Abstract
Reversing brain aging may be possible through systemic interventions such as exercise. We found that administration of circulating blood factors in plasma from exercised aged mice transferred the effects of exercise on adult neurogenesis and cognition to sedentary aged mice. Plasma concentrations of glycosylphosphatidylinositol (GPI)–specific phospholipase D1 (Gpld1), a GPI-degrading enzyme derived from liver, were found to increase after exercise and to correlate with improved cognitive function in aged mice, and concentrations of Gpld1 in blood were increased in active, healthy elderly humans. Increasing systemic concentrations of Gpld1 in aged mice ameliorated age-related regenerative and cognitive impairments by altering signaling cascades downstream of GPI-anchored substrate cleavage. We thus identify a liver-to-brain axis by which blood factors can transfer the benefits of exercise in old age.
The ability to reverse or delay the effects of aging on the brain through systemic interventions such as exercise could help to mitigate vulnerability to age-related neurodegenerative diseases (1–3). Despite the evident benefit of exercise, its application is hindered in the elderly, as physical frailty or poor health can decrease a person’s ability or willingness to exercise (4). Thus, it is critical to identify accessible therapeutic approaches that may confer the benefits of exercise.
In animal models, exercise reverses age-related declines in adult neurogenesis and cognitive function in the aged hippocampus (5–8), a brain region sensitive to the detrimental effects of aging (9). Similarly, transfer of blood from young animals, either by heterochronic parabiosis (in which young and old circulatory systems are joined) or by administration of young blood plasma, improves regenerative capacity and cognition in aged mice (10–15). Given parallels between the effects of exercise and young blood, we tested whether exercise-induced circulating blood factors could confer the beneficial effects of exercise on regenerative and cognitive function in the aged brain. We found that systemic administration of blood plasma derived from mice that exercised ameliorates age-related impairments in adult neurogenesis and cognitive function in the aged hippocampus. Furthermore, we identify glycosylphosphatidylinositol (GPI)–specific phospholipase D1 (Gpld1) as a liver-derived, exercise-induced circulating blood factor sufficient to improve function in the hippocampus of aged mice.
Systemic blood plasma administration transfers the benefits of exercise to the aged hippocampus
We characterized the effect of direct exercise on the aged hippocampus. As a control, we assessed age-related cellular and cognitive impairments in the hippocampus of aged (18 months) relative to young (3 months) mice (fig. S1, A to G). Subsequently, an independent cohort of aged mice was given continuous access to a running wheel for 6 weeks, while age-matched sedentary control mice were provided nesting material (fig. S2A). Direct exercise resulted in increased adult neurogenesis (fig. S2B), increased expression of brain-derived neurotrophic factor (BDNF) (fig. S2C), and improved hippocampal-dependent learning and memory (fig. S2, D to H) in aged mice (5, 16).
We tested whether these effects of exercise on the aged hippocampus could be transferred through administration of exercise-induced circulating blood factors. After exercise, blood was collected and plasma was isolated from exercised and sedentary aged mice and pooled by group. An independent cohort of naïve aged mice was then intravenously injected with plasma from exercised or sedentary aged mice eight times over 3 weeks (Fig. 1A). We analyzed adult neurogenesis by immunohistochemical analysis. Although no difference in the number of neural stem cells expressing Sox2 (sexdetermining region Ybox 2) and glial fibrillary acidic protein (GFAP) was observed (Fig. 1B), we detected an increase in the number of newly born neurons containing doublecortin (Dcx) in the dentate gyrus region of the hippocampus in aged animals that were administered plasma from exercised mice (Fig. 1B). We assessed neuronal differentiation and survival by 5-bromo-2-deoxyuridine (BrdU) incorporation. Mature differentiated neurons express both BrdU and the neuronal marker NeuN. Naïve aged mice that were administered plasma from exercised mice showed an increase in the number of mature neurons expressing both BrdU and NeuN in the dentate gyrus (Fig. 1B). We examined the expression of BDNF by Western blot and observed an increase in hippocampal expression in naïve aged mice that were administered plasma from exercised mice (Fig. 1C). Together, these data indicate that systemic administration of plasma from exercised aged animals can transfer the beneficial effect of exercise on regenerative capacity in the aged hippocampus.
Fig. 1. Systemic administration of exercise-induced circulatory blood factors ameliorates impaired neurogenesis and cognition in the aged hippocampus.
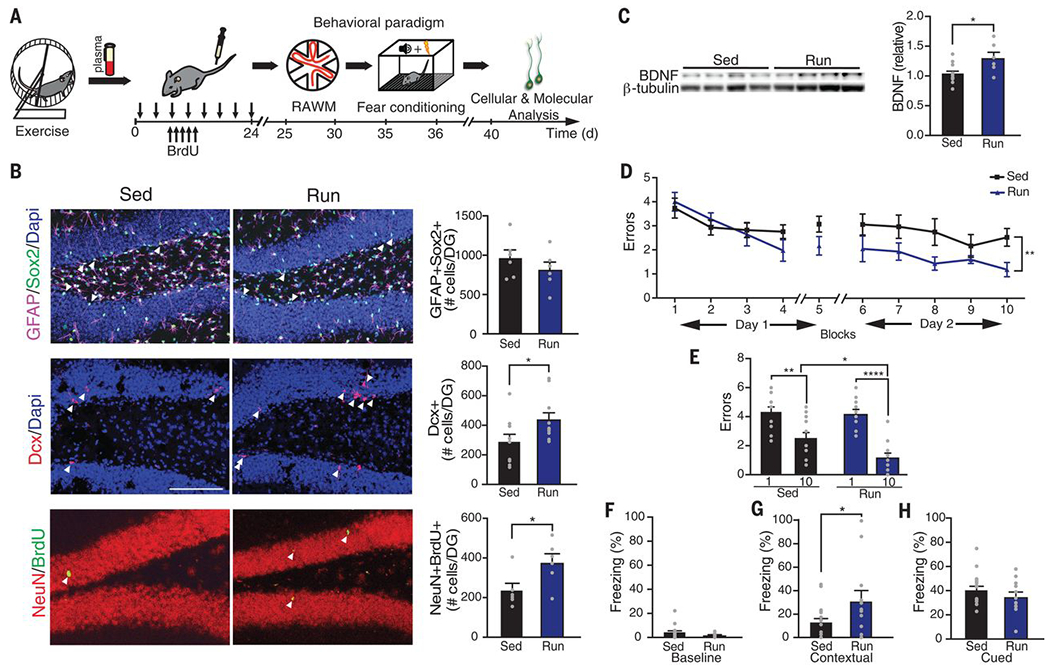
(A) Plasma was collected from exercised or sedentary aged (18 months) mice and administered to sedentary aged mice 8 times over 24 days (100 μl per intravenous injection). Schematic illustrates chronological order of plasma administration from exercised aged mice and cognitive testing. (B) Representative microscopic fields and quantification of GFAP/Sox2 double-positive, Dcx-positive, and NeuN/BrdU double-positive cells in the dentate gyrus (DG) of the hippocampus of naïve aged mice administered plasma from sedentary (Sed) or exercised (Run) aged mice (n = 10 or 11 per group; arrowheads point to individual cells; scale bar, 100 μm). Dapi, 4′,6-diamidino-2-phenylindole. (C) Western blot and quantification of BDNF in the hippocampus of naïve aged mice administered plasma from sedentary or exercised aged mice (n = 6 to 10 per group). Quantification is normalized to β-tubulin. (D and E) Spatial learning and memory were assessed by RAWM as the number of entry errors committed during the training and testing phases. Overall learning and memory were analyzed between block 1 and block 10 (1 block = 3 trials; n = 12 to 15 per group). (F to H) Associative fear memory was assessed using contextual (G) and cued (H) fear conditioning as percent time spent freezing 24 hours after training. Baseline freezing (F) was assessed as the percentage of time spent freezing prior to fear conditioning (n = 12 to 19 per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ****P < 0.0001 [t test in (B), (C), (F), (G), and (H); repeated-measures analysis of variance (ANOVA) with Bonferroni post hoc test in (D); ANOVA with Tukey post hoc test in (E)].
To assess the potential of plasma from exercised mice to rescue age-related impairments in hippocampal-dependent learning and memory, we used the radial-arm water maze (RAWM) and contextual fear conditioning paradigms (Fig. 1A). In the training phase of the RAWM paradigm, all mice showed similar spatial learning capacity (Fig. 1D). When naïve aged mice were administered plasma from aged mice that exercised, they demonstrated improved learning and memory for the platform location during the testing phase of the task relative to animals treated with plasma from sedentary aged mice (Fig. 1, D and E). During fear conditioning training, all mice exhibited similar baseline freezing regardless of treatment (Fig. 1F). However, aged mice receiving plasma from exercised aged mice demonstrated increased freezing in contextual (Fig. 1G), but not cued (Fig. 1H), memory testing. These data indicate that exercise-induced circulating blood factors in plasma can ameliorate impairments in hippocampal-dependent learning and memory in aged mice.
Exercise enhances regenerative capacity in young (17–19) and aged (5–8) animals. Correspondingly, we investigated whether the beneficial effects of exercise observed in mice at younger ages could also be transferred to aged mice through circulating blood factors. We administered plasma derived from exercised or sedentary mature (6 to 7 months) mice to aged mice. As a control, we examined the effect of direct exercise on the hippocampus of mature mice (fig. S3A). Exercise promoted neurogenic and cognitive enhancements in the hippocampus of mature mice (fig. S3, B to H). Next, we collected blood and isolated plasma from exercised and sedentary mature mice and pooled the plasma by group. Naïve aged mice were intravenously injected with the plasma (fig. S4A). To account for any potential benefit of blood from mature animals, we administered saline to an additional aged control group. No significant changes were observed between aged mice that were administered plasma from sedentary mature mice or were given saline. However, administration of plasma from exercised mature mice resulted in increased adult neurogenesis relative to controls (fig. S4B). Thus, exercise-induced circulating blood factors across ages can confer the benefits of exercise on the aged hippocampus.
Gpld1 ameliorates age-related regenerative and cognitive decline in mice
Given that plasma from both aged and mature exercised mice alleviated age-related impairments in the hippocampus of naïve aged mice, we sought to identify individual circulating blood factors that mediated these effects. We used isobaric tagging together with liquid chromatography–tandem mass spectrometry to measure relative amounts of soluble proteins in the plasma from exercised or sedentary aged and mature mice (table S1). The abundance of 30 factors increased after exercise in aged mice (Fig. 2A and fig. S5A), and 33 factors increased after exercise in mature mice (Fig. 2A and fig. S5B). According to the Tabula Muris compendium of single-cell transcriptome data from mice (20) and the protein atlas (21), 63% and 67% of exercise-induced factors in aged and mature mice, respectively, are predominantly expressed in the liver. The abundance of 12 factors—Gpld1, Bche (cholinesterase), Napsa (napsin A), Pon1 (serum paraoxonase 1), Gpx3 (glutathione peroxidase 3), Mbl2 (mannose binding protein C), Ica (inhibitor of carbonic anhydrase), Itih2 (inter-α-trypsin inhibitor heavy chain H2), Pltp (phospholipid transfer protein), Ca2 (carbonic anhydrase 2), Serpinala (α1-antitrypsin 1-1), and Serpinald (α1-antitrypsin 1-4)—was increased in plasma from exercised aged and mature animals (Fig. 2A). Functional enrichment analysis of these factors using STRING, a search tool for the retrieval of interacting genes and proteins, identified largely metabolic processes, in which Gpld1 and Pon1 were overrepresented (Fig. 2B). We elected to investigate Gpld1, a GPI-degrading enzyme (22) not previously linked to aging, neurogenesis, or cognition.
Fig. 2. Exercise increases systemic levels of Gpld1 in mature and aged mice and healthy elderly humans.
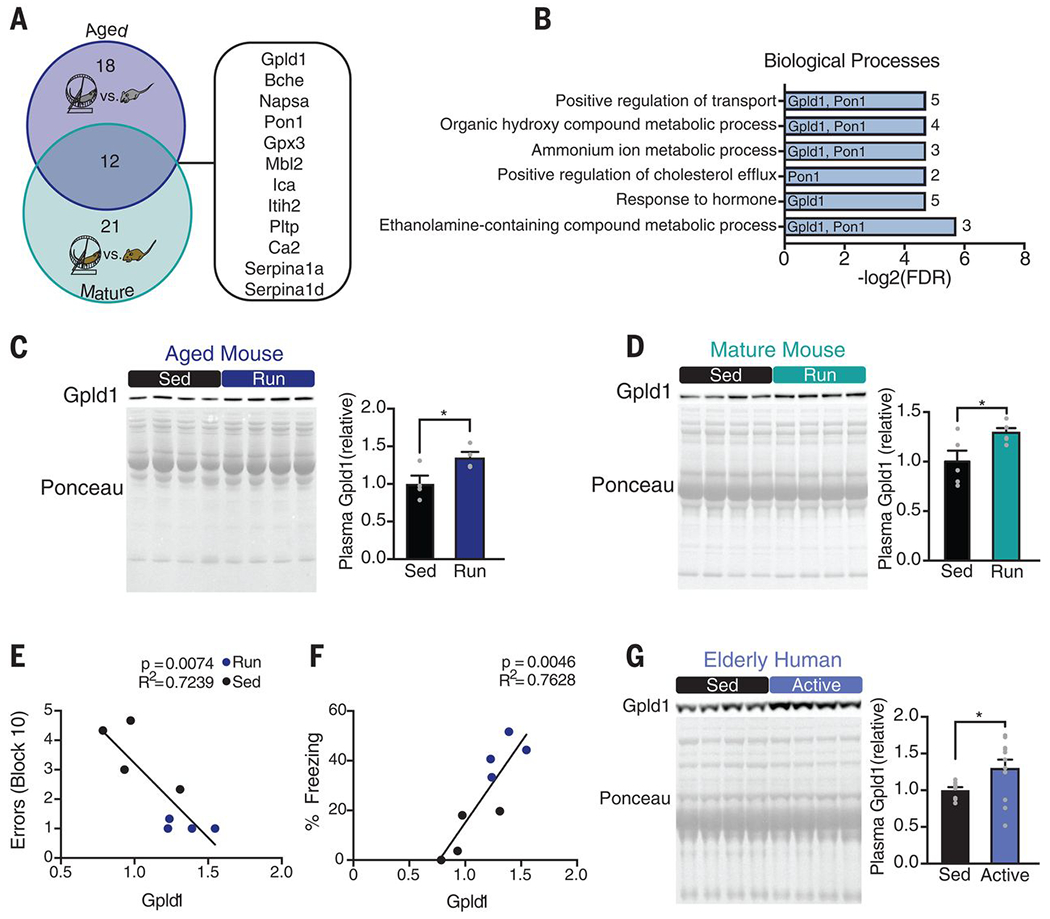
(A) Venn diagram of results from proteomic screens of aged (18 months) and mature (7 months) exercised mice. Numbers of proteins whose concentrations increase with exercise in aged and mature mice are shown in the blue and teal regions, respectively. Proteins common to both groups are listed at the right. (B) Enrichment analysis of the 12 proteins up-regulated by exercise in mature and aged mice. Gpld1 and Pon1 are listed next to the processes in which they are implicated. Numerals at far right are numbers of proteins represented in each process. (C and D) Western blots with corresponding Ponceau S stains and quantification of Gpld1 in equal volumes of blood plasma from individual aged (C) and mature (D) sedentary and exercised mice (n = 4 or 5 per group). (E and F) Correlation between plasma Gpld1 levels in exercised and sedentary aged mice and number of errors committed during the final block of RAWM (E) or time spent freezing in contextual fear conditioning (F). (G) Western blot and quantification of Gpld1 in equal volumes of blood plasma from individual sedentary (<7100 steps per day) and active (>7100 steps per day) healthy elderly humans (aged 66 to 78 years; n = 8 to 12 per group). Data are means ± SEM; *P < 0.05 [t test in (C), (D), and (G); linear regression in (E) and (F)].
We confirmed that concentrations of Gpld1 increased in plasma of individual aged (Fig. 2C) and mature (Fig. 2D) exercised mice relative to those in plasma from sedentary age-matched controls. In exercised and sedentary aged mice, we observed a significant correlation between increased Gpld1 concentrations in plasma and improved cognitive performance in the RAWM and contextual fear conditioning behavioral tests (Fig. 2, E and F). Furthermore, we detected an increase in Gpld1 in plasma from active, healthy elderly human individuals relative to their sedentary counterparts (Fig. 2G). These data identify Gpld1 as an exercise-induced circulating blood factor in aged mice and humans with potential relevance to cognitive function in mice.
To identify the potential source of exercise-induced systemic Gpld1, we characterized Gpld1 mRNA expression in mouse liver, lung, fat, spleen, skin, kidney, heart, muscle, cortex, hippocampus, and cerebellum (Fig. 3A). We detected the highest Gpld1 expression in the liver (Fig. 3A), consistent with previous reports identifying the liver as the primary source of circulating Gpld1 (23). We examined whether Gpld1 mRNA expression changed in the liver as a function of aging, exercise, or plasma administration. No changes in Gpld1 expression were detected during aging or after plasma administration (fig. S6, A and B). Gpld1 expression was increased in the liver of exercised aged mice relative to that in sedentary animals (Fig. 3B). As a control, we evaluated Gpld1 expression in muscle and hippocampus and observed no changes under any condition (fig. S6, C to I). We also observed no change in circulating levels of Gpld1 in plasma with age (fig. S6J). These data are consistent with a role of Gpld1 as a liver-derived, exercise-induced circulating factor in aged mice.
Fig. 3. Increased systemic GPLD1 ameliorates impaired neurogenesis and cognition in the aged hippocampus.
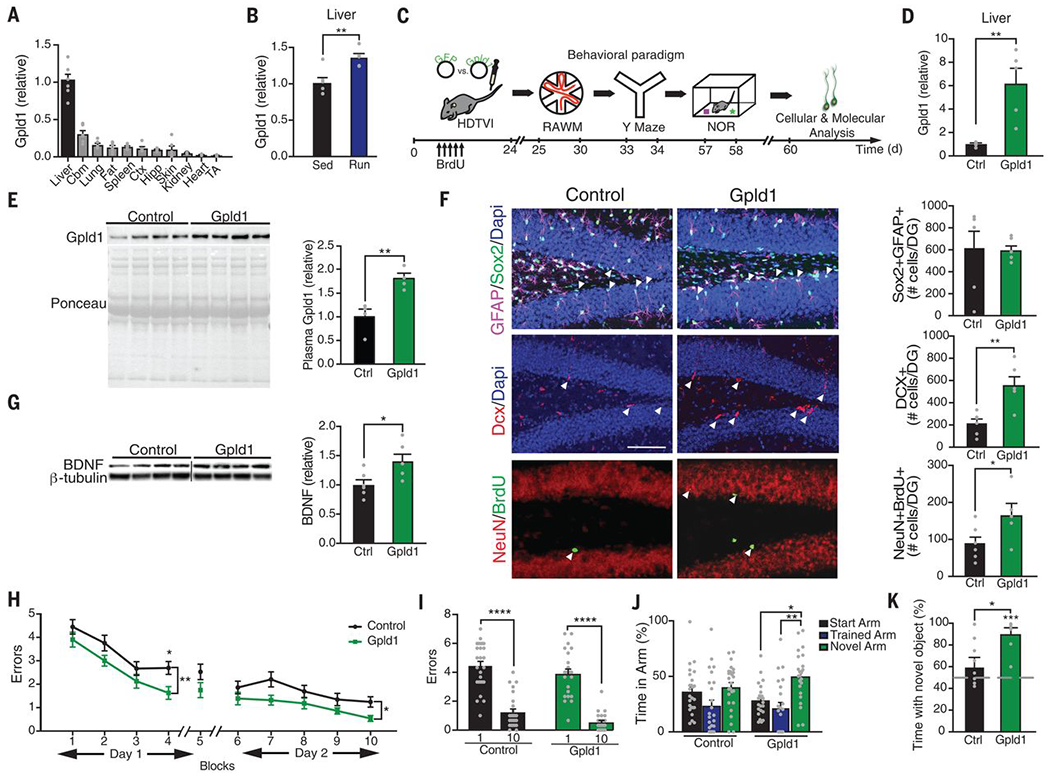
(A and B) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) of Gpld1 across tissues in sedentary aged mice (A) and in liver of exercised and sedentary aged mice (B). Gene expression is measured relative to Gapdh (n = 5 or 6 per group). Abbreviations: Cbm, cerebellum; Ctx, cortex; Hipp, hippocampus; TA, tibialis anterior muscle. (C) Aged (18 months) mice were given HDTVI of expression constructs encoding either Gpld1 or GFP control. Schematic illustrates chronological order of HDTVI, cognitive testing, and cellular and molecular analysis. (D) qRT-PCR of Gpld1 in liver of aged mice expressing Gpld1 or GFP control. Gene expression is measured relative to Gapdh (n = 5 per group). (E) Western blot with corresponding Ponceau S stain and quantification of Gpld1 in equal volumes of blood plasma from individual aged mice expressing Gpld1 or GFP control (n = 4 per group). (F) Representative microscopic fields and quantification of GFAP/Sox2 double-positive, Dcx-positive, and NeuN/BrdU double-positive cells in the DG of the hippocampus of aged mice expressing Gpld1 or GFP control (n = 6 per group; arrowheads point to individual cells; scale bar, 100 μm). (G) Western blot and quantification of BDNF in the hippocampus of aged mice expressing Gpld1 or GFP control (n = 6 per group). Quantification is normalized to β-tubulin. (H and I) Spatial learning and memory were assessed by RAWM as number of entry errors committed during the training and testing phases. Overall learning and memory was analyzed between block 1 and block 10 (1 block = 3 trials; n = 26 per group). (J) Spatial working memory was assessed by YMaze as time spent in the start, trained, and novel arms during the testing phase (n = 23 to 25 per group). (K) Object recognition memory was assessed by NOR as time spent exploring a novel object 24 hours after training (n = 8 to 12 per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 [t test in (B), (D), (E), (F), (G), and (K); repeated-measures ANOVA with Bonferroni post hoc test in (H); ANOVA with Tukey post hoc test in (I) and (J); one-sample t test versus 50% in (K)].
To test the effect of Gpld1 on the aged hippocampus, we used hydrodynamic tail vein injection (HDTVI)–mediated in vivo transfection to overexpress Gpld1 in the liver. Aged mice were injected with expression constructs encoding either Gpld1 or green fluorescent protein (GFP) control, and analysis was done in a time frame consistent with our previous plasma administration experiments (Fig. 3C). Increased Gpld1 mRNA expression in the liver and increased Gpld1 plasma concentrations were confirmed after HDTVI in aged mice (Fig. 3, D and E). By immunohistochemistry and Western blot analysis, we detected an increase in adult neurogenesis (Fig. 3F) and expression of BDNF (Fig. 3G) in the hippocampus of aged mice overexpressing Gpld1 in liver. To assess the effect of Gpld1 overexpression on hippocampal-dependent learning and memory, we used RAWM, forced alternation (Y maze), and novel object recognition (NOR) tests (Fig. 3C). Aged animals overexpressing Gpld1 committed significantly fewer errors in locating the target platform during the RAWM training and testing phases (Fig. 3, H and I) relative to controls. During Y maze and NOR testing, aged mice overexpressing Gpld1 spent significantly more time in the novel arm (Fig. 3J) and with the novel object (Fig. 3K). We also tested whether increasing Pon1, the other liver-derived circulating factor overrepresented in our exercise proteomic functional enrichment analysis (Fig. 2B), ameliorated age-related impairments in hippocampal-dependent cognitive function. Aged mice were given HDTVI with expression constructs encoding either Pon1 or GFP control (fig. S7, A and B); however, no cognitive improvements were observed in a time frame consistent with Gpld1 experiments (fig. S7, C to F). Together, these data indicate that selectively increasing liver-derived systemic concentrations of Gpld1 is sufficient to improve adult neurogenesis and cognitive function in the aged hippocampus.
Coagulation and complement signaling cascades are altered in response to Gpld1
We sought to delineate central versus peripheral mechanisms of action of liver-derived Gpld1. To evaluate the potential of Gpld1 to cross the blood-brain barrier, we generated expression constructs encoding a high-affinity nanoluciferase binary technology (HiBiT)–tagged version of Gpld1 (fig. S8A). HiBiT is a small peptide with high affinity to large BiT (LgBiT), with which it forms a complex that produces a luminescent signal (24), allowing for sensitive and quantitative detection of tagged proteins. Aged mice were given HDTVI with expression constructs encoding HiBiT Gpld1, which we detected in plasma (fig. S8B). We characterized HiBiT activity in mice in plasma, liver, cortex, hippocampus, and cerebellum (fig. S8, B and C). Luminescent signal was detected in plasma and liver (fig. S8, B and C). However, the signal detected in the brain (fig. S8C) was several orders of magnitude lower than that in plasma. Thus, liver-derived systemic Gpld1 appears not to readily enter the brain.
In its canonical role, Gpld1 hydrolyzes GPI anchors that have acylated inositol, releasing membrane-bound GPI-anchored proteins from the cell surface (25–28). Although the physiological role of Gpld1 is not fully understood, cleavage of its substrates regulates signaling cascades important in biological processes such as differentiation and inflammation (27, 29). Therefore, we measured relative amounts of soluble proteins in the plasma of aged mice given HDTVI with expression constructs encoding Gpld1 or GFP control by label-free mass spectrometry (table S2). We surveyed the top 20 up- and down-regulated proteins (Fig. 4A) for known signaling cascades associated with Gpld1 substrates (27) and detected changes in the urokinase-type plasminogen activator receptor (uPAR) signaling pathway. uPAR is a Gpld1 GPI-anchored substrate whose proteolytic function regulates the plasminogen (Plg) activation system involved in coagulation. Nonproteolytic function of uPAR regulates extracellular matrix proteins through interactions with vitronectin (Vtn) (29, 30). On Western blots, we observed decreased amounts of both Plg and Vtn in plasma of aged animals with increased systemic Gpld1 relative to those from control animals (Fig. 4, B and C). We surveyed our previous exercise proteomic analysis (table S1) for proteins that decrease in plasma of aged mice after exercise and also identified Plg (Fig. 4D). We compared functional enrichment analysis using STRING of factors identified to decrease in aged plasma after either Gpld1 overexpression (Fig. 4A) or exercise (Fig. 4D). Consistent with changes in the uPAR signaling pathway, we identified biological processes involved in coagulation as well as the complement system under both conditions (Fig. 4, E and F). Of the factors surveyed after increased systemic Gpld1 and exercise, we identified 80% and 71%, respectively, to be predominantly expressed in the liver according to Tabula Muris (20) and the protein atlas (21). Together, systemic changes in signaling cascades downstream of GPI-anchored substrate cleavage correlate with beneficial effects of Gpld1 and exercise.
Fig. 4. Increased systemic Gpld1 alters signaling cascades downstream of GPI-anchored substrate cleavage in the aging systemic milieu.
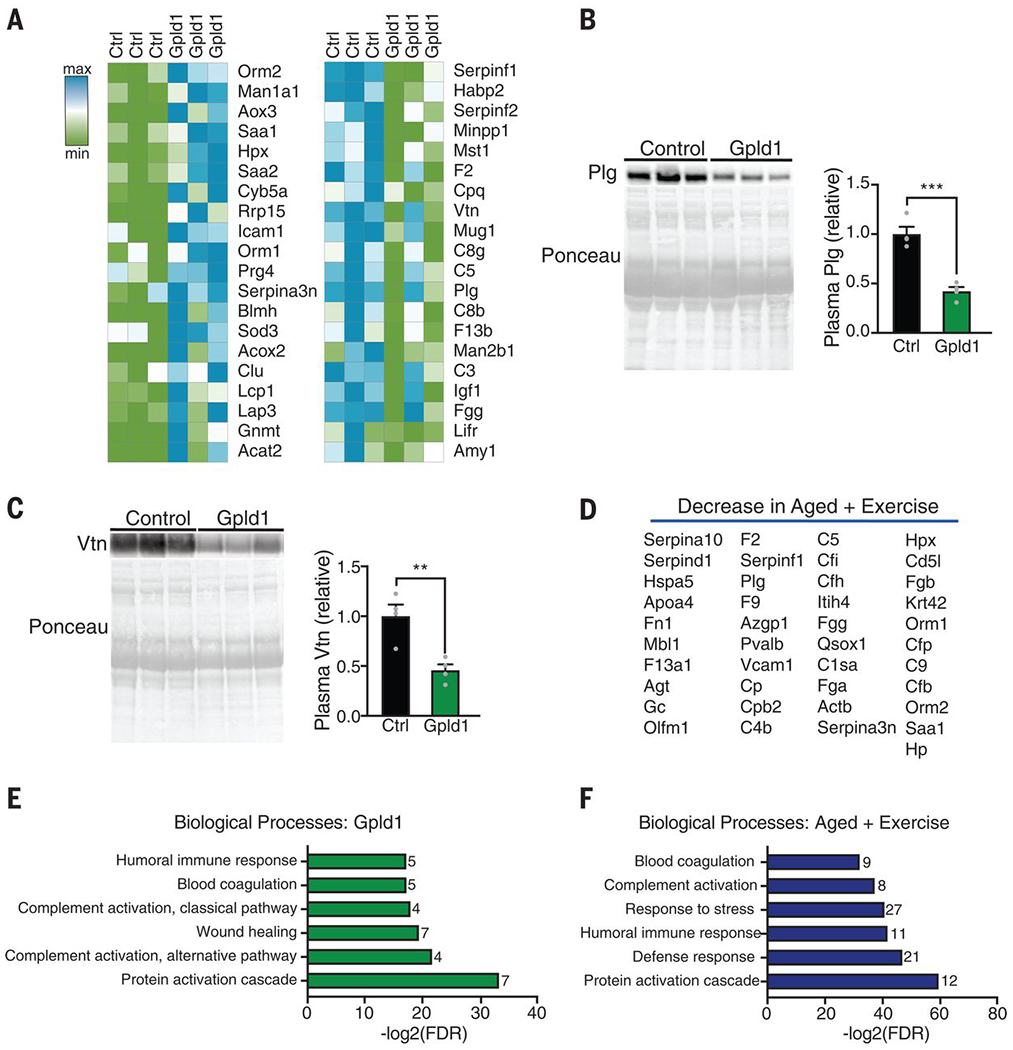
(A) Heat maps of top 20 proteins up- and down-regulated in blood plasma of aged mice after Gpld1 HDTVI relative to GFP HDTVI control, identified by mass spectrometry. (B and C) Western blot with corresponding Ponceau S stain and quantification of plasminogen [Plg; (B)] and vitronectin [Vtn; (C)] in equal volumes of blood plasma from individual aged mice 24 hours after HDTVI of Gpld1 or GFP control (n = 4 per group). (D) List of 41 proteins down-regulated in blood plasma from aged mice after exercise. (E and F) Enrichment analysis of plasma proteins down-regulated with Gpld1 HDTVI (E) or exercise (F) in aged mice, as identified by mass spectrometry. The number of proteins represented in each process is listed to the right of each bar. Data are means ± SEM; **P < 0.01, ***P < 0.001 [t test in (B) and (C)].
GPI-anchored substrate cleavage is necessary for the effects of Gpld1 on the aged hippocampus
We tested whether the enzymatic activity of liver-derived systemic Gpld1, and presumed subsequent GPI-anchored substrate cleavage, directly mediates its effects on adult neurogenesis and cognitive function in the aged hippocampus. The catalytic activity of Gpld1 is dependent on His133 and His158, and mutations at either site abrogate enzymatic activity (31). We generated expression constructs encoding Gpld1 with site-directed His → Asn mutations, and abrogation of GPI-anchored substrate cleavage was validated in vitro (Fig. 5A). Aged mice were injected with expression constructs encoding Gpld1, catalytically inactive His133 → Asn (H133N) Gpld1, or GFP control (Fig. 5B), and plasma concentrations were measured (Fig. 5C). We observed increased adult neurogenesis (Fig. 5D), increased BDNF expression (Fig. 5E), and cognitive improvements in the RAWM and NOR tasks (Fig. 5, F to H) in aged mice with increased expression of Gpld1. However, no differences were observed in aged mice with increased expression of catalytically inactive H133N Gpld1 (Fig. 5, D to H). These data indicate that the enzymatic activity of liver-derived systemic Gpld1 is necessary for its effects on the aged hippocampus, and are consistent with signaling cascades activated after GPI-anchored substrate cleavage as possible molecular mediators of these beneficial effects.
Fig. 5. GPI-anchored substrate cleavage is associated with restorative effects of Gpld1 on the aged hippocampus.
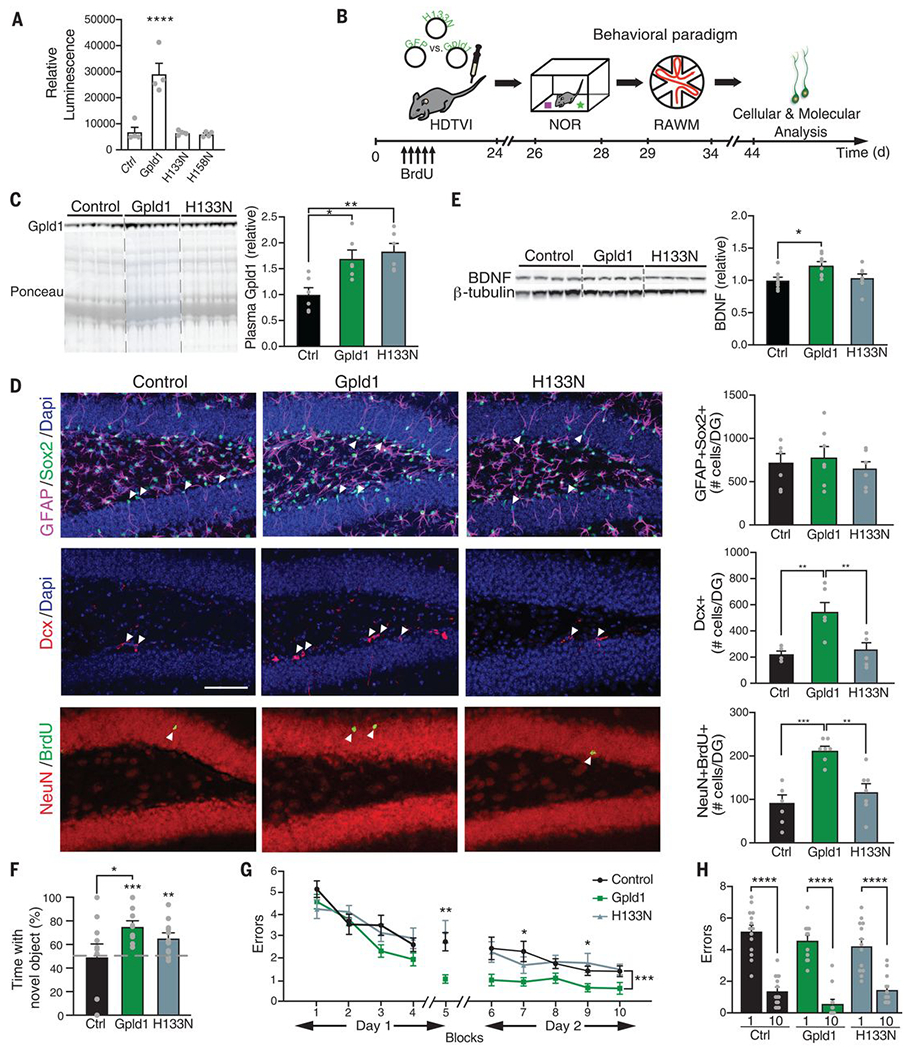
(A) Luminescence-based quantification of alkaline phosphatase activity in cell culture supernatant 48 hours after transfection with ubiquitin-lox-stop-lox-PLAP (GPI-anchored alkaline phosphatase) and EF1a-Cre, in combination with GFP, Gpld1, H133N-Gpld1, or H158N-Gpld1 (n = 3 samples per group). (B) Aged (18 months) mice were given HDTVI of expression constructs encoding Gpld1, catalytically inactive H133N-Gpld1, or GFP control. Schematic illustrates chronological order of HDTVI, cognitive testing, and cellular and molecular analysis. (C) Western blot with corresponding Ponceau S stain and quantification of Gpld1 in equal volumes of blood plasma from individual aged mice expressing Gpld1, Gpld1-H133N, or GFP control (n = 6 per group). (D) Representative microscopic fields and quantification of GFAP/Sox2 double-positive, Dcx-positive, and NeuN/BrdU double-positive cells in the DG of the hippocampus of aged mice expressing Gpld1, Gpld1-H133N, or GFP control (n = 7 per group; arrowheads point to individual cells; scale bar, 100 μm). (E) Western blot and quantification of BDNF in the hippocampus of aged mice expressing Gpld1, Gpld1-H133N, or GFP control (n = 8 per group). Quantification is normalized to β-tubulin. (F) Object recognition memory was assessed by NOR as time spent exploring a novel object 24 hours after training (n = 9 to 11 per group). (G and H) Spatial learning and memory were assessed by RAWM as number of entry errors committed during the training and testing phases. Overall learning and memory were analyzed between block 1 and block 10 (1 block = 3 trials; n = 12 to 14 per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 [repeated-measures ANOVA with Bonferroni post hoc test in (G); ANOVA with Tukey post hoc test in (C), (F), (G), and (H); one-sample t test versus 50% in (F)].
Discussion
Cumulatively, our data show that beneficial effects of exercise on the aged brain can be transferred through administration of blood components. We identified the liver-derived factor Gpld1 as one such factor, and we suspect that signaling cascades activated by GPI-anchored substrate cleavage activity may also participate. Our results identify a liver-to-brain axis by which circulating blood factors confer the beneficial effects of exercise in old age.
Adult neurogenesis in humans remains controversial (32). Nonetheless, adult neurogenesis is reported in the human hippocampus through the ninth decade of life, with age-related decline exacerbated in Alzheimer’s disease (AD) patients (33) and correlating with cognitive dysfunction (34). In the context of dementia-related neurodegenerative diseases, exercise is correlated with reduced risk for cognitive decline in the elderly, improves cognition in populations at risk for AD, and is associated with better neurobehavioral outcomes even in autosomal dominant AD (35–37). Exercise ameliorates impairments in learning and memory in animal models of AD (38, 39) by increasing adult neurogenesis and abundance of BDNF in the aged hippocampus (39)—benefits that we found to be transferred with injected plasma.
Our data identify decreased uPAR signaling, and associated changes in the coagulation and complement system cascades, as potential proaging molecular targets. The effects of liver-derived Gpld1 and exercise are likely the result of changes in multiple signaling cascades. However, a prominent role is emerging for the coagulation and complement pathways in aging. Changes in the coagulation pathway have been identified as part of the senescence-associated secretory phenotype (SASP) (40), and blood-derived complement Clq promotes age-related regenerative decline in peripheral tissues (41). The benefits of targeting members of the coagulation pathway modulated by Gpld1 have been reported in the context of neurodegeneration (42). Genetic mouse models deficient for Plg were protected from demyelination and paralysis in a mouse model of multiple sclerosis (43). Moreover, targeting blood-derived Plg through oligonucleotide technologies decreased amyloid β plaque deposition and neuropathology in a mouse model of AD (42). Given that transfer of young blood simultaneously elicits central (3, 9) and peripheral (44–46) enhancements in regenerative capacity in aged mice, our data raise the possibility that the beneficial effects of exercise could be promoted broadly across tissues through circulating blood factors.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Brack and J. Sneddon for critically reading the manuscript.
Funding: Supported by Hillblom Foundation predoctoral (A.M.H.) and postdoctoral (K.B.C.) fellowships, an Irene Diamond AFAR postdoctoral fellowship (G.G.), a National Institute on Aging (NIA) Ruth L. Kirschstein NRSA fellowship (AG064823, A.B.S.), NIA [AG058752 (K.B.C.), AG023501 (J.H.K.), AG053382 (S.A.V.), AG067740 (S.A.V.)], and a gift from M. and L. Benioff (S.A.V.).
Footnotes
Competing interests: The authors declare no conflict of interest. A.M.H., X.F., and S.A.V. are named as inventors on a patent application arising from this work.
Data and materials availability: All data needed to understand and assess the conclusions of this study are included in the text, figures, and supplementary materials.
REFERENCES AND NOTES
- 1.Erickson KI et al. , Proc. Natl. Acad. Sci. U.S.A 108, 3017–3022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maass A et al. , Mol. Psychiatry 20, 585–593 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Horowitz AM, Villeda SA, F1000 Res. 6, 1291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes RE et al. , Sports Med. 28, 397–411 (1999). [DOI] [PubMed] [Google Scholar]
- 5.van Praag H, Shubert T, Zhao C, Gage FH, J. Neurosci 25, 8680–8685 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Rani A, Tchigranova O, Lee W-H, Foster TC, Neuroblol. Aging 33, 828.e1–828.e17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK, Brain Behav. Immun 28, 25–43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto I et al. , PLOS Biol. 13, e1002279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Wheatley EG, Villeda SA, Annu. Rev. Neurosci 40, 251–272 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Villeda SA et al. , Nature 477, 90–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villeda SA et al. , Nat. Med 20, 659–663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsimpardi L et al. , Science 344, 630–634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellano JM et al. , Nature 544, 488–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khrimian L et al. , J. Exp. Med 214, 2859–2873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gontier G et al. , Cell Rep. 22, 1974–1981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C-W et al. , J. Appl. Physiol 105, 1585–1594 (2008). [DOI] [PubMed] [Google Scholar]
- 17.van Praag H, Christie BR, Sejnowski TJ, Gage FH, Proc. Natl. Acad. Sci. U.S.A 96, 13427–13431 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugert S et al. , Cell Stem Cell 6, 445–456 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ, Proc. Natl. Acad. Sci. U.S.A 107, 2367–2372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabula Muris Consortium Nature 562, 367–372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlén M et al. , Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Scallon BJ et al. , Science 252, 446–448 (1991). [DOI] [PubMed] [Google Scholar]
- 23.Maguire GA, Gossner A, Ann. Clin. Biochem 32, 74–78 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Schwinn ΜK et al. , ACS Chem. Biol 13, 467–474 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Davitz MA et al. , Science 238, 81–84 (1987). [DOI] [PubMed] [Google Scholar]
- 26.Metz CN et al. , EMBO J. 13, 1741–1751 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujihara Y, Ikawa M, J. Lipid Res 57, 538–545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low MG, Prasad ARS, Proc. Natl. Acad. Sci. U.S.A 85, 980–984 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Rosso M et al. , Curr. Pharm. Des 17, 1924–1943 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Blasi F, Sidenius N, FEBS Lett. 584, 1923–1930 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Raikwar NS, Bowen RF, Deeg MA, Biochem. J 391, 285–289 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorrells SF et al. , Nature 555, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Jiménez EP et al. , Nat. Med 25, 554–560 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Tobin MK et al. , Cell Stem Cell 24, 974–982.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lautenschlager NT et al. , JAMA 300, 1027–1037 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Geda YE et al. , Arch. Neurol 67, 80–86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller S et al. , Alzheimers Dement. 14, 1427–1437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Intlekofer KA, Cotman CW, Neurobiol. Dis 57, 47–55 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Choi SH et al. , Science 361, eaan8821 (2018).30190379 [Google Scholar]
- 40.Wiley CD et al. , Cell Rep. 28, 3329–3337.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naito AT et al. , Cell 149, 1298–1313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker SK et al. , Proc. Natl. Acad. Sci. U.S.A 115, E9687–E9696 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw MA et al. , J. Neurosci 37, 3776–3788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conboy IM et al. , Nature 433, 760–764 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Sinha M et al. , Science 344, 649–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baht GS et al. , Nat. Commun 6, 7131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


