Abstract
Background and purpose:
Dorema ammoniacum D. Don (Apiaceae family) is a perennial plant whose oleo- gum resin is used as a natural remedy for various diseases, especially chronic bronchitis, and asthma. In the present study, hydromethanolic extract of D. ammoniacum root was subjected to phytochemical analyses and α-glucosidase inhibitory potentials of the isolated compounds were assessed.
Experimental approach:
Silica gel (normal and reversed phases) and Sephadex® LH-20 column chromatographies were used for the isolation and purification of the compounds. Structures of the compounds were characterized by 1D and 2D nuclear magnetic resonance (NMR) techniques. All the isolated compounds were assessed for their in vitro α-glucosidase inhibitory activity in comparison with acarbose, a standard drug.
Findings/Results:
Two phloroacetophenone glycosides; echisoside (1) and pleoside (2), along with dihydroferulic acid-4-O-β-D-glucopyranoside (3), and β-resorcylic acid (4), and two caffeoylquinic acid derivatives; chlorogenic acid (5) and 1, 5-dicaffeoylquinic acid (cynarin, 6) were isolated. Among the isolated compounds, the α-glucosidase inhibitory effect of 1,5-dicaffeoylquinic acid was found as 76.9% of the acarbose activity at 750 μM (IC50 value of acarbose).
Conclusion and implications:
Considerable α-glucosidase inhibitory effect of 1,5-dicaffeoylquinic acid makes it an appropriate candidate for further studies in the development of new natural antidiabetic drugs.
Keywords: α-glucosidase inhibitor, Caffeoylquinic acid, Dorema ammoniacum D. Don, Phloroacetophenone glycoside
INTRODUCTION
Dorema ammoniacum D. Don (D. ammoniacum) from Apiaceae family is one of the seven Dorema species represented in the flora of Iran (1). The oleo-gum resin of this species (gum ammoniacum) is traditionally used for different medicinal purposes, especially as antispasmodic and expectorant for the treatment of chronic bronchitis and asthma (2). It has also been reported that gum ammoniacum is sold in Jordanian herbal markets as a traditional natural drug for the reduction of blood sugar (3). Previous biological studies have demonstrated antimicrobial, cytotoxic, anti-inflammatory, and anticonvulsant activity of gum ammoniac (4,6,7). In 2013, Adhami et al. reported the isolation of ammoresinol, dshamirone, and doremin A from this oleo-gum resin as compounds with acetylcholinesterase inhibitory effects (8). The root of this species has also been found to possess antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) methods, as well as considerable antimicrobial activity against Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus (9). There are some reports on the essential oil composition of D. ammoniacum root (9,10).
The gas chromatography-mass spectrometry (GC-MS) analysis of the essential oil of D. ammoniacum root from Kashan region (Isfahan province, Iran) resulted in the identification of β-bisabolene, hexadecanal and (E)-nerolidol with the relative percentages of 15.1, 13.2, and 11.3%, respectively, as the major compounds (9). In another study, Takallo et al. reported the presence of 3-n-butyl phthalide (62.49%), benzyl butanoate (6.57%), and liguloxide (5.15 %) in the essential oil of D. ammoniacum root collected from Rayen region (Kerman province, Iran) (10). In the present study, phenolic constituents of the hydromethanolic extract obtained from D. ammoniacum root were investigated. In view of the α-glucosidase inhibitory potentials reported for phenolic natural compounds in the literature (11,12), in vitro α-glucosidase inhibitory activity of the isolated compounds was also assessed.
MATERIALS AND METHODS
Plant material
The roots of D. ammoniacum were collected in June 2017 from Kashan district (Isfahan province, central Iran) was deposited at the research herbarium of Kashan Botanical Garden, Kashan, Iran under the voucher specimen (No. 1890-KBGH)
Extraction
The air-dried and comminuted roots (1.0 kg) were macerated with chloroform and 70% methanol in water (3 × 5 L each), successively at the lab temperature. The extract was concentrated with a rotary evaporator under 45 °C and dried using a vacuum oven.
Isolation and purification of compounds 1-6
Compound 1 (echisoside, 1.7 g) was obtained as a white precipitate following the addition of methanol (50 mL) to the hydromethanolic extract (50 g). The dried methanol soluble portion was then suspended in water (0.5 L) and partitioned with n-butanol (3 × 0.5 L). Four g of n-butanol fraction was subjected to Sephadex® LH-20 column chromatography with methanol to get nine fractions (A-I). Fraction D (2.1 g) was chromatographed on a silica gel (mesh 230400, Merck) column using a gradient mixture of CHCh:MeOH (95:5 to 50:50) to give twenty fractions (D1-D20), of which fraction D6 yielded compound 2 (pleoside, 42 mg). Reversed-phase (RP) chromatography of fraction D9 (350 mg) on a RP-18 (mesh 230-400, Fluka) column using ACN-H2O (2:8) yielded compound 3 (18 mg). Fraction G (360 mg) was divided into seven fractions (G1-G7) via Sephadex® LH-20 (GE Healthcare, USA) column chromatography (MeOH:H2O, 8:2), among them fraction G2 was compound 4 (β-resorcylic acid, 21 mg). Compound 5 (chlorogenic acid, 12 mg) was isolated from fraction G3 (136 mg) on a RP-18 column using ACN-H2O (1:9) as eluent. Column chromatography of fraction J (230 mg) on Sephadex® LH-20 (MeOH:H2O, 8:2) afforded compound 6 (5-dicaffeoylquinic acid, cynarin, 24 mg).
Thin-layer chromatography (Pre-coated Si gel GF254 and Si gel 60 RP-18 F254s plates, Merck, Germany) was applied for the monitoring of column chromatography and fractions with similar spots under 254 and 366 nm UV wavelengths were combined. The structures of the isolated compounds were elucidated by proton nuclear magnetic resonance (1H-NMR), carbon-13 (13C)-NMR, heteronuclear single quantum correlation (HSQC), and heteronuclear multiple bond correlation (HMBC) spectral analysis (Bruker Avance 400 DRX, USA, 400 MHz for 1H and 100 MHz for 13C), as well as by comparison with published data.
α-Glucosidase inhibition assay
An in vitro colorimetric assay was used for the evaluation of α-glucosidase inhibitory potentials of the isolated compounds (13). Twenty μL of α-glucosidase enzyme (EC3.2.1.20, S. cerevisiae, 20 U/mg) solution, 20 μL of sample solution (750 μM), and 135 μL of potassium phosphate buffer (50 mM, pH 6.8) were added to a 96-well plate. After incubation of the plate at 37 °C for 10 min, 25 μL of p-nitrophenylglucopyranoside (Sigma-Aldrich, Switzerland) (4 mM) was added to the mixture and the plate was incubated for 20 min at 37 °C. Finally, the absorbance changes were recorded at 405 nm by a spectrophotometer (Gen5, PowerWave XS2, BioTek, USA), and the percentage of enzyme inhibition was determined. Dimethyl sulfoxide (DMSO, 10% final concentration) and acarbose were used as a control and standard drug, respectively. Each sample was tested in triplicate.
Statistical analysis
Data were analyzed using Sigmaplot 11.0 software and expressed as mean ± SD.
RESULTS
Phytochemical analysis
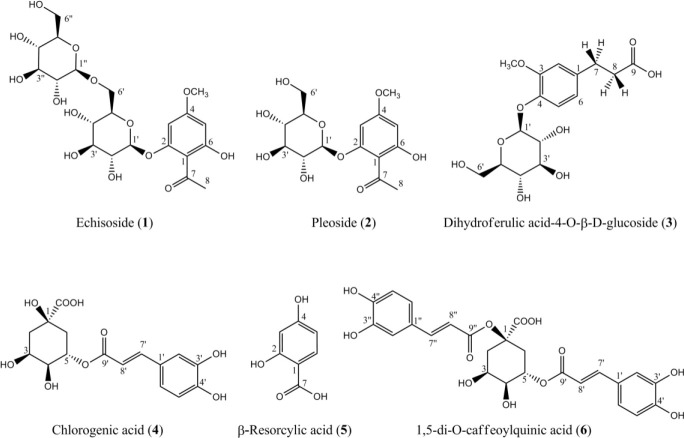
The extraction of D. ammoniacum roots yielded 13.1 and 15.0% for chloroform and hydromethanolic extracts, respectively. Phytochemical analysis of the hydromethanolic extract obtained from D. ammoniacum roots using silica gel (normal and reversed-phase) and Sephadex® LH-20 column chromatography resulted in the isolation of six phenolic compounds (1-6). Structures of compounds were identified as echisoside (1), pleoside (2), dihydroferulic acid-4-O-β-D-glucopyranoside (3) , β-resorcylic acid (4-hydroxy salicylic acid) (4) , 5-O-caffeoylquinic acid (5-CQA, chlorogenic acid) (5), and cynarin (6) using 1H- NMR, 13C-NMR, HSQC, and HMBS spectral analysis, as well as by comparison with those reported in the literature (14,15,16,17,18,19) (Fig. 1).
Fig. 1.
Structures of the compounds isolated from Dorema ammoniacum root.
Spectroscopic data of the isolated compounds
Echisoside (1)
1H-NMR (DMSO-d6, δ/ppm, J/Hz): 13.68 (1H, s, OH-6), 6.34 (1H, d, J = 2.0, H-3), 6.12 (1H, d, J = 2.0, H-5), 5.05 (1H, d, J = 7.6, H- 1’), 4.17 (1H, d, J= 7.6, H-1”), 3.80 (3H, s, OCH3), 2.90-3.75 (12H, overlapped signals, H- 2’-6’, H-2”-6”), 2.66 (3H, s, H-8); 13C-NMR (DMSO-dg, δ/ppm): 204.0 (C-7), 166.2 (C-4), 166.0 (C-6) 161.0 (C-2), 106.3 (C-1), 104.1 (C- 1’'), 100.9 (C-1’), 95.5 (C-5), 93.9 (C-3), 77.4 (C-3’'), 77.2 (C-3’), 77.1 (C-5’'), 76.0 (C-5’), 74.0 (C-2’'), 73.6 (C-2’), 70.5 (C-4’'), 70.1 (C- 4’), 69.4 (C-6’), 61.5 (C-6’'), 56.2 (OCH3), 33.6 (C-8) (14).
Pleoside (2)
1H-NMR (DMSO-de, δ/ppm, J/Hz): 13.69 (1H, s, OH-6), 6.28 (1H, d, J = 1.9, H-3), 6.13 (1H, d, J = 1.9, H-5), 5.00 (1H, d, J = 7.3, H- 1’), 3.80 (3H, s, OCH3), 3.1-3.5 (6H, overlapped signals, H-2’-6’), 2.66 (3H, s, H-8); 13C-NMR (DMSO-dg, δ/ppm): 204.0 (C-7), 166.1 (C-4 and C-6), 161.1 (C-2), 106.3 (C-1), 101.1 (C-1’), 95.4 (C-5), 93.9 (C-3), 77.8 (C-3’), 77.2 (C-5’), 73.6 (C-2’), 70.2 (C-4’), 61.1 (C-6’), 56.1 (OCH3), 33.6 (C-8) (15).
Dihydroferulic acid-4-O-β-D-glucopyranoside (3)
1H-NMR (DMSO-dg, δ/ppm, J/Hz): 7.01 (1H, d, J = 8.3, H-5), 6.70 (1H, d, J = 2.2, H-2), 6.48 (1H, dd, J = 8.3, 2.2, H-6), 4.78 (1H, d, J = 7.0, H-1’), 3.70 (3H, s, OCH3), 3.0-3.6 (6H, overlapped signals, H-2’-6’), 2.76 (2H, m, H-7), 2.56 (2H, m, H-8); 13C-NMR (DMSO-d6, δ/ppm): 175.4 (C-9), 159.1 (C-4), 156.6 (C-3), 130.3 (C-5), 122.5 (C-1), 107.0 (C-2), 102.06 (C6), 101.5 (C-1’), 77.6 (C-3’), 77.2 (C-5’), 73.8 (C-2’), 70.4 (C-4’), 61.3 (C-6’), 55.4 (OCH3), 35.7 (C-7), 25.4 (C-8) (16).
5-O-Caffeoylquinic acid (chlorogenic acid) (4)
1H-NMR (DMSO-de, δ/ppm, J/Hz): 7.43 (1H, d, J = 15.8, H-7’), 7.05 (1H, d, J = 1.5, H- 2’), 6.98 (1H, dd, J = 8.1, 1.5, H-6’), 6.75 (1H, d, J = 8.1, H-5’), 6.20 (1H, d, J = 15.8, H-7’), 5.13 (1H, m, H-5), 3.90 (1H, m, H-3), 3.48 (1H, br d, J = 8.4, H-4), 1.6-2.0 (4H, overlapped signals, H-2,6); 13C-NMR (DMSO-d6, δ/ppm): 176.7 (C-7), 166.7 (C-9’), 148.9 (C-4’), 146.2 (C-7’), 145.1 (C-3’), 126.0 (C-1’), 121.7 (C-5’), 116.3 (C-6’), 115.2 (C-2’), 115.0 (C-8’), 75.0 (C-1), 74.1 (C-4), 71.9 (C-5,3), 39.8 (C-2), 38.2 (C-6) (17).
β-Resorcylic acid (4-hydroxy salicylic acid) (5)
1H-NMR (DMSO-de, δ/ppm, J/Hz): 7.52 (1H, d, J = 8.7, H-6), 6.38 (1H, dd, J = 8.7, 2.2, H-5), 6.29 (1H, d, J = 2.2, H-3); 13C-NMR (DMSO-dg, δ/ppm): 191.2 (C-7), 165.8 (C-2), 164.0 (C-4), 133.8 (C-6), 111.4 (C-1), 109.2 (C- 5), 102.9 (C-3) (18).
1,5-dicaffeoylquinic acid (6)
1H-NMR (DMSO-de, δ/ppm, J/Hz): 5 7.47 (1H, d, J = 15.8, H-7’), 7.43 (1H, d, J = 15.9, H- 7’'), 7.11 (1H, br s, H-2’), 7.05 (1H, br s, H-2’'), 6.94 (1H, br d, J = 8.1, H-6’), 6.92 (1H, br d, J = 8.1, H-6’'), 6.75 (1H, d, J = 8.1, H-5’), 6.74 (1H, d, J = 8.1, H-5’'), 6.22 (2H, d, J = 15.8, H- 8’), 6.20 (2H, d, J = 15.9, H-8’'), 5.27 (1H, m, H-5), 4.05 (1H, m, H-3), 3.54 (1H, br d, J = 8.2, H-4), 1.7-2.5 (4H, m, H-2,6); 13C-NMR (DMSO-dg, δ/ppm): 174.6 (C-7), 166.8 (C-9’), 165.7 (C-9”), 149.6 (C-4’), 148.8 (C-4”), 146.5 (C-3’), 145.8 (C3”), 145.4 (C7’), 144.4 (C7’'), 126.2 (C1”), 125.8 (C1’), 121.8 (C-6’), 120.7 (C-6”), 116.6 (C-8”), 116.4 (C-5’), 116.3 (C- 5”), 115.6 (C-2’), 115.6 (C-2’'), 114.7 (C-8’), 82.1 (C-1), 72.6 (C-4), 70.9 (C-5), 69.1 (C-3), 37.9 (C-6), 35.3 (C-2) (19).
Evaluation of α-glucosidase inhibitory activity
The isolated compounds (1-6) were evaluated for their in vitro α-glucosidase inhibitory activity and compared with acarbose as the standard drug. The result of α-glucosidase inhibitory assay has been summarized in Table 1.
Table 1.
α-Glucosidase inhibitory effects of the isolated compounds from D. ammoniacum root. Data represent mean ± SD, n = 3.
| Samples a | Inhibition (%) |
|---|---|
| Echisoside (1) | 13.5 ± 0.7 |
| Pleoside (2) | 17.2 ± 0.9 |
| Dihydroferulic acid-4-O-β-D-glucopyranoside (3) | 8.1 ± 0.9 |
| Chlorogenic acid (4) | 19.0 ± 1.0 |
| β-Resorcylic acid (5) | 13.6 ± 1.5 |
| 1,5-Dicaffeoylquinic acid (Cynarin) (6) | 40.0 ± 1.0 |
| Acarbose | 52.0 ± 1.0 |
a Compounds were tested in a concentration of 750 μM, equimolar to IC50 value determined for acarbose.
DISCUSSION
The structure of compound 2 was characterized as 2-O-β-D-g lucopyranosyl-4- O-methyl-phloroacetophenone (pleoside) based on 1H-NMR and 13C-NMR spectral data and comparing the data with those reported in the literature, as well (15). The 1H-NMR spectrum of compound 2 showed two doublet signals at δH 6.28 and 6.12 with a coupling constant of 2.0 Hz, characteristic for a pair of meta-coupled aromatic protons, H-3 and H-5, respectively. A methyl singlet at δH 3.80 was assigned to the methoxy group. The presence of glucosyl moiety was confirmed by observation of a series of overlapped signals at the range of 3.1-3.5 ppm, along with an oxymethine doublet at δH 5.00 (J = 7.2 Hz), characteristic for H-1’ anomeric proton. An upfield methyl singlet at δH 2.66 was also assigned to H-8 of acetophenone skeleton. In the 13C-NMR spectrum of compound 2, thirteen signals were observed. A downfield quaternary carbon signal at δc 204.0 was assigned to ketone carbonyl at C-7. Resonances of aromatic ring carbons attached to phenolic hydroxy groups were observed at δc 166.0 (C-4 and C-6) and 161.1 (C-2). Signals appeared at 106.3, 95.4, and 93.9 were also assigned to C-1, C-5, and C- 3, respectively. The signal of an anomeric carbon atom of glucosyl moiety (C-1’) was found further downfield (δc 101.1) rather than the resonances of C-2’ to C-6’ (δc 61.0-78.0). Signals corresponding for methoxy and C-8 methyl carbons were also observed at 5c 56.08 and 33.58, respectively.
The 1H-NMR and 13C-NMR spectra of compound 1 displayed close similarities with those of 2. The diagnostic difference was the presence of one additional doublet (J = 7.6 Hz) oxymethine resonance at δH 4.18 and observation of more overlapped signals in sugar protons region (δH 3.0-3.5), in comparison with 2. Therefore, the presence of a disaccharide residue was proposed for 1, which was confirmed by its 13C-NMR data. A comparison of the NMR data acquired for compound 2 with those published in the literature for acetophenone glycosides resulted in the elucidation of the structure of 2-O-[β-D-glucopyranosyl-(1’→6’)-β-D-glucopyranosyl]- 4-O-methyl-phloroacetophenone (echisoside) (14).
Compound 4 was isolated as a yellowish amorphous solid. This compound was identified as 5-O-caffeoyl quinic acid (chlorogenic acid) by 1H-NMR and 13C-NMR spectral analysis (17). The 1H-NMR of compound 4 displayed an ABX system at 6.6-7.1 ppm, characteristic for a 1,2,4-trisubtituted benzene ring. Two doublet signals at δH 7.43 and 6.20 with the large splitting of 15.8 Hz, typical for trans orientated olefinic protons of an α-β unsaturated ester, were assigned to H-7’ and H-8’, respectively. Resonance of the oxymethine H-5 adjacent to caffeoyl moiety was found more downfield (δH 5.13), rather than H-3 (δH 3.90) and H-4 (δH 3.49) signals of the quinic acid skeleton. Overlapped signals appeared at the range of δH 1.6 to 2.0, were also assigned to aliphatic methylene protons of H-2 and H-6. The structure of chlorogenic acid for 4 was also supported by a comparison of 13C- NMR data with literature data (17).
The 1H-NMR spectrum of compound 6 was closely related to 4, with an additional caffeoyl moiety. The partial similarity was observed between chemical shifts of H-3, H-4, and H-5 signals in 6 and 4, which is indicative to substitution of second caffeoyl moiety at C-1 of the quinic acid skeleton. Therefore, the structure of cynarin was proposed for compound 6. The 1H-NMR and 13C-NMR data of this compound were also identical to those reported for cynarin in the literature (19).
The structure of compound 3 was elucidated as dihydroferulic acid-4-O-β-D-glucopyranoside on the basis of 1H-NMR and 13C-NMR spectral analysis. In 1H-NMR of compound 3, signals at δH 7.01 (d, J = 8.3 Hz), 6.70 (d, J = 2.2 Hz) and 6.48 (dd, J = 8.3 Hz, 2.2, H-6), were assigned to aromatic protons of H-5, H-2, and H-6, respectively. The signal corresponding to an anomeric proton of glucosyl moiety (H-1’) appeared as a doublet at δH 4.78 (d, J = 7.0 Hz). Six other protons of glucosyl moiety (H-2’-H-6’) were also found as overlapped signals at the range of δH 3.0 to 3.6. A methyl signet at δH 3.70 was also assigned to the methoxy group. In comparison with cinnamic acid derivatives, two methylene multiplet signals at δH 2.76 and 2.56 were found in 1H-NMR of compound 3, instead of typical doublets signals of α/β vinylic methines, suggesting the saturation of C-7/C-8 bond in compound 3. This was also confirmed by 13C- NMR data analysis, trough the observation of C-7 and C-8 signals at δC 35.4 and 25.5. Other 13C-NMR data were in agreement with those reported for dihydroferulic acid-4-O-β-D-glucopyranoside in the literature (16).
The 1H-NMR of compound 5 showed three methine signals at δH 5.9-7.5, characteristic for a 1,2,4-trisubstituted benzene ring. In 13C-NMR of 5, seven carbon signals were observed including four quaternary carbons (δC 191.2, 165.8, 164.0, and 111.3) and three tertiary carbons (δC 133.8, 109.2, and 102.9). Therefore, the structure of a dihydroxy benzoic acid was proposed for compound 5. The positions of hydroxy groups were determined at C-2 and C-4 by HSQC and HMBC experiments. The key HMBC was found between H-6 (δH 7.42) and C-7 (191.2). Further 2J and 3J HMBC correlations were observed between H-6 (δH 7.52) and C-2 (165.8) and C-4 (164.0), H-5 (5H 6.38) and C-1 (111.4) and C-3 (102.9), H-3 (δH 6.29) and C-5 (109.2), C-1 (111.4) and C-4 (164.0). Accordingly, 4-hydroxy salicylic acid (β-resorcylic acid) was deduced for compound 5 (18).
All compounds (1-6) from the roots of D. ammoniacum are being reported for the first time. This is also the first report on the isolation of β-resorcylic acid (5) from the genus Dorema D. Don. Chlorogenic acid and some dicaffeoylquinic acid isomers (1,5-DCQA, 3,5- DCQA, and 4,5-DQCA) have been reported as potent free radical scavenging principles of the roots and aerial parts of D. glabrum by Delnavazi et al. (16). Echisoside and pleoside are also two phloroacetophenone glycosides, previously isolated from the roots of some Dorema species such as D. glabrum, D. hyrcanum, and D. aitchisonii (14,16,20,21). Inhibitory effects of echisoside, pleoside, chlorogenic acid, 4,5- and 1,5-dicaffeoylquinic acids on the proliferation of adenocarcinoma gastric cell line have been shown by Jafari et al. (22).
The presence and distribution of the isolated compounds 1-4 and 5 in some other Dorema species, especially D. glabrum, is indicative to close chemotaxonomic correlation between the members of Dorema genus from Apiaceae family. Among the mentioned compounds, echisoside, has only been reported from the genus Dorema (14,16,21), and can be considered as a chemotaxonomic marker of the plants classified in the genus Dorema. In in vitro α-glucosidase inhibitory assay, among the isolated compounds, α-glucosidase inhibitory effect of 1,5-dicaffeoylquinic acid was found as 76.9% of the acarbose activity in a concentration of 750 μM (IC50 value of acarbose, Fig. 2). Enzyme inhibition potentials of the other compounds were determined at the range of 15.5 to 36.5% of the acarbose activity (Table 1). Although myrciaphenone B, a phloroacetophenone glycoside from Myrcia multiflora DC. leaves has been reported to possess a potent inhibitory effect on maltase (IC50: 440 μM) and sucrase (IC50: 310 μM) (23), phloroacetophenone glycosides isolated from D. ammoniacum roots (echisoside and pleoside) showed a weak α-glucosidase inhibitory effect.
Fig. 2.
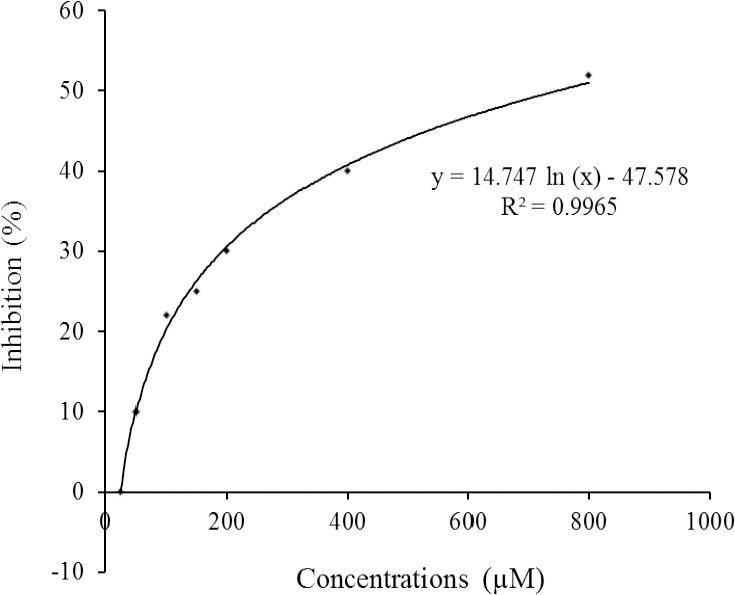
Dose-response curve for acarbose in α-glucosidase inhibition assay.
Comparing the inhibition activity of 1,5- dicaffeoylquinic acid (76.9%) and chlorogenic acid (36.5%) shows that the substitution of OH- 1 group in chlorogenic acid by a caffeoyl moiety increases the α-glucosidase inhibitory effects. In an enzyme assay guided fractionation study on methanolic extract of 50 traditional Chinese herbs, Gao et al. reported 3.4- dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid as active principles of Tussilago farfara L. buds with the potent maltase inhibitory activity (IC50: 0.89-0.91 mM) (24). The maltase inhibitory activity of quinic acid and caffeic was also reported less than 20% at 1 mM (24). Chen et al. demonstrated that yeast α-glucosidase inhibitory activity of the methyl ester derivatives of 3,4-dicaffeoylquinic acid and 3.5-dicaffeoylquinic acid isolated from the aerial parts of Gynura divaricata is about ten times more potent than their analogues (25). In another study on α-glucosidase inhibitory potentials of caffeoylquinic acid derivatives of Brazilian propolis, 1,3,5-dicaffeoylquinic acid was found to be more active than isolated dicaffeoylquinic acids (3,4-DCQA and 3,5-DCQA) (26). The mechanism of α-glucosidase inhibitory activity of caffeoylquinic acids isolated from Ilex kudingcha was clarified by Xu and colleagues (27). They showed that caffeoylquinic acids (3,4-DCQA, 4,5-DCQA, and 3,5-DCQA) inhibit the α-glucosidase enzyme in a non- competitive mode, mainly through hydrophobic interaction which is led to decreasing the catalytic activity via altering the molecular conformation of the enzyme (27). Cynarin isolated in this work, is a polyphenol present in some functional foods such as artichoke and sunflower sprouts (28,29). Cynarin has been reported as an active constituent of sunflower sprouts with a potent inhibitory effect against the formation of advanced glycation end products (EC50: 9.38 μg/mL) (29). The considerable α-glucosidase inhibitory activity of cynarin illustrates the possible beneficial impact of these functional foods for diabetic patients.
CONCLUSION
Phytochemical analyses of the hydromethanolic extract of D. ammoniacum root resulted in the isolation and identification of the six phenolic compounds, namely, echisoside (1), pleoside (2), dihydroferulic acid-4-O-β-D-glucopyranoside (3), β-resorcylic acid (4), chlorogenic acid (5), and 1, 5-dicaffeoylquinic acid (6) of which compound 6 was exhibited considerable α-glucosidase inhibitory effect. The results of this study showed the close chemotaxonomic relationship between D. ammoniacum and other Dorema speciesand suggesting 1,5-dicaffeoylquinic acid as a potent α-glucosidase inhibitor which has the great potential to be considered as an appropriate candidate in antidiabetic drug development researches.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest in this study.
AUTHORS’ CONTRIBUTION
N. Etemadi-Tajbakhsh acquired and analyzed the experimental data. M.A. Faramarzi designed and analyzed the obtained data from the biological study. M.R. Delnavazi accomplished the concept, design, and data interpretation in the phytochemical study, the definition of intellectual content, literature search, manuscript preparation, manuscript editing, and manuscript review.
ACKNOWLEGMENTS
This research was financially supported, through the Grant No. 38329, by the Tehran University of Medical Sciences and Health Services, Tehran, I.R. Iran.
REFERENCES
- 1.Mozaffarian V. Umbelliferae. In: Assadi M, Khatamsaz M, Maasoumi AA, editors. Flora of Iran, No.54. Tehran: Publication of Research Institute of Forests and Rangelands; 2007. pp. 368–374. [Google Scholar]
- 2.Mobeen A, Siddiqui MA, Quamri MA, Itrat M, Imran-Khan MD. Therapeutic potential of Ushaq (Dorema ammoniacum D Don): a unique drug of Unani medicine. Int J Unani Integ Med. 2018;2(1):11–16. [Google Scholar]
- 3.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J Ethnopharmacol. 2002;82(2-3):131–145. doi: 10.1016/s0378-8741(02)00182-4. DOI: 10.1016/S0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 4.Rajani M, Saxena N, Ravishankara MN, Desai N, Padh H. Evaluation of the antimicrobial activity of ammoniacum gum from Dorema ammoniacum. Pharm Biol. 2002;40(7):534–541. DOI: 10.1076/phbi.40.7.534.14686. [Google Scholar]
- 5.Shahidi GH, Moein MR, Foroumadi AR, Rokhbakhsh Zamin F. Cytotoxic activity of medicinal plants used in Iranian traditional medicine on two strains of Saccharomyces cerevisiae. DARU. 2002;10(4):162–164. [Google Scholar]
- 6.Pandpazir M, Kiani A, Fakhri S, Mousavi Z. Anti- Inflammatory effect and skin toxicity of aqueous extract of Dorema ammoniacum gum in experimental animals. Res J Pharmacogn. 2018;5(4):1–8. DOI: 10.22127/rjp.2018.69199. [Google Scholar]
- 7.Motevalian M, Mehrzadi S, Ahadi S, Shojaii A. Anticonvulsant activity of Dorema ammoniacum gum: evidence for the involvement of benzodiazepines and opioid receptors. Res Pharm Sci. 2017;12(1):53–59. doi: 10.4103/1735-5362.199047. DOI: 10.4103/1735-5362.199047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhami HR, Lutz J, Kaehlig H, Zehl M, Krenn L. Compounds from gum ammoniacum with acetylcholinesterase inhibitory activity. Sci Pharm. 2013;81(3):793–805. doi: 10.3797/scipharm.1306-16. DOI: 10.3797/scipharm.1306-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delnavazi MR, Tavakoli S, Rustaei A, Batooli H, Yassa N. Antioxidant and antibacterial activities of the essential oils and extracts of Dorema ammoniacum roots and aerial parts. Res J Pharmacogn. 2014;1(4):11–18. [Google Scholar]
- 10.Sadeghei Takallo M, Sajjadifar S, Mansouji Avval M. Chemical composition of the essential oils from flowers, stems and roots of Dorema ammoniacum D Don from Iran. Res J Pharm Biol Chem Sci. 2014;4(4):640–644. [Google Scholar]
- 11.Xiao J, Kai G, Yamamoto K, Chen X. Advance in dietary polyphenols as α-glucosidases inhibitors: a review on structure-activity relation-ship aspect. Crit Rev Food Sci Nutr. 2013;53(8):818–836. doi: 10.1080/10408398.2011.561379. DOI: 10.1080/10408398.2011.561379. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Narwal S, Kumar V, Prakash O. a- Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011;5(9):19–29. doi: 10.4103/0973-7847.79096. DOI: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadi-Khanaposhtani M, Rezaei S, Khalifeh R, Imanparast S, Faramarzi MA, Bahadorikhalili S, et al. Design, synthesis, docking study, α-glucosidase inhibition, and cytotoxic activities of acridine linked to thioacetamides as novel agents in treatment of type 2 diabetes. Bioorg Chem. 2018;80:288–295. doi: 10.1016/j.bioorg.2018.06.035. DOI: 10.1016/j.bioorg.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Bukreeva TV, Pimenov MG. 2,6-Dihydroxy-4- methoxyacetophenone 2-O-β-D-gentiobioside from the roots of Dorema aitchisonii. Chem Nat Compd. 1991;27:638–639. DOI: 10.1007/BF00630378. [Google Scholar]
- 15.Chevalley I, Marston A, Hostettmann K. Liquid chromatography-Electrospray mass spectrometry for detection and isolation of an antifungal acetophenone from Ribes rubrum (Saxifragaceae) Chromatographia. 2001;54:274–277. DOI: 10.1007/BF02492257. [Google Scholar]
- 16.Delnavazi MR, Hadjiakhoondi A, Delazar A, Ajani Y, Yassa N. Azerosides A and B: two new phloroacetophenone glycosides from the roots of Dorema glabrum Fisch &. CA Mey Med Chem Res. 2015;24:787–796. DOI: 101007/s00044-014-1138-2. [Google Scholar]
- 17.Chan EWC, Lim YY, Ling SK, Tan SP, Lim KK, Khoo MGH. Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae) LWT-Food Sci Technol. 2009;42(5):1026–1030. DOI: 10.1016/j.lwt.2009.01.003. [Google Scholar]
- 18.Taka MJM, Topic DV, Govorcinovic T. FT-IR and NMR spectroscopic studies of salicylic acid derivatives I Gentisamide-a metabolite of salicylamide. Acta Pharm. 2004;54(3):163–176. [PubMed] [Google Scholar]
- 19.Carnat A, Heitz A, Fraisse D, Carnat AP, Lamaison JL. Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia. 2000;71(5):587–589. doi: 10.1016/s0367-326x(00)00163-5. DOI: 10.1016/s0367-326x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 20.Nurmukhamedova MR, Nikonov GK. Glycosides of Dorema hyrcanum. Chem Nat Compd. 1976;12:92–93. DOI: 10.1007/BF00570207. [Google Scholar]
- 21.Naghibi F, Ghafari S, Esmaeili S, Jenett-Siems K. Naghibione; a novel sesquiterpenoid with antiplasmodial effect from Dorema hyrcanum Koso- Pol root, a plant used in traditional medicine. Iran J Pharm Res. 2015;14(3):961–968. [PMC free article] [PubMed] [Google Scholar]
- 22.Jafari N, Zargar SJ, Delnavazi MR, Yassa N. Cell cycle arrest and apoptosis induction of phloroacetophenone glycosides and caffeoylquinic acid derivatives in gastric adenocarcinoma (AGS) cells. Anticancer Agents Med Chem. 2018;18(4):610–616. doi: 10.2174/1871520618666171219121449. DOI: 10.2174/1871520618666171219121449. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Nishida N, Yoshikawa M. Antidiabetic principles of natural medicines V Aldose reductase inhibitors from Myrcia multiflora DC (2): structures of myrciacitrins III, IV, and V. Chem Pharm Bull (Tokyo) 2002;50(3):429–431. doi: 10.1248/cpb.50.429. DOI: 101248/cpb50429. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Huang YN, Gao B, Xu PY, Inagaki C, Kawabata J. a-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008;106(3):1195–1201. DOI: 10.1016/j.foodchem.2007.07.064. [Google Scholar]
- 25.Chen J, Mangelinckx S, Ma L, Wang Z, Li W, Kimpe ND. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast a- glucosidase and PTP1B inhibitory activity. Fitoterapia. 2014;99:1–6. doi: 10.1016/j.fitote.2014.08.015. DOI: 10.1016/j.fitote.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Matsui T, Ebuchi S, Fujise T, Abesundara KJM, Doi S, Yamada H, et al. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol Pharm Bull. 2004;27(11):1797–1803. doi: 10.1248/bpb.27.1797. DOI: 10.1248/bpb.27.1797. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Wang Q, Zhang W, Hu B, Zhou L, Zeng X, et al. Inhibitory activities of caffeoylquinic acid derivatives from Ilex kudingcha CJ Tseng on a- glucosidase from Saccharomyces cerevisiae. J Agric Food Chem. 2015;63(14):3694–3703. doi: 10.1021/acs.jafc.5b00420. DOI: 101021/acsjafc5b00420. [DOI] [PubMed] [Google Scholar]
- 28.Gezer C. Potential health effects of the popular compound of artichoke: Cynarin (CYN) Prog Nutr. 2017;19(S-1):5–9. DOI: 10.23751/pn.v19i1-S.4967. [Google Scholar]
- 29.Sun Z, Chen J, Ma J, Jiang Y, Wang M, Ren G, et al. Cynarin-rich sunflower (Helianthus annuus) sprouts possess both antiglycative and antioxidant activities. J Agric Food Chem. 2012;60(12):3260–3265. doi: 10.1021/jf300737y. DOI: 10.1021/jf300737y. [DOI] [PubMed] [Google Scholar]