Abstract
Neurogenic inflammation results from the release of biologically active agents from the peripheral primary afferent terminal. This release reflects the presence of releasable pools of active product and depolarization-exocytotic coupling mechanisms in the distal afferent terminal and serves to alter the physiologic function of innervated organ systems ranging from the skin and meninges to muscle, bone, and viscera. Aside from direct stimulation, this biologically important release from the peripheral afferent terminal can be initiated by antidromic activity arising from five anatomically distinct points of origin: (i) afferent collaterals at the peripheral-target organ level, (ii) afferent collaterals arising proximal to the target organ, (iii) from mid-axon where afferents lacking myelin sheaths (C fibers and others following demyelinating injuries) may display crosstalk and respond to local irritation, (iv) the dorsal root ganglion itself, and (v) the central terminals of the afferent in the dorsal horn where local circuits and bulbospinal projections can initiate the so-called dorsal root reflexes, i.e., antidromic traffic in the sensory afferent.
Keywords: Antidromic, Neurogenic inflammation, Ephaptic connection, Mid-axonal activation, Dorsal root reflex
Introduction
The traditional way of thinking about the role of the sensory afferent is that following application of any nociceptive stimulus, its energy is transduced by a receptor (nociceptor) into a local generator potential expressed on the sensory afferent terminal. If the generator potential achieves threshold, action potentials are produced and conveyed orthodromically to the spinal cord. However, an important property of nerve conduction is that the excitable membrane can generate action potentials in both directions from a point of depolarization. Thus, when primary afferent nerve fibers are activated distant from the receptor/terminal (mid-axonally or dorsal root ganglia (DRG)), they will generate action potentials that are conveyed bi-directionally, orthodromically to the spinal cord, and antidromically away from the cord. Orthodromic input to the spinal cord depolarizes central terminals leading to a calcium-dependent extracellular release of neurotransmitters into the tripartite synapse, onto second-order neurons as well as proximal glia. The antidromic signal travels peripherally and generates a comparable excitation in the peripheral terminals of the sensory axon, resulting in the calcium-dependent release of terminal contents, neurotransmitters, and trophic factors, into the peripheral extravascular/extracellular space. Importantly, at axonal branch points, action potentials can be conveyed along more than one axon collateral; thus, the antidromic message may be transmitted throughout the entire peripheral receptive field of the axon. As reviewed elsewhere in this themed volume, these released products have a substantial impact upon local vascular perfusion, capillary permeability, tissue integrity/growth, and immune function as well as the trophic state of the innervated organ.
In this review, we wish to focus specifically on the points of origin of afferent activity leading to the depolarization of the peripheral afferent terminal. Broadly speaking, there are two mechanisms accounting for peripheral terminal activation: (i) activation of receptor/channels on the peripheral terminal itself by agents in the immediate extracellular milieu and (ii) invasion of the peripheral terminal from antidromically conducted action potentials. In this latter case, we will consider the several mechanisms by which antidromic activity is generated: (i) collateralization of the distal or proximal afferent axon, (ii) antidromic activity arising from along the axon either through local irritation and/or cross-talk, (iii) the cell body of the afferent axon in the dorsal root ganglion, and (iv) antidromic activity arising from the spinal terminals though the classic dorsal root reflex.
Afferent terminal transmitters and their release
Terminal transmitter context
Afferent transmitter release is typically considered to originate at the terminals of the axon. However, extracellular movement of transmitters may occur from the cell body (DRG) [1] and has been demonstrated to occur mid axon after local injury [1]. Most neurotransmitters/neuromodulators are typically synthesized and packaged into vesicles in the dorsal root ganglion cell body and then transported to both the central and peripheral terminals of the primary afferent axon where they await release. Small molecules such as glutamate may be synthesized and packaged at the terminal [2]. Larger molecules (peptides) may undergo translational or post-translational processing after vesicular packaging, and even synthesis has been demonstrated within the terminal [3].
Transmitter release profile
Glutamate is the most common excitatory neurotransmitter in all afferent axons. Specifically, vesicular glutamate, identified with immunohistochemistry, is reported in about 65–80% of spinal dorsal root ganglion (DRG) neurons as well as in trigeminal neurons. This percentage varies with the spinal or medullary level, and although both large and small neurons may contain glutamate, a greater percentage of small cell bodies are positive for this excitatory amino acid. Increased extracellular glutamate, acting via both ionotropic and metabotropic receptors, elicits further neuronal activity and sensitization of peripheral Aδ and C terminals [4, 5]. Aside from glutamate, defined subsets of these afferent axons also contain and release a rich variety of neuromodulators (ATP) and neuropeptides; these include substance P (sP), calcitonin gene-related peptide (CGRP), galanin, vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), neuropeptide Y (NPY), and somatostatin (Fig. 1). Other released products, based on the expression of the respective synthetic pathways in the DRG, may include modulators of excitability, such as prostaglandins and nitric oxide. Calcitonin gene-related peptide is found in roughly half of the somatic axons while the majority of terminals with CGRP containing vesicles are associated with unmyelinated axons, others are associated with both Aβ and Aδ fibers. In uninjured tissue, about half of the CGRP containing fibers, primarily the unmyelinated fibers, contain sP. Substance P is not expressed in the absence of CGRP within primary afferent fibers.
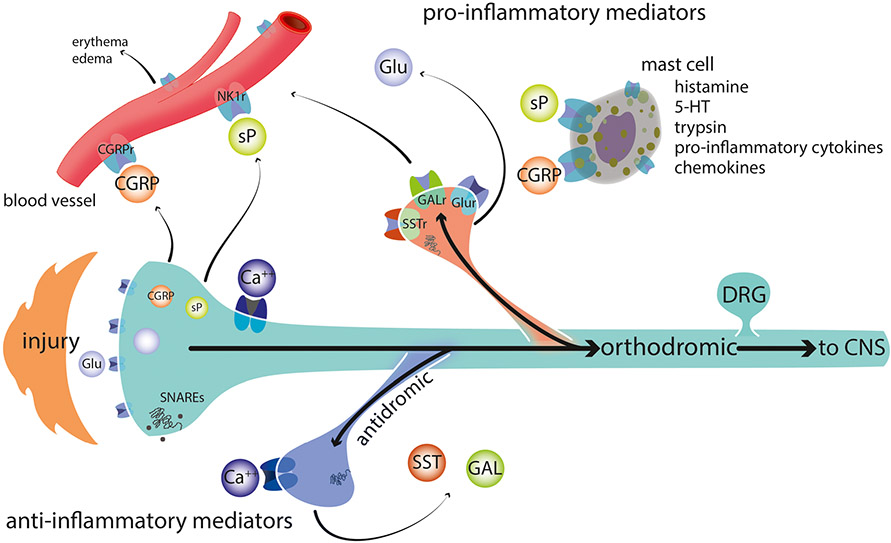
Fig. 1.
Injury and/or injury products triggers an orthodromic action potential that also invades local collaterals and terminal endings unaffected by the original insult. When these action potentials enter the terminal, they trigger voltage-gated calcium channels, Ca++ entry, activation of SNAREs, and neurotransmitter release. Neurotransmitters may be excitatory and cause excitation of nearby terminals from other afferents (not shown), increased capillary permeability and degranulation of mast cells, or they may be inhibitory and thus pre-synaptically reduce neurotransmitter release from nearby terminals. Excitatory and inhibitory neurotransmitters are released from different axons, but have been shown on the top and bottom, respectively, in this schematic. NK1r neurokinin1 receptor, SST(r) somatostatin and its receptor, Gal(r) galanin and its receptor, Glu glutamate GluR any of several glutamate receptors including both ionotropic and metabotropic receptors, 5-HT serotonin, SNAREs soluble NSF attachment protein receptor
Terminal release mechanisms
In the face of terminal depolarization by a local agent acting upon a membrane receptor (such as capsaicin or a myriad of active factors present in the local milieu after injury) [6] or when an action potential invades the peripheral terminal, voltage-gated calcium channels are activated, leading to increased intracellular calcium serving to initiate intracellular signaling leading to neurotransmitter release. Local injection of botulinum toxin, which cleaves membrane and vesicular SNAREs (soluble NSF attachment protein receptor), blocks local peripheral terminal release, emphasizing the role of these synaptic proteins in this exocytotic process [7].
While increased intracellular calcium mediates release after terminal depolarization, there is controversy concerning which type of voltage-gated calcium channel plays the predominant role in vesicular neurotransmitter release in different compartments of the peripheral nerve. Claims have been made that N-type calcium channel predominates within the exocytotic process in the central terminal, while T-type channels are functionally more important in the peripheral terminal [8]. However, N- and L-type calcium channel blockade has been shown to prevent evoked neuropeptide release in isolated skin-nerve (peripheral terminal) preparations [9]. One complicating factor in data interpretation is that there are N-type calcium channels on the sympathetic efferent fiber terminals that control the release of norepinephrine and NPY [10] presynaptic to the peripheral terminals. Just and Heppelmann [11] showed that electrically evoked antidromic activation of joint afferents at low frequencies sensitizes almost half of the nociceptive fibers in an N-type calcium channel-dependent fashion. However, at higher stimulus frequencies, antidromic activation predominantly resulted in desensitization. They concluded that all of the N-type channels of interest were on the sympathetic fiber terminals. As will be reviewed below, axonal compartments may display different calcium channel profiles. Mid-axonal release of CGRP induced by elevated extracellular potassium is regulated by L- and T-type, but not N-type calcium channels [1], and release from the cell body in the DRG is controlled by L-type channels [12]. Regardless of the calcium channel type involved, antidromic action potentials that invade the peripheral terminal evoke release of glutamate and peptides appear to reflect a conventional calcium dependent exocytotic coupling.
Peripheral effects of products released from the peripheral afferent terminals
Peripheral release of afferent transmitters plays a pivotal role in neurogenic inflammation. The specific role played by this release in different systems is reviewed in other papers in this volume. As a general comment, several overarching effects are noted.
Peripheral vasomotor effects
The peripheral release of pro-inflammatory mediators results in signs such as redness and edema, the hallmarks of neurogenic inflammation, which are virtually overlapping with the sensory receptive field for any individual axon [13-15] (Fig. 1). Release of the peptides sP and CGRP results in dilation and increased permeability of pre-capillary arterioles. Diffusion of blood products, such as bradykinin and serotonin, out of the vasculature may directly excite additional peripheral nociceptors. Direct acute excitatory effects of the peripheral terminal on immune cells are sparse; however, electrically induced antidromic afferent activity has been shown to induce mast cell degranulation and to have trophic effects on mast cell density [16]. The extent of the resulting changes in local perfusion and terminal excitability depends upon not only the examined species but also on specific characteristics of the innervated tissue [13]. Neurogenic inflammation is present not only in skin and joints but also observed in viscera, the trachea, heart, bladder, and reproductive organs [17].
Small versus large afferents
Not surprisingly, in naïve tissue, neurogenic inflammation is mediated by a combination of finely myelinated and unmyelinated fibers with different effector functions [15]. Thus, electrical stimulation of fine fibers, but not large fibers, elicits plasma extravasation into the peripheral tissue, including structures such as the skin and joint. Exclusive antidromic activity in Aδ fibers produces vasodilation, while stimulation sufficient to antidromically activate both A and C fibers appears to be necessary to induce plasma extravasation and edema [13]. The relevant afferent axons may also include small afferent axons classified as “sleeping” or “silent” nociceptors. Products released into the local milieu by tissue injury and inflammation readily sensitize these normally unresponsive receptors. Under these conditions, threshold for terminally mediated activation falls to very low levels, and accordingly, the propensity for silent nociceptors to initiate terminal release contributing to neurogenic inflammation would be similarly lowered [18].
Heterogeneity of the peripheral effect
Not all C fibers have peripheral vasodilator actions. In rat and rabbit, this action seems limited to a subpopulation of polymodal nociceptors with relatively high mechanical thresholds. In pigs, the relevant C fibers are selectively activated by heat. In man, flare due to axon reflex appears to be mediated by C-fibers that are insensitive to mechanical stimulation, rather than by the common polymodal nociceptors [19].
Pro versus anti-inflammatory actions
Finally, under an important subset of conditions, including bacterial infection or immune complex-induced arthritis, peripheral terminals of nociceptors may also release anti-inflammatory neuropeptides, such as somatostatin and galanin (Fig. 1). These agents can modulate peripheral lymph nodes and may directly reduce the amount of pro-inflammatory cytokines released by infiltrating macrophages [20]. These inhibitory peptides also inhibit peripheral release of pro-inflammatory neuropeptides from C-fiber terminals and thus can inhibit plasma extravasation and edema [21]. The changing balance of antidromic excitatory and inhibitory effects complicates interpretation of experimental results.
Origins of antidromic activity in afferent axons
Neurogenic inflammation reflects the release of products from the peripheral afferent. This terminal depolarization may arise from several sources. We will review these sources and the cascades that lead to this antidromic invasion of the peripheral terminal to initiate terminal release.
Peripheral afferent collaterals
As noted above, explanations of neurogenic inflammation typically focus on the effects arising from direct activation of the terminal. Such activation leads to orthodromic activity in the afferent to the spinal cord. However, the peripheral afferent displays significant arborization leading to depolarization at branch points, which leads to activity traveling antidromically along these axon collaterals to the peripheral terminal. While the arborization area of a single axon is frequently small, in many cases, cutaneous receptive fields extend over distances of several centimeters and/or consist of more than one, non-contiguous areas [22]. Receptive fields on the trunk are the largest, but are relatively sparsely innervated as evidenced by larger 2-point discrimination thresholds. Cutaneous innervation density typically appears to increase and receptive field size to decrease in more distal receptive fields.
A non-cutaneous example that beautifully illustrates non-contiguous receptive fields has been demonstrated within the pelvic viscera where a significant number of DRG neurons may each innervate more than one organ. Indeed, within upper lumbar and lumbosacral DRG neurons, up to 20% of neurons innervate both the colon and urinary bladder [23]; the numbers vary substantially among ganglia. Of these dually projecting neurons, 10–30% were positive for IB4, implying that they were mechanical nociceptors; others responded to capsaicin (indicative of being TRPV1(+) unmyelinated afferents). It is not known if these DRG neurons achieve their dual receptive fields because they have two separate axons, or if a single axon develops two widely projecting collaterals after it reaches the spinal nerve. Either way, following irritation/stimulation to the colon, the bladder may be invaded by antidromic activity. Similar pairings of dissimilar structures innervated by single DRG neurons have been shown for the uterus and urinary bladder. Other occurrences of nociceptive DRG neurons projecting through different peripheral nerves to separate structures have been reported for lumbar facet joints and sciatic nerve [24], lumbar muscles and knee [25], and lumbar discs and groin skin [26]; as for the viscera, the vast majority of these afferent fibers are thought to be nociceptive. This atypical wiring has been postulated to contribute to referred pain and sensitization of the second, non-directly stimulated, structure via neurogenic inflammation.
Proximal afferent collaterals
Early studies indicated an equal number of dorsal root ganglia neurons and emerging axons in proximal nerve segments; later studies employing electron microscopy, which allows clear visualization and quantification of unmyelinated fibers, indicate that there is a significant increase (2.3 axons/neuron) in fiber counts in the nerve taken 1–2 mm distal to the ganglia in sacral segments of the rat [27]. This implies that significant branching occurs shortly after the axons emerge from the DRG. Studies of the dorsal root indicate a higher degree of branching in unmyelinated compared to myelinated fibers [28]; equivalent studies do not appear to have been performed on the peripheral side of the ganglia. However, electrophysiological examination of the C-fiber subset indicates that up to 30% of the unmyelinated axons branch within a few centimeters of their cell bodies [29]. This observation does not preclude additional branching as the axons continue towards their peripheral termini.
DRG
The cell body of the sensory afferent is connected to the main trunk of the afferent by the glomerulus. This sinuous structure can support conducted potentials traveling to and from the main trunk to the cell body. Depolarization of the DRG can thus lead to both orthodromic and antidromic action potentials in the main axon trunk. Importantly, it is appreciated that after nerve injury and inflammation, the DRG may display trophic changes that lead to ongoing cellular depolarization that will initiate action potentials that travel orthodromically and antidromically. The origin of the ectopic DRG depolarization has been widely considered [30-32]. Several of the relevant mechanisms are further cataloged here (Fig. 2).
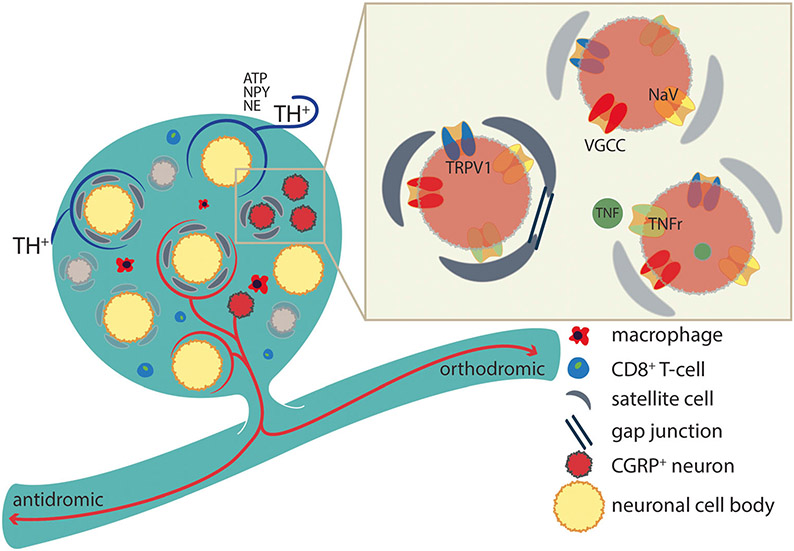
Fig. 2.
Following various nociceptive insults, neurons in the DRG may increase expression of excitatory channels and receptors and decrease expression of inhibitory channels. Satellite cells increase expression of gap junctions, signs of activation, and release of cytokines and other excitatory agents. Sprouting can occur from postganglionic sympathetic efferent fibers, which stain for TH and/or intrinsic peptidergic neurons, which envelop some of the larger cell bodies. Immune cells frequently infiltrate the DRG and release a selection of pro-inflammatory cytokines and other agents. TH tyrosine hydroxylase, NPY neuropeptide Y, NE norepinephrine, TNF(r) tumor necrosis factor and its receptor, VGCC voltage-gated calcium channel, NaV sodium channel TRPV1
Increased expression of excitatory (NaV/VGCC) and decreased expression of inhibitory channels (K+) [33].
Increased excitatory receptor expression on DRG neurons (e.g., TRPV1) [34].
Increased gap junction expression between satellite cells and neurons [35], associated with increases in neuronal activation [36]. After nerve injury, DRG neurons may develop ectopic activity, subthreshold voltage oscillations, and decreased firing thresholds [37, 38]. Local administration of gap junction antagonists blocks or prevents the cellular coupling and electrophysiological changes [39]. Activity in DRG neurons may evoke release of ATP, which can also activate local satellite cells, which in turn release TNF to activate DRG neurons [12].
Migration of inflammatory cells including macrophages and CD8+ T-lymphocytes which secrete a variety of cytokines increasing activity in DRG neurons [40].
Sprouting of local peptidergic (CGRP) neurons producing axon collaterals, which form pericellular baskets around predominantly large DRG neurons [41].
Sprouting of post-ganglionic sympathetic terminals following nerve injury to form basket-like structures around DRG neurons [42-44]. Many of these tyrosine hydroxylase (TH)-staining fibers extend between the soma and the layer of surrounding satellite cells [43]; a likely consequence of satellite cell increased neurotrophin production [45]. Stimulation of postganglionic sympathetic fibers indeed leads to the activation of DRG neurons and neuromas (see below) [42]. The populations of neurons surrounded by TH positive and CGRP positive fibers are overlapping, but the majority of enveloped neurons are surrounded by one set of fibers or the other. There is no co-localization of TH and CGRP staining in any fiber, indicative of their different origins (afferent versus post-ganglionic sympathetic).
Mid-axonal activation
In the normal sensory nerve trunk, many C-fiber axons have some degree of electrical linkage with other fibers. Griffin reported that up to 10% of C-fibers in Remak bundles in the dorsal root are not separated from neighboring axons [46]. Composition of these Remak bundles vary, e.g., axons “jump” from bundle to bundle, along the course of the nerve, and an individual Remak bundle can contain axons from more than one dorsal root ganglion [47, 48]; this implies that anatomically distinct structures can project through axons in the same Remak bundle. In rats, these polyaxonal bundles, containing a mean of 9 or more (depending on distance from spinal cord) axons/bundle, occur throughout the extent of the sciatic nerve. The authors speculate that electrical activity within any one of these non-insulated adjacent fibers is likely to increase the probability of action potentials in the others and that this probability would increase significantly in the presence of diffusible injury products such as occurs after partial nerve injury (Fig. 3). Mid-axonal activation would, by its nature, result in bidirectional ectopic activity in the affected axon. As channels and receptors in the nerve are upregulated and downregulated as a consequence of the injury, new foci of ectopic activity develop in the uninjured fibers, first in the periphery near the receptor and then at later time points in the DRG (see below) [49-51].
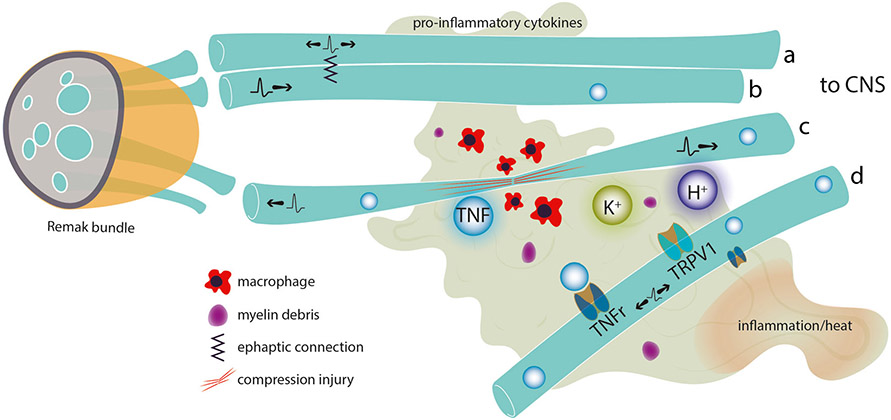
Fig. 3.
An inflammatory environment, including increased K+ from damaged tissue, macrophage infiltration, and release of pro-inflammatory cytokines, may develop after local tissue injury or damage to (compression) or degeneration of fibers in the fascicle (c). In such an environment, adjacent unmyelinated C fibers in a Remak bundle may develop an ephaptic connection such that orthodromic activity in one fiber (b) initiates bidirectional activity in the other (a). If concentration of pro-inflammatory agents builds up to sufficient levels, receptor-mediated events may also result in bidirectional activity in nearby fibers (d) resulting in apparent “spontaneous activity”
Early work identified the development of ephaptic connections between pairs of sensory axons or sensory and motor axons in dorsal roots of dystrophic mice, between sprouts in neuromas, and within nerve trunks following crush [52, 53]. Activity in the excited fiber from a point of depolarization is usually bidirectional and does not seem to depend on local extracellular K+ ion concentrations [53]. In the dystrophic animals, the fibers initiating activity conducted at C-fiber velocities although this could be due to demyelination, and the fiber being excited always had faster conduction velocities, usually within the A-fiber range. The exciting fiber often displays spontaneous activity. Interestingly, this connection is stable and transmission in the excited fiber can follow bursting activity in the initiating fiber of over 70 Hz.
In the several days following sectioning of the L5 spinal nerve or the L5 ventral root, cutaneous C-fibers within the L4 spinal nerve display a high incidence of spontaneous activity, 100 and 40%, respectively [54, 55]. This incidence increases over time, but is present as soon as 1 day following the nerve injury. Ectopic activity is generated first within the peripheral nerve, and later (in an L4 intact animal), the irritative focus moves to the DRG. It is to be expected, but is not yet demonstrated, that action potentials generated mid-axonally or from the ganglia in this manner are bidirectional. The most common explanation for the generation of the ectopic activity is that the injured axons are subjected to Wallarian degeneration, leading to macrophage infiltration, local Schwann cell activation, and local release of various growth factors, pro-inflammatory cytokines, and short-acting intermediaries. Interestingly, non-myelinating Schwann cells associated with uninjured (C-fiber) axons also become activated and release diffusible mitogens [46]. Together, these factors alter the external milieu surrounding the co-mingled injured and uninjured sensory fibers in the mixed sciatic nerve. Alteration of each of these elements individually alters development of pain behavior and ectopic activity. Animals with delayed infiltration of macrophages following nerve injury develop a similarly delayed pain behavior [56]. The pro-inflammatory cytokine tumor necrosis factor (TNF), when applied to the otherwise uninjured sciatic nerve trunk elicits ectopic activity in C-fibers within sural nerve, i.e., antidromic activity [57]. Similar results have been demonstrated for ATP, serotonin, norepinephrine [58], and interleukin-1β [59]. Importantly, this activation results in lowered mechanical withdrawal thresholds of the innervated structures (paw).
Roughly equivalent situations exist when a nerve travels through an area of tissue inflammation with increased temperature, acidity, and/or pro-inflammatory agents. Mid-axonal application of pro-inflammatory cytokines such as TNF elicits both orthodromic and antidromic action potentials and pain behavior, implicating the inflammatory milieu in the process [57, 60, 61]. Locally applied thermal stimuli also produce bidirectional ectopic activity along the axons in about two thirds of C nociceptors [62] as does application of capsaicin to the nerve [63]. Interestingly, Reeh and colleagues showed that heat, K+, and capsaicin applied to axons of passage in the sciatic nerve elicit a Ca++-dependent local release of CGRP mid-axonally at the site of stimulation [64]. They convincingly showed that the capsaicin and thermal effects are mediated via TRPV1 receptors located on the axon; thus, the acidity, in conjunction with the heat present in inflammatory sites, could synergistically activate TRPV1 and perhaps additional receptors to contribute to the mid-axonal generation of ectopic activity. This is likely to hold for human subjects, as radiant heat applied to the superficial radial nerve results in reports of thermal pain referred to the center of the stimulated nerve’s receptive field, but not to the tissue in or around the stimulation site [65]. During the course of neuritis, but not in naive animals, axons associated with Aδ and C nociceptors develop ectopic activity as well as mechanosensitivity resulting in action potential generation [66]. Taken together, this implies that a nerve passing through an inflammatory environment may fire bi-directionally resulting in not only referred pain to the innervated structure but also an antidromically generated corresponding neurogenic inflammation of the same structure.
Spinal dorsal horn
Outflow (antidromic activity) along the dorsal roots in response to strong afferent activation was originally described by Gotch and Horsley in 1891 [67]. However, the concept was not well accepted until the late 1930s. Toennies [67] postulated that this slow recognition was due to the inherent conflict between reflexive efferent output along the dorsal roots and the long-standing law of Bell and Magendie, which states that all dorsal root traffic is sensory afferent in nature. Today, the dorsal root reflex (DRR) is recognized as a common component of dorsal root function, resulting in antidromic activity following intense peripheral activation of nociceptive afferent fibers.
It is now established that ongoing orthodromic activity in nociceptive afferent fibers activates ascending circuitry projecting to the brain and, in addition, triggers intraspinal processing within the superficial dorsal horn, including activation of γ-aminobutyric acid (GABA) containing inhibitory neurons (Fig. 4). These GABAergic neurons not only receive monosynaptic input from nociceptive Aδ and C primary afferent fibers but also have collaterals that form axo-axonic synapses on GABAA receptors located on the central terminals of afferent Aβ (low threshold mechanoreceptive), Aδ (guard hairs), and C (non-peptidergic) fibers implying that the afferent signal is processed by the GABAergic neuron, which then reflexively modifies the afferent drive [68-72]. Reciprocal synapses, with rectified GABAergic signaling onto presumed primary afferent terminals, have been demonstrated in primate superficial dorsal horn (lamina II) [70]. Due to the presence of an electrically neutral Na+K+Cl− pump, primary afferent neurons, including their terminals, have unusually high levels of intracellular Cl−. Following low to moderate amounts of nociceptive input, GABA-induced opening of the GABAA receptor channels in the membrane of the central terminal of the afferent fiber allows Cl− to flow outward, slightly depolarizing the terminal and leading to a phenomena known as primary afferent depolarization (PAD), see [73]. Low levels of PAD by a presynaptic action through partial inactivation of excitatory channels paradoxically inhibits the transmission of the afferent signal due to the partial inactivation (desensitization of voltage-gated sodium and calcium channels). In contrast, in the presence of substantial suprathreshold or maintained nociceptive input, as occurs after tissue inflammation or injury (excessive PAD), the magnitude of the afferent traffic induces significantly more central GABA release from the inhibitory interneurons and consequently a significantly greater degree of terminal depolarization that actually achieves membrane activation threshold and subsequently generates action potentials (Fig. 4). Under certain high-intensity stimulus conditions, there is also a K+ component [74]. If DRRs occur, the spinally generated action potential travels antidromically from the central terminal in the spinal cord, over the dorsal roots and back to the peripheral terminal in the injured tissue where it releases neurotransmitters into the extracellular milieu [75]. Primary afferent depolarization has been recorded on all sizes of afferent fibers, large myelinated Aβ fibers, as well as Aδ and C-fibers [76].
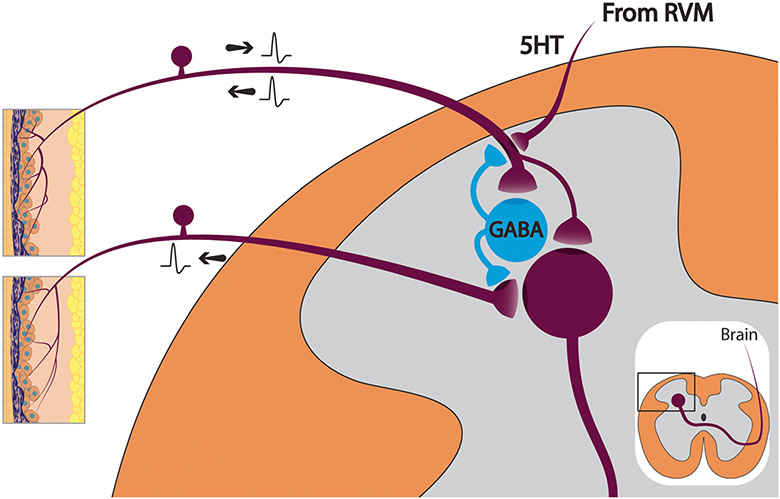
Fig. 4.
Following a maintained barrage of afferent activity, activation of GABAergic neurons in the dorsal horn elicits suprathreshold levels of depolarization in GABAA receptor containing primary afferent terminals sufficient to trigger antidromic actions potentials (DRR). Depending on the system, these may be axons of any caliber. Dorsal root reflexes may also be triggered by any descending activity that normally elicits primary afferent depolarization. The illustrated example is serotonergic bulbospinal fibers from the raphe in RVM that are presynaptic to 5HT3 receptors on the primary afferent terminal. RVM rostroventral medulla
Subcutaneous injection of capsaicin (activating TRPV1 bearing peptidergic C-fibers) results in primary afferent neurotransmitter release in the skin, not only within the injection site but also in the surrounding area. This latter effect has been attributed to antidromic activity in Aδ and C-fibers, but not in the larger Aβ fibers [75, 77]. This release results in neurogenic inflammation, i.e., increased blood flow, edema, cellular infiltrates, and enhanced nociception. In contrast, continuous irritation of joints in several models of arthritis results in warmth and edema of the affected joint which is attributed to DRRs in groups II, III, and IV fibers within the joint afferents, indicating that DRRs can be generated in the large myelinated group II fibers as well as the fine fibers [78]. This difference is commonly considered to be due to differences between cutaneous and joint innervation; although the exact underlying anatomy is not clearly defined, it is possible that the difference lies in the initiating stimuli employed to initiate the original afferent barrage. Thus, although high levels of activity in Aδ and C group III and IV nociceptors are necessary to trigger the conditions permissive for generation of dorsal root reflexes, once this sensitization has occurred, Aβ fiber activation appears to be sufficient to generate continued DRR activity [79, 80].
Pharmacological studies confirm that the antidromic discharge following joint inflammation is dependent on activation of GABAA and non-NMDA receptors in the dorsal horn [81]. Unexpectedly, despite the presence of presynaptic NMDA receptors, there does not appear to be an NMDA receptor component, in spite of the prominent role of this receptor in dorsal horn neuronal sensitization. Similar pharmacological results have been observed following induction of experimental arthritis using joint heat, edema, and pain behavior as outcomes [82, 83].
In addition to an afferent barrage of nociceptive activity, primary afferent depolarization can be initiated by descending modulation from a variety of brainstem sites including loci known to be involved in the so-called endogenous analgesia system. This activity is relatively specific for generating PAD and DRR in nociceptive and thermally sensitive afferents [84]. Primary afferent depolarization is induced in all sizes of myelinated fibers following stimulation of the ventromedial medulla [85]. Stimulation of supraspinal motor sites such as the red nucleus/rubrospinal tract and various cerebellar nuclei [74, 86-88] are also documented to produce PAD and DRR in afferent sensory fibers. Multiple studies have been performed to determine the spinal pharmacology for some descending PAD/DRR-producing pathways. In all instances where it was examined, a GABAergic link was demonstrated, presumably the GABAergic interneuron mentioned above. There is also a 5HT pathway, independent of the GABAergic interneuron (Fig. 4). This system effect is thought to be due predominantly to a direct action of 5HT acting on the 5HT3 ligand gated ion channel located on primary afferent terminals of some myelinated and unmyelinated nociceptors [89-92]. This serotonergic linkage has been demonstrated within the trigeminal system as well as in the lumbar cord [90]. Activation of bulbospinal systems through stimulation of the periaqueductal gray is clearly seen to elicit DRR fibers of the sural nerve [89]. Oddly, transection of the ventral quadrant at the upper cervical level eliminated the PAD/DRR induced by supraspinal stimulation implying that this descending pathway differs from that of the descending inhibitory pathway originating in the ventromedial medulla and which travels in the dorsolateral funiculus [87]. Surprisingly, dorsal root reflexes can be triggered bilaterally following a unilateral insult. This is consistent with the thought that focal inflammation on one limb produces a mirror image inflammation on the contralateral side [89]. This phenomenon appears to be mediated via a neuronal polysynaptic pathway and may involve sympathetic efferent fibers and/or capsaicin sensitive primary afferent fibers [93]. Sympathetic fibers sprout into contralateral DRGs and form baskets around individual neuronal somata following a unilateral lesion; following establishment of this connection, sympathetic activation elicits an α1-dependent activity in the DRG neurons [42]. This activity should be conveyed peripherally as well as centrally.
Concluding commentary
The release of active factors from the peripheral terminals of the sensory afferent is a common phenomenon with a broad impact upon the physiology of peripheral systems, as reviewed in the other papers in this journal volume. The present commentary emphasizes that this phenomena can be initiated not just by activation of the peripheral terminal but also through at least five principal points of origin: (i) afferent collaterals distal to the cell body (e.g., at the peripheral level), (ii) collaterals arising in the vicinity of the DRG cell body, (iii) from the mid axon where afferents lacking myelin sheaths may display crosstalk and following injuries wherein demyelination has occurred, (iv) the dorsal root ganglion itself, and finally (v) the central terminals of the afferent in the dorsal horn. In the dorsal horn, local circuits and bulbospinal projection can initiate the so-called dorsal root reflexes that represent antidromic traffic on the sensory afferent. Importantly, as reviewed, each of these originating circuits is subject to activating cascades that will yield antidromic potentials influencing the physiological function of the local organ systems receiving this innervation.
Footnotes
This article is a contribution to the special issue on Neurogenic Inflammation - Guest Editors: Tony Yaksh and Anna Di Nardo
References
- 1.Spitzer MJ, Reeh PW, Sauer SK (2008) Mechanisms of potassium- and capsaicin-induced axonal calcitonin gene-related peptide release: involvement of L- and T-type calcium channels and TRPV1 but not sodium channels. Neuroscience 151(3):836–842. 10.1016/j.neuroscience.2007.10.030 [DOI] [PubMed] [Google Scholar]
- 2.Kvamme E (1998) Synthesis of glutamate and its regulation. Prog Brain Res 116:73–85. 10.1016/S0079-6123(08)60431-8 [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP (2008) Local translation in primary afferent fibers regulates nociception. PLoS One 3(4):e1961 10.1371/journal.pone.0001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du J, Koltzenburg M, Carlton SM (2001) Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain 89(2):187–198. 10.1016/S0304-3959(00)00362-6 [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Komak S, Du J, Carlton SM (2001) Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res 913(1):18–26. 10.1016/S0006-8993(01)02747-0 [DOI] [PubMed] [Google Scholar]
- 6.Hucho T, Levine JD (2007) Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55(3):365–376. 10.1016/j.neuron.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Pellett S, Yaksh TL, Ramachandran R (2015) Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 7(11):4519–4563. 10.3390/toxins7114519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP (2009) Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev 60(1):84–89. 10.1016/j.brainresrev.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kress M, Izydorczyk I, Kuhn A (2001) N- and L- but not P/Q-type calcium channels contribute to neuropeptide release from rat skin in vitro. Neuroreport 12(4):867–870. 10.1097/00001756-200103260-00048 [DOI] [PubMed] [Google Scholar]
- 10.Saxena VK, de Deyn PP, Schoups AA, Coen EP, de Potter WP (1989) Relationship between external calcium concentration and noradrenaline- and neuropeptide Y-evoked release from perfused dog spleen. Brain Res 486(2):310–315. 10.1016/0006-8993(89)90517-9 [DOI] [PubMed] [Google Scholar]
- 11.Just S, Heppelmann B (2002) Frequency dependent changes in mechanosensitivity of rat knee joint afferents after antidromic saphenous nerve stimulation. Neuroscience 112(4):783–789. 10.1016/S0306-4522(02)00125-2 [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Chen Y, Wang C, Huang LY (2007) Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 104(23):9864–9869. 10.1073/pnas.0611048104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gee MD, Lynn B, Cotsell B (1997) The relationship between cutaneous C fibre type and antidromic vasodilatation in the rabbit and the rat. J Physiol 503(Pt 1):31–44. 10.1111/j.1469-7793.1997.031bi.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter SE, Lynn B (1981) Vascular and sensory responses of human skin to mild injury after topical treatment with capsaicin. Br J Pharmacol 73(3):755–758. 10.1111/j.1476-5381.1981.tb16812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrell WR, Russell NJ (1986) Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetized cat. J Physiol 379(1):407–416. 10.1113/jphysiol.1986.sp016260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine JD, Coderre TJ, Covinsky K, Basbaum AI (1990) Neural influences on synovial mast cell density in rat. J Neurosci Res 26(3):301–307. 10.1002/jnr.490260306 [DOI] [PubMed] [Google Scholar]
- 17.Lundberg JM, Brodin E, Hua X, Saria A (1984) Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand 120(2):217–227. 10.1111/j.1748-1716.1984.tb00127.x [DOI] [PubMed] [Google Scholar]
- 18.Michaelis M, Habler HJ, Jaenig W (1996) Silent afferents: a separate class of primary afferents? Clin Exp Pharmacol Physiol 23(2):99–105. 10.1111/j.1440-1681.1996.tb02579.x [DOI] [PubMed] [Google Scholar]
- 19.Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO (2000) Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 11(3):645–648. 10.1097/00001756-200002280-00041 [DOI] [PubMed] [Google Scholar]
- 20.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501(7465):52–57. 10.1038/nature12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinter E, Helyes Z, Nemeth J, Porszasz R, Petho G, Than M, Keri G, Horvath A, Jakab B, Szolcsanyi J (2002) Pharmacological characterisation of the somatostatin analogue TT-232: effects on neurogenic and non-neurogenic inflammation and neuropathic hyperalgesia. Naunyn Schmiedeberg's Arch Pharmacol 366(2):142–150. 10.1007/s00210-002-0563-9 [DOI] [PubMed] [Google Scholar]
- 22.Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN (1991) Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res 561(2):252–261. 10.1016/0006-8993(91)91601-V [DOI] [PubMed] [Google Scholar]
- 23.Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI (2006) Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18(10):936–948. 10.1111/j.1365-2982.2006.00807.x [DOI] [PubMed] [Google Scholar]
- 24.Sameda H, Takahashi Y, Takahashi K, Chiba T, Ohtori S, Moriya H (2001) Primary sensory neurons with dichotomizing axons projecting to the facet joint and the sciatic nerve in rats. Spine (Phila Pa 1976) 26(10):1105–1109. 10.1097/00007632-200105150-00003 [DOI] [PubMed] [Google Scholar]
- 25.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H (2003) Calcitonin gene-related peptide immunoreactive neurons with dichotomizing axons projecting to the lumbar muscle and knee in rats. Eur Spine J 12(6):576–580. 10.1007/s00586-003-0573-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sameda H, Takahashi Y, Takahashi K, Chiba T, Ohtori S, Moriya H (2003) Dorsal root ganglion neurones with dichotomising afferent fibres to both the lumbar disc and the groin skin. A possible neuronal mechanism underlying referred groin pain in lower lumbar disc diseases. J Bone Joint Surg Br 85(4):600–603. 10.1302/0301-620X.85B4.13306 [DOI] [PubMed] [Google Scholar]
- 27.Langford LA, Coggeshall RE (1981) Branching of sensory axons in the peripheral nerve of the rat. J Comp Neurol 203(4):745–750. 10.1002/cne.902030411 [DOI] [PubMed] [Google Scholar]
- 28.Chung K, Coggeshall RE (1984) The ratio of dorsal root ganglion cells to dorsal root axons in sacral segments of the cat. J Comp Neurol 225(1):24–30. 10.1002/cne.902250104 [DOI] [PubMed] [Google Scholar]
- 29.McCarthy PW, Prabhakar E, Lawson SN (1995) Evidence to support the peripheral branching of primary afferent C-fibres in the rat: an in vitro intracellular electrophysiological study. Brain Res 704(1):79–84. 10.1016/0006-8993(95)01107-2 [DOI] [PubMed] [Google Scholar]
- 30.Ramer MS, Thompson SW, McMahon SB (1999) Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain Suppl 6:S111–S120 [DOI] [PubMed] [Google Scholar]
- 31.Xu Q, Yaksh TL (2011) A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 24(4):400–407. 10.1097/ACO.0b013e32834871df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanani M (2012) Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res 1487:183–191. 10.1016/j.brainres.2012.03.070 [DOI] [PubMed] [Google Scholar]
- 33.Waxman SG, Zamponi GW (2014) Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 17(2):153–163. 10.1038/nn.3602 [DOI] [PubMed] [Google Scholar]
- 34.Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, Plee-Gautier E, Carre JL, Lefeuvre L, Misery L, Le Garrec R (2017) TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 8(9):644–661. 10.1007/s13238-017-0395-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warwick RA, Hanani M (2013) The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain 17(4):571–580. 10.1002/j.1532-2149.2012.00219.x [DOI] [PubMed] [Google Scholar]
- 36.Dublin P, Hanani M (2007) Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun 21(5):592–598. 10.1016/j.bbi.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 37.Huang TY, Hanani M (2005) Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol 289(4):G670–G678. 10.1152/ajpgi.00028.2005 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Mei X, Zhang P, Ma C, White FA, Donnelly DF, Lamotte RH (2009) Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia 57(15):1588–1599. 10.1002/glia.20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TY, Belzer V, Hanani M (2010) Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain 14(49):e1–11 [DOI] [PubMed] [Google Scholar]
- 40.Hu P, Bembrick AL, Keay KA, McLachlan EM (2007) Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 21(5):599–616. 10.1016/j.bbi.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 41.McLachlan EM, Hu P (1998) Axonal sprouts containing calcitonin gene-related peptide and substance P form pericellular baskets around large diameter neurons after sciatic nerve transection in the rat. Neuroscience 84(4):961–965. 10.1016/S0306-4522(97)00680-5 [DOI] [PubMed] [Google Scholar]
- 42.McLachlan EM, Janig W, Devor M, Michaelis M (1993) Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 363(6429):543–546. 10.1038/363543a0 [DOI] [PubMed] [Google Scholar]
- 43.Chung K, Yoon YW, Chung JM (1997) Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res 751(2):275–280. 10.1016/S0006-8993(96)01408-4 [DOI] [PubMed] [Google Scholar]
- 44.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM (2006) Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience 142(3):809–822. 10.1016/j.neuroscience.2006.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramer MS, Bisby MA (1997) Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain 70(2):237–244. 10.1016/S0304-3959(97)03331-9 [DOI] [PubMed] [Google Scholar]
- 46.Murinson BB, Griffin JW (2004) C-fiber structure varies with location in peripheral nerve. J Neuropathol Exp Neurol 63(3):246–254. 10.1093/jnen/63.3.246 [DOI] [PubMed] [Google Scholar]
- 47.Griffin JW, Thompson WJ (2008) Biology and pathology of nonmyelinating Schwann cells. Glia 56(14):1518–1531. 10.1002/glia.20778 [DOI] [PubMed] [Google Scholar]
- 48.Murinson BB, Hoffman PN, Banihashemi MR, Meyer RA, Griffin JW (2005) C-fiber (Remak) bundles contain both isolectin B4-binding and calcitonin gene-related peptide-positive axons. J Comp Neurol 484(4):392–402. 10.1002/cne.20506 [DOI] [PubMed] [Google Scholar]
- 49.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH (2003) Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 89(3):1588–1602. 10.1152/jn.00855.2002 [DOI] [PubMed] [Google Scholar]
- 50.Xie Y, Zhang J, Petersen M, LaMotte RH (1995) Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol 73(5):1811–1820. 10.1152/jn.1995.73.5.1811 [DOI] [PubMed] [Google Scholar]
- 51.Kajander K, Waikaka S, Bennett G (1992) Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett 138(2):225–228. 10.1016/0304-3940(92)90920-3 [DOI] [PubMed] [Google Scholar]
- 52.Seltzer Z, Devor M (1979) Ephaptic transmission in chronically damaged peripheral nerves. Neurology 29(7):1061–1064. 10.1212/WNL.29.7.1061 [DOI] [PubMed] [Google Scholar]
- 53.Rasminsky M (1980) Ephaptic transmission between single nerve fibres in the spinal nerve roots of dystrophic mice. J Physiol 305(1):151–169. 10.1113/jphysiol.1980.sp013356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA (2002) Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci 22(17):7746–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA (2001) Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 21:RC140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers RR, Heckman HM, Rodriguez M (1996) Reduced hyperalgesia in nerve-injured WLD mice: relationship to nerve fiber phagocytosis, axonal degeneration, and regeneration in normal mice. Exp Neurol 141(1):94–101. 10.1006/exnr.1996.0142 [DOI] [PubMed] [Google Scholar]
- 57.Sorkin LS, Xiao WH, Wagner R, Myers RR (1997) Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81(1):255–262. 10.1016/S0306-4522(97)00147-4 [DOI] [PubMed] [Google Scholar]
- 58.Moalem G, Grafe P, Tracey DJ (2005) Chemical mediators enhance the excitability of unmyelinated sensory axons in normal and injured peripheral nerve of the rat. Neuroscience 134(4):1399–1411. 10.1016/j.neuroscience.2005.05.046 [DOI] [PubMed] [Google Scholar]
- 59.Zelenka M, Schafers M, Sommer C (2005) Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 116(3):257–263. 10.1016/j.pain.2005.04.018 [DOI] [PubMed] [Google Scholar]
- 60.Sorkin L, Doom C (2000) Epineurial application of TNF elecits an acute mechanical hyperalgesia in the awake rat. J Peripheral Nervous Sys 5(2):96–1000. 10.1046/j.1529-8027.2000.00012.x [DOI] [PubMed] [Google Scholar]
- 61.Bove GM, Leem J-G (2002) Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain 3:45. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann T, Sauer SK, Horch RE, Reeh PW (2008) Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci 28(24):6281–6284. 10.1523/JNEUROSCI.1627-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung JM, Lee KH, Hori Y, Willis WD (1985) Effects of capsaicin applied to a peripheral nerve on the responses of primate spinothalamic tract cells. Brain Res 329(1-2):27–38. 10.1016/0006-8993(85)90509-8 [DOI] [PubMed] [Google Scholar]
- 64.Sauer SK, Reeh PW, Bove GM (2001) Noxious heat-induced CGRP release from rat sciatic nerve axons in vitro. Eur J Neurosci 14(8):1203–1208. 10.1046/j.0953-816x.2001.01741.x [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann T, Sauer SK, Horch RE, Reeh PW (2009) Projected pain from noxious heat stimulation of an exposed peripheral nerve–a case report. Eur J Pain 13(1):35–37. 10.1016/j.ejpain.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 66.Bove GM, Ransil BJ, Lin HC, Leem JG (2003) Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol 90(3):1949–1955. 10.1152/jn.00175.2003 [DOI] [PubMed] [Google Scholar]
- 67.Toennies JF (1938) Reflex discharge from the spinal cord over the dorsal roots. J Neurophysiol 1(4):378–390. 10.1152/jn.1938.1.4.378 [DOI] [Google Scholar]
- 68.Alvarez FJ, Kavookjian AM, Light AR (1992) Synaptic interactions between GABA-immunoreactive profiles and the terminals of functionally defined myelinated nociceptors in the monkey and cat spinal cord. J Neurosci 12(8):2901–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer E, Placheta P (1980) Reduction of [3H]muscimol binding sites in rat dorsal spinal cord after neonatal capsaicin treatment. Brain Res 202(2):484–487. 10.1016/0006-8993(80)90160-2 [DOI] [PubMed] [Google Scholar]
- 70.Carlton SM, Hayes ES (1990) Light microscopic and ultrastructural analysis of GABA-immunoreactive profiles in the monkey spinal cord. J Comp Neurol 300(2):162–182. 10.1002/cne.903000203 [DOI] [PubMed] [Google Scholar]
- 71.Todd AJ, Lochhead V (1990) GABA-like immunoreactivity in type I glomeruli of rat substantia gelatinosa. Brain Res 514(1):171–174. 10.1016/0006-8993(90)90454-J [DOI] [PubMed] [Google Scholar]
- 72.Todd AJ (2015) Plasticity of inhibition in the spinal cord. Handb Exp Pharmacol 227:171–190. 10.1007/978-3-662-46450-2_9 [DOI] [PubMed] [Google Scholar]
- 73.Schmidt RF (1971) Presynaptic inhibition in the vertebrate nervous system. Rev Physiol Biochem Pharm 63:21–101 [DOI] [PubMed] [Google Scholar]
- 74.Jimenez I, Rudomin P, Solodkin M (1987) Mechanisms involved in the depolarization of cutaneous afferents produced by segmental and descending inputs in the cat spinal cord. Exp Brain Res 69(1):195–207 [DOI] [PubMed] [Google Scholar]
- 75.Willis WD Jr (1999) Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124(4):395–421. 10.1007/s002210050637 [DOI] [PubMed] [Google Scholar]
- 76.Fitzgerald M, Woolf CJ (1981) Effects of cutaneous nerve and intraspinal conditioning of C-fibre afferent terminal excitability in decerebrate spinal rats. J Physiol 318(1):25–39. 10.1113/jphysiol.1981.sp013848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Q, Zou X, Willis WD (2000) Adelta and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol 84(5):2695–2698. 10.1152/jn.2000.84.5.2695 [DOI] [PubMed] [Google Scholar]
- 78.Sluka KA, Rees H, Westlund KN, Willis WD (1995) Fiber types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol 74(3):981–989. 10.1152/jn.1995.74.3.981 [DOI] [PubMed] [Google Scholar]
- 79.Cervero F, Laird JM (1996) Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. Neuroreport 7(2):526–528. 10.1097/00001756-199601310-00036 [DOI] [PubMed] [Google Scholar]
- 80.Cervero F, Laird JM (1996) Mechanisms of touch-evoked pain (allodynia): a new model. Pain 68(1):13–23. 10.1016/S0304-3959(96)03165-X [DOI] [PubMed] [Google Scholar]
- 81.Rees H, Sluka KA, Westlund KN, Willis WD (1995) The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol Lond 484(2):437–445. 10.1113/jphysiol.1995.sp020676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sluka KA, Westlund KN (1993) Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain 55(2):217–225. 10.1016/0304-3959(93)90150-N [DOI] [PubMed] [Google Scholar]
- 83.Sluka KA, Jordan HH, Westlund KN (1994) Reduction in joint swelling and hyperalgesia following post-treatment with a non-NMDA glutamate receptor antagonist. Pain 59(1):95–100. 10.1016/0304-3959(94)90052-3 [DOI] [PubMed] [Google Scholar]
- 84.Garcia-Ramirez DL, Calvo JR, Hochman S, Quevedo JN (2014) Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9(2):e89999 10.1371/journal.pone.0089999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin RF, Haber LH, Willis WD (1979) Primary afferent depolarization of identified cutaneous fibers following stimulation in medial brain stem. J Neurophysiol 42(3):779–790. 10.1152/jn.1979.42.3.779 [DOI] [PubMed] [Google Scholar]
- 86.Vinay L, Brocard F, Fellippa-Marques S, Clarac F (1999) Antidromic discharges of dorsal root afferents in the neonatal rat. J Physiol Paris 93(4):359–367. 10.1016/S0928-4257(00)80063-7 [DOI] [PubMed] [Google Scholar]
- 87.Owen MP, Hodge CJ Jr (1973) Positive dorsal root potentials evoked by stimulation of the brain stem reticular formation. Brain Res 54:305–308. 10.1016/0006-8993(73)90051-6 [DOI] [PubMed] [Google Scholar]
- 88.Lovick TA (1981) Primary afferent depolarization of tooth pulp afferents by stimulation in nucleus raphe magnus and the adjacent reticular formation in the cat: effects of bicuculline. Neurosci Lett 25(2):173–178. 10.1016/0304-3940(81)90327-X [DOI] [PubMed] [Google Scholar]
- 89.Peng YB, Wu J, Willis WD, Kenshalo DR (2001) GABA(a) and 5-HT(3) receptors are involved in dorsal root reflexes: possible role in periaqueductal gray descending inhibition. J Neurophysiol 86(1):49–58. 10.1152/jn.2001.86.1.49 [DOI] [PubMed] [Google Scholar]
- 90.Lovick TA (1983) The role of 5-HT, GABA and opioid peptides in presynaptic inhibition of tooth pulp input from the medial brainstem. Brain Res 289(1-2):135–142. 10.1016/0006-8993(83)90014-8 [DOI] [PubMed] [Google Scholar]
- 91.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI (2002) The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22(3):1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khasabov SG, Lopez-Garcia JA, Asghar AU, King AE (1999) Modulation of afferent-evoked neurotransmission by 5-HT3 receptors in young rat dorsal horn neurones in vitro: a putative mechanism of 5-HT3 induced anti-nociception. Br J Pharmacol 127(4):843–852. 10.1038/sj.bjp.0702592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine JD, Dardick SJ, Basbaum AI, Scipio E (1985) Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci 5(5):1380–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]