Abstract
Genetic defects that accumulate in haematopoietic stem cells (HSCs) are thought to be responsible for age-related changes in haematopoiesis that include a decline in lymphopoiesis and skewing towards the myeloid lineage. This HSC-centric view is based largely on studies showing that HSCs from aged mice exhibit these lineage biases following transplantation into irradiated young recipient mice. In this Opinion article, we make the case that the reliance on this approach has led to inaccurate conclusions regarding the effects of ageing on blood-forming stem cells; we suggest instead that changes in the environment contribute to haematopoietic system ageing. We propose that a complete understanding of how ageing affects haematopoiesis depends on the analysis of blood cell production in unperturbed mice. We describe how this can be achieved using in situ fate mapping. This approach indicates that changes in downstream progenitors, in addition to any HSC defects, may explain the reduced lymphopoiesis and sustained myelopoiesis that occur during ageing.
Ageing is associated with changes in the patterns of blood cell production in both mice1,2 and humans3, including a decline in B cell development and an increased propensity for myeloid cell production4–9. The prevailing model10,11 — which is based largely on studies showing that total bone marrow-derived cells or purified haematopoietic stem cells (HSCs) from old mice exhibit a myeloid-biased pattern of differentiation and attenuated lymphopoiesis following transplantation into young, irradiated recipients9,12,13 — is that these and other alterations in blood cell development are the result of intrinsic defects that accumulate in HSCs over time. This conclusion is further supported by studies showing that old HSCs exhibit various genetic and functional changes compared with their young counterparts (BOX 1), and it is thought that correction of these ‘defects’ may allow the cells to function normally following transplantation into young recipients14,15.
Box 1 |. Effects of ageing on haematopoietic stem cells.
Compared with haematopoietic stem cells (HSCs) from young humans or mice, HSCs from aged individuals have been reported to have several changes; these include DNA damage, impaired DNA repair, altered cell polarity, increased production of reactive oxygen species, augmented myeloid potential with attenuated lymphoid potential on transplantation and alterations in gene expression2,10,11,68,79. Differences in patterns of gene expression have been observed in total HSCs80–85, as well as in young and old lymphoid-biased HSCs and myeloid-biased HSCs (My-HSCs)46. Some of the changes in gene expression in old HSCs may be due to a loss of epigenetic regulation81. It is also noteworthy that an increase in the expression of myeloid-lineage genes and a downregulation of genes that specify lymphoid cell production have been observed at the stem cell population level12. Such findings in total HSCs may result from an increase in the number of My-HSCs (see BOX 2). The effects of these alterations in old HSCs on blood cell production are not fully understood.
In this Opinion article, we raise several challenges to the current view of haematopoietic system ageing. We first emphasize that measuring the developmental potential of old stem cells in irradiated hosts has led to an emphasis on stem cell-intrinsic changes when explaining haematopoietic system ageing, as well as to an underappreciation of how changes that may occur in the bone marrow microenvironment drive that process. Indeed, recent studies have highlighted the role of perturbations in the bone marrow environment in haematopoietic system ageing16–18. We then make the case that a complete understanding of haematopoiesis in ageing will require the analysis of unmanipulated old mice and that in situ fate-mapping approaches can be used to do so. The use of this approach indicates that the focus of ageing research should not be on HSCs alone but also on changes occurring in downstream progenitors that maintain constant blood cell production.
Irradiation and inflammation
The developmental potential of HSCs has been routinely measured by transplanting them into recipients that have been pre-conditioned by irradiation19. Because long-term reconstitution of the lymphoid and myeloid lineages occurs, this transplantation model has been widely used for decades. However, it is increasingly appreciated that stem cell dynamics are different in irradiated compared with non-irradiated mice19. Moreover, the conditions in irradiated versus non-irradiated recipients may alter lineage fate decisions by the transplanted HSCs20. A point of particular relevance to studies of HSC ageing is that irradiation triggers the production of pro-inflammatory cytokines21–24 (FIG. 1a).
Fig. 1 |. Recipient conditioning and the composition of the haematopoietic stem cell compartment affect reconstitution.
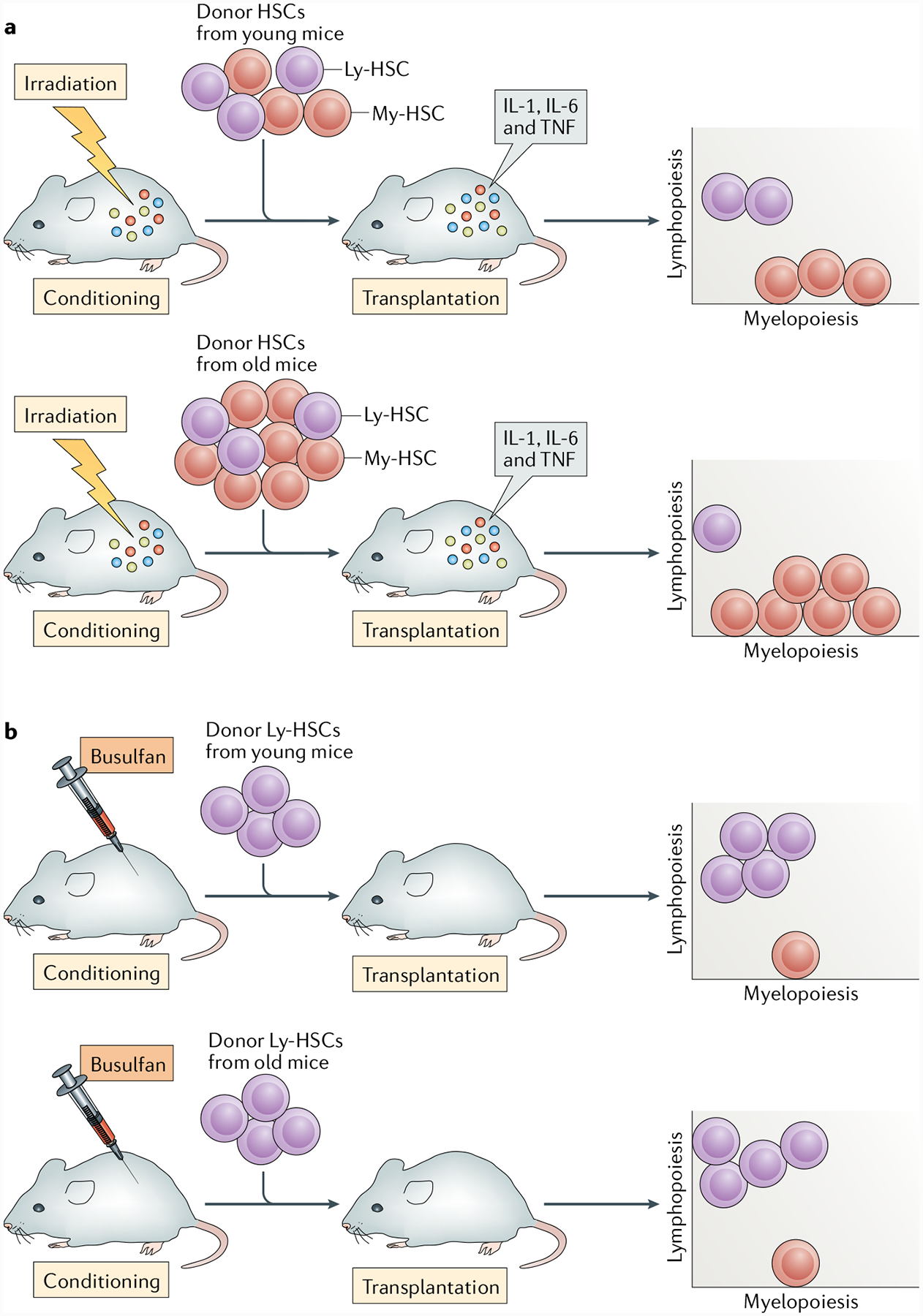
a | Haematopoietic stem cells (HSCs) include lymphoid-biased HSC (Ly-HSC) and myeloid-biased HSC (My-HSC) subsets. The bone marrow of young animals has approximately equal numbers of Ly-HSCs and My-HSCs. Ly-HSC numbers are maintained with age, but the total number of My-HSCs increases so they become the predominant stem cell population in aged mice43,44,46,47. Thus, when total HSCs from young mice are transplanted, the recipients receive similar numbers of Ly-HSCs and My-HSCs, whereas HSCs from old donors would have a majority of cells that are myeloid biased. When either young or old HSCs are transplanted into irradiated mice, they are transferred into an inflammatory environment that has been induced by irradiation. b | Recipient mice conditioned with 1,4-butanediol dimethanesulfonate (busulfan) have low levels of inflammation21–24, thus allowing assessment of the lymphoid potential of young and old Ly-HSCs in a salutary environment and revealing no deficit in the lymphoid potential of Ly-HSCs46. TNF, tumour necrosis factor.
Irradiation-induced inflammation promotes myelopoiesis.
HSCs and haematopoietic progenitor cells were once thought to be refractory to external signals, but it is now recognized that they express receptors that allow them to respond to external cues such as inflammatory factors25–29. In general, inflammation stimulates myelopoiesis and inhibits lymphopoiesis25,30–37. This response may be beneficial to the host in the short term, by promoting the production of mature myeloid cells that provide an initial response against infection38. However, ageing is associated with a state of chronic inflammation, termed inflammageing39, and the inflammatory milieu of old bone marrow may suppress lymphocyte development.
Several published studies have shown that the production of inflammatory cytokines, such as IL-1, IL-6 and tumour necrosis factor (TNF), is increased systemically and/or in the bone marrow microenvironment following irradiation and that levels remain increased for at least 3 months21–24. In addition, irradiation may induce additional long-term changes in mice40. Thus, when old HSCs are transplanted into irradiated recipients, the HSCs are transferred into an environment that is rich in myelopoietic cytokines (FIG. 1a). It is therefore not surprising that myelopoiesis predominates and lymphopoiesis is inhibited under these conditions. In addition, the difference in lymphoid potential between young and old HSCs is compounded by the fact that they are not comparable populations.
It is now recognized that the HSC compartment is heterogeneous and includes lymphoid-biased HSC (Ly-HSC) and myeloid-biased HSC (My-HSC) subsets41,42, which can be distinguished on the basis of phenotypic differences43–45 (BOX 2). Ly-HSCs have reduced self-renewal potential compared with My-HSCs44. Thus, when total HSCs are transplanted into a myelo-ablated host, the Ly-HSC subset of stem cells may not be maintained, leading to diminished production of lymphoid progeny.
Box 2 |. Stem cell subsets.
Haematopoietic stem cells (HSCs) can be subdivided into subsets based on lineage output41–44,47,86. These include lymphoid-biased HSC (Ly-HSC) and myeloid-biased HSC (My-HSC) subpopulations that primarily, but not exclusively, produce lymphoid and myeloid progeny, respectively. These stem cell subsets can be prospectively identified on the basis of levels of CD150 expression within the Lin−SCA1+Kit+ fraction43–45. CD150low HSCs are lymphoid biased, and CD150hi cells are myeloid biased. In addition to these markers, Hoechst dye efflux has been used to purify Ly-HSCs and My-HSCs44.
Following purification using flow cytometry from the bone marrow of young or old mice and transplantation into young, irradiated recipient mice, Ly-HSCs and My-HSCs exhibit lymphoid and myeloid reconstitution biases, respectively43,44,46. The same developmental bias also occurs when these cells are cultured in vitro46. The observation that Ly-HSCs and My-HSCs retain their lineage bias in these assays supports the view that they are naturally occurring stem cell subpopulations, yet their differentiation fates under physiological conditions in situ remain to be understood. Recent barcoding experiments have shown evidence for lineage-biased HSCs in situ58; however, it is not clear whether these HSCs correspond to the lineage-biased HSCs described in transplantation experiments.
The Ly-HSC self-renewal deficiency may be exacerbated by the fact that the proportion of Ly-HSCs and My-HSCs changes over time in some mouse strains43,44,46,47. We found that Ly-HSCs do not decline in number with age in either C57BL/6 or BALB/c mice46. Other groups have also reported that stem cells with lymphoid potential are maintained with age48,49. However, the number of My-HSCs increases over time, such that My-HSCs outnumber Ly-HSCs by up to six times in old C57BL/6 mice46, a strain commonly used in ageing studies. Thus, when total old HSCs are transplanted, most of the donor stem cells are myeloid biased44 (FIG. 1a). By contrast, Ly-HSCs constitute approximately 50% of the total HSCs in a young C57BL/6 mouse46, so it is not surprising that they generate lymphoid progeny in irradiated recipients (FIG. 1a). However, given their limited self-renewal capacity, it is not clear whether young Ly-HSCs generate lymphoid progeny as efficiently in irradiated hosts as they do in situ in young mice.
Lymphoid potential of aged HSCs.
The observations that inflammation induces myelopoiesis and that the proportions of Ly-HSCs and My-HSCs change over time, along with the self-renewal differences in HSC subsets, indicate that the effects of irradiation conditioning on the recipient and of the composition of the donor HSC population need to be considered when transplantation is used to compare the developmental potentials of young and old HSCs.
To better understand the issues surrounding lymphopoiesis during ageing, we transplanted equal numbers of young and old Ly-HSCs into mice treated with 1,4-butanediol dimethanesulfonate (busulfan). Busulfan is an alkylating conditioning agent that is given before transplantation and has been shown to allow high levels of donor chimerism50,51 as well as low to non-detectable levels of inflammation21–24 (FIG. 1b). Total HSCs52,53 and Ly-HSCs43 from aged animals exhibit poor homing to the bone marrow and donor chimerism following transplantation into irradiated recipients. However, a recently published paper from our laboratory showed that young and old Ly-HSCs exhibited equivalent levels of donor chimerism as well as equivalent numbers of donor-derived Ly-HSCs in the bone marrow of mice pre-conditioned with busulfan. There were also no deficits in primary lymphopoiesis, as the reconstitution of B lineage cells was similar in recipients of young and old Ly-HSCs46. Old Ly-HSCs show a myeloid-biased pattern of gene expression46 but, despite this, myelopoiesis was equivalent in recipients of the young and old stem cells. Although we have not yet carried out limiting dilution or competitive transplantation experiments in busulfan-treated mice, these results support the views that conditions in the irradiated environment inhibit lymphopoiesis and that old stem cells retain normal developmental potential when transferred to a salutary milieu. These observations are not consistent with the view that intrinsic defects in HSCs permanently impair their developmental potential in ageing. The observations also do not fit with studies indicating that intrinsic defects must be corrected in old HSCs for them to function normally following transplantation14,15, although there are possible explanations for these contradictions (BOX 3).
Box 3 |. Reconciling different models of haematopoietic stem cell ageing.
Haematopoietic stem cells (HSCs) from aged individuals exhibit alterations in gene expression, and there is evidence that interventions that change the expression of even a single gene or the activity of its product can attenuate various ageing phenotypes14,15,87,88. For example, expression of the transcription factor Krüppel-like factor 5 (KLF5) is increased in old HSCs, and stem cells in which levels of KLF5 are reduced exhibit increased lymphoid cell output and decreased myeloid cell output following transplantation into irradiated recipients15. The activity of cell division control protein 42 homologue (CDC42), which is involved in the formation of actin-linked cytoskeletal structures, is increased in old HSCs, and treatment with agents that inhibit CDC42 activity also leads to improved stem cell function following transplantation into irradiated mice14,89.
We suggest that simply removing HSCs from the old inflammatory environment might downregulate KLF5 and CDC42 activity. If the old stem cells are then transferred to a salutary, non-inflammatory environment, such as would exist in recipients preconditioned with 1,4-butanediol dimethanesulfonate (busulfan), KLF5 and CDC42 activity would remain low, and the stem cells would show improved function. By contrast, if old HSCs are transplanted into the inflammatory milieu of an irradiated mouse, KLF5 and CDC42 activity would be maintained, and stem cell function would be impaired. However, if the old HSCs were genetically or pharmacologically manipulated to suppress KLF5 or CDC42 activity, they would be able to function more efficiently in irradiated recipients, thus explaining the findings of Mann et al.15 and Florian and colleagues14. The fact that KLF5 (REF.15) and CDC42 (REFS90–92) activity and/or expression is increased in response to inflammation is consistent with these predictions, but they need to be experimentally tested.
We do not dispute the possibility that some of the changes that accumulate in HSCs and progenitors over time could inhibit their lymphoid developmental potential in situ. However, our transplantation data suggest that these changes are reversible following transfer of cells to a salutary milieu. The fact that epigenetic changes arise in old HSCs (BOX 1) and may be reset suggests a mechanism by which this could occur48. Thus, a priority of future studies should be to better understand how changes in the ageing environment affect haematopoiesis, in particular lymphopoiesis. There has been considerable focus on the role of inflammation in this regard, and we suggest that differences in the induction of inflammatory cytokine production explain why reconstitution is distinct in irradiation-conditioned versus busulfan-conditioned mice. However, this remains to be formally demonstrated.
Unperturbed haematopoiesis in vivo
The discussion above strongly supports the use of alternatives to irradiation conditioning in transplantation studies that compare the developmental potentials of young and old HSCs. However, the use of busulfan or other treatments to condition recipients may still create an environment that differs from the physiological steady state. The various caveats associated with transplantation have not always been fully appreciated, but they are critical to consider when studying ageing of the haematopoietic system. Thus, the application of recently developed systems to assess haematopoiesis in situ54–58 may provide a more accurate view of how ageing affects blood and immune cell production, as this approach allows measurement of the output from HSCs as well as from downstream progenitors under physiological conditions in situ.
HSC output in unperturbed bone marrow.
We have previously shown that time-resolved HSC fate mapping allows measurement of HSC output during steady-state haematopoiesis56. In this approach, adult HSCs are labelled by an HSC-specific inducible Cre system, and downstream progenitors inherit the label, which allows tracing, first, of short-term repopulating HSCs (ST-HSCs) and then of multipotent progenitors (MPPs), followed by lineage-restricted progenitors and, eventually, all mature blood and immune cell types. In our studies56, the long-term repopulating HSCs (LT-HSCs) were CD48−CD150+Lin− SCA1+Kit+ (LSK) cells. The ST-HSCs were CD48−CD150−LSK cells; these cells have been designated as MPP1–MPP3 (REF.59) or MPPs60 in other studies. In addition, we designated CD48+CD150−LSK cells as MPPs56, whereas cells of this phenotype have been referred to as haematopoietic progenitor cells fraction 1 (HPC-1)59 or MPP3/4 (REF.60) in other reports.
In this fate-mapping approach, the frequency of labelled cells in downstream progenitor populations will steadily increase towards the frequency of the labelled HSCs (FIG. 2a). The rate at which the frequency of labelled cells increases in the population immediately downstream of LT-HSCs, the ST-HSCs, is proportional to the difference in frequency between the labelled HSCs and ST-HSCs, the cellularity ratio of HSCs to ST-HSCs, and the output rate at which HSCs generate ST-HSCs. Thus, the HSC output rate can be calculated on the basis of the time-dependent increase in the frequency of labelled ST-HSCs and the cellularity ratio61. When analysing TIE2+ tip HSCs (so called because they are said to reside at the ‘tip’ of the stem cell hierarchy, of which we label about 25%62,63), we found the output rate to be remarkably low: approximately 1% of TIE2+ tip HSCs differentiate per day in an adult mouse, whereas over 6 months, at least 30% of HSCs contribute to the generation of differentiated cells56. Sawai et al.57 reported an HSC output rate of 2.6% per day, albeit with broader labelling of HSCs and haematopoietic progenitor cells by their Cre driver. The very low HSC output in native haematopoiesis has also been noted in an independent analysis of the kinetic propagation of transposon-based barcodes in situ54. In further support of this finding, near-complete ablation of HSCs in the bone marrow did not reduce the numbers of red and white blood cells for at least 7 months64,65.
Fig. 2 |. Haematopoietic stem cell and progenitor output in situ can be measured by fate mapping.
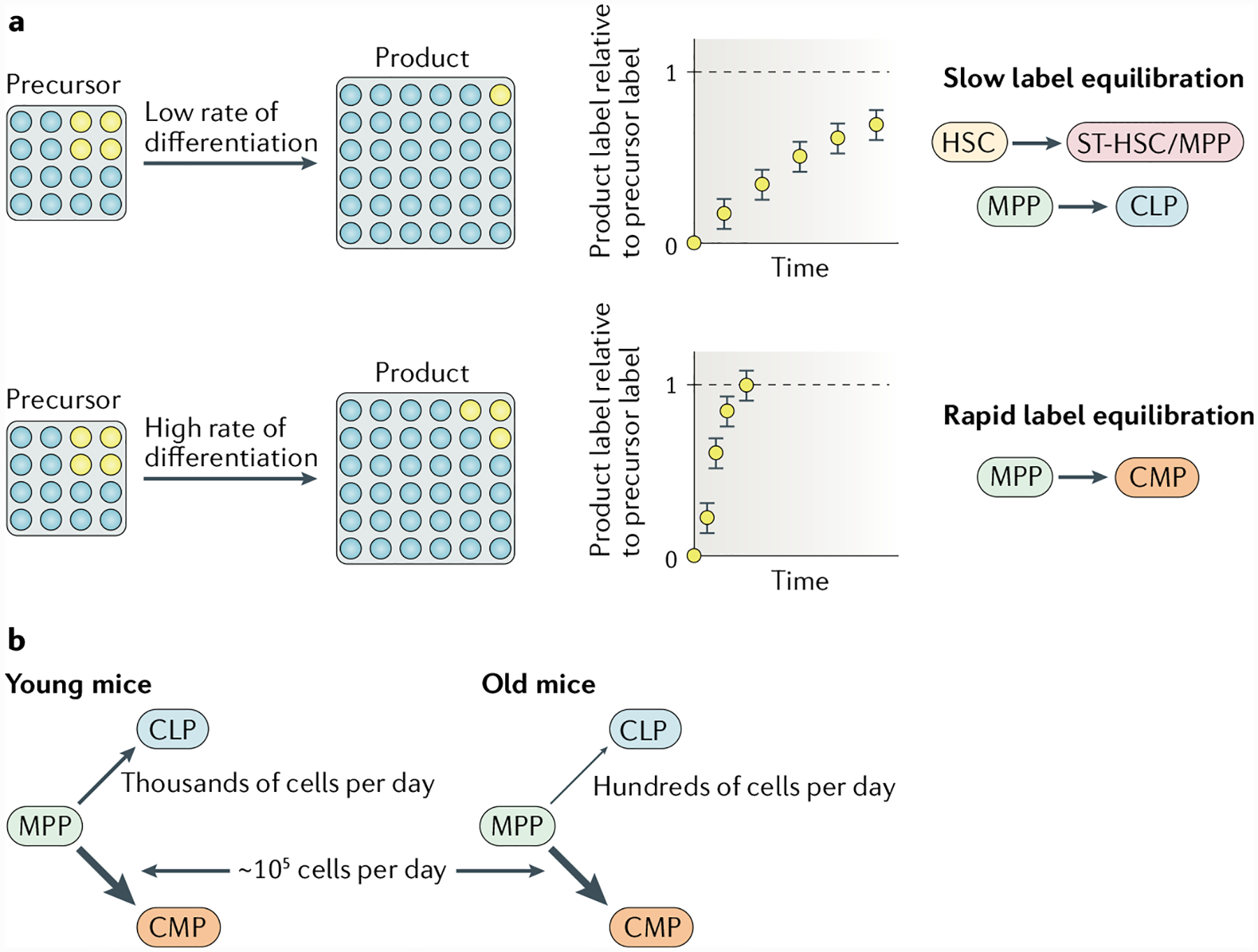
a | During steady-state haematopoiesis, the rate at which a heritable cell label propagates from a source to its product is proportional to the precursor-to-product differentiation rate. Fate-mapping experiments with selective haematopoietic stem cell (HSC) labelling have shown slow propagation of a label from HSCs to short-term repopulating HSCs (ST-HSCs) and multipotent progenitors (MPPs), and further downstream, also from MPPs to common lymphoid progenitors (CLPs), which corresponds to low differentiation rates for both differentiation steps (top row). By contrast, label propagation from MPPs to common myeloid progenitors (CMPs) is fast, indicating a high differentiation rate (bottom row). b | Measurements of cell fluxes into the myeloid and lymphoid branches of the haematopoietic system in situ show that the strong myeloid bias that is already present in young mice becomes accentuated with age, owing to a relative decline in lymphoid differentiation. The figure is based on data published in REF.56.
The lessons learned from these types of analyses provide further evidence that transplantation models do not accurately replicate in situ haematopoiesis. For example, comparatively few HSCs proliferate and differentiate vigorously following transplantation66,67. Furthermore, compared with HSCs, ST-HSCs and MPPs yield only transient haematopoietic reconstitution after transplantation. Nevertheless, ST-HSCs and MPPs are long lived in the steady state in situ and indeed are the major source of haematopoiesis under unperturbed conditions in vivo54,56,64,65. Given the newly recognized importance of long-lived progenitors in vivo, it may also be important to consider their roles in determining myeloid versus lymphoid output during ageing.
HSC output over the mouse lifespan.
As described above, we used time-resolved HSC fate mapping under unperturbed conditions, inducing HSC labelling in young adult mice and measuring HSC output at different ages, in animals up to 25 months of age. These data (based on the analysis of more than 100 mice) indicate that HSC output is maintained and remains approximately constant over the lifespan of the mouse56. Given that the absolute number of HSCs increases with age in mice, this suggests that the average output rate per HSC decreases with age (M. Barile, A.-K. Fanti, K. Busch, A. Greco, X. Wang, Q. Zhang, H.-R. Rodewald and T. Höfer, unpublished observations). Whether the various ‘defects’ reported in old HSCs68 (BOX 1) contribute to this reduction remains to be determined.
Our studies of HSC output are based on the selective induction of label expression in TIE2+ HSCs, which represent the tip HSC fraction with the highest repopulating activity62,63. Other inducible Cre systems, such as Pdzk1ip1–CreER mice or Fgd5–CreERT2 mice, have been used to label HSCs, but they also label downstream cells55,57,60,69,70. Using young Fgd5–CreERT2 mice, Säwen et al.60 observed marked initial Cre labelling of ST-HSCs (~50% of HSC labelling frequency), in addition to labelling of HSCs. Under these experimental conditions, it is difficult to draw conclusions regarding the relative HSC versus ST-HSC output towards MPPs in young mice. In old mice, Säwen et al. specifically labelled HSCs, and the contribution of these cells to MPPs was low, which is consistent with our data56.
In summary, we suggest that in the steady state, HSCs proliferate very little, on average about once every month71. MPPs have higher proliferation rates, dividing on average once in a few days59, while retaining considerable self-renewal capacity in unperturbed bone marrow54,56. Hence, the rapidly dividing MPP pool continuously receives low-level input from slowly dividing HSCs throughout life.
Myeloid and lymphoid output in young adult mice.
Fate-mapping experiments in which label expression is induced in HSCs also allow measurement of the output rates and differentiation rates of downstream progenitor compartments56,57. Analysis of the progression from MPPs to common myeloid progenitors (CMPs) or common lymphoid progenitors (CLPs) in young mice indicated that differentiation to CMPs was ~100-fold higher than differentiation to CLPs (FIG. 2b). This observation may be explained by preferential differentiation of MPPs to CMPs and/or by faster proliferation of cells differentiating into CMPs than cells differentiating into CLPs56. Hence, there is a marked imbalance favouring myeloid over lymphoid progenitor production in normal bone marrow in situ. Given that many myeloid cells, notably granulocytes, are short-lived (in the order of hours) and that lymphoid cells are long lived (in the order of years), these production rates may correspond to differential demands of lineage progenitors for the maintenance of innate and adaptive effectors.
It is unclear whether all myeloid lineages and all lymphoid lineages are generated normally via the CMP and CLP stages, respectively. With this caveat in mind, we still consider it useful to estimate flow based on MPP, CMP and CLP phenotypes at the presumed myeloid–lymphoid bifurcation point, given that these cells have prospectively well-defined properties as intermediates in haematopoiesis72,73. Regardless, these recent data suggest that a myeloid bias, resulting in a 100-fold greater flow from MPPs to myeloid progenitors than to lymphoid progenitors, is an inbuilt property of haematopoiesis, regardless of age56,58.
CMP and CLP production with age.
To understand the potential effects of ageing on MPP differentiation to myeloid or lymphoid lineages, we studied mice aged from 12 to 25 months. Surprisingly, the flow from MPPs towards CMPs remained comparable to that observed in young adult mice (up to 9 months old)56 (FIG. 2b). These results challenge the long-held view that myeloid-biased haematopoiesis is a feature of ageing. The data also suggest that the increased number of My-HSCs in old mice has little impact on steady-state myelopoiesis, which is consistent with minimal input from HSCs into MPPs. Instead, the predominance of My-HSCs within the HSC compartment of aged mice appears to be most relevant in transplantation studies, in which it leads to myeloid-biased reconstitution in irradiated mice.
Although flow from MPPs towards CMPs did not seem to be affected by ageing, we show that the flow from MPPs towards CLPs is reduced (approximately fivefold to tenfold) in old mice compared with younger mice56 (FIG. 2b). Other studies are consistent with our measurements and show that phenotypically defined numbers of lymphoid-primed MPPs and CLPs decline with age, despite MPP numbers remaining constant over time49. Whether the flow from HSCs to lymphoid-biased MPPs is also reduced with age remains to be determined. Thus, the fundamental basis for the myeloid bias in old mice is a relative reduction in lymphoid output while myeloid output is retained. Ageing is associated with chronic inflammation, and increased production of inflammatory cytokines in the bone marrow23 could result in heightened levels of myelopoiesis18,28. Further studies will be needed to reconcile our observation that CMP production rates are similar in young and old mice with the finding that inflammation stimulates myelopoiesis.
Recent in vivo barcoding experiments have shown that a myelo-erythroid arm and a lymphoid arm of haematopoiesis are retained when HSCs are barcoded at embryonic or young-adult stages58. Similar experiments in older mice may address whether the fates emerging in situ from old HSCs are different from those of young stem cells.
Concluding remarks
In this Opinion article, we propose that the analysis of old HSCs in irradiated recipients has led to an inaccurate picture of how ageing affects their developmental potential. Most of these studies have used γ-ray irradiators (with caesium137 and cobalt60), but X-ray irradiators are in increasing use. Transplantation models will still have applications, but the caveats associated with transplantation into irradiated hosts must be appreciated, particularly when comparing the productivity and developmental potential of young and old haematopoietic stem and progenitor cells. The use of busulfan provides one alternative method for conditioning recipients. Although we are not aware of other studies that have used this approach to study ageing haematopoiesis, several reports suggest that lymphoid output is robust in recipients conditioned with this agent50,51,74. However, differences in how busulfan is metabolized by individual recipients may lead to variability in conditioning and impact reconstitution efficiency. Thus, further work will be needed to standardize regimens for busulfan conditioning.
We propose that a complete understanding of how ageing affects haematopoiesis will depend on the analysis of intact, unmanipulated animals, such as through the use of in situ fate mapping, as we have presented here. An in situ fate-mapping approach has revealed that HSCs provide only low-level input to haematopoiesis, whereas progenitor cells, which yield only transient haematopoietic reconstitution after transplantation, are major drivers of blood cell production under unperturbed conditions19,54,58. Because many types of mutation originate or become fixed during DNA replication, HSCs should have comparatively fewer mutations than downstream progenitor cells, although to our knowledge this issue has not been examined experimentally to date. Thus, the slow, steady input from HSCs could serve to rejuvenate the genome of MPPs and to prevent or delay the emergence of leukaemia, in much the same way as input from bone marrow progenitor cells into the thymus prevents the development of T cell acute lymphoblastic leukaemia75. According to this view, lifelong maintenance of HSC output is a constituent of normal ageing, and its perturbation may predispose to disease. The observation that progenitors maintain steady-state haematopoiesis suggests that further analysis of downstream populations, not just of HSCs, will be of interest. Recent studies showing that ageing affects gene expression in and the function of lymphoid76 and myeloid18 progenitors are relevant in this regard. For example, Young et al.49 demonstrated that lymphoid-primed MPPs exhibited deficient expression of lymphoid-priming genes as a consequence of ageing, as well as a reduction in lymphoid differentiation potential. In addition, pro-B cells from old mice exhibit defects in proliferation and survival, compared with their young counterparts8, and express the senescence-associated gene Cdkn2a. CLPs express low (or undetectable) levels of Cdkn2a, suggesting that some age-related changes occur in B lineage-specified precursors, independent of those in upstream precursors76. Finally, the number of pro-B cells that express the transcription factor E47, which is required for normal B lymphopoiesis, declines with age77. Further studies will be needed to determine the degree to which these cell-intrinsic changes are triggered by the effects of the old environment.
Fate-mapping studies have demonstrated that myeloid bias is an inherent property of haematopoiesis, regardless of age; it is only further accentuated with age by a moderate decline in lymphopoiesis. This reduced production of lymphoid cells occurs even though the number of Ly-HSCs is not reduced in old mice46. The finding that Ly-HSCs function relatively normally when removed from the old environment suggests that the age-related decline in lymphocyte development, which on the basis of fate-mapping studies likely occurs at the MPP-to-CLP transition, is driven by environmental changes. This conclusion is consistent with a previous report that bone marrow cells from old mice efficiently generated B lineage cells in young recipients78. Inflammation may play an important role in this regard, but a recent study from our laboratory, showing that reducing the level of inflammation had no effect on the rejuvenation of lymphocyte development, suggests that other factors are also involved18. Identification of these factors will be critical to understanding how ageing affects declines in lymphopoiesis.
Acknowledgements
The authors’ research presented here was supported by grants to K.D. from the US National Institutes of Health (AG056480), Deutsche Forschungsgemeinschaft DFG-SFB 873-B11 to T.H. and H.-R.R., and European Research Council Advanced Grant 742883 and DFG Leibniz program to H.-R.R.
Glossary
- Barcoding
Genetic tagging of cells with large numbers of distinct, and ideally unique, labels. Originally this was achieved by analyses of highly diverse integration sites of viral DNA in the genomes of cells infected in vitro, followed by cell transplantation and tracking of barcodes in progeny. More recently, endogenous barcoding has been developed, in which cells can be tagged in whole organisms without the need for cell manipulations in vitro. Examples of endogenous techniques include CRISPR–Cas9-based methods, transposon integration site analysis and Cre-dependent Polylox barcoding. When the probability of induction of a given barcode is smaller than the target population, it becomes highly likely that single cells are being labelled. Hence, endogenous barcoding can reveal precursor–product relationships emerging from single stem cells and can provide insights into lineage relationships.
- Conditioning
The treatment of recipients before transplantation in order to create space in the bone marrow to allow for donor bone marrow cells to engraft. Conditioning regimens include drugs, such as 1,4-butanediol dimethanesulfonate (busulfan), and/or ionizing irradiation that kills endogenous stem and progenitor cells.
- Fate-mapping approaches
Experimental approaches in which a single genetic switch, usually lineage-specific or stage-specific and sometimes inducible, is used to turn on a heritable marker in stem or progenitor cells. All progeny that arise from labelled cells can be tracked, which yields information on precursor–product relationships and on the flow of differentiation. Combined with mathematical analysis and modelling, fate mapping can reveal in situ frequencies of differentiating stem cells and progenitors and differentiation rates, independent of the vagaries of cell transplantation. The inducible Cre systems referred to in this article include Tie2–MerCreMer mice, Pdzk1ip1–CreER mice and Fgd5–CreERT2 mice.
- Irradiation
Process in which a subject is exposed to radiation. Ionizing radiation can be delivered from caesium137 or cobalt60 γ-irradiators or X-ray devices. Both γ radiation and X-rays are highly penetrating and can travel into tissue, and as a result these forms of radiation are frequently used to deplete haematopoietic cells from the bone marrow before transplantation.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Immunology thanks F. Camargo, S. Morrison and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Geiger H, de Haan G & Florian M The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol 13, 376–389 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Berent-Maoz B & Dorshkind K Causes, consequences and reversal of immune system aging. J. Clin. Invest 123, 958–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang W et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley R, Blomberg B & Frasca D B cells, E2A, and aging. Immunol. Rev 205, 30–47 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Min H, Montecino-Rodriguez E & Dorshkind K Effects of aging on early B- and T-cell development. Immunol. Rev 205, 7–17 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Miller JP & Allman D The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol 171, 2326–2330 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Johnson KM, Owen K & Witte PL Aging and developmental transitions in the B cell lineage. Int. Immunol 14, 1313–1323 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Min H, Montecino-Rodriguez E & Dorshkind K Effects of aging on the common lymphoid progenitor to pro-B cell transition. J. Immunol 176, 1007–1012 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Dykstra B, Olthof S, Schreuder J, Ritsema M & De Haan G Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med 208, 2691–2703 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Haan G & Lazare S Aging of hematopoietic stem cells. Blood 131, 479–487 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Wahlestedt M, Pronk C & Bryder D Concise review: hematopoietic stem cell aging and the prospects for rejuvenation. Stem Cells Transl Med. 4, 186–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi DJ et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudo K, Ema H, Morita Y & Nakauchi H Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med 192, 1273–1280 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florian M et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann M et al. Heterogeneous responses of hematopoietic stem cells to inflammatory stimuli are altered with age. Cell Rep. 25, 2992–3005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maryanovich M et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med 24, 782–791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho Y et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiologic aging. Cell Stem Cell 25, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pioli P, Casero D, Montecino-Rodriguez E, Morrison S & Dorshkind K Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity 51, 351–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busch K & Rodewald H-R Unperturbed vs. post-transplantation hematopoeisis: both in vivo but different. Curr. Opin. Hematol 23, 295–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu R, Czechowica A, Seita J, Jiang D & Weissman I Clonal-level lineage commitment pathways of hematopoietic stem cells in vivo. Proc. Natl Acad. Sci. USA 116, 1447–1456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson F et al. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther 21, 868–876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xun C, Thompson J, Jennings C, Brown S & Widmer M Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood 83, 2360–2367 (1994). [PubMed] [Google Scholar]
- 23.Henry C et al. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J. Clin. Invest 125, 4666–4680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youshani A et al. Non-myeloablative busulfan chimeric mouse models are less pro-inflammatory than head-shielded irradiation for studying immune cell interactions in brain tumors. J. Neuroinflammation 16, 25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King K & Goodell M Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol 11, 685–692 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirantes C, Passegue E & Pietras E Pro-inflammatory cytokines: emerging players regulating HSC function in normal and diseased hematopoiesis. Exp. Cell Res 329, 248–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovtonyuk L, Fritsch K, Feng X, Manz M & Takizawa H Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front. Immunol 7, 502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldridge M, King K & Goodel M Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 32, 57–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietras E Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood 130, 1693–1698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda Y, Kondo M & Kelsoe G Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med 201, 1771–1780 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietras E et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol 18, 607–618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorshkind K Interleukin-1 inhibition of B lymphopoiesis is reversible. Blood 72, 2053–2055 (1988). [PubMed] [Google Scholar]
- 34.Ergen A, Boles N & Goodell M Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500–2509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy D & Knight K Inflammatory changes in bone marrow microenvironment associated with declining B lymphopoiesis. J. Immunol 198, 3471–3479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorshkind K IL-1 inhibits B cell differentiation in long term bone marrow cultures. J. Immunol 141, 531–538 (1988). [PubMed] [Google Scholar]
- 37.Maeda K et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood 106, 879–885 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takizawa H, Boettcher S & Manz M Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119, 2991–3002 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Fransceschi C, Garagnani P, Parini P, Giuliani C & Santoro A Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol 14, 576–590 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Schaue D, Kackikwu E & McBride W Cytokines in radiobiological responses: a review. Radiat. Res 178, 505–523 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller-Sieburg C, Cho R, Karlsson L, Huang J & Sieburg H Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood 103, 4111–4118 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Dykstra B et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218–229 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Beerman I et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Challen G, Boles N, Chambers S & Goodell M Distinct hematopoietic stem cells subtypes are differentially regulated by TGF-β1. Cell Stem Cell 6, 265–278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita Y, Ema H & Nakauchi H Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med 207, 1173–1182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montecino-Rodriquez E et al. Lymphoid biased hematopoietic stem cells are maintained with age and efficiently generate lymphoid progeny. Stem Cell Rep. 12, 584–596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho R, Sieburg H & Muller-Sieburg C A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not the individual stem cells. Blood 111, 5553–5561 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahlestedt M et al. Clonal reversal of ageing-associated stem cell lineage bias via a pluripotent intermediate. Nat. Commun 8, 14533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young K et al. Progressive alterations in multipotent hematopoietic progenitors underlie lymphoid cell loss in aging. J. Exp. Med 213, 2259–2287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeager A, Shinn C & Pardoll D Lymphoid reconstitution after transplantation of congenic hematopoietic cells in busulfan-treated mice. Blood 78, 3312–3316 (1991). [PubMed] [Google Scholar]
- 51.Hsieh M et al. Low-dose parenteral busulfan provides an extended window for the infusion of hematopoietic stem cells in murine hosts. Exp. Hematol 35, 1415–1420 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang Y, Van Zant G & Szilvassy S Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1480 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison SJ, Wandycz AM, Akashi K, Globerson A & Weissman IL The aging of hematopoietic stem cells. Nat. Med 2, 1011–1016 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Sun J et al. Clonal dynamics of native haematopoiesis. Nature 514, 322–327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gazit R et al. Fgd5 identifies hematopoietic stem cells in the murine bone marrow. J. Exp. Med 211, 1315–1331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busch K et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518, 542–546 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Sawai C et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals.Immunity 45, 597–609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pei W et al. Polylox barcoding reveals haematopoeitic stem cell fates realized in vivo. Nature 548, 456–460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oguro H, Ding L & Morrison S SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Säwen P et al. Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. eLife 7, e41258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hofer T, Busch K, Klapproth K & Rodewald H-R Fate mapping and quantitation of hematopoiesis in vivo. Annu. Rev. Immunol 34, 4490478 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Hofer T & Rodewald H-R Differentiation-based model of hematopoietic stem cell functions and lineage pathways. Blood 132, 1106–1113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito K et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354, 1156–1160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoedel K et al. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 128, 2285–2296 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Sheikh B et al. MOZ (KAT6A) is essential for the maintenance of classically defined adult hematopoietic stem cells. Blood 128, 2307–2318 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Lu R, Neff N, Quake S & Weissman I Tracking single hematopoietic stem cells in vivo using highthroughput sequencing in conjunction with viral genetic barcoding. Nat. Biotechnol 29, 928–933 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerrits A et al. Cellular barcoding tool for clonal analysis in the hematopoietic system. Blood 115, 2610–2618 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Geiger H, Denkiger M & Schimbeck R Hematopoietic stem cell aging. Curr. Opin. Immunol 29, 86–92 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Chapple R et al. Lineage tracing of murine adult hematopoietic stem cells reveals active contribution to steady-state hematopoiesis. Blood Adv. 2, 1220–1228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Upadhaya S, Reizis B & Sawai C New genetic tools for the in vivo study of hematopoietic stem cell function. Exp. Hematol 61, 26–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson A et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–11129 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Akashi K, Traver D, Miyamoto T & Weissman IL A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Kondo M, Weissman IL & Akashi K Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997). [DOI] [PubMed] [Google Scholar]
- 74.Anam K, Black A & Hale D Low dose busulfan facilitates chimerism and tolerance in a murine model. Transpl. Immunol 15, 199–204 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Martins V et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 509, 465–470 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Signer RAJ, Montecino-Rodriguez E, Witte ON & Dorshkind K Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 22, 3115–3120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King AM, Van der Put E, Blomberg BB & Riley RL Accelerated notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J. Immunol 178, 3521–3529 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Labrie JE, Sah AP, Allman DM, Cancro MP & Gerstein RM Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J. Exp. Med 200, 411–423 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geiger H & Van Zant G The aging of lympho-hematopoietic stem cells. Nat. Immunol 3, 329–333 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Sun D et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14, 673–688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chambers S et al. Aging hematopoietic stem cells decline in function and exhibit epigentic dysregulation. PLOS Biol. 5, e201 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kowalczyk M et al. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 25, 1860–1872 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grover A et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat. Commun 7, 11075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rundberg Nilsson A, Soneji S, Adolfsson S, Bryder D & Pronk C Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLOS ONE 11, e0158369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirschner K et al. Proliferation drives aging-related functional decline in a subpopulation of the hematopoietic stem cell compartment. Cell Rep. 19, 1503–1511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benz C et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10, 273–283 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Mohrin M et al. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen CY, Liu Y & Zheng P mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal 2, ra75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leins H et al. Aged murine hematopoietic stem cells drive aging-associated immune remodeling. Blood 132, 565–576 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghosh S, Twarri R, Dixit D & Sen E TNF α induced oxidative stress dependent Akt signaling affects actin cytoskeletal organization in glioma cells. Neurochem. Int 56, 194–201 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Gadea G et al. TNFα induces sequenctial activation of Cdc42- and p38/p53-dependent pathways that antagonistically regulate filopodia formation. J. Cell Sci 117, 6355–6364 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Razidlo G, Burton K & McNiven M Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J. Biol. Chem 293, 11143–11153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


