Supplemental Digital Content is Available in the Text.
Key Words: HIV/AIDS, task shifting, antiretroviral therapy, nurse, clinician
Background:
With countries moving toward the World Health Organization's “Treat All” recommendation, there is a need to initiate more HIV-infected persons into antiretroviral therapy (ART). In resource-limited settings, task shifting is 1 approach that can address clinician shortages.
Setting:
Uganda.
Methods:
We conducted a randomized controlled trial to test if nurse-initiated and monitored ART (NIMART) is noninferior to clinician-initiated and monitored ART in HIV-infected adults in Uganda. Study participants were HIV-infected, ART-naive, and clinically stable adults. The primary outcome was a composite end point of any of the following: all-cause mortality, virological failure, toxicity, and loss to follow-up at 12 months post-ART initiation.
Results:
Over half of the study cohort (1,760) was women (54.9%). The mean age was 35.1 years (SD 9.51). Five hundred thirty-three (31.6%) participants experienced the composite end point. At 12 months post-ART initiation, nurse-initiated and monitored ART was noninferior to clinician-initiated and monitored ART. The intention-to-treat site-adjusted risk differences for the composite end point were −4.1 [97.5% confidence interval (CI): = −9.8 to 0.2] with complete case analysis and −3.4 (97.5% CI: = −9.1 to 2.5) with multiple imputation analysis. Per-protocol site-adjusted risk differences were −3.6 (97.5% CI: = −10.5 to 0.6) for complete case analysis and −3.1 (−8.8 to 2.8) for multiple imputation analysis. This difference was within hypothesized margins (6%) for noninferiority.
Conclusions:
Nurses were noninferior to clinicians for initiation and monitoring of ART. Task shifting to trained nurses is a viable means to increase access to ART. Future studies should evaluate NIMART for other groups (e.g., children, adolescents, and unstable patients).
INTRODUCTION
Sub-Saharan Africa (SSA) has more than two-thirds of the global population of persons living with HIV (PLHIV) and the lowest rates of physicians per capita.1,2 Uganda has less than 2 medical doctors per 10,000 population. The Joint United Nations Program on HIV/AIDS embarked on a strategy to end the AIDS epidemic by 2030 with a target to achieve 90% of all PLHIV who know their status to be on antiretroviral therapy (ART) by 2020 and enhance this to 95% by 2030.3 Uganda is a low-income country with a generalized epidemic and a high prevalence of HIV (6.2%).4 Despite the absence of a formal task-shifting policy in health, the country has adopted the World Health Organization's (WHO) “Treat All” recommendation to initiate all HIV-infected persons into ART.5
WHO has recommended task shifting as a means to increase access to HIV/AIDS care services amid workforce shortages.6 Task shifting involves the movement of specific tasks, where appropriate, from highly trained to lower-level health care workers (HCWs) who receive shorter training and supportive supervision to accomplish the tasks.6 The US Institute of Medicine recommends task sharing, acknowledging a knowledge-based requirement for delegated roles and responsibilities and emphasizes collaboration of HCWs when providing care.7
Task shifting for ART in HIV-infected adults has been studied in SSA and found to be efficacious and cost-effective in a variety of settings. A systematic review of task shifting from doctors to nondoctors for the initiation and maintenance of ART found 4 randomized controlled trials (RCTs) and 6 cohort studies conducted in SSA.8–12 The review found only 1 RCT by Fairall et al,9 conducted in South Africa, that included ART initiation by nurses. The other 3 RCTs had all patients initiated on ART by doctors.10–12 Three of the RCTs compared nurse-led care to doctor-led care (standard of care).9,11,12 One RCT compared trained community health workers to doctors for ART maintenance.10 The review found high-quality evidence from RCTs indicating no difference in death and lower rates of loss to follow-up (LTFU) at 1 year among patients initiated and monitored on ART by trained, supported nurses when compared with those initiated and monitored by doctors.
Notably, the RCT that evaluated nurse-initiated and monitored ART (NIMART) excluded patients with CD4 <50 cells/µL, WHO clinical stage 4 AIDS, or who were pregnant, bed-ridden, on concomitant medication other than cotrimoxazole or vitamins, had a weight of ≤40 kg, or a body mass index of >28.9 In addition, these RCT results, conducted in the high-middle income setting of South Africa, may not be generalizable to low-income countries, such as Uganda. Thus, there is a need for more country-specific evidence to inform task-shifting policy for NIMART. We found only 1 small RCT (N = 85) conducted in Uganda by Kiweewa et al12 that compared nurse-maintained ART to physician-maintained ART among HIV-positive women at a prevention of mother-to-child transmission of HIV clinic; physician initiated ART for both study arms. We conducted a study that compared patients' outcomes between NIMART and clinician-initiated and monitored ART (CIMART).
METHODS
Study Design
We performed a parallel, unblinded RCT using a noninferiority design to test if NIMART is noninferior to CIMART for clinically stable, HIV-infected adults in Uganda (Sharing HIV/AIDS Responsibilities and Efforts, SHARE).
Study Population
We enrolled study participants from February 2015 to September 2016. Eligible patients were those who were HIV-infected, ART-naive, and clinically stable adults (18 years or older) eligible for ART according to the 2013 Ministry of Health (MoH) national HIV treatment guidelines.13
Participants were excluded if they had more than grade 3 (the 2009 US National Institutes of Health–Division of AIDS table for grading the severity of adult and pediatric adverse events) laboratory test results for hematology and liver and renal function tests (LFTs and RFTs); were unwilling or unable to give written informed consent; or resided outside a 40-km radius (more than 1-hour drive) from the study site or anticipated moving from their current residence (to beyond a 40-km radius) in the subsequent 12 months.14
Study Setting
The study was conducted in HIV treatment clinics at 8 of the 12 public regional referral hospitals (ie, Arua, Gulu, Lira, Mbale, Kabale, Fort Portal, Hoima, and Mubende).15 Lower-level facilities did not meet the staffing and laboratory criteria (see Supplemental Digital Content 1, http://links.lww.com/QAI/B571, site selection criteria).
Sample Size Calculation
The sample size calculation was based on showing noninferiority for a composite end point of treatment-limiting events comprising all-cause mortality, virological failure (VF), treatment-limiting ART toxic effects, and LTFU at 12 months.
We used a noninferiority margin of 6% based on results from a similar noninferiority study conducted by Sanne et al11 in South Africa. We set the failure proportion for the physician arm to 24% and that of the nurse arm to 30% with a noninferiority margin of 6% after 12 months of follow-up.
We computed a minimum sample size of 627 participants per arm using the Power Analysis and Sample Size software (PASS 13, NCSS, LLC, Kaysville, UT) with the following null hypothesis: the nurse arm was inferior to the clinician arm. We used a one-sided unpooled Z test, with a 5% level of significance and 80% power.
To account for any clustering effects, we considered a design effect of 1.25, and this yielded a sample size of 784 per arm. We adjusted for an attrition rate of 12% giving a recruitment target of 878 per arm.
Health Care Workers Selection and Training
Hospitals nominated HCWs from HIV treatment clinics for training. Nurses were registered nursing officers as per Uganda MoH (ie, diploma or university degree in nursing and licensed by the Uganda Nurses and Midwives Council),with at least 3 months of hands-on ART experience. Clinicians were either medical doctors (a 5-year university bachelor's degree in medicine and surgery, plus 1 year of internship and licensed by the Uganda Medical and Dental Practitioners Council) or clinical officers (3-year diploma in clinical medicine from an accredited paramedical school in Uganda and licensed by the Allied Health Professionals Council).
We obtained voluntary written consent from each participating HCW. Eligible HCWs received 1-week training in the WHO's Integrated Management of Adolescent and Adult Illnesses chronic HIV care module and the Uganda ART guidelines.13,16,17 Participating HCWs were also trained on the data collection tools.
Participant Recruitment
Study staff approached patients in the HIV clinic and provided them with study information. We obtained voluntary, written informed consent from each patient before screening them for the study.
HCWs in both study arms determined if the participant was eligible for ART.13 Determination of eligibility included the following: medical history, physical examination, complete blood count (CBC), LFTs (alanine and aspartate aminotransferases), RFTs (creatinine and blood urea nitrogen), CD4 count, and a checklist based on inclusion and exclusion criteria. Additional consent was obtained from eligible participants before their enrollment.
Randomization
We used simple randomization to allocate participants at each facility to either the NIMART (intervention) or CIMART (control) arm with an allocation probability of 0.5 for each arm. The randomization code was generated offsite, and assignments were sealed in opaque envelopes. After consenting for screening, participants were asked to select an envelope from a box containing the sealed envelopes. Subsequently, they were escorted to the HCW (nurse or clinician) to determine ART eligibility and study enrollment (see Supplemental Digital Content 2, http://links.lww.com/QAI/B571, eligibility checklist).
HIV Care Models and Patient Follow-Up
All participants had a baseline plasma HIV-1 RNA viral load (VL) performed at the MoH Central Public Health Laboratories using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, v2.0 (Roche Molecular Systems Inc, Pleasanton, CA) and received standardized antiretroviral (ARV) and cotrimoxazole prophylaxis regimens, HIV counseling, and support services as per the MoH guidelines.5,13 Each participant had monthly clinic visits scheduled for a period of 1 year, whereby they underwent a clinical assessment including a self-reported pill count, to gauge their medication adherence and received monthly medication refills. To monitor treatment success and safety, each participant had a VL test, CBC, LFTs, RFTs, and CD4 counts scheduled at 6 and 12 months post-ART initiation.
Although participants were required to remain in the same treatment arm throughout their participation, they were not necessarily attended to by the same HCW at each follow-up visit. To avoid crossovers, participants began and ended their visit in the study office and staff guided them through the clinician and nurse rooms, laboratory, and pharmacy. In addition, study staff used color-coded participant files and arranged them by arm ahead of the visits. HCWs were free to consult with study and nonstudy providers on the management of participants. For safety purposes, the protocol provided for irreversible crossovers from the nurse to the clinician arm when a participant failed first-line ART (part of the composite end point).
Data Collection
All site study staff received training on the protocol, good clinical practice, and human subjects' protection. At the baseline visit, each participant was assigned a unique enrollment number. Staff recorded participant data on case report forms and then scanned and faxed them into a central database (NIH DataFax).
Data Analysis Plan
The primary study outcome was a composite end point of any of the following: all-cause mortality, VF, treatment-limiting toxic effects, and LTFU at 12 months post-ART initiation. We defined VF as 2 successive VL measures of ≥1000 copies/mL at 6 or more months after ART initiation, taken at least 4 weeks apart. Toxicity failure was defined as any study participant in whom treatment was discontinued or changed because of intolerance of the prescribed medicines. We defined LTFU (according to the Current PEPFAR Monitoring, Evaluation and Reporting Indicator reference guide (MER 2.0) version 2.3 September 2018, LFTU is defined as patient not receiving ARVs within 4 weeks (28 days) of their last missed drug pick-up.) as a participant's failure to return for care for 3 consecutive scheduled monthly visits.
We examined the composite end point at 6 and 12 months since ART initiation. Because of challenges with incomplete VL testing, VF at 12 months was redefined post hoc to include VL ≥ 1000 copies/mL at 12 months since ART start or having VL ≥ 1000 copies/mL at 6 months if VL measurement was missing at 12 months. VL data were considered missing if VL at both 6 and 12 months were unavailable (see Supplemental Digital Content 3, http://links.lww.com/QAI/B571, study protocol).
Statistical Methods
We performed statistical analysis using STATA version 14.2 (StataCorp LLC, College Station, TX) software. Participants' characteristics were summarized as frequencies and percentages, for categorical factors, and compared across study arms using the Pearson χ2 test. Continuous factors were summarized as mean and SDs and median and interquartile ranges.
The primary end point analyses were based on both intention-to-treat (ITT) and per-protocol (PP) analysis. Noninferiority was established only if both analyses (PP and ITT) supported it.18,19 The proportions of participants with composite outcome and individual event, that is, VF, toxicity failure, LTFU, and death, were determined and compared between the 2 study arms. Statistical noninferiority was concluded when the upper bound of the 97.5% confidence interval (CI) limits of the difference in the proportion of composite outcome between the 2 study arms was within less than or equal to a 6% margin. Risk differences and their 97.5% CIs between the nurse and clinician arms were determined using a binomial generalized linear regression model with an identity link function, with site included as a fixed effects term to control for confounding.
The ITT population included all participants randomized but excluded those randomized in error. We excluded 74 participants who were HIV stage 3 or 4 at enrollment and thus ineligible. The PP analysis included all participants in the ITT population but excluded those who withdrew consent, were discontinued by investigators because of protocol noncompliance, or migrated out of the study area. Data on primary end point (composite outcome) were missing for 17% (clinician arm = 18%, nurse arm = 17%) of patients. We performed multiple imputation analysis (MIA) of missing values on the composite end point in both ITT and PP, as sensitivity analysis to examine the impact of missingness on comparison with the primary end point between the 2 arms. MIA was performed using the chained equations method, with 30 imputations. We selected employment, age, religion, disclosure of HIV status, and site as auxiliary variables in the imputation models. This was because of their association with missingness on the composite outcome and thus made missing at random assumption more plausible, desired for using multiple imputation. Study arm and gender were also included as auxiliary variables because of their inherent importance.
Finally, we performed a secondary analysis of incidence and time-to-event for a composite outcome of either death, LTFU, or ARV-associated-toxicity, with survival time censored at either withdraw or closure of follow-up (12 months), and the comparison of study arms was performed using Kaplan–Meier graphs and log-rank test. Notwithstanding, secondary analysis excluded VF because it was measured at predetermined time points.
Ethical Considerations
The study received ethical approval from the Uganda Virus Research Institute and the Centers for Disease Control and Prevention institutional review board. Regulatory approval was obtained from the Uganda National Council for Science and Technology. The study was registered on the US National Library of Medicine website ClinicalTrials.gov (Identifier: NCT02417636) and used an independent Data and Safety Monitoring Board.
RESULTS
A total of 1974 participants consented to screening and were randomized (Fig. 1). Of those screened, 1760 (89.2%) were enrolled in the study. Of the 214 participants that were not enrolled, 137 (64.0%) did not meet study eligibility criteria (see Supplemental Digital Content 4, http://links.lww.com/QAI/B571, reasons for ineligibility by arm), 42 (19.6%) were eligible but did not return for the enrollment visit, 21 (9.8%) declined to consent for enrollment, and 11 (5.1%) did not complete the screening process because of breakdown of site laboratory equipment. In addition, 2 (1.0%) left the site before completing the screening visit procedures and 1 (0.5%) was eligible but the study had fully enrolled by the time they returned to the clinic. Seventy-four participants (40 in the clinician arm and 34 in the nurse arm) who were WHO stage 3 or 4 and thus ineligible were excluded from the ITT and PP analyses. Another 68 (30 in the clinician arm and 38 in the nurse arm) were excluded from the PP analysis because of migration out of the study area, withdraw of consent, and protocol noncompliance. Subsequently, 1686 participants (845 in the clinician arm and 841 in the nurse arm) were included in the ITT analysis while 1618 (815 in the clinician arm and 803 in the nurse arm) were included in PP analysis.
FIGURE 1.
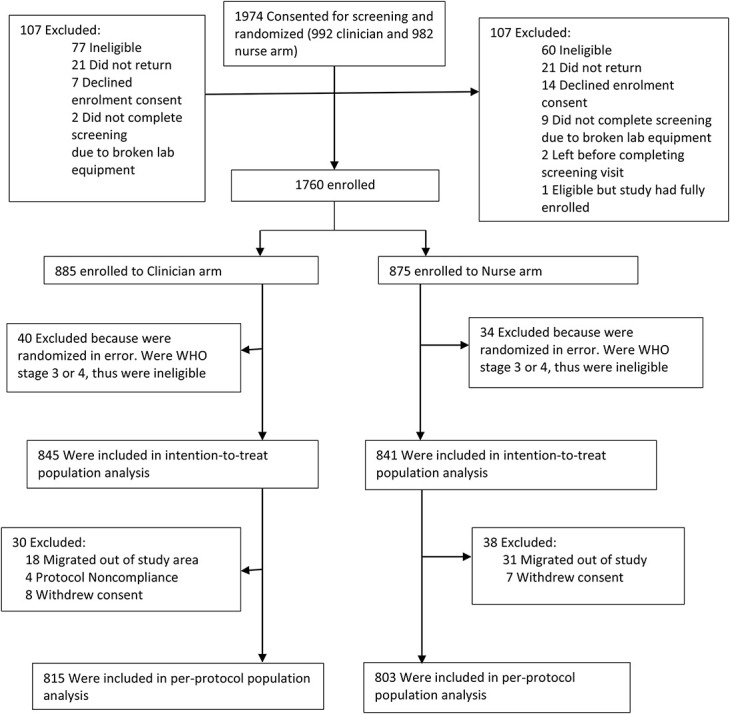
Participant flow.
Baseline characteristics are shown in Table 1 (see Supplemental Digital Content 5, http://links.lww.com/QAI/B571, ART regimens by arm). More than half of the study cohort was women (54.9%), with a mean age of 35.1 years (SD 9.51). Less than 5% of the study participants had advanced HIV disease (WHO clinical stage III and IV). The median baseline VL was 38,924 copies/mL (interquartile range 7428–131640). One hundred eighty-three (10.4%) participants had a baseline VL of <1000 copies/mL. Forty-six (2.6%) participants had an undetectable baseline VL. The 2 arms were generally similar at baseline save for 7.4% participants in the nurse arm compared with 3.7% in the clinician arm with Karnofsky scores of <90%.
TABLE 1.
Participant Baseline Characteristics
| Characteristic | Clinician (N = 885) | Nurse (N = 875) | Total (N = 1760) |
| Age (mean, SD) | 34.9 (9.14) | 35.3 (9.88) | 35.1 (9.51) |
| Gender | |||
| Female | 487 (55.0%) | 479 (54.7%) | 966 (54.9%) |
| Male | 398 (45.0%) | 396 (45.3%) | 794 (45.1%) |
| Education | |||
| None | 67 (7.6%) | 67 (7.7%) | 134 (7.6%) |
| Primary | 491 (55.5%) | 485 (55.4%) | 976 (55.5%) |
| Secondary or higher | 327 (36.9%) | 323 (36.9%) | 650 (36.9%) |
| Marital status | |||
| Single | 126 (14.2%) | 124 (14.2%) | 250 (14.2%) |
| Married/Cohabiting | 498 (56.3%) | 505 (57.7%) | 1003 (57.0%) |
| Divorced/Separated/Widowed | 261 (29.5%) | 246 (28.1%) | 507 (28.8%) |
| HIV disclosure at baseline (yes) | 848 (95.8%) | 846 (96.7%) | 1694 (96.3%) |
| WHO staging | |||
| Stage I or II | 845 (95.5%) | 841 (96.1%) | 1686 (95.8%) |
| Stage III or IV | 40 (4.5%) | 34 (3.9%) | 74 (4.2%) |
| CD4 (cells/µL) | |||
| Mean (SD) | 322.0 (160.4) | 304.0 (145.9) | 314.1 (153.53) |
| Categories | |||
| <100 | 99 (11.2%) | 100 (11.4%) | 199 (11.3%) |
| 101–249 | 177 (20.0%) | 187 (21.4%) | 364 (20.7%) |
| 250–349 | 173 (19.5%) | 194 (22.2%) | 367 (20.9%) |
| 350–499 | 403 (45.5%) | 372 (42.5%) | 775 (44.0%) |
| >500 | 31 (3.5%) | 19 (2.2%) | 50 (2.8%) |
| Missing* | 2 (0.2%) | 3 (0.3%) | 5 (0.3%) |
| Karnofsky score | |||
| Mean (SD) | 96.6 (5.62) | 95.1 (6.67) | 95.9 (6.21) |
| Categories | |||
| Below 90 | 33 (3.7%) | 65 (7.4%) | 98 (5.6%) |
| 90 and above | 841 (95.0%) | 807 (92.2%) | 1648 (93.6%) |
| Missing | 11 (1.2%) | 3 (0.3%) | 14 (0.8%) |
| Baseline VL copies/mL | |||
| Median | 35,504 | 42,058 | 38,924 |
| Interquartile range | 6180–110476 | 8930–142156 | 7428–131640 |
| Mean (SD) | 137548.1 (376961.9) | 165110.3 (424783.9) | 151085.6 (401267.9) |
| Categories | |||
| Undetected (<20) | 23 (2.6%) | 23 (2.6%) | 46 (2.6%) |
| 21 to <1000 | 77 (8.7%) | 60 (6.9%) | 137 (7.8%) |
| 1000 to 99,999 | 471 (53.2%) | 423 (48.3%) | 894 (50.8%) |
| >100,000 | 206 (23.3%) | 244 (27.9%) | 450 (25.6%) |
| Missing | 108 (12.2%) | 125 (14.3%) | 233 (13.2%) |
| Baseline VL log (base e) copies/mL | |||
| Median (IQR) | 10.5 (8.7–11.6) | 10.7 (9.1–11.9) | 10.6 (8.9–11.8) |
| Mean (SD) | 9.9 (2.59) | 10.2 (2.50) | 10.1 (2.55) |
Five participants who were either breastfeeding, pregnant, or a seropositive partner in a serodiscordant couple did not have a baseline CD4 and were determined to be eligible for the study.
ARV, antiretroviral; ; IQR, interquartile range, VL, viral load.
Five hundred thirty-three (31.6%) participants experienced the study end point (ITT). These include 404 (24%) LTFU, 94 (5.6%) VFs, 19 (1.1%) deaths, and 16 (0.95%) toxicity failures (Table 2).
TABLE 2.
Risk, Risk Differences, and Their 97.5% CI of Primary End Points at 12 months
| Endpoints | Clinician (MD) (N = 845), Number (%) | Nurse (NS) (N = 841), Number (%) | Risk Difference (NS-MD), (Point Estimate, 97.5% CI) |
| Individual Outcomes | |||
| Died | 8/845 (0.9) | 11/841 (1.3) | 0.4 (−1.1 to 0.9) |
| LTFU | 213/845 (25.2) | 191/841 (22.7) | 1.7 (−1.9 to 4.9) |
| Toxicity | 8/845 (0.9) | 8/841 (0.9) | −0.1 (−1.1 to 0.9) |
| VF* | 46/548 (8.4) | 48/570 (8.4) | 0.4 (−3.3 to 4.0) |
| Composite outcome | |||
| Intention-to-treat analysis (complete cases analysis) | 259/692 (37.4) | 233/699 (33.3) | −4.1 (−9.8 to 0.2) |
| Intention-to-treat analysis (MI) | 314/845 (37.2) | 284/841 (33.8) | −3.4 (−9.1 to 2.5) |
| Per-protocol analysis | 257/685 (37.5) | 232/685 (33.9) | −3.6 (−10.5 to 0.6) |
| Per-protocol analysis (MI) | 303/815 (37.2) | 274/803 (34.1) | −3.1 (−8.8 to 2.8) |
Risk difference = Risk in nurse arm − Risk among clinician arm.
Total individual events may not add up to total composite events because a patient could have multiple events, thus were counted once. Seventy-four participants in WHO stage 3/4 enrolled in error, thus excluded from both intention-to-treat and per-protocol analyses.
VF = virological failure.
Missing data; VF: (clinician = 297/845, nurse = 271/841), composite (clinician = 153/845, nurse = 142/841).
At 12 months, the ITT site-adjusted risk differences for the composite end point were −4.1 (97.5% CI: = −9.8 to 0.2) with complete case analysis and −3.4 (97.5% CI: = −9.1 to 2.5) with MIA. The PP site-adjusted risk differences were −3.6 (97.5% CI: = −10.5 to 0.6) for complete case analysis and −3.1 (−8.8 to 2.8) for MIA. Notably, at 12 months, the upper 97.5% CI for the ITT and PP analyses was below the hypothesized noninferiority margin of 6% (Table 2 and Fig. 2).
FIGURE 2.
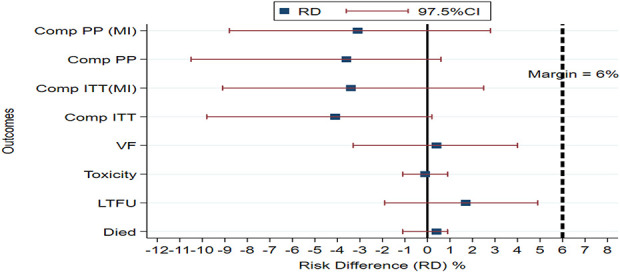
Site-adjusted risk difference for end points and 97.5% CIs between nurse arm and clinician arm at 12 months. Dotted vertical line indicates the a priori noninferiority margin (<6%). Composite PP analysis (Comp PP), composite PP analysis with multiple imputation [Comp PP (MI)], composite intention-to-treat analysis (Comp ITT), composite intention-to-treat analysis with multiple imputation [Comp ITT (MI)], and individual outcomes (VF, toxicity, death, and LTFU).
At 6 months, the ITT site-adjusted risk difference for the composite outcome was 2.1 (97.5% CI: −3.5 to 7.7). Notably, at 6 months, the upper 97.5% CI is above the hypothesized noninferiority margin of 6% (see Supplemental Digital Content 6, http://links.lww.com/QAI/B571, outcomes at 6 months). There was no statistically significant difference between the 2 arms for composite and individual events (death, LTFU, toxicity, and viral nonsuppression).
Overall, the Kaplan–Meier survival graphs and log-rank test indicated no statistically significant difference between the 2 arms for the time to the composite end point of death, LTFU, or ARV toxicity—as shown in Fig. 3.
FIGURE 3.
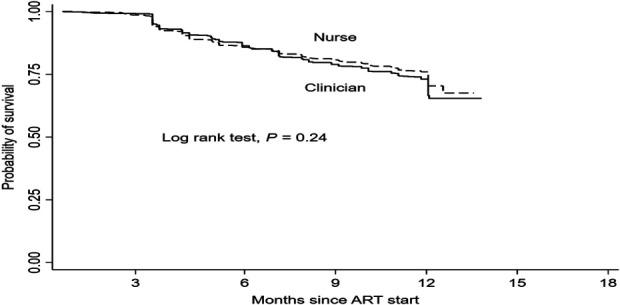
Kaplan–Meier survival from composite events. Time to first event was about 9 days in clinician arm and 14 days in nurse arm. Event included death, LTFU, and toxicity, but not VF.
DISCUSSION
The results demonstrate that NIMART is not inferior to CIMART in the key patient outcomes achieved after initiation and monitoring of ART among stable, HIV-infected adults. The findings show that noninferiority was achieved by 12 months, but not at 6 months post-ART initiation. It is likely that participating nurses were acquiring knowledge and competencies in their new role and thus were more likely to become noninferior to clinicians over time.
Results are supported by findings from observational studies and trials on the effectiveness of task shifting for ART delivery in resource-limited settings.8 Sanne et al11 demonstrated noninferiority for mortality and LTFU at 12 months. Fairall et al found no difference in mortality (in a superiority analysis) and viral suppression (<400 copies/mL, equivalence analysis) at 12 months, and a lower LTFU at 12 months in the nurse arm.9 Both the Sanne and Kiweewa studies found noninferiority for VF (>1000 copies/mL and >400 copies/mL, respectively) at 12 months.11,12
These results add to the existing body of knowledge that supports the use of NIMART to increase access to ART as a key strategy to achieve epidemic control.3 The results also support the 2016 Consolidated Guidelines for Prevention and Treatment of HIV in Uganda in which nurses are allowed to initiate and follow adults, although they are not permitted to manage complicated cases, such as cryptococcal meningitis, ART regimen switches, and initiation of tuberculosis (TB) treatment based on chest radiograph interpretation.5
Although NIMART has been shown to be noninferior to CIMART, previous research has found gaps in training, mentoring, and competency among nurses managing PLHIV in East, Central, and Southern Africa. Naikoba et al20 showed that one-on-one mentoring of clinical officers, registered nurses, and midwives improves knowledge, competency, and efficacy in HIV and TB indicators of facility performance in Uganda. A focus group on task shifting in Uganda by Spies et al21 found that nurses experienced a lack of appropriate and consistent support, factors that lead to reduced performance. In South Africa, Jones et al found low rates of mentoring for nurses participating in NIMART.22 Binagwaho and Eyal both recommended that physicians roles should evolve into specialized medicine and skills in mentorship, research, and management.23,24 However, medical doctor shortages preclude implementation unless carefully planned. Previous studies have also found NIMART to be acceptable to patients and health care providers. Assefa et al25 found evidence of patient acceptability and satisfaction with NIMART in terms of reduced waiting time and more comfortable and friendlier interactions with health workers. Patients noted that nurses gave them more time to discuss their health problems. In a focus group discussion, they also found that health care providers agreed that nurses can provide high-quality ART when given adequate training and supervision. Humphreys et al26 also found evidence of patient satisfaction in terms of reduced transport costs, better care, and shorter queues with decentralized ART maintenance from doctors at hospitals to nurses at health centers.
There were study limitations. Notably, 10.4% of participants had a baseline VL of <1000 copies/mL and, thus, were not likely to have been ART-naive. These participants are almost evenly distributed between the 2 arms and are unlikely to have significantly affected the overall result. In addition, although nurses who participated in the study received additional training, further studies are needed to define a generalizable training and supervisory support framework for NIMART. Furthermore, the HCWs who participated in the study may have been different from those that did not in terms of perceived ability, availability, and willingness to participate. Although such a selection bias could potentially limit the generalizability of our findings, it is unlikely to have differed by study arm. Similarly, the patients who chose to take part in our study might have been healthier, more educated, of a higher socioeconomic status, and more likely to adhere to treatment than those who declined to join hence potentially affecting the generalizability of the results. However, such an occurrence would still not affect the internal validity of the study.
Despite continuous engagement with HCWs and site laboratory teams, the study witnessed incomplete VL testing. This, in part, reflects the challenges with VL testing at the public hospitals where the study was based. We however performed 2 sensitivity analyses that included multiple imputation of the outcome in both the ITT and PP analyses.
Although the 2 arms were comparable for LTFU at 12 months, the study rate of LTFU of 24% is higher than the 13.1% reported in the July 2016 to June 2017 Uganda HIV/AIDS country progress report.27 However, routine data reports likely underestimate the true LTFU rate.
Although regional referral hospitals provided a favorable setting to execute the study, their staffing levels, training, supervisory and mentorship support, and laboratory capacity are different from those of lower-level facilities. Therefore, our findings may be less generalizable to nurses working at peripheral facilities; they may need more intensive training and support than was provided in our study. More so with more nurses operating at peripheral units than at regional hospitals. Lufuno et al have identified continuous training, enhanced support supervision, and improved relationships with colleagues as important factors for NIMART-trained nurses to adhere to treatment guidelines.28
Overall, this study demonstrates that NIMART can be successfully deployed in resource-limited settings for clinically stable, HIV-infected adults. With task shifting, doctors may have more time to attend to more complex HIV patients and supervise nonclinical staff who are attending to stable patients. Our findings should be used by the MoH to develop a formal task-shifting policy in support of NIMART to meet the demand for HIV treatment services in the context of the ongoing shortages of clinicians and the need for mentoring nonclinical staff. There is need for countries to implement a task-sharing policy to ensure collaboration among HCWs in the care and management of PLHIV. Task sharing may also be helpful in generating more patients and community support as well as a better supervisory and regulatory framework for NIMART.
In conclusion, this study shows that nurses were not inferior in initiating and monitoring ART in HIV-infected stable adults in a low-income country. Future studies should evaluate the role of nurses for other patients (children, adolescents, and unstable adults), particularly in differentiated service delivery models where clinic visits and pharmacy refills are less frequent. Future studies should also generate country-specific evidence on the supervisory and regulatory framework needed for NIMART. Given the high rate of LTFU and VF, more study is needed to identify those at risk of these end points.
ACKNOWLEDGMENTS
The authors thank the SHARE study staff, participating patients, nurses, clinicians, and hospital teams for their time and efforts. All the authors contributed to the study design, study conduct, data analysis and interpretation, or manuscript writing.
Footnotes
Supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention cooperative agreement number 3U2GPS002918-04S1 Revised.
The authors have no conflicts of interest to disclose.
Substantial contributions to conception and design (B.J.T., K.A., Musinguzi J., B.B., D.S., A.S.B., E.M.R., and S.S.) or acquisition of data (A.S.B., N.M.S., B.E., M.P.N., K.A., and S.E.), or analysis and interpretation of data (Musaazi J., A.N.K., M.P.N., S.S., A.S.B., N.M.S., K.A., B.E., E.M.R., D.S., and B.B.). Drafting the paper or revising it critically for important intellectual content (S.A.B., B.E., E.M.R., S.S., M.P.N., N.M.S., A.N.K., Musaazi J., Musinguzi J., S.E., B.B., D.S., B.J.T., and K.A.). Final approval of the version to be published (A.S.B., B.E., E.M.R., S.S., M.P.N., N.M.S., A.N.K., Musaazi J., Musinguzi J., S.E., B.B., D.S., B.J.T., and K.A.).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or other funding agencies.
REFERENCES
- 1.UNAIDS. Global HIV and AIDS statistics. Available at: http://unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed March 06, 2019; 2018. [Google Scholar]
- 2.The 2017 update, Global Health Workforce Statistics: World Health Organisation; 2017. Available at: http://who.int/hrh/statistics/hwfstats/. Accessed March 06, 2019. [Google Scholar]
- 3.UNAIDS. Fast-Track Ending the AIDS Epidemic by 2030. 2014. Available at: http://unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed March 06, 2019. [Google Scholar]
- 4.Ministry of Health. Uganda Population-Based HIV Impact Assessment (UPHIA) 2016-2017: Final Report. Kampala, Uganda: Ministry of Health; 2019. [Google Scholar]
- 5.Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Ministry of Health; 2016. [Google Scholar]
- 6.World Health Organisation. Task Shifting: Global Recommendations and Guidelines. Available at: https://who.int/workforcealliance/knowledge/resources/taskshifting_guidelines/en. Accessed July 15, 2019; 2008. [Google Scholar]
- 7.Quinn TC, Serwadda D. The future of HIV/AIDS in Africa: a shared responsibility. Lancet. 2011;377:1133–1134. [DOI] [PubMed] [Google Scholar]
- 8.Kredo T, Adeniyi FB, Bateganya M, et al. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. Cochrane Database Syst Rev. 2014:Cd007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffar S, Amuron B, Foster S, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiweewa FM, Wabwire D, Nakibuuka J, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. J Acquir Immune Defic Syndr. 2013;63:e125–32. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health. Addendum to the National Antiretroviral Treatment Guidelines. Kampala, Uganda: Ministry of Health; 2013. [Google Scholar]
- 14.National Institutes of Health. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0, December 2004. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 15.Online List of Regional Referral Hospital. Available at: http://health.go.ug/affiliated-institutions/hospitals/regional-referral-hospitals. Accessed March 06, 2019. [Google Scholar]
- 16.World Health Organization. Chronic HIV Care with ARV Therapy and Prevention–Integrated Management of Adolescent and Adult Illnesses Interim Guidelines for Workers at Health Centre and District Hospital Outpatient Clinic. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 17.Ministry of Health. Uganda IMAI District Clinician Manual: Hospital Care for Adolescents and Adults. Kampala, Uganda; 2014. [Google Scholar]
- 18.Snapinn S. Noninferiority trials. Curr Control Trial Cardiovasc Med. 2000;1:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head SJ, Kaul S, Bogers AJ, et al. Non-inferiority study design: lessons to be learned from cardiovascular trials. Eur Heart J. 2012;33:1318–1324. [DOI] [PubMed] [Google Scholar]
- 20.Naikoba S, Senjovu KD, Mugabe P, et al. Improved HIV and TB knowledge and competence among mid-level providers in a cluster-randomized trial of one-on-one mentorship for task shifting. J Acquir Immune Defic Syndr. 2017;75:e120–e127. [DOI] [PubMed] [Google Scholar]
- 21.Spies LA, Gray J, Opollo J, et al. HIV and nurses: a focus group on task shifting in Uganda. J Assoc Nurses AIDS Care. 2016;27:312–321. [DOI] [PubMed] [Google Scholar]
- 22.Jones M, Cameron D. Evaluating 5 years' NIMART mentoring in South Africa's HIV treatment programme: successes, challenges and future needs. S Afr Med J. 2017;107:839–842. [DOI] [PubMed] [Google Scholar]
- 23.Binagwaho A, Sarriera G, Eagan A. The evolution of the physician role in the setting of increased non-physician clinicians in sub-saharan Africa: an insistence on timing and culturally-sensitive, purposefully selected skill development; comment on “non-physician clinicians in sub-saharan Africa and the evolving role of physicians”. Int J Health Pol Manag. 2017;6:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyal N, Cancedda C, Kyamanywa P, et al. Non-physician clinicians in sub-saharan Africa and the evolving role of physicians. Int J Health Pol Manag. 2015;5:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assefa Y, Kiflie A, Tekle B, et al. Effectiveness and acceptability of delivery of antiretroviral treatment in health centres by health officers and nurses in Ethiopia. J Health Serv Res Pol. 2012;17:24–29. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys CP, Wright J, Walley J, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: a controlled prospective study in Swaziland. BMC Health Serv Res. 2010;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uganda AIDS Commission. Uganda HIV & AIDS Country Progress Report July 2016–June 2017. Kampala, Uganda: Uganda AIDS Commission; 2017. [Google Scholar]
- 28.Makhado L, Davhana-Maselesele M, Lebese RT, et al. Factors facilitating trained NIMART nurses' adherence to treatment guidelines: a vital matter in the management of TB/HIV treatment in South Africa. BMC Nurs. 2020;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]


