Abstract
Purpose of Review
The global burden of non-alcoholic steatohepatitis (NASH) as a major cause of chronic liver disease continues to rise. Cardiovascular disease (CVD) is a leading cause of morbidity and mortality in this patient population. The current review summarizes recent advances in the understanding of CVD in NASH and strategies for screening and management.
Recent findings
Large genetic epidemiological studies support the intricate role of the metabolic syndrome in the pathophysiology of CVD risk in patients with NASH. Atherosclerotic CVD risk scores can predict elevated CV risk in NASH, but additional work is necessary to refine risk stratification and to guide optimal management. New antidiabetic agents may offer benefit in treating steatosis and reducing CV morbidity in NASH.
Summary
Achieving improved outcomes in patients with NASH requires that future efforts focus on optimizing methods for CVD screening and designing clinical trials with long-term cardiovascular endpoints in mind.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, cirrhosis, cardiovascular disease, atherosclerosis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition that is intricately associated with the metabolic syndrome. Mirroring the rising global burden of obesity, NAFLD is rapidly becoming one of the most common etiologies of chronic liver disease, affecting one out of every four individuals worldwide [1, 2]. Non-alcoholic steatohepatitis (NASH), a subtype of NAFLD characterized by inflammation and hepatocyte injury, can ultimately lead to cirrhosis and hepatocellular carcinoma and is now the second-most common indication for liver transplantation (LT) in the United States [3, 4]. However, cardiovascular disease (CVD), rather than liver decompensation, remains the leading cause of morbidity and mortality in patients with NAFLD and NASH [5]. While a significant proportion of the cardiovascular risk in NASH is likely attributable to shared metabolic risk factors, there is much interest in understanding the independent contribution of hepatic steatosis and associated inflammation and fibrosis. Extensive research documents a high prevalence of various sub-clinical and clinical manifestations of atherosclerotic CVD (ASCVD) in NAFLD, and yet there remains an ongoing need for evidence-based guidance on the optimal approach to CVD screening, prevention, and treatment in this high-risk population.
The purpose of this review is to provide an up-to-date summary focused on highlighting recent advances in the understanding of mechanisms, screening, prevention and management of CVD in patients with NAFLD.
Pathophysiologic mechanisms linking NAFLD and CVD
One of the principal pathologic underpinnings of CVD is the formation of the atherosclerotic plaque [6, 7]. Briefly, plaque forms when endothelial dysfunction allows oxidized low-density lipoprotein (LDL) to deposit within subendothelial macrophages. In turn, inflammatory cytokine release and vascular smooth muscle proliferation create a “vulnerable” plaque that is prone to rupture and occlusion, particularly in pro-thrombotic states. In individuals with NASH, numerous pro-atherogenic derangements in these processes are proposed to contribute to increased cardiovascular risk (Figure 1).
Figure 1 –
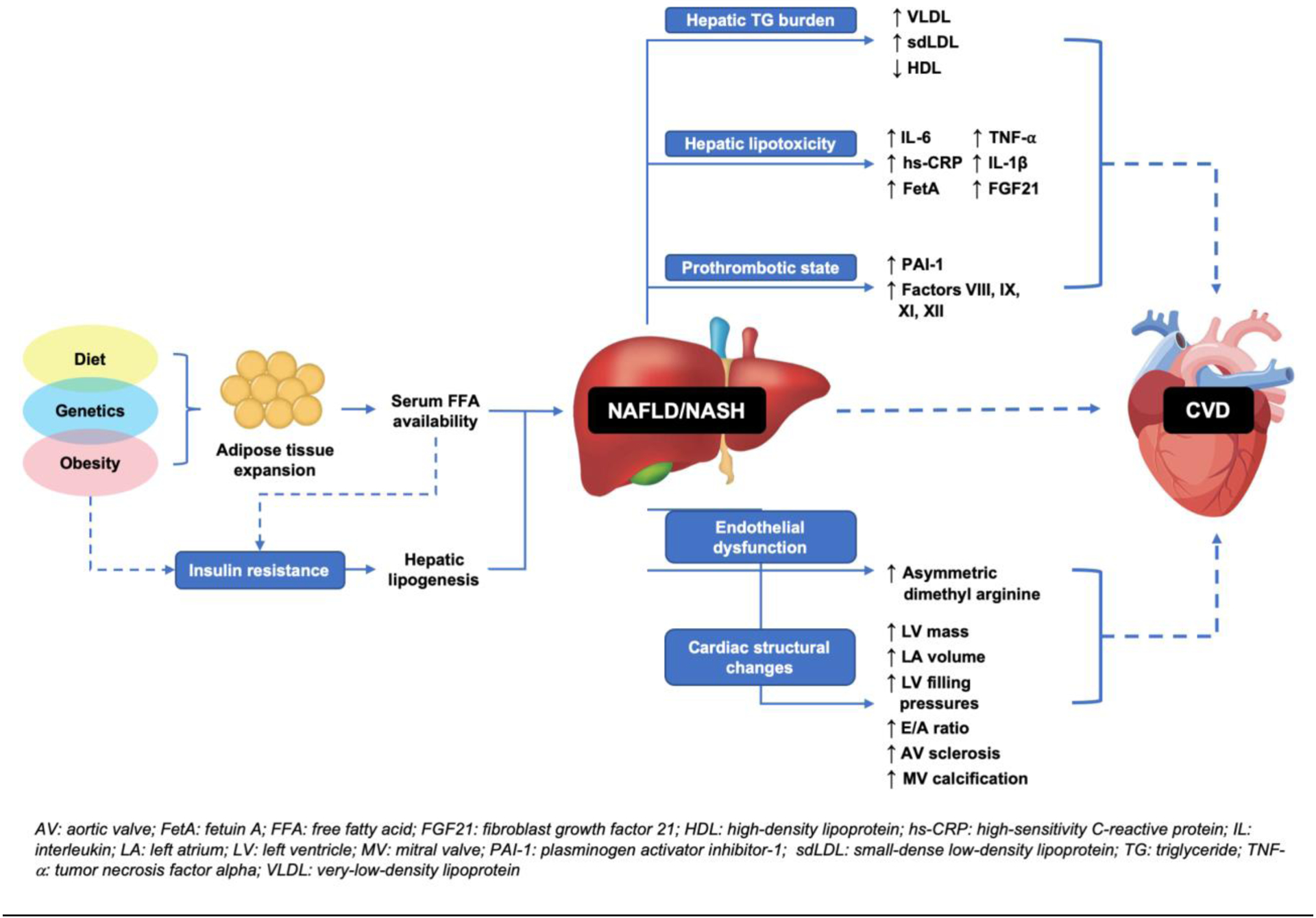
Pathophysiologic mechanisms linking hepatic steatosis to CVD
Dyslipidemia
Adipose tissue expansion and insulin resistance (IR), both precursors for the formation of hepatic steatosis, cause triglyceride (TG) accumulation within hepatocytes. Hepatic TG burden causes an atherogenic serum lipoprotein profile by increasing production of very-low-density lipoprotein (VLDL) and small-dense LDL (sdLDL) and decreasing high-density lipoprotein (HDL) levels [8–10].
Oxidative stress and systemic inflammation
“Lipotoxicity” within hepatocytes induces oxidative stress, upregulating hepatic inflammatory cascades. The result is increased serum levels of cytokines including interleukin-6 (IL-6), high sensitivity C-reactive protein (hs-CRP), IL-1β, and tumor necrosis factor alpha (TNF-α) and liver-specific proteins including Fetuin-A (FetA) and fibroblast growth-factor 21 (FGF21) [11–13]. Abundant research links these proteins to development of CVD, including stroke and coronary artery disease (CAD) [14–20].
Hemostatic and endothelial dysfunction
Hepatic fat accumulation alters hemostasis by increasing prothrombotic factors and plasminogen activator inhibitor type 1 (PAI-1) and exacerbates endothelial dysfunction through elevated serum levels of asymmetric dimethyl arginine (ADMA), a nitric oxide synthase (NOS) antagonist that is also a known biomarker of CVD [21–23].
Taken together, individuals with NAFLD exhibit a state of dyslipidemia in a milieu of chronic inflammation, oxidative stress, hemostatic and fibrinolytic alterations, and endothelial dysfunction – an environment ripe for atherogenesis and CVD. However, as IR is a sine qua non in NAFLD, it remains difficult to disentangle the effects of IR and metabolic syndrome from the independent contribution of processes occurring in and around the liver when studying CVD risk. Recently, however, investigators have turned to evaluating known NAFLD susceptibility genes in an effort to help answer this perplexing question.
Genetic studies
Two of the most robust genetic polymorphisms associated with NAFLD development and progression are patatin-like phospholipase domain-containing protein 3 (PNPLA3) and transmembrane 6 super family 2 (TM6SF2). PNPLA3 I148M and TM6SF2 E167K are variants that interfere with hepatic TG metabolism [24, 25]. Both are consistently shown to predispose to steatosis and associate with increased severity of NAFLD at all stages [26].
In a meta-analysis of 48 genome-wide association studies, Simons et al. interestingly showed that both PNPLA3 and TM6SF2 conferred a small, but statistically significant, protective effect against CAD; this has previously been demonstrated for TM6SF2 alone [27, 28]. In addition, in a Mendelian randomization study of PNPLA3 in a Dutch cohort, despite a clear association between PNPLA3 and hepatic fat content, there was no association between the presence of the PNPLA3 variant and CAD (OR per allele 0.98, 95% CI 0.95–1.02) [29].
These genetic epidemiologic studies question the notion that hepatic steatosis directly and independently contributes to CV risk. Still, these studies are unable to completely untangle the connection between hepatic physiology and metabolic regulation. Indeed, a commonly posited theory for the above findings are that the cardioprotective effects of the PNPLA3 and TM6SF2 gene variants may be explained by a secondary effect of decreased circulating VLDL [27].
While the evidence thus far clearly supports a powerful supporting role of the liver in many pathophysiologic processes that lead to CVD, a direct and independent link between NAFLD and CVD, in particular CAD and atherosclerosis, remains difficult to isolate.
Subclinical and clinical CVD in patients with NAFLD
Subclinical disease
A variety of validated non-invasive measures of underlying ASCVD exist in clinical practice. Coronary artery calcium (CAC), a widely studied measure of subclinical atherosclerosis, has high sensitivity and specificity in detecting CAD [30]. Carotid intima-media thickness (CIMT) and brachial artery flow-mediated vasodilation (FMD) are also predictive of CV events, including myocardial infarction (MI) and stroke [31, 32]. In two recent meta-analyses, participants with NAFLD had significantly higher CAC scores, CIMT, and FMD than controls [33, 34].
Individuals with NAFLD also demonstrate cardiac structural changes associated with preclinical ventricular remodeling and dysfunction that may predispose to clinical heart failure (HF). Studies demonstrate increased left ventricular (LV) mass and left atrial (LA) volume, lower early diastolic relaxation velocity, higher LV filling pressures, and lower trans-mitral peak early to late ventricular filling ratios [35–41]. Many of these echocardiographic parameters are also shown to worsen over five years in persons with NAFLD [42]. Furthermore, the presence of underlying NASH or advanced fibrosis correlate with increased severity of the observed cardiac geometric changes and indices of diastolic dysfunction [37, 40].
Coronary artery disease
Individuals with NAFLD experience higher rates of clinical CAD and worse outcomes after coronary events. Patel et al. prospectively studied 228 patients undergoing coronary angiography as part of LT evaluation [43]. After adjusting for traditional CAD risk factors, individuals with NASH had significantly higher rates of severe CAD compared to those with hepatitis C or alcohol-related cirrhosis. Patients with NAFLD also have higher prevalence of coronary lesions requiring percutaneous coronary intervention, in-hospital mortality during an acute coronary syndrome episode, and 3-year mortality after acute ST-segment elevation MI [44–46].
Cerebrovascular disease
Whether NAFLD is associated with clinical cerebrovascular disease is less clear. A 2016 meta-analysis by Haddad et al. of six studies with 5,953 NAFLD patients demonstrated a 2-fold increase in the relative risk of ischemic stroke versus controls (RR 2.09, 95% CI 1.46–1.98) [47]. Furthermore, in a cohort of 306 patients with acute brainstem infarctions, NAFLD was associated with stroke severity, as determined by National Institutes of Health Stroke Scale scores >7 (HR 2.24, 95% CI 1.25–4.01), and with stroke progression [48].
In contrast, in a population-based European cohort of over 120,000 patients with NAFLD followed for up to five years, there was no association between NAFLD and stroke [49]. Finally, a population-based study of over 30,000 individuals in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort found an inverse association between stroke risk and NAFLD (diagnosed by fatty liver index [FLI] > 60) in men [50]. Of note, a positive correlation was noted only in women with FLI above the 90th percentile.
There is, however, data to suggest that the presence of fibrosis in NAFLD may be associated with cerebrovascular disease. In the United States National Health and Nutrition Examination Survey (NHANES) 2005 to 2014, persons with NAFLD-fibrosis (defined using the FIB-4 index) experienced higher rates of stroke compared to those without fibrosis (OR 1.87, 95% CI 1.00–3.50) [51]. Baik et al. also described 395 patients with stroke or transient ischemia attack (TIA) undergoing transient elastography, noting that only the degree of fibrosis, not steatosis, predicted mortality [52].
Structural and functional cardiac disease
There is an increased incidence of cardiac valvular disease in NAFLD. Patients with type 2 diabetes (T2DM) and NAFLD have increased rates of aortic valve sclerosis and/or mitral annular calcification after adjustment for traditional CV risk factors and diabetes variables (adjusted OR 2.70, 95% CI 1.23–7.38) [53].
Despite knowledge of increased subclinical indices of ventricular dysfunction in patients with NAFLD, data on occurrence of HF events and its sequelae are sparse. In a study of 102 inpatients with HF undergoing liver ultrasound in Beijing, those with NAFLD had higher LV mass indices and LV fibrosis, but no significant difference in subsequent major adverse cardiac events (MACE) or readmission rates over one year [54]. In contrast, Valbusa et al. have reported data from Italy among patients admitted for decompensated HF, showing that NAFLD is associated with higher rates of 1-year all-cause (aHR 5.05, 95% CI 2.78–9.10) and cardiac rehospitalizations (aHR 8.05, 95% CI 3.77–15.8) and all-cause mortality (aHR 1.82, 95% CI 1.22–2.81) over a two-year period [55, 56].
Cardiac arrhythmias
The risk of atrial fibrillation (AF) has been studied extensively in NAFLD, with conflicting findings [57–60]. However, a 2019 meta-analysis incorporating nine cross-sectional and longitudinal studies of over 360,000 middle-aged and elderly individuals demonstrated a significant association between NAFLD and prevalent AF (OR 2.07, 95% CI 1.38–3.10), independent of other risk factors, and an increased risk of incident AF in those with NAFLD and T2DM [61].
Additional conduction abnormalities observed in patients with NAFLD include QTc prolongation, ventricular arrhythmias, and various forms of heart block [62–64].
Overall cardiovascular events and cardiovascular mortality
To date, the question of whether NAFLD can be considered an independent risk factor for the development of CV events or CVD-related mortality remains controversial. A number of recent cohort studies and meta-analyses have added to the cumulative trove of evidence and help shed additional light on the subject.
Regarding CV events, Zeb et al. studied 4,119 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) over a median follow-up of 7.6 years and noted that NAFLD (diagnosed by computed tomography [CT]) was associated with incident non-fatal CAD events, defined as first occurrence of MI, resuscitated cardiac arrest, or angina with or without revascularization (HR 1.74, 95% CI 1.25–2.41) [65]. Additional studies of NAFLD patients with or without T2DM show a similar degree of increase in risk of CV events [66, 67].
Regarding CV mortality, the picture is less clear. Table 1 summarizes four large meta-analyses published over the past five years. In 2016, Targher et al. evaluated 16 studies of 34,043 individuals (36.3% with NAFLD, defined by imaging or histology) and noted a higher risk of fatal and/or non-fatal CVD events (pooled OR 1.63, 95% CI 1.26–2.13) [5]. However, when the analysis was restricted to studies evaluating CV mortality alone, there was no significant association (pooled OR 1.31, 95% CI 0.87–1.97). In the same year, Wu et al. published a meta-analysis of 34 cross-sectional and cohort studies with 164,494 participants, showing an association between NAFLD (defined by imaging, histology, or liver enzymes) and both prevalent (pooled HR 1.81, 95% CI 1.23–2.66) and incident (pooled HR 1.37, 95% CI 1.10–1.72) CVD, but again there was no association with CVD-related mortality (pooled HR 1.10, 95% CI 0.86–1.41) [68]. In 2019, Liu et al. included 498,501 patients in 14 studies, of which seven included data on CVD-specific mortality. In patients with NAFLD (defined by imaging or histology), there was no significant association with the risk of death from CVD (pooled HR 1.13, 95% CI 0.92–1.38) [69]. These three meta-analyses collectively suggest against a significant association between NAFLD and CV mortality. A fourth meta-analysis, published by Haddad et al. in 2017 and including six studies with over 25,000 individuals, seems at first glance to offer contrary evidence [47]. In two out of the six studies in this meta-analysis with necessary outcome data, Haddad reported higher CV mortality in individuals with NAFLD (pooled RR 1.46, 95% CI 1.31–1.64). Importantly, Haddad et al. used unadjusted RRs in their analysis. However, neither of the original studies, which consisted of overlapping NHANES-III samples, were able to demonstrate increased CV mortality in NAFLD after adjust ment for major confounders [70, 71].
Table 1 –
Published meta-analyses as of 2016 investigating the association between NAFLD and cardiovascular events and/or mortality
| Authors, year [Ref.] | Inclusion/exclusion criteriaa | NAFLD diagnosis | Number of studies (Total, reporting CV mortality) | Sample size (Total, with NAFLD) | Heterogeneity (I2 statistic)b | Pooled point estimate for CVD-related mortality, (95% CI) |
|---|---|---|---|---|---|---|
| Targher et al., 2016 [5] |
|
Imaging or histology only | Total: 16 CV mortality: 7 | Total participants: 34,043 No. with NAFLD: 12,381 | 90% | OR 1.31, (0.87–1.97) |
| Wu et al., 2016 [68] |
|
Imaging, histology, or labs | Total: 34 CV mortality: 5 | Total participants: 164.494 No. with NAFLD: not reported | 64.9% | HR 1.10, (0.86–1.41) |
| Haddad et al., 2017 [47] |
|
Imaging only | Total: 6 CV mortality: 2 | Total participants: 25,837 No. with NAFLD: 5,953 | 0% | RR 1.46, (1.30–1.64)c |
| Liu et al., 2019 [69] |
|
Imaging or histology | Total: 14 CV mortality: 7 | Total participants: 498,501 No. with NAFLD: 95,111 | 57.5% | HR 1.13, (0.92–1.38) |
The following inclusion/exclusion criteria were common to all meta-analyses: adult (>18 years) participants only and included studies reported association between NAFLD and CV mortality,
I2 statistic only for subset of studies with outcome of CV mortality
Reported RRs are unadjusted. The original studies reported adjusted HRs and showed no statistical increase in CV mortality.
An important consideration is the role of NAFLD disease stage in cause-specific outcomes. In the aforementioned Targher et al. meta-analysis, more “severe” NAFLD was associated with an overall higher risk of fatal and/or non-fatal CVD events [5]. However, in the included studies, “severe” was defined in a variety of disparate ways, including elevated gamma-glutamyl transferase, high hepatic 18F-fluoro-2-deoxyglucose uptake on positron emission tomography, high NAFLD fibrosis score, or histology. Despite these limitations, the association between CV endpoints and NAFLD-fibrosis, rather than simple steatosis, is supported by other studies as well [72–74].
To summarize, the evidence to-date supports the notion that NAFLD, and in particular advanced fibrosis, is associated with increased risk of CV events independent of metabolic comorbidities. However, investigators have been unable to definitively demonstrate an increase in CV mortality in this patient population.
Screening
There is limited guidance on CVD screening and risk-stratification specific to the NAFLD/NASH population. The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend comprehensive work-up of CVD risk factors but offer no more detailed guidance [3, 75]. The American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on CVD prevention include a major emphasis on calculation of 10-year ASCVD risk in the general popluation [76].
The Framingham Risk Score and the ASCVD risk score are two such commonly used tools. The severity of hepatic steatosis correlates with estimated CVD risk using both scores [77]. Furthermore, in NHANES-III from 1988 to 1994, participants with NAFLD and a high-risk ASCVD score (≥7.5%) had a two-fold increase in CV mortality (aHR 2.02, 95% CI 1.12–3.65) compared to NAFLD participants with scores <7.5% [78]. Notably, the cardiac-specific mortality risk was reduced by approximately 45% if the 10-year ASCVD score was lowered to below 7.5%. Interestingly, the presence of advanced fibrosis (as determined by high NFS scores) did not add to CV risk determination in this study.
The available evidence suggests that established risk scores can estimate risk of ASCVD risk in patients with NAFLD; however, many unanswered questions remain. In younger patients with NAFLD (e.g., age < 40 years), in whom available risk scores are not validated, how can ASCVD risk be estimated? In these patients, should metabolic assessment occur more frequently than every 4 to 6 years as currently recommended by ACC/AHA guidelines? Is there a role for routine assessments of subclinical ASCVD, such as the use of CAC scoring or CRP to re-classify risk? Can newly developed HF risk scores predict HF events among persons with NAFLD [79]? Finally, does the presence of steatohepatitis or fibrosis alter CV risk such that they should be incorporated into CV risk scores in persons with NAFLD? These and other questions represent important future directions for research in the field.
Management
There is significant overlap in many aspects of management of NAFLD and CVD. Below, and in Table 2, we summarize the important components of management for each condition with a focus on strategies to optimize CV morbidity and mortality.
Table 2 -.
Summary of management of metabolic risk factors and cardiovascular disease in patients with NAFLD
| Intervention | Guidance for CVD | Guidance for NASH |
|---|---|---|
| Dietary modification |
|
|
| Exercise |
|
|
| Alcohol |
|
|
| Aspirin |
|
|
| Statins |
|
|
| Metformin |
|
|
| Pioglitazone |
|
|
| GLP-1 agonists |
|
|
| SGLT-2 inhibitors |
|
|
| Obeticholic acid |
|
|
ASCVD: atherosclerotic cardiovascular disease; GLP-1: glucagon-like peptide 1; HDL: high-density lipoprotein; HF: heart failure; LDL: low-density lipoprotein; MACE: major adverse cardiovascular events; NAFLD: nonalcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; SGLT-2: sodium-glucose cotransporter 2; T2DM: type 2 diabetes mellitus.
Lifestyle interventions
Lifestyle modification, including dietary intervention and exercise, is universally recommended as first-line therapy in both NAFLD and CVD [3, 75, 76]. Specific ACC/AHA guidelines endorse Mediterranean or plant-based diets and recommend specific minimum durations of moderate or vigorous intensity aerobic exercise per week [76]. In NAFLD, the well-known benefits of weight loss were recently re-demonstrated by Vilar-Gomez et al., showing that loss of 5% or greater of body weight improved all histological features of NAFLD [80]. No specific diets have proven superior in NAFLD; however, various small-scale studies of the Mediterranean diet suggest a modest decrease in steatosis in a proportion of patients [81].
The role of alcohol in NAFLD is hotly debated. Prior retrospective studies indicated a possible protective benefit of moderate (non-heavy) alcohol use in NAFLD, which is of interest in particular because of the possible CV benefit of moderate alcohol use [82]. The AASLD currently does not make a recommendation on non-heavy alcohol consumption in NAFLD due to insufficient data [3]. However, recent studies suggest that even light alcohol consumption may be detrimental in NAFLD. In a cohort of over 58,000 Korean adults with NAFLD, light (up to 9.9g/day) or moderate (women: <20g/day, men: <30g/day) drinking worsened fibrosis scores [83]. In a Mendelian randomization study by Sookoian et al., carriers of a polymorphism in the alcohol dehydrogenase gene that is robustly associated with decreased alcohol consumption served as a control group with reliably low levels of long-term alcohol exposure [84]. Non-carrier “moderate” drinkers (mean alcohol use 8.2 +/− 21g/day), in comparison to carriers (mean 2.3 +/ 5.3g/day), demonstrated higher degrees of steatosis, lobular inflammation, and NAFLD activity scores on biopsy. More recently, in persons with longitudinally assessed biopsy-proven NASH from the NASH Clinical Research Network (NASH-CRN), modest alcohol use was actually associated with less improvement in markers of NASH severity and lower odds of NASH resolution [85]. Finally, among black and white middle-age adults with CT-defined NAFLD in the population-based Coronary Artery Risk Development in Young Adults (CARDIA) study, prospectively-assessed moderate alcohol use was not associated with established risk factors for CVD or subclinical markers of CVD [86]. In sum, we believe that the evidence to-date suggests a possible harm of moderate alcohol consumption in NAFLD and no apparent CV benefit in this subpopulation.
Aspirin
While the benefits of aspirin for primary prevention of CVD are questioned, its use is well-established for secondary CV prevention [76]. Significantly less data exists in patients with NAFLD. Prior studies suggest a lower prevalence of fibrosis (determined by fibrosis scores and transient elastography) in NAFLD patients on aspirin therapy, however the cross-sectional nature of these studies prevents any causal inferences [87, 88]. A recent prospective study conducted by Simon et al. followed 361 adults with biopsy-proven NAFLD over a median of 7.4 years. Daily aspirin use was associated with a significantly lower incidence of worsening fibrosis indices (aHR 0.63, 95% CI 0.43–0.85) [89].
Future research should aim to elucidate the degree of CV risk reduction that patients with NAFLD experience with aspirin. Further, in more advanced stages of liver disease, the possible benefit of aspirin due to more prevalent clinical CVD needs to be balanced with the concerns of higher bleeding risks.
Statins
ACC/AHA guidelines recommend that decisions for statin therapy be determined by lipoprotein profile, presence of T2DM, and 10-year ASCVD risk or presence of clinical CVD [90]. The liver disease community does not make specific recommendations other than to advise that NAFLD does not increase the risk of liver injury from statins and that practitioners should follow current lipid guidelines [3, 75]. However, the story is more complex.
First and foremost, despite the clear prevalence of CVD in NAFLD and substantial evidence to suggest against statin hepatotoxicity in these patients, statins are under-prescribed in patients with fatty liver disease [91, 92]. Furthermore, post-hoc analyses of multiple large randomized controlled trials (RCTs) of the effect of statins on CV morbidity and mortality demonstrate that in the subgroups of patients with abnormal liver chemistries and NAFLD, statin therapy also improves liver chemistries, steatosis, and the risk of CV events [93–95].
More recently, smaller studies using histology also suggest the possibility of liver-related benefit in NAFLD. Kargiotis et al. prospectively studied 20 individuals with biopsy-proven NASH and dyslipidemia treated with rosuvastatin (10mg/day) and showed complete resolution of NASH in 95% after 1 year of therapy [96]. One criticism of this paper was the lack of reporting of other confounding parameters such as weight changes. In another cross-sectional study of patients undergoing liver biopsy for suspected NASH, Dongionvanni et al. noted that the subgroup of patients on a statin at the time of biopsy were significantly less likely to show steatosis, NASH, or advanced fibrosis [97]. Acknowledging the limitations of these two studies, at a minimum they suggest a need for ongoing research into the potential benefits of statins in NAFLD.
Future investigation must also focus on whether indications for statin therapy in NAFLD should extend beyond current ACC/AHA guidelines. Is a greater degree of CV risk reduction achievable by prescribing statins to a wider cohort of patients with NAFLD? Should NAFLD be considered amongst the “risk-enhancing” factors described by the ACC/AHA to help guide treatment decisions in intermediate risk patients?
Until more data is available, clinicians should at least feel comfortable initiating statin therapy in those individuals with clear indications based on the lack of hepatotoxicity and the known CV benefits in the general population.
Diabetic medications
The agents used to treat T2DM in NAFLD can be viewed from two perspectives: those shown to affect CVD risk and those with potential efficacy in treating NAFLD.
Metformin is a well-established first-line therapy for newly-diagnosed patients with T2DM [98]. It also continues to be recommended by the ACC/AHA, citing previous studies showing improvement in late CV outcomes, however it has not been associated with improved liver histology in NAFLD [99, 3].
Pioglitazone improves liver histology in patients with NASH, regardless of the presence of T2DM [100]. As a result, the AASLD and EASL both endorse consideration of pioglitazone in persons with NASH and T2DM after a careful risk assessment given the known risk of weight gain and HF [3, 75]. In a recent meta-analysis, Liao et al. reviewed 9 studies of CV outcomes with pre-diabetes or T2DM, finding pioglitazone to be associated with a significantly lower risk of MACE but increased weight gain and HF [101].
There is increasing evidence that glucagon-like peptide 1 (GLP-1) agonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors are effective antidiabetic agents in reducing CV risk [102–104]. The American Diabetes Association now recommends use of these agents independent of hemoglobin A1C levels in persons with high ASCVD risk or established CVD [105]. Evolving research also suggests a potential role in treating NASH. In 2016, Armstrong et al. published the Liraglutide Efficacy and Action in NASH (LEAN) study, a multicenter RCT of 52 patients with biopsy-proven NASH over 48 weeks. The GLP-1 agonist liraglutide was superior to placebo for NASH resolution (RR 4.3, 95% CI 1.0–17.7) with less progression of fibrosis (RR 0.2, 95% CI 0.1–1.0) [106]. Multiple RCTs comparing SGLT2 inhibitors to placebo in patients with T2DM and NAFLD show significant improvements in hepatic fat content as measured by controlled attenuation parameter (CAP) or magnetic resonance imaging proton density fat fraction (MRI-PDFF) [107–110]. While these have been relatively small (approximate sample sizes 50–80 patients) with short-term follow-up of 12–24 weeks, the data is nonetheless promising, and clinical trials with both GLP-1 agonists (clinicaltrials.gov ID: NCT03648554) and SGLT2 inhibitors are ongoing (clinicaltrials.gov ID: NCT03723252).
Obeticholic acid
Obeticholic acid (OCA) is a farnesoid X receptor (FXR) agonist that has gained recent press due to the expected upcoming Food and Drug Administration (FDA) approval for treatment of NASH. In a multicenter, double-blinded, phase III RCT by Younossi et al., compared to placebo, OCA significantly improved fibrosis without worsening NASH (OCA 10mg: RR 1.5, 95% CI 1.0–2.2; OCA 25mg: RR 1.9, 95% CI 1.4–2.8) [111]. However, a notable side effect of OCA is increased LDL and decreased HDL. Future studies will help to understand the overall impact of OCA on CVD incidence and progression in patients with NASH.
Conclusion
With the rising global burden of NAFLD, in which cardiovascular disease persists as the major contributor to morbidity and mortality, it is imperative that clinicians understand the metabolic risk factors and subsequent manifestations of CVD in this population. Lifestyle modification and weight loss remain cornerstones of treatment in NAFLD and NASH and in primary and secondary prevention of CVD. Significant work has been done thus far to elucidate underlying mechanisms of disease and to study management paradigms. Going forward, it will be necessary to develop and validate more precise methods for screening for CVD in NAFLD. Ultimately, improving overall morbidity and mortality in NAFLD will require that clinical trials of new medications be designed with cardiovascular endpoints in mind.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
Dr. Shroff has no conflicts to disclose. Dr. VanWagner reports grants and personal fees from W.L. Gore & Associates, personal fees from Gilead Sciences, personal fees from Salix Pharmaceuticals, and non-financial support from AMRA Medical, outside the submitted work.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of Nonalcoholic Fatty Liver Disease. Hepatology. 2020. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- ••5.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]; This meta-analysis convincingly demonstrated the increased incidence of CV events in individuals with NAFLD while suggesting a lack of assocation with CV mortality, a finding that was subsequently supported by mulitple other investiagtions.
- 6.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW et al. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109(21):2617–25. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arab JP, Arrese M, Trauner M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu Rev Pathol. 2018;13:321–50. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 9.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429–36. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey KE, Misdraji J, Gelrud L, Zheng H, Chung RT, Krauss RM. Nonalcoholic steatohepatitis is associated with an atherogenic lipoprotein subfraction profile. Lipids Health Dis. 2014;13:100. doi: 10.1186/1476-511X-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Ben M, Polimeni L, Carnevale R, Bartimoccia S, Nocella C, Baratta F et al. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014;14:81. doi: 10.1186/1471-230X-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato M, Kamada Y, Takeda Y, Kida S, Ohara Y, Fujii H et al. Fetuin-A negatively correlates with liver and vascular fibrosis in nonalcoholic fatty liver disease subjects. Liver Int. 2015;35(3):925–35. doi: 10.1111/liv.12478. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53(5):934–40. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103(6):1372–9. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamirani YS, Katz R, Nasir K, Zeb I, Blaha MJ, Blumenthal RS et al. Association between inflammatory markers and liver fat: The Multi-Ethnic Study of Atherosclerosis. J Clin Exp Cardiolog. 2014;5. doi: 10.4172/2155-9880.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond). 2005;108(3):205–13. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 18.Stoner L, Lucero AA, Palmer BR, Jones LM, Young JM, Faulkner J. Inflammatory biomarkers for predicting cardiovascular disease. Clin Biochem. 2013;46(15):1353–71. doi: 10.1016/j.clinbiochem.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MK, Bartz TM, Mukamal KJ, Djousse L, Kizer JR, Tracy RP et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes Care. 2013;36(5):1222–8. doi: 10.2337/dc12-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol. 2013;12:124. doi: 10.1186/1475-2840-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietilainen KH et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31(2):176–83. doi: 10.1111/j.1478-3231.2010.02375.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell PT, VanWagner LB, Colangelo LA, Lewis CE, Henkel A, Ajmera VH et al. Association between plasminogen activator inhibitor-1 in young adulthood and nonalcoholic fatty liver disease in midlife: CARDIA. Liver Int 2020. doi: 10.1111/liv.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc. 2015;4(6):e001833. doi: 10.1161/JAHA.115.001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trepo E, Romeo S, Zucman-Rossi J, Nahon P. PNPLA3 gene in liver diseases. J Hepatol. 2016;65(2):399–412. doi: 10.1016/j.jhep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–6. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anstee QM, Day CP. The Genetics of Nonalcoholic Fatty Liver Disease: Spotlight on PNPLA3 and TM6SF2. Semin Liver Dis. 2015;35(3):270–90. doi: 10.1055/s-0035-1562947. [DOI] [PubMed] [Google Scholar]
- ••27.Simons N, Isaacs A, Koek GH, Kuc S, Schaper NC, Brouwers M. PNPLA3, TM6SF2, and MBOAT7 Genotypes and Coronary Artery Disease. Gastroenterology. 2017;152(4):912–3. doi: 10.1053/j.gastro.2016.12.020. [DOI] [PubMed] [Google Scholar]; The authors performed a GWAS of NAFLD susceptibility genes, PNPLA3 and TM6SF2, and indicated a protective effect against CAD, further clouding any evidence of a direct association between NAFLD or NASH and atherosclerotic CVD.
- 28.Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: A meta-analysis. Hepatology. 2015;62(6):1742–56. doi: 10.1002/hep.28142. [DOI] [PubMed] [Google Scholar]
- ••29.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Kober L, Nordestgaard BG et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39(5):385–93. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]; This is a Mendelian randomization study assessing the correlation between the NAFLD susceptibility gene PNPLA3 and ischemic heart disease (IHD). The results showed no association between the gene variant and the risk of IHD in this large genetic epidemiologic study, again casting doubt on the true association betweent NAFLD and CVD.
- 30.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. 2018;72(4):434–47. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 32.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaruvongvanich V, Wirunsawanya K, Sanguankeo A, Upala S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig Liver Dis. 2016;48(12):1410–7. doi: 10.1016/j.dld.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhou YY, Zhou XD, Wu SJ, Fan DH, Van Poucke S, Chen YP et al. Nonalcoholic fatty liver disease contributes to subclinical atherosclerosis: A systematic review and meta-analysis. Hepatol Commun. 2018;2(4):376–92. doi: 10.1002/hep4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62(3):773–83. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graner M, Nyman K, Siren R, Pentikainen MO, Lundbom J, Hakkarainen A et al. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging. 2015;8(1). doi: 10.1161/CIRCIMAGING.114.001979. [DOI] [PubMed] [Google Scholar]
- 37.Petta S, Argano C, Colomba D, Camma C, Di Marco V, Cabibi D et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62(4):928–33. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Trovato FM, Martines GF, Catalano D, Musumeci G, Pirri C, Trovato GM. Echocardiography and NAFLD (non-alcoholic fatty liver disease). Int J Cardiol. 2016;221:275–9. doi: 10.1016/j.ijcard.2016.06.180. [DOI] [PubMed] [Google Scholar]
- 39.Jung JY, Park SK, Ryoo JH, Oh CM, Kang JG, Lee JH et al. Effect of non-alcoholic fatty liver disease on left ventricular diastolic function and geometry in the Korean general population. Hepatol Res. 2017;47(6):522–32. doi: 10.1111/hepr.12770. [DOI] [PubMed] [Google Scholar]
- 40.Simon TG, Bamira DG, Chung RT, Weiner RB, Corey KE. Nonalcoholic Steatohepatitis is Associated with Cardiac Remodeling and Dysfunction. Obesity (Silver Spring). 2017;25(8):1313–6. doi: 10.1002/oby.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68(4):764–72. doi: 10.1016/j.jhep.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 42.VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME et al. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9(4):e014279. doi: 10.1161/JAHA.119.014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel SS, Nabi E, Guzman L, Abbate A, Bhati C, Stravitz RT et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl. 2018;24(3):333–42. doi: 10.1002/lt.25012. [DOI] [PubMed] [Google Scholar]
- 44.Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology. 2016;63(3):754–63. doi: 10.1002/hep.28253. [DOI] [PubMed] [Google Scholar]
- 45.Perera N, Indrakumar J, Abeysinghe WV, Fernando V, Samaraweera WM, Lawrence JS. Non alcoholic fatty liver disease increases the mortality from acute coronary syndrome: an observational study from Sri Lanka. BMC Cardiovasc Disord. 2016;16:37. doi: 10.1186/s12872-016-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keskin M, Hayiroglu MI, Uzun AO, Guvenc TS, Sahin S, Kozan O. Effect of Nonalcoholic Fatty Liver Disease on In-Hospital and Long-Term Outcomes in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017;120(10):1720–6. doi: 10.1016/j.amjcard.2017.07.107. [DOI] [PubMed] [Google Scholar]
- 47.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209–S16. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Hu B, Wei L, Zhou L, Zhang L, Lin Y et al. Non-alcoholic fatty liver disease is associated with stroke severity and progression of brainstem infarctions. Eur J Neurol. 2018;25(3):577–e34. doi: 10.1111/ene.13556. [DOI] [PubMed] [Google Scholar]
- 49.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. doi: 10.1136/bmj.l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander KS, Zakai NA, Lidofsky SD, Callas PW, Judd SE, Tracy RP et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One. 2018;13(3):e0194153. doi: 10.1371/journal.pone.0194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parikh NS, VanWagner LB, Elkind MSV, Gutierrez J. Association between nonalcoholic fatty liver disease with advanced fibrosis and stroke. J Neurol Sci. 2019;407:116524. doi: 10.1016/j.jns.2019.116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baik M, Kim SU, Kang S, Park HJ, Nam HS, Heo JH et al. Liver Fibrosis, Not Steatosis, Associates with Long-Term Outcomes in Ischaemic Stroke Patients. Cerebrovasc Dis. 2019;47(1–2):32–9. doi: 10.1159/000497069. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Valbusa F et al. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism. 2015;64(8):879–87. doi: 10.1016/j.metabol.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Wang P, Guo F, Liu X, Luo T, Guan Y et al. Chronic heart failure in patients with nonalcoholic fatty liver disease: prevalence, clinical features, and relevance. J Int Med Res. 2018;46(9):3959–69. doi: 10.1177/0300060518782780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valbusa F, Bonapace S, Agnoletti D, Scala L, Grillo C, Arduini P et al. Nonalcoholic fatty liver disease and increased risk of 1-year all-cause and cardiac hospital readmissions in elderly patients admitted for acute heart failure. PLoS One. 2017;12(3):e0173398. doi: 10.1371/journal.pone.0173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valbusa F, Agnoletti D, Scala L, Grillo C, Arduini P, Bonapace S et al. Non-alcoholic fatty liver disease and increased risk of all-cause mortality in elderly patients admitted for acute heart failure. Int J Cardiol. 2018;265:162–8. doi: 10.1016/j.ijcard.2018.04.129. [DOI] [PubMed] [Google Scholar]
- 57.Markus MR, Meffert PJ, Baumeister SE, Lieb W, Siewert U, Schipf S et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population: The Study of Health in Pomerania (SHIP). Atherosclerosis. 2016;245:123–31. doi: 10.1016/j.atherosclerosis.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Karajamaki AJ, Patsi OP, Savolainen M, Kesaniemi YA, Huikuri H, Ukkola O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study). PLoS One. 2015;10(11):e0142937. doi: 10.1371/journal.pone.0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long MT, Yin X, Larson MG, Ellinor PT, Lubitz SA, McManus DD et al. Relations of Liver Fat With Prevalent and Incident Atrial Fibrillation in the Framingham Heart Study. J Am Heart Assoc. 2017;6(5). doi: 10.1161/JAHA.116.005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitsett M, Wilcox J, Yang A, Zhao L, Rinella M, VanWagner LB. Atrial fibrillation is highly prevalent yet undertreated in patients with biopsy-proven nonalcoholic steatohepatitis. Liver Int. 2019;39(5):933–40. doi: 10.1111/liv.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantovani A, Dauriz M, Sandri D, Bonapace S, Zoppini G, Tilg H et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. 2019;39(4):758–69. doi: 10.1111/liv.14044. [DOI] [PubMed] [Google Scholar]
- 62.Hung CS, Tseng PH, Tu CH, Chen CC, Liao WC, Lee YC et al. Nonalcoholic Fatty Liver Disease Is Associated With QT Prolongation in the General Population. J Am Heart Assoc. 2015;4(7). doi: 10.1161/JAHA.115.001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G et al. Nonalcoholic Fatty Liver Disease Is Associated With Ventricular Arrhythmias in Patients With Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39(8):1416–23. doi: 10.2337/dc16-0091. [DOI] [PubMed] [Google Scholar]
- 64.Mantovani A, Rigolon R, Pichiri I, Bonapace S, Morani G, Zoppini G et al. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS One. 2017;12(10):e0185459. doi: 10.1371/journal.pone.0185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeb I, Li D, Budoff MJ, Katz R, Lloyd-Jones D, Agatston A et al. Nonalcoholic Fatty Liver Disease and Incident Cardiac Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965–6. doi: 10.1016/j.jacc.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 66.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67(5):1726–36. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B et al. Cardiovascular Disease, Cancer, and Mortality Among People With Type 2 Diabetes and Alcoholic or Nonalcoholic Fatty Liver Disease Hospital Admission. Diabetes Care. 2018;41(2):341–7. doi: 10.2337/dc17-1590. [DOI] [PubMed] [Google Scholar]
- 68.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Zhong GC, Tan HY, Hao FB, Hu JJ. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Sci Rep. 2019;9(1):11124. doi: 10.1038/s41598-019-47687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646–50. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 72.Mangla N, Ajmera VH, Caussy C, Sirlin C, Brouha S, Bajwa-Dulai S et al. Liver Stiffness Severity is Associated With Increased Cardiovascular Risk in Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol. 2020;18(3):744–6 e1. doi: 10.1016/j.cgh.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N et al. Nonalcoholic Fatty Liver Disease and Fibrosis Associated With Increased Risk of Cardiovascular Events in a Prospective Study. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 74.Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51(7):728–36. doi: 10.1111/apt.15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JI, Kim MC, Moon BS, Song YS, Han EN, Lee HS et al. The Relationship between 10-Year Cardiovascular Risk Calculated Using the Pooled Cohort Equation and the Severity of Non-Alcoholic Fatty Liver Disease. Endocrinol Metab (Seoul). 2016;31(1):86–92. doi: 10.3803/EnM.2016.31.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality Risk Detected by Atherosclerotic Cardiovascular Disease Score in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2019;3(8):1050–60. doi: 10.1002/hep4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. 2019;73(19):2388–97. doi: 10.1016/j.jacc.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–78 e5; quiz e14–5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Gelli C, Tarocchi M, Abenavoli L, Di Renzo L, Galli A, De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23(17):3150–62. doi: 10.3748/wjg.v23.i17.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55(13):1339–47. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Chang Y, Cho YK, Kim Y, Sung E, Ahn J, Jung HS et al. Nonheavy Drinking and Worsening of Noninvasive Fibrosis Markers in Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology. 2019;69(1):64–75. doi: 10.1002/hep.30170. [DOI] [PubMed] [Google Scholar]
- 84.Sookoian S, Flichman D, Castano GO, Pirola CJ. Mendelian randomisation suggests no beneficial effect of moderate alcohol consumption on the severity of nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2016;44(11–12):1224–34. doi: 10.1111/apt.13828. [DOI] [PubMed] [Google Scholar]
- •85.Ajmera V, Belt P, Wilson LA, Gill RM, Loomba R, Kleiner DE et al. Among Patients With Nonalcoholic Fatty Liver Disease, Modest Alcohol Use Is Associated With Less Improvement in Histologic Steatosis and Steatohepatitis. Clin Gastroenterol Hepatol. 2018;16(9):1511–20 e5. doi: 10.1016/j.cgh.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides prospective, longitudinal histologic data to show that compared to nondrinkers, modest (<=2 drinks/day) alcohol consumers had lower reductions in steatosis, transaminase levels, and NASH. Individuals with NASH must be cautioned against the possible harmful effects of alcohol use on progression of disease.
- •86.VanWagner LB, Ning H, Allen NB, Ajmera V, Lewis CE, Carr JJ et al. Alcohol Use and Cardiovascular Disease Risk in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;153(5):1260–72 e3. doi: 10.1053/j.gastro.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a longitudinal cohort of patients, VanWagner et al. demonstrate a lack of benefit of alcohol use on CVD risk factors or markers of subclinical CVD in individuals with NAFLD. In light of other studies suggesting a detriment of alcohol in NAFLD and NASH, the lack of apparent CV benefit in this study serves to remove any perceived benefit of moderate alcohol use in NAFLD
- 87.Jiang ZG, Feldbrugge L, Tapper EB, Popov Y, Ghaziani T, Afdhal N et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016;43(6):734–43. doi: 10.1111/apt.13515. [DOI] [PubMed] [Google Scholar]
- 88.Schwarzkopf K, Bojunga J, Ruschenbaum S, Martinez Y, Mucke MM, Seeger F et al. Use of Antiplatelet Agents Is Inversely Associated With Liver Fibrosis in Patients With Cardiovascular Disease. Hepatol Commun. 2018;2(12):1601–9. doi: 10.1002/hep4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT et al. Daily Aspirin Use Associated With Reduced Risk For Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17(13):2776–84 e4. doi: 10.1016/j.cgh.2019.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Statins Chalasani N. and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690–5. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 92.Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins Are Underutilized in Patients with Nonalcoholic Fatty Liver Disease and Dyslipidemia. Dig Dis Sci. 2016;61(6):1714–20. doi: 10.1007/s10620-015-4000-6. [DOI] [PubMed] [Google Scholar]
- 93.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 94.Tikkanen MJ, Fayyad R, Faergeman O, Olsson AG, Wun CC, Laskey R et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168(4):3846–52. doi: 10.1016/j.ijcard.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 95.Athyros VG, Giouleme O, Ganotakis ES, Elisaf M, Tziomalos K, Vassiliadis T et al. Safety and impact on cardiovascular events of long-term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7(5):796–805. doi: 10.5114/aoms.2011.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kargiotis K, Athyros VG, Giouleme O, Katsiki N, Katsiki E, Anagnostis P et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21(25):7860–8. doi: 10.3748/wjg.v21.i25.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705–12. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 98.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 100.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •101.Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7(1):e013927. doi: 10.1136/bmjopen-2016-013927. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liao et al.’s meta-analysis presents data in support of a decrease in risk of MACE in individuals with impaired glucose tolerance while also noting well-known risks of weight gain, heart failure, and edema. Still, the reduction in MACE is an important finding for the NAFLD population.
- 102.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 103.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- •104.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139(17):2022–31. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]; In this systematic review and meta-analysis, the authors show that both GLP-1 agonists and SGLT-2 inhibitors offer similar protections against MACE in individuals with established CVD, but that SGLT-2 inhibitors are more likely to reduce HF hospitalizations and kidney disease progression. The study offers evidence of CV benefits that should be taken into consideration for individuals with NAFLD and T2DM.
- 105.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 107.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41(8):1801–8. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 108.Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnstrom M, Moris L et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61(9):1923–34. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):285–92. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 110.Kahl S, Gancheva S, Strassburger K, Herder C, Machann J, Katsuyama H et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. 2020;43(2):298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- ••111.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]; This pivotal RCT demonstrates the benefit of OCA in individuals with biopsy-proven NASH in improving fibrosis without worsening of histologic steatohepatitis. Importantly, OCA, which is expected to be FDA-approved for treatment of NASH, has potential adverse lipoprotein effects that will require further study in high-CV-risk patients.


