Abstract
Introduction:
The mortality of coronavirus disease 2019 (COVID-19) is frequently driven by an injurious immune response characterized by the development of acute respiratory distress syndrome (ARDS), endotheliitis, coagulopathy, and multi-organ failure. This spectrum of hyperinflammation in COVID-19 is commonly referred to as cytokine storm syndrome (CSS).
Areas covered:
Medline and Google Scholar were searched up until 15th of August 2020 for relevant literature. Evidence supports a role of dysregulated immune responses in the immunopathogenesis of severe COVID-19. CSS associated with SARS-CoV-2 shows similarities to the exuberant cytokine production in some patients with viral infection (e.g.SARS-CoV-1) and may be confused with other syndromes of hyperinflammation like the cytokine release syndrome (CRS) in CAR-T cell therapy. Interleukin (IL)-6, IL-8, and tumor necrosis factor-alpha have emerged as predictors of COVID-19 severity and in-hospital mortality.
Expert opinion:
Despite similarities, COVID-19-CSS appears to be distinct from HLH, MAS, and CRS, and the application of HLH diagnostic scores and criteria to COVID-19 is not supported by emerging data. While immunosuppressive therapy with glucocorticoids has shown a mortality benefit, cytokine inhibitors may hold promise as ‘rescue therapies’ in severe COVID-19. Given the arguably limited benefit in advanced disease, strategies to prevent the development of COVID-19-CSS are needed.
Keywords: Coronavirus disease 2019 (COVID-19), hyperinflammation, cytokine storm syndrome, cytokine release syndrome, hemophagocytic lymphohistiocytosis
1. Introduction
The Coronavirus disease 2019 (COVID-19) pandemic is an ongoing threat to global health. COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported to have emerged in humans in December 2019 and has since spread rapidly worldwide [1]. To date, the numbers of people infected by the SARS-CoV-2 and deaths directly attributable to COVID-19 continue to increase or are again rising in areas with previously declining new case burden. As of October 2020, more than 36 million individuals worldwide were diagnosed with COVID-19 and more than 1 million deaths were reported [2].
A major cause of morbidity and mortality in SARS-CoV-2-associated pneumonia is the progression to acute respiratory distress syndrome (ARDS) [1]. Data from both experimental animal models and clinical studies of other viral syndromes suggest a model of immune-mediated damage caused by virus-associated dysregulation of immune responses in the genetically or immunologically susceptible host [3-5]. In infection with two other highly pathogenic coronaviruses, SARS-CoV-1 and MERS-CoV, this state of hyperinflammation has been likened to a cytokine storm syndrome (CSS). Evidence suggests that COVID-19 is similarly characterized by a deleterious activation of proinflammatory pathways, potentially related to dysregulated T cell responses, delayed type I interferon (IFN) responses, and exuberant production of cytokines, accompanied by increases in morbidity and mortality [3,4]. Targeting immune dysregulation with the goal to ameliorate ARDS and prevent multi-organ failure holds some promise as a potential therapeutic pathway. However, caution must be exercised, as immunomodulatory therapy may blunt host innate and adaptive responses during active viral replication. In this review, we summarize the evolving evidence supporting hyperinflammation as a pathogenetic mechanism for severe COVID-19 infection as well as therapeutic strategies currently in use and under investigation.
Given the rapid evolving changes in Covid19 knowledge, the authors chose to use Google search in addition to a Medline search using ‘Covid-19, SARS-CoV-2, Cytokine storm.’ The last search date was 15 August 2020.
2. Hyperinflammation in COVID-19 and infection with other virulent coronaviruses
2.1. Immune dysregulation in severe coronavirus infection
2.1.1. Animal and in vitro models of severe respiratory coronavirus infection
Coronaviruses are a family of enveloped non-segmented positive-sense RNA viruses broadly distributed in humans and animals. Of the coronaviruses known to cause infection in humans, three are now recognized to cause fatal respiratory disorders: the severe acute respiratory syndrome coronavirus (SARS-CoV-1) and SARS-COV-2, and Middle East respiratory syndrome coronavirus (MERS-CoV) [5].
Although no direct evidence implicates proinflammatory cytokines as the cause of lung damage, the observation of patterns of proinflammatory cytokine production in animal and in-vitro models of SARS-CoV-1 and MERS-CoV infection supports the idea of a shared pathophysiology of immune dysregulation. Chu et al. examined the comparative abilities of MERS-CoV and SARS-CoV-1 to trigger a cytokine response in monocyte-derived dendritic cells and found that both induced upregulation of TNF-α and IL-6 during the same time frame that peak viral titers were observed in culture [6]. Zhou et al. similarly examined infectivity and innate immune response-related cytokines in monocyte-derived macrophages (MDMs) and found that MERS-CoV and SARS-CoV-1 showed a sustained induction of MCP-1, MIP-1α, and IL-8, whereas MERS-CoV-infected MDMs produced higher levels of these chemokines [7]. As expected, TNF-α and IL-6 were induced at high levels by both MERS-CoV and SARS-CoV-1. Interestingly, IFN-γ induction in MDMs was much more prominent than IFN-α and IFN-β [7]. Lau et al. examined cytokine profiles in MERS-CoV- and SARS-CoV-1-infected polarized airway epithelial Calu-3 cells and found that both viruses caused a delayed induction of IL-1β, IL-6, and IL-8, with MERS-CoV triggering a stronger response than SARS-CoV-1 [8]. Animal models of MERS and SARS are more heterogeneous in terms of symptom severity, limiting the usefulness of rodent models. However, in more rapidly lethal and systemically symptomatic models, elevations of pro-inflammatory cytokines such as IL-6, IP-10, and IL-8 were observed in lung and brain tissues [9-11].
Animal models that model human COVID-19 are emerging. Mouse models – without genetic modification – do not support SARS-CoV-2 infection due to the inability of spike protein to bind the murine ortholog of its cognate receptor human angiotensin-converting enzyme 2 (hACE2) [12]. Recent efforts to overcome these limitations using transgenic and adeno-associated virus-mediated expression of hACE2 in mice allow for SARS-CoV-2 infection and replicate features of COVID-19 [12-14]. Israelow et al. found expansion of infiltrating myeloid-derived inflammatory cells and inflammatory monocyte-derived macrophages (CD64+ CD11c-CD11b+Ly6C+) in the diseased lungs at days 2 and 4 post-infection, which was paralleled by increases in activated CD4+ and CD8 + T cells as well as natural killer (NK) cells. These cellular changes in the lungs were associated with increased cytokines and interferon-stimulated gene signatures, including a subset of 45 genes specific to type I IFN signaling, similar to what has been observed in the lungs of patients with COVID-19 [12]. IFN-α receptor- and IFN regulatory transcription factor 3/7-deficient mice showed markedly decreased recruitment of monocytes/macrophages, and activation of CD4 + T cells, CD8 + T cells, and NK cells in infected lungs, highlighting the role of type I IFNs in the immunopathology of SARS-CoV-2 pneumonia [12]. Unlike the sequelae observed in human disease, this model of SARS-CoV-2 infection in young mice did not recapitulate the mortality observed in COVID-19-ARDS and CSS. In another model of hACE2 transgenic mice, SARS-CoV-2 infection resulted in isolated pulmonary pathology with interstitial pneumonia and was associated with transient weight loss, but otherwise did not recapitulate COVID-19-CSS [14]. By contrast, HFH4-hACE2 transgenic C3B6 mice showed evidence of a multi-system disease (including interstitial pneumonia, elevation in CPK and AST, cardiac muscle edema and myocyte necrosis, sporadic neuro-invasion) and variably showed weight loss, respiratory distress, neurological symptoms, and mortality, reminiscent of human disease [13]. Cynomolgus macaques were permissive to SARS-CoV-2 infection and showed limited pulmonary lesions with evidence of diffuse alveolar damage but remained asymptomatic at day 4 post-infection [15]. It remains unclear whether SARS-CoV-2-related hyperinflammation is replicated in non-human primate models, similar to the cytokine storm observed during lethal infection with 1918 influenza virus in macaques [16]. Animal models that comprehensively replicate the dysregulated immune responses and associated immunopathology of COVID-19, can model the response to prophylactic and immunosuppressive therapies, and inform their timing will remain a priority.
2.1.2. Proinflammatory cytokines are associated with disease severity in coronavirus infection
Macrophage infiltration of the lungs, their presence in bronchoalveolar lavage (BAL) fluid, high concentrations of proinflammatory mediators, and hemophagocytosis suggest that host responses contribute to the immunopathogenesis of SARS and MERS [3,4,17-20]. Understanding this hyperinflammatory state and relative contributions of host immune responses to morbidity and mortality is of interest in identifying targets for potential treatment. Several pro-inflammatory cytokines have been found to be elevated in patients with severe pneumonia associated with SARS-CoV-1 and MERS-CoV, including IL-1β, IL-6, IL-12, IL-15, IL-17, IFN-γ, IP-10, and MCP-1 [4,21]. More severe courses of SARS and MERS are associated with persistent fever, lung infiltrates, and higher plasma levels of proinflammatory cytokines including IL-6, MCP-1, and IP-10 [3,4]. The difference is most pronounced in the second week of illness, days after peak viral loads were observed. Elevated levels of IL-6 and IL-8 were observed in SARS patients, and IL-6 and IP-10 in MERS patients, suggesting that a dysregulated immune response phase occurs after the viral replication stage. Similar findings of elevated levels of pro-inflammatory cytokines have been reproducibly demonstrated in COVID-19 [3,22]. IL-6 elevation in particular has received considerable attention as a marker of disease severity and in-hospital mortality [23-29]. Several other cytokines have been associated with disease severity in COVID-19, including IL-2 R, IL-8, IL-10, TNF-α, IP-10, MCP-3, IL-1RA, but not IL-1β [27,28,30,31]. In a large cohort of 1,484 patients TNF-α and IL-6 were the only two cytokines that significantly and independently predicted mortality [27]. In an early study, patients requiring ICU admission had higher concentrations of IL-2, IL-7, IL-10, IL-12, G-CSF, MCP-1, MIP-1A, and TNF-α than did those not requiring ICU admission [32]. Elevation of other chemokines and cytokines has been reported in target tissues and circulation however, elevation of IL-6 and other cytokine levels alone may not reliably differentiate moderate from severe COVID-19 or even signify a hyperinflammatory state [33,34]. One study of peripheral blood mononuclear cells (PBMCs) found increased polyclonal granulocyte-macrophage colony-stimulating factor (GM-CSF)+ CD4 T cells in COVID-19 patients compared to healthy controls and GM-CSF-responsive CD14+ CD16+ monocytes capable of producing IL-6, suggesting a mechanism by which dysregulation of T cell responses can contribute to the overproduction of cytokines seen in hyperinflammation [35].
Despite the association between high levels of pro-inflammatory cytokines and increased disease severity, it should be emphasized that this observation does not establish a causal relationship. Conversely, while the cryopyrin-associated periodic syndromes (CAPS) are driven by deregulated release of IL-1β and can be treated effectively with anakinra or other IL-1 blocking agents [36] Serum levels of IL-1β are below the level of detection even during CAPS flares. More mechanistic research will be needed to determine the roles of specific cytokines in COVID-19 pathophysiology.
2.1.3. Delayed type I IFN responses may lead to more severe coronavirus infection
IFNs are produced during a viral infection and are critical to orchestrate innate and adaptive antiviral immune responses [5]. IFNs induce the production of antiviral effector proteins, thereby inhibiting intracellular viral replication. Type 1 IFNs (IFN-I) activate interferon-stimulated genes (ISG) that are involved in inflammation, signaling, and immunomodulation. A deficient IFN-I response can lead to reduced antiviral activity. In addition, IFN-I treatment has been studied in MERS-CoV and SARS-CoV-1 infection alone or in combination with other antivirals, glucocorticoids, or IFN-γ, although studies in patients have thus far been disappointing [5,6]. Some have used IFN-α2b sprays to reduce the infection rate of SARS-CoV-1 in children [37]. In an elegant demonstration, Channappanavar et al. showed that a robust viral replication and delayed IFN-I responses were detrimental in SARS-CoV-1-infected mice due to influx of inflammatory monocytes-macrophages into target tissue, which led to severe forms of SARS [37]. Additionally, they found that inflammatory monocytes-macrophages were the predominant source of the proinflammatory cytokines CCL2, TNF-α, and IL-6 in SARS-CoV-1-infected lungs. A strong and persistent expression of IFN and ISGs, associated with impaired T cell and antibody responses, was also associated with fatal cases of SARS [38].
Emerging information implicates impairment of the type I interferon response in the pathogenesis of severe COVID-19 infection. Blanco-Melo et al. found that SARS-CoV-2 impairs expression of type I and III IFN genes in a SARS-CoV-2 animal model as well as in lung tissue and sera from patients with COVID-19 [39]. Trouillet-Assant et al. examined the kinetics of the plasma IFN-I response in 26 critically ill patients with COVID-19 and found that all (5 out of 5) patients with sustained lack of IFN-α2 production required invasive ventilation compared to 9 out of 21 patients with the expected peak of IFN-α2 8–10 days after onset of symptoms; all patients had sustained elevation of C-reactive protein (CRP) and IL-6 levels [40]. Hadjadj et al. reported that plasma levels of IFN-α2 at 8–12 days after symptom onset were lower in 18 critically ill patients with COVID-19 than in 15 patients with mild-to-moderate symptoms [41]. Gene expression analysis also showed that ISGs such as MX1, IFITM1, and IFIT2 were down-regulated, but serial measurements were not reported [41]. Taken together, these findings support the concept of delayed or impaired IFN responses causing delayed viral clearance and increased proinflammatory cytokine release, ultimately causing severe disease. While we are still striving to fully understand the pathophysiology of COVID-19, this model could explain part of the wide range of clinical presentations of SARS-CoV-2 infection.
2.2. Clinical presentation of COVID-19
2.2.1. Progression of COVID-19
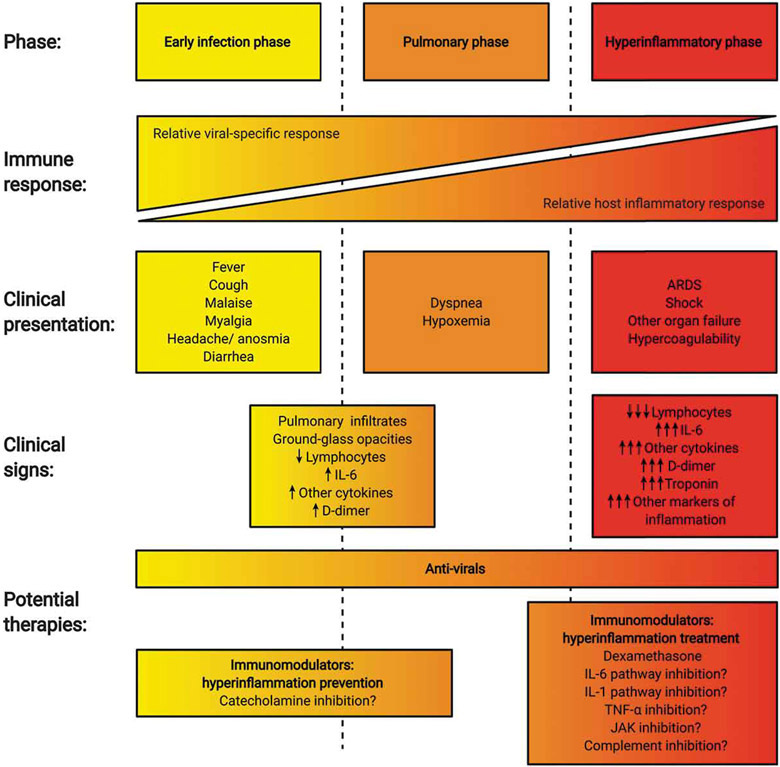
Infection with SARS-CoV-2 leads to a wide range of clinical manifestations ranging from asymptomatic to severe, life-threatening disease with a median time from illness onset to clinical resolution of 22 · 0 days (IQR 18 · 0–25 · 0). In the adaptive immune phases of infection, COVID-19 may be complicated by ARDS, multi-organ failure (including heart and kidney failure), sepsis/septic shock, and death [1,42]. The outcome of COVID-19 is thought to be largely governed by the interplay between the virus and host antiviral immune responses [43]. Although more studies are needed to establish a reliable clinical staging system, the initial state of COVID-19 infection appears to be relatively mild, with nonspecific symptoms such as malaise, fever, myalgia, dry cough, and diarrhea. While the virus multiplies in the host cells, primarily affecting the lower respiratory system but also the gut and nasopharynx, mild respiratory and systemic symptoms predominate (stage 1). In a second stage of the infection, patients develop a viral pneumonia with bilateral infiltrates and ground-glass opacities, leading in some patients to dyspnea (7 · 0 (IQR 4 · 0–9 · 0) days from illness onset) or hypoxemia requiring hospitalization (stage 2) [1]. A minority of patients will progress to the most severe form of illness, characterized by ARDS with or without an extrapulmonary systemic hyperinflammation syndrome, commonly referred to as CSS (stage 3) [44] (Figure 1).
Figure 1.
Clinical phases of COVID-19 and potential strategies for the prevention and treatment of hyperinflammation (‘cytokine storm’). Disease progression in COVID-19 can be categorized based on the severity of clinical signs and symptoms in addition to the development of objective imaging and laboratory abnormalities. A hyperinflammatory state – following an initial viral replication phase – can be present in patients with severe disease. Potential approaches to ameliorate COVID-19 include strategies to reduce binding of SARS-CoV-2 to its cognate receptors (not shown), antiviral therapy (e.g. remdesivir), and the prevention and treatment of hyperinflammation and immune-mediated end-organ damage. ARDS: acute respiratory distress syndrome. ARDS: acute respiratory distress syndrome. IFN: interferon. IL: interleukin. JAK: Janus kinase. TNF: tumor necrosis factor.
2.2.2. Clinical and laboratory features of severe COVID-19
The severe presentation of COVID-19 peaks around 7–14 days after onset of illness and is associated with respiratory distress, and hyperinflammation [1,45]. Compared to moderate cases, patients with severe cases of COVID-19 have more chest tightness, tachypnea, and dyspnea with frequent hypoxemia requiring invasive mechanical ventilation [46] (see Table 1).
Table 1.
Clinical and laboratory features of COVID-19-CSS in comparison to other states of hyperinflammation/immune dysregulation. COVID-19-CSS shares clinical and laboratory features with HLH, MAS, CRS, and sepsis but does not recapitulate any one of these related conditions entirely. ARDS: acute respiratory distress syndrome. CD: cluster of differentiation. CRS: cytokine-release syndrome. CSS: cytokine storm syndrome. HLH: hemophagocytic lymphohistiocytosis. IL: interleukin. MAS: macrophage activation syndrome. NK: natural killer.
| HLH-04 Criteria* | HScore | MAS Criteria | CRS features |
Sepsis features |
COVID-19-CSS features | |
|---|---|---|---|---|---|---|
| Fever | x | x | x** | x | x (or hypothermia) | x |
| Organomegaly | splenomegaly hepatomegaly (may be driven by congestion and thrombosis) | splenomegaly and | hepatomegaly | splenomegaly and hepatomegaly | ||
| Cytopenias | ||||||
| Anemia | x | x | x | x | x | |
| Thrombocytopenia | x | x | ≤181 x10^9/mL | x | x | x |
| Neutropenia | x | x | x | x | x | |
| Hypertriglyceridemia or hypofibrinogenemia | x | ≥132.7 mg/dL | >156 mg/dL | Unknown | ||
| x | ≤250 mg/dL | ≤360 mg/dL | x | x | x | |
| Hemophagocytosis on biopsy | x | x | x | x | x | Limited evidence |
| Low/absent NK cell activity | x | Unknown | ||||
| Hyperferritinemia | x | ≥2000 ng/mL | >684 ng/mL** | x | x | x |
| Elevated soluble CD25 (soluble IL-2 receptor) | x | x | x | x | ||
| Organ system dysfunction | x | x | x | x | ||
| Liver enzyme elevation | Elevated AST≥30 IU/L | AST>48 IU/L | AST/ALT elevated | AST/ALT elevated | AST/ALT elevated | |
| Other evidence of organ system dysfunction | Acute kidney injury not attributable to other cause; respiratory failure; altered mental status; hypotension and potential cardiac failure; coagulopathy. | Similar to CRS, HLH, MAS | Similar to CRS, HLH, MAS with lung-predominant organ system dysfunction (hypoxemia, ARDS); prominent coagulopathy. | |||
| Genetic testing supporting the diagnosis | Genetic mutations in PRF1, UNC13D, STX11, STXBP2 gene, among others. | Genetic mutations in primary HLH-associated genes (most commonly PRF1, UNC13D) are found in MAS: at least 1 heterozygous variant is found in 45% of MAS; >1 variant is identified in a majority of those. | Unknown | Unknown | Unknown |
Five out of eight criteria must be fulfilled unless family history or molecular diagnosis is consistent with HLH.
Required in patients with known or suspected sJIA, in addition to any two of the other laboratory abnormalities.
Despite an increase in white blood cell count, severe SARS-CoV-2 infection is associated with lymphopenia (particularly in CD4 + T cells and CD8 + T cells but not in B cells). As we discussed, the levels of proinflammatory cytokines IL-6, IL-2 R, IL-10, and TNFα are markedly elevated in severe cases. Elevated levels of transaminases, creatinine, creatine kinase, LDH, D-dimer, ferritin, cardiac troponin, and NT-pro-BNP were all more frequently seen in deceased patients [1,33]. In our experience, elevations of these acute phase proteins and pro-inflammatory cytokines correspond to COVID-19 disease progression. In particular, some patients with severe disease appear to have a hyperinflammatory state characterized by very high D-dimer levels, elevated CRP, and elevated ferritin. Coagulopathy with pulmonary thrombosis, microangiopathy, and multi-organ system dysfunction, including acute kidney injury and cardiomyopathy, can also be seen [47].
2.2.3. Pathological studies of COVID-19
There are relatively few reports of histopathological data from patients with COVID-19. In autopsies of 75 fatal cases of COVID-19 pneumonia, the main pulmonary findings included diffuse alveolar damage with hyaline membrane formation, fibrin exudates, epithelial damage, and diffuse type II pneumocyte hyperplasia [34,35,48]. Interstitial lymphocytic inflammatory infiltrates were seen in only one patient; in the same patient, multinucleated syncytial cells with atypical enlarged pneumocytes and viral cytopathic-like changes in the intraalveolar spaces were also visible [34]. Another case series revealed evidence of predominantly thrombotic injury without similar fibroproliferative or viral cytopathic changes in the lung [49]. Thrombotic microvascular injury with complement C5b-9 deposition was seen in skin tissue of three and lung tissue of two of these five patients [49]. Similarly, lung pathology of four patients who died of COVID-19 revealed small, firm thrombi in sections of the peripheral parenchyma, platelets, and thrombi in small vessels, and foci of hemorrhage [50]. Similar findings were seen in the form of deep venous thrombosis and pulmonary thromboembolism in a series highlighting COVID-19-associated coagulopathy [51]. In patients who had their hearts examined, there was scarce evidence of inflammation. While some had only few interstitial mononuclear inflammatory infiltrates and no other substantial damage, others also had evidence of scattered individual cell myocyte necrosis [34,35,50]. None had a significant lymphocytic inflammatory infiltrate consistent with the typical pattern of viral myocarditis [34,35,50]. Reports of hemophagocytosis among autopsy series are relatively sparse, especially from bone marrow biopsy specimens [52,53]. While the presence or absence of hemophagocytosis by itself does not define cytokine storm, this sparsity may suggest a divergence between COVID-19-CSS and other hyperinflammatory syndromes.
2.2.4. Predictors of mortality and severity in severe COVID-19
Age, male sex, chronic hypertension, and other cardiovascular comorbidities are risk factors for death due to COVID-19 [33]. Leukocytosis, persistent and severe lymphopenia, as well as elevated transaminases, creatinine, creatine kinase, LDH, D-dimer, ferritin, cardiac troponin, and NT-pro-BNP were all more frequently seen in patients who died from COVID-19 [1,33]. In a multivariable logistic regression model, older age, higher Sequential Organ Failure Assessment (SOFA) score, and elevated D-dimer were associated with increased odds of death [1]. Concentrations of IL-6, IL-8, IL-10, TNF-α, and IL-2-receptor were also significantly higher in patients who died than those who recovered [1,33]. In a study from Germany, the authors found that IL-6 levels above 80 pg/mL were strongly associated with the need for mechanical ventilation and may identify patients at highest risk of respiratory failure [54]. While it remains unclear whether IL-6 directly contributes to organ-damage in severe COVID-19 or merely represents a biomarker, its elevation in severe COVID-19 patients has raised suspicion that it can trigger or sustain cytokine storm. Hyperinflammatory syndromes such as MAS, HLH, CRS, and the viral response to SARS-CoV-2, while similar in their cytokine profile and associated laboratory parameters, should be differentiated and compared with caution.
3. Comparison of severe COVID-19 to other syndromes with hyperinflammation
3.1. HLH and MAS
HLH is the prototypical hyperinflammatory syndrome that is driven by T and NK cells and associated with an often fatal cytokine storm [55,56]. HLH is subdivided into two categories. Familial or primary HLH is associated with various genetic defects in the perforin cytotoxic pathway and commonly autosomal recessive in inheritance. Patients with secondary or reactive HLH (rHLH) can also have underlying mutations and/or polymorphisms affecting genes of the perforin pathway and have identifiable triggers including cancer or infection, commonly Epstein Barr virus (EBV), cytomegalovirus (CMV), human immunodeficiency virus (HIV), or influenza [56,57]. When triggered by an autoinflammatory/autoimmune disorder, the term macrophage activation syndrome (MAS) is commonly applied in the literature [58,59].
Clinical manifestations of HLH include fever, lymphadenopathy, hepatosplenomegaly, and at times complicating neurological symptoms. Laboratory features include marked cytopenia, elevated liver enzymes, hypertriglyceridemia, hyperferritinemia, and hypofibrinogenemia. Bone marrow findings include many well-differentiated macrophages phagocytosing other hematopoietic elements (e.g. erythrocytes, platelets, or granulocytes). A decrease in ESR elevation has been proposed as a characteristic feature that is secondary to fibrinogen consumption and liver dysfunction [60]. Marked elevation in ferritin is seen and reflects activation and active production by macrophages. NK cell function is abnormal due to cytotoxic pathway defects and soluble IL-2 R is elevated due to ineffective immune cell activation when target cell killing is impaired [61]. Despite many clinical and laboratory abnormalities, there is no pathognomonic feature of this cytokine storm and several classifications have been proposed (Table 1). Unfortunately, classification criteria for HLH and MAS most likely cannot be directly applied to COVID-19-CSS [62,63].
3.2. Cytokine release syndrome (CRS)
Cytokine-release syndrome (CRS) is a dysregulated systemic inflammatory response seen as a common and severe complication of cancer immunotherapy as well as other forms of immunomodulatory therapy [64-66]. More recently, CRS has drawn attention as a complication of chimeric antigen receptor (CAR) T cell therapy, an approved treatment for multiple B cell malignancies [67-70]. In these cases, simultaneous activation of a large number of CAR T cells by engagement with their cognate antigen on cancer cells causes release of inflammatory cytokines and chemokines, triggering further cytokine release from monocytes, macrophages, dendritic cells, and other immune cells [71,72]. Biomarkers can include expansion of CAR T cell numbers and elevation in a variety of inflammatory proteins, chemokines, and cytokines including IL-6 [73,74].
CRS can manifest clinically on a spectrum of acuity ranging from constitutional symptoms of fever, fatigue, headache, and myalgia to severe end-organ toxicity, hemodynamic shock, respiratory failure, and death [67,68,70]. In rare cases, CAR T cell therapy has been linked to the induction of an rHLH/MAS-like syndrome, which may be a progression to the most severe end of the CRS spectrum [75,76]. Common features of CRS as well as HLH and MAS are shown in Table 1. Both treatment factors such as the CAR construction, target, and dose, as well as patient characteristics such as age, comorbidities, and tumor burden are believed to affect the risk, severity, and presentation of CRS.
3.3. Management of HLH, MAS, and CRS
3.3.1. Management of pHLH and rHLH/MAS
In all cases of HLH, early recognition and treatment initiation is key to reduce morbidity and mortality. In children, the primary goal of pHLH is to suppress the life-threatening inflammatory process, often using an etoposide-based treatment induction regimen (dexamethasone, etoposide, intrathecal methotrexate, and cyclosporine) [77]. The goal of aggressive induction therapy in pHLH is to achieve remission and bridge to allogeneic hematopoietic stem cell transplantation (HSCT) [78]. In adults, tailored treatment approaches with dose reduction and duration as well as a modified diagnostic approach that factors in age and potential alternative drivers of the disease needs to be considered [79].
For rHLH secondary to malignancy, the underlying malignancy is usually targeted, with additional HLH-specific treatment including glucocorticoids and etoposide. In rHLH secondary to infection, treatment of the underlying infection is balanced with additional HLH-specific treatments including glucocorticoids, etoposide, and cytokine targeted therapy, including IL-1 and/or IL-6 pathway inhibition, as well as rituximab in the case of EBV-related rHLH [79,80]. Emapalumab, an interferon-γ neutralizing antibody, has also been successfully used in the treatment of refractory HLH as well as in a case series of patients with rHLH/MAS [81,82].
A targeted treatment approach is also followed by both pediatric and adult rheumatologists when treating MAS. Due to low event rates, phase 3 clinical trials in patients with MAS are difficult to perform, and most treatments used are extrapolated from our understanding of systemic juvenile idiopathic arthritis (sJIA), one of the most common underlying disorders associated with MAS. IL – 1β, IL – 6, and IL-18 have been implicated in the immunopathogenesis of sJIA and possible therapeutic targets in MAS. Both IL-1 pathway inhibition with anakinra, a non-glycosylated form of human IL – 1Ra that competitively inhibits binding of IL-1α and/or IL-1β, and canakinumab, a human monoclonal anti-IL-1β antibody, as well as IL-6 R inhibition with tocilizumab have shown promising results in treating sJIA, but do not prevent the development of MAS in all patients that achieve control of their arthritis [59,83,84]. Anakinra is also used in the treatment of MAS despite difficulties in performing clinical trials in MAS and lack of phase III clinical trials, it can be effective in patients who fail to achieve remission with glucocorticoids and cyclosporine alone [85]. Higher doses of anakinra may be required to treat MAS than sJIA. Interestingly, phase III trials of canakinumab in sJIA did not show a dramatic reduction in the incidence of MAS, which was usually triggered by infection [84]. Indeed, the occurrence of new-onset MAS in patients with sJIA in clinical trials of tocilizumab and canakinumab demonstrates that the inhibition of either IL-1 or IL-6 alone does not provide full protection against MAS [59]. While IL-1 and IL-6 may be contributing factors in the development of MAS, other cytokines may be critical drivers of immunopathogenesis when these cytokine signaling pathways are inhibited. IL-1- or IL-6-targeted therapies may also benefit those with hypomorphic genetic variants, as they are susceptible to MAS triggered by infection.
3.3.2. Management of CRS
Management strategies for CRS with CAR T cell therapies have traditionally weighed the need to mitigate CRS effects against the potential for immunosuppression to abrogate anti-tumor efficacy and increase infection susceptibility. As such, mild or moderate CRS is managed with supportive care and often resolves without the need for pharmacologic intervention. Informed by the elevation of IL-6 in CRS, high-grade CRS is managed with anti-IL-6 or anti-IL-6 R blocking antibodies [68,70]. Tocilizumab is an FDA-approved anti-IL-6 R monoclonal antibody for treating CRS. Glucocorticoids are also administered, typically only when severe CRS is refractory to tocilizumab [68,70,86,87]. In current practice, immunosuppressive therapies are generally withheld until the presentation of severe CRS with the intention of preserving immune treatment efficacy. However, emerging evidence suggests that targeted therapies inhibiting IL-6 signaling and glucocorticoids may not decrease response rates or durability [88]. Whether immunosuppressive treatments can be applied earlier in the course of CRS without compromising efficacy remains an active area of investigation.
3.3.3. Consideration for the treatment of hyperinflammatory syndrome secondary to SARS-CoV-2
While considering similarities between hyperinflammation in CRS and COVID-19, it is also important to appreciate distinctions in pathophysiology, clinical setting, and treatment goals that may influence the potential translatability between treatments and biomarkers for COVID-19-CSS and CRS. CSS in COVID-19 develops after a prolonged period of crosstalk between the innate and adaptive arms of the immune system in response to viral dynamics, whereas CRS is triggered by the abrupt infusion of a large number of activated T cells [89]. In CRS, synthetic constructs drive signaling pathways that are qualitatively and quantitatively distinct from signaling driven through natural TCRs [90]. Lymphodepleting regimens administered prior to CAR T cell therapy infusion influence cytokine signaling along with immune cell interactions [91]. Treatment goals and acceptable downsides also differ between CRS and COVID-19-CSS. For example, anti-IL-6(R) therapies may not decrease anti-cancer activity for CAR T therapies but could blunt antiviral innate and adaptive immunity at a time of active viral replication in the infected host [92]. Thus, caution should be used in considering evidence of hyperinflammation in cases of patients with COVID-19 prior to recommending immunomodulatory therapy.
4. Approaches in the treatment of COVID-19-CSS
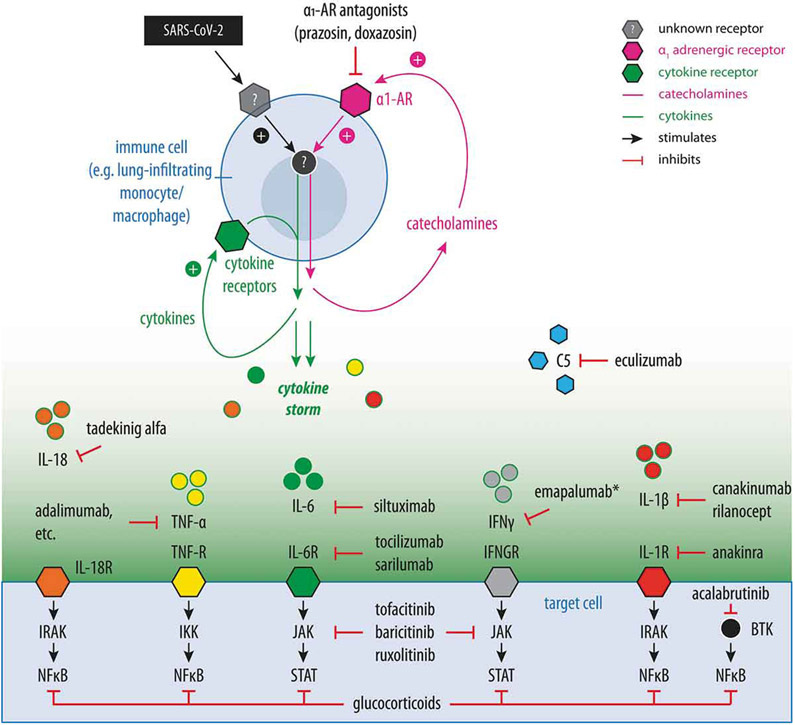
There is a paucity of published randomized controlled trials that can inform the use and timing of immunosuppressive therapy in patients with severe COVID-19. Clinical trial registries reflect the large and ever-growing spectrum of approaches hypothesized to ameliorate COVID-19-CSS. While the ‘compassionate’ off-label use of some of these medications may be appropriate in critically ill patients (especially when clinical trials are not available to treating physicians and supportive care is insufficient), cautious and judicious use of these drugs is paramount until reliable data regarding their efficacy from clinical trials becomes available and their safety in this unique patient population is established. A list of current prospective clinical trials on immunosuppressive and immunomodulatory agents in COVID-19 can be found through a variety of COVID-19 clinical trial trackers [93-96]. Figure 2 summarizes the mechanisms of many of the targeted therapeutic strategies discussed below.
Figure 2.
Summary of key therapeutic strategies investigated for the prevention or treatment of COVID-19-CSS and ARDS. Model of an inflammatory feed-forward loop in immune cells (e.g. lung-infiltrating monocytes and macrophages) in response to SARS-CoV-2 infection that results in exuberant cytokine production and cytokine storm. Drugs currently investigated for the treatment or prevention of severe COVID-19 and their respective targets are shown. Catecholamine and cytokine feed-forward loops are shown in magenta and green, respectively. α-1 AR: α-1 adrenergic receptor. BTK: Bruton tyrosine kinase. C5: Complement factor 5. IFN: interferon. IKK: I kappa B kinase. IL: interleukin. IRAK: Interleukin-1 receptor-associated kinase. JAK: Janus kinase. NFκB: Nuclear Factor kappa-light-chain-enhancer of activated B cells. R: receptor. STAT: signal transducers and activators of transcription. TNF: tumor necrosis factor.
4.1. Biologics
4.1.1. IL-6 pathway inhibition
The observation of systemically elevated IL-6 levels in patients with severe COVID-19 suggests a potential role for anti-IL-6 or anti-IL-6 R antibodies in the treatment of COVID-19-ARDS and CSS. Drawing from immuno-oncology, the success of tocilizumab in managing CAR-T cell-related CRS provides a further clinical rationale for studying therapeutics modulating the IL-6 axis in COVID-19-CSS [68,70,97]. Candidate agents include tocilizumab and sarilumab, both of which target the IL-6 R, and siltuximab, which neutralizes IL-6 directly.
Several studies have reported anti-IL-6/IL-6 R treatment in COVID-19. The National Clinical Trial (NCT) registry reflects tocilizumab’s position as the main IL-6/IL-6 R antagonist under investigation (see www.clinicaltrials.gov), with most trials studying IL-6/IL-6 R antagonists at dosing approved for CAR-T-associated CRS. Primary endpoints for these trials range from acute changes in vital signs and laboratory parameters of uncertain clinical significance (e.g. defervescence or reduction in CRP) to ICU length of stay and mortality measures (e.g. 28-day survival). Tocilizumab was the first monoclonal antibody of this class reported to be of potential benefit in severe COVID-19, mirroring its success in treating CRS associated with CAR-T cell therapy [68,70,98]. Notably, however, a randomized-controlled trial of tocilizumab in severe COVID-19-associated pneumonia reported no difference in clinical status or mortality in patients who received tocilizumab compared to those who received placebo [99]. Another randomized controlled trial of sarilumab in patients with COVID-19 receiving mechanical ventilation also did not reveal a significant mortality benefit [100]. However, in these studies, treatment was neither given nor assessed based on the inflammatory state, leaving open the possibility that hyperinflammation could respond to inhibition of the IL-6 pathway [101]. Moreover, trends toward potential benefit in more severe disease were noted.
A historically controlled study of 172 patients with COVID-19-associated hyperinflammation found a benefit in respiratory status, length of hospital stay, and mortality in patients treated with tocilizumab and glucocorticoids compared to glucocorticoids alone, in addition to low-quality evidence from case series. The largest published series of 100 tocilizumab-treated patients with hyperinflammatory syndrome or ARDS related to COVID-19 found an improvement in respiratory status in 77%, though 20% died despite treatment [102]. Smaller case series of moderately to critically ill patients have reported similar findings of improvement in respiratory status after one to three doses of tocilizumab [98,103,104]. Data from another uncontrolled case series of siltuximab in 21 patients with COVID-19-ARDS revealed that 7 (33%) were clinically improved, 9 (43%) had no change in clinical status, and 5 (24%) had a deterioration in condition after a median of 8 days of follow-up [105]. A potential disconnect in effect sizes reported in cases series and uncontrolled studies on one hand and those observed in placebo-controlled clinical trials of IL-6 signaling pathway inhibitors on the other is noted.
While some of these preliminary studies show promise in select populations, care must be taken in critically assessing measures of clinical response used in these studies. As a reduction in CRP and other acute-phase reactants is expected with IL-6/IL-6 R inhibitor therapy, these changes may not reflect normalization on the target tissue level and cannot be equated with meaningful clinical improvement. Treatment with anti-IL-6/IL-6 R therapies in the setting of clinical trials is recommended, especially given the lack of established efficacy of these agents in severe COVID-19-associated pneumonia. When such trials are not available, decisions on compassionate use of these drugs are best made in an interdisciplinary team of infectious disease physicians, pulmonologists, rheumatologists, hematologists, and other primary team members following local guidance documents.
4.1.2. IL-1 pathway inhibition
Data examining the use of anakinra in patients with sepsis and severe organ dysfunction showed some benefit in a subgroup of patients with sepsis and concurrent features of HLH/MAS [106]. Based on its safety profile in sepsis and its efficacy in rHLH/MAS, IL-1 inhibition has been proposed as a treatment of COVID-19-CSS [59,107,108].
While anakinra and canakinumab are being used off-label in the treatment of severe COVID-19, we are limited to observational studies on the safety and efficacy of IL-1 antagonism in COVID-CSS. A proof of concept study using anakinra in nine patients with moderate to severe COVID-19 pneumonia showed improvement in oxygen requirements, markers of inflammation, and clinical outcomes [109]. Another retrospective cohort study comparing intravenous (IV) and lower dose subcutaneous (SC) anakinra to standard of care demonstrated that IV anakinra slowed systemic inflammation and improved respiratory function in those with moderate to severe COVID-19 ARDS in a non-ICU setting, but no evidence of benefit for SC anakinra in this study [110]. Another larger case–control study compared outcomes in 52 consecutive hospitalized patients with COVID-19 receiving SC anakinra at a single center in France with 44 historical controls who received standard of care. Anakinra use was associated with significantly lower risk of admission to the ICU and death (25% vs.73%, HR:0.22; 95% CI 0.11–0.41) [111]. Major limitations of these studies are the use of historical comparator groups and small cohort sizes.
The effects of IL-1 inhibition on antiviral immunity and clearance of SARS-CoV-2 are not known, and safety profiles may vary independent of mechanism of action based on pharmacokinetics alone, as half-lives are significantly longer for canakinumab (26 days) and rilonacept (7 days) compared to anakinra (4–6 hours) [112-114]. The observational studies above cannot be interpreted for safety outcomes. Consequently, the results of ongoing clinical trials will be helpful in evaluating efficacy and safety of IL-1 inhibitors alone or in combination with other agents compared to standard of care in COVID-19-CSS (NCT04324021).
4.1.3. Tumor necrosis factor-α inhibition
Anti-TNF-α therapies are widely used in the treatment of autoimmune diseases including rheumatoid arthritis and inflammatory bowel disease. In these conditions, TNF-α appears to represent a critical signaling node, and inhibition has proven effective in a subset of patients despite upregulation of numerous cytokines. Case reports describe effective use of the anti-TNF-α agent etanercept in MAS, although other case reports suggest that etanercept can induce or exacerbate MAS [115--115-120]. Feldmann et al. has postulated that a single infusion of an anti-TNF-α agent may be able to reduce lung inflammation in COVID-19 [121,122]. Registry data from the COVID-19 Global Rheumatology Alliance showed that in 600 patients the adjusted odds ratio for hospitalization was 0.40 (95% CI 0.19–-0.81) compared to no disease-modifying anti-rheumatic drug [123]. Data from the SECURE-Inflammatory Bowel Disease Registry demonstrated that anti-TNF reduced the adjusted odds of hospitalization or death 0.60 (95% CI 0.38–0.96), but not death alone or the composite outcome of ICU, hospitalization, or death [124]. However, these registries do not address COVID-19-associated hyperinflammation. The prospect of using anti-TNF for the treatment of coronavirus infection has also been proposed prior to the current SARS-CoV-2 pandemic on the basis of its potential to reduce severe inflammatory sequelae [125]. While they should be interpreted with caution there are also eight reported cases of COVID-19 being treated with anti-TNF and showing clinical improvement [126-128]. The effects of anti-TNF-α therapies on viral replication in SARS-CoV-2 infection are not known [129]. However, a signal for improved outcomes from two large registries of patients with autoimmune disease and COVID-19 is reassuring. Notably, patients with these conditions are treated with anti-TNF-α therapies at baseline for their underlying inflammatory disease (i.e. prior to infection), suggesting that these drugs may have a role in the prevention of severe COVID-19.
4.1.4. Interferon-γ inhibition
The involvement of IFN-γ in the pathogenesis of the hyperinflammatory syndrome is complex and potentially complicated by its antiviral role. Emapalumab, an interferon-γ neutralizing antibody, has been approved for the treatment of refractory HLH. Remarkably, in one case report of refractory HLH in the setting of multiple viremias, viral clearance was not negatively impacted [81]. Emapalumab was also used in a series of patients with rHLH/MAS related to sJIA who had not improved on high-dose glucocorticoids; patients had improvement in clinical and laboratory parameters [130]. Potential interference with antiviral host immune responses may be expected in early use of anti-INF-γ therapy, and the safety of this approach needs to be established. Emapulumab is currently being studied along with anakinra in reducing hyperinflammation in COVID-19 (NCT04324021). However, in light of conflicting data on levels of IFN-γ in severe COVID-19, emapalumab should not be used outside of clinical trials and controlled studies are needed to evaluate its efficacy, optimal timing of administration, and safety.
4.1.5. GM-CSF inhibition
GM-CSF has pleiotropic and complex roles in homeostasis and inflammation, leading to varied hypotheses on the effects of increasing or decreasing GM-CSF signaling as a therapeutic strategy for COVID-19 [131,132]. The role of GM-CSF in promoting proliferation of pulmonary epithelial cells and maintenance of alveolar macrophage function supports the hypothesis that increasing GM-CSF signaling may be beneficial in early stages of COVID-19. A trial investigating the administration of the recombinant GM-CSF sargramostim is underway. In contrast, GM-CSF also has roles in inflammatory signaling cascades, suggesting that inhibition of GM-CSF signaling may ameliorate COVID-19-CSS. A prospective cohort study of patients with severe COVID-19 treated with the anti-GM-CSF receptor antibody mavrilumab found that treatment was associated with improved clinical outcomes. Compared to 26 patients in a standard of care control group, 13 patients in the mavrilumab treatment group had a decreased risk of death and a shorter time to clinical improvement [133]. Additional trials of mavrilumab for patients with COVID-19 are ongoing, as are trials of the anti-GM-CSF agents gimsilumab, lenzilumab, namilumab, otilimab, and TJM2. Data from these larger and randomized trials will be necessary to determine the efficacy of this potentially promising strategy for COVID-19-CSS.
4.1.6. Complement factor 5 inhibition
Drugs that inhibit the complement pathway can reduce immune-mediated damage in complementopathies and certain autoimmune rheumatic diseases [134]. Not surprisingly, complement pathway activation has been observed to regulate inflammatory responses in animal models of SARS-CoV-1 and influenza infection [135,136]. Inhibition of complement C5 has been found to attenuate CSS and inflammatory lung injury in animal models [136,137]. In addition, reports of microangiopathy associated with complement deposition in patients with COVID-19 suggest a potential pathogenetic role for complement pathway activation, and role for complement inhibition, in COVID-19 [49]. A recently developed C3 inhibitor (AMY-101), the anti-C5 antibody eculizumab, and BDB-001, another C5 antagonist currently in development, have been used in small case series of one, four, and two patients with severe COVID-19, respectively, with reported improvement in clinical and laboratory parameters. C5 antagonists are currently being studied in COVID-19 (NCT04288713, 2020L00003) [138-140].
4.2. Small molecules
4.2.1. Janus kinase (JAK) inhibition
Small molecules that inhibit intracellular signaling through the JAK/signal transducers and activators of transcription (JAK-STAT) pathway, including baricitinib, tofacitinib, and ruxolitinib, reduce downstream production of cytokines and thereby inflammation [141]. Tofacitinib and baricitinib are commonly used in the treatment of autoimmune disorders and rheumatic diseases [142]. The JAK1/2 inhibitor ruxolitinib has shown promise in rHLH [143]. Beyond its effects on JAK-STAT signaling, baricitinib was predicted to have a direct antiviral activity by interrupting viral entry into cells by interfering with AP2-associated protein kinase 1 (AAK1) which is involved in receptor-mediated endocytosis [144,145]. How these in-silico predictions translate into clinical practice remains to be determined. In an exploratory open-label, non-randomized study of patients with moderate COVID-19 pneumonia, 12 consecutive patients who received baricitinib plus ritonavir-lopinavir for two weeks were compared to a prior cohort of 12 consecutive patients who received ritonavir-lopinavir plus hydroxychloroquine. Fever, oxygenation, and CRP significantly improved in the baricitinib-treated group compared with historical controls, and no escalation of therapy to ICU level care was required in the former group [146]. Clinical trials examining the efficacy and safety of JAK inhibitors in COVID-19 are ongoing (see www.clinicaltrials.gov). Ruxolitinib in addition to standard of care was evaluated in a small (n = 14) pilot study; 11/14 patients showed sustained clinical improvement, and particularly improvement in markers of inflammation, without significant short term toxicity. A multicenter phase-II clinical trial has been initiated (NCT04338958). Despite these promising preliminary data, caution should be used with JAK inhibitors due to their association with increased risk of thromboembolic events, which is of particular concern in patients with severe COVID-19, in addition to the well-characterized activity of all JAK inhibitors in suppressing antiviral interferons [1,147].
4.3. Other approaches
4.3.1. Glucocorticoids
As an immunosuppressive therapy, glucocorticoids could confer a benefit of attenuating hyperinflammation in COVID-19-CSS patients, though potential benefits must be considered in light of the risks of broad immunosuppression and other specific adverse effects. Prior evidence has shown that glucocorticoids are not associated with reduced mortality but are associated with delayed viral clearance in SARS-CoV, MERS-CoV, and influenza infection, and an analysis in ARDS of any cause found insufficient evidence to support glucocorticoids use [148-150]. Retrospective analyses of glucocorticoids use in COVID-19 patients have reported conflicting results. Some studies have found associations between glucocorticoids use and increased risk of mortality or worsened clinical courses [151-154]. Other studies have suggested that glucocorticoids use was associated with reduced mortality risk or better clinical courses, and others still have found no association between glucocorticoids use and outcomes [32,153-160] [161-164]. In the COVID-19 Global Rheumatology Alliance physician-reported registry, chronic use of prednisone dose ≥10 mg/day was associated with higher odds of hospitalization [123]. Timing of administration in the disease course, doses, and patient population characteristics are likely contributing to the contrasting data. In fact, a preliminary analysis of the controlled, open-label, RECOVERY trial found that in patients hospitalized with COVID-19, the use of dexamethasone (at a dose of 6 mg daily for up to 10 days) resulted in lower 28-day mortality among those who were receiving oxygen or invasive mechanical ventilation than usual care. For patients on ventilators, the treatment was shown to reduce mortality by about one-third, and for patients requiring only oxygen, mortality was reduced by about one-fifth. Importantly, this was not observed in patients who did not require oxygen or respiratory support [163]. Based on this report, the WHO is currently revising its guidelines which until now indicated that glucocorticoids should not be routinely given for the treatment of patients with COVID-19 requiring respiratory support 164].
4.3.2. Colchicine
Colchicine is a nonselective inhibitor of NLRP3 inflammasome that reduces the production of IL-1β [165]. The NLRP3 inflammasome is thought to play a role in the development of ARDS and acute lung injury and viroporin E, a protein expressed by SARS-CoV-1, was previously shown to activate the inflammasome [165,166 167 168] . In SARS-CoV-2, viroporin E is thought to play a major role in viral replication. The possibility of improving vascular inflammation and endothelial dysfunction has prompted 4 trials studying colchicine use in COVID-19 [168]. Two studies of colchicine use in COVID-19 have been reported; however, data on patients’ hyperinflammation status were not reported. In a randomized trial of 105 hospitalized patients with relatively mild COVID-19 infection, colchicine was given as a loading dose followed by 1 mg daily. Patients in the colchicine group had less risk of clinical deterioration (1.8% vs 14%) and better cumulative event-free 10-day survival rate (97% vs 83%) while there was no difference in CRP or troponin levels between the groups [169,170]. In the other series, 122 patients were given 1 mg daily, reduced to 0.5 mg daily for severe diarrhea (occured in 7.4%); the historical comparison standard of care group had 37% mortality while the colchicine group had 16% mortality [171]. However, 58% of the colchicine group received dexamethasone, compared to 32% of the historical comparison group. This limited evidence is not yet sufficient to support use of colchicine in COVID-19-CSS.
4.3.3. Intravenous immunoglobulin (IVIG)
IVIG therapy can exert immunomodulatory effects to reduce end-organ damage in autoimmunity and hyperinflammation. IVIG has been used in attempts to reduce CSS due to HLH, although there is little evidence for its efficacy [172,173]. A prior case series also suggested a role for IVIG in the treatment of severe SARS and associated cytopenias [174]. Thus far, evidence supporting the use of IVIG in severe COVID-19 is limited to case reports [175,176]. Hyperimmune IVIG and convalescent plasma have been pursued as a treatment for COVID-19 based on prior success in influenza A and small studies in COVID-19 [176,177]. To date, evidence from controlled trials has been conflicting as to their success in severe COVID-19, and treatment is not given based on hyperinflammation [178,179]. Due to the current paucity of supporting evidence and risk of thromboembolic events associated with IVIG therapy, especially due to reports of hypercoagulability in severe COVID-19, IVIG, hyperimmune globulin, and convalescent plasma remain investigational [1,180].
4.3.4. Plasma exchange
Therapeutic plasma exchange (TPE) has previously been proposed as a rescue therapy in patients with inflammatory conditions in order to remove pathogenic cytokines and other drivers of inflammation in plasma, and specifically in COVID-19-ARDS [181]. Use of TPE has been reported in a case series of three critically ill patients with COVID-19 with subsequent improvement in inflammatory markers; however, one of the three died from complications of the illness [182]. A larger pilot study showed laboratory and clinical improvement among 10 patients with COVID-19-associated ARDS enrolled in a pilot study of TPE without any documented adverse effects [183]. Another non-randomized study of 11 critically ill patients treated with TPE in addition to standard of care showed improved laboratory and clinical parameters and a possible benefit in 14 and 28 day mortality compared to patients who did not receive TPE; however, due to differences in treatments and the low number of subjects enrolled, these results should not yet be extrapolated to larger settings without further confirmation [184]. Prior experience in patients with acute lung injury due to the 2009 pH1N1 influenza A virus showed improvement in hemodynamic status and PaO2/FiO2 ratio after TPE [185]. Another retrospective observational study showed improvement in adjunctive use of TPE in patients with septic shock due to pneumonia [186]. Due to the limited evidence, TPE should be further investigated regarding a possible role in treatment of COVID-19-CSS, ideally in randomized clinical trials.
4.3.5. Anticoagulation
Coagulopathy, resulting in both venous thrombosis and microangiopathy, is a prominent feature in some patients with severe COVID-19. A prospective study of 150 patients with COVID-associated ARDS found a high incidence of thromboembolic complications (18%) compared to a non-COVID-19-ARDS cohort (6%); pulmonary embolism was the most common (16.7%), followed by cerebrovascular ischemic attacks and deep venous thrombosis [187]. Although the presence of antiphospholipid antibodies and lupus anticoagulant has been reported, the significance of these levels, as well as the nature of this coagulopathic state and its relationship to hyperinflammation in COVID-19 remains unknown [187,188]. Most studies on anticoagulation in COVID-19 outcomes are retrospective and reference the use of prophylactic heparin or low molecular weight heparin; a pilot study of 5 patients with severe COVID-19 treated with tirofiban and fondaparinux along with clopidogrel and acetylsalicylic acid had improved respiratory outcomes; however, control patients were treated with prophylactic or therapeutic heparin only [189]. Given the strong association of D-dimer and fibrin split products elevation with poor prognosis and mortality in COVID-19, the use of thromboprophylaxis and anticoagulation is currently being investigated and can be considered in patients at risk and with a hyperinflammatory phenotypes, though randomized clinical trials are needed [1,190].
5. Novel approaches in the treatment of hyperinflammation
5.1. Tyrosine kinase inhibitors
A role for the tyrosine kinase inhibitor dasatinib has been proposed in management of CRS associated with CAR-T cell therapy. Studies in mice demonstrated that dasatinib can act as an on/off switch, temporarily halt CAR T cell activity, and protect against fatal CRS [191,192]. Due to its rapid reversibility and titratable effect, dasatinib may have a role in interrupting the cascade of hyperinflammation and associated injury. The short terminal half-life of dasatinib may mitigate the risk of secondary infection observed with prolonged immunosuppression. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib has been hypothesized to protect against pulmonary injury in COVID-19-infected patients [193]. A newer BTK inhibitor acalabrutinib may have a role in interfering with dysregulated BTK-dependent macrophage signaling. In a study of 19 patients with severe COVID-19, treatment with acalabrutinib was associated with improved oxygenation and reduced inflammation [194]. Acalabrutinib is being investigated in a randomized, open-label trial compared to standard of care alone in patients with COVID-19 hospitalized due to respiratory complications [195]. Implementation of any of these experimental strategies will require clinical validation.
5.2. Mesenchymal stem cells (MSC)
Novel, experimental approaches for treating hyperinflammation may be complementary in mechanism to immunomodulatory or immunosuppressive drugs. One emerging area of interest is cellular therapies. Mesenchymal stem cells (MSC) may reduce immunopathology through complex mechanisms which include the secretion of anti-inflammatory cytokines and the promotion of endogenous tissue repair. Although their efficacy is not yet established in humans, clinical trials in ARDS have demonstrated that treatment with MSCs is relatively safe [196]. Early reports of MSCs and MSC-derived extracellular vesicles in COVID-19 suggest limited toxicity and a reduction of inflammation [197-199]. Additional trials in the treatment of COVID-19 are ongoing [200].
6. Approaches in the prevention of hyperinflammation
6.1. Catecholamine inhibition
Preclinical studies have shown that hyperinflammation in CRS, lipopolysaccharide-induced cytokine storm, and polymicrobial sepsis coincides with a surge in catecholamines [201]. Catecholamines augment cytokine production in immune cells, generating a feed-forward loop [201,202]. Prophylactic inhibition of catecholamine synthesis or blockade of catecholamine signaling with the α-1-adrenergic receptor (α1-AR) antagonist prazosin markedly reduced cytokine storm and mortality in mouse models [201]. These data are consistent with other studies suggesting that excessive catecholamine signaling increases cytokine production and immune-mediated damage, whereas catecholamine antagonism protects against injuries [203,204]. Doxazosin has been shown to abrogate catecholamine-induced IL-6 production in PBMCs from patients with juvenile rheumatoid arthritis [202]. Prazosin is a first-line treatment for scorpion envenomation, which involves dysregulated inflammation and can progress to ARDS [205]. In a retrospective study of >400,000 patients diagnosed with pneumonia or acute respiratory distress, the risk of requiring mechanical ventilation and death was significantly lower if patients were taking an α1-AR antagonist prior to hospitalization [206]. Because prazosin, doxazosin, and other α1-AR antagonists are widely used in the adult population and have a well-established safety profile, they may be uniquely suited for the prevention of severe COVID-19 in patients who have mild symptoms (rather than treatment of severe complications once developed). Prospective controlled clinical trials are currently ongoing to evaluate whether catecholamine antagonism through prophylactic administration of prazosin can prevent ARDS, cytokine storm, and death in hospitalized patients with COVID-19 [207,208].
7. Conclusion
Although the first cases of COVID-19 were only reported a few months ago, the SARS-CoV-2 outbreak is proving to be the most challenging pandemic of the 21st century. While the major feature of COVID-19 is that of severe pneumonia leading to ARDS in some cases, some, but not all, patients with COVID-19 enter a hyperinflammatory state characterized by highly elevated D-dimer, elevated CRP, and elevated ferritin. Development of hyperinflammation corresponds to a worsening clinical status including oxygen requirements and respiratory status, multi-organ failure, and too often death despite maximal supportive care.
Though similar to HLH/MAS in its deleterious activation of proinflammatory pathways and exuberant production of cytokines and associated morbidity and mortality, the hyperinflammatory state associated with SARS-CoV-2 infection has a unique fingerprint. For example, organomegaly, elevated LDH, or triglyceride levels are not a prominent feature. D-dimer can, on the other hand, be extremely elevated, and is probably a reflection of the hypercoagulable state and microthrombi that commonly complicate severe COVID-19 and is observed in autopsy studies. Targeting this immune dysregulation with the goal of preventing the development of ARDS and multi-organ failure holds some promise as a potential therapeutic pathway. However, caution must be used as immunomodulatory therapy may blunt beneficial host innate and adaptive responses during active viral replication. While the ‘compassionate’ off-label use of some of these medications may be appropriate in critically ill patients (especially when clinical trials are not available to treating physicians and supportive care is insufficient), cautious and judicious use of these drugs is paramount until reliable data regarding efficacy from clinical trials become available and their safety in this unique patient population is established. This is even more important at a time where clinical trial results are commonly reported in pre-print or in the form of preliminary press releases, making it difficult to scrutinize the primary data.
8. Expert opinion
Increasing evidence suggests that poor outcomes in COVID-19 are associated with a hyperinflammatory state (often termed ‘cytokine storm syndrome’), typically occurring at least a week following initial infection and characterized by marked elevation in inflammatory markers and pro-inflammatory cytokines. This is consistent with experimental models of delayed hyperinflammation in SARS-CoV-1 infection and suggest that dysregulated host response to SARS-CoV-2 are responsible for much of the detrimental immunopathology in this phase of the disease, including localized and systemic macrophage activation which drive worsening oxygenation and an ARDS phenotype. While these features resemble other hyperinflammatory states such as HLH, they are not uniformly similar: organomegaly is not a prominent feature of severe COVID-19, LDH, triglycerides, and liver enzymes are only modestly elevated, and cytopenias, most prominently lymphopenia and to a lesser extent thrombocytopenia, are frequently observed.
It is not fully clear how this hyperinflammatory phase relates to SARS-CoV-2 viral loads in an absolute sense, but the use of treatments to suppress the host response may be helpful in controlling excessive inflammation and reducing organ dysfunction in individual patients. As with other hyperinflammatory states, including MAS, rHLH, and viral-induced HLH, cytokine inhibitors have been used in management. Emerging data from the inflammatory bowel disease and rheumatology patient registries suggest that those taking immunosuppressive agents are not at dramatically increased risk of severe COVID-19 infection, and raises the possibility that some patients may have milder disease due to baseline suppression of specific inflammatory pathways. Immunomodulatory and immunosuppressive drugs used in these conditions could thus act to dampen the abnormal host response to SARS-CoV-2 although optimal timing in relation to viral replication remains uncertain [123,209-212].
To date, no blinded, placebo-controlled data have been published, but there are anecdotal reports suggesting efficacy of inhibitors of the IL-6 and IL-1 signaling axes. Randomized controlled trials of these and other immunomodulatory drugs discussed above are ongoing, both individually in pharmaceutical industry-sponsored studies as well as in multi-arm adaptive platform studies. The latter may provide head-to-head data and allow comparison of the efficacy of these agents. Given the pandemic nature of COVID-19, it is essential that inexpensive, widely accessible, and ideally oral agents are explored fully in addition to the more costly inhibitors of IL-1, IL-6, JAK signaling, or complement activation. While the short-term safety of IL-1 inhibitors in COVID-19 has been demonstrated in small case series, the safety of other immunosuppressive agents including IL-6 and JAK inhibitors is yet to be demonstrated [110,213]. Long-term follow-up of trial cohorts and matched controls will be important to delineate the natural history of the infection and its sequelae. Data from these trials could help cement the role of cytokine inhibitors in other hyperinflammatory states, such as rHLH and MAS, where trial data has been largely lacking.
Immunomodulatory agents have long been posited to transform outcomes in other infections. Studies in sepsis have been disappointing; however, induced hyperinflammatory states, such as CRS, can be rapidly and effectively treated with targeted cytokine inhibition, suggesting that timing of immunomodulation is crucial. Trials in COVID-19 currently focusing on immunomodulation have been treating patients with advanced and severe illness in critical care environments (generally late in the disease course), where organ dysfunction of the lungs and other organ systems is established. However, the reports of macro- and microvascular thrombosis causing or aggravating end-organ damage suggests that, in the case of inflammation leading to endothelial dysfunction, immunomodulation at this stage may be too late. Use of early immunomodulatory treatment strategies – in combination with antiviral therapy – is important to consider, but in the absence of clinical data remains experimental and may blunt the host antiviral response.
Trials not just of efficacy but of the timing of intervention with immunomodulatory therapy are essential and may inform not just the treatment of COVID-19 but also other forms of hyperinflammation. While ongoing investigative efforts have primarily focused on identifying effective therapies for patients who developed severe COVID-19, an emphasis on prioritizing outpatient clinical trials of preventative treatment approaches that are safe and scalable to the population level is overdue and critically needed.
Article highlights.
Some patients with COVID-19 enter a hyperinflammatory state characterized by highly elevated D-dimer, elevated CRP, and elevated ferritin.
Development of hyperinflammation corresponds to a worsening clinical status including oxygen requirements and respiratory status, multi-organ failure, and death.
Despite similarities, COVID-19-CSS appears to be distinct from HLH, MAS, and CRS, and the application of HLH diagnostic scores and criteria to COVID-19 without further study is not supported by emerging data and discouraged.
While glucocorticoids have shown a mortality benefit, more studies are needed to evaluate cytokine inhibitors in severe COVID-19.
Strategies to prevent the development of COVID-19-CSS are needed.
Acknowledgments
The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance, and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism, or any other organization.
Funding
This paper was not funded.
Declaration of interest
I. Amigues reports personal fees from Abbvie unrelated to this manuscript. A. Kim was supported by grants from NIH/NIAMS and Rheumatology Research Foundation, and personal fees from Exagen Diagnostics, Inc. and GlaxoSmithKline, unrelated to this manuscript. A. Patel reports personal fees from Abbvie, Celgene and Eli Lilly unrelated to this manuscript. M. Konig was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award no. T32AR048522, and received personal fees from Bristol-Myers Squibb and Celltrion, unrelated to this manuscript. P. Robinson reports personal fees from Abbvie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche and UCB, research grants from Janssen, Novartis, Pfizer and UCB, and non-financial support from BMS, all unrelated to this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet]. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. Available from: https://coronavirus.jhu.edu/map.html.
- 3.Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Sci [Internet]. 2016;31:1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol [Internet]. 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol [Internet]. 2017;39:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu H, Zhou J, Wong BH-Y, et al. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology [Internet]. 2014;454–455:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Chu H, Li C, et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis [Internet]. 2014;209:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau SKP, Lau CCY, Chan K-H, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol [Internet]. 2013;94:2679–2690. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal AS, Garron T, Tao X, et al. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol [Internet]. 2015;89:3659–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol [Internet]. 2010;84(3):1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CW, Baric R, Cai SX, et al. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology [Internet]. 2009;395:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israelow B, Song E, Mao T, et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. bioRxiv: Preprint Serv Biol [Internet]. 2020. DOI: 10.1101/2020.05.27.118893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang R-D, Liu M-Q, Chen Y, et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2 [Internet]. Cell. 2020; Available from: http://www.sciencedirect.com/science/artide/pii/S009286742030622X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao L, Deng W, Huang B, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature [Internet]. 2020;1–6. Available from: https://www.nature.com/articles/s41586-020-2312-y [DOI] [PubMed] [Google Scholar]
- 15.Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science [Internet]. 2020;368:1012–1015. Available from: https://science.sciencemag.org/content/368/6494/1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature [Internet]. 2007;445:319–323. Available from: http://www.nature.com/articles/nature05495 [DOI] [PubMed] [Google Scholar]
- 17.Franks TJ, Chong PY, Chui P, et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol [Internet]. 2003;34:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholls JM, Poon LLM, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet [Internet]. 2003;361:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guery B, Poissy J, El Mansouf L, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet [Internet]. 2013;381:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong K-H, Choi J-P, Hong S-H, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax [Internet]. 2018;73:286–289. [DOI] [PubMed] [Google Scholar]
- 21.Mahallawi WH, Khabour OF, Zhang Q, et al. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine [Internet]. 2018;104:8–13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7129230/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W-K, Chen S-Y, Liu I-J, et al. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis [Internet]. 2004;39:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis [Internet]. 2020. DOI: 10.1093/cid/ciaa449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med [Internet]. 2020;e12421 DOI: 10.15252/emmm.202012421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong J, Dong H, Xia SQ, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv [Internet]. 2020. DOI: 10.1101/2020.02.25.20025643v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol [Internet]. 2020. DOI: 10.1002/jmv.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Valle DM, Kim-Schulze S, Hsin-Hui H, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv: The Preprint Server for Health Sciences [Internet]. 2020. DOI: 10.1101/2020.05.28.20115758 [DOI] [Google Scholar]
- 28.Luo M, Liu J, Jiang W, et al. IL-6 combined with CD8+ T cell count early predict in-hospital mortality for patients with COVID-19. JCI Insight [Internet]. 2020. DOI: 10.1172/jci.insight.139024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol [Internet]. DOI: 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol [Internet]. 2020. DOI: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome \textbar medRxiv [Internet]. DOI: 10.1101/2020.03.02.20029975v1 [DOI] [Google Scholar]
- 32.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [Internet]. 2020;395:497–506. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ [Internet]. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med [Internet]. 2020:420–422. DOI: 10.1016/s2213-2600(20)30076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol [Internet]. 2020;33:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol [Internet]. 2018;9:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Channappanavar et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016. February 10;19(2):181–93.••In an animal model of SARS-CoV-1, robust virus replication and delayed IFN-I signaling promote severe disease. IFN-I promotes the immunopathology of SARS through accumulation of monocyte-macrophages in the lung.
- 38.Cameron MJ, Ran L, Xu L, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol [Internet]. 2007;81:8692–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–45.•• Cell and animal models of SARS-COV-2 infection show that the transcriptional response to the virus is abnormal, with high chemokine levels and a limited IFN-I and IFN-III response.
- 40.Trouillet-Assant S, Viel S, Gaymard A, et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol [Internet]. 2020;146:206–208.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients [Internet]. DOI: 10.1101/2020.04.19.20068015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China. JAMA [Internet]. 2020;1239 DOI: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 43.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplantation [Internet]. 2020;405–407. DOI: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson LA, Canna SW, Schulert GS, et al. On the Alert for Cytokine Storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. Internet . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest [Internet]. 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019 [Internet]. DOI: 10.1101/2020.02.16.20023903 [DOI] [Google Scholar]
- 47.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City [Internet]. DOI: 10.1101/2020.04.08.20057794 [DOI] [Google Scholar]
- 48.Barton LM, Duval EJ, Stroberg E, et al. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol [Internet]. 2020;153:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res [Internet]. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans [Internet]. DOI: 10.1101/2020.04.06.20050575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wichmann D, Sperhake J-P, Lülgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med [Internet]. 2020. DOI: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. Pathology [Internet]. 2020; medRxiv. Available from: https://www.medrxiv.org/content/10.1101/2020.05.18.20099960v1.full [Google Scholar]
- 53.Prilutskiy A, Kritselis M, Shevtsov A, et al. SARS-CoV-2 Infection-Associated Hemophagocytic Lymphohistiocytosis. Am J Clin Pathol [Internet]. 2020. DOI: 10.1093/ajcp/aqaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herold T, Jurinovic V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients [Internet]. DOI: 10.1101/2020.04.01.20047381 [DOI] [Google Scholar]
- 55.Schulert GS, Canna SW. Convergent pathways of the hyperferritinemic syndromes. Int Immunol [Internet]. 2018;30:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehmberg K, Nichols KE, Henter J-I, et al. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica [Internet]. 2015;100:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouphael NG, Talati NJ, Vaughan C, et al. Infections associated with haemophagocytic syndrome. Lancet Infect Dis [Internet]. 2007:814–822. D0I: 10.1016/s1473-3099(07)70290-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology [Internet]. 2019;58:5–17. [DOI] [PubMed] [Google Scholar]
- 59.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol [Internet]. 2016;12:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood [Internet]. 2015;125:2908–2914. [DOI] [PubMed] [Google Scholar]
- 61.Jenkins MR, Rudd-Schmidt JA, Lopez JA, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med [Internet]. 2015;212:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lerkvaleekul B, Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key. Open Access Rheumatol [Internet]. 2018;10:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn SS, Yoo B-W, Jung SM, et al. Application of the 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome in patients with adult-onset still disease. J Rheumatol [Internet]. 2017;44:996–1003. [DOI] [PubMed] [Google Scholar]
- 64.Chatenoud L, Ferran C, Legendre C, et al. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation [Internet]. 1990;49:697–702. [DOI] [PubMed] [Google Scholar]
- 65.Winkler U, Jensen M, Manzke O, et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood [Internet]. 1999;94:2217–2224. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10498591 [PubMed] [Google Scholar]
- 66.Wing MG, Moreau T, Greenwood J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest [Internet]. 1996;98:2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant [Internet]. 2019;25:625–638. [DOI] [PubMed] [Google Scholar]
- 68.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev [Internet]. 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer [Internet]. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol [Internet]. 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med [Internet]. 2018:739–748. DOI: 10.1038/s41591-018-0036-4. . [DOI] [PubMed] [Google Scholar]
- 72.Giavridis T, SJC VDS, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med [Internet]. 2018:731–738. DOI: 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood [Internet]. 2017;130:2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov [Internet]. 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase i anti-CD22 CAR T-cell trial. J Clin Oncol [Internet]. 2020;38:1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashmi H, Bachmeier C, Chavez JC, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol [Internet]. 2019;187:e35–e38. [DOI] [PubMed] [Google Scholar]
- 77.Bergsten E, Horne A, Aric0 M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood [Internet]. 2017;130:2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horne A, Janka G, Maarten Egeler R, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. British J Haematol [Internet]. 2005:622–630. DOI: 10.1111/j.1365-2141.2005.05501.x. [DOI] [PubMed] [Google Scholar]
- 79.La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood [Internet]. 2019;133:2465–2477. [DOI] [PubMed] [Google Scholar]
- 80.Kumar B, Aleem S, Saleh H, et al. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol [Internet]. 2017;37:638–643. [DOI] [PubMed] [Google Scholar]
- 81.Lounder DT, Bin Q, de Min C, et al. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv [Internet]. 2019;3:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med [Internet]. 2020;382:1811–1822. [DOI] [PubMed] [Google Scholar]
- 83.Quartier P, Allantaz F, Cimaz R, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis [Internet]. 2011;70:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med [Internet]. 2012;367:2396–2406. [DOI] [PubMed] [Google Scholar]
- 85.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheumatol [Internet]. 2020;72:326–334. [DOI] [PubMed] [Google Scholar]
- 86.Teachey DT, Bishop MR, Maloney DG, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL”. Nat Rev Clin Oncol [Internet]. 2018:218 DOI: 10.1038/nrclinonc.2018.19 [DOI] [PubMed] [Google Scholar]
- 87.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med [Internet]. 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antil-eukemic efficacy. Blood [Internet]. 2019;134:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheih A, Voillet V, Hanafi L-A, et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat Commun [Internet]. 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salter AI, Ivey RG, Kennedy JJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal [Internet]. 2018;11 DOI: 10.1126/scisignal.aat6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood [Internet]. 2011;118:4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol [Internet]. 2017;13:399–409. [DOI] [PubMed] [Google Scholar]
- 93.covid-trials.org [Internet]. cited 2020 Aug 18 Available from: https://www.covid-trials.org/.
- 94.COVID-19 Vaccine and Therapeutic Drugs Tracker [Internet]. cited 2020 Aug 18 Available from: https://biorender.com/covid-vaccine-tracker
- 95.Milken Institute’s COVID-19 Treatment and Vaccine Tracker [Internet]. cited 2020 Aug 18 Available from: https://covid-19tracker.milkeninstitute.org/
- 96.https://covid19.trialstracker.net/[Internet]. cited 2020 Aug 18 Available from: https://covid19.trialstracker.net/
- 97.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract [Internet]. 2019;25:551–557. [DOI] [PubMed] [Google Scholar]
- 98.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [Internet]. In Proceedings of the National Academy of Sciences. 2020. p. 10970–10975. DOI: 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia [Internet]. 2020. Available from: https://www.roche.com/media/releases/med-cor-2020-07-29.htm.
- 100.Sanofi and Regeneron provide update on Kevzara® (sarilumab) Phase 3 U.S. trial in COVID-19 patients [Internet]. 2020. Available from: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00.
- 101.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents [Internet]. 2020;55:105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev [Internet]. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol [Internet]. 2020. DOI: 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814–818.Internet . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gritti G, Raimondi F, Ripamonti D, et al. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support [Internet]. DOI: 10.1101/2020.04.01.20048561 [DOI] [Google Scholar]
- 106.Shakoory B, Carcillo JA, Chatham WW. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: re-analysis of a. Crit Care [Internet]. 2016. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc5378312/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Opal SM, Fisher CJJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med [Internet]. 1997;25:1115–1124. [DOI] [PubMed] [Google Scholar]
- 108.Wampler Muskardin TL. Intravenous anakinra for macrophage activation syndrome may hold lessons for treatment of cytokine storm in the setting of Coronavirus Disease 2019. ACR Open Rheumatol [Internet]. 2020;2:283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis [Internet]. 2020. DOI: 10.1136/annrheumdis-2020-217706 [DOI] [PubMed] [Google Scholar]
- 110.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol [Internet]. 2020;2:e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol [Internet]. 2020;2: e393–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ilaris (canakinumab) dose, indications, adverse effects, interactions [Internet]. cited 2020 Aug 15 Available from: https://www.pdr.net/drug-summary/Ilaris-canakinumab-434.
- 113.Arcalyst (rilonacept) dose, indications, adverse effects, interactions [Internet]. cited 2020 Aug 15 Available from: https://www.pdr.net/drug-summary/Arcalyst-rilonacept-494
- 114.Kineret (anakinra) dose, indications, adverse effects, interactions [Internet]. cited 2020 Aug 15 Available from: https://www.pdr.net/drug-summary/Kineret-anakinra-2061
- 115.Makay B, S Y, TCrkyilmaz Z, et al. Etanercept for therapy-resistant macrophage activation syndrome. Pediatr Blood Cancer [Internet]. 2008;50:419–421. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/pbc.21019?casa_token=1fcmmYi6r8gAAAAA:1rtVSVkXItEL3vBfe1KUhf5zL-2lEDeuCO4T7dpbyGVE0wm3C938mBWBpdfCOUEqFbB0wGn6PkY5R2DzBg [DOI] [PubMed] [Google Scholar]
- 116.Flammiger A, Fiedler W, Bacher U, et al. Critical imbalance of TNF-α and soluble TNF receptor 1 in a patient with macrophage activation syndrome: potential implications for diagnostics and treatment. Acta Haematol [Internet]. 2012;128:69–72. [DOI] [PubMed] [Google Scholar]
- 117.Prahalad S, Bove KE, Dickens D, et al. Etanercept in the treatment of macrophage activation syndrome. J Rheumatol [Internet]. 2001;28:2120–2124. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11550985. [PubMed] [Google Scholar]
- 118.Sterba G, Sterba Y, Stempel C, et al. Macrophage activation syndrome induced by etanercept in a patient with systemic sclerosis. Isr Med Assoc J [Internet]. 2010;12:443–445. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20862830. [PubMed] [Google Scholar]
- 119.Ramanan AV, Schneider R. Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol [Internet]. 2003;30:401–403. Availablr from: https://www.ncbi.nlm.nih.gov/pubmed/12563702. [PubMed] [Google Scholar]
- 120.Stern A, Riley R, Buckley L. Worsening of Macrophage Activation Syndrome in a Patient with Adult Onset Still’s Disease after Initiation of Etanercept Therapy. JCR [Internet]. 2001;252–256. DOI: 10.1097/00124743-200108000-00013 [DOI] [PubMed] [Google Scholar]
- 121.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet [Internet]. 2020;395:1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robinson PC, Richards D, Tanner HL, et al. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol [Internet]. 2020. Available from: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(20)30309-X/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020. Available from: https://ard.bmj.com/content/early/2020/05/29/annrheumdis-2020-217871?utm_term=consumer&utm_content=062020covid&utm_campaign=covidtrendmd&utm_medium=cpc&utm_source=trendmd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: results From an International Registry. Gastroenterology [Internet]. 2020. DOI: 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tobinick E TNF-alpha inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr Med Res Opin [Internet]. 2004;20:39–40. [DOI] [PubMed] [Google Scholar]
- 126.Stallmach A, Kortgen A, Gonnert F, et al. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure—a cautionary case series. Crit Care [Internet]. 2020;24:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bezzio C, Manes G, Bini F, et al. Infliximab for severe ulcerative colitis and subsequent SARS-CoV-2 pneumonia: a stone for two birds. Gut [Internet]. 2020. DOI: 10.1136/gutjnl-2020-321760 [DOI] [PubMed] [Google Scholar]
- 128.Dolinger MT, Person H, Smith R, et al. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated With Infliximab. J Pediatr Gastroenterol Nutr [Internet]. 2020;71:153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol [Internet]. 2020;20:271–272. Available from: http://www.nature.com/articles/s41577-020-0312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Benedetti F, Brogan P, Grom AA, et al. Interferon-gamma (IFN-gamma) Neutralization with Emapalumab and Time to Response in Patients with Macrophage Activation Syndrome (MAS) Complicating Systemic Juvenile Idiopathic Arthritis (s-JIA) who failed High-Dose Glucocorticoids. WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. Arthritis & Rheumatology. 2019. [Google Scholar]
- 131.Lang FM, Lee KM-C, Teijaro JR, et al. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol [Internet]. 2020;20:507–514.• GM-CSF may act to enhance tissue repair and active anti-viral immune responses, but dysregulation of GM-CSF expression in patients with COVID-19 could induce production of pro-inflammatory mediators and infiltration of inflammatory cells into the lungs and other tissues. GM-CSF administration and inhibition in COVID-19 should be investigated with caution with respect to timing of use.
- 132.Bonaventura A, Vecchie A, Wang TS, et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front Immunol [Internet]. 2020;11:1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyper-inflammation: a single-centre, prospective cohort study. Lancet Rheumatol [Internet]. 2020;2:e465–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov [Internet]. 2015;14:857–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio [Internet]. 2018;9 DOI: 10.1128/mBio.01753-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun S, Zhao G, Liu C, et al. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol [Internet]. 2013;49:221–230. [DOI] [PubMed] [Google Scholar]
- 137.Hellerud BC, Orrem HL, Dybwik K, et al. Combined inhibition of C5 and CD14 efficiently attenuated the inflammatory response in a porcine model of meningococcal sepsis. J Intensive Care Med [Internet]. 2017;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mastaglio S, Ruggeri A, Risitano AM, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol [Internet]. 2020;215:108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Diurno F, Numis FG, Porta G, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci [Internet]. 2020:4040–4047. DOI: 10.26355/eurrev_202004_20875 [DOI] [PubMed] [Google Scholar]
- 140.Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation [Internet]. DOI: 10.1101/2020.03.29.20041962 [DOI] [Google Scholar]
- 141.Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17:78. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jamilloux Y, El Jammal T, Vuitton L, et al. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev [Internet]. 2019;18:102390. [DOI] [PubMed] [Google Scholar]
- 143.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol [Internet]. 2019;6:e630–e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis [Internet]. 2020:400–402. DOI: 10.1016/s1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet [Internet]. 2020;395:e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect [Internet]. 2020:318–356. DOI: 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: need for vigilance regarding increased inherent thrombotic risk. Eur Respir J [Internet]. 2020. DOI: 10.1183/13993003.01919-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med [Internet]. 2018;197:757–767. [DOI] [PubMed] [Google Scholar]
- 149.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med [Internet]. 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ni Y-N, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care [Internet]. 2019;23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA [Internet]. 2020. DOI: 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 152.Lewis SR, Pritchard MW, Thomas CM, et al. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev [Internet]. 2019;7:CD004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang Z, Liu J, Zhou Y, et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect [Internet]. 2020;81:e13–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang D, Wang J, Jiang Q, et al. No Clear Benefit to the Use of Corticosteroid as Treatment in Adult Patients with Coronavirus Disease 2019: A Retrospective Cohort Study [Internet]. DOI: 10.1101/2020.04.21.20066258 [DOI] [Google Scholar]
- 155.Lu X, Chen T, Wang Y, et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19 [Internet]. DOI: 10.1101/2020.04.07.20056390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu S, Zhou Q, Huang L, et al. Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis. Ann Transl Med [Internet]. 2020;8:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y, Jiang W, He Q, et al. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China [Internet]. DOI: 10.1101/2020.03.06.20032342 [DOI] [Google Scholar]
- 158.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med [Internet].. 2020. DOI: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA [Internet]. 2020. [cited 2020 Aug 15];323:1061–1069. Available from: https://jamanetwork.com/journals/jama/fullarticle/2761044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gangopadhyay KK, Mukherjee JJ, Sinha B, et al. The role of corticosteroids in the management of critically ill patients with corona-virus disease 2019 (COVID-19): a meta-analysis [Internet]. DOI: 10.1101/2020.04.17.20069773. [DOI] [Google Scholar]
- 161.Fang X, Mei Q, Yang T, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect [Internet]. 2020:147–178. DOI: 10.1016/j.jinf.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID −19). Med J Australia [Internet]. 2020; 416–420. DOI: 10.5694/mja2.50577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med [Internet]. 2020; Available from: 10.1056/NEJMoa2021436•• Use of the glucocorticoid dexamethasone resulted in reduced 28-day mortality among hospitalized patients with COVID-19 who receiving invasive mechanical ventilation or oxygen alone, but not in patients with less severe disease.
- 164.Clinical management of COVID-19 [Internet]. World Health organisation; cited 2020 Aug 15 Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Google Scholar]
- 165.Martinez GJ, Robertson S, Barraclough J, et al. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc [Internet]. 2015;4:e002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grailer JJ, Canning BA, Kalbitz M, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol [Internet]. 2014;192:5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med [Internet]. 2012;185:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Castano-Rodriguez C, Honrubia JM, Gutierrez-Alvarez J, et al. Role of severe acute respiratory syndrome coronavirus Viroporins E, 3a, and 8a in replication and pathogenesis. mBio [Internet]. 2018. DOI: 10.1128/mbio.02325-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Network Open [Internet]. 2020;3:e2013136–e2013136. Available from: https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2767593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis [Internet]. 2020. Available from. https://ard.bmj.com/content/early/2020/07/30/annrheumdis-2020-217712.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Deftereos S, Giannopoulos G, Vrachatis DA, et al. Colchicine as a potent anti-inflammatory treatment in COVID-19: can we teach an old dog new tricks? Eur Heart J Cardiovasc Pharmacother [Internet]. 2020;6:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Henderson LA, Cron RQ. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr Drugs [Internet]. 2020;22:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Halyabar O, Chang MH, Schoettler ML, et al. Calm in the midst of cytokine storm: a collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome. Pediatr Rheumatol Online J [Internet]. 2019;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang J-T, Sheng W-H, Fang C-T, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis [Internet]. 2004;10:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis [Internet]. 2020;7:ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hung IFN, To KKW, Lee C-K, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest [Internet]. 2013;144:464–473. [DOI] [PubMed] [Google Scholar]
- 177.Hartman WR, Hess AS, Connor JP. Hospitalized COVID-19 Patients treated with Convalescent Plasma in a Mid-size City in the Midwest. Res Sq [Internet];2020. DOI: 10.21203/rs.3.rs-39447/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abolghasemi H, Eshghi P, Cheraghali AM, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci [Internet]. 2020;102875 DOI: 10.1016/j.transci.2020.102875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li L, Zhang W, Hu Y, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA [Internet]. 2020. DOI: 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ovanesov MV, Menis MD, Scott DE, et al. Association of immune globulin intravenous and thromboembolic adverse events. Am J Hematol [Internet]. 2017;92:E44–E45. [DOI] [PubMed] [Google Scholar]
- 181.Keith P, Day M, Perkins L, et al. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care [Internet]. 2020;24:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ma J, Xia P, Zhou Y, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol [Internet]. 2020;214:108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Faqihi F, Alharthy A, Alodat M, et al. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: A pilot study. J Crit Care [Internet]. 2020. DOI: 10.1016/j.jcrc.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Khamis F, Al-Zakwani I, Al Hashmi S, et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis [Internet]. 2020. DOI: 10.1016/j.ijid.2020.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Patel P, Nandwani V, Vanchiere J, et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med [Internet]. 2011;12:e87 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6328374/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keith P, Wells AH, Hodges J, et al. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single center retrospective review. 2020. http://www.researchsquare.com/article/rs-16022/latest.pdf [DOI] [PMC free article] [PubMed]
- 187.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med [Internet]. 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med [Internet]. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Viecca M, Radovanovic D, Forleo GB, et al. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res [Internet]. 2020;158:104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia [Internet]. J Thromb Haemost. 2020;844–847. DOI: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med [Internet]. 2019;11 DOI: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weber EW, Lynn RC, Sotillo E, et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv [Internet]. 2019;3:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood [Internet]. 2020;135:1912–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol [Internet]. 2020;5 DOI: 10.1126/sciimmunol.abd0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. CALAVI (Calquence Against the Virus) [Internet]. Clinicaltrials.gov; cited 2020 Aug 15 Available from: https://clinicaltrials.gov/ct2/show/NCT04346199 [Google Scholar]
- 196.Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med [Internet]. 2019;7:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sanchez-Guijo F, Garcia-Arranz M, L0pez-Parra M, et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine [Internet]. 2020:100454 DOI: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia [Internet]. Aging Dis. 2020:216 DOI: 10.14336/ad.2020.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sengupta V, Sengupta S, Lazo A, et al. exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev [Internet]. 2020;29:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Can A, Coskun H. The rationale of using mesenchymal stem cells in patients with COVID-19-related acute respiratory distress syndrome: what to expect. Stem Cells Transl Med [Internet]. 2020. DOI: 10.1002/sctm.20-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Staedtke V, Bai R-Y, Kim K, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature [Internet]. 2018;564:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Heijnen CJ, Rouppe van der Voort C, Wulffraat N, et al. Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol [Internet]. 1996;71:223–226. [DOI] [PubMed] [Google Scholar]
- 203.Flierl MA, Rittirsch D, Nadeau BA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature [Internet]. 2007;449:721–725. [DOI] [PubMed] [Google Scholar]
- 204.Flierl MA, Rittirsch D, Nadeau BA, et al. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One [Internet]. 2009;4:e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chippaux J-P. Emerging options for the management of scorpion stings. Drug Des Devel Ther [Internet]. 2012;6:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Koenecke A, Powell M, Xiong R, et al. Alpha-1 adrenergic receptor antagonists to prevent acute respiratory distress syndrome and death from cytokine storm syndrome [Internet]. arXiv [q-bio.TO]. 2020. Available from: http://arxiv.org/abs/2004.10117 [Google Scholar]
- 207.Konig MF, Powell M, Staedtke V, et al. Preventing cytokine storm syndrome in COVID-19 using α−1 adrenergic receptor antagonists. J Clin Invest [Internet]. 2020;130:3345–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Prazosin to Prevent COVID-19 (PREVENT-COVID Trial) [Internet]. Clinicaltrials.gov; cited 2020 Aug 15 Available from: https://clinicaltrials.gov/ct2/show/NCT04365257 [Google Scholar]
- 209.Higgins PDR, Ng S, Danese S, et al. The Risk of SARS-CoV-2 in Immunosuppressed IBD Patients. Crohns Colitis 360 [Internet]. 2020;2:otaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol [Internet]. 2020;32:434–440. [DOI] [PubMed] [Google Scholar]
- 211.Gianfrancesco MA, Hyrich KL, Gossec L. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet [Internet]. 2020; Available from: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(20)30095-3/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Konig MF, Kim AH, Scheetz MH, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis [Internet]. 2020. DOI: 10.1136/annrheumdis-2020-217690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Navarro-Millan I, Sattui SE, Lakhanpal A, et al. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol [Internet]. 2020. DOI: 10.1002/art.41422 [DOI] [PMC free article] [PubMed] [Google Scholar]