Abstract
Background and purpose:
To investigate the pharmacokinetics of piperine after single oral doses of capsules containing Sahastara (SHT) remedy dried ethanolic extracts in healthy Thai volunteers.
Experimental approach:
Twenty-four healthy volunteers were divided into two dosage groups. They received a single oral dose of SHT remedy extract capsules of 100 or 200 mg. Blood was collected at time intervals of 0, 0.5, 1, 2, 4, 6, 8, 12, 24, and 48 h. Acute clinical safety was monitored by complete physical examination and laboratory tests during the study period. Piperine concentration in blood and urine was determined by liquid chromatography tandem-mass spectrometry.
Findings/Results:
No serious adverse events were detected, only one volunteer had abdominal pain that was self-limiting. The pharmacokinetics of piperine following SHT remedy extract capsule administration demonstrated a mean peak concentration (Cmax) of piperine of 3.77 μg/mL and 6.59 μg/mL after dosing with 100 and 200 mg, respectively. Interestingly, a secondary maximum concentration of piperine was observed in this study, which might be related to enterohepatic recirculation. Negligible amounts of unchanged piperine were detected in urine.
Conclusion and implication:
The systemic exposure of piperine after SHT remedy ethanolic extract demonstrated dose proportionality after single oral dosing of 100-200 mg. Piperine was detectable in plasma for at least 48 h with evidence of enterohepatic recirculation. Metabolism and excretion profiles of piperine after administration of SHT remedy extract capsule need to be further explored for phytopharmaceutical product development.
Keywords: Pharmacokinetics, Piperine, Sahastara remedy
INTRODUCTION
The Sahastara (SHT) remedy is a traditional Thai medicine most commonly administered orally to treat muscle and joint pain. The SHT remedy is published in the National List of Essential Medicine of Thailand and assumes it is generally recognized as safe in humans. The SHT remedy consists of 21 medicinal plants (Table 1). There is a report demonstrating that SHT remedy ethanolic extract has anti-inflammatory activity through the inhibition of nitric oxide and prostaglandin E2 with IC50 values of 2.81μg/mL and 16.97 μg/mL, respectively (1).
Table 1.
The medicinal plants in Sahastara remedy formulation (for 1,000 g of the powdered drug).
| Thai name | Scientific name | Voucher specimen | Part used | Weight (g) | Area collected |
|---|---|---|---|---|---|
| Prik-Thai | Piper nigrum Linn. | SKP146161401 | Fruit | 240 | Chanthaburi |
| Jet-Ta-Mul-Plerng-Dang | Plumbago indica Linn. | SKP148160901 | Root | 224 | Thailand |
| Sa-mhor-thai | Terminalia chebula Retz. | SKP049200301 | Fruit | 104 | Laos |
| Dee-Plee | Piper retrofractum Vahl. | SKP146160301 | Fruit | 96 | Sakaeo |
| Tong-Tank | Baliospermum montanum Muell.A. | SKP121021301 | Root | 80 | Thailand |
| Wan-Nam | Acorus calamus Linn. | SKP015010301 | Rhizome | 88 | Chanthaburi, |
| Has-sa-khun-tade | Kleinhovia hospita Linn. | SKP183110801 | Root | 48 | Thailand |
| Ka-ra-boon | Cinnamomum camphora Linn. | SKP096030301 | - | 14 | Kanchanaburi, |
| Dok-Chan | Myristica fragrans Houtt. | SKP121130601 | Aril of seed | 13 | Thailand |
| Luk-Chan | Myristica fragrans Houtt. | SKP121130601 | Seed | 12 | Nonthaburi, |
| Tien-Dang | Lepidiums ativum Linn. | SKP057121901 | Seed | 11 | Thailand |
| Tien-Ta-Tuk-Ka-Tan | Anethum graveolens Linn. | SKP199010701 | Fruit | 10 | Kanchanaburi |
| Ma-Ha-Hing | Ferula assafoetida Linn. | SKP199060101 | Resin | 10 | Thailand |
| Tien-Sut-Ta-But | Pimpinella anisum Linn. | SKP199160101 | Fruit | 9 | China |
| Tien-Khao | Cuminum cyminum Linn. | SKP199030301 | Fruit | 8 | China |
| Jing-Jor | Merremia vitifolia (Burm.f.) Hallier f. | SKP054132201 | Root | 8 | China |
| Tien-Dum | Nigella sativa Linn. | SKP160141901 | Seed | 7 | India |
| Kote-Kag-Kra | Anacyclus pyrethrum (L.) DC. | SKP051011601 | Root | 6 | India |
| Kote-Ka-Mao | Atractylodes lancea (Thunb) DC. | SKP051011201 | Rhizome | 5 | India |
| Kote-Kan-Prao | Picrorhiza kurroa Benth. | SKP177161101 | Root | 4 | China |
| Kote-Pung-Pla | Terminalia chebula Retz. | SKP019200301 | Gall | 3 | India |
A clinical study of SHT remedy in a powdered formulation could reduce osteoarthritis knee pain and improve quality of life that is equivalent to the nonsteroidal anti-inflammatory drug diclofenac. This study also reported that SHT remedy has no toxicity on the liver or renal function and showed no effect on blood pressure (2). However, the powdered SHT formulation leads to suboptimal patient compliance and adherence as it required many capsules administered per day. Therefore, the SHT remedy has the potential to be developed as a modern therapeutic entity in the form of an extract for treating muscle and joint pain with fewer daily capsules administred and an optimized dosing schedule.
Piper species including pepper (Piper nigrum Linn.) and long pepper (Piper retrofractum Vahl.) are the main ingredients in SHT, accounting for approximately 34% of the formulation by weight. Piperine, the major constituent of piper species, is one of the bioactives of SHT remedy. Piperine is well-known in plants but there are no reports of its pharmacokinetic disposition after SHT remedy for phytopharmaceutical product development. The administration of a 100 and 200 mg SHT oral capsule was selected for this study based on a safe and efficacious dose in osteoarthritis knee patients (2,3,4). The objective of this study was to, for the first time, investigate the pharmacokinetic profile of piperine after single oral doses of SHT remedy in healthy volunteers as a part of phytopharmaceutical development.
MATERIALS AND METHODS
Drug and test chemicals
The SHT remedy extract was prepared by the Center of Excellence in Applied Thai Traditional Medicine Research (CEATMR). The SHT extract was subjected to quality control of biological activity by nitric oxide production inhibitions (IC50 < 30 μg/mL) and piperine content of the extract. The capsule of SHT was also prepared by CEATMR. The SHT remedy extract capsules passed quality control including, loss on drying, weight variation, a contamination test, and disintegration time. Piperine (purity > 99%) as an analytical standard was purchased from Merck, Thailand. Methanol high-pressure liquid chromatography (HPLC) grade for the mobile phase was purchased from Labscan, Thailand.
Research design
This phase 1 clinical trial study investigated the pharmacokinetics of a single oral dose of SHT remedy extract in healthy volunteers. The study included two doses of SHT extract (100 or 200 mg, respectively) in each group. The study was approved by the Medical Ethics Committee of the Faculty of Medicine, Thammasat University, which is accredited by the Thai FDA (Registry No. MTU-EC-TM6-121/58), and the study was registered at the Thai Clinical Trial Registry (RegistryNo. TCTR20160729002).
Subjects
Twenty-four healthy Thai volunteers between 20 and 45 years old were screened by personal history, complete physical examinations, and laboratory tests including complete blood count, lipid profile, renal function, liver function, blood electrolytes, and urine analysis. Healthy female volunteers were required to be non-lactating and non-pregnant, to be included in the study. Volunteers with severe peptic ulcer, high blood pressure (systolic > 140 mm Hg and diastolic > 90 mm Hg), impaired renal and/or liver functions were excluded from the study.
Drug administration and sample collection
The healthy volunteers received information on the study, and were advised to report to the study investigator, and sign consent forms. Eligible volunteers were recruited into the study and were divided into 2 groups, 6 males and 6 females each, for both 100 mg and 200 mg doses. The volunteers were admitted to the Thammasat University hospital after fasting for at least 8 h before admission to the hospital. All volunteers underwent a patient history, complete physical examinations, and laboratory tests for baseline data. Then, the volunteers received a single oral dose of SHT remedy capsules depending on which dose they were randomly assigned, 1 capsule for 100 mg dose, and 2 capsules for 200 mg dose. Drinking water was limited to no more than 240 mL upon administration. Ten mL of blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes at the following times: 0.5, 1, 2, 4, 6, 8, 12, 18, 24, and 48 h. All urine was collected over 24 h after taking SHT capsules. Then, a 100 mL urine sample was taken for piperine measurement.
The whole blood at each time point was centrifuged at 1,500 g for 10 min at 4 °C to collect plasma and red blood cells. All samples were stored at -20 °C until analysis with liquid chromatography-mass spectrometry (LC-MS)/MS .
Sample preparation and determination of piperine in biological samples
Analytic procedures were slightly modified from the previous report of Li et al. (5). In brief, the concentration of piperine in biological samples was analyzed as the unchanged form. The protein precipitation technique was used to treat all biological samples as follows, the plasma or urine samples (50 μL) were mixed with 200 μL of methanol containing 10 μg of puerarin as the internal standard. Then the mixture was centrifuged at 1,500 g for 10 min, and the supernatant was collected for further analysis by LC-MS/MS.
The concentration of piperine was analyzed by Eksigent ekspert UHPLC 100 liquid chromatograph equipped with a QTRAP 6500 mass spectrometer, controlled by Analyst software version 1.6 (AB Sciex, USA). The UHPLC system was equipped with Synergi Fusion-RP C18 column as the stationary phase (Phenomenex, USA). One hundred percent methanol and 0.2% formic acid in water (pH 2.5) was applied in the mobile phase.
The gradient elution was started at 10% methanol for 0.5 min, then increased to 90% at 1.5 min until 3.0 min; after that it decreased to 10% methanol at 4.0 until 4.5 min. The retention times of piperine was 1.9 min. MS analysis was conducted by positive mode ionization. Parent ion and daughter ion of piperine were 286.3 and 201.0 dalton, respectively. Optimized MS conditions including decluttering potential, entrance potential, collision energy, and collision exit potential were 80, 5, 25, and 13 volts, respectively. The calibration curves of piperine showed good correlation coefficients (R2 > 0.99) over the concentration range from 0.5 to 1000 ng/mL (1000, 500, 250, 125, 62.50, 31.25, 15.64, 7.81, 3.90, 1.95, 0.97, and 0.48 ng/mL). Samples were rerun or diluted when necessary to be within the calibration range.
Data analysis
The pharmacokinetic parameters were calculated by non-compartmental analysis using PK Solution 2.0 (Summit Research Service, USA). The maximum concentration of piperine (Cmax) and time to reach the maximum concentration of piperine (Tmax) were obtained directly from the plasma concentration-time profile for each individual. The area under the concentration-time curve (AUC0-t) was obtained by the trapezoidal method from the concentration-time profile of piperine from time zero to observed time (0-t). The AUCt-∞ was estimated by dividing the plasma concentration of piperine at the last sampling time to the elimination rate constant. The mean residence time (MRT) and elimination half-life (T1/2) were also reported. The data represent as mean ± SD. A comparison of pharmacokinetic parameters between the two dosages of SHT remedy, basic characteristics of the volunteers, and volunteers’ laboratory parameters were performed by the Mann-Whitney U test or chi- square test, with P < 0.05 indicating a significant difference. SPSS software (version 16.0, USA) was used to analyze the data.
RESULTS
Baseline characteristics: tolerability
Twenty-four eligible volunteers were recruited into the study. All volunteers were healthy, verified by complete physical examinations and laboratory results including renal function, liver function, haematology, blood chemistry, and urinary analysis. No volunteer was excluded during this study. A comparison of baseline characteristics did not show a significant difference between the two dosage groups (Table 2).
Table 2.
Baseline characteristics of volunteers recruited for two groups. Data represented as mean ± SD.
| Characteristics | Sahastara (100 mg) | Sahastara (200 mg) | P-value |
|---|---|---|---|
| Subjects | 12 | 12 | |
| Male | 6 | 6 | 1.000c |
| Female | 6 | 6 | |
| Age (years) | 26.78 ± 3.69 | 27.33 ± 5.45 | |
| Body mass index (kg/m2) | 22.95 ± 3.91 | 22.39 ± 3.83 | 1.000m |
| Systolic blood pressure (mmHg) | 107.50 ± 10.55 | 112.5 ± 10.55 | 0.729m |
| Diastolic blood pressure (mmHg) | 75.00 ± 5.22 | 73.33 ± 9.85 | 0.271m |
| Blood urea nitrogen (mg/dL) | 12.38 ± 2.47 | 11.58 ± 2.30 | 0.449m |
| Creatinine (mg/dL) | 0.84 ± 0.17 | 0.79 ± 0.18 | 0.340m |
| Aspartate transaminase (U/L) | 19.08 ± 7.35 | 22.92 ± 15.25 | 0.488m |
| Alanine transaminase (U/L) | 37.17 ± 23.47 | 28.08 ± 8.66 | 0.602m |
| Alkaline phosphatase (U/L) | 62.00 ± 12.48 | 63.17 ± 19.97 | 0.309m |
c Chi-square-test, m Mann-Whitney U test.
Tolerability
Clinical safety for adverse events was monitored with the following parameter, CBC, blood chemistry, renal, and liver functions. The adverse events were collected before, during, and after the study for 24 h. The results showed no serious adverse events. Only one volunteer had mild abdominal pain after taking a 200 mg SHT extract capsule. The discomfort was relieved after 2 h when the volunteer had a meal. There were significant decreases of creatinine and increases of glucose in 100 mg group while RBC and Hb were significantly increased within the 200 mg group compared with the baseline. These changes seem to be statistically but not clinically significant since the values were still in the normal range. In addition, there was no statistically significant difference between the two dosage groups (Table 3).
Table 3.
Safety monitoring of healthy volunteers in the pharmacokinetic study. The data were presented as mean ± SD, n = 12. *Denotes to the statistical analysis between groups; †P < 0.05 and ††P < 0.01indicate significant differences from time 0 h within-group which analyzed using the Mann-Whitney U test.
| Clinical data | Time (h) | Sahastara (100 mg) | Sahastara (200 mg) | P-value* | Reference values |
|---|---|---|---|---|---|
| 0 | 86.33 ± 6.81 | 84.17 ± 5.97 | 0.450 | ||
| Glucose | 24 | 92.00 ± 6.37†† | 89.25 ± 6.51† | 0.487 | 74-106 mg/dL |
| 0 | 60.75 ± 14.96 | 61.08 ± 13.17 | 0.795 | ||
| High-density lipoprotein-cholesterol | 24 | 60.25 ± 13.29 | 59.58 ± 14.95 | 0.931 | 40-60 mg/dL |
| 0 | 217.08 ± 44.28 | 202.00 ± 27.63 | 0.506 | ||
| Total cholesterol | 24 | 226.58 ± 45.86 | 195.58 ± 26.83 | 0.060 | 0-200 mg/dL |
| 0 | 139.67 ± 39.98 | 128.50 ± 20.52 | 0.665 | ||
| Low-density lipoprotein-cholesterol | 24 | 145.00 ± 39.69 | 119.42 ± 19.92 | 0.126 | 0-100 mg/dL |
| 0 | 106.42 ± 75.05 | 83.42 ± 39.92 | 0.583 | ||
| Triglycerides | 24 | 105.08 ± 59.23 | 85.00 ± 39.24 | 0.453 | 0-150 mg/dL |
| 0 | 12.38 ± 2.47 | 11.58 ± 2.30 | 0.347 | ||
| Blood urea nitrogen | 24 | 12.00 ± 1.94 | 11.57 ± 1.68 | 0.887 | 7.0-18.0 mg/dL |
| 0 | 0.84 ± 0.17 | 0.79 ± 1.78 | 0.488 | ||
| Creatinine | 24 | 0.80 ± 0.17† | 0.75 ± 0.17 | 0.487 | 0.7-1.3 mg/dL |
| 0 | 8.00 ± 0.27 | 7.85 ± 0.35 | 0.257 | ||
| Total protein | 24 | 8.03 ± 0.35 | 8.03 ± 0.50 | 0.908 | 6.4-8.2 g/dL |
| 0 | 4.44 ± 0.37 | 4.29 ± 0.27 | 0.368 | ||
| Albumin | 24 | 4.53 ± 0.29 | 4.38 ± 0.33 | 0.336 | 3.4-5.0 g/dL |
| 0 | 3.54 ± 0.24 | 3.58 ± 0.35 | 1.000 | ||
| Globulin | 24 | 3.52 ± 0.36 | 3.67 ± 0.41 | 0.242 | 1.5-3.5 g/dL |
| 0 | 0.60 ± 0.28 | 0.49 ± 0.20 | 0.379 | ||
| Total bilirubin | 24 | 0.65 ± 0.21 | 0.54 ± 0.22 | 0.241 | 0.2-1.0 mg/dL |
| 0 | 0.17 ± 0.07 | 0.13 ± 0.05 | 0.188 | ||
| Direct bilirubin | 24 | 0.19 ± 0.07 | 0.14 ± 0.05 | 0.062 | 0.0-0.2 mg/dL |
| 0 | 19.08 ± 7.35 | 22.92 ± 15.25 | 0.602 | ||
| Aspartate transaminase (U/L) | 24 | 18.25 ± 7.26 | 19.67 ± 9.16 | 0.908 | 15-37 U/L |
| 0 | 37.17 ± 23.47 | 28.08 ± 8.66 | 0.309 | ||
| Alanine transaminase (U/L) | 24 | 35.50 ± 24.85 | 32.33 ± 19.78 | 0.707 | 30-65 U/L |
| 0 | 62.00 ± 12.48 | 63.17 ± 19.97 | 0.931 | ||
| Alkaline phosphatase (U/L) | 24 | 58.53 ± 10.48 | 57.41 ± 16.25 | 0.862 | 50-136 U/L |
| White blood cells | 0 | 5.79 ± 1.42 | 5.48 ± 1.18 | 0.525 | |
| 24 | 5.88 ± 1.78 | 5.86 ± 1.37 | 0.667 | 4.0-11.0 K/cumm | |
| Neutrophil | 0 | 52.20 ± 7.38 | 59.24 ± 5.81 | 0.019 | 45-75% |
| 24 | 51.11 ± 9.47 | 53.14 ± 11.36 | 0.667 | ||
| 0 | 37.91 ± 6.47 | 33.20 ± 6.18 | 0.069 | ||
| Lymphocyte | 24 | 39.82 ± 8.94 | 38.29 ± 10.76 | 0.805 | 20-45% |
| 0 | 4.79 ± 1.46 | 3.80 ± 1.51 | 0.106 | ||
| Monocyte | 24 | 4.80 ± 2.24 | 3.80 ± 1.26 | 0.388 | 2-10% |
| 0 | 4.53 ± 2.65 | 3.33 ± 2.09 | 0.194 | ||
| Eosinophil | 24 | 3.65 ± 1.76 | 4.40 ± 1.97 | 0.268 | 4-6% |
| 0 | 0.58 ± 0.24 | 0.43 ± 0.16 | 0.143 | ||
| Basophil | 24 | 0.63 ± 0.53 | 0.37 ± 0.30 | 0.204 | 0-1% |
| 0 | 4.80 ± 0.41 | 4.83 ± 0.45 | 0.954 | 4.50-6.00 × | |
| Red blood cell count | 24 | 4.93 ± 0.39 | 5.00 ± 0.59†† | 0.878 | 106/cumm |
| 0 | 13.18 ± 1.48 | 12.44 ± 0.95 | 0.354 | ||
| Hemoglobin | 24 | 13.62 ± 1.12 | 12.92 ± 0.97†† | 0.103 | 14.0-18.0 gm/dL |
Pharmacokinetic profiles of piperine in SHT capsules
Volunteers received 100 and 200 mg of SHT extract in a single-oral dose. The plasma concentration of piperine in 100 mg SHT extract slightly increased from time zero and reached a Cmax of 3.77 μg/mL at the time to maximum (Tmax) of 2.1 h (Fig. 1). Meanwhile, the plasma concentration of piperine in 200 mg SHT extract reached a maximum level of 6.59 μg/mL at 1.98 h. The plasma concentration of piperine gradually decreased until 48 h after doses. The elimination half-life of piperine had a significant variance between 8.74-19.48 h, respectively.
Fig. 1.
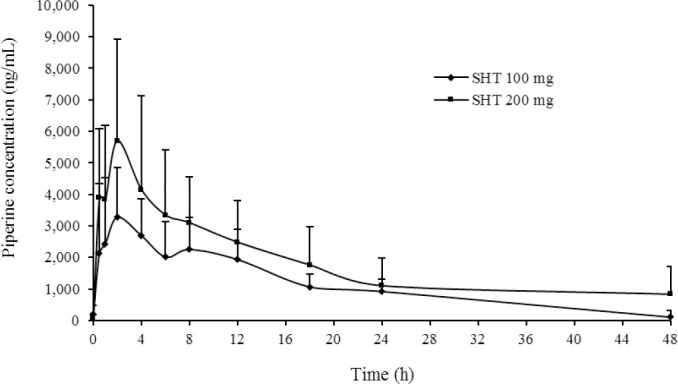
Plasma concentration-time profile of piperine following administration of 100 and 200 mg capsules of SHT remedy. SHT, Sahastara.
The pharmacokinetics parameters of piperine including Cmax and AUC demonstrated dose- proportionality when the dose increased from 100 mg to 200 mg of SHT extract (Table 4). Negligible amounts of unchanged piperine were detected in urine 24 h after dosing.
Table 4.
Pharmacokinetics parameters of Sahastara remedy extract capsules in healthy volunteers. Data presented as the mean ± SD, n = 12. Mann-Whitney U test has been used to statistically compared the data.
| Parameters | Sahastara (100 mg) | Sahastara (200 mg) | P-value |
|---|---|---|---|
| Cmax (ng/mL) | 3.77 ± 1.63 | 6.59 ± 2.86 | 0.008 |
| Tmax (h) | 2.15 ± 1.21 | 1.88 ± 1.19 | 0.160 |
| AUC0-48 (μg.h/mL) | 54.58 ± 21.00 | 77.73 ± 45.40 | 0.178 |
| AUC0-inf (μg.h/mL) | 58.41 ± 23.50 | 106.64 ± 87.07 | 0.178 |
| Mean residence time (h) | 16.76 ± 8.24 | 28.27 ± 34.38 | 0.590 |
| Half-life (h) | 8.74 ± 8.95 | 19.48 ± 29.53 | 0.977 |
Cmax, the maximum concentration of piperine; Tmax, time to reach the maximum concentration.
DISCUSSION
This is the first investigation of the pharmacokinetics of piperine following the administration of SHT remedy in humans. The administration of 100 and 200 mg doses was based on safety and efficacy in osteoarthritis knee patients. There were no severe clinical adverse events evident, and only one volunteer had mild self-limiting abdominal pain. This adverse event profile in healthy volunteers was similar to the SHT powder drug in osteoarthritis knee patients. Moreover, all the laboratory results including renal and liver function indices did not show significant changes after oral administration. These results indicate good tolerability of healthy volunteers to SHT remedy extract.
Following SHT administration, piperine exhibited a peak concentration between 2-4 h after oral dosing in healthy volunteers. This absorption was similar to the previous study in rats where the SHT extract also showed rapid absorption after oral administration (6,7). In addition, the pharmacokinetic study of the SHT remedy powdered drug 1,000 mg/kg and piperine 50 mg/kg in Wistar rats demonstrated a similar Tmax at 1.67 and 3.83 h, respectively (8). This finding confirms that piperine in SHT remedy formulation was absorbed more rapidly than piperine standard, which may be due to the SHT extract containing other constituents and excipients that can enhance piperine absorption. However, a previous study on the pharmacokinetics of piperine in humans following the Benjakul remedy extract found the maximum concentration at about 1 h, which was more rapid than piperine in SHT remedy extract (9). The difference in absorption rate constants and expressed Tmax findings might be a consequence of different constituent ingredients and formulation between both remedies. The SHT remedy contains 21 medicinal plants, which has more constituents than the Benjakul remedy. Furthermore, piperine is lipophilic with Log P of 3.5 and demonstrated poor aqueous solubility and dissolution-limited rate-controlled absorption.
The previous study on the pharmacokinetics of piperine in Benjakul observed a secondary Cmax. This phenomenon may imply that piperine undergoes enterohepatic recirculation. In addition, multiple peaking phenomena in pharmacokinetic disposition could be due to other mechanisms including lymphatic absorption and transport proteins, etc. (10,11). The present study also reveals the same phenomenon in some volunteers (6 of 12 in 100 mg and 9 of 12 in 200 mg). This finding might assume that piperine in SHT remedy has a similar pharmacokinetic profile to piperine in Benjakul, which also demonstrated some secondary peaking. Interestingly, the pharmacokinetic study of piperine standard in Wistar rats did not show the secondary Cmax but it was a good model to study the SHT remedy (8). A possible explanation is that not only piperine in the SHT remedy underwent enterohepatic recirculation but also SHT remedy has other components with structural similarity to piperine such as piperanine (12) and piperidine which are metabolized to piperine and might be reabsorbed via enterohepatic recirculation. The presence of unchanged piperine following SHT remedy was not detectable in urine which was similar to a previous study in rats (13). This present study confirmed that piperine was not excreted in an unchanged form through urine, but it might be found in the feces (7). Piperine metabolites could be excreted in urine (6,7) with major metabolic pathways including reduction, hydroxylation, glucuronidation, and sulfation (14). Follow up studies examining the presence and excretion of metabolites after SHT administration should be undertaken.
The pharmacokinetics parameter of piperine following the SHT administration was a dose- linear phenomenon. Doubling the dose created a 2-fold change in Cmax, AUC0-48, and AUC0-∞.
This may indicate that piperine following SHT extract administration was dose-proportional because Cmax and AUC0-∞ increased 2 folds with doubling the dose. Furthermore, there is a report that shows piperine is a bioenhancer through inhibition of hepatic enzyme CYP3A4 and p-glycoprotein (15). The inhibition effect of piperine on CYP3A4 decreased hepatic metabolism that led to an increase of unchanged piperine available for absorption. It was similar to the inhibition effect of piperine on p-glycoprotein, which was an efflux transporter of many drugs in the intestine. This current study focused on piperine following SHT administration; the SHT contained not only piperine but also other constituents. Further characterization of the terminal elimination phase may also be possible through longer sampling times in future studies.
CONCLUSION
Following SHT extract administration, piperine was rapidly absorbed reaching peak concentrations at approximately 2 h. Piperine in SHT extract showed a linear pharmacokinetic profile in the dose range studied. The pharmacokinetic study of piperine following SHT extract administration provides data which suggests the clinical application and the need for further well-designed studies of SHT remedy extract in healthy volunteers and patients.
CONFLICT OF INTEREST STATEMENT
The authors declared that there is no conflict of interest in this study.
AUTHORS’ CONTRIBUTION
P. Kanokkangsadal undertook the entire research project, P. Khemawoot contributed to this study design of pharmacokinetics and participated in revising the manuscript; P. Wanichsetakul supervised and participated in the clinical diagnosis of volunteers and in revising the manuscript; A. Itharat produced the SHT ethanolic extract, was involved in chemical analysis, participated in revising the manuscript, and acted as a project manager and principal investigator for grant funding. N.M. Davies hosted A. Itharat at the University of Alberta and assisted in interpreting the data and revising the manuscript and calculations and statistical analysis..
ACKNOWLEDGMENTS
This research was supported by the Thailand National Research Fund and Center of Excellence in Applied Thai Traditional Research, Faculty of Medicine, Thammasat University, Thailand.
REFERENCES
- 1.Kakatum N, Jaiarree N, Makchucit S, Itharat A. Antioxidant and anti-inflammatory activities of Thai medicinal plants in Sahasthara remedy for muscle pain treatment. J Med Assoc Thai. 2012;95(1):S120–S126. [PubMed] [Google Scholar]
- 2.Pinsornsak P, Kanokkangsadal P, Itharat A. The clinical efficacy and safety of the sahastara remedy versus diclofenac in the treatment of osteoarthritis of the knee: a double-blind, randomized, and controlled trial. Evid Based Complement Alternat Med. 2015;2015:103046,1–8. doi: 10.1155/2015/103046. DOI: 10.1155/2015/103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakpakdeejaroen I, Itharat A. Tablet formulation and stability test of thai traditional remedy for muscle pain treatment called sahasthara. Planta Medica. 2013;79(13):PN95. D0I:10.1055/s-0033-1352437. [Google Scholar]
- 4.Kanokkangsadal P, Wanichsetakul P, Itharat A. The clinical safety of sahastara remedy ethanolic extract capsules in healthy volunteers. J Med Assoc Thai. 2018;101:1429–1436. [Google Scholar]
- 5.Li C, Wang Q, Ren T, Zhang Y, Lam CWK, Chow MSS, et al. Non-linear pharmacokinetics of piperine and its herb-drug interactions with docetaxel in Sprague-Dawley rats. J Pharm Biomed Anal. 2016;128:286–293. doi: 10.1016/j.jpba.2016.05.041. D0I:10.1016/j .jpba.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Bhat BG, Chandrasekhara N. Studies on the metabolism of piperine: absorption, tissue distribution and excretion of urinary conjugates in rats. Toxicology. 1986;40(1):83–92. doi: 10.1016/0300-483x(86)90048-x. D0I:10.1016/0300-483x(86)90048-x. [DOI] [PubMed] [Google Scholar]
- 7.Suresh D, Srinivasan K. Tissue distribution and elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682–691. [PubMed] [Google Scholar]
- 8.Booranasubkajorn S, Huabprasert S, Wattanarangsan J, Chotitham P, Jutasompakorn P, Laohapand T, et al. Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine. 2017;24:148–156. doi: 10.1016/j.phymed.2016.11.013. DOI: 10.1016/j.phymed.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Jumpa-ngern P, Kietinun S, Sakpakdeejaroen I, Cheomung A, Na-Bangchang K. Pharmacokinetics of piperine following single oral dose administration of benjakul formulation in healthy Thai subjects. Afr J Pharm Pharmacol. 2013;7(10):560–566. DOI: 10.5897/AJPP2013.3469. [Google Scholar]
- 10.Davies NM, Takemoto JK, Brocks DR, Yáñez JA. Multiple peaking phenomena in pharmacokinetic disposition. Clin Pharmacokinet. 2010;49:351–377. doi: 10.2165/11319320-000000000-00000. DOI: 10.2165/11319320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Brocks DR, Davies NM. Lymphatic drug absorption via the enterocytes: pharmacokinetic simulation, modeling, and considerations for optimal drug development. J Pharm Pharm Sci. 2018;21(1s):254s–270s. doi: 10.18433/jpps30217. DOI: 10.18433/jpps30217. [DOI] [PubMed] [Google Scholar]
- 12.Liu HL, Luo R, Chen XQ, Ba YY, Zheng L, Guo WW, et al. Identification and simultaneous quantification of five alkaloids in Piper longum L by HPLC-ESI-MS(n) and UFLC-ESI-MS/MS and their application to Piper nigrum. L Food Chem. 2015;177:191–196. doi: 10.1016/j.foodchem.2015.01.033. DOI:101016/jfoodchem201501033. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Bi Y, Luo R, Wu X. Simultaneous UFLC-ESI- MS/MS determination of piperine and piperlonguminine in rat plasma after oral administration of alkaloids from Piper longum L: application to pharmacokinetic studies in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(27):2885–2890. doi: 10.1016/j.jchromb.2011.08.018. DOI:101016/jjchromb201108018. [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Xue H, Lu L, Zhang T, Han H. Characterization of piperine metabolites in rats by ultra-high-performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2017;31(11):901–910. doi: 10.1002/rcm.7864. DOI: 10.1002/rcm.7864. [DOI] [PubMed] [Google Scholar]
- 15.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302(2):645–650. doi: 10.1124/jpet.102.034728. DOI: 10.1124/jpet. 102.034728. [DOI] [PubMed] [Google Scholar]


