Abstract
The current pandemic of coronavirus disease 19 (COVID-19) is a global issue caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Studies have revealed that this virus results in poorer consequences and a higher rate of mortality in older adults and those with comorbidities such as cardiovascular disease, hypertension, diabetes and prolonged respiratory illness. In this review, we discuss in detail the potential groups at risk of COVID-19 and outline future recommendations to mitigate community transmission of COVID-19. The rate of COVID-19 was high in healthcare workers, smokers, older adults, travellers and pregnant women. Furthermore, patients with severe medical complications such as heart disease, hypertension, respiratory illness, diabetes mellitus and cancer are at higher risk of disease severity and mortality. Therefore, special effort and devotion are needed to diminish the threat of SARS-CoV-2 infection. Proper vaccination, use of sanitizers for handwashing and complete lockdown are recommended to mitigate the chain of COVID-19 transmission.
Keywords: Cardiovascular disease, coronavirus disease, diabetes, hypertension, severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) is a persistent pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The epidemic began in the city of Wuhan, the capital of Hubei province, China, in late 2019 and was declared a public health emergency of international concern on 30 January 2020 [1]. More than 48 136 225 COVID-19 cases have been identified globally, resulting in more than 1 225 913 deaths; over 31 919 360 patients have recovered [2]. This pandemic has caused socioeconomic mayhem worldwide, with rescheduling and cancellation of outings and sporting events as well as political, religious and traditional events [3]. In more than 193 countries, schools and universities have been closed at a local or national level – about 99.4% of the worldwide student population [3].
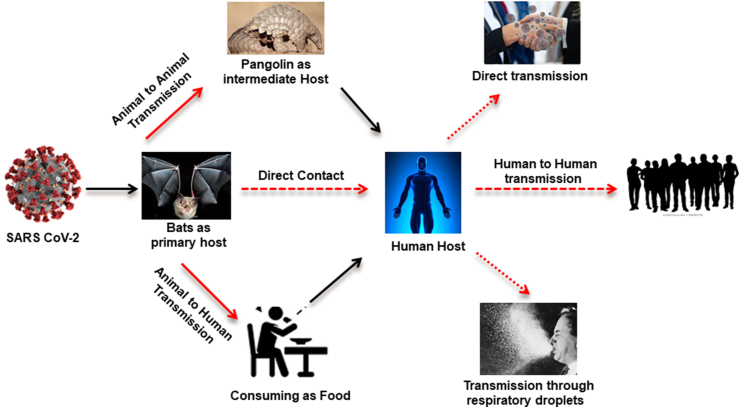
COVID-19 is a highly transmittable and pathogenic viral infection caused by SARS-CoV-2. Genomic analysis has revealed that SARS-CoV-2 is phylogenetically related to severe acute respiratory syndrome (SARS)-like bat viruses, with approximately 96% genome sequence similarity [4]. Therefore, bats could be the possible primary reservoir for human coronavirus (CoV) [5,6]. A growing body of research has identified bats as the evolutionary sources of SARS and Middle East respiratory syndrome (MERS) CoVs [7,8] and as the source of progenitors of human CoV [9]. The emergence of SARS-CoV-2 further underscores the importance of bat-origin CoVs to global health, and understanding their origin and cross-species transmission is a high priority for pandemic preparedness [10,11]. Scientists have also proposed pangolins as a potential intermediate species for SARS-CoV-2 emergence, suggesting that they are a potential reservoir species [12,13]. A metagenomic study identified pangolin-associated CoVs that belong to two sublineages of SARS-CoV-2–related CoVs, including one that exhibits strong similarity in the receptor-binding domain to SARS-CoV-2. The discovery of multiple lineages of pangolin CoV and their similarity to SARS-CoV-2 suggests that pangolins should be considered as possible hosts in the emergence of new CoVs [14]. It was concluded from the abovementioned theories that CoV may be transmitted directly from bats to humans, or it may transmit into pangolins as an intermediate host (Fig. 1). Among humans, it is mainly spread through coughing, sneezing, respiratory droplets and contact routes (Fig. 1) [1,15]. Droplet transmission occurs via the infected person (respiratory illness) to an adjacent person as a result of the high risk of exposure when infectious droplets are inhaled through mucosa or conjunctiva. Transmission may also occur through fomites in the atmosphere near the infected person [16]. Subsequently, the transmission of the COVID-19 virus can occur through direct contact with a person with disease and through indirect contact with surfaces in the nearby atmosphere or with items used by an infected person, like stethoscopes or thermometers [17].
Fig. 1.
Coronavirus disease 2019 (COVID-19) transmission cycle.
Patients with COVID-19 generally experience nausea, dry or painful cough, sore throat, tiredness or myalgia, headache, shortness of breath and general weakness [18,19]. Although most COVID-19 patients are thought to have a good prognosis, older patients and those with chronic illness may experience poorer outcomes [20]. Patients with chronic underlying conditions can experience viral pneumonia, hypoxaemia and dyspnoea within 1 week of disease onset, which may lead to respiratory or end-organ failure and even death [21]. Patient-reported loss of smell and taste has been related to COVID-19 infection, although no clinical olfactory tests have been conducted on a cohort of COVID-19 patients. It has been reported that quantitative odor testing shows that decreased odor activity is a significant marker for SARS-CoV-2 infection and suggests the possibility that odor testing may help in recognizing COVID-19 patients in need of early treatment [22].
COVID-19 is often more serious in people over the age of 60 or those who have health problems such as lung or heart disease, cancer, diabetes or immune-affecting disorders [23]. The basic rates of mortality vary, ranging from 5.6% to 15.2% throughout the world [24], with a greater risk of dying for the elderly and those with comorbid conditions like diabetes mellitus (DM) and hypertension [25]. A global study of 46 248 cases showed that the most prevalent comorbidities are obesity, cardiovascular disease, DM and respiratory morbidity [26]. In COVID-19, smoking appears to increase the probability of adverse outcomes [16]. It can increase angiotensin-converting enzyme expression 2 (ACE2) in current Asian smokers and make them vulnerable to COVID-19 [27]. Likewise, air pollution also increases the risk of extreme COVID-19 [28]. Air pollution such as particulate matter acts as a vector to carry the virus and increases its transmission. These particulate matters may damage the lung cells, resulting in severe inflammation [29].
Here we summarize the potential groups at risk of COVID-19 and propose future recommendations to control the transmission of COVID-19 among the community and healthcare workers (HCWs).
COVID-19 risk groups
According to the World Health Organization, most virus-infected people will develop mild or uncomplicated COVID-19, which comprises 80% of cases; the remaining 20% may develop its extreme version, requiring hospitalization (14%) or care in an intensive care unit (ICU) (6%) [30]. Well-described risk factors for severe disease among patients with COVID-19 in China include HCWs [31], older age [32,33] and serious medical conditions such as diabetes [32,34], cardiovascular disease [32,35], hypertension [32,35], cancer [32,36], chronic respiratory disease [32] and cerebrovascular disease [33,35]. The United States and Europe recently confirmed these risk features and suggested new ones, such as obesity, chronic kidney disease, smoking and asthma [37].
Healthcare workers
The well-being of healthcare professionals is the backbone of every health system that works well. As a result of the pandemic, healthcare providers are under immense strain from a heavy workload coupled with increased overall health expenses. The excessive burden of COVID-19 may lead to stress in HCWs. The main causes of psychologic distress in Health care workers (HCWs) include long hours of work, sleep disturbances and exhaustion as well as the possibility of becoming infected and placing their families at risk of a life-threatening condition [38].
According to the China National Health Commission, by late February, more than 3000 HCWs were reportedly infected in China, and many have died [39]. Additionally, in Iran, about 40 HCWs died of COVID-19, and several have been placed under observation after showing signs and symptoms of COVID-19 [38]. However, according to official online records, it was reported that 10% of HCWs in Italy were infected – a total of 7763 patients. HCWs are at greater risk of infection according to the Italian National Federation of Medicine Surgery and Odontology; anesthetists and surgeons are highly susceptible to exposure. It has been proposed that a community-centred or home care programme for COVID-19 could minimize disease transmission and physician exposure, rather than a hospital-focused system [31]. Dental HCWs are typically at a higher risk of transmitting this infectious disease, and oral HCWs may spread disease [40]. It has also been demonstrated that patients may visit dental settings for daily or emergency treatments, with dental HCWs serving as reservoirs for microbes that lead to a variety of diseases, including COVID-19 [41]. Dentists are exposed to oral secretions for prolonged periods, and their high-speed handpieces and ultrasonic devices aerosolize body fluids. It has been reported that dentists comprise about 5% of the total deaths [42].
Public health administrators worldwide [43,44], including the Ministry of Health in Saudi Arabia, are working diligently to prevent the transmission of COVID-19 by disseminating timely educational videos, instructional brochures and social networking notifications to HCWs [45]. All healthcare specialists from psychiatry to urology have died of work-related infection with COVID-19. Doctors whose specialties include the airway, such as anesthesiologists, dentists and otorhinolaryngologists, are susceptible to COVID-19; this group accounted for 12% of all doctor deaths. During the course of their work, ophthalmologists are also around oronasal secretions for long periods. Dr Li Wenliang, the Chinese ophthalmologist who first notified the globe of COVID-19, died at the age of 33 from this disease [46]. Two more ophthalmologists at Li's workstation died of COVID-19, possibly because of prolonged exposure to the airways of infected patients during ophthalmoscopy or nasolacrimal manipulation or from virus transmission via tears [47]. Countries like Singapore, South Korea and Taiwan, which have taken decisive measures to prevent travel from affected areas, strictly implemented quarantines and engaged in widespread testing, managed to control the epidemic; there are very few reports of physician deaths in these countries in social media [48].
In South Korea, until 10 weeks after the disease outbreak, there was no death of a physician or nurse reported from COVID-19 [49]. Because the onset of the disease in the United Kingdom and North America lagged behind Asia and Europe, there may be an increase in COVID-19–related physician deaths in these areas in the coming weeks. At least 12 COVID-19–related physician deaths occurred in North America on 5 April 2020. A rising number of North American doctors have contracted COVID-19 and are receiving critical care [50]. Senior doctors and those with comorbidities should preferably not be allocated to frontline duties with COVID-19 patients but rather should be given roles such as video or phone reviews, consultation or public liaison [51]. Finally, international communities should learn early how to protect their HCWs, especially in low- and middle-income countries with potential COVID-19 outbreaks. The increase in knowledge of personal safety, adequate personal protective equipment (PPE) and proper preparedness and response will play an essential role in decreasing the risk of infection for medical professionals.
Patients with medical complications
According to the World Health Organization, COVID-19 infects people of all ages. However, it has been suggested that people with severe medical complications such as cardiovascular disease, chronic respiratory disease, diabetes and cancer are at higher risk of disease morbidity and mortality [52].
Heart disease
COVID-19 interacts with the circulatory system at multiple levels, increasing morbidity and causing myocardial injury and dysfunction in patients with underlying cardiovascular disorders [53]. SARS-CoV-2 infection is caused by binding the surface spike protein of the virus to the human ACE2 receptor after the spike protein activates by the transmembrane protease serine 2 [54]. ACE2 is found in the lung (mainly alveolar type II cells) and tends to be the dominant portal of entry [55]. ACE2 is also highly expressed in the heart, countering the angiotensin II effects with extreme activation of the renin–angiotensin system, such as hypertension, atherosclerosis and congestive heart failure [56]. In addition to heart and lung, ACE2 is also found in intestinal epithelium, kidney and vascular endothelium, providing a basis for the multiorgan dysfunction observed with SARS-CoV-2 infection [55,56].
There is cumulative evidence that COVID-19 is linked with increased severity of and mortality from cardiovascular disease. Comorbidities including the cardiovascular system are common in patients with COVID-19, and these patients are at higher risk of disease severity and mortality. However, it is unknown whether cardiovascular comorbid conditions alone influence the risk of COVID-19 or whether some other factors are involved, such as age [53]. According to the US Centers for Disease Control and Prevention (CDC), 44 000 cases of laboratory-confirmed COVID-19 were reported. It was shown that cardiovascular disease, older age, diabetes, cancer, chronic respiratory disease and hypertension were all associated with an increased risk of morbidity and mortality [32]. Similar factors were also associated with 191 severe COVID-19 cases in China, with 54 deaths recorded while 137 patients were discharged [57]. It was observed that the risk of mortality was strongly associated with coronary heart disease, hypertension, DM and multiple organ failure.
Respiratory illness
The presence of a respiratory illness such as asthma and tuberculosis (TB) has been listed as a potential risk factor indicating COVID-19 severity and mortality, as described by the CDC [58]. Asthma is widely recognized as an important risk factor for serious respiratory disease in patients with COVID-19, especially in the United States. There is some evidence that patients with asthma who have COVID-19 are overrepresented among adult patients who have been admitted to hospital [59]. This overrepresentation may occur because SARS-CoV-2 causes exacerbations of asthma, as do other viruses, which is why asthma is identified as a risk factor for COVID-19 comorbidity [59]. Another study revealed that a 37-year-old man with asthma had postmortem lung findings which met the clinical criteria for severe acute respiratory distress syndrome; he died of COVID-19 less than 2 weeks after seeking care at the hospital. His lungs displayed plugging of mucus and other asthma-related histologic changes as well as early diffuse alveolar damage and fibrinous pneumonia. Diffuse alveolar damage is common in patients diagnosed with influenza virus, including influenza A/H1N1, and is often followed by a combination of haemorrhage, acute bronchopneumonia in which neutrophils predominate, peripheral pulmonary macroscopic thrombosis and haemophagocytosis [60]. The most frequent symptoms associated with COVID-19 – fever, dry cough and shortness of breath – are also normal during acute asthma exacerbation. A study showed a 14% prevalence of asthma in COVID-19 patients [58]. The use of appropriate PPE should be recommended to asthma patients to reduce the risk of disease.
Similarly, TB is also a severe bacterial disease which mainly affects the lungs of infected patients [2]. There is evidence that patients with latent TB infection and TB disease are at increased risk of infection with SARS-CoV-2 and are predisposed to severe COVID-19 pneumonia [61]. Because the portal of entry for both TB and COVID-19 is the upper respiratory tract, the relationship between serious influenza coinfection and pulmonary TB disease has remained unclear [62,63]. Similarly, a case report noted that the disease course of older TB patients easily progressed to the severe type of COVID-19, and patients had a long recovery process. These case reports notify us of the risk of COVID-19 patients with poor recovery being coinfected with TB; they also show the importance of the effective use of steroids for case management [64]. The importance of primary prevention measures should be emphasized, especially in TB patients. Hospitals and TB care centres need to be prepared for early detection and management of serious COVID-19.
Diabetes mellitus
Diabetes is an important cause of mortality and morbidity globally. The condition is related to a few macrovascular and microvascular complexities, which eventually affect patient survival [65]. A relationship between diabetes and infection, especially influenza A (H1N1) [66], SARS-CoV [67], MERS-CoV [68] and pneumonia is normal relation but it could effect the older people with DM (T2DM) [62,69,70]. According to current information, patients with T2DM do not seem to be at a higher risk of contracting SARS-CoV-2 than the overall population. However, the features of patients with diabetes at high risk for developing severe and serious forms of COVID-19, as well as the prognostic effect of diabetes on the course of COVID-19, are currently under examination [70]. However, it was reported from China and Italy that elderly people with chronic diseases (e.g. diabetes) were shown to have a high risk of dying from COVID-19 [32,71,72].
SARS-CoV-2 infection in patients with diabetes probably stimulates higher stress via the release of hyperglycemic hormones – for example catecholamines and glucocorticoids – leading to an increase in blood glucose levels as well as glucose inconsistency [34]. Furthermore, a retrospective study from Wuhan noted that about 10% of the patients with T2DM and COVID-19 experienced at least one bout of hypoglycaemia (<3.9 mmol/L) [10]. As the pandemic progresses to other continents, data from Europe and the Americas have shown a perturbing relationship between DM and COVID-19 prognosis. A study of 1099 COVID-19 patients was conducted in China, with results indicating that 16.2% of the patients had DM, 23.7% hypertension, 2.3% cerebrovascular disease and 5.8% coronary heart disease [71]. Similarly, another study including 140 patients showed a similar rate of hypertension (30%) and DM (12%) [73]. In Belgium, more than half a million individuals were identified with T2DM, and 20% of them were older than 65 [74,75]. Similarly, a French study indicated that diabetes affects more than 3 million individuals, mostly as T2DM, with 25% of them older than 75 years [2,76]. In Hong Kong, the first three deaths related to COVID-19 were reported in people with diabetes. A study of 1099 laboratory-confirmed COVID-19 cases found that 173 patients (16%) had severe disease with diabetes, while the remaining 926 patients (5.7%) had mild disease with diabetes. Similarly, 24% of those with severe disease had hypertension compared to 13% among those with mild disease, thus highlighting the increased risk of infection among those with chronic diseases like diabetes [71]. DM has been related to death and disease severity in patients with COVID-19.
Cancer
The outbreak of COVID-19 has infected thousands of people in at least 186 countries, with the cancer care delivery system affected in addition to affecting the overall health system. Cancer patients are more vulnerable to COVID-19 than cancer-free people because of their suppressed immune systems, the result of malignancy or cancer therapy [77]. Furthermore, cancer patients are frequently older (60 years of age) with one or more significant comorbidities, placing them at greater risk of COVID-19–related morbidity and mortality [78]. In addition, as a result of frequent physician visits for anticancer treatment, cancer screening and preventive and supportive care, they also have high rates of interaction with the healthcare system. Therefore, the concern is that the potential coexistence of a cancer diagnosis and an infection with COVID-19 may produce a synergistic negative prognostic effect.
The rates of COVID-19 mortality and morbidity were significantly higher among individuals with a cancer history (39%) than those without such a history (8%) (p 0.0003) [36]. Similarly, a 57-year-old Chinese male patient with lung cancer was reported to be COVID-19 positive, with disease manifesting symptoms such as fever, shortness of breath, cough, diarrhoea and myalgia [73]. A recent retrospective study of 28 cancer patients with confirmed COVID-19 was conducted in Wuhan to measure the risk factors associated with COVID-19 severity and mortality. It was reported that cancer patients at high risk of COVID-19 developed severe complications such as acute respiratory distress syndrome (28.6%), acute myocardial infarction (3.6%) and septic shock (3.6%) [79]. However, cancer patients require ongoing treatment, so it is not a privilege to undergo medical testing or clinical treatments; their future exposure to COVID-19 may be risky or even fatal. Remarkable strides are being made to identify the unique characteristics of patients with cancer who contract the novel CoV to address diagnostic and therapeutic obstacles and to develop guidelines to protect this vulnerable population from both virus exposure and disease progression resulting from testing and treatment delays.
Smoking
Smoking is considered a possible risk factor associated with negative disease prognosis. Extensive evidence has demonstrated the adverse effect of tobacco use on the lungs and its fundamental association with a plethora of respiratory illnesses [80]. Smoking is also harmful to the immune system and increases its sensitivity to infection, making smokers more susceptible to infectious diseases [81]. Previous studies have demonstrated that smokers are 2-fold more likely to contract influenza than nonsmokers and have more severe symptoms, while smokers were also more likely to have higher severity and mortality to the previous outbreak of MERS-CoV [82,83].
According to recent literature reported by the World Health Organization, smoking is associated with increased severity of disease and mortality of hospitalized COVID-19 patients [84]. Similar results have been described, mostly from China, suggesting that smoking is a potential risk factor associated with progression and adverse consequences of COVID-19 [71]. An epidemiologic study of 191 COVID-19–infected individuals reported 54 deaths while the remaining 137 patients survived. Among those who died, 9% were recent smokers compared to 4% among those who lived. There was no statistically significant difference between the smoking rates of survivors and nonsurvivors (p 0.21) to mortality from COVID-19 [81]. Similarly, a meta-analysis was conducted describing 11 590 COVID-19 patients showing that the risk of disease progression was almost double in smokers compared to nonsmokers. However, the rate of COVID-19 mortality among smokers was 25.8% [35]. Smoking involves the close contact of fingers and polluted cigarettes tubes with the lips, which increases the risk of COVID-19 transmission from hand to mouth [85]. Therefore, smoking is a possible mode of virus transmission for both direct and indirect smokers. Smokers with COVID-19 have a 3.25 times higher chance of getting serious forms of the disease compared to nonsmokers [71]. A study of 78 COVID-19 patients was conducted which showed adverse outcomes in patients with a history of smoking (27.3%) compared to nonsmokers (3.0%). The results were statistically significant (p 0.018), indicating that a history of smoking is considered a potential risk factor in disease progression [15].
Older adults
COVID-19 appears to follow a pattern like that of influenza and previous SARS-CoV outbreaks: the frequency and mortality of the disease are higher among the elderly age group [25]. In most countries, older people are at a significantly higher risk of developing serious disease as a result of physiologic changes occurring during ageing and possibly underlying health conditions [86]. A study of 44 672 COVID-19–positive cases conducted in China showed that the mortality rate was 2.3%, which differed according to age and disease severity. Death occurred in 14.8% of patients over 80 years of age, 8.0% between 70 and 79 years of age and 49.0% of serious cases. For those who died who had chronic diseases, 10.5% had cardiovascular disease, 6.3% had a chronic respiratory disease, 7.3% had DM, 5.6% had cancer and 6.0% had hypertension [32]. A similar analysis of 72 314 cases showed an overall case fatality rate (CFR) of 2.3%, while an 8% CFR was observed in patients aged 70 to 79 years and was 14.5% in patients older than 80 [32]. A study of 355 COVID-19–positive patients showed a high mortality rate in patients whose average age was 79.5 years [87]. Another multi–age group study of 4226 cases was reported in the United States, showing a less than 1% CFR in patients younger than age 54, while an older group aged 65 to 84 showed 3% to 11% CFR, with 10% to 27% CFR shown in patients older than 85 [78]. The COVID-19 pandemic has resulted in a much higher rate of mortality in older adults, especially older adults with comorbidities.
Travellers
The current outbreak of the novel CoV has resulted in important concerns. Continuously and rapidly rising numbers of COVID-19 infections threaten the globe. Every old or new disease or infection has certain risk factors, including COVID-19, which has many risk factors, with one important one being travel. There has been a noticeable increase in travel over the last decade [88], with tourism and international travel at a peak [89]. This trend of tourism and travel will lead to a greatly increased risk of illness and infections [88]. Research has repeatedly shown that people are creatures of habit and custom; tourism and travel also depend on habits and routines [90]. Despite its high risk factor, travel is considered to be an important part of religious life, as with Catholic pilgrims and Holy Week travellers in Latin America [91] as well as the Umrah and Hajj pilgrims [5]; people also travel for business [92]. People have travelled around the world not only for religious and business activities but also for educational purposes; indeed, China is the biggest source of international students [93]. During the first 2 months after the epidemic began in Wuhan, the disease quickly travelled out of China to other countries through domestic and international travel [94]. By late February, many countries had developed large-scale epidemics, raising international concern [95]. As a result of such concern, the Australian government announced travel restrictions and denied entry to anyone who had been in China within 14 days of arrival [93]. In Australia, where there are more than 100 000 Chinese students [93], the travel ban prevented almost all students from continuing their studies [81].
One study revealed that most COVID-19 cases were related to a travel history to China [96]. The results of this study indicated that a lower proportion of COVID-19 infection was noted in nontravellers compared to travellers. Another study reported that on 19 January 2020, a man sought care at a clinic in Snohomish Country, Washington State, with a history of coughing and fever. He was evaluated by doctors, and he disclosed that he had returned from Wuhan a few days before. Further investigation by real-time reverse transcriptase PCR revealed that the man was infected with COVID-19 [97]. Similarly, a 32-year-old student returned from the Wuhan University of Technology to Nepal with a heavy cough, sneezing and elevated temperature with mild breathing difficulties after some time [98]. The same scenario occurred in Pakistan when students returned there from China during the winter break. One case of COVID-19 infection discovered on 24 January 2020 occurred in a person with a travel history from China [99]. It is therefore concluded that travel is a main risk factor for transmission of COVID-19 infection. Many studies concluded that there should be a ban on travel, and a hard lockdown should be applied to prevent the current outbreak from escalating [96]. This conclusion is supported by another study which reported that there was a ban on travel to and from Wuhan during the epidemic, and this travel ban led to a COVID-19–free environment in Wuhan within 50 days [100]. The above studies mainly focused on the fact that travel is a risk factor for COVID-19; some studies further suggested that lockdowns and travel bans are better tactics to tackle the COVID-19 infection. However, we suggest that there are consequences of lockdowns and travel bans which must be carefully analysed.
Pregnant women
COVID-19 affects people of any age, with the elderly most affected, but cases have also been identified in newborns and young children, although these groups of patients tend to develop a milder disease course [32]. Pregnant women may be more vulnerable to COVID-19 because they are more susceptible to viral infections as a result of the physiologic immunosuppression that characterizes pregnancy; they also have a higher risk of developing a more serious type of disease [21]. The previous MERS-CoV and SARS-CoV outbreaks indicated that pregnant women are specifically susceptible to adverse outcomes, including ICU admission, renal failure and death and need for endotracheal intubation [101,102]. Pregnancy is characterized by some changes involving both the immune system and pulmonary physiology, thus exposing the pregnant woman to a greater susceptibility to viral infections and more serious complications [103]. Evaluation of COVID-19 during pregnancy is in progress. Research groups want to learn about the influence of COVID-19 on pregnant and nonpregnant women to see whether or not it is different. The limited data available indicate that there is no significantly higher influence of COVID-19 on pregnant women compared to the everyday person [38,104]. For the maintenance of foetal semiallografts, pregnant women have special immunologic adaptations. The immune system is temporarily suppressed because of the suppression of T-cell activity, and consequently pregnant women are predisposed to viral infections [105,106]. Moreover, the clinical outcomes of viral infections might be worse in pregnant women as a result of the physiologic and hormonal changes that occur in the circulatory and respiratory systems [104].
With pregnancy, many physiologic changes occur compared to nonpregnant women. In cases of infection of the lower respiratory tract, pregnant women have worse outcomes and need to be treated in the ICU. Most women were affected in the third trimester and had the same clinical findings as nonpregnant women. Preterm deliveries and foetal distress were observed in some cases [104,107]. The history of other CoVs indicates that pregnant women are at more risk than nonpregnant women. Due to diabetes in pregnant women, their bodies respond in the form of suppression of TH1 and high expression of ACE2 receptors, which are responsible for the attachment of SARS-CoV and SARS-CoV-2, so they are more susceptible to the viral infection [55,108].
The common symptoms of pregnant women were cough, fever shortness of breath, myalgia and fatigue. From 0 to 14% of the woman were reported have severe pneumonia and needed to be admitted to the ICU [109]. To date, studies have shown that pregnant women are not at a higher risk than the general population, but they may be considered a high-risk group because of the results of studies of other viral infections demonstrating their detrimental impact on pregnant women. Approximate data indicate that 85% of pregnant women had mild disease; severe disease was reported in 10% and critical disease in 5% [110,111]. A comparison of pregnant and nonpregnant women with SARS-CoV-2 revealed a lower lymphocyte count in pregnant women. Other diagnostic parameters, like white blood cell count, haemoglobin level and platelet and liver enzyme counts, were reported to be in the normal range [112]. It has been reported that pregnant women with COVID-19 are not susceptible to its complications, and the outcomes of pregnancy are not significantly affected [113]. Pregnancy outcomes between symptomatic and asymptomatic patients show that the former group has a high rate of severe disease and preterm birth than the latter. Our results indicate that asymptomatic pregnant patients may not be at high risk of severe illness, at least relative to symptomatic pregnant women. The small sample size was the major limitation of the above study, so we cannot generalize any findings based on this study [114]. In seven confirmed cases from New York City, five patients were symptomatic, with symptoms like cough, myalgia, fever, chest pain and headache. This is not much different from their nonpregnant counterparts [110]. Comparing clinical courses and outcomes of pregnant and nonpregnant women found no association between pregnancy and severity of diseases, virus clearance time or length of hospital stay [115]. A survey of these studies reveals that pregnant women are not significantly more susceptible to COVID-19 than nonpregnant women. Scientific research and exploration of COVID-19 is currently in its infancy; more research needs to be done to gain insight into the risk posed to infected pregnant women.
It is well known that some virus species can cause congenital infections and affect the health status of the foetus – for instance, rubella virus infection [116]. The effects and consequences of COVID-19 transmission from mother to foetus remain poorly understood [117]. Testing for the presence of SARS-CoV-2 by reverse transcriptase PCR has repeatedly failed to identify the presence of the virus's genome in maternal and neonatal specimens including placenta, umbilical cord blood, amniotic fluid or amniotic swab, maternal blood, vaginal secretions or breast milk [21,118]. The detection of SARS-CoV-2 RNA in placental or membrane samples supports the possibility of vertical transmission [119]. One detected virus RNA in three of 11 samples from COVID-19–positive women, all of whom had moderate to severe disease at the time of delivery. However, none of the infants tested positive for SARS-CoV-2 from postnatal days 1 through 5, and none showed symptoms of COVID-19 infection [117]. More recently, a case report has been published regarding placental transmission of COVID-19 [120]. A case occurred in a woman in her third trimester of pregnancy who had critical COVID-19; amniotic fluid, umbilical cord blood, placenta and neonatal gastric fluid were retained during Cesarean section. The COVID-19 nucleic acid test results of the specimens revealed no COVID-19 infection. There is no evidence of intrauterine vertical transmission during delivery in the third trimester, but the data are limited and need to be further explored.
Hypertension
Hypertension has been reported to greatly increase the incidence and morbidity of patients with the novel CoV. Nevertheless, early COVID-19 studies reported mixed findings regarding hypertension [121]. Hypertension was found to be associated with an ∼2.5-fold increased risk of both increased incidence and mortality and generally occurs in people over age 60 [121]. Likewise, the incidence of hypertension is high in patients affected by COVID-19 and tends to be associated with an increased risk of mortality and morbidity [122]. Early evidence from the United States and China shows that hypertension tends to be the most prevalent comorbidity in at least 30% to 49% of COVID-19–admitted patients [123,124]. Patients with hypertension whose COVID-19 improves are more likely to be admitted to hospital than normotensive persons [125]. Guan et al. [71] collected data from 1099 confirmed COVID-19 patients, with 15% reporting hypertension as the single highest risk factor of infection. Similarly, Zhang et al. [73] identified 140 patients with COVID-19 and found that 30% of all patients and 37.9% of those with severe illness had hypertension. Hypertension was found in 23.7% to 30% of patients admitted with COVID-19 associated with a more serious infection. Similarly, research from Italy that looked at patients admitted to the ICU found that 49% had hypertension.
Similar to influenza and SARS-CoV outbreaks, the incidence and mortality of COVID-19 is higher in the elderly. Nevertheless, because hypertension is strongly associated with age, an alternative definition is end-organ damage in patients with hypertension. As a result of hypertension, a number of pathophysiologic changes occur in the cardiovascular system, such as fibrosis and left ventricular hypertrophy, which make the hypertensive heart susceptible to SARS-CoV-2 [25]. The scientific and epidemiologic characteristics of COVID-19 have been repeatedly published, which show specific comorbidities associated with COVID-19 severity and mortality. It has been reported that the most common comorbidities are hypertension (30%), coronary heart disease (8%) and diabetes (19%) [57]. Another study reported that the most common comorbidities in COVID-19 patients with established acute respiratory distress syndrome were hypertension (27%), cardiovascular disease (6%) and diabetes (19%) [20]. In Italy, of 1591 ICU patients treated between February and March 2020, 49% had systemic hypertension [37]. Hypertension in older patients should be considered a clinical indicator of COVID-19 severity and mortality.
Future recommendations
The current COVID-19 pandemic caused by SARS-CoV-2 is of global concern. Environmental factors such as contaminated air and smoking as well as comorbid conditions (e.g. DM, hypertension and fundamental cardiorespiratory illness) may likely increase the mortality rate of COVID-19. Therefore, special attention and effort are required to mitigate the risk of SARS-CoV-2 infection. Some recommendations follow that indicate how to fight COVID-19 and how to reduce the risk of this disease at both national and international levels.
-
•
The novel CoV spreads among the community through respiratory droplets, contacts and fomites [19,126]. Therefore, it is important to implement restrictive measures at the social level to reduce virus spread. Proper handwashing on a regular basis, sneeze and cough sanitization and the use of PPE for HCWs who treat COVID-19 patients are probably helping to mitigate the spread of this contagious infection [127,128].
-
•
Similarly, complete lockdowns and strict limitations on travel and social interactions may help control the COVID-19 transmission chain. This approach is often effective for the elderly and those with prolonged comorbid conditions [129].
-
•
It is important to develop a policy for mitigating local transmission of the virus and for providing mass-scale separation and treatment. It is also crucial to develop well-thought-out approaches for distributed care, including home-based diagnosis and quarantine, arrangements with medical technology companies and the strong leadership of local authorities in encouraging social distancing throughout the pandemic.
-
•
Frontline HCWs represent are at high risk of COVID-19 infection, so it is important to support HCWs during outbreaks. It is essential to take immediate measures to train HCWs to safely manage the burden of treating this disease. This must include guidance and training on screening, infection prevention protocols and case management.
-
•
It is important to pursue extra manufacturing capability and strengthen the existing PPE supply chain and other critical medical supplies. It is also crucial to evaluate the availability of essential PPE and its supply chain for national and international use during a COVID-19 respiratory outbreak.
-
•
Important guidelines from the CDC and the US National Institutes of Health must be issued; experts use these venues to communicate to the public regularly and to diminish the fear, panic and spread of misinformation among the community, as well as to provide updates to the public on planning for COVID-19's spread worldwide.
-
•
As COVID-19 becomes an international matter, it is important to share policies internationally and to improve coordination for mitigating its transmission.
Conclusions
COVID-19 is an emerging disease that was first diagnosed in Wuhan, China, and has consequently spread throughout the world. HCWs are at a potential risk for COVID-19 mortality because they serve as the backbone of every healthcare system. Hypertension may be associated with an up to 2.5-fold higher risk of severe and lethal COVID-19, particularly among older people. Moreover, smokers, travellers, pregnant women and patients with severe health complications (heart disease, respiratory illness, diabetes and cancer) are also at a greater risk of COVID-19 morbidity and mortality. Proper handwashing, sneeze and cough sanitization, the use of PPE and complete lockdowns are probably helpful in mitigating the spread of this contagious infection. Future research is urgently needed to provide a better understanding of the basic pathophysiologic mechanisms of the association between COVID-19 and comorbidities.
Conflict of interest
None declared.
Acknowledgements
We are thankful to all the experts who provided insights during the writing process.
References
- 1.World Health Organization (WHO) WHO; Geneva: 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) [Google Scholar]
- 2.World Health Organization (WHO) https://www.who.int/diabetes/country-profiles/irq_en.pdf
- 3.Scipioni J. 5 June 2019. CNBC.https://www.cnbc.com/jade-scipioni/ [Google Scholar]
- 4.Kakakhel M.A., Wu F., Khan T.A., Feng H., Hassan Z., Anwar Z. The first two month epidemiological study of COVID-19, related public health preparedness and response to the ongoing epidemic in Pakistan. New Microbe. New Infect. 2020;37:100734. doi: 10.1016/j.nmni.2020.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tambo E., El-Dessouky A.G., Khater E.I.M., Xianonng Z. Enhanced surveillance and response approaches for pilgrims and local Saudi populations against emerging Nipah, Zika and Ebola viral diseases outbreaks threats. J Infect Public Health. 2020;13:674–678. doi: 10.1016/j.jiph.2020.01.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni M.F., Lemey P., Jiang X., Lam T.T., Perry B.W., Castoe T.A. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. 2020;(5):1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- 7.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 8.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman V.M., Baldwin H.J., Tateno A.F., Zerbinati R.M., Annan A., Owusu M. Evidence for an ancestral association of human coronavirus 229E with bats. J Virol. 2015;89:11858–11870. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J., Tan J. Diabetes patients with COVID-19 need better care. Metabolism. 2020;107:154216. doi: 10.1016/j.metabol.2020.154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.J. Isolation of SARS-CoV-2–related coronavirus from Malayan pangolins. Nature. 2020;583(7815):286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- 13.Liu P., Jiang J.Z., Wan X.F., Hua Y., Li L., Zhou J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H.H., Jiang J.F., Zhu H.C. Identifying SARS-CoV-2–related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Tao Z.W., Lei W. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020 doi: 10.1097/cm9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong S.W., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) WHO; Geneva: 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020. No. WHO/2019-nCoV/Sci_Brief/Transmission modes/2020.1. [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) 2019. Global tuberculosis report.https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf [Google Scholar]
- 20.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Public Health England . 30 May 2019. Seasonal influenza vaccine uptake in GP patients: winter season 2018 to 2019.https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-gp-patients-winter-2018-to-2019 [Google Scholar]
- 24.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comunian S., Dongo D., Milani C., Palestini P. Air pollution and Covid-19: the role of particulate matter in the spread and increase of Covid-19’s morbidity and mortality. Int J Environ Res Public Health. 2020;17:4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing H., Wang X., Zhang N., Zheng K., Du K., Zheng M. The effect of fine particulate matter on the inflammatory responses in human upper airway mucosa. Am J Respir Crit Care Med. 2019;200:1315–1318. doi: 10.1164/rccm.201903-0635LE. [DOI] [PubMed] [Google Scholar]
- 30.Zhonghua L., Xing B.X., Za Z. Novel coronavirus pneumonia emergency response epidemiology team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) China. 2020;41:145–151. [Google Scholar]
- 31.Nacoti M., Ciocca A., Giupponi A., Brambillasca P., Lussana F., Pisano M. At the epicenter of the Covid-19 pandemic and humanitarian crises in Italy: changing perspectives on preparation and mitigation. NEJM Catalyst. 21 March 2020;1(2) https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0080 [Google Scholar]
- 32.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648.32091533. [DOI] [PubMed] [Google Scholar]
- 33.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020:108118. doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:E181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUS of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J., Zhou M., Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;105(1) doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoVand controls in dental practice. Int J Oral Sci. 2020;12:9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volgenant C.M.C., de Soet J.J. Cross-transmission in the dental office: does this make you ill? Curr Oral Health Rep. 2018;5:221–228. doi: 10.1007/s40496-018-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99:481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarwar Shah S.G. A commentary on World Health Organization declares globalemergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020 doi: 10.1016/j.ijsu.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell L.N., Charles A.G. An invited commentary on World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19): emergency or new reality? Int J Surg. 2020:111. doi: 10.1016/j.ijsu.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ministry of Health (MOH) 2020. Kingdom of Saudi Arabia. Events and activities guide on COVID-19.https://www.moh.gov.sa/en/Ministry/MediaCenter/Pages/default.aspx [Google Scholar]
- 46.Lee K.J. American Academy of Opthalmology; 10 February 2020. Coronavirus kills Chinese whistleblower ophthalmologist.https://www.aao.org/headline/coronavirus-kills-chinese-whistleblower-ophthalmol [Google Scholar]
- 47.Ing E.B., Xu Q.A., Salimi A., Torun N. Physician deaths from corona virus (COVID-19) disease. Occup Med (Lond) 2020;70:370–374. doi: 10.1093/occmed/kqaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xinghui K. 28 March 2020. Why are there so few coronavirus infections in Singapore’s health workers? Coronavirus Pandemic.https://www.scmp.com/week-asia/health-environment/article/3077345/coronavirus-why-so-few-infections-singapores-health [Google Scholar]
- 49.Yoon D. How South Korea Solved Its Acute Hospital-Bed Shortage. Wall St J. 2020 https://www.wsj.com/articles/how-south-korea-solved-its-acute-hospital-bed-shortage-11584874801 [Google Scholar]
- 50.Kirsch T. 24 March 2020. What happens if health-care workers stop showing up? Atlantic.https://www.theatlantic.com/ideas/archive/2020/03/were-failing-doctors/608662/ [Google Scholar]
- 51.Buerhaus P.I., Auerbach D.I., Staiger D.O. Older clinicians and the surge in novel coronavirus disease 2019 (COVID-19) JAMA. 2020;323:1777–1778. doi: 10.1001/jama.2020.4978. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization (WHO) December 2020. Novel coronavirus (2019-nCoV) situation report – 22.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- 53.Kevin J.C., Justin A.F., Jayant R., Gabriel S., Jan M.G., Amirali M. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E. Prevalence and characterization of asthma in hospitalized and non-hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abrams E.T., Jong G., Yang C. Canadian Paediatric Society; 8 September 2020. Paediatric asthma and COVID-19.https://www.cps.ca/en/documents/position/paediatric-asthma-and-covid-19 updated. [Google Scholar]
- 60.Harms P.W., Schmidt L.A., Smith L.B. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol. 2010;134:27–35. doi: 10.1309/AJCP35KOZSAVNQZW. [DOI] [PubMed] [Google Scholar]
- 61.Marimuthu Y., Nagappa B., Sharma N., Basu S., Chopra K.K. COVID-19 and tuberculosis: a mathematical model based forecasting in Delhi, India. Indian J Tuberc. 2020;67:177–181. doi: 10.1016/j.ijtb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S., Wang J., Zhang B., Li X., Liu Y. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J. 2019;43:319–341. doi: 10.4093/dmj.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walaza S., Cohen C., Tempia S., Moyes J., Nguweneza A., Madhi S.A. Influenza and tuberculosis co-infection: a systematic review. Influenza Other Respir Virus. 2020;14(14):77–91. doi: 10.1111/irv.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He G., Wu J., Shi J., Dai J., Gamber M., Jiang X. COVID-19 in tuberculosis patients: a report of three cases. J Med Virol. 2020 doi: 10.1002/jmv.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams R., Karuranga S., Malanda B., Saeedi P., Basit A., Besançon S. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2020;13:108072. doi: 10.1016/j.diabres.2020.108072. 9th edition. [DOI] [PubMed] [Google Scholar]
- 66.Schoen K., Horvat N., Guerreiro N.F.C., de Castro I., de Giassi K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMI Infect Dis. 2019;19:964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetic Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 68.Banik G.R., Alqahtani A.S., Booy R., Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sinica. 2016;31:81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald H.I., Nitsch D., Millett E.R., Sinclair A., Thomas S.L. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabetic Med. 2014;31:606–614. doi: 10.1111/dme.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orioli L., Hermans M.P., Thissen J.P., Maiter D., Vandeleene B., Yombi J.C. COVID-19 in diabetic patients: related risks and specifics of management. Ann Endocrinol. 2020;81:101–109. doi: 10.1016/j.ando.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 74.Sciensano . 2018. Health interview survey.https://his.wiv-isp.be [Google Scholar]
- 75.Gamble A., Pham Q., Goyal S., Cafazzo J.A. The challenges of COVID-19 for people living with diabetes: considerations for digital health. JMIR Diabetes. 2020;5 doi: 10.2196/19581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santé publique, France. https://www.santepubliquefrance.fr
- 77.Shankar A., Saini D., Roy S., Mosavi Jarrahi A., Chakraborty A., Bharti S.J. Cancer care delivery challenges amidst coronavirus disease-19 (COVID-19) outbreak: specific precautions for cancer patients and cancer care providers to prevent spread. Asian Pac J Cancer Prev. 2020;21:569–573. doi: 10.31557/APJCP.2020.21.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) – United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H., Huang Y., Xie C. The treatment and outcome of a lung cancer patient infected with SARS-CoV-2. J Thorac Oncol. 2020:10. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tonnesen P., Marott J.L., Nordestgaard B., Bojesen S.E., Lange P. Secular trends in smoking in relation to prevalent and incident smoking-related disease: a prospective population-based study. Tob Induced Dis. 2019;17(10) doi: 10.18332/tid/112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Z., Chen P., Peng H. Are healthy smokers really healthy? Tob Induc Dis. 2016;14(November) doi: 10.1186/s12971-016-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park J.E., Jung S., Kim A. MERS transmission and risk factors: a systematic review. BMC Public Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch Intern Medicines. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 84.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induced Dis. 2020;18 doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sabino-Silva R., Jardim A.C., Siqueira W.L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Invest. 2020;24(4):1619–1621. doi: 10.1007/s00784-020-03248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(2):505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iqbal M.Z., Zohra B. 2020. COVID-19 and diabetes mellitus: prevalence and precautions. Annals of King Edward Medical University; pp. 218–225. 26(Special Issue) [Google Scholar]
- 88.Saleh Abdullah A.M., Johnson Hedley A., Fielding R. Prevalence of travel related illness amongst a group of Chinese undergraduate students in Hong Kong. J Travel Med. 2000;7:125–132. doi: 10.2310/7060.2000.00043. [DOI] [PubMed] [Google Scholar]
- 89.Hiemstra S.J. World tourism outlook for 1990s. World Travel Tourism Rev. 1991;1:62. [Google Scholar]
- 90.Schönfelder S., Axhausen K.W. Ashgate; Farnham, UK: 2010. Urban rhythms and travel behaviour: spatial and temporal phenomena of daily travel. [Google Scholar]
- 91.Rodriguez-Morales A.J., Sah R., Paniz-Mondolfi A. Should the Holy Week 2020 be cancelled in Latin America due to the COVID-19 pandemic? Travel Med Infect Dis. 2020:101633. doi: 10.1016/j.tmaid.2020.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Unger O., Uriely N., Fuchs G. The business travel experience. Ann Tourism Res. 2016;61:142–156. [Google Scholar]
- 93.Ma T., Heywood A., MacIntyre C.R. Travel health risk perceptions of Chinese international students in Australia – implications for COVID-19. Infect Dis Health. 2020 doi: 10.1016/j.idh.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Day M. Covid-19: surge in cases in Italy and South Korea makes pandemic look more likely. BMJ. 2020;368:m751. doi: 10.1136/bmj.m751. [DOI] [PubMed] [Google Scholar]
- 96.Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Ichii H., Schubert J. Internationally lost COVID-19 cases. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bastola A., Sah R., Rodriguez-Morales A.J., Lal B.K., Jha R., Ojha H.C. The first 2019 novel coronavirus case in Nepal. Lancet Infect Dis. 2020;20:279–280. doi: 10.1016/S1473-3099(20)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmad T., Khan M., Khan F.M., Hui J. Are we ready for the new fatal coronavirus: scenario of Pakistan? Hum Vaccines Immunother. 2020;16:736–738. doi: 10.1080/21645515.2020.1724000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian H., Liu Y., Li Y., Wu C.H., Chen B., Kraemer M.U.G. The impact of transmission control measures during the first 50 days of the COVID-19 epidemic in China. MedRxiv. 2020 doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 102.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forestieri S., Marcialis M.A., Migliore L., Panisi C., Fanos V. Relationship between pregnancy and coronavirus: what we know. J Matern Fetal Neonatal Med. 2020:1–12. doi: 10.1080/14767058.2020.1771692. [DOI] [PubMed] [Google Scholar]
- 104.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pazos M., Sperling R.S., Moran T.M., Kraus T.A. The influence of pregnancy on systemic immunity. Immunol Res. 2012;54:254–261. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weetman A.P. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol. 2010;6:311. doi: 10.1038/nrendo.2010.46. [DOI] [PubMed] [Google Scholar]
- 107.Elwood C., Boucoiran I., VanSchalkwyk J., Money D., Yudin M., Poliquin V. Updated SOGC committee opinion – COVID-19 in pregnancy. J Obstet Gynaecol Can. 2020 [Google Scholar]
- 108.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 109.Juan J., Gil M., Rong Z., Zhang Y., Yang H., Poon L. Effects of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol. 2020:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Breslin N., Baptiste C., Miller R., Fuchs K., Goffman D., Gyamfi-Bannerman C. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020;2:100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryean G.A., Purandare N.C., McAuliffe F.M., Hod M., Purandare C.N. Clinical update on COVID-19 in pregnancy: a review article. J Obstet Gynaecol Res. 2020:1235–1245. doi: 10.1111/jog.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohr-Sasson A., Chayo J., Bart Y., Meyer R., Sivan E., Mazaki-Tovi S. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet. 2020;302:629–634. doi: 10.1007/s00404-020-05655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruggiero M., Somigliana E., Tassis B., Piani L.L., Renteria S.U., Barbara G. Covid-19 in the second half of pregnancy: prevalence and clinical relevance. Europe PMC. 2020 doi: 10.21203/rs.3.rs-271806/v1. [DOI] [Google Scholar]
- 114.London V., McLaren R., Jr., Atallah F., Cepeda C., McCalla S., Fisher N. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020;37:991. doi: 10.1055/s-0040-1712164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiancheng X., Jian S., Lingling P., Lei H., Xiaogan J., Weihua L. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. 2020;95:376–383. doi: 10.1016/j.ijid.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–49. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V. SARS-CoV-2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020:193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Penfield C.A., Brubaker S.G., Limaye M.A. Detection of SARS-CoV-2 in placental and 436 fetal membrane samples. Am J Obstet. 2020:100133–100438. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lv Y., Gu B., Chen Y., Hu S., Ruan T., Xu G. No intrauterine vertical transmission in pregnancy with COVID-19: a case report. J Infect Chemother. 2020;26:1313–1315. doi: 10.1016/j.jiac.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lippi G., Wong J., Henry B.M. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Polish Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 122.Ruocco G., Feola M., Palazzuoli A. Hypertension prevalence in human coronavirus: the role of ACE system in infection spread and severity. Int J Infect Dis. 2020;95:373–375. doi: 10.1016/j.ijid.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.European Alliance of Associations for Rheumatology (EULAR) EULAR guidance for patients COVID-19 outbreak. https://www.eular.org/eular_guidance_for_patients_covid19_outbreak.cfm Updated 17 March 2020.
- 128.Australian Rheumatology Association (ARA) Guidance for patients during Covid-19 outbreak. https://arthritisaustralia.com.au/advice-regarding-coronavirus-covid-19-from-th australian-rheumatology-association/ Updated 17 March 2020.
- 129.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27:taaa020. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]