1 (9.4 to 10.8) |
| Immunocompromised from organ transplantation | 21 | 0.1 (0.1 to 0.2) |
| Heart disease | 1114 | 9.3 (8.7 to 10.0) |
| Sickle cell disease | 11 | 0.1 (0.0 to 0.1)† |
| Any comorbidity in the past 24 months* | 4473 | 37.9 (36.6 to 39.2) |
| Age | ||
| ≥50 yrs | 6129 | 51.6 (50.4 to 52.8) |
| ≥65 yrs | 993 | 8.3 (7.5 to 9.0) |
| HIV control during the past 24 mo | ||
| Lowest CD4 count <200 cells/µL | 1539 | 13.2 (12.2 to 14.2) |
| Any HIV RNA ≥200 copies/mL | 3070 | 27.6 (26.1 to 29.2) |
| Any AIDS-defining illness‡ | 727 | 5.9 (5.5 to 6.4) |
| Poorly controlled HIV (any CD4 count <200 cells/µL, any HIV RNA ≥200 copies/mL, or any AIDS-defining illness) | 3990 | 35.8 (34.4 to 37.2) |
| Combinations of any comorbidity, age, and poorly controlled HIV | ||
| Any comorbidity or age ≥50 yrs | 7614 | 64.5 (63.3 to 65.7) |
| Any comorbidity or age ≥65 yrs | 4868 | 41.2 (40.0 to 42.4) |
| Any comorbidity or poorly controlled HIV | 6898 | 61.3 (59.9 to 62.7) |
| Any comorbidity, age ≥50 years, or poorly controlled HIV | 9125 | 79.7 (78.7 to 80.7) |
| Any comorbidity, age ≥65 years, or poorly controlled HIV | 7203 | 63.9 (62.7 to 65.1) |
Defined as documented diagnosis in the medical record at the usual place of HIV care.
Estimate is unstable. Coefficient of variation is ≥ 0.3.
AIDS-defining opportunistic illness defined by CDC during the past 24 months: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a1.htm
CD4, CD4+ T-lymphocyte cell.
TABLE 3.
Weighted Prevalence and Prevalence Differences of Having ≥1 Diagnosed Comorbidities Associated With Severe COVID-19 Among People Receiving HIV Care in the United States, 2014–2019, the Medical Monitoring Project
| Sociodemographic Characteristic | Sample Size | Weighted Row % (95% CI) | Prevalence Difference % (95% CI) | P | Adjusted Prevalence difference* % (95% CI) | P |
| Total | 4473 | 37.9 (36.6 to 39.2) | ||||
| Race/ethnicity | ||||||
| American Indian/Alaska Native, non-Hispanic | 22 | 32.0 (19.5 to 44.4)† | −5.7 (−18.8 to 7.4)† | 0.390 | −4.2 (−16.5 to 8.2)† | 0.507 |
| Asian, non-Hispanic | 25 | 17.7 (10.0 to 25.3) | −20.1 (−27.8 to −12.3) | <0.001 | −13.7 (−22.3 to −5.0) | 0.002 |
| Black or African American, non-Hispanic | 2063 | 41.3 (39.5 to 43.1) | 3.6 (1.2 to 6.0) | 0.004 | 7.8 (5.7 to 10.0) | <0.001 |
| Hispanic or Latino of any race | 843 | 32.9 (31.0 to 34.8) | −4.8 (−7.6 to −2.0) | 0.001 | −0.1 (−2.7 to 2.5) | 0.952 |
| Native Hawaiian/Other Pacific Islander, non-Hispanic | 10 | 45.0 (16.7 to 73.4)† | 7.3 (−21.2 to 35.8)† | 0.615 | 18.5 (−4.1 to 41.0)† | 0.108 |
| White, non-Hispanic | 1270 | 37.7 (35.7 to 39.8) | Reference | Reference | ||
| Multiracial, non-Hispanic | 239 | 37.6 (33.1 to 42.1) | −0.1 (−5.2 to 5.0) | 0.964 | 3.8 (−1.1 to 8.8) | 0.132 |
| Incomeठ| ||||||
| Above poverty threshold | 2156 | 35.1 (33.4 to 36.8) | Reference | |||
| At or below poverty threshold | 1998 | 42.4 (40.6 to 44.1) | 7.3 (5.1 to 9.4) | <0.001 | ||
| Health insurance║ | ||||||
| Any private insurance | 1354 | 33.1 (31.3 to 35.0) | Reference | |||
| Public insurance alone | 2809 | 43.4 (41.5 to 45.3) | 10.2 (8.3 to 12.2) | <0.001 | ||
| RWHAP assistance alone | 237 | 24.8 (21.9 to 27.6) | −8.4 (−11.5 to −5.2) | <0.001 | ||
| Unspecified insurance | 21 | 47.9 (29.2 to 66.6) | 14.8 (−3.5 to 33.1) | 0.114 | ||
| No insurance or coverage | 28 | 27.1 (18.1 to 36.2) | −6.0 (−15.4 to 3.4) | 0.210 | ||
| RWHAP-funded facility¶ | ||||||
| Yes | 3090 | 37.7 (35.9 to 39.5) | −0.4 (−2.9 to 2.2) | 0.789 | ||
| No | 1346 | 38.1 (36.3 to 39.9) | Reference |
Model adjusted for age (continuous variable).
Estimate is unstable. Absolute confidence interval is ≥30.
During the past 12 months.
Poverty guidelines as defined by the Department of Health and Human Services: https://aspe.hhs.gov/frequently-askedquestions-related-poverty-guidelines-and-poverty
Participants could select more than 1 response for health insurance or coverage for medications (including antiretroviral medications).
Any RWHAP funding (parts A, B, C, D, or F).
FIGURE 2.

Prevalence difference of having ≥1 diagnosed comorbidities associated with severe COVID-19 among people receiving HIV care in the United States, 2014–2019, the Medical Monitoring Project, N = 4473. A, Race/ethnicity, adjusted for age, reference group is White, non-Hispanic adults. B, Income at or below the poverty threshold, unadjusted, reference is income above the poverty threshold. C, Type of health insurance, unadjusted, reference is any private insurance. D, Ryan White HIV/AIDS Program funding of the usual place of care (health care facility), unadjusted, reference is no funding. Dashed red line is the meaningful threshold of increased prevalence. Dashed blue line is the meaningful threshold of decreased prevalence. Solid black line is the null threshold. aUnstable estimate (coefficient of variation is ≥0.3, absolute confidence interval is ≥30, and/or relative confidence interval is >130%). bPoverty guidelines as defined by the Department of Health and Human Services: https://aspe.hhs.gov/frequently-askedquestions-related-poverty-guidelines- and-poverty. cParticipants could select more than 1 response for health insurance or coverage for medications. dAny RWHAP funding (parts A, B, C, D, or F).
DISCUSSION
More than one-third of adults receiving HIV care in the United States (37.9%) had ≥1 diagnosed comorbidity associated with an increased risk for severe illness from COVID-19. Those with low income or public insurance alone and Blacks (adjusting for age) with income either above or below the poverty threshold were more likely to have ≥1 diagnosed comorbidity.
To the best of our knowledge, these are the first population-based data exploring these issues among people with HIV. In the general adult population, based on data from the Behavioral Risk Factor Surveillance System, a slightly higher percent (40.7%) of adults reported having ≥1 of these comorbidities.34 However, methodologic differences between the 2 surveys (self-reported data for the Behavioral Risk Factor Surveillance System and medical record review for the MMP) prevent direct comparison of the prevalence of comorbidities in the general population and people receiving HIV care.
Our finding that Blacks (but not Hispanics) receiving HIV care were more likely than Whites to have ≥1 diagnosed comorbidity is consistent with an analysis of surveillance data exploring racial/ethnic disparities in progression of COVID-19 from mild illness to hospitalization and death.35 Holtgrave constructed a continuum of COVID-19 outcomes, modeled on the HIV care continuum,36 and found that although infection rates were higher for Blacks and Hispanics than Whites, hospitalization rates (given infection) were higher for Blacks but not Hispanics. Together, our findings suggest that different interventions may be needed to build COVID-19 health equity in Black and Hispanic communities. Reducing the burden of severe illness from COVID-19 in both communities may require addressing factors related to exposure, eg, housing density, food and housing insecurity, dependence on public transportation, and reliance on front-line jobs that makes physical distancing difficult. To reduce burden in Black communities, structural changes to mitigate the higher prevalence of underlying health conditions may also be needed, including increased access to medical care and supportive services, healthy food options, and recreational spaces.37,38 Findings from a study of 6916 patients with COVID-19 at the Kaiser Permanente of Southern California support this assumption.39 In contrast to most population-based studies, the risk of death from COVID-19, once diagnosed, was comparable among Black and White adults, suggesting that equalized access to health care within an integrated health care system may mitigate the high COVID-19 case fatality rate among Blacks.
Although many of the structural barriers that underlie the higher prevalence of comorbidities among Blacks receiving HIV care are linked to poverty, we found a higher prevalence of comorbidities among Blacks with incomes above and below the poverty threshold, suggesting that factors in addition to poverty (eg, segregation and discrimination) may limit access to health services, health messaging, and healthy lifestyle options in Black communities.
The intersection of poverty with HIV and COVID-19 is complex. People with low income are at increased risk for acquiring and having poor outcomes from both illnesses, for many of the reasons discussed above. In turn, COVID-19 has caused unemployment and loss of employer-based health insurance for millions of Americans,40 potentially weakened the health safety net,41 and challenged recent efforts to end the HIV epidemic.42 Interrupting the mutually reinforcing cycle of HIV, COVID-19, and poverty may require sustained economic support to prevent worsening of income and health disparities.
About half of US adults with HIV have Medicaid and/or Medicare as their only health insurance,43 and this proportion may increase as people who lose employer-based insurance during the pandemic become newly eligible for Medicaid.44 We found that adults receiving HIV care who had public insurance alone were more likely than those with private insurance to have ≥1 diagnosed comorbidity (43.4% vs. 33.1%), suggesting Medicare and Medicaid programs may bear increased responsibility as payers of care for adults with HIV at increased risk of severe illness from COVID-19. Conversely, we found that those whose only health coverage was RWHAP assistance were less likely than those with private insurance to have comorbidities, at least in part because they are, on average, younger than others.
Because a large proportion of patients receiving HIV care at Ryan White–funded facilities have social determinants associated with poor health outcomes,45 we expected a higher percentage of patients at those facilities to have comorbidities. However, there was no difference, again at least in part, because RWHAP-funded facilities serve younger patients. However, any conclusion about the burden of severe illness from COVID-19 among patients receiving HIV care at RWHAP-funded facilities should be tempered by knowledge that Blacks and Hispanics, who comprise three-quarters of HIV patients at these facilities,45 die from COVID-19 at younger ages than Whites.8
LIMITATIONS
For several reasons, our primary outcome likely underestimates the increased risk of severe illness from COVID-19 among adults receiving HIV care. First, to measure comorbidities, we used only diagnosis data instead of supplementing with medical record information that can identify some conditions, even when not diagnosed, eg, laboratory data for CKD and height and weight for obesity. Our estimate of diagnosed obesity (12.3%) is lower than a previous MMP estimate that calculated the prevalence of body mass index ≥30 kg/m2 using height and weight documented in the medical record (19% of men and 42% of women were obese).46 Because some conditions (eg, COPD) can only be identified using diagnosis data, for consistency, we measured all comorbidities in the same way to avoid information bias of the prevalence of comorbidities, relative to each other.
Second, we included comorbidities known to increase the risk of severe illness as of July 15, 2020, and there may have been subsequent additions to the list. We did not include diagnosed conditions that might be associated with the risk for severe illness, but for which there is currently insufficient evidence to support inclusion on the CDC list of conditions that definitely increase the risk,16 including poorly controlled HIV, which affects one in 3 adults receiving HIV care.
Third, we did not include older age as a risk factor for severe illness from COVID-19. Because the relationship between age and risk is continuous,33 we did not include an age cut-off when formulating the primary outcome.
For these reasons, our estimate that 37.9% of adults receiving HIV care had ≥1 diagnosed comorbidity should be considered a minimum estimate of disease burden. Underestimation of risk could have biased our estimates of PDs across race/ethnicities, income levels, and types of health insurance. However, because several of the comorbidities we assessed are more prevalent among Blacks than Whites with HIV,32,46,47 underestimation of their prevalence would likely have biased differences toward the null.
Strengths of our study included population-based sampling and weighting designed to produce nationally representative estimates of a wide range of sociodemographic and clinical characteristics of adults with diagnosed HIV, of which those receiving HIV care is a subset. The large sample size allows reasonably precise estimates of subgroup characteristics. Although not all sampled persons participated, results were adjusted for nonresponse using the standard methodology.48,49 Even with suboptimal response rates, there is still value in results obtained from unbiased sampling methods.50
CONCLUSIONS
Among adults receiving HIV care, those with low income or public insurance alone and Blacks with income either above or below the poverty threshold were more likely to have ≥1 diagnosed comorbidity associated with severe illness from COVID-19. Building health equity during the co-occurring epidemics of COVID-19 and HIV may require structural changes to mitigate the burden of these underlying health conditions in Black and low-income communities, including increased access to medical care and supportive services, healthy food options, and recreational spaces.
ACKNOWLEDGMENTS
The authors thank MMP participants, providers, facilities, and project area staff as well as members of the MMP advisory boards. The authors also acknowledge the contributions of the Clinical Outcomes Team and the Behavioral and Clinical Surveillance Branch at the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Funding for the MMP is provided by a cooperative agreement (PS09-937) from the CDC.
Footnotes
Supported by Centers for Disease Control and Prevention.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Single overriding communication objective: Among adults in HIV care, those with low income and Blacks with income either above or below the poverty level were more likely to have comorbidities associated with severe illness from COVID-19, potentially compounding health disparities in these populations.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the authors' affiliated institutions.
Contributor Information
Yunfeng Tie, Email: hzu3@cdc.gov.
Linda Beer, Email: gur0@cdc.gov.
Robyn Neblett Fanfair, Email: iyo5@cdc.gov.
Roy Luke Shouse, Email: zxz3@cdc.gov.
REFERENCES
- 1.Cuomo A (@NYGovCuomo). The Virus Is the Great Equalizer. Twitter; 2020. Available at https://twitter.com/NYGovCuomo/status/1245021319646904320. Accessed September 18, 2020. [Google Scholar]
- 2.Azar K, Shen Z, Romanelli R, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). 2020;39:1253–1262. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, National Center for Health Statistics. Health Disparities: Race and Hispanic Origin. Available at: https://www.cdc.gov/nchs/nvss/vsrr/covid19/health_disparities.htm. Accessed September 18, 2020. [Google Scholar]
- 4.Centers for Disease Control and Prevention. COVIDView. A weekly surveillance summary of U.S. COVID-19 activity. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-31-2020.pdf. Accessed September 18, 2020.
- 5.Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on Black communities. Ann Epidemiol. 2020;47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentsch CT, Kidwai-Khan F, Tate JP, et al. COVID-19 by race and ethnicity: a national cohort study of 6 million United States veterans. Available at: https://www.medrxiv.org/content/medrxiv/early/2020/05/18/2020.05.12.20099135.full.pdf. Accessed September 18, 2020.
- 7.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wortham JM, Lee JT, Althomsons S, et al. Characteristics of persons who died with COVID-19—United States, February 12–May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:923–929. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. COVID Data Tracker. Available at: https://www.cdc.gov/covid-data-tracker/index.html#demographics. Accessed October 19, 2020. [Google Scholar]
- 10.Tenforde MW, Rose E, Lindsell CJ, et al. Characteristics of adult outpatients and inpatients with COVID-19—11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koma W, Artiga S, Neuman T, et al. Low-income and Communities of Color at Higher Risk of Serious Illness if Infected with Coronavirus. Kaiser Family Foundation; Available at: https://www.kff.org/coronavirus-covid-19/issue-brief/low-income-and-communities-of-color-at-higher-risk-of-serious-illness-if-infected-with-coronavirus. Accessed September 18, 2020. [Google Scholar]
- 12.Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382:2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics associated with hospitalization among patients with COVID-19—metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LF, Moran CA, Oliver NT, et al. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS. 2020;34:1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerowitz EA, Kim AY, Ard KL, et al. Disproportionate burden of COVID-19 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS. 2020;34:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). People with certain medical conditions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed September 18, 2020.
- 17.Byrd KM, Beckwith CG, Garland JM, et al. SARS-CoV-2 and HIV coinfection: clinical experience from Rhode Island, United States. J Int AIDS Soc. 2020;23:e25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:2276–2278. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmen-Tuohy S, Carlucci PM, Zervou FN, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigel K, Swartz T, Golden E, et al. Covid-19 and People with HIV infection: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71:2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalev N, Scherer M, LaSota ED, et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID-19. Clin Infect Dis. 2020;71:2294–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadi YB, Naqvi SFZ, Kupec JT, et al. Characteristics and outcomes of COVID-19 in patients with HIV: a multi-center research network study. AIDS. 2020;34:F3–F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National HIV Curriculum. HIV in Racial and Ethnic Minority Populations. University of Washington; Available at: https://www.hiv.uw.edu/go/key-populations/minority-populations/core-concept/all. Accessed September 19, 2020. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2014–2018. In: HIV Surveillance Supplemental Report 2020;25(No. 1). 2020. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed September 19, 2020. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2017. In: HIV Surveillance Supplemental Report 2019;24(No. 3). 2019. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental- report-vol-24-3.pdf. Accessed September 19, 2020. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project US, 2018 Cycle (June 2018–May 2019). In: HIV Surveillance Special Report 25. 2020. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed September 19, 2020. [Google Scholar]
- 28.Shiau S, Krause KD, Valera P, et al. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020;24:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services. Code of federal regulations, title 45 public welfare, part 46: protection of human subjects. 2009. Available at: https://www.hhs.gov/ohrp/sites/default/files/ohrp/policy/ohrpregulations.pdf. Accessed October 20, 2020.
- 30.Health Resources and Services Administration. HRSA ryan white HIV/AIDS program. HRSA website. Available at: https://hab.hrsa.gov/about-ryan-white-hivaids-program/about-ryan-white-hivaids-program. Accessed September 19, 2020.
- 31.Schneider E, Whitmore S, Glynn MK, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57:1–12. [PubMed] [Google Scholar]
- 32.Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5:e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention, National Center for Health Statistics Reporting System. COVID-NET. Available at: https://www.cdc.gov/coronavirus/2019-ncov/images/need-extra-precautions/high-risk-age.jpg. Accessed September 19, 2020. [Google Scholar]
- 34.Razzaghi H, Wang Y, Lu H, et al. Estimated county-level prevalence of selected underlying medical conditions associated with increased risk for severe COVID-19 Illness—United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtgrave DR, Barranco MA, Tesoriero JM, et al. Assessing racial and ethnic disparities using a COVID-19 outcomes continuum for New York State. Ann Epidemiol. 2020;48:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV Care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essien U, Venkataramani A. Data and policy solutions to address racial and ethnic disparities in the COVID-19 pandemic. JAMA Health Forum. Available at: https://jamanetwork.com/channels/health-forum/fullarticle/2765498. Accessed September 19, 2020. [DOI] [PubMed] [Google Scholar]
- 38.Wen L, Sadeghi N. Addressing Racial Health Disparities in the COVID-19 pandemic: immediate and long-term policy solutions, Health Aff Blog, 2020. Accessed September 19, 2020. doi: 10.1377/hblog20200716.620294. [DOI] [Google Scholar]
- 39.Tartof S, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banthin J, Holahan J. Making Sense of Competing Estimates: The COVID-19 Recession's Effects on Health Insurance Coverage. Urban Institute, Robert Wood Johnson Foundation; 2020. Available at: https://www.urban.org/research/publication/making-sense-competing-estimates-covid-19-recessions-effects-health-insurance-coverage. Accessed September 19, 2020. [Google Scholar]
- 41.Van Slyke A. The collapse of health care: the effects of COVID-19 on U.S. Community health centers. In: Lerner Center for Public Health Promotion. Syracuse University; 2020. Available at: https://lernercenter.syr.edu/2020/08/10/ib-38. Accessed September 19, 2020. [Google Scholar]
- 42.Hoff T, Kates J, Dawson L, et al. Managing HIV During COVID-19: Working to End One Epidemic While Confronting Another. Kaiser Family Foundation; 2020. Available at: https://www.kff.org/policy-watch/managing-hiv-during-covid-19-working-to-end-one-epidemic-while-confronting-another. Accesssed September 19, 2020. [Google Scholar]
- 43.Dawson L, Kates J. An Update on Insurance Coverage Among People with HIV in the United States. Kaiser Family Foundation; 2019. Available at: https://www.kff.org/hivaids/issue-brief/an-update-on-insurance-coverage-among-people-with-hiv-in-the-united-states. Accessed September 19, 2020. [Google Scholar]
- 44.Garfield R, Claxton G, Damico A, et al. Eligibility for ACA Health Coverage Following Job Loss. Kaiser Family Foundation; 2020. Available at: https://www.kff.org/coronavirus-covid-19/issue-brief/eligibility-for-aca-health-coverage-following-job-loss. Accessed September 19, 2020. [Google Scholar]
- 45.Weiser J, Beer L, Frazier EL, et al. Service delivery and patient outcomes in Ryan White HIV/AIDS Program–funded and –nonfunded health care facilities in the United States. JAMA Intern Med. 2015;175:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson-Paul AM, Wei SC, Mattson CL, et al. Obesity among HIV-infected adults receiving medical care in the United States: data from the cross-sectional medical monitoring project and national health and nutrition examination survey. Medicine. 2015;94:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi AI, Rodriguez RA, Bacchetti P, et al. Racial differences in end-stage renal disease rates in HIV Infection versus diabetes. J Am Soc Nephrol. 2007;18:2968–2974. [DOI] [PubMed] [Google Scholar]
- 48.Iachan R, Johnson CH, Harding RL, et al. Design and weighting methods for a nationally representative sample of HIV-infected adults receiving medical care in the United States-Medical Monitoring Project. Open AIDS J. 2016;10:164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beer L, Johnson CH, Fagan JL, et al. A national behavioral and clinical surveillance system of adults with diagnosed HIV (The Medical Monitoring Project): protocol for an annual cross-sectional interview and medical record abstraction survey. JMIR Res Protoc. 2019;8:e15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groves RM. Nonresponse rates and nonresponse bias in household surveys. Public Opin Q. 2006;70:646–675. [Google Scholar]


